Abstract
Excess nitrogen (N) and phosphorus (P) inputs to streams occur globally, and affect not only stream autotrophs, but also heterotrophic microbes and detrital carbon processing. Detrital carbon, such as leaf litter, supports stream food webs and their connectivity via downstream detritus fluxes. Nutrient enrichment increases litter decomposition rates across multiple scales and trophic levels by stimulating activity of microbial decomposers and enhancing interactions among microbial decomposers, detritivores, and physical abrasion. Nutrient effects on microbial and detritivore-mediated decomposition are typically greater for recalcitrant vs. labile litter, especially when coupled to low initial nutrient concentrations. Recent studies and syntheses show that (1) dissolved N and P affect litter by stimulating fungal activity and nutrient immobilization, thus, increasing detrital nutrient content, (2) nutrient effects are greatest with N and P together (vs. individually) and when detritivores are present, and (3) ecosystem-level effects of nutrient enrichment can be predicted from small-scale measurements. Despite extensive studies of leaf litter decomposition, its application as a tool to manage nutrient enrichment issues trails comparable tools for autotrophic (i.e., algal) pathways. Thus, better understanding of the consequences of nutrient enrichment on leaf litter and other detrital carbon is important to predict how nutrients will affect stream ecosystem functioning.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
1 Pathways of Nutrient Enrichment Effects in Streams
Human activities have increased nutrient concentrations in streams and rivers worldwide (Dodds & Smith, 2016; Wurtsbaugh et al., 2019) modifying critical ecosystem functions, including leaf litter decomposition. The effects of excessive nitrogen (N) and phosphorus (P) concentrations have been studied most extensively, because these two elements often co-limit growth and subsequent biological activity of both autotrophic and heterotrophic biota in freshwater ecosystems (Ferreira et al., 2015; Harpole et al., 2011). Nitrogen and P enter streams via both point sources, and diffuse, non-point pollution. Land use changes, fertilizer application, atmospheric deposition (Fowler et al., 2004; Linker et al., 2013), and animal or human wastes (e.g., livestock manure, wastewater) all contribute to increasing nutrients in streams, and each source can yield distinct patterns of nutrient loading and resultant streamwater nutrient concentrations and ratios (Manning et al., 2020; Stets et al., 2020). Excessive concentrations of total N (TN) and total P (TP) are estimated to affect 41 and 46% of total stream length in the United States (US EPA, 2016), and less than 2% of U.S. streams have TP concentrations indicative of reference conditions (TP <10 µg L-1; Stoddard et al., 2016; Fig. 16.1). Other parts of the globe are not immune to this problem; elevated N and P concentrations are pervasive across streams and rivers in Europe, Asia, Oceania, Africa, and Central and South America (McDowell et al., 2020).
Kernel density plots based on Gaussian kernel density estimators at each x-value representing the distribution of dissolved inorganic N (DIN; a) and dissolved inorganic P (DIP; b) concentrations observed in publicly available data sets (USA [dark grey areas] and global [light grey areas]). Concentrations generally span several orders of magnitude, and concentrations near the lower end of the spectrum (i.e., near pristine) are rare. We also indicate the mean of half-saturation constants (Km) for litter decomposition rates reported in the literature for both N and P (red vertical lines in a and b; Ferreira et al., 2006; Kominoski et al., 2015; Rosemond et al., 2002). About 94 and 100% of concentration values were above the DIN thresholds in the U.S. and global datasets, respectively, compared to 59 and 56% for DIP. Data were collected and made available by the U.S. Geological Survey, as part of the U.S. National Water Quality Assessment (dark grey density curves; n =7,653), and global sites from the Global River Chemistry dataset (GLORICH; Hartmann et al., 2014, 2019) with streamflow <20 m3 s-1 (light grey density curves; n = 14,097 [DIN] and 53,861 [DIP]).
The effects of nutrient pollution in streams and rivers can vary spatially and temporally, because of the multiple sources of N and P, and their interactions with the physical attributes and biological activity of streams. For example, nutrient availability may vary according to stream flow regimes controlled by climate and land use, where floods and droughts can episodically deplete, dilute, or elevate nutrient concentrations (Kaushal et al., 2014; Moss et al., 2011; Rose et al., 2018). Similarly, seasonal patterns of nutrient availability may occur because of the timing of fertilizer application, seasonality of riparian or in-stream nutrient uptake, animal migrations, or climatic variables (e.g., interactions among precipitation and wet/dry nutrient deposition; Mulholland & Hill, 1997). The spatial distribution of excessive nutrient concentrations in streams is affected by the prevalence of nutrient sources within watersheds. For instance, the dominant land use in the corn-belt region of the Midwest USA is row-crop agriculture, and streams in this region tend to exhibit higher N concentrations compared to regions with other prevailing types of land use (Hill et al., 2011).
The spatial and temporal variation of stream N and P concentration highlights the need for metrics that can integrate their effects in time and space. The spatial and temporal dynamics of nutrient availability in streams interact with seasonal pulses of terrestrial organic matter inputs that are the base of food webs in most forest streams as well as with the seasonal patterns in activity of plant litter decomposers. Thus, the processes through which this organic matter is broken down offer several integrative metrics that can be used to understand how both acute and chronic nutrient enrichment modify stream ecosystem functions. Specifically, leaf litter decomposition is well suited to quantify the multiple effects of nutrient enrichment in streams because leaf litter from terrestrial sources remains within streams for long periods of time (months to years; Webster & Benfield, 1986), and involves multiple facets of physical, chemical and biological attributes of stream ecosystems (Frainer et al. [Chapter 21 in this volume]).
1.1 Nutrient Effects Are Less Understood in Heterotrophic vs. Autotrophic Systems
Some of the most obvious impacts of nutrient pollution in freshwaters include increased algal biomass, blooms of harmful algae and associated hypoxic zones, fish kills, and drinking water contamination (McDowell et al., 2020; Smith et al., 2006). Increased nutrient availability can stimulate ecosystem productivity (i.e., eutrophication), and can increase the importance of primary producers as the energy base for the food web when light is not a limiting factor. This process of anthropogenic eutrophication and the build-up of within-system carbon (C) via increased photosynthesis has justifiably received considerable attention (Conley et al., 2009 and references therein). However, in forest streams, solar irradiation is limited by the riparian vegetation, and aquatic food webs derive most of their C and energy from terrestrial organic matter (i.e., allochthonous C, such as leaf litter, detailed below; Cebrian & Lartigue, 2004; Fisher & Likens, 1973; Moore et al., 2004; Wallace et al., 1997). Nutrient effects on these heterotrophic, “brown” food web pathways have received less attention than autotrophic, “green” food web pathways, particularly for management. For example, efforts to define ecosystem trophic state in streams first focused on relationships between streamwater TN and TP and benthic algae (Dodds, 2006) while more recent work has begun to encompass both green and brown pathways to define ecosystem responses to nutrient enrichment (Arroita et al., 2019; Dodds & Cole, 2007). Whereas nutrient enrichment leads to increased biomass and biomass-specific rates of primary production in autotroph-based ecosystems, it can increase microbial respiration rates, stimulate detritivore activity and result in ecosystem-level C losses in detritus-based systems (Benstead et al., 2009). However, heterotrophic responses to nutrient enrichment, such as decomposition of coarse particulate organic matter (i.e., leaf litter), remain largely absent from conventional strategies to monitor and manage the problems associated with nutrient enrichment of streams and rivers.
Food webs in forest streams are fueled by plant litter (leaves and wood) derived from terrestrial ecosystems (Wallace et al., 1997; Walther & Whiles, 2011). The decomposition of leaf litter is a key ecosystem process that has been studied extensively for several decades (Abelho, 2001; Chauvet et al., 2016; Marks, 2019; Tank et al., 2010; Webster & Benfield, 1986). Moreover, leaf litter decomposition integrates biological activity across multiple trophic levels (microbial decomposers, such as aquatic hyphomycetes, to predators; Gessner et al., 2010) and patterns of patch- or reach-scale decomposition rates can inform models addressing decomposition rates at catchment to river network scales (Rosemond et al., 2015; Webster, 2007). The stimulation of leaf litter decomposition by nutrients results in accelerated loss of stream C altering its availability to stream consumers, and affecting climate feedbacks via increased respiratory loss of leaf litter C to CO2 (Follstad-Shah [Chapter 12 in this volume]; Manning et al., 2018; Rosemond et al., 2015). As both N and P can limit the growth of key stream biota, and especially those that play an outsized role in leaf litter processing, increased stream nutrient concentrations can lead to rapid turnover of leaf litter in streams, and may ultimately result in reduced C standing stock and availability to in-stream biota. Indeed, a recent meta-analysis of observational and experimental studies suggest that moderate increases of nutrient concentrations can increase leaf litter decomposition rates by 50%, on average (Ferreira et al., 2015). Consistent with this finding, Rosemond et al. (2015) found comparable increases (~50%) for whole-stream leaf litter loss rates from experimentally enriched streams. However, when streams are not nutrient limited (e.g., due to underlying geology or diffuse non-point source pollution), further increases in nutrient concentrations may have no effect on litter decomposition (Baldy et al., 2007; Chadwick & Huryn, 2003). Also, litter decomposition can be inhibited at high nutrient concentrations due to toxic effects of high concentrations of nitrite or ammonia to detritivores (but not microorganisms), or to the concomitant changes in other environmental factors (e.g., decrease in dissolved oxygen concentration or increase in fine sediment load and pesticide or other contaminant concentrations) that may negatively affect both microbial and invertebrate activity (Lecerf et al., 2006; Woodward et al., 2012).
In this chapter, we describe the pathways and explore the mechanisms through which streamwater nutrient enrichment can affect leaf litter decomposition rates. We develop a conceptual model based on current evidence for the effects of nutrient enrichment on leaf litter decomposition driven by microbial decomposers and detritivores. These explorations and conceptualizations are not exhaustive (see Ferreira et al., 2015), but rather focus on both observational and experimental evidence that illustrate the emerging mechanisms of how leaf litter decomposition responds to nutrient enrichment. We also discuss potential interactions of nutrient effects on litter decomposition and other global change stressors, such as rising stream temperatures. We conclude with an overview of consequences for whole ecosystems, including C residence times, and offer perspectives on the need to promote efforts to fully incorporate leaf litter decomposition responses into strategies to monitor and manage nutrient pollution at extensive (i.e., continental) scales.
2 Mechanisms of Nutrient Effects on Leaf Litter Decomposition
2.1 Microbially Mediated Litter Processing
Microbial communities associated with decaying leaf litter in streams encompass fungi, including so-called aquatic hyphomycetes that are specially adapted to stream environments, as well as bacteria. Fungi dominate these microbial communities in terms of biomass (88–99.9%) and production (up to 627 × higher) while the importance of bacteria in leaf litter decomposition is rather minor (Gessner et al., 2007; Pascoal & Cássio, 2004; Pascoal et al., 2005; Suberkropp et al., 2010; Tant et al., 2013; Weyers & Suberkropp, 1996). For example, fungi contributed 95–99.7% of total microbial biomass and 88–95% of total microbial production on submerged leaf litter in southern Appalachian streams at Coweeta Hydrologic Laboratory, NC, USA (Gulis & Suberkropp, 2003a; Suberkropp et al., 2010). Fungal hyphae are capable of penetrating inside the leaf litter matrix and directly accessing plant polymers while bacteria are restricted to leaf surfaces. In addition, bacteria may rely to a greater extent on dissolved organic C from streamwater than fungi, rather than participating in leaf C processing. While leaf-associated fungi are strongly stimulated by elevated nutrient concentrations in water (see below), bacteria are either only slightly affected or the effect of dissolved nutrients is lacking (Suberkropp et al., 2010). Thus, we will focus on the nutrient effects on litter-associated fungi that drive increases in litter decomposition rates under nutrient enrichment.
In contrast to decomposition in terrestrial ecosystems, fungi associated with submerged leaf litter are capable of obtaining N and P from both the substrate and the water column (Cheever et al., 2013; Suberkropp, 1995). Thus, in streams, decomposition of leaf litter may depend not only on the nutrient content of the substrate but also the availability of N and P from the water column that can modify the activity of microbial decomposers (Suberkropp & Chauvet, 1995). Since plant litter C:N and C:P ratios are considerably higher than those of fungal biomass (Danger & Chauvet, 2013; Grimmett et al., 2013; Gulis et al., 2017), fungi have to alleviate the stoichiometric imbalance by either retaining N and P from leaf litter more efficiently than C or by immobilizing N and P from streamwater. Production of extracellular enzymes to obtain N and P from leaf litter is energetically costly, thus, fungi should preferentially use dissolved inorganic nutrients from the water column. This notion is supported by findings that higher concentrations of dissolved inorganic nutrients in laboratory studies and in whole-stream nutrient addition experiments or due to anthropogenic activities stimulate fungal activity, leading to nutrient immobilization and faster plant litter decomposition (e.g., Ferreira et al., 2006; Gulis, Ferreira et al., 2006; Gulis & Suberkropp, 2003a). Stimulation of fungal activity and plant litter decomposition by inorganic nutrients should be theoretically more pronounced for substrates with high initial C:N and C:P ratios, such as wood or rhododendron (Rhododendron maximum) leaves, due to more severe nutrient limitation of microbial activity on these substrates; on the other hand, external nutrients should have a less pronounced effect on leaf litter with initially high N or P content (e.g., leaves of N-fixing alder species). This pattern has been frequently reported in streams (e.g., Ferreira et al., 2006; Gulis et al., 2004, 2006; Gulis & Suberkropp, 2003a; Stelzer et al., 2003), though it can be complicated by variable lignin content of plant litter (Jabiol et al., 2019).
Early studies testing the effects of nutrient addition on microbially driven decomposition of plant litter in streams produced variable results (Elwood et al., 1981; Newbold, Elwood, Schulze, et al., 1983). In the last decades, however, multiple experiments in lab microcosms simulating stream conditions clearly demonstrated stimulation of microbial activity (fungal biomass accrual, growth efficiency, sporulation rate and cumulative spore production, respiration) and leaf litter decomposition by dissolved inorganic nutrients (Ferreira & Chauvet, 2011; Gulis & Suberkropp, 2003a, 2003b; Sridhar & Bärlocher, 1997; Suberkropp, 1998). Short-term whole-stream nutrient addition experiments have also shown positive effects of dissolved nutrients on microbial activity (fungal biomass accrual, sporulation, respiration) and plant litter decomposition rates (Ferreira et al., 2006; Rosemond et al., 2002). Multi-year nutrient enrichments provided additional fine details (Gulis et al., 2004; Gulis & Suberkropp, 2003a; Gulis et al., 2008; Rosemond et al., 2015; Tant et al., 2013, 2015), including uncovering important ecosystem-level consequences of elevated microbial activity and decomposition rates, namely accelerated C loss from the system due to downstream export of fine particulate organic matter (FPOM) and CO2 evolution (Benstead et al., 2009). Since leaf litter decomposition rates, fungal biomass and sporulation rates in higher-order streams can be similar to those found in headwater streams (Baldy et al., 1995), the importance of aquatic fungi in regulating leaf litter decomposition extends beyond the reaches of headwater streams. Recently, manipulative experiments in streams also addressed the relative importance of dissolved N and P (Kominoski et al., 2015; Manning et al., 2015, 2016). From the microbial perspective, it appears that nitrate-N has stronger effect on fungal activity and microbial decomposition rates while excess soluble reactive phosphorus (SRP) results in luxury P immobilization by fungi and sharp decreases in leaf litter C:P ratios (Gulis et al., 2017; Manning et al., 2015).
The relationship between dissolved inorganic nutrients and parameters of fungal activity or plant litter decomposition rates can be described in some cases by asymptotic saturation-type models (Ferreira et al., 2006; Gulis, Ferreira, et al., 2006; Gulis, Kuehn et al., 2006; Rosemond et al., 2002). In such models, large increases in fungal activity or decomposition rates occur with relatively small increases in nutrients and at low concentration levels suggesting that microbial nutrient demands can be easily satisfied by moderate nutrient enrichment. The half-saturation constants Km (concentration at which half of the maximum decomposition rate or activity is reached) for DIN was estimated at <300 μg L-1 and was as low as <20 μg L-1 for SRP.
There are several possible mechanisms, some of them operating concurrently, that translate elevated fungal activity into faster leaf litter decomposition under nutrient addition scenarios (Fig. 16.2):
Conceptual diagram depicting leaf litter decomposition in low nutrient streams (left diagram) and effects of nutrient (N, P) enrichment of streamwater on leaf litter decomposition and associated microbial decomposers (right diagram). In general, nutrient enrichment of streamwater with N and P stimulates fungal biomass accrual and activity on leaf litter with the release of C as CO2 and fine particulate organic matter (FPOM; including fungal spores), both of which occur throughout the decomposition sequence. Fungal-mediated dissolved nutrient immobilization and litter softening lead to changes in key litter attributes, such as decreases in litter C:N and C:P ratios and litter toughness, which result in increased litter palatability for detritivores. Increased detritivore biomass and activity promotes further litter mass loss by incorporation of litter C into secondary production or release as FPOM (i.e., small leaf fragments and feces). Red arrows in the right diagram indicate stimulation with increased nutrient concentrations. Effects of nutrient enrichment on microbes, detritivores and litter decomposition are likely modified by temperature, discharge, and changes in riparian vegetation. Figure modeled after Cummins and Klug (1979) and Marks (2019). Images of Pycnopsyche and Taeniopteryx from Macroinvertebrates.org (CC BY-NC 4.0). Maple leaf vector images: Tracy Saxby, Integration and Application Network, University of Maryland Center for Environmental Science (ian.umces.edu/imagelibrary/)
-
i.
Previous studies have shown that, in general, the greater metabolic energy spent by microbes on acquisition of N and P, such as nutrient mining by extracellular enzymes, the less energy is directed towards the acquisition of C (Gallo et al., 2009; Linkins et al., 1990). Therefore, readily accessible external inorganic N and P should stimulate the activity of extracellular enzymes involved in sequestration of C from plant polymers (Güsewell & Freeman, 2005). Indeed, the activity of beta-glucosidase involved in degradation of cellulose from leaf litter was positively affected by dissolved inorganic N (but not P) availability (Gulis et al., unpublished).
-
ii.
A related mechanism involves nutrient stimulation of the activity of fungal pectin-degrading enzymes that are crucial for litter mass loss resulting in enhanced maceration of leaf litter by releasing whole plant cells as FPOM (Jenkins & Suberkropp, 1995).
-
iii.
Elevated nutrient concentrations stimulate fungal production including conversion of plant C into fungal spores (e.g., Ferreira et al., 2006; Gulis & Suberkropp, 2003a, 2003b, 2003c; Suberkropp et al., 2010) that are released into the current, and fungi can channel up to 80% of production (or 8–12% of leaf mass loss) into sporulation (Suberkropp, 1991).
-
iv.
Elevated nutrients are known to stimulate microbial respiration associated with decaying submerged leaf litter resulting in increased litter C losses as CO2 to the atmosphere (Benstead et al., 2009; Gulis & Suberkropp, 2003a, 2003b, 2003c; Suberkropp et al., 2010).
-
v.
As leaf litter decomposes, fungal biomass accrual and nutrient immobilization from the water column lead to changes in litter stoichiometry (decreases in C:N and C:P ratios) and an increase in nutritional quality and palatability of decaying leaf litter to detritivores (Bärlocher, 1985; Gessner et al., 2007) that in turn stimulate detritivore feeding and furthers leaf litter decomposition (see below).
The ability of aquatic fungi to control and homogenize detrital stoichiometry as plant litter decomposes may have important consequences to stream detritivores, which rely on plant-associated microbial biomass rather than plant material alone as a major source of nutrients (Chung & Suberkropp, 2009a, 2009b; Suberkropp, 1992). Thus, litter-associated fungi are important intermediaries in energy and nutrient transfer in streams while their activity and role in leaf litter decomposition can be modified by inorganic N and P availability.
2.2 Detritivore-Mediated Litter Processing
Detritivores colonizing leaf litter promote decomposition via feeding activity and fragmentation and due to using leaf litter to make cases (e.g., case-building caddisflies of the families Calamoceratidae, Lepidostomatidae, Limnephilidae) (Azevedo-Pereira et al., 2006; Moretti et al., 2009; Rincón & Martínez, 2006; Sanpera-Calbet et al., 2009). Detritivores have been shown to contribute up to 63.5% of total leaf litter mass loss in streams (Cornut et al., 2010; Hieber & Gessner, 2002; Taylor & Chauvet, 2014). The fine particles released by shredding detritivore activities (i.e., small leaf fragments and feces) are used by invertebrate collectors (Cummins & Klug, 1979), while shredders and collectors serve as food for predators (e.g., invertebrates, fish) (Flecker & Townsend, 1994; Yule et al., 2010). Thus, detritivores play an important role in mediating energy and matter transfer from the litter to higher trophic levels with the effects of nutrient enrichment modifying invertebrate-dominated food webs and nutrient cycles (Cross et al., 2003, 2006; Davis et al., 2010).
The colonization of submerged leaf litter by microbes, with the accumulation of microbial biomass and litter softening, generally increases susceptibility to physical abrasion in high flows (Manning et al., 2015), and its palatability to invertebrate detritivores (i.e., shredders; reviewed by Bärlocher & Sridhar, 2014; Graça, 2001). Thus, the stimulation of microbial biomass accumulation and activity on submerged leaf litter by increased nutrient availability in streamwater (see above, Microbially mediated litter processing) may facilitate and promote detritivore colonization of the litter. For instance, Gulis, Ferreira et al. (2006) found higher macroinvertebrate abundance and taxa richness on oak (Quercus robur) and alder (Alnus glutinosa) leaves decomposing in nutrient enriched streams (216–2996 μg NO3-N L-1 and 25–56 μg SRP L-1) than in paired reference streams with ambient nutrient concentration (42–483 μg NO3-N L-1 and 3–16 μg SRP L-1) in central Portugal, and Greenwood et al. (2007) found higher detritivore biomass on rhododendron and maple (Acer rubrum) leaves decomposing in a stream experimentally enriched with N and P (~400 μg DIN L-1 and ~45 μg SRP L-1) than in a reference stream (<30 μg DIN L-1 and <10 μg SRP L-1) in the Appalachian Mountains (North Carolina, USA). Ferreira et al. (2006), however, did not find an effect of experimental N enrichment (214–983 μg NO3-N L-1 vs. 33–104 μg NO3-N L-1 in reference conditions) on macroinvertebrate abundance associated with decomposing litter in a forest stream in the protected area of Açor Mountain (central Portugal). Effects of nutrient enrichment on benthic detritivores associated with decomposing litter are, thus, likely to be context dependent. The facilitation of detritivore colonization of litter under nutrient enriched conditions may be more pronounced on low-nutrient leaf species (e.g., oak, rhododendron), where microbial activity may be nutrient limited to a greater extent and, therefore, more responsive to dissolved N and P than on nutrient-rich leaf species (e.g., alder, maple), which translates into a stronger stimulation of microbial biomass accrual and nutrient immobilization (Greenwood et al., 2007; Gulis, Ferreira et al., 2006; but see Ardón et al., 2006). Also, detritivore contribution to litter decomposition may be greater under moderate nutrient enrichment compared to reference conditions (Gulis, Ferreira et al., 2006), while it may decrease under high nutrient concentrations (Lecerf et al., 2006; Woodward et al., 2012) due to toxicity or other negative effects of concomitant pollutants.
Correlative and experimental tests of the mechanisms through which increased stream nutrient concentrations affect detritivore communities are becoming more common. Available evidence suggests that interactions among streamwater nutrients, microbial immobilization of nutrients on leaf litter, and subsequent reductions to imbalances between detritivore nutrient demands and resource nutrient content are important (Cornut et al., 2015; Demi et al., 2018; Frainer et al., 2016; Manning et al., 2016). Thus, a useful framework that has advanced our understanding of nutrient enrichment-detritivore interactions is ecological stoichiometry theory (Cross et al., 2005 and references therein; Danger et al. [Chapter 3 in this volume]), which considers the mass balance between consumer nutrient demand (typically in terms of biomass C:nutrient ratio) and nutrients supplied in resources (C:nutrient ratio of leaf litter). In detritus-based ecosystems such as forest streams under reference conditions, detritivore nutrient demands can far exceed nutrients contained in autumn-shed leaf litter, which tends to be nutrient poor (Cross et al., 2003; Hladyz et al., 2009). The consequences of these drastic imbalances between nutrient demand and litter nutrient resources for detritivores likely include combinations of reduced growth, reproduction and survival. Thus, since nutrient enrichment modulates litter nutrient concentrations, these constraints on detritivore populations can be reduced, which may lead to increases in the consumption efficiency, individual mass, body condition, and abundance of key detritivore taxa (Connolly & Pearson, 2013; Danger et al., 2013; Halvorson et al., 2018).
Increased P content of leaf litter after nutrient enrichment appears to be a critical factor for detritivore responses to nutrients in some cases, especially given the relatively high and potentially flexible body P of some detritivore taxa (e.g., Tallaperla sp. ~1% P; Cross et al., 2003; Prater et al., 2020), and the importance of dietary P for rapid growth (Demi et al., 2018; Halvorson et al., 2016; Prater et al., 2015), and detritivore fitness (Connolly & Pearson, 2013). In an experimental study of five streams continuously enriched with varying concentrations of N and P, Demi et al. (2019) showed strong positive detritivore community responses, with 30–300% increases in detritivore biomass under nutrient-enriched conditions. The taxa that responded to nutrient enrichment (especially stoneflies of the genera Allocapnia, Leuctra, and Tallaperla and the caddisfly genus Pycnopsyche) also showed increased biomass with nutrient-induced decreases in litter C:P ratios. The relationships between experimental nutrient enrichment, reduced litter C:P ratios, and detritivore biomass found by Demi et al. (2019) are also consistent with correlative evidence from a landscape-scale study that dealt with the effects of a streamwater nutrient (TN, TP) and corresponding litter nutrient (C:N, C:P) gradient (Prater et al., 2015). In this study, detritivores with low body C:P ratios (e.g., caddisflies of the genus Pycnopsyche) tended to have higher abundance and biomass in streams that exhibited higher P concentrations, and corresponding higher quality leaf litter (lower C:P ratios).
2.3 Comparing the Magnitude of Microbial Decomposer vs. Detritivore Effects on Decomposition
The relative importance of microbial and detritivore contributions to leaf litter decomposition changes as decomposition progresses (Hieber & Gessner, 2002) with the initial microbial colonization and conditioning of leaf litter being critical for the subsequent consumption by detritivores (Fig. 16.2; see above Microbially mediated litter processing and Detritivore mediated litter processing). The different traits and functions of microbial decomposers, detritivores, and their interactions can result in different outcomes for their cumulative contributions to leaf litter decomposition under nutrient-enriched conditions. For instance, because microbial decomposers can obtain nutrients from both leaves and continuously renewed dissolved nutrients in flowing water, their responses to elevated dissolved nutrients can be rapid, however, microbial demands will be likely met at relatively low dissolved nutrient concentrations. Detritivores exclusively rely on nutrients from leaf litter (including associated microbial biomass), so their responses to nutrient enrichment are mediated by microbial immobilization of N and P from the water column, especially when freshly fallen leaf litter has low nutrient content. Using field data from an experimental enrichment of a forest stream, combined with microbial assimilation and invertebrate feeding models, Tant et al. (2015) quantified the relative contributions of fungi, bacteria, and detritivores to decomposition rates under reference and nutrient-enriched conditions. Their findings largely confirmed that microbial decomposers contribute 3.9–6.9× more than detritivores at early stages of rhododendron leaf decay (days 0–49) under reference conditions, with contributions of detritivores outweighing those of microbial decomposers at later stages of decay (days 49–108). Under nutrient-enriched conditions, the relative importance of detritivores was greatly increased, so they contributed more to decomposition than microbial decomposers by earlier stages of rhododendron decay (days 7–49) due to an early and relatively high peak of fungal biomass (Tant et al., 2015). This modeling approach to quantify the relative importance of microbial decomposers vs. detritivores in response to nutrient enrichment underscores the possibility that detritivore-mediated decomposition may respond strongly to nutrients, particularly when leaf litter is nutrient poor.
Another common method to separate the effects of macroinvertebrates and microorganisms for litter processing is to enclose leaf litter in mesh bags of different mesh size. Typically, mesh aperture <1 mm is sufficient to exclude larger detritivores, allowing for comparisons between litter decomposition mediated by microbial decomposers alone (kfine) vs. litter processing by shredding detritivores and microbial decomposers together (kcoarse or ktotal). For example, Gulis, Ferreira et al. (2006) showed that litter type was an important driver of differential response of litter decomposition rates to nutrient enrichment when invertebrates were excluded (kfine) vs. present (kcoarse). The relative importance of each group of decomposers can be compared by examining the ratio of kcoarse/kfine (i.e., decomposition due to microbial and detritivore activity together relative to decomposition due to microbial activity only). In Gulis, Ferreira et al. (2006), kcoarse/kfine ratio was 2.54 and 3.59 for alder leaves in reference and nutrient-enriched conditions, respectively, while for slower-decomposing oak, kcoarse/kfine was 1.92 and 3.72 in reference vs. nutrient-enriched streams. Both cases illustrate that kcoarse responded to nutrient enrichment to a greater extent, especially for nutrient-poor oak, demonstrating that nutrient effects on decomposition rates are amplified when detritivores are present. In contrast to this finding, an exhaustive meta-analysis (Ferreira et al., 2015) of nutrient enrichment effects in correlative studies found that litter decomposition rates in coarse and fine mesh bags were stimulated to a comparable degree (26 and 21%, respectively), while in manipulative studies nutrient effects were significant for litter decomposition rates in fine mesh bags (35% increase) but not in coarse mesh bags (Fig. 16.3a). This finding is inconsistent with the prediction that nutrient enrichment should stimulate total decomposition rates more than microbial decomposition rates; however, as noted previously, leaf litter traits, such as C quality and C:nutrient ratios, likely have a modulating effect on the magnitude of microbial and detritivore responses to nutrient enrichment.
Response ratios (knutrient-enriched/kreference) and 95% confidence intervals (CI [vertical bars]) of microbially mediated (i.e., fine mesh) and total (i.e., coarse mesh) decomposition rates from Ferreira et al. (2015) (a), and of microbially (i.e., fine mesh) and invertebrate-mediated (i.e., coarse–fine mesh) decomposition rates as a function of initial litter lignin concentration (%) from a multi-year experimental enrichment of 5 streams (Manning et al., 2016) (b). The horizontal dashed line in (a) and (b) indicates a response ratio of 1 (knutrient-enriched = kreference). In (a), nutrient enrichment stimulated both microbially and total (microbially and invertebrate-mediated) decomposition rates in correlative studies, but only microbially mediated decomposition showed a response in manipulative stream channel studies (the 95% CI around the mean response ratio does not cross 1); response ratios did not differ across treatments (the 95% CIs overlap). In (b), invertebrate-mediated decomposition rate response ratios (filled squares) increased as a function of initial lignin concentration (linear regression; kcoarse–fine response ratio = 2.4 × initial lignin – 18.3; R2=0.75, P = 0.003), whereas microbially mediated decomposition response ratios showed no relationship with initial lignin concentration
Insights from experimental studies and watershed- to regional-scale surveys of litter decomposition rates have been important for gaining a firm understanding of the mechanisms that stimulate leaf litter decomposition in nutrient-rich streams. However, these studies often face inherent shortcomings in terms of the narrow range of nutrient concentrations that are tractable to achieve, in addition to the idiosyncrasies of limited spatial scales (e.g., effects of regional climate or land use). Thus, continental-scale experiments that involve standardized methods and extensive spatial replication offer considerable promise for testing hypotheses related to nutrient enrichment that cannot be addressed by small-scale studies. These continental-scale experiments are especially effective because they reflect the spatial scale of pervasive nutrient enrichment. The pan-European RivFunction project addressed the effects of nutrient enrichment on decomposition rates of oak and alder leaf litter enclosed in both coarse and fine mesh bags that were incubated in 100 streams spanning a broad nutrient gradient (Woodward et al., 2012). Decomposition rates of both oak and alder leaf litter showed a hump-shaped relationship with dissolved nutrient concentration, especially for detritivore-mediated litter decomposition (Woodward et al., 2012). Notably, decomposition rates responded in this way to both dissolved N and P concentrations. Slow leaf litter decomposition in low nutrient streams was likely due to nutrient limitation of microbial activity, while low decomposition rates at extreme nutrient enrichment were likely due to toxic effects to detritivores (e.g., high ammonium or nitrite concentrations) or concomitant changes in other environmental characteristics (e.g., oxygen depletion, smothering by fine sediments, other toxic pollutants). Thus low-to-moderate nutrient enrichment should elicit the strongest response of litter decomposition rates in streams affected by anthropogenic pollution. This generalization was made possible due to the extremely wide ranges of nutrient concentrations that occurred in the 100 study streams across Europe (1–926 µg SRP L-1, 14–21,641 µg DIN L-1). Similar extreme ranges in nutrient concentrations have been documented on other continents, including North and South America, Australia, and Asia (McDowell et al., 2020). However, continental-scale studies investigating nutrient enrichment effects on leaf litter decomposition in these regions are nonexistent, such that it remains unclear whether similar patterns in leaf litter decomposition rates exist across other continents. The sampling bias toward temperate regions is especially important to address given that leaf litter species and benthic communities in tropical regions may respond differently to nutrient enrichment due to the unique phenologies of riparian forests, their phylogenetic context (e.g., LeRoy et al., 2020), and the biogeography of microbial decomposers and detritivores in these catchments (Boyero, Pearson, Dudgeon et al., 2011; Boyero et al., 2015; Seena et al., 2019).
2.4 Litter C Quality and C:Nutrient Stoichiometry
Multiple initial characteristics of autumn-shed leaf litter affect their decomposition rates (Enríquez et al., 1993; Webster & Benfield, 1986). Among these characteristics, we will focus on leaf litter C quality, or abundance of structural plant polymers (e.g., lignin), in addition to leaf litter nutrient stoichiometry (i.e., C:nutrient ratios) and their interactions with streamwater nutrient supply. Litter types with high concentration of recalcitrant compounds (e.g., lignin) have been shown to consistently respond to nutrient enrichment to a greater extent than more labile litter types (Ferreira et al., 2015; Manning et al., 2016), with some exceptions (Ardón et al., 2006). A recent study showed that for standardized C substrates that differed in structural compounds (recalcitrant wood veneers vs. labile cellulose sponge), microbial respiration and decomposition were stimulated on both substrates, but decomposition had a greater response to nutrients on recalcitrant wood veneers (Usher et al., 2020).
A key predictor of slower leaf litter decomposition, beyond bulk C:nutrient concentrations, is leaf litter structural and defense compounds that can delay initial colonization by fungi, and impede detritivore consumption. Specifically, structural plant polymers, like lignin and aromatic compounds related to plant defenses (e.g., polyphenols, tannins), are often associated with slower decomposition rates (Ardón et al., 2006; Jabiol et al., 2019; LeRoy & Marks, 2006). Lignin is especially difficult for many microbial decomposers to break down, requiring the ability to produce ligninolytic enzymes (Hendel et al., 2020). Slow-decomposing leaf litter that contains higher amounts of these structural compounds is often also nutrient poor (i.e., high C:nutrient ratios). However, the relative importance of litter C:nutrient concentration vs. lignin or polyphenol concentration in modulating the effects of dissolved nutrient enrichment remains understudied. In a microcosm experiment investigating the interactions between litter nutrient and lignin concentration across 38 litter species, Jabiol et al. (2019) found that lignin concentration was a stronger predictor of microbial responses to dissolved nutrient enrichment than initial litter nutrient concentration; leaf litter decomposition rates increased 2.9× for lignin-poor leaf litter vs. 1.4× for lignin-rich leaf litter. However, it is well established that microbial activity and decomposition rates of wood that has much higher lignin content than leaf litter (but also lower nutrients) nevertheless respond more strongly to dissolved nutrients than those of leaf litter (Ferreira et al., 2006; Gulis et al., 2004; Stelzer et al., 2003). Thus, it appears that relaxed nutrient limitation due to availability of dissolved nutrients may alleviate limitations specific to structural C compounds as well.
Some detritivore taxa harbor gut microbiota that aid in the digestion of complex C compounds, perhaps reducing the importance of lignin as a barrier to its consumption by detritivores (Canhoto & Graça, 2006). Detritivore consumption of bulk leaf material and non-consumptive uses (i.e., case-building by caddisflies) implies that detritivores may respond differently to the interactive effects of high-lignin litter and nutrient enrichment because of enhanced conditioning that changes the mechanical features of the litter (reduced leaf toughness; Foucreau et al., 2013). To further examine the interplay between leaf litter lignin and nutrient enrichment, we explored responses (response ratio = nutrient-enriched/reference) of 4 leaf types (red maple, tulip poplar [Liriodendron tulipifera], chesnut oak [Quercus prinus], and rhododendron) with variable initial lignin concentration to nutrient enrichment using data from Manning et al. (2016). Litter was enclosed in litterbags with fine and coarse meshes, to allow for comparison between microbial (kfine) and detritivore-mediated (kcoarse–kfine) decomposition rates. These data illustrate increasing response magnitude for decomposition rates mediated by detritivores (plus physical abrasion), compared to microbially mediated decomposition, as a function of initial lignin concentration (Fig. 16.3b). This evidence, combined with previous findings that indicate differential effects of lignin on microbial decomposers vs. detritivores, suggests that microbial decomposition of lignin-rich litter species will respond to a lesser degree to nutrient enrichment than detritivore-mediated litter decomposition.
As mentioned previously (see Microbially mediated litter processing, above), aquatic fungi that colonize leaf litter are able to use both dissolved nutrients and nutrients contained in leaf litter (e.g., Cheever et al., 2013; Pastor et al., 2014; Suberkropp, 1998). This ability can allow fungi to immobilize dissolved inorganic nutrients and alter litter C:nutrient ratios as decomposition progresses (Cheever et al., 2013; Cornut et al., 2015; Gulis et al., 2017). As a result, patterns of nutrient immobilization and the ratio of C:nutrients in decomposing leaf litter are expected to vary through time (from initial to late stages of decomposition), and with litter type, where initially nutrient poor species likely gain disproportionate amounts of nutrients relative to nutrient-rich species (Manzoni et al., 2010; Scott et al., 2013).
Landscape-scale relationships between stream nutrient concentrations and the C:nutrient ratio of leaf litter have been observed, consistent with the prediction that the degree of nutrient immobilization in leaf litter is driven partly by dissolved nutrient availability. Across landscape-scale gradients of stream P concentrations, Scott et al. (2013) observed a negative relationship between litter C:P ratio and increasing stream P. A similar relationship was observed by Prater et al. (2015), with associated consequences for shredding macroinvertebrate communities (see Detritivore-mediated litter processing, above). In experimental contexts, fungal-mediated changes to leaf litter C:nutrient stoichiometry have been shown to be an important link between streamwater nutrients and decomposition rates, especially for invertebrate-mediated decomposition (Manning et al., 2015).
2.5 Mechanistic Effects of N vs. P
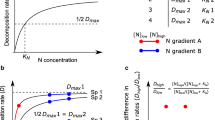
The mechanisms of effects of streamwater N vs. P via effects on fungal biomass, associated change in litter nutrient content, and effects on detritivores are somewhat similar (Manning et al., 2015), with a key difference. A structural equation analysis was used to discern differences in the effects of N vs. P in an experiment in which both nutrients were increased in 5 streams, but with nutrient gradients running in opposite directions (i.e., in high P streams, N was low and in high N streams, P was low). This analysis revealed that dissolved P had effects on decomposition not only through fungal uptake and effects on litter stoichiometry, but that there was additional variance in litter stoichiometry explained by streamwater P concentrations alone, suggesting storage of P when it was available (Fig. 16.4). Nitrogen effects on litter stoichiometry were explained by fungal biomass, with no additional evidence of storage. The ability of fungi to store P but not N, affecting fungal and detrital stoichiometry, has been corroborated in laboratory studies (Gulis et al., 2017). These findings may have implications for management, as fungi may be able to store excess P in relation to its availability in streamwater, which is temporally variable.
Structural equation models showing relationships among streamwater dissolved inorganic nitrogen (DIN; a) and soluble reactive phosphorus (SRP; b) concentrations and drivers of leaf litter decomposition rates. Standardized path coefficients are shown, where the sign of the coefficient indicates the direction of the effect between variables. Arrow weights correspond to path coefficients adjusted based on standard deviations, with the strength of the correlations indicated by arrow width (small, medium, and large arrows denote adjusted coefficients <0.30, >0.30 and <0.45, >0.45, respectively). Path coefficients not significantly different from zero are shown with dashed arrows. Figure redrawn from Manning et al. (2015), with permission.
3 Consequences of Nutrient Effects on Litter Decomposition for Aquatic Ecosystems
3.1 Other Global Change Drivers Interact with Nutrients: Nutrient × Temperature Effects on Leaf Litter Decomposition
The effects of nutrient enrichment on litter decomposition in streams may be modified by concomitant changes in other environmental variables. For instance, nutrient enrichment of streamwater in agricultural contexts can be accompanied by increases in water temperature if the riparian vegetation is removed, which increases the amount of solar irradiation reaching the stream (Gomi et al., 2006; Johnson & Jones, 2000; Kiffney et al., 2003). Also, higher water temperatures in a global warming context can be accompanied by increases in dissolved nutrient concentration because of increased nutrient mineralization and decreased water volume (Moss et al., 2011; Murdoch et al., 2000).
The last decade has witnessed an increase in the number of studies addressing possible interactive effects of nutrient enrichment and increased temperature on litter decomposition. While moderate increases in each factor generally stimulate litter decomposition, their interaction is difficult to predict with synergistic, antagonistic and additive effects reported. For instance, Ferreira and Chauvet (2011) reported synergistic effects between nutrient concentration (low: 1390 μg NO3-N L-1 and 10 μg PO4-P L-1; high: 13860 μg NO3-N L-1 and 100 μg PO4-P L-1) and water temperature (5, 10 and 15 °C) on alder leaf litter decomposition in microcosms, with stronger effects of nutrient enrichment in warm than cold water conditions and stronger effects of warming in high than in low nutrient conditions. Fernandes et al. (2014) also found an interaction between the effects of nutrient concentration (0.09–5 mg NO3-N L-1) and water temperature (12 and 18 °C) on alder and oak leaf litter decomposition in microcosms with stronger effects of temperature at low nutrient concentrations. Piggott et al. (2012) found an interaction between the effects of nutrient concentration and water temperature (ambient and elevated by 1.4 °C) on mahoe (Melicytus ramiflorus) leaf litter tensile strength loss in mesocosms, whereas Piggott et al. (2015) found no significant interaction between the effects of nutrient concentration and water temperature (6 levels, 0.7–6 °C above ambient temperature) on seven mahoe litter decomposition measures in mesocosms. Manning et al. (2018) found additive effects of nutrient concentration (82–517 μg DIN L-1 and 93–9.7 μg SRP L-1, N and P varied in opposite directions) and water temperature (0.8–19.5 °C) on microbial respiration rates in streams under experimental nutrient enrichment over a seasonal temperature gradient. Leaf litter AFDM-specific respiration rates were 1.24–1.51× higher under nutrient enriched conditions and were positively related with temperature, but no interaction was found between factors (Manning et al., 2018). When fungal biomass-specific respiration rates were considered for leaf litter, no effect of nutrient enrichment was detected, while the effect of temperature was positive.
Studies addressing the interaction between nutrient concentration and water temperature are still scarce and use distinct methodological approaches, which make comparisons and predictions difficult. The distinct types of interaction found may reflect different methods or suggest that other factors may modify the interaction between nutrients and temperature (e.g., litter characteristics, biotic communities). For instance, while both temperature and nutrients are generally predicted to increase microbially mediated respiration and leaf processing (Follstad-Shah [Chapter 12 in this volume], Tiegs et al., 2019), higher temperatures likely inhibit stream detritivores over the short term, or potentially extirpate cold-adapted detritivores over the long term, decreasing litter decomposition rates (Boyero, Pearson, Dudgeon et al., 2011; Boyero et al., 2016). Effects of temperature on growth and activity of aquatic fungi also vary across temperatures commonly found in streams (Dang et al., 2009) and can be further complicated by temperature-driven changes in fungal community structure. Nevertheless, considering possible interaction between nutrient concentrations and water temperature could be critical for water resource managers in the face of climate and land use changes that couple higher temperatures and nutrient enrichment. For instance, low nutrient concentration in an oligotrophic mountain stream likely mitigated the effect of experimental stream warming on litter decomposition (Ferreira & Canhoto, 2015), while low water temperature reduced the potential stimulatory effect of high nutrient concentration on litter decomposition in insular streams (Ferreira et al., 2016).
3.2 Nutrient Enrichment Results in Shorter C Residence Time in Streams
Managing nutrient pollution requires consideration of how leaf litter in streams contributes to ecosystem functions that benefit and support ecosystem health and human well-being (i.e., ecosystem services; Frainer et al. [Chapter 21 in this volume], Richardson et al. [Chapter 22 in this volume]); with the understanding that nutrient enrichment may modify the availability (timing, retention and export) of leaf litter resources that fuel stream food webs, as well as stream nutrient uptake rates and export (Newbold, Elwood, O’Neill et al., 1983; Robbins et al., 2019), and feedbacks to the global C cycle and climate change (Follstad-Shah [Chapter 12 in this volume]). Models and observations that target site- to catchment-scale understanding of how nutrients speed the sequence of leaf litter depletion from annual peaks to annual minima could provide several useful benchmarks that link stream leaf litter to its important roles as a driver of other critical stream ecosystem functions. Fortunately, measuring stream ‘decomposition potential’ via litterbag experiments generally mirrors rates of detritus loss at the stream-reach scale (Rosemond et al., 2015) such that litterbag studies remain a viable and economical option to parameterize models and make predictions about annual depletion of leaf litter standing stocks for any stream of interest with relatively few data points (Fig. 16.5). With litter decomposition rate data in hand, investigators could simulate multiple scenarios, including the effects of nutrient enrichment, and apply them to predict how quickly leaf litter would be processed within a given stream (e.g., time to 50% mass loss [T50], mean residence time, etc.). In addition, estimates of the temporal dynamics of leaf litter standing stocks at stream-reach scale could potentially be used within hierarchical models to predict other ecosystem functions that are coupled to the amount of organic matter in the stream, such as nutrient uptake (Robbins et al., 2019), consumer production (Venarsky et al., 2018; Walther & Whiles, 2011), and ecosystem metabolism (Bernot et al., 2010).
Modeled benthic leaf litter mass (g AFDM m-2) within hypothetical streams under reference and nutrient-enriched conditions (after Rosemond et al., 2015) (a). We simulated peak benthic leaf litter mass within the stream from a normal distribution with mean 1000 g AFDM m-2 and standard deviation of 250 g AFDM m-2. We then used random draws from decay coefficients observed for 4 leaf litter species (Acer rubrum [Maple], Quercus prinus [Oak], Liriodendron tulipifera [Tulip poplar], Rhododendron maximum [Rhododendron]) under reference (n = 80) and nutrient-enriched (n = 160) conditions from an experimental nutrient enrichment of 5 streams in the Southern Appalachian mountains, North Carolina, USA (Manning et al., 2016). Solid and dotted curved lines indicate the mean leaf litter mass at time t from 1000 simulations under reference and nutrient-enriched conditions, respectively; gray and light gray areas indicate ± 1SD under reference and nutrient-enriched conditions, respectively. Solid and dotted vertical lines show the time to 50% mass remaining (T50) for reference and nutrient-enriched, respectively. On average, T50 was reduced by about half with nutrient enrichment (T50 = 108 days at reference; T50 = 49 days at nutrient-enriched). In (b), we show how measuring litter decomposition rates using litterbags for these same four litter species can approximate whole-stream litter loss rates. Whole-stream leaf litter loss rates are shown from corresponding streams and years for the four leaf litter types across the five streams under reference (open circles) and years 1 (gray circles) and 2 (black circles) of experimental N and P enrichment. Litterbag rates were quantified from coarse-mesh bags and represent microbial + detritivore-mediated decomposition. The dashed line in each panel represents a 1:1 relationship, the solid line indicates the linear relationship between litterbag and whole-stream litter loss rates
4 Management Implications of Nutrient Enrichment Effects on Leaf Litter Decomposition
4.1 Litterbag-Scale Measurements Are Predictive of Whole Stream Reach Processes
Nutrient enrichment has been shown to have effects on standing stocks of detrital C at whole stream-reach scales (Rosemond et al., 2015). Because both small-scale litterbag measurements and stream-reach measurements were made concurrently, Rosemond et al. (2015) provide evidence that litterbag measurements accurately predict larger scale processes (Fig. 16.5b). Further, decomposition rates from litterbags of medium-quality litter (in terms of both recalcitrance and nutrient content—relative to other litter types tested) were most closely aligned (closest to a 1:1 relationship; maple slope = 0.46, oak slope = 0.40) with stream-reach scale dynamics, whereas low quality litter (rhododendron) had the best predictive relationship (R2= 0.56) with stream-reach rates (Fig. 16.5b). The better predictive power may have been due to the resistance to physical fragmentation (such that biologically-driven processes dominated) for these litterbag measurements. The ability to predict whole-stream standing stocks of detritus, which has been linked to other important stream ecosystem functions, from small scale measurements is a critical step toward fully incorporating heterotrophic processes into management programs devoted to mitigating nutrient enrichment in streams.
4.2 Using Decomposition Rates to Assess Nutrient Effects on Stream Ecosystems
Litterbags can predict larger scale processes and previous work has summarized the characteristics of litter that are best-suited for management applications (Chauvet et al., 2016). Among these characteristics are several noted in this chapter, including the potential utility of exploiting differences in intrinsic leaf litter traits (e.g., lignin, C:nutrient stoichiometry) that greatly affect responses to nutrient enrichment. Alternatively, minimizing variability among leaf litter to isolate the effects of nutrient enrichment using standardized substrates can be a useful approach. For example, standardized cotton-strip assays have been used to generate comparable decomposition rates to understand the global patterns that drive detrital C processing (Tiegs et al., 2019), but they have yet to be applied to understand the effects of nutrient enrichment at such extensive scales (Colas et al., 2019). Further, litter decomposition responses to nutrient enrichment can often be non-linear, complicating efforts to detect and effectively manage the effects of nutrient pollution in streams (Dodds et al., 2010; Jarvie et al., 2013; Woodward et al., 2012). In general, nutrient enrichment is predicted to increase rates of leaf litter processing according to the asymptotic model (e.g., Monod- or Michaelis-Menten-type relationships; Ferreira et al., 2006; Kominoski et al., 2015; Rosemond et al., 2002), but as mentioned throughout this chapter, certain conditions can result in unchanged or slower leaf litter decomposition than would be predicted based on nutrient concentrations alone (e.g., Royer & Minshall, 2001; Woodward et al., 2012). These challenges may require the use of additional standardized substrates, in tandem with substrates like cotton strips, that are sensitive to both microbial and detritivore-mediated decomposition, in order to adequately quantify their interaction in response to nutrient enrichment. Recent evidence from a study that exploited landscape scale nutrient concentration gradient suggests that wood veneer substrates can be predictive of nutrient enrichment effects, and notably, sensitive to relatively narrow ranges of nutrient concentrations (Usher et al., 2020). While wood veneers are unlike leaf litter in many ways, they have several similar intrinsic characteristics that may make them a suitable substrate for detecting either N or P enrichment effects: they have low nutrient content and high lignin concentrations, are consumed by detritivores (e.g., Eggert & Wallace, 2007), and are resistant to physical abrasion. Thus, the combination of standardized cellulose substrates (such as cotton strips) and standardized substrates that are recalcitrant (e.g., wood veneers) could be a powerful tool for predicting nutrient enrichment effects on leaf litter across landscape-scale gradients that either dampen or enhance interactions among nutrients, microbial decomposers, and stream detritivore communities.
5 Conclusions
Nutrient enrichment will continue to threaten freshwater resources that provide for crucial ecological and societal needs. The extent of the problem suggests that many ecosystem functions and the services they provide will be affected, with undesirable consequences for stream ecosystem health. Among the ecosystem functions that occur within streams, leaf litter decomposition is an established metric that is currently and will continue to be affected by nutrient enrichment. Stimulation of heterotrophic pathways via elevated nutrient concentrations has been shown to involve multiple levels of organization, from microorganisms to invertebrates, can affect respiration (CO2 flux), and ecosystem-scale processing of litter-derived C and nutrients. Collectively, experimental enrichment studies, micro- and mesocosm experiments and landscape-scale studies indicate that (1) dissolved N and P affect litter by stimulating fungal activity and nutrient immobilization, thus, increasing detrital nutrient content, (2) the joint effects of N and P together (compared to N and P alone) as well as the presence of detritivores result in greater effects, and (3) the whole-stream reach effects of nutrient enrichment can be predicted from small scale measurements. With the caveat that watershed land use and ecological context are important (e.g., are nutrients limiting to stream heterotroph growth and activity? Are high nutrient concentrations occurring alongside water-quality issues such as low oxygen and sedimentation?), the combined effects of nutrient enrichment on multiple levels of stream food webs increase litter decomposition rates by ~50% on average, reducing residence time of this important resource that forms the energy base of most stream ecosystems. The importance of terrestrial organic matter, and specifically leaf litter, for stream food webs has been documented in numerous contexts; thus, any changes to the timing of its availability could also modify functions that are mediated by stream biota (e.g., nutrient uptake and retention, secondary production, insect emergence). As our mechanistic understanding of leaf litter decomposition and its responses to nutrient enrichment continues to solidify, future efforts should endeavor to fully incorporate this integrative measure of stream ecosystem functioning into nutrient monitoring and management strategies.
References
Abelho, M. (2001). From litterfall to breakdown in streams: A review. The Scientific World Journal, 1, 656–680. https://doi.org/10.1100/tsw.2001.103.
Ardón, M., Stallcup, L. A., & Pringle, C. M. (2006). Does leaf quality mediate the stimulation of leaf breakdown by phosphorus in Neotropical streams? Freshwater Biology, 51(4), 618–633. https://doi.org/10.1111/j.1365-2427.2006.01515.x.
Arroita, M., Elosegi, A., & Hall, R. O. (2019). Twenty years of daily metabolism show riverine recovery following sewage abatement. Limnology and Oceanography, 64(S1), S77–S92. https://doi.org/10.1002/lno.11053.
Azevedo-Pereira, H. V. S., Graça, M. A. S., & González, J. M. (2006). Life history of Lepidostoma hirtum in an Iberian stream and its role in organic matter processing. Hydrobiologia, 559(1), 183–192. https://doi.org/10.1007/s10750-005-1267-1.
Baldy, V., Gessner, M. O., & Chauvet, E. (1995). Bacteria, fungi and the breakdown of leaf litter in a large river. Oikos, 74(1), 93–102. JSTOR. https://doi.org/10.2307/3545678.
Baldy, V., Gobert, V., Guerold, F., Chauvet, E., Lambrigot, D., & Charcosset, J.-Y. (2007). Leaf litter breakdown budgets in streams of various trophic status: Effects of dissolved inorganic nutrients on microorganisms and invertebrates. Freshwater Biology, 52(7), 1322–1335. https://doi.org/10.1111/j.1365-2427.2007.01768.x.
Bärlocher, F. (1985). The role of fungi in the nutrition of stream invertebrates. Botanical Journal of the Linnean Society, 91(1–2), 83–94. https://doi.org/10.1111/j.1095-8339.1985.tb01137.x.
Bärlocher, F., & Sridhar, K. R. (2014). 19. Association of animals and fungi in leaf decomposition. In E. B. G. Jones, K. D. Hyde, & K.-L. Pang (Eds.), Freshwater Fungi. De Gruyter. https://doi.org/10.1515/9783110333480.413.
Benstead, J. P., Rosemond, A. D., Cross, W. F., Wallace, J. B., Eggert, S. L., Suberkropp, K., Gulis, V., Greenwood, J. L., & Tant, C. J. (2009). Nutrient enrichment alters storage and fluxes of detritus in a headwater stream ecosystem. Ecology, 90(9), 2556–2566. https://doi.org/10.1890/08-0862.1.
Bernot, M. J., Sobota, D. J., Hall, R. O., Mulholland, P. J., Dodds, W. K., Webster, J. R., Tank, J. L., Ashkenas, L. R., Cooper, L. W., Dahm, C. N., Gregory, S. V., Grimm, N. B., Hamilton, S. K., Johnson, S. L., Mcdowell, W. H., Meyer, J. L., Peterson, B., Poole, G. C., Valett, H. M., … Wilson, K. (2010). Inter-regional comparison of land-use effects on stream metabolism. Freshwater Biology, 55(9), 1874–1890. https://doi.org/10.1111/j.1365-2427.20.
Boyero, L., Pearson, R. G., Dudgeon, D., Graça, M. A. S., Gessner, M. O., Albariño, R. J., Ferreira, V., Yule, C. M., Boulton, A. J., Arunachalam, M., Callisto, M., Chauvet, E., Ramírez, A., Chará, J., Moretti, M. S., Gonçalves, J. F., Helson, J. E., Chará-Serna, A. M., Encalada, A. C., … Pringle, C. M. (2011). Global distribution of a key trophic guild contrasts with common latitudinal diversity patterns. Ecology, 92(9), 1839–1848. https://doi.org/10.1890/10-2244.1.
Boyero, L., Pearson, R. G., Gessner, M. O., Barmuta, L. A., Ferreira, V., Graça, M. A. S., Dudgeon, D., Boulton, A. J., Callisto, M., Chauvet, E., Helson, J. E., Bruder, A., Albariño, R. J., Yule, C. M., Arunachalam, M., Davies, J. N., Figueroa, R., Flecker, A. S., Ramírez, A., … West, D. C. (2011). A global experiment suggests climate warming will not accelerate litter decomposition in streams but might reduce carbon sequestration. Ecology Letters, 14(3), 289–294. https://doi.org/10.1111/j.1461-0248.2010.01578.x.
Boyero, L., Pearson, R. G., Hui, C., Gessner, M. O., Pérez, J., Alexandrou, M. A., Graça, M. A. S., Cardinale, B. J., Albariño, R. J., Arunachalam, M., Barmuta, L. A., Boulton, A. J., Bruder, A., Callisto, M., Chauvet, E., Death, R. G., Dudgeon, D., Encalada, A. C., Ferreira, V., … Yule, C. M. (2016). Biotic and abiotic variables influencing plant litter breakdown in streams: A global study. Proceedings of the Royal Society B: Biological Sciences, 283(1829), 20152664. https://doi.org/10.1098/rspb.2015.2664.
Boyero, L., Pearson, R. G., Swan, C. M., Hui, C., Albariño, R. J., Arunachalam, M., Callisto, M., Chará, J., Chará-Serna, A. M., Chauvet, E., Cornejo, A., Dudgeon, D., Encalada, A. C., Ferreira, V., Gessner, M. O., Gonçalves, J., Graça, M. A. S., Helson, J. E., Mathooko, J. M., … Yule, C. M. (2015). Latitudinal gradient of nestedness and its potential drivers in stream detritivores. Ecography, 38(9), 949–955. https://doi.org/10.1111/ecog.00982.
Canhoto, C., & Graça, M. A. S. (2006). Digestive tract and leaf processing capacity of the stream invertebrate Tipula lateralis. Canadian Journal of Zoology, 84(8), 1087–1095. https://doi.org/10.1139/z06-092.
Cebrian, J., & Lartigue, J. (2004). Patterns of herbivory and decomposition in aquatic and terrestrial ecosystems. Ecological Monographs, 74(2), 237–259. https://doi.org/10.1890/03-4019.
Chadwick, M. A., & Huryn, A. D. (2003). Effect of a whole-catchment N addition on stream detritus processing. Journal of the North American Benthological Society, 22(2), 194–206. https://doi.org/10.2307/1467992.
Chauvet, E., Ferreira, V., Giller, P. S., McKie, B. G., Tiegs, S. D., Woodward, G., Elosegi, A., Dobson, M., Fleituch, T., Graça, M. A. S., Gulis, V., Hladyz, S., Lacoursière, J. O., Lecerf, A., Pozo, J., Preda, E., Riipinen, M., Rîşnoveanu, G., Vadineanu, A., … Gessner, M. O. (2016). Litter decomposition as an indicator of stream ecosystem functioning at local-to-continental scales: Insights from the European RivFunction project. In A. J. Dumbrell, R. L. Kordas, & G. Woodward (Eds.), Advances in ecological research (pp. 99–182). Academic Press. https://doi.org/10.1016/bs.aecr.2016.08.006.
Cheever, B. M., Webster, J. R., Bilger, E. E., & Thomas, S. A. (2013). The relative importance of exogenous and substrate-derived nitrogen for microbial growth during leaf decomposition. Ecology, 94(7), 1614–1625. https://doi.org/10.1890/12-1339.1.
Chung, N., & Suberkropp, K. (2009a). Contribution of fungal biomass to the growth of the shredder, Pycnopsyche gentilis (Trichoptera: Limnephilidae). Freshwater Biology, 54(11), 2212–2224. https://doi.org/10.1111/j.1365-2427.2009.02260.x.
Chung, N., & Suberkropp, K. (2009b). Effects of aquatic fungi on feeding preferences and bioenergetics of Pycnopsyche gentilis (Trichoptera: Limnephilidae). Hydrobiologia, 630(1), 257–269. https://doi.org/10.1007/s10750-009-9820-y.
Colas, F., Woodward, G., Burdon, F. J., Guérold, F., Chauvet, E., Cornut, J., Cébron, A., Clivot, H., Danger, M., Danner, M. C., Pagnout, C., & Tiegs, S. D. (2019). Towards a simple global-standard bioassay for a key ecosystem process: Organic-matter decomposition using cotton strips. Ecological Indicators, 106, 105466. https://doi.org/10.1016/j.ecolind.2019.105466.
Conley, D. J., Paerl, H. W., Howarth, R. W., Boesch, D. F., Seitzinger, S. P., Havens, K. E., Lancelot, C., & Likens, G. E. (2009). Ecology: Controlling eutrophication: Nitrogen and phosphorus. Science, 323(5917), 1014–1015. https://doi.org/10.1126/science.1167755.
Connolly, N. M., & Pearson, R. G. (2013). Nutrient enrichment of a heterotrophic stream alters leaf litter nutritional quality and shredder physiological condition via the microbial pathway. Hydrobiologia, 718(1), 85–92. https://doi.org/10.1007/s10750-013-1605-7.
Cornut, J., Elger, A., Lambrigot, D., Marmonier, P., & Chauvet, E. (2010). Early stages of leaf decomposition are mediated by aquatic fungi in the hyporheic zone of woodland streams. Freshwater Biology, 55(12), 2541–2556. https://doi.org/10.1111/j.1365-2427.2010.02483.x.
Cornut, J., Ferreira, V., Gonçalves, A. L., Chauvet, E., & Canhoto, C. (2015). Fungal alteration of the elemental composition of leaf litter affects shredder feeding activity. Freshwater Biology, 60(9), 1755–1771. https://doi.org/10.1111/fwb.12606.
Cross, W. F., Benstead, J. P., Frost, P. C., & Thomas, S. A. (2005). Ecological stoichiometry in freshwater benthic systems: Recent progress and perspectives. Freshwater Biology, 50(11), 1895–1912. https://doi.org/10.1111/j.1365-2427.2005.01458.x.
Cross, W. F., Benstead, J. P., Rosemond, A. D., & Wallace, J. B. (2003). Consumer-resource stoichiometry in detritus-based streams. Ecology Letters, 6(8), 721–732. https://doi.org/10.1046/j.1461-0248.2003.00481.x.
Cross, W. F., Wallace, J. B., Rosemond, A. D., & Eggert, S. L. (2006). Whole-system nutrient enrichment increases secondary production in a detritus-based ecosystem. Ecology, 87(6), 1556–1565. https://doi.org/10.1890/0012-9658(2006)87%5b1556:wneisp%5d2.0.co;2.
Cummins, K. W., & Klug, M. J. (1979). Feeding ecology of stream invertebrates. Annual Review of Ecology and Systematics, 10(1), 147–172. https://doi.org/10.1146/annurev.es.10.110179.001051.
Dang, C. K., Schindler, M., Chauvet, E., & Gessner, M. O. (2009). Temperature oscillation coupled with fungal community shifts can modulate warming effects on litter decomposition. Ecology, 90(1), 122–131. https://doi.org/10.1890/07-1974.1.
Danger, M., & Chauvet, E. (2013). Elemental composition and degree of homeostasis of fungi: Are aquatic hyphomycetes more like metazoans, bacteria or plants? Fungal Ecology, 6(5), 453–457. https://doi.org/10.1016/j.funeco.2013.05.007.
Danger, M., Funck, J. A., Devin, S., Heberle, J., & Felten, V. (2013). Phosphorus content in detritus controls life-history traits of a detritivore. Functional Ecology, 27(3), 807–815. https://doi.org/10.1111/1365-2435.12079.
Davis, J. M., Rosemond, A. D., Eggert, S. L., Cross, W. F., & Wallace, J. B. (2010). Nutrient enrichment differentially affects body sizes of primary consumers and predators in a detritus-based stream. Limnology and Oceanography, 55(6), 2305–2316. https://doi.org/10.4319/lo.2010.55.6.2305.
Demi, L. M., Benstead, J. P., Rosemond, A. D., & Maerz, J. C. (2018). Litter P content drives consumer production in detritus-based streams spanning an experimental N: P gradient. Ecology, 99(2), 347–359. https://doi.org/10.1002/ecy.2118.
Demi, L. M., Benstead, J. P., Rosemond, A. D., & Maerz, J. C. (2019). Experimental N and P additions alter stream macroinvertebrate community composition via taxon-level responses to shifts in detrital resource stoichiometry. Functional Ecology, 33(5), 855–867. https://doi.org/10.1111/1365-2435.13289.
Dodds, W. K. (2006). Eutrophication and trophic state in rivers and streams. Limnology and Oceanography, 51, 671–680. https://doi.org/10.4319/lo.2006.51.1_part_2.0671.
Dodds, W. K., Clements, W. H., Gido, K., Hilderbrand, R. H., & King, R. S. (2010). Thresholds, breakpoints, and nonlinearity in freshwaters as related to management. Journal of the North American Benthological Society, 29(3), 988–997. https://doi.org/10.1899/09-148.1.
Dodds, W. K., & Cole, J. J. (2007). Expanding the concept of trophic state in aquatic ecosystems: It’s not just the autotrophs. Aquatic Sciences, 69(4), 427–439. https://doi.org/10.1007/s00027-007-0922-1.
Dodds, W., & Smith, V. (2016). Nitrogen, phosphorus, and eutrophication in streams. Inland Waters, 6(2), 155–164. https://doi.org/10.5268/IW-6.2.909.
Eggert, S. L., & Wallace, J. B. (2007). Wood biofilm as a food resource for stream detritivores. Limnology and Oceanography, 52(3), 1239–1245. https://doi.org/10.4319/lo.2007.52.3.1239.
Elwood, J. W., Newbold, J. D., Trimble, A. F., & Stark, R. W. (1981). The limiting role of phosphorus in a woodland stream ecosystem: Effects of P enrichment on leaf decomposition and primary producers. Ecology, 62(1), 146–158. https://doi.org/10.2307/1936678.
Enríquez, S., Duarte, C. M., & Sand-Jensen, K. (1993). Patterns in decomposition rates among photosynthetic organisms: The importance of detritus C:N: P content. Oecologia, 94(4), 457–471. https://doi.org/10.1007/BF00566960.
Fernandes, I., Seena, S., Pascoal, C., & Cássio, F. (2014). Elevated temperature may intensify the positive effects of nutrients on microbial decomposition in streams. Freshwater Biology, 59(11), 2390–2399. https://doi.org/10.1111/fwb.12445.
Ferreira, V., & Canhoto, C. (2015). Future increase in temperature may stimulate litter decomposition in temperate mountain streams: Evidence from a stream manipulation experiment. Freshwater Biology, 60(5), 881–892. https://doi.org/10.1111/fwb.12539.
Ferreira, V., Castagneyrol, B., Koricheva, J., Gulis, V., Chauvet, E., & Graça, M. A. S. (2015). A meta-analysis of the effects of nutrient enrichment on litter decomposition in streams. Biological Reviews, 90(3), 669–688. https://doi.org/10.1111/brv.12125.
Ferreira, V., & Chauvet, E. (2011). Synergistic effects of water temperature and dissolved nutrients on litter decomposition and associated fungi. Global Change Biology, 17(1), 551–564. https://doi.org/10.1111/j.1365-2486.2010.02185.x.
Ferreira, V., Gulis, V., & Graça, M. A. S. (2006). Whole-stream nitrate addition affects litter decomposition and associated fungi but not invertebrates. Oecologia, 149(4), 718–729. https://doi.org/10.1007/s00442-006-0478-0.
Ferreira, V., Raposeiro, P. M., Pereira, A., Cruz, A. M., Costa, A. C., Graça, M. A. S., & Gonçalves, V. (2016). Leaf litter decomposition in remote oceanic island streams is driven by microbes and depends on litter quality and environmental conditions. Freshwater Biology, 61(5), 783–799. https://doi.org/10.1111/fwb.12749.
Fisher, S. G., & Likens, G. E. (1973). Energy flow in Bear Brook, New Hampshire: An integrative approach to stream ecosystem metabolism. Ecological Monographs, 43(4), 421–439. JSTOR. https://doi.org/10.2307/1942301.
Flecker, A. S., & Townsend, C. R. (1994). Community-wide consequences of trout introduction in New Zealand streams. Ecological Applications, 4(4), 798–807. https://doi.org/10.2307/1942009.
Foucreau, N., Puijalon, S., Hervant, F., & Piscart, C. (2013). Effect of leaf litter characteristics on leaf conditioning and on consumption by Gammarus pulex. Freshwater Biology, 58(8), 1672–1681. https://doi.org/10.1111/fwb.12158.
Fowler, D., O’Donoghue, M., Muller, J. B. A., Smith, R. I., Dragosits, U., Skiba, U., Sutton, M. A., & Brimblecombe, P. (2004). A chronology of nitrogen deposition in the UK between 1900 and 2000. Water, Air, & Soil Pollution: Focus, 4(6), 9–23. https://doi.org/10.1007/s11267-004-3009-1.
Frainer, A., Jabiol, J., Gessner, M. O., Bruder, A., Chauvet, E., & McKie, B. G. (2016). Stoichiometric imbalances between detritus and detritivores are related to shifts in ecosystem functioning. Oikos, 125(6), 861–871. https://doi.org/10.1111/oik.02687.
Gallo, M. E., Porras-Alfaro, A., Odenbach, K. J., & Sinsabaugh, R. L. (2009). Photoacceleration of plant litter decomposition in an arid environment. Soil Biology & Biochemistry, 41(7), 1433–1441. https://doi.org/10.1016/j.soilbio.2009.03.025.
Gessner, M. O., Gulis, V., Kuehn, K., Chauvet, E., & Suberkropp, K.. (Eds.). (2007). Fungal decomposers of plant litter in aquatic ecosystems. In C. P. Kubicek, & I. S. Druzhinina (eds.), Environmental and microbial relationships (pp. 301–324). Springer. https://doi.org/10.1007/978-3-540-71840-6_17.
Gessner, M. O., Swan, C. M., Dang, C. K., McKie, B. G., Bardgett, R. D., Wall, D. H., & Hättenschwiler, S. (2010). Diversity meets decomposition. Trends in Ecology & Evolution, 25(6), 372–380. https://doi.org/10.1016/j.tree.2010.01.010.
Gomi, T., Moore, R. D., & Dhakal, A. S. (2006). Headwater stream temperature response to clear-cut harvesting with different riparian treatments, coastal British Columbia, Canada. Water Resources Research, 42(8). https://doi.org/10.1029/2005wr004162.
Graça, M. A. S. (2001). The role of invertebrates on leaf litter decomposition in streams—A review. International Review of Hydrobiology, 86(4–5), 383–393. https://doi.org/10.1002/1522-2632(200107)86:4/5%3c383:AID-IROH383%3e3.0.CO;2-D.
Greenwood, J. L., Rosemond, A. D., Wallace, J. B., Cross, W. F., & Weyers, H. S. (2007). Nutrients stimulate leaf breakdown rates and detritivore biomass: Bottom-up effects via heterotrophic pathways. Oecologia, 151(4), 637–649. https://doi.org/10.1007/s00442-006-0609-7.
Grimmett, I. J., Shipp, K. N., Macneil, A., & Bärlocher, F. (2013). Does the growth rate hypothesis apply to aquatic hyphomycetes? Fungal Ecology, 6(6), 493–500. https://doi.org/10.1016/j.funeco.2013.08.002.
Gulis, V., Ferreira, V., & Graça, M. A. S. (2006). Stimulation of leaf litter decomposition and associated fungi and invertebrates by moderate eutrophication: Implications for stream assessment. Freshwater Biology, 51(9), 1655–1669. https://doi.org/10.1111/j.1365-2427.2006.01615.x.
Gulis, V., Kuehn, K., & Suberkropp, K. (2006). The role of fungi in carbon and nitrogen cycles in freshwater ecosystems. In G. M. Gadd (Ed.), Fungi in biogeochemical cycles (pp. 404–435). Cambridge University Press. https://doi.org/10.1017/cbo9780511550522.018.
Gulis, V., Kuehn, K. A., Schoettle, L. N., Leach, D., Benstead, J. P., & Rosemond, A. D. (2017). Changes in nutrient stoichiometry, elemental homeostasis and growth rate of aquatic litter-associated fungi in response to inorganic nutrient supply. The ISME Journal, 11(12), 2729–2739. https://doi.org/10.1038/ismej.2017.123.
Gulis, V., Rosemond, A. D., Suberkropp, K., Weyers, H. S., & Benstead, J. P. (2004). Effects of nutrient enrichment on the decomposition of wood and associated microbial activity in streams. Freshwater Biology, 49(11), 1437–1447. https://doi.org/10.1111/j.1365-2427.2004.01281.x.
Gulis, V., & Suberkropp, K. (2003a). Leaf litter decomposition and microbial activity in nutrient-enriched and unaltered reaches of a headwater stream. Freshwater Biology, 48(1), 123–134. https://doi.org/10.1046/j.1365-2427.2003.00985.x.
Gulis, V., & Suberkropp, K. (2003b). Effect of inorganic nutrients on relative contributions of fungi and bacteria to carbon flow from submerged decomposing leaf litter. Microbial Ecology, 45(1), 11–19. https://doi.org/10.1007/s00248-002-1032-1.
Gulis, V., & Suberkropp, K. (2003c). Interactions between stream fungi and bacteria associated with decomposing leaf litter at different levels of nutrient availability. Aquatic Microbial Ecology, 30, 149–157.
Gulis, V., Suberkropp, K., & Rosemond, A. D. (2008). Comparison of fungal activities on wood and leaf litter in unaltered and nutrient-enriched headwater streams. Applied and Environmental Microbiology, 74(4), 1094–1101. https://doi.org/10.1128/AEM.01903-07.
Güsewell, S., & Freeman, C. (2005). Nutrient limitation and enzyme activities during litter decomposition of nine wetland species in relation to litter N: P ratios. Functional Ecology, 19(4), 582–593. https://doi.org/10.1111/j.1365-2435.2005.01002.x.
Halvorson, H. M., Fuller, C. L., Entrekin, S. A., Scott, J. T., & Evans-White, M. A. (2018). Detrital nutrient content and leaf species differentially affect growth and nutritional regulation of detritivores. Oikos, 127(10), 1471–1481. https://doi.org/10.1111/oik.05201.
Halvorson, H. M., White, G., Scott, J. T., & Evans-White, M. A. (2016). Dietary and taxonomic controls on incorporation of microbial carbon and phosphorus by detritivorous caddisflies. Oecologia, 180(2), 567–579. https://doi.org/10.1007/s00442-015-3464-6.
Harpole, W. S., Ngai, J. T., Cleland, E. E., Seabloom, E. W., Borer, E. T., Bracken, M. E. S., Elser, J., Gruner, D. S., Hillebrand, H., Shurin, J. B., & Smith, J. E. (2011). Nutrient co-limitation of primary producer communities. Ecology Letters, 14(9), 852–862. https://doi.org/10.1111/j.1461-0248.2011.01651.x.
Hartmann, J., Lauerwald, R., & Moosdorf, N. (2014). A brief overview of the GLObal RIver Chemistry Database, Glorich. Procedia Earth and Planetary Science, 10, 23–27. https://doi.org/10.1016/j.proeps.2014.08.005.
Hartmann, J., Lauerwald, R., & Moosdorf, N. (2019). GLORICH—Global river chemistry database [Data set]. Supplement to: Hartmann, J et al. (2014): A Brief Overview of the GLObal RIver Chemistry Database, GLORICH. Procedia Earth and Planetary Science, 10, 23–27. https://doi.org/10.1016/j.Proeps.2014.08.005. PANGAEA. https://doi.org/10.1594/pangaea.902360.
Hendel, B., Sinsabaugh, R. L., & Marxsen, J. (2020). Lignin-degrading enzymes: Phenoloxidase and Peroxidase. In F. Bärlocher, M. O. Gessner, & M. A. S. Graça (Eds.), Methods to study litter decomposition: A practical guide (pp. 425–431). Springer International Publishing. https://doi.org/10.1007/978-3-030-30515-4_46.
Hieber, M., & Gessner, M. O. (2002). Contribution of stream detritivores, fungi, and bacteria to leaf breakdown based on biomass estimates. Ecology, 83(4), 1026–1038. https://doi.org/10.2307/3071911.
Hill, B. H., Bolgrien, D. W., Herlihy, A. T., Jicha, T. M., & Angradi, T. R. (2011). A synoptic survey of nitrogen and phosphorus in tributary streams and Great Rivers of the upper Mississippi, Missouri, and Ohio River basins. Water, Air, and Soil pollution, 216(1), 605–619. https://doi.org/10.1007/s11270-010-0556-0.
Hladyz, S., Gessner, M. O., Giller, P. S., Pozo, J., & Woodward, G. (2009). Resource quality and stoichiometric constraints on stream ecosystem functioning. Freshwater Biology, 54(5), 957–970. https://doi.org/10.1111/j.1365-2427.2008.02138.x.
Jabiol, J., Lecerf, A., Lamothe, S., Gessner, M. O., & Chauvet, E. (2019). Litter quality modulates effects of dissolved nitrogen on leaf decomposition by stream microbial communities. Microbial Ecology, 77(4), 959–966. https://doi.org/10.1007/s00248-019-01353-3.
Jarvie, H. P., Sharpley, A. N., Withers, P. J. A., Scott, J. T., Haggard, B. E., & Neal, C. (2013). Phosphorus mitigation to control river eutrophication: Murky waters, inconvenient truths, and “postnormal” science. Journal of Environmental Quality, 42(2), 295–304. https://doi.org/10.2134/jeq2012.0085.
Jenkins, C. C., & Suberkropp, K. (1995). The influence of water chemistry on the enzymatic degradation of leaves in streams. Freshwater Biology, 33(2), 245–253. https://doi.org/10.1111/j.1365-2427.1995.tb01165.x.
Johnson, S. L., & Jones, J. A. (2000). Stream temperature responses to forest harvest and debris flows in western Cascades, Oregon. Canadian Journal of Fisheries and Aquatic Sciences, 57(S2), 30–39. https://doi.org/10.1139/f00-109.
Kaushal, S. S., Mayer, P. M., Vidon, P. G., Smith, R. M., Pennino, M. J., Newcomer, T. A., Duan, S., Welty, C., & Belt, K. T. (2014). Land use and climate variability amplify carbon, nutrient, and contaminant pulses: A review with management implications. Journal of the American Water Resources Association, 50(3), 585–614. https://doi.org/10.1111/jawr.12204.
Kiffney, P. M., Richardson, J. S., & Bull, J. P. (2003). Responses of periphyton and insects to experimental manipulation of riparian buffer width along forest streams. Journal of Applied Ecology, 40(6), 1060–1076. https://doi.org/10.1111/j.1365-2664.2003.00855.x.
Kominoski, J. S., Rosemond, A. D., Benstead, J. P., Gulis, V., Maerz, J. C., & Manning, D. W. P. (2015). Low-to-moderate nitrogen and phosphorus concentrations accelerate microbially driven litter breakdown rates. Ecological Applications, 25(3), 856–865. https://doi.org/10.1890/14-1113.1.
Lecerf, A., Usseglio-Polatera, P., Charcosset, J.-Y., Lambrigot, D., Bracht, B., & Chauvet, E. (2006). Assessment of functional integrity of eutrophic streams using litter breakdown and benthic macroinvertebrates. Archiv Für Hydrobiologie, 165(1), 105–126. https://doi.org/10.1127/0003-9136/2006/0165-0105.
LeRoy, C. J., Hipp, A. L., Lueders, K., Shah, J. J. F., Kominoski, J. S., Ardón, M., Dodds, W. K., Gessner, M. O., Griffiths, N. A., Lecerf, A., Manning, D. W. P., Sinsabaugh, R. L., & Webster, J. R. (2020). Plant phylogenetic history explains in-stream decomposition at a global scale. Journal of Ecology, 108(1), 17–35. https://doi.org/10.1111/1365-2745.13262.
LeRoy, C. J., & Marks, J. C. (2006). Litter quality, stream characteristics and litter diversity influence decomposition rates and macroinvertebrates. Freshwater Biology, 51(4), 605–617. https://doi.org/10.1111/j.1365-2427.2006.01512.x.
Linker, L. C., Dennis, R., Shenk, G. W., Batiuk, R. A., Grimm, J., & Wang, P. (2013). Computing atmospheric nutrient loads to the Chesapeake Bay watershed and tidal waters. Journal of the American Water Resources Association, 49(5), 1025–1041. https://doi.org/10.1111/jawr.12112.
Linkins, A. E., Sinsabaugh, R. L., Mcclaugherty, C. A., & Melillo, J. M. (1990). Comparison of cellulase activity on decomposing leaves in a hardwood forest and woodland stream. Soil Biology & Biochemistry, 22(3), 423–425. https://doi.org/10.1016/0038-0717(90)90123-H.
Manning, D. W. P., Rosemond, A. D., Benstead, J. P., Bumpers, P. M., & Kominoski, J. S. (2020). Transport of N and P in U.S. streams and rivers differs with land use and between dissolved and particulate forms. Ecological Applications. https://doi.org/10.1002/eap.2130.
Manning, D. W. P., Rosemond, A. D., Gulis, V., Benstead, J. P., & Kominoski, J. S. (2018). Nutrients and temperature additively increase stream microbial respiration. Global Change Biology, 24(1), e233–e247. https://doi.org/10.1111/gcb.13906.
Manning, D. W. P., Rosemond, A. D., Gulis, V., Benstead, J. P., Kominoski, J. S., & Maerz, J. C. (2016). Convergence of detrital stoichiometry predicts thresholds of nutrient-stimulated breakdown in streams. Ecological Applications, 26(6), 1745–1757. https://doi.org/10.1890/15-1217.1.
Manning, D. W. P., Rosemond, A. D., Kominoski, J. S., Gulis, V., Benstead, J. P., & Maerz, J. C. (2015). Detrital stoichiometry as a critical nexus for the effects of streamwater nutrients on leaf litter breakdown rates. Ecology, 96(8), 2214–2224. https://doi.org/10.1890/14-1582.1.
Manzoni, S., Trofymow, J. A., Jackson, R. B., & Porporato, A. (2010). Stoichiometric controls on carbon, nitrogen, and phosphorus dynamics in decomposing litter. Ecological Monographs, 80(1), 89–106. https://doi.org/10.1890/09-0179.1.
Marks, J. C. (2019). Revisiting the fates of dead leaves that fall into streams. Annual Review of Ecology, Evolution, and Systematics, 50(1). https://doi.org/10.1146/annurev-ecolsys-110218-024755.
McDowell, R. W., Noble, A., Pletnyakov, P., Haggard, B. E., & Mosley, L. M. (2020). Global mapping of freshwater nutrient enrichment and periphyton growth potential. Scientific Reports, 10(1), 3568. https://doi.org/10.1038/s41598-020-60279-w.
Moore, J. C., Berlow, E. L., Coleman, D. C., Ruiter, P. C., de, Dong, Q., Hastings, A., Johnson, N. C., McCann, K. S., Melville, K., Morin, P. J., Nadelhoffer, K., Rosemond, A. D., Post, D. M., Sabo, J. L., Scow, K. M., Vanni, M. J., & Wall, D. H. (2004). Detritus, trophic dynamics and biodiversity. Ecology Letters, 7(7), 584–600. https://doi.org/10.1111/j.1461-0248.2004.00606.x.
Moretti, M. S., Loyola, R. D., Becker, B., & Callisto, M. (2009). Leaf abundance and phenolic concentrations codetermine the selection of case-building materials by Phylloicus sp. (Trichoptera, Calamoceratidae). Hydrobiologia, 630(1), 199–206. https://doi.org/10.1007/s10750-009-9792-y.
Moss, B., Kosten, S., Meerhoff, M., Battarbee, R. W., Jeppesen, E., Mazzeo, N., Havens, K., Lacerot, G., Liu, Z., de Meester, L., Paerl, H., & Scheffer, M. (2011). Allied attack: Climate change and eutrophication. Inland Waters, 1(2), 101–105. https://doi.org/10.5268/IW-1.2.359.
Mulholland, P. J., & Hill, W. R. (1997). Seasonal patterns in streamwater nutrient and dissolved organic carbon concentrations: Separating catchment flow path and in-stream effects. Water Resources Research, 33(6), 1297–1306. https://doi.org/10.1029/97WR00490.
Murdoch, P. S., Baron, J. S., & Miller, T. L. (2000). Potential effects of climate change on surface-water quality in North America. Journal of the American Water Resources Association, 36(2), 347–366. https://doi.org/10.1111/j.1752-1688.2000.tb04273.x.
Newbold, J. D., Elwood, J. W., O’Neill, R. V., & Sheldon, A. L. (1983). Phosphorus dynamics in a woodland stream ecosystem: A study of nutrient spiralling. Ecology, 64(5), 1249–1265. https://doi.org/10.2307/1937833.
Newbold, J. D., Elwood, J. W., Schulze, M. S., Stark, R. W., & Barmeier, J. C. (1983). Continuous ammonium enrichment of a woodland stream: Uptake kinetics, leaf decomposition, and nitrification. Freshwater Biology, 13(2), 193–204. https://doi.org/10.1111/j.1365-2427.1983.tb00671.x.
Pascoal, C., & Cássio, F. (2004). Contribution of fungi and bacteria to leaf litter decomposition in a polluted river. Applied and Environmental Microbiology, 70(9), 5266–5273. https://doi.org/10.1128/AEM.70.9.5266-5273.2004.
Pascoal, C., Cássio, F., Marcotegui, A., Sanz, B., & Gomes, P. (2005). Role of fungi, bacteria, and invertebrates in leaf litter breakdown in a polluted river. Journal of the North American Benthological Society, 24(4), 784–797. https://doi.org/10.1899/05-010.1.
Pastor, A., Compson, Z. G., Dijkstra, P., Riera, J. L., Martí, E., Sabater, F., Hungate, B. A., & Marks, J. C. (2014). Stream carbon and nitrogen supplements during leaf litter decomposition: Contrasting patterns for two foundation species. Oecologia, 176(4), 1111–1121. https://doi.org/10.1007/s00442-014-3063-y.
Piggott, J. J., Lange, K., Townsend, C. R., & Matthaei, C. D. (2012). Multiple stressors in agricultural streams: A mesocosm study of interactions among raised water temperature, sediment addition and nutrient enrichment. PLoS ONE, 7(11), https://doi.org/10.1371/journal.pone.0049873.
Piggott, J. J., Niyogi, D. K., Townsend, C. R., & Matthaei, C. D. (2015). Multiple stressors and stream ecosystem functioning: Climate warming and agricultural stressors interact to affect processing of organic matter. Journal of Applied Ecology, 52(5), 1126–1134. https://doi.org/10.1111/1365-2664.12480.
Prater, C., Bumpers, P. M., Demi, L. M., Rosemond, A. D., & Jeyasingh, P. D. (2020). Differential responses of macroinvertebrate ionomes across experimental N: P gradients in detritus-based headwater streams. Oecologia. https://doi.org/10.1007/s00442-020-04720-x.
Prater, C., Norman, E. J., & Evans-White, M. A. (2015). Relationships among nutrient enrichment, detritus quality and quantity, and large-bodied shredding insect community structure. Hydrobiologia, 753(1), 219–232. https://doi.org/10.1007/s10750-015-2208-2.
Rincón, J., & Martínez, I. (2006). Food quality and feeding preferences of Phylloicus sp. (Trichoptera: Calamoceratidae). Journal of the North American Benthological Society, 25(1), 209–215. https://doi.org/10.1899/0887-3593(2006)25%5b209:fqafpo%5d2.0.co;2.
Robbins, C. J., Matthaeus, W. J., Cook, S. C., Housley, L. M., Robison, S. E., Garbarino, M. A., LeBrun, E. S., Raut, S., Tseng, C.‐Y., & King, R. S. (2019). Leaf litter identity alters the timing of lotic nutrient dynamics. Freshwater Biology. https://doi.org/10.1111/fwb.13410.
Rose, L. A., Karwan, D. L., & Godsey, S. E. (2018). Concentration–discharge relationships describe solute and sediment mobilization, reaction, and transport at event and longer timescales. Hydrological Processes, 32(18), 2829–2844. https://doi.org/10.1002/hyp.13235.
Rosemond, A. D., Benstead, J. P., Bumpers, P. M., Gulis, V., Kominoski, J. S., Manning, D. W. P., Suberkropp, K., & Wallace, J. B. (2015). Experimental nutrient additions accelerate terrestrial carbon loss from stream ecosystems. Science, 347(6226), 1142–1145. https://doi.org/10.1126/science.aaa1958.
Rosemond, A. D., Pringle, C. M., Ramírez, A., Paul, M. J., & Meyer, J. L. (2002). Landscape variation in phosphorus concentration and effects on detritus-based tropical streams. Limnology and Oceanography, 47(1), 278–289. https://doi.org/10.4319/lo.2002.47.1.0278.
Royer, T. V., & Minshall, G. W. (2001). Effects of nutrient enrichment and leaf quality on the breakdown of leaves in a hardwater stream. Freshwater Biology, 46(5), 603–610. https://doi.org/10.1046/j.1365-2427.2001.00694.x.
Sanpera-Calbet, I., Lecerf, A., & Chauvet, E. (2009). Leaf diversity influences in-stream litter decomposition through effects on shredders. Freshwater Biology, 54(8), 1671–1682. https://doi.org/10.1111/j.1365-2427.2009.02216.x.
Scott, E. E., Prater, C., Norman, E., Baker, B. C., Evans-White, M., & Scott, J. T. (2013). Leaf-litter stoichiometry is affected by streamwater phosphorus concentrations and litter type. Freshwater Science, 32(3), 753–761. https://doi.org/10.1899/12-215.1.
Seena, S., Bärlocher, F., Sobral, O., Gessner, M. O., Dudgeon, D., McKie, B. G., Chauvet, E., Boyero, L., Ferreira, V., Frainer, A., Bruder, A., Matthaei, C. D., Fenoglio, S., Sridhar, K. R., Albariño, R. J., Douglas, M. M., Encalada, A. C., Garcia, E., Ghate, S. D., … Graça, M. A. S. (2019). Biodiversity of leaf litter fungi in streams along a latitudinal gradient. Science of the Total Environment, 661, 306–315. https://doi.org/10.1016/j.scitotenv.2019.01.122.
Smith, V. H., Joye, S. B., & Howarth, R. W. (2006). Eutrophication of freshwater and marine ecosystems. Limnology and Oceanography, 51, 351–355. https://doi.org/10.4319/lo.2006.51.1_part_2.0351.
Sridhar, K. R., & Bärlocher, F. (1997). Water chemistry and sporulation by aquatic hyphomycetes. Mycological Research, 101(5), 591–596. https://doi.org/10.1017/S0953756296003024.
Stelzer, R. S., Heffernan, J., & Likens, G. E. (2003). The influence of dissolved nutrients and particulate organic matter quality on microbial respiration and biomass in a forest stream. Freshwater Biology, 48(11), 1925–1937. https://doi.org/10.1046/j.1365-2427.2003.01141.x.
Stets, E. G., Sprague, L. A., Oelsner, G. P., Johnson, H. M., Murphy, J. C., Ryberg, K., Vecchia, A. V., Zuellig, R. E., Falcone, J. A., & Riskin, M. L. (2020). Landscape drivers of dynamic change in water quality of U.S. rivers. Environmental Science & Technology, 54(7), 4336–4343. https://doi.org/10.1021/acs.est.9b05344.
Stoddard, J. L., Van Sickle, J., Herlihy, A. T., Brahney, J., Paulsen, S., Peck, D. V., Mitchell, R., & Pollard, A. I. (2016). Continental-scale increase in lake and stream phosphorus: Are oligotrophic systems disappearing in the United States? Environmental Science & Technology, 50(7), 3409–3415. https://doi.org/10.1021/acs.est.5b05950.
Suberkropp, K. (1991). Relationships between growth and sporulation of aquatic hyphomycetes on decomposing leaf litter. Mycological Research, 95(7), 843–850. https://doi.org/10.1016/S0953-7562(09)80048-8.
Suberkropp, K. (1992). Interactions with invertebrates. In F. Bärlocher (Ed.), The ecology of aquatic hyphomycetes (pp. 118–134). Springer. https://doi.org/10.1007/978-3-642-76855-2_6.
Suberkropp, K. (1995). The influence of nutrients on fungal growth, productivity, and sporulation during leaf breakdown in streams. Canadian Journal of Botany, 73(S1), 1361–1369. https://doi.org/10.1139/b95-398.
Suberkropp, K. (1998). Effect of dissolved nutrients on two aquatic hyphomycetes growing on leaf litter. Mycological Research, 102(8), 998–1002. https://doi.org/10.1017/S0953756297005807.
Suberkropp, K. & Chauvet, E. (1995). Regulation of leaf breakdown by fungi in streams: Influences of water chemistry. Ecology, 76(5), 1433–1445. https://doi.org/10.2307/1938146.
Suberkropp, K., Gulis, V., Rosemond, A. D., & Benstead, J. P. (2010). Ecosystem and physiological scales of microbial responses to nutrients in a detritus-based stream: Results of a 5-year continuous enrichment. Limnology and Oceanography, 55(1), 149–160. https://doi.org/10.4319/lo.2010.55.1.0149.
Tank, J. L., Rosi-Marshall, E. J., Griffiths, N. A., Entrekin, S. A., & Stephen, M. L. (2010). A review of allochthonous organic matter dynamics and metabolism in streams. Journal of the North American Benthological Society, 29(1), 118–146. https://doi.org/10.1899/08-170.1.
Tant, C. J., Rosemond, A. D., & First, M. R. (2013). Stream nutrient enrichment has a greater effect on coarse than on fine benthic organic matter. Freshwater Science, 32(4), 1111–1121. https://doi.org/10.1899/12-049.1.
Tant, C. J., Rosemond, A. D., Helton, A. M., & First, M. R. (2015). Nutrient enrichment alters the magnitude and timing of fungal, bacterial, and detritivore contributions to litter breakdown. Freshwater Science, 34(4), 1259–1271. https://doi.org/10.1086/683255.
Taylor, B. R., & Chauvet, E. E. (2014). Relative influence of shredders and fungi on leaf litter decomposition along a river altitudinal gradient. Hydrobiologia, 721(1), 239–250. https://doi.org/10.1007/s10750-013-1666-7.
Tiegs, S. D., Costello, D. M., Isken, M. W., Woodward, G., McIntyre, P. B., Gessner, M. O., Chauvet, E., Griffiths, N. A., Flecker, A. S., Acuña, V., Albariño, R., Allen, D. C., Alonso, C., Andino, P., Arango, C., Aroviita, J., Barbosa, M. V. M., Barmuta, L. A., Baxter, C. V., … Zwart, J. A. (2019). Global patterns and drivers of ecosystem functioning in rivers and riparian zones. Science Advances, 5(1), eaav0486. https://doi.org/10.1126/sciadv.aav0486.
US Environmental Protection Agency. (2016). National rivers and streams assessment 2008–2009 report [Reports and Assessments]. https://www.epa.gov/national-aquatic-resource-surveys/national-rivers-and-streams-assessment-2008-2009-report.
Usher, R. L., Wood, J., Bumpers, P. M., Wenger, S. J., & Rosemond, A. D. (2020). Streamwater nutrients stimulate respiration and breakdown of standardized detrital substrates across a landscape gradient: Effects of nitrogen, phosphorus, and carbon quality. Freshwater Science, 39(1), 101–114. https://doi.org/10.1086/707598.
Venarsky, M. P., Benstead, J. P., Huryn, A. D., Huntsman, B. M., Edmonds, J. W., Findlay, R. H., & Bruce Wallace, J. (2018). Experimental detritus manipulations unite surface and cave stream ecosystems along a common energy gradient. Ecosystems, 21(4), 629–642. https://doi.org/10.1007/s10021-017-0174-4.
Wallace, J. B., Eggert, S. L., Meyer, J. L., & Webster, J. R. (1997). Multiple trophic levels of a forest stream linked to terrestrial litter inputs. Science, 277(5322), 102–104. https://doi.org/10.1126/science.277.5322.102.
Walther, D. A., & Whiles, M. R. (2011). Secondary production in a southern Illinois headwater stream: Relationships between organic matter standing stocks and macroinvertebrate productivity. Journal of the North American Benthological Society, 30(2), 357–373. https://doi.org/10.1899/10-006.1.
Webster, J. R. (2007). Spiraling down the river continuum: Stream ecology and the U-shaped curve. Journal of the North American Benthological Society, 26(3), 375–389. https://doi.org/10.1899/06-095.1.
Webster, J. R., & Benfield, E. F. (1986). Vascular plant breakdown in freshwater ecosystems. Annual Review of Ecology and Systematics, 17(1), 567–594. https://doi.org/10.1146/annurev.es.17.110186.003031.
Weyers, H. S., & Suberkropp, K. (1996). Fungal and bacterial production during the breakdown of yellow poplar leaves in 2 Streams. Journal of the North American Benthological Society, 15(4), 408–420. https://doi.org/10.2307/1467795.
Woodward, G., Gessner, M. O., Giller, P. S., Gulis, V., Hladyz, S., Lecerf, A., Malmqvist, B., McKie, B. G., Tiegs, S. D., Cariss, H., Dobson, M., Elosegi, A., Ferreira, V., Graça, M. A. S., Fleituch, T., Lacoursière, J. O., Nistorescu, M., Pozo, J., Risnoveanu, G., … Chauvet, E. (2012). Continental-scale effects of nutrient pollution on stream ecosystem functioning. Science, 336(6087), 1438–1440. https://doi.org/10.1126/science.1219534.
Wurtsbaugh, W. A., Paerl, H. W., & Dodds, W. K. (2019). Nutrients, eutrophication and harmful algal blooms along the freshwater to marine continuum. WIREs Water, 6(5), https://doi.org/10.1002/wat2.1373.
Yule, C. M., Boyero, L., & Marchant, R. (2010). Effects of sediment pollution on food webs in a tropical river (Borneo, Indonesia). Marine & Freshwater Research, 61(2), 204. https://doi.org/10.1071/MF09065.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Manning, D.W.P., Ferreira, V., Gulis, V., Rosemond, A.D. (2021). Pathways, Mechanisms, and Consequences of Nutrient-Stimulated Plant Litter Decomposition in Streams. In: Swan, C.M., Boyero, L., Canhoto, C. (eds) The Ecology of Plant Litter Decomposition in Stream Ecosystems. Springer, Cham. https://doi.org/10.1007/978-3-030-72854-0_16
Download citation
DOI: https://doi.org/10.1007/978-3-030-72854-0_16
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-72853-3
Online ISBN: 978-3-030-72854-0
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)