Abstract
Meat is one of the major food groups which is frequently adulterated for economic reasons. In line with halal certification, the presence of non-halal meats in food products must be identified. Mislabelling and undeclaring meat types are typically seen in meat-based products such as meatballs, burgers, sausages and Salami. Therefore, the application of analytical methods for the detection, identification, and confirmation of non-halal meats is a must. This chapter highlighted the application of spectroscopic and chromatographic-based methods combined with several chemometrics for the identification and confirmation of non-halal meats in food products. Spectroscopy offered more accelerating methods with higher accuracy rates throughout the screening process for non-halal meats. Further confirmation can be done using chromatography by identifying specific markers present in analyzed non-halal meats.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
5.1 Introduction
Meat is considered one of the most commonly consumed foods worldwide due to the nutritional composition found in meats, especially protein. Meats contain essential healthy nutrients and are an excellent source of protein (Hassoun et al., 2020). From an Islamic perspective, meats could be classified as halal-meats that are allowed to be consumed and non-halal meats that are prohibited to be consumed according to Syariah law (Islamic jurisprudence) (Rohman & Windarsih, 2020).
With the increased awareness among the Muslim population to only consume halal and tayyib (good or pure) foods, the demand for halal food products also increased, emplacing a huge responsibility on the government, jurisprudence and manufacturers to assure the halalness of food products through halal certifications (Martuscelli et al., 2020). Indonesia has the regulation (Indonesian Act) No. 33 established in 2014 that mandates a halal certification for all halal products which are declared halal. Indeed, meat is one of most essential food components in the food industry and due to the big discrepancy between halal and non-halal meats, some unethical producers intentionally replace halal with non-halal meat (Nakyinsige et al., 2012).
At present, it is estimated that the Muslim population will increase from 1.8 billion in 2014 to 2.2 billion by 2030, with an approximate increased growth of 26.4%. The Muslim population occupies a quarter part of the world; therefore, it is not surprising if the Halal market exhibited a lucrative and significant impact on international business (Adiarni & Fortunella, 2018). However, there are several issues regarding Halal as non-halal meats either because of their source (such as pork) or from the way the animals were slaughtered. Disputes regarding the halal status of animal sources typically arise from thoughts’ scholar (madzhab) to be followed by Muslim communities (Rohman et al., 2020), while debates on the halal status of various slaughtering methods arise due to the possibility that animals’ slaughtering processes did not meet the Halal requirements determined by the Syariah law (Ali et al., 2020).
5.2 Meat’s Authentication Using Spectroscopic and Chromatographic-Based Methods
In recent years, consumers have become more concerned about the quality, halalness, and safety of meat-based food products, especially the traceability and authenticity of meat sources (Hassoun et al., 2020). The analytical methods for meat-species identification and detection of adulteration are always needed for quality control and the safety of consumers. The Gold standard method used for halal meat authentication is a type of DNA-based methods, especially analytical methods applying polymerase chain reaction (PCR) with its development and revolution, including real-time PCR using primer species-specific, TaqMan probe and multiplex (Ali et al., 2014; Erwanto et al., 2018a, 2018b; Fajardo et al., 2010; Kumar et al., 2015; Rodríguez-Ramírez et al., 2011). However, PCR techniques involve more complex steps such as starting primer selection, denaturation of DNA, primer annealing and amplification. Therefore, a range of uncomplicated and quick methods were developed and used for analyzing non-halal meats in food products. Some reviews on the application of molecular spectroscopic (Esteki, Shahsavari, et al., 2018; Li et al., 2019; Rodriguez-Saona & Allendorf, 2011) and chromatographic methods (Abbas et al., 2018; Bosque-Sendra et al., 2012; Esteki, Simal-Gandara, et al., 2018) for food authentication and traceability have existed. In this chapter, two groups of methods (spectroscopy and chromatography) were critically assessed for the authentication analysis of non-halal meats in fresh and meat-based food products.
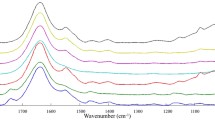
Among the molecular spectroscopic-based methods, Fourier transform infrared (FTIR) spectroscopy is the most applied technique for the analysis of non-halal meats either in fresh meats or meats in food-based products. The analysis of raw meats and meat-based foods is done by extracting the lipid fractions of meats using several extraction methods and then subjecting them to FTIR spectral measurement (Rohman, 2019). Typical FTIR spectra of lipid components extracted from meats are depicted in Fig. 5.1. The intensity is represented by absorbance values (not in transmittance mode) because absorbance can contain quantitative information according to Lambert–Beer law. Each peak and shoulder with specific wavenumbers in FTIR spectra represented functional groups present in lipids which can be correlated with compounds or a group of compounds composing the lipid. The main components of lipids extracted from meats which is represented by functional groups of methyl- (CH3-) present in wavenumbers (1/λ) of 2953 cm−1 (str asymmetric), 2875 cm−1 (str symmetric), 1376 cm−1 (ben), methylene (-CH2-) at 1/λ 2922 cm−1 (str asymmetric), 2853 cm−1 (str symmetric), 1462 cm−1 (ben), carbonyl- (C = O) at 1741 cm−1 (str) 1654 cm−1 corresponding to cis C = C str, 1417 cm−1 from = C–H cis disubstituted olefins str, and C–O (ether) at 1/λ 1117 and 1098 cm−1, 965 cm−1 due to -HC = CH-(trans) in which str stands for stretching, while ben stands for bending vibration (Lerma-García et al., 2010).
The peaks and shoulders in FTIR spectra are rather complex, and they are used as variables for extracting the qualitative and quantitative information regarding the presence and the quantity of certain meats in food products. Therefore, data management using chemometrics was normally used (Nunes, 2014). Table 5.1 compiled the application of different spectroscopic techniques used for the analysis of pork fresh meat or in meat-based food products.
5.3 Analysis of Non-halal Meats in Fresh and Meat-Based Food Products
There are some non-halal meats that are not allowed to be consumed by Muslim communities, namely, pork (pig meat), wild boar meat (WBM), dog meat (DM), monkey meat, cat meat, snake meat, as well as meats from some amphibians and other animals which live in the water and on the ground such as frogs and crocodiles (Ceranic & Bozinovic, 2009).
5.3.1 Analysis of Pork in Fresh and Meat-Based Food
Pork is the most used non-halal meat in food products, and the analytical methods intended for detecting pork in raw components in the mixture with other meats and in meat-based food products such as hamburger, meatballs, and sausages are widely reported. Near-infrared (NIR), mid-infrared (MIR) spectroscopy, gas chromatography and liquid chromatography in combination with some chemometrics techniques have been validated and applied in the analysis of pork for halal authentication. MIR spectra combined with PLS-Kernel have been successfully used for quantifying pork in beef with a limit of detection reaching up to 1.4% wt/wt. The chemometrics of the PLS-Kernel algorithm exhibited good performance results for treating many variables in complex spectral data due to the lower memory storage needed with fewer computation cycles. The absorbance values at wavenumbers of 1900–900 cm−1 were used as variables while preparing the calibration curve for the relationship between actual values and MIR-PLS Kernel predicted values, resulting in the statistical performance of R2 of 0.9994 and a relative error of prediction (REP) of 3.7%. High R2 and low REP indicated that MIR spectroscopy combined with PLS-Kernel provided an accurate and precise technique for quantifying pork (non-halal meat) in beef (halal meat) for halal authentication purposes (Abu-Ghoush et al., 2017).
Fourier transform near-infrared spectroscopy (FT-NIR) used in combination with chemometrics of PCA, Partial Least-Squares Discriminant Analysis (PLS-DA) and PLSR has been currently used for the analysis of pork in other meats (lamb, chicken, mutton, beef, camel). PCA was utilized for exhibiting the similarities and differences of pork and other types of meat. PLS-DA was used for the discrimination of pork and other types of meats, while the multivariate calibration of PLSR was applied for the prediction of pork levels in the mixture with other meats. This study involved a large number of samples (>5900 samples from different countries) scanned at wavenumbers of 10,000–4000 cm−1. PCA using full NIR spectra that resulted in PC-1 and PC-2 explaining 87 and 8% of the total spectral variability. From PC1 and PC2 curves, it is clear that PCA revealed a complete segregation between pork and other types of meat. All pork samples were grouped in two different categories corresponding to fresh pork and treated pork (dried and smoked pork samples). PLS-DA could distinguish pork from other meats successfully where the lowest level of pork that could be detected is 10%. The prediction of pork levels in the mixture with other meats was optimized using full NIR spectra preprocessed with standard normal variate (SNV) and unit vector normalization transformation. This better model showed a good correlation between actual values and FT-NIR predicted values with R2 of 0.9774, RMSECV of 1.08% and RMSEP of 1.835%. From these results, it is evident that the combination of NIR spectra and chemometrics of PCA, PLS-DA and PLSR is an effective mechanism for the authentication analysis of distinguishing meats from non-halal meat containing pork (Mabood et al., 2020).
Infrared spectroscopy in mid-region (MIR) combined with chemometrics of PCA, SIMCA and PLS has statistically reported that fats extracted from pork (pig meat) are used as an adulterant in pure ghee (heat clarified milk fat) either qualitatively or quantitatively. Optimizing wavenumbers is capable of providing a good classification using PCA and SIMCA. The combined wavenumbers region of 3030–2785, 1786–1680, and 1490–919 cm−1 which exhibit strong intensities (absorbances) were utilized for these analytical tasks. SIMCA, using this combined region could classify lard, pure ghee and lard mixed with ghee with efficiency of 100%. PCA, using PC1 accounting of 92% and PC2 accounting of 8% variances applying absorbance values at 3030–2785 cm−1 as variables, was capable of clustering pure pork, pure ghee (cow and buffalo), and pure ghee adulterated with pork fat (lard) with different concentrations as shown in Fig. 5.2. The presence of lard of about 5% in pure ghee revealed a clear separation, while lard with a lower rate of 5% (especially 3%) displayed an overlapping separation. The wavenumbers used for PCA were also successfully applied for the prediction of lard in pure ghee with R2 for the correlation between the actual and FTIR predicted value of > 0.99 and a detection limit of 3% (Upadhyay et al., 2018).
PCA based on principal component (PC1) and second principal component (PC2) using absorbance values of 3030–2785 cm−1 for clustering pure pork (PBF), pure mutton ghee (PMG), and PMG adulterated with PBF with different concentrations. Adopted from (Upadhyay et al., 2018)
Raman spectroscopy combined with PCA is also a successful approach for a swift identification of pork meat and other meats (sheep, cattle, fish, goat, poultry, and buffalo) and in salami products in a shorter period of time (about 30 s) after the extraction of fats from the corresponding meats. PCA was performed using variables of absorbance values at 200–2000 cm−1. The first two PCs (PC1 and PC2) having the highest variances with 65.86 and 20.98% were chosen, and the plotting of PC1 and PC2 was capable of identifying pork and other meats in Salami products (Boyaci et al., 2014).
5.3.2 Analysis of Wild Boar Meat as Meat Adulterants
Currently, Wild boar meat, which is similar to pork, has been reported to be used as an adulterant in beef meatballs in Indonesia and Malaysia. Therefore, FTIR spectroscopy is developed for the identification and quantification of WBM in meatballs. The selection of fingerprint regions in FTIR spectra was typically carried out before modeling using chemometrics techniques. Some studies usually involve the addition of WBM into beef meatballs with varying concentrations. PCA using PC1 and PC2 based on absorbance values at combined wavenumbers of 999–1481 and 1650–1793 cm−1 has successfully distinguished WBM and beef meatballs. In addition, PLSR using the first derivative spectra at the combined wavenumbers of 999–1481 and 1650–1793 cm−1 could predict the levels of WBM with R2 value of 0.9991, RMSEC of 1.028%, and RMSECV of 0.30%, respectively. This result indicated that FTIR spectroscopy combined with PCA and PLSR is effective for authenticating halal meatballs from WBM (Ahda et al., 2020).
5.3.2.1 Analysis of Frog Meats
Frog meat (FM) is a popular food also known as “swike” (in Indonesia and Malaysia) and is considered a non-halal meat according to madzhab Syafi’i. FTIR spectroscopy using unsupervised pattern recognition of PCA was applied for fingerprinting profiling of fats extracted from FM and other animal fats, marine oils and vegetable oils. PCA using PC1 and PC2, which are accounting for 88 and 7% variances, could classify fats extracted from FM and others with a distinct separation. The absorbance values at wavenumbers of 2922 (corresponding to -CH2-), 2853 (CH2-), 1745 (carbonyl), 1158 cm−1 (C–O), and 721 cm−1 (cis–CH = CH) were considered as the most discriminating variables that are capable of separating fats extracted from FM and others, as explained in the loading plot. This fundamental study has clear implications on the identification of FM fat from its marine and vegetable oils for the potential detection of FM adulteration in various fat-based food products (Ali et al., 2015).
5.3.2.2 Analysis of Dog Meat
Dog meat (DM) is occasionally added into beef meatballs intentionally or unintentionally. FTIR spectroscopy in combination with PLSR is used for the quantification of DM in beef meatballs. During the preparation of the prediction and validation samples, DM was added into beef meatballs in the range of 0–100% wt/wt. All samples were subjected to fat extraction using the Folch method applying chloroform–methanol (2:1 v/v) as extracting solvents, and the lipid extracts obtained were scanned at 4000–650 cm−1. PLSR applying absorbance values at combined wavenumbers region of 1782–1623 and 1485–659 cm−1 using normal spectra with a high accuracy as indicated by high R2 either in the calibration model (0.993) or in the validation model (0.995). The errors were also low in both calibration (RMSEC of 1.63%) and in cross validation using the leave-one-out technique with a RMSECV of 2.68%. FTIR spectroscopy combined with PLSR provided an accurate and precise technique for the quantitative analysis of DM in beef meatballs (Rahayu, Rohman, et al., 2018).
The classification and quantification of DM in beef sausages have been carried out. Based on the optimization in terms of FTIR spectra model and wavenumbers region, the authors discovered that the normal spectra at wavenumbers of 1124–688 cm−1 was suitable for the quantification of DM, which produced the equation of predicted value = 0.9999 × actual value + 0.0004, with statistical parameters of R2 of 0.9999, RMSEC of 0.30%, RMSEP of 0.05% and RMSECV of 0.05%. Based on PCA results, samples 1 and 5 (sausages obtained from a commercial market) had the closest point to that of DM (Guntarti & Ayu Purbowati, 2019).
Rahayu et al. have compared two extraction techniques, namely Bligh-Dyer and Folch methods for fat extraction from dog meat in meatballs formulation. The lipids obtained were scanned in FTIR spectrophotometer at the mid-IR region (4000–650 cm−1). FTIR spectral bands correlated with fats of beef, dog and the mixture of DM and beef were analyzed. The small variations among spectra were utilized as basic tools to differentiate between DM and beef. PCA using variables of absorbance values at 1700–700 cm−1 was capable of identifying DM in meatballs (Rahayu, Martono, et al., 2018).
5.3.2.3 Analysis of Rat Meat
Our group has reported the application of FTIR spectroscopy combined with chemometrics of PCA and PLSR for qualitative (identification) and quantitative analysis of rat meat (RM) in beef meatballs. Lipids in meatballs were extracted using Soxhlet, applying hexane as an extracting solvent according to the AOAC method. Lipids components scanned at mid-IR region (4000–650 cm−1) were classified using PCA for grouping the meatballs composed of beef and rat meat. In this study, some wavenumber regions were optimized for getting to acquire the best classification and prediction models. Finally, absorbances at wavenumbers of 750–1000 cm−1 were preferred for PCA PLSR. PCA using PC1 and PC2 could classify beef and rat meatballs. In addition, for the quantitative analysis, the R2 value showing the correlation between the actual values and FTIR predicted values was 0.993 with the obtained equation for such relationship of predicted value = 0.9417 × actual value + 2.8410 with RMSEC of 1.79% (Rahmania et al., 2015).
Assisted with chemometrics of PCA and multivariate calibrations, FTIR spectroscopy is reported to have been used for the identification and quantification of rat meat (RM) in food products such as sausages. Some lipid extraction techniques were carried out during this task, including Bligh and Dyer, Folch, and Soxhlet. The lipid extracted was subjected to FTIR measurement at the wavenumbers region of 4000–650 cm−1. PCA was successfully applied for the classification of fats extracted from RM sausages and fats extracted with different extraction methods from beef sausages using variables of FTIR spectral absorbances at 1/λ 1800–750 cm−1 with PC1 and PC2 contribution and Soxhlet was 97.57% and 1.28% (Bligh and Dyer), 85.50 and 10.64% (Folch) and 97.86 and 2.02% (Soxhlet). In addition, quantitative analysis of RM in sausages extracted using three extraction techniques revealed good accuracy results as indicated by a high coefficient of determination (R2) between actual values of RM prepared in sausages and FTIR predicted values, namely using the Soxhlet method. The results were 0.945 (Bligh and Dyer), 0.991 (Folch), and 0.992 (Soxhlet). The precision method was also acceptable as indicated by the low values of error with RMSEC of 2.73, 1.73 and 1.69%, respectively, using Bligh and Dyer, Folch, and Soxhlet. In validation models using sausage samples extracted by Folch and Soxhlet, the R2 values and RMSEP values obtained were 0.458 and 18.90% (Folch), and 0.983 and 4.21 (Soxhlet). Guntarty et al. also used a similar method (FTIR spectra combined with PCA and PLSR) for the identification and quantification of RM in meatball samples using absorbance values at wavenumbers of 1800–750 cm−1. The extraction method used was Soxhlet using hexane as an extracting solvent (Guntarti & Prativi, 2017). Meatballs from RM and beef could also be differentiated using FTIR spectroscopy combined with two unsupervised pattern recognitions of PCA and cluster analysis employing variables of absorbance values at 4000–400 cm−1 (Rosyidi & Khamidinal, 2019).
The analysis of fatty acid composition using gas chromatography with a flame ionization detector exhibited significant differences between the contents of fatty acids in fats extracted from beef and rat meat sausages. The contents of fatty acids of C12:0, C16:0, C16:1 cis 9, and C18:0 in rat lipid were higher than those in beef lipid, and the contrary was observed for fatty acids of C14:1 cis 9, C15:0, C17:0, C17:1 cis 10, unsaturated C18, and C21:0. The difference in fatty acid compositions can be used as complementary data for determining the presence of RM in food products (Pebriana et al., 2017). Fatty acid compositions determination by gas chromatography with a mass spectrometric detector combined with PCA's chemometrics were successfully applied for the differentiation of fats extracted from RM and other fats from pigs, cows, chickens, wild boars, dogs, and goats. The dominant fatty acids extracted from Wistar RM were oleate (40.48%), followed by linoleate (30.14%), palmitate (19.08%), stearate (2.55%), palmitoleate (0.73%) and myristate (0.1509%) (Guntarti et al., 2020). A similar procedure was also applied for the analysis of fats extracted from RM strain Sprague Dawley (Guntarti et al., 2021) and black rats (Utami et al., 2018). The combination of FTIR spectroscopy and fatty acid composition could be an effective method for detecting the adulteration of rat meat into beef meatballs (Damayanti et al., 2020).
5.3.2.4 Analysis of Donkey Meat
In China, the adulteration of donkey meat products with a similar species, namely horse meat is becoming a widespread concern. Therefore, the availability of analytical methods for detecting donkey meat is a must (Wang et al., 2020). The analysis of donkey meat as an adulterant in beef meatballs was carried out using FTIR spectroscopy combined with chemometrics of hierarchical cluster analysis (HCA). Before being used for clustering, FTIR spectra were subjected to some spectral treatments. Finally, FTIR spectra were preprocessed using first derivatization, first derivatization + vector normalization and vector normalization at wavenumbers region of 1480–1425 cm−1 which were capable of distinguishing donkey-adulterated beef meatballs, donkey meatballs and beef meatballs with 100% sensitivity and specificity (Candoğan et al., 2020).
The combination of near-infrared (NIR) spectroscopy and chemometrics has been used for differentiating donkey meat (167 samples) from different parts of the donkey’s body, beef, pork and mutton with reflectance models at wavenumbers of 12,500–4000 cm−1. The chemometrics of soft independent modeling of class analogy (SIMCA) and least squares-support vector machine (LS-SVM) were applied. Some NIR spectral treatments, including Savitzky-Golay smoothing and derivative spectra (first and second-derivative spectra), multiplicative scatter correction and standard normal variate, were compared and used. LS-SVM using the first 6PCs and SIMCA with the first 8PCs could accurately classify donkey meat and other meats with an accuracy of 100% in the calibration set and 98.96% in prediction sets (LS-SVM) and 100% in calibration and 97.53% in prediction sets (SIMCA). These results indicated that NIR spectra combined with chemometrics methods offered fast and reliable tools for distinguishing DM from other meats (Xiao-ying et al., 2014).
References
Abbas, O., Zadravec, M., Baeten, V., Mikuš, T., Lešić, T., Vulić, A., Prpić, J., Jemeršić, L., & Pleadin, J. (2018). Analytical methods used for the authentication of food of animal origin. Food Chemistry, 246(October 2017), 6–17. https://doi.org/10.1016/j.foodchem.2017.11.007.
Abu-Ghoush, M., Fasfous, I., Al-Degs, Y., Al-Holy, M., Issa, A. A., Al-Reyahi, A. Y., & Alshathri, A. A. (2017). Application of mid-infrared spectroscopy and PLS-Kernel calibration for quick detection of pork in higher value meat mixes. Journal of Food Measurement and Characterization, 11(1), 337–346. https://doi.org/10.1007/s11694-016-9402-4.
Adiarni, N., & Fortunella, A. (2018). The analysis of halal assurance system implementation (HAS 23000) in fried chicken flour product: A case study on XXX brand. In (Icosat 2017) (pp. 57–61). https://doi.org/10.2991/icosat-17.2018.13.
Ahda, M., Guntarti, A., Kusbandari, A., & Melianto, Y. (2020). Authenticity analysis of beef meatball adulteration with wild boar using FTIR spectroscopy combined with chemometrics. Journal of Microbiology, Biotechnology and Food Sciences, 9(5), 937–940. https://doi.org/10.15414/jmbfs.2020.9.5.937-940.
Ali, M. E., Nina Naquiah, A. N., Mustafa, S., & Hamid, S. B. A. (2015). Differentiation of frog fats from vegetable and marine oils by Fourier Transform Infrared Spectroscopy and chemometric analysis. Croatian Journal of Food Science and Technology, 7(1), 1–8. https://doi.org/10.17508/cjfst.2015.7.1.03.
Ali, M. E., Razzak, M. A., & Hamid, S. B. A. (2014). Multiplex PCR in species authentication: Probability and prospects—A review. Food Analytical Methods, 7(10), 1933–1949. https://doi.org/10.1007/s12161-014-9844-4.
Ali, N. S. M., Zabidi, A. R., Manap, M. N. A., Zahari, S. M. S. N. S., & Yahaya, N. (2020). Identification of metabolite profile in halal and non-halal broiler chickens using Fourier-transform infrared spectroscopy (FTIR) and ultra high performance liquid chromatography-time of flight-mass spectrometry (UHPLC-TOF-MS). Malaysian Applied Biology, 49(3), 87–93.
Bosque-Sendra, J. M., Cuadros-Rodríguez, L., Ruiz-Samblás, C., & de la Mata, A. P. (2012). Combining chromatography and chemometrics for the characterization and authentication of fats and oils from triacylglycerol compositional data-A review. Analytica Chimica Acta, 724, 1–11. https://doi.org/10.1016/j.aca.2012.02.041.
Boyaci, I. H., Uysal, R. S., Temiz, T., Shendi, E. G., Yadegari, R. J., Rishkan, M. M., Velioglu, H. M., Tamer, U., Ozay, D. S., & Vural, H. (2014). A rapid method for determination of the origin of meat and meat products based on the extracted fat spectra by using of Raman spectroscopy and chemometric method. European Food Research and Technology, 238(5), 845–852. https://doi.org/10.1007/s00217-014-2168-1.
Candoğan, P. D. K., Deniz, E., Güneş Altuntaş, E., Iğci, N., & Özel Demiralp, D. (2020). SiğirEti̇ Karişimlarinda Domuz, At Ve EşekEtTağşi̇şi̇ni̇n Fourier DönüşümlüKizilötesiSpektroskopi̇si̇İlBeli̇rlenmesi̇. Gida/The Journal of Food, 45, 369–379. https://doi.org/10.15237/gida.gd19146.
Ceranic, S., & Bozinovic, N. (2009). Possibilities and significance of has implementation (Halal assurance system) in existing quality system in food industry. Biotechnology in Animal Husbandry, 25(3–4), 261–266. https://doi.org/10.2298/bah0904261c.
Damayanti, A., Djalil, A. D., Susanti, A. T., Apriani, B., & Astuti, I. Y. (2020). Analysis of rat adulteration in beef meatball using Fourier transform infrared spectroscopy and gas chromatography-mass spectrometry for halal authentication. In IRCPAS/2020/OP-219, 8916 (Ircpas).
Erwanto, Y., Rohman, A., Arsyanti, L., & Pranoto, Y. (2018a). Identification of pig DNA in food products using polymerase chain reaction (PCR) for halal authentication—A review. International Food Research Journal, 25(4), 1322–1331.
Erwanto, Y., Rohman, A., Arsyanti, L., & Pranoto, Y. (2018b). Use of polymerase chain reaction to test for presence of pig derivatives in halal authentication studies. International Food Research Journal, 25(August), 1322–1331.
Esteki, M., Shahsavari, Z., & Simal-Gandara, J. (2018). Use of spectroscopic methods in combination with linear discriminant analysis for authentication of food products. Food Control, 91, 100–112. https://doi.org/10.1016/j.foodcont.2018.03.031.
Esteki, M., Simal-Gandara, J., Shahsavari, Z., Zandbaaf, S., Dashtaki, E., & Vander Heyden, Y. (2018, April). A review on the application of chromatographic methods, coupled to chemometrics, for food authentication. Food Control, 93, 165–182. https://doi.org/10.1016/j.foodcont.2018.06.015.
Fajardo, V., González Isabel, I., Rojas, M., García, T., & Martín, R. (2010). A review of current PCR-based methodologies for the authentication of meats from game animal species. Trends in Food Science and Technology, 21(8), 408–421. https://doi.org/10.1016/j.tifs.2010.06.002.
Guntarti, A., & Ayu Purbowati, Z. (2019). Analysis of dog fat in beef sausage using FTIR (Fourier Transform Infrared) combined with chemometrics. Pharmaciana, 9(1), 21–28. https://doi.org/10.12928/pharmaciana.v%vi%i.10467.
Guntarti, A., Gandjar, I. G., & Jannah, N. M. (2020). Authentication of wistar rat fats with gas chromatography mass spectometry combined by chemometrics. Potravinarstvo Slovak Journal of Food Sciences, 14(December 2019), 52–57. https://doi.org/10.5219/122910.5219/1229.
Guntarti, A., Martono, S., Yuswanto, A., & Rohman, A. (2015). FTIR spectroscopy in combination with chemometrics for analysis of wild boar meat in meatball formulation. Asian Journal of Biochemistry, 10(4). https://doi.org/10.3923/ajb.2015.165.172.
Guntarti, A., Ningrum, K. P., Gandjar, I. G., & Salamah, N. (2021). Authentication of sprague dawley rats (Rattus norvegicus) fat witH GC-MS (Gas chromatography-mass spectrometry) combined with chemometrics. International Journal of Applied Pharmaceutics, 13(2), 1–6. https://innovareacademics.in/journals/index.php/ijap/article/view/40130.
Guntarti, A., & Prativi, S. R. (2017). Application method of Fourier Transform Infrared (FTIR) combined with chemometrics for analysis of rat meat (Rattus Diardi) in meatballs beef. Pharmaciana, 7(2), 133. https://doi.org/10.12928/pharmaciana.v7i2.4247.
Hassoun, A., Måge, I., Schmidt, W. F., Temiz, H. T., Li, L., Kim, H. Y., Nilsen, H., Biancolillo, A., Aït-Kaddour, A., Sikorski, M., Sikorska, E., Grassi, S., & Cozzolino, D. (2020). Fraud in animal origin food products: Advances in emerging spectroscopic detection methods over the past five years. Foods, 9(8). https://doi.org/10.3390/foods9081069.
Jiang, H., Ru, Y., Chen, Q., Wang, J., & Xu, L. (2021). Near-infrared hyperspectral imaging for detection and visualization of offal adulteration in ground pork. Spectrochimica Acta—Part A: Molecular and Biomolecular Spectroscopy, 249, 1–9. https://doi.org/10.1016/j.saa.2020.119307.
Kumar, A., Kumar, R. R., Sharma, B. D., Gokulakrishnan, P., Mendiratta, S. K., & Sharma, D. (2015). Identification of species origin of meat and meat products on the DNA basis: A review. Critical Reviews in Food Science and Nutrition, 55(10), 1340–1351. https://doi.org/10.1080/10408398.2012.693978.
Kurniawati, E., Rohman, A., & Triyana, K. (2014). Analysis of lard in meatball broth using Fourier transform infrared spectroscopy and chemometrics. Meat Science, 96(1), 94–98. https://doi.org/10.1016/j.meatsci.2013.07.003.
Kuswandi, B., Cendekiawan, K. A., Kristiningrum, N., & Ahmad, M. (2015). Pork adulteration in commercial meatballs determined by chemometric analysis of NIR Spectra. Journal of Food Measurement and Characterization, 9(3), 313–323. https://doi.org/10.1007/s11694-015-9238-3.
Kuswandi, B., Putri, F. K., Gani, A. A., & Ahmad, M. (2015). Application of class-modelling techniques to infrared spectra for analysis of pork adulteration in beef jerkys. Journal of Food Science and Technology, 52(12), 7655–7668. https://doi.org/10.1007/s13197-015-1882-4.
Lamyaa, M. A. (2013). Discrimination of pork content in mixtures with raw minced camel and buffalo meat using FTIR spectroscopic technique. International Food Research Journal, 20(3), 1389–1394.
Lerma-García, M. J., Ramis-Ramos, G., Herrero-Martínez, J. M., & Simó-Alfonso, E. F. (2010). Authentication of extra virgin olive oils by Fourier-transform infrared spectroscopy. Food Chemistry, 118(1), 78–83. https://doi.org/10.1016/j.foodchem.2009.04.092.
Li, Q., Chen, J., Huyan, Z., Kou, Y., Xu, L., Yu, X., & Gao, J. M. (2019). Application of Fourier transform infrared spectroscopy for the quality and safety analysis of fats and oils: A review. In Critical reviews in food science and nutrition (Vol. 59, Issue 22, pp. 3597–3611). Taylor and Francis Inc. https://doi.org/10.1080/10408398.2018.1500441.
Mabood, F., Boqué, R., Alkindi, A. Y., Al-Harrasi, A., Al Amri, I. S., Boukra, S., Jabeen, F., Hussain, J., Abbas, G., Naureen, Z., Haq, Q. M. I., Shah, H. H., Khan, A., Khalaf, S. K., & Kadim, I. (2020). Fast detection and quantification of pork meat in other meats by reflectance FT-NIR spectroscopy and multivariate analysis. Meat Science, 163(September 2019), 108084. https://doi.org/10.1016/j.meatsci.2020.108084.
Martuscelli, M., Serio, A., Capezio, O., & Mastrocola, D. (2020). Meat products, with particular emphasis on salami: A review. Foods, 9, 1–19.
Morsy, N., & Sun, D. W. (2013). Robust linear and non-linear models of NIR spectroscopy for detection and quantification of adulterants in fresh and frozen-thawed minced beef. Meat Science, 93(2), 292–302. https://doi.org/10.1016/j.meatsci.2012.09.005.
Nakyinsige, K., Man, Y. B. C., & Sazili, A. Q. (2012). Halal authenticity issues in meat and meat products. Meat Science, 91(3), 207–214. https://doi.org/10.1016/j.meatsci.2012.02.015.
Nunes, C. A. (2014). Vibrational spectroscopy and chemometrics to assess authenticity, adulteration and intrinsic quality parameters of edible oils and fats. Food Research International, 60, 255–261. https://doi.org/10.1016/j.foodres.2013.08.041.
Pebriana, R. B., Rohman, A., Lukitaningsih, E., & Sudjadi. (2017). Development of FTIR spectroscopy in combination with chemometrics for analysis of rat meat in beef sausage employing three lipid extraction systems. International Journal of Food Properties, 20(Supplement 1), 1995–2005. https://doi.org/10.1080/10942912.2017.1361969.
Rady, A., & Adedeji, A. (2018). Assessing different processed meats for adulterants using visible-near- infrared spectroscopy. Meat Science, 136(October 2017), 59–67. https://doi.org/10.1016/j.meatsci.2017.10.014.
Rahayu, S. W., Martono, S., Sudjadi, S., & Rohman, A. (2018). The potential use of infrared spectroscopy and multivariate analysis for differentiation of beef meatball from dog meat for Halal authentication analysis. Journal of Advanced Veterinary and Animal Research, 5(3), 307–314. https://doi.org/10.5650/jos.ess15294.
Rahayu, W. S., Rohman, A., Martono, S., & Sudjadi. (2018). Application of FTIR spectroscopy and chemometrics for halal authentication of beef meatball adulterated with dog meat. Indonesian Journal of Chemistry, 18(2), 376–381. https://doi.org/10.22146/ijc.27159.
Rahmania, H., Sudjadi, & Rohman, A. (2015). The employment of FTIR spectroscopy in combination with chemometrics for analysis of rat meat in meatball formulation. Meat Science, 100, 301–305.
Rodríguez-Ramírez, R., González-Córdova, A. F., & Vallejo-Cordoba, B. (2011). Review: Authentication and traceability of foods from animal origin by polymerase chain reaction-based capillary electrophoresis. Analytica Chimica Acta, 685(2), 120–126. https://doi.org/10.1016/j.aca.2010.11.021.
Rodriguez-Saona, L. E., & Allendorf, M. E. (2011). Use of FTIR for rapid authentication and detection of adulteration of food. Annual Review of Food Science and Technology, 2(1), 467–483. https://doi.org/10.1146/annurev-food-022510-133750.
Rohman, A. (2019). The employment of Fourier transform infrared spectroscopy coupled with chemometrics techniques for traceability and authentication of meat and meat products. Journal of Advanced Veterinary and Animal Research, 6(1). https://doi.org/10.5455/javar.2019.f306.
Rohman, A., Himawati, A., Triyana, K., Sismindari, & Fatimah, S. (2017). Identification of pork in beef meatballs using Fourier transform infrared spectrophotometry and real-time polymerase chain reaction. International Journal of Food Properties, 20(3), 654–661. https://doi.org/10.1080/10942912.2016.1174940.
Rohman, A., & Windarsih, A. (2020). The application of molecular spectroscopy in combination with chemometrics for halal authentication analysis: A review. International Journal of Molecular Sciences, 21(14), 1–18. https://doi.org/10.3390/ijms21145155.
Rohman, A., Windarsih, A., Erwanto, Y., & Zakaria, Z. (2020). Review on analytical methods for analysis of porcine gelatine in food and pharmaceutical products for halal authentication. Trends in Food Science and Technology, 101(May), 122–132. https://doi.org/10.1016/j.tifs.2020.05.008.
Rosyidi, N. N., & Khamidinal. (2019). Analisis Lemak Bakso Tikus dalam Bakso Sapi di Sleman Menggunakan Spektroskopi Inframerah (Fourier Transform Infrared). Indonesian Journal of Halal Science, 001(01), 18–29.
Sari, T. N. I., & Guntarti, A. (2018). Wild boar fat analysis in beef sausage using Fourier Transform Infrared method (FTIR) combined with chemometrics. Jurnal Kedokteran Dan Kesehatan Indonesia, 9(4), 16–23.
Schmutzler, M., Beganovic, A., Böhler, G., & Huck, C. W. (2015). Methods for detection of pork adulteration in veal product based on FT-NIR spectroscopy for laboratory, industrial and on-site analysis. Food Control, 57, 258–267. https://doi.org/10.1016/j.foodcont.2015.04.019.
Silva, L. C. R., Folli, G. S., Santos, L. P., Barros, I. H. A. S., Oliveira, B. G., Borghi, F. T., dos Santos, F. D., Filgueiras, P. R., & Romão, W. (2020, April). Quantification of beef, pork, and chicken in ground meat using a portable NIR spectrometer. Vibrational Spectroscopy, 111. https://doi.org/10.1016/j.vibspec.2020.103158.
Upadhyay, N., Jaiswal, P., & Jha, S. N. (2018). Application of attenuated total reflectance Fourier Transform Infrared spectroscopy (ATR–FTIR) in MIR range coupled with chemometrics for detection of pig body fat in pure ghee (heat clarified milk fat). Journal of Molecular Structure, 1153, 275–281. https://doi.org/10.1016/j.molstruc.2017.09.116.
Utami, P. I., Rahayu, W. S., Nugraha, I., & Rochana, A. N. (2018). Fatty acid analysis of lipid extracted from rats by gas chromatography-mass spectrometry method. IOP Conference Series: Materials Science and Engineering, 288(1). https://doi.org/10.1088/1757-899X/288/1/012115.
Vichasilp, C., & Poungchompu, O. (2014). Feasibility of detecting pork adulteration in halal meatballs using near infrared spectroscopy (NIR). Chiang Mai University Journal of Natural Sciences, 13(1), 497–507. https://doi.org/10.12982/cmujns.2014.0052.
Wang, D., Wang, L., Xue, C., Han, Y., Li, H., Geng, J., & Jie, J. (2020). Detection of meat from horse, donkey and their hybrids (mule/hinny) by duplex real-time fluorescent PCR. PLoS One, 15(12 December), 1–9. https://doi.org/10.1371/journal.pone.0237077.
Xiao-ying, N., Li-min, S., Fang, D., Zhi-lei, Z., & Yan, Z. (2014). Discrimination of donkey meat by NIR and chemometrics. Spectroscopy and Spectral Analysis, 10, 1. http://en.cnki.com.cn/Article_en/CJFDTOTAL-GUAN201410032.htm.
Xu, L., Cai, C. B., Cui, H. F., Ye, Z. H., & Yu, X. P. (2012). Rapid discrimination of pork in Halal and non-Halal Chinese ham sausages by Fourier transform infrared (FTIR) spectroscopy and chemometrics, 92, 506–510. https://doi.org/10.1016/j.meatsci.2012.05.019.
Yang, L., Wu, T., Liu, Y., Zou, J., Huang, Y., Babu, S. V., & Lin, L. (2018). Rapid identification of pork adulterated in the beef and mutton by infrared spectroscopy. Journal of Spectroscopy, 2018. https://doi.org/10.1155/2018/2413874.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Rohman, A., Fadzillah, N.A. (2021). Application of Spectroscopic and Chromatographic Methods for the Analysis of Non-halal Meats in Food Products. In: Amid, A. (eds) Multifaceted Protocols in Biotechnology, Volume 2. Springer, Cham. https://doi.org/10.1007/978-3-030-75579-9_5
Download citation
DOI: https://doi.org/10.1007/978-3-030-75579-9_5
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-75578-2
Online ISBN: 978-3-030-75579-9
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)