Abstract
Background:
Oxidative stress that occurs as a consequence of the imbalance between antioxidant activity and free radicals can contribute in the pathogenesis of metabolic disorders, such as type 2 diabetes mellitus (T2DM). Antioxidant therapies have been proposed as possible approaches to treat and attenuate diabetic complications. The purpose of this study was to evaluate potential antioxidant effects of trehalose on oxidative indices in a streptozotocin (STZ)-induced diabetic rat model.
Methods:
Diabetic rats were divided randomly into five treatment groups (six rats per group). One test group received 45 mg/kg/day trehalose via intraperitoneal injection, and another received 1.5 mg/kg/day trehalose via oral gavage for 4 weeks. Three control groups were also tested including nondiabetic rats as a normal control (NC), a nontreated diabetic control (DC), and a positive control given 200 mg/kg/day metformin. Levels of thiol groups (-SH), and serum total antioxidant capacity were measured between control and test groups. In addition, superoxide dismutase (SOD) and glutathione peroxidase (GPx) enzyme activities were assessed.
Results:
In both oral and injection trehalose-treated groups, a marked increase was observed in serum total antioxidant capacity (TAC) (p > 0.05) and thiol groups (-SH) (p < 0.05). Also, SOD and GPx activities were increased after 4 weeks of treatment with trehalose.
Conclusion:
In conclusion, the present results indicate ameliorative effects of trehalose on oxidative stress, with increase antioxidant enzyme activities in STZ-induced diabetic rats.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Trehalose
- Diabetes mellitus
- Oxidative stress
- Total antioxidant capacity
- Malondialdehyde
- Superoxide dismutase
1 Introduction
Type 2 diabetes mellitus (T2DM) is defined as a permanent condition of hyperglycemia with predominant impacts on multiple metabolic pathways and physiologic functions of organs, caused by beta-cell dysfunction and insulin deficiency, tissue insulin resistance, or other metabolic alterations such as disruption of the redox balance and stress [1,2,3]. Oxidative stress has the potential to induce cell death mechanisms associated with tissue damage and multiple diabetic complications, including diabetic cardiomyopathy, retinopathy, and nephropathy [4]. This can occur via activation of nuclear factor kappa B (NF-κB), p38 MAPK, and c-jun NH2-terminal kinase/stress-activated protein kinase (JNK/SAPK) signaling pathways [5]. Indeed, there is an association between hyperglycemia-induced oxidative stress and local or systemic inflammation via increased pro-inflammatory cytokine production and macrophage infiltration [6]. Due to the deleterious outcomes of oxidative stress on diabetes complications, application of antioxidant therapies has been considered as a potential means of reducing T2DM pathogenesis through a decrease in free radicals and an increase in antioxidant enzyme activities [7,8,9].
Trehalose (mycose) is a carbohydrate with a disaccharide structure naturally produced by a wide range of organisms from prokaryotes to plants, except humans [10]. This sweetener molecule is frequently applied in food and drug industries and has been found to exert important biological impacts and modulate several metabolic pathways after consumption [11,12,13,14]. Experimental studies have indicated trehalose functions as an antioxidant, anti-inflammatory, and autophagy enhancer, which suppresses oxidative stress, inflammation, and autophagy-related disorders such as diabetes [15,16,17], atherosclerosis [18, 19], and Parkinson [20], Alzheimer [21, 22], and Huntington [23] diseases. Antidiabetic effects of trehalose can be linked to improving pathophysiological mechanisms such as inflammation and oxidative stress, pancreatic islet function, and lipid profile correction [24]. The role of trehalose as a natural antioxidant has been reported in in vitro and in vivo studies [25,26,27,28]. Here, we have attempted to determine the antioxidant effects of intraperitoneal (IP) and oral trehalose administration on total antioxidant capacity (TAC) and total thiols, along with the activities of the antioxidant enzymes superoxide dismutase (SOD) and glutathione peroxidase (GPx) as markers of oxidative stress in a streptozotocin (STZ)-induced diabetes rat model. In addition, antioxidant effects of trehalose were compared to those of the standard T2DM medication, metformin. The results showed that both oral and IP routes of trehalose administration suppressed oxidative stress, confirming the trehalose therapeutic potential in controlling oxidative stress-induced complications of diabetes in animal models.
2 Material and Methods
2.1 Animal
Male Wistar albino rats (8 weeks old, 180–200 g) were bred and housed in the Laboratory Animal Research Center of Medicine Faculty, Mashhad University of Medical Sciences, Mashhad, Iran. All animal experiments were approved by the Institutional Ethics Committee and Research Advisory Committee of the Mashhad University of Medical Sciences and the National Institute for Medical Research Development (NIMAD). The animals were maintained using a 12:12-h day-night cycle, at a constant 22 ± 2 °C, and humidity of 45–64%. Over the entire experimental procedure, the rats were fed with a standard rodent diet and water ad libitum. All rats were anesthetized with IP injections of thiopental sodium and blood samples collected after 4 weeks of treatment at study termination.
2.2 Induction of Rat T2DM Model
Non-insulin-dependent diabetes mellitus was induced by intravenous injection of single 60 mg/kg dose of streptozotocin in overnight-fasted rats (Masiello et al., 1998). STZ was dissolved in citrate-buffered saline (0.1 M, pH 4.5). Hyperglycemia was confirmed with blood glucose levels >180 mg/dL, determined at 72 h and then on day 7 after injection, and diabetic rats were included in this study. Two groups of diabetic rats (six rats per group) were treated daily with 45 mg/kg/day trehalose via i.p. injection and 1.5 g/kg/day via oral gavage for 4 weeks. Nondiabetic rats (n = 6) were used as the normal control (NC) group that received citrate buffer (i.p.). The diabetic (DC) and positive control groups received saline buffer and metformin (200 mg/kg/day), respectively.
2.3 Total Thiol (-SH) Group
Total thiol groups (-SH) were measured using the Kiazist kit according to the manufacturer’s instructions. In this assay, 5,5′-dithiobis-(2-nitrobenzoic acid) (DTNB) reacts with reduced sulfhydryl (-SH) groups in the serum, resulting in a yellow-colored complex, which is detectable at 405 nm.
2.4 Total Antioxidant Capacity (TAC)
The potential of samples for reducing ferric (Fe+3) to the ferrous form (Fe+2) was considered as the total antioxidant capacity (TAC) and measured by a colorimetric method. For this assay, 150 μL Kiazist TAC reagent was added to 30 μL sample or standard and incubated at room temperature for 45 min. The absorbance was read in 450 nm.
2.5 Antioxidant Enzyme Activity Assay
The levels of antiperoxidative enzymes, including GPx and SOD, were determined in the serum of diabetic rats using specific assay kits (Kiazist, Iran). The measurement of SOD and GPx activities was based on reducing free radicals produced by the xanthine/xanthine oxidase system and conversion of hydrogen peroxide to water, accompanied by glutathione oxidation, respectively.
2.6 Statistical Analysis
Statistical analysis was performed with Microsoft Excel (2019) and GraphPad Prism version 8 software. The results were analyzed using one-way analysis of variance (ANOVA) and the Tukey’s multiple comparison posttest to evaluate the significance of differences between treatment groups. Results with p < 0.05 were considered as statistically significant.
3 Results
3.1 Evaluation of Reduced (Free) Thiol (-SH) Groups and Total Antioxidant Capacity
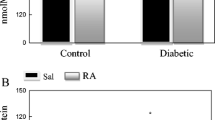
IP and oral administration of trehalose led to an increase in TAC and thiols, with lower levels in diabetic rats than the healthy control group (nondiabetic). Although TAC alterations did not reach statistical significance (Fig. 1), total thiol groups were increased significantly (p < 0.05) in treated groups compared to nontreated diabetic control, and the effect of IP trehalose administration was more potent than the oral route (Fig. 2).
3.2 Evaluation of SOD and GPx Antioxidant Enzyme Activities
For studying the effect of trehalose to induce enzymes that counteract free radical production, we measured the activities of SOD and GPx. These enzymes were increased in both the IP and oral trehalose-treated groups with a stronger effect of oral trehalose when compared with diabetic control rats. Differences in oral (P = 0.07) and IP trehalose (P = 0.89) groups were not significant for SOD (Fig. 3), whereas a significant increase was observed in GPx activity (P < 0.05) (Fig. 4).
4 Discussion
Diabetes is a chronic disease characterized by hyperglycemia resulting from deficiency of insulin secretion or insulin resistance, leading to microvascular and macrovascular complications that can damage different organs and tissues [29]. Hyperglycemia causes oxidative stress through multiple pathways, which is considered as a trigger for developing vascular complications of T2DM [30, 31]. High glucose levels promote the activity of some enzymes, including protein kinase C and nicotinamide adenine dinucleotide phosphate (NADPH) oxidase, leading to augmentation of reactive oxygen species (ROS) and oxidative stress, which in turn promote cell damage and tissue injuries [15]. Free radicals may attack cell membranes resulting in lipid peroxidation and an increase in MDA as a sensitive index of the systemic redox status and potential disease progression [32]. Besides lipid oxidation effects, ROS can oxidize free thiols and decrease circulating sulfhydryl (SH) concentrations, leading to a reduction in total antioxidant capacity [33]. Moreover, the alterations of antioxidant enzyme patterns are a characteristic feature of the uncontrolled diabetic state associated with a higher incidence of diabetic complications [34]. Since oxidative stress is a critical pathogenic factor for secondary complications of diabetes, the antioxidant therapy approach may be a useful strategy to treat diabetes by controlling free radical production; increasing intracellular antioxidant defenses, along with protective mechanisms against oxidative stress-induced apoptosis; and preserving β-cell function [35,36,37]. This study aimed to evaluate the antioxidant effects of trehalose as a natural antioxidant compound in T2DM . The changes in antioxidant markers such as serum thiol levels, and TAC, as well as the activity of GPx and SOD, were determined following 4 weeks of trehalose administration in STZ-induced diabetic rats.
Trehalose is a nonreducing disaccharide consisting of two glucose units in an α,α-1,1-glycosidic linkage, synthesized in numerous organisms from plants and bacteria to invertebrates and yeast [38]. Recent studies indicate that trehalose may decrease blood glucose and ameliorate insulin sensitivity and, thereby, may serve as a potential non-pharmacological agent for the management of diabetes [24]. We evaluated this possibility in our previous animal study and confirmed trehalose antidiabetic effects in a rat model of type 2 diabetes. The antioxidant effects of trehalose have also been assessed in different in vitro and in vivo studies [39, 40]. Treatment with trehalose in preclinical studies revealed that this antioxidant molecule significantly decreased the amount of ROS and H2O2 levels in a dose-dependent manner [15, 25] and upregulated antioxidant gene expression of SOD , glutathione (GSH), and catalase (CAT) via promotion of nuclear translocation of Nrf2 [25, 41]. Although antioxidant enzyme-dependent defenses play a crucial role in scavenging free radicals produced under oxidative stress [42, 43], there have been conflicting reports on SOD and GPx activity in diabetes mellitus. Both increased and decreased antioxidant enzyme activities have been reported [44,45,46,47,48,49], while some studies have shown no change in comparison to nondiabetic healthy controls [50, 51]. In diabetes, impaired pancreatic β-cells may express low physiological levels of the antioxidant enzymes SOD and GPx [52,53,54]. On the other hand, elevated ROS levels and increased production of O2− may increase the total antioxidant enzyme activity, suggesting a possible adaptive response to oxidative status [55]. Our results indicated a marked decrease in GPX activity in the diabetic rats, whereas this activity was significantly increased in both trehalose-treated groups compared with the DC group. A similar trend was found for SOD activity after 4 weeks of trehalose intervention, though the differences were not statistically significant. Experimental models have determined that antioxidant compounds can change TAC in serum or plasma; therefore, monitoring plasma TAC may be a valuable index for oxidative burden [56, 57]. However, no prior study has investigated the effects of trehalose on plasma TAC levels; our research reported that TAC and the amount of free thiol increased during the treatment process. Differences in TAC marker was significant between the IP-treated trehalose group and DC group. Intraperitoneal administration of trehalose had greater potential efficacy than oral administration, which could be due to the higher bioavailability of trehalose in the IP route.
As mentioned earlier, previous studies displayed in vitro antioxidant activities of trehalose, and here we carried out the in vivo experimental study to support an antioxidant effect of trehalose in T2DM model during 4 weeks of treatment. The obtained results suggest that trehalose might be regarded as a safe antioxidant supplement for diabetic subjects in clinical studies over a longer timeframe.
In conclusion, regarding the importance of oxidative stress in activating intracellular signaling pathways and the pathogenesis of multiple disorders, natural antioxidant products could be a potential therapeutic strategy to manage and reduce oxidative damage. The findings of our study demonstrated that trehalose administration could enhance antioxidant capacities, and protect antioxidant enzyme activity slightly; however, a clear and comprehensive understanding of the effect of trehalose on antioxidant enzymes needs further investigation.
Conflict of Interests
None.
References
Bertoluci, M. C., Salles, J. E. N., Silva-Nunes, J., Pedrosa, H. C., Moreira, R. O., da Silva Duarte, R. M. C., et al. (2020). Portuguese-Brazilian evidence-based guideline on the management of hyperglycemia in type 2 diabetes mellitus. Diabetology & Metabolic Syndrome, 12, 1–30.
Li, Y.-Y., Yang, X.-F., Gu, H., Snellingen, T., Liu, X.-P., & Liu, N.-P. (2018). The relationship between insulin resistance/β-cell dysfunction and diabetic retinopathy in Chinese patients with type 2 diabetes mellitus: the Desheng Diabetic Eye Study. International Journal of Ophthalmology, 11(3), 493.
Rehman, K., & Akash, M. S. H. (2017). Mechanism of generation of oxidative stress and pathophysiology of type 2 diabetes mellitus: How are they interlinked? Journal of Cellular Biochemistry, 118(11), 3577–3585.
Giacco, F., & Brownlee, M. (2010). Oxidative stress and diabetic complications. Circulation Research, 107(9), 1058–1070.
Evans, J. L., Goldfine, I. D., Maddux, B. A., & Grodsky, G. M. (2002). Oxidative stress and stress-activated signaling pathways: A unifying hypothesis of type 2 diabetes. Endocrine Reviews, 23(5), 599–622.
Oguntibeju, O. O. (2019). Type 2 diabetes mellitus, oxidative stress and inflammation: Examining the links. International Journal of Physiology, Pathophysiology and Pharmacology, 11(3), 45–63.
Li, C., Shi, X., Shen, Q., Guo, C., Hou, Z., & Zhang, J. (2018). Hot topics and challenges of regenerative nanoceria in application of antioxidant therapy. Journal of Nanomaterials, 2018, 1–12.
Panahi, Y., Khalili, N., Sahebi, E., Namazi, S., Karimian, M. S., Majeed, M., et al. (2017). Antioxidant effects of curcuminoids in patients with type 2 diabetes mellitus: A randomized controlled trial. Inflammopharmacology, 25(1), 25–31.
Yaribeygi, H., Mohammadi, M. T., & Sahebkar, A. (2018). Crocin potentiates antioxidant defense system and improves oxidative damage in liver tissue in diabetic rats. Biomedicine & Pharmacotherapy, 98, 333–337.
Elbein, A. D., Pan, Y., Pastuszak, I., & Carroll, D. (2003). New insights on trehalose: A multifunctional molecule. Glycobiology, 13(4), 17R–27R.
Figueroa, C. M., Feil, R., Ishihara, H., Watanabe, M., Kölling, K., Krause, U., et al. (2016). Trehalose 6–phosphate coordinates organic and amino acid metabolism with carbon availability. The Plant Journal, 85(3), 410–423.
Eleutherio, E., Panek, A., De Mesquita, J. F., Trevisol, E., & Magalhães, R. (2015). Revisiting yeast trehalose metabolism. Current Genetics, 61(3), 263–274.
Wang, X., Du, Y., & Yu, D. (2019). Trehalose phosphate synthase 5-dependent trehalose metabolism modulates basal defense responses in Arabidopsis thaliana. Journal of Integrative Plant Biology, 61(4), 509–527.
Hosseinpour-Moghaddam, K., Caraglia, M., & Sahebkar, A. (2018). Autophagy induction by trehalose: Molecular mechanisms and therapeutic impacts. Journal of Cellular Physiology, 233(9), 6524–6543.
Lin, C. F., Kuo, Y. T., Chen, T. Y., & Chien, C. T. (2016). Quercetin-rich guava (Psidium guajava) juice in combination with Trehalose reduces autophagy, apoptosis and Pyroptosis formation in the kidney and pancreas of type II diabetic rats. Molecules, 21(3), 334.
Mizote, A., Yamada, M., Yoshizane, C., Arai, N., Maruta, K., Arai, S., et al. (2016). Daily intake of trehalose is effective in the prevention of lifestyle-related diseases in individuals with risk factors for metabolic syndrome. Journal of Nutritional Science and Vitaminology, 62(6), 380–387.
Yoshizane, C., Mizote, A., Yamada, M., Arai, N., Arai, S., Maruta, K., et al. (2017). Glycemic, insulinemic and incretin responses after oral trehalose ingestion in healthy subjects. Nutrition Journal, 16(1), 9.
Stachowicz, A., Wiśniewska, A., Kuś, K., Kiepura, A., Gębska, A., Gajda, M., et al. (2019). The influence of Trehalose on atherosclerosis and hepatic Steatosis in Apolipoprotein E knockout mice. International Journal of Molecular Sciences, 20(7), 1552.
Sahebkar, A., Hatamipour, M., & Tabatabaei, S. A. (2019). Trehalose administration attenuates atherosclerosis in rabbits fed a high-fat diet. Journal of Cellular Biochemistry, 120(6), 9455–9459.
Khalifeh, M., Barreto, G. E., & Sahebkar, A. (2019). Trehalose as a promising therapeutic candidate for the treatment of Parkinson’s disease. British Journal of Pharmacology, 176(9), 1173–1189.
Portbury, S. D., Hare, D. J., Sgambelloni, C., Perronnes, K., Portbury, A. J., Finkelstein, D. I., et al. (2017). Trehalose improves cognition in the transgenic Tg2576 mouse model of Alzheimer’s disease. Journal of Alzheimer’s Disease, 60, 549–560.
Khalifeh, M., Read, M. I., Barreto, G. E., & Sahebkar, A. (2020). Trehalose against Alzheimer’s disease: Insights into a potential therapy. BioEssays, 42(8), e1900195.
He, Q., Koprich, J. B., Wang, Y., Yu, W.-B., Xiao, B.-G., Brotchie, J. M., et al. (2016). Treatment with Trehalose prevents Behavioral and neurochemical deficits produced in an AAV α-Synuclein rat model of Parkinson’s disease. Molecular Neurobiology, 53(4), 2258–2268.
Yaribeygi, H., Yaribeygi, A., Sathyapalan, T., & Sahebkar, A. (2019). Molecular mechanisms of trehalose in modulating glucose homeostasis in diabetes. Diabetes & Metabolic Syndrome: Clinical Research & Reviews, 13(3), 2214–2218.
Mizunoe, Y., Kobayashi, M., Sudo, Y., Watanabe, S., Yasukawa, H., Natori, D., et al. (2018). Trehalose protects against oxidative stress by regulating the Keap1-Nrf2 and autophagy pathways. Redox Biology, 15, 115–124.
Alvarez-Peral, F. J., Zaragoza, O., Pedreno, Y., & Argüelles, J.-C. (2002). Protective role of trehalose during severe oxidative stress caused by hydrogen peroxide and the adaptive oxidative stress response in Candida albicans. Microbiology, 148(8), 2599–2606.
Tang, Q., Zheng, G., Feng, Z., Chen, Y., Lou, Y., Wang, C., et al. (2017). Trehalose ameliorates oxidative stress-mediated mitochondrial dysfunction and ER stress via selective autophagy stimulation and autophagic flux restoration in osteoarthritis development. Cell Death & Disease, 8(10), e3081–e3081.
Echigo, R., Shimohata, N., Karatsu, K., Yano, F., Kayasuga-Kariya, Y., Fujisawa, A., et al. (2012). Trehalose treatment suppresses inflammation, oxidative stress, and vasospasm induced by experimental subarachnoid hemorrhage. Journal of Translational Medicine, 10(1), 80.
Cade, W. T. (2008). Diabetes-related microvascular and macrovascular diseases in the physical therapy setting. Physical Therapy, 88(11), 1322–1335.
King, G. L., & Loeken, M. R. (2004). Hyperglycemia-induced oxidative stress in diabetic complications. Histochemistry and Cell Biology, 122(4), 333–338.
Dos Santos, J. M., Tewari, S., & Mendes, R. H. (2019). The role of oxidative stress in the development of diabetes mellitus and its complications. Hindawi.
Ayala, A., Muñoz, M. F., & Argüelles, S. (2014). Lipid peroxidation: Production, metabolism, and signaling mechanisms of malondialdehyde and 4-hydroxy-2-nonenal. Oxidative Medicine and Cellular Longevity, 2014, 360438.
Opara, E. C., Abdel-Rahman, E., Soliman, S., Kamel, W. A., Souka, S., Lowe, J. E., et al. (1999). Depletion of total antioxidant capacity in type 2 diabetes. Metabolism, 48(11), 1414–1417.
Godin, D. V., Wohaieb, S. A., Garnett, M. E., & Goumeniouk, A. D. (1988). Antioxidant enzyme alterations in experimental and clinical diabetes. Molecular and Cellular Biochemistry, 84(2), 223–231.
Ceriello, A., & Testa, R. (2009). Antioxidant anti-inflammatory treatment in type 2 diabetes. Diabetes Care, 32(suppl 2), S232–S236.
Rajendiran, D., Packirisamy, S., & Gunasekaran, K. (2018). A review on role of antioxidants in diabetes. Asian Journal of Pharmaceutical and Clinical Research, 11(2), 48–53.
Li, C., Miao, X., Li, F., Wang, S., Liu, Q., Wang, Y., et al. (2017). Oxidative stress-related mechanisms and antioxidant therapy in diabetic retinopathy. Oxidative Medicine and Cellular Longevity, 2017, 1–15.
Lee, H.-J., Yoon, Y.-S., & Lee, S.-J. (2018). Mechanism of neuroprotection by trehalose: Controversy surrounding autophagy induction. Cell Death & Disease, 9(7), 1–12.
Benaroudj, N., & Goldberg, A. L. (2001). Trehalose accumulation during cellular stress protects cells and cellular proteins from damage by oxygen radicals. Journal of Biological Chemistry, 276(26), 24261–24267.
Oku, K., Kurose, M., Kubota, M., Fukuda, S., Kurimoto, M., Tujisaka, Y., et al. (2005). Combined NMR and quantum chemical studies on the interaction between trehalose and dienes relevant to the antioxidant function of trehalose. The Journal of Physical Chemistry B, 109(7), 3032–3040.
Sun, L., Zhao, Q., Xiao, Y., Liu, X., Li, Y., Zhang, J., et al. (2020). Trehalose targets Nrf2 signal to alleviate d-galactose induced aging and improve behavioral ability. Biochemical and Biophysical Research Communications, 521(1), 113–119.
Mahboob, M., Rahman, M., & Grover, P. (2005). Serum lipid peroxidation and antioxidant enzyme levels in male and female diabetic patients. Singapore Medical Journal, 46(7), 322.
Harris, E. D. (1992). Regulation of antioxidant enzymes 1. The FASEB Journal, 6(9), 2675–2683.
Maritim, A., Sanders, A., & Watkins Iii, J. (2003). Diabetes, oxidative stress, and antioxidants: A review. Journal of Biochemical and Molecular Toxicology, 17(1), 24–38.
Sailaja, Y., Baskar, R., & Saralakumari, D. (2003). The antioxidant status during maturation of reticulocytes to erythrocytes in type 2 diabetics. Free Radical Biology and Medicine, 35(2), 133–139.
Ozkilic, A. C., Cengiz, M., Ozaydin, A., Cobanoglu, A., & Kanigur, G. (2006). The role of N-acetylcysteine treatment on anti-oxidative status in patients with type II diabetes mellitus. Journal of Basic and Clinical Physiology and Pharmacology, 17(4), 245–254.
Matkovics, B., Varga, S. I., Szabo, L., & Witas, H. (1982). The effect of diabetes on the activities of the peroxide metabolism enzymes. Hormone and Metabolic Research, 14(02), 77–79.
Palanduz, S., Ademoğlu, E., Gökkuşu, C., & Tamer, S. (2001). Plasma antioxidants and type 2 diabetes mellitus. Research Communications in Molecular Pathology and Pharmacology, 109(5–6), 309.
Gunawardena, H. P., Silva, R., Sivakanesan, R., Ranasinghe, P., & Katulanda, P. (2019). Poor glycaemic control is associated with increased lipid peroxidation and glutathione peroxidase activity in type 2 diabetes patients. Oxidative Medicine and Cellular Longevity, 2019, 9471697.
Kesavulu, M., Giri, R., Rao, B. K., & Apparao, C. (2008). Lipid peroxidation and antioxidant enzyme levels in type 2 diabetics with microvascular complications. Diabetes & Metabolism, 26(5), 387.
Şekeroğlu, M. R., Sahin, H., Dülger, H., & Algün, E. (2000). The effect of dietary treatment on erythrocyte lipid peroxidation, superoxide dismutase, glutathione peroxidase, and serum lipid peroxidation in patients with type 2 diabetes mellitus. Clinical Biochemistry, 33(8), 669–674.
Tanaka, Y., Tran, P. O. T., Harmon, J., & Robertson, R. P. (2002). A role for glutathione peroxidase in protecting pancreatic β cells against oxidative stress in a model of glucose toxicity. Proceedings of the National Academy of Sciences, 99(19), 12363–12368.
Welsh, N., Margulis, B., Borg, L. H., Wiklund, H. J., Saldeen, J., Flodström, M., et al. (1995). Differences in the expression of heat-shock proteins and antioxidant enzymes between human and rodent pancreatic islets: Implications for the pathogenesis of insulin-dependent diabetes mellitus. Molecular Medicine, 1(7), 806–820.
Suh, K. S., Choi, E. M., Jung, W.-W., Kim, Y. J., Hong, S. M., Park, S. Y., et al. (2017). Deoxyactein protects pancreatic β-cells against methylglyoxal-induced oxidative cell damage by the upregulation of mitochondrial biogenesis. International Journal of Molecular Medicine, 40(2), 539–548.
Bandeira Sde, M., Guedes Gda, S., da Fonseca, L. J., Pires, A. S., Gelain, D. P., Moreira, J. C., et al. (2012). Characterization of blood oxidative stress in type 2 diabetes mellitus patients: Increase in lipid peroxidation and SOD activity. Oxidative Medicine and Cellular Longevity, 2012, 819310.
Rani, A. J., & Mythili, S. (2014). Study on total antioxidant status in relation to oxidative stress in type 2 diabetes mellitus. Journal of Clinical and Diagnostic Research: JCDR, 8(3), 108.
Kambayashi, Y., Binh, N. T., Asakura, H. W., Hibino, Y., Hitomi, Y., Nakamura, H., et al. (2009). Efficient assay for total antioxidant capacity in human plasma using a 96-well microplate. Journal of Clinical Biochemistry and Nutrition, 44(1), 46–51.
Funding
This study was supported by a grant from the National Institute for Medical Research Development (NIMAD), Tehran, Iran (Grant no: 987820).
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Radbakhsh, S., Ganjali, S., Moallem, S.A., Guest, P.C., Sahebkar, A. (2021). Antioxidant Effects of Trehalose in an Experimental Model of Type 2 Diabetes. In: Sahebkar, A., Sathyapalan, T. (eds) Natural Products and Human Diseases. Advances in Experimental Medicine and Biology(), vol 1328. Springer, Cham. https://doi.org/10.1007/978-3-030-73234-9_32
Download citation
DOI: https://doi.org/10.1007/978-3-030-73234-9_32
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-73233-2
Online ISBN: 978-3-030-73234-9
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)