Abstract
Neuroendocrine neoplasms (NENs) originate from the diffuse neuroendocrine system and, therefore, can arise in every part of the body. In two-thirds of cases, they rise in the gastroenteropancreatic (GEP) tract.
NENs represent a heterogeneous group with variable biological and clinical characteristics. They are usually considered rare neoplasms when compared in terms of incidence with the corresponding non-neuroendocrine neoplasms. NENs represent about 1–2% of the total neoplasms of the gastrointestinal (GI) tract but are of particular diagnostic, prognostic, and therapeutic interest.
The main negative prognostic factors of NENs are the site of the primary tumor [pancreatic NENs (PanNENs) generally have a worse prognosis than NENs in the rectum], the stage according to TNM and the World Health Organization (WHO) histopathological classification, which expresses both the morphological aspect of the tumor cells and their proliferative activity in terms of the number of mitoses or proliferation index (Ki-67).
In PanNENs, for example, Ki-67 labeling index correlates significantly with overall survival and also with disease progression in patients with advanced neoplasms and with tumor recurrence in patients undergoing curative surgery. GEP-NENs are all potentially malignant and are distinguished in tumors and carcinomas.
Tumor grading is defined by both morphology and the proliferative index of the tumor (mitotic index and Ki-67).
According to the aforesaid grading rule, WHO 2019 distinguishes: G1-NETs (well-differentiated morphology, <2 mitosis/10 HPF, and/or Ki-67 <3%); G2-NETs (well-differentiated morphology, 2–20 mitosis/10 HPF, and/or Ki-67 between 3% and 20%); G3-NET (well-differentiated morphology, >20 mitosis/10 HPF, and/or Ki-67 >20%); and NEC (poorly differentiated morphology, >20 mitosis/10 HPF, and/or Ki-67 >20%).
MiNENs (mixed neuroendocrine non-neuroendocrine neoplasms) are neoplasms in which the two components, neuroendocrine and non-neuroendocrine, are both represented in at least 30% of the entire tumor.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Classifications at Present
1.1 Gastroenteropancreatic Neuroendocrine Neoplasms (GEP-NENs): World Health Organization (WHO) 2019 Rules (Fig. 3.1a)
The highest percentage of neuroendocrine neoplasms (NENs) arise in gastroenteropancreatic (GEP) system [1]. GEP-NENs represent a heterogeneous tumor group described by variable biological and clinical characteristics. Histological grading drives GEP-NEN’s clinical outcome and therapeutic strategy.
(a) The World Health Organization (WHO) 2019 classification distinguishes gastro-entero-pancreatic neuroendocrine neoplasms (GEP-NENs) on the basis of morphological aspects (well differentiated and poorly differentiated) and the cyto-proliferative activity of the tumor, expressed as grading (G). The G is based on the proliferative index of the tumor (number of mitoses on 10 high-magnification fields—HPF, High Power Field, with a minimum magnification of 40×) or as a value of Ki-67 (immunohistochemical parameter obtained by measuring the percentage of MIB-1 antibody positive cells out of 2000 cells, evaluated in the area of greatest nuclear labeling). Based on the assessment of the mitotic count and the proliferation index with Ki-67, the G of the GEP-NENs is defined: neuroendocrine tumor (NET) G1, NET G2, NET G3, and neuroendocrine carcinoma (NEC). The proposed cut-off to distinguish NET G1 from NET G2 is 2 mitosis/10 HPF and 3% Ki-67 index. The category of NET G3, characterized by well-differentiated neoplasms but with a Ki-67 proliferative index >20%, includes NENs characterized by high proliferative activity, but well-differentiated morphology, typical of NETs. Finally, a mitotic count >20/10 HPF and a Ki-67 index >20%, but with poorly differentiated morphology, define the NECs. The aforementioned principles, initially proposed in the WHO 2010 classification, were partially modified in the WHO 2017 classification which concerned only the pancreatic site (pancreatic NENs). The result of the changes made to the WHO 2010 classification in the 2017 version for the pancreatic site alone has been condensed, incorporated, and extended to the entire GEP system in the WHO 2019 classification. (b) The terminology to be used to describe lung NENs (LU-NENs) is that contained in the WHO classification, 2015 edition, which identifies four morphological variants: typical carcinoid (CT), atypical carcinoid (CA), large cell neuroendocrine carcinoma (LCNEC), and small cell lung carcinoma (SCLC). CT and CA have well-differentiated morphology, whereas LCNEC and SCLC poorly differentiated. Based on the assessment of the mitotic count and presence/absence of necrosis, the G of the LU-NENs is defined: CT, CA, LCNEC, and SCLC. The proposed cut-off to distinguish CT from AC is <2 mitosis/10 HPF and absence of necrosis. The category of poorly differentiated is defined by a mitotic count >10/10 HPF and presence of necrosis. Cytological features such as cell size, nuclear morphology, and architecture are additional characteristics useful to distinguish between LCNEC and SCLC
GEP-NENs grading is given by their morphological features and proliferative activity evaluation. In contrast to ordinary carcinomas where “grade (G)” represents the histological parameter based on histologic resemblance between neoplastic cells and their normal counterpart, GEP-NENs grading has to be considered properly a prognostic parameter; when G increases, GEP-NENs patients clinical outcome became poorer. Since 2010, WHO classifications defined rigid rules to define the GEP-NENs grading system [2].
1.1.1 Current WHO 2019 Classification Classes
GEP-NENs 2019 WHO classification (hereinafter called simply WHO 2019) defines the following prognostic categories, since 2017 associated only to pancreatic NENs [3, 4] (Fig. 3.1a):
-
A.
Well-Differentiated Neuroendocrine Tumor (NET).
-
NET G1: well-differentiated neuroendocrine tumor, Ki-67 index <3%, and/or mitotic count <2/2 mm2 or 10 higher power fields (HPF);
-
NET G2: well-differentiated neuroendocrine tumor, Ki-67 index 3–20%, and/or mitotic count 2–20/2 mm2 or 10 HPF;
-
NET G3: well-differentiated neuroendocrine tumor, Ki-67 index >20%, and/or mitotic count >20/2 mm2 or 10 HPF.
-
-
B.
Poorly Differentiated Neuroendocrine Carcinoma (NEC).
Poorly differentiated neuroendocrine carcinoma, Ki-67 index >20%, and/or mitotic count >20/ 2 mm2 or 10 HPF. Further distinguished in:
-
Large cells NEC;
-
Small cells NEC.
The importance of separating NEC according to the neoplastic cells size features takes origin from bronchopulmonary NEC and so we will discuss it in paragraph 2.
-
-
C.
Mixed Neuroendocrine–Non-neuroendocrine Neoplasm (MiNEN).
The coexistence of neuroendocrine and non-neuroendocrine components in the same neoplasm is a rare but a well-known phenomenon in the digestive system.
WHO 2019 described this phenomenon as “mixed neuroendocrine–non-neuroendocrine neoplasm (MiNEN)” using the same term just proposed by 2017 WHO pancreatic neoplasm classifications [5]. In more details, MiNENs represent mixed neoplasms composed by the association between well or poorly neuroendocrine and other (non-neuroendocrine) neoplasms only when each counterpart covers at least 30% (≥30%) within the whole neoplasm [6, 7]. MiNENs enclose the previous mixed neoplasm categories: mixed adeno-neuroendocrine tumor (MANET) [8] and mixed adeno-neuroendocrine carcinoma (MANEC) [2, 9, 10].
MANET and MANEC are discussed extensively in further paragraph “From MANEC to MiNEN.”
1.1.2 GEP-NENs Morphological Examination Rules
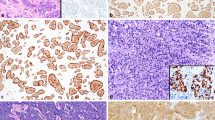
NETs (Fig. 3.2a) are composed by neoplastic cells, uniform in size and features, arranged in trabecular, organoid, gyriform, or ribbon architecture, and cytoplasm is intensively and diffusely (100% of neoplastic cells) stained by general neuroendocrine markers [Synaptophysin (Syn) and Chromogranin A (CgA)] because it is rich in secretory granules. Nuclear chromatin is regular with inconspicuous nucleoli, without atypia. Mitoses are uncommon or at least rare.
The identification of the neuroendocrine phenotype involves the use of immunohistochemical markers capable of defining the neuroendocrine nature of the neoplasm: Chromogranin A (CgA) and Synaptophysin (Syn). (a) NETs show intense positivity for Syn and CgA. (b) NECs preserve positivity for Syn but may show reduced expression of CgA
NEC’s cells (Fig. 3.2b), if small cell (SC) or if large cell (LC) (see Table 3.1), arranged in solid growth pattern, show pleomorphic and highly atypical nuclei rich in mitotic figures intermingled by abundant nonischemic necrosis that may be focal (punctate or spot) or diffuse (geographic or map). Syn and CgA positivity confirmed at immunohistochemistry (IHC) analysis are mandatory; even if Syn staining has to be maintained in whole neoplasm, CgA expression usually in the highest grade tumors. Criteria for distinguishing SC from LC and NETs from NECs are deeply listed in Table 3.1 [11, 12, 18,19,20].
1.1.3 Proliferative Indices: Mitotic Index (MI) and Ki-67 Labeling Index (Ki-67 LI)
-
Mitotic index (MI): Understood as the number of mitoses on 10 high-magnification fields [high power field (HPF), with minimum magnification 40×] corresponding to a tumoral area of 2 mm2 [2].
-
Ki-67 labeling index (LI): An immunohistochemical parameter obtained by measuring the percentage of Ki-67 (MIB-1 antibody) nuclear positivity in tumoral cells out of 500–2000 cells (corresponding to a tumoral area of 2 mm2), evaluated in the area of greatest nuclear marking, the so called hot spot [2].
Some tips and tricks are useful to properly define the tumoral area where proliferative indices will be evaluated. The aforesaid 2 mm2 has to be searched in the so-called specimen’s “hot-spots” in depth areas where at panoramic (larger microscopic fields) observation the higher proportion of stained nuclei and/or mitotic figures could be detected. According to WHO classification since 2010, the aforesaid 2 mm2 areas could be properly covered by 10 high power optical microscopic field (HPF) at 40× magnification considering that each HPF could be sized at 0.5 mm [2]. Of note, HPF real size in the current microscopes available is not uniform covering a range between 0.096 and 0.31 mm2. As concluding remark considering 10 HPF, according to WHO, could not be precisely reproducible in daily practice, otherwise could be better to consider HPF final number according to the specific microscope considering that each manufacturer should indicate the HPF size in mm2 [21].
MI is the quantitative expression of M phase’s cell cycle. The M phase is the shortest of the cell cycle and is therefore very fleeting. As a result, MI underestimates proliferating cells; otherwise Ki-67, a nuclear antigen expressed in proliferative cells (both S and M phases), has been proven as the powerful independent tool in predicting NENs clinical outcome [22,23,24,25,26,27,28].
1.2 Lung Neuroendocrine Neoplasms (LU-NENs) WHO 2015 Rules (Fig. 3.1b)
Lung neuroendocrine neoplasms (LU-NENs) represent a heterogeneous group of tumors showing different morphological features and clinical aggressiveness. According to 2015 WHO classification, LU-NENs are distinguished in four morphological and prognostic categories namely typical carcinoid (TC) and atypical carcinoid (AC), well-differentiated NENs, respectively, large-cell neuroendocrine carcinoma (LCNEC) and small-cell lung carcinoma (SCLC), still called microcitoma (Fig. 3.1b), poorly differentiated NENs, respectively [29, 30]. LU-NENs classification, similarly to GEP-NENs, considered as main skill the distinction between well or poorly differentiated NENs, using tumoral cells proliferation, exclusively identified by mitotic index, and necrosis assessment (Fig. 3.1b).
Different past terminologies are not recommended, deeply “carcinoma” and/or “malignant carcinoid,” to collectively indicate TC and AC have to be carefully avoided because they could lead to inappropriate treatments [30].
Among the main well and poorly differentiated LU-NENs classes, differential diagnosis is based on the presence/absence of necrosis and the mitotic index (MI) per 2 mm2 (Fig. 3.3). In more details, TC does not show necrosis and MI is <2 mitosis per 2 mm2, while AC group shows necrosis, even if focal and/or MI between 2 and 10 per 2 mm2 finally poorly differentiated carcinomas must have >10 mitosis per 2 mm2 and extensive necrotic areas [30]. Cytological features such as cell size, nuclear morphology, and architecture are additional characteristics useful to distinguish between LCNEC and SCLC, but not between AC and TC that, according to their common well-differentiated morphology, always share similar cyto-architectural features [30]. LU-NENs, confirmation is given by immunohistochemical markers such as CgA, Syn, and NCAM/CD56 [30]. WHO indicated CgA and Syn as reliable neuroendocrine markers (Fig. 3.4), NCAM/CD56, cell adhesion molecule, as helpful but not mandatory marker [29] and neuron-specific enolase, has been not recommended, because it lacked reproducibility [30].
Criteria for diagnosis of lung neuroendocrine neoplasms (LU-NENs). Terminologies used in the past are not recommended, indeed should be carefully avoided, in particular the use of the term “carcinoma” to collectively indicate typical carcinoid (CT) and atypical carcinoid (CA) or that of “malignant carcinoid,” because they could lead to inappropriate therapeutic treatments or not be consistent with the classification criteria. Based on the morphological and immunophenotypic similarities between the neoplastic cells of CT (A) and CA (B) and the normal cellular counterpart of the diffuse NE system of the respiratory system, they represent a group of well-differentiated tumors (NETs) as opposed to large cell neuroendocrine carcinoma (C) and small cell carcinoma (D) which are poorly differentiated carcinomas
Immunohistochemical criteria for the diagnosis of lung neuroendocrine neoplasms (LU-NENs). Chromogranin A (CgA) and synaptophysin (Syn) are the most helpful neuroendocrine lung tumors immunohistochemical markers. A low proliferation rate is seen in typical carcinoid by Ki-67 staining compared with atypical carcinoid where it is usually between 5% and 20%. Large cell neuroendocrine carcinoma and small cell carcinoma have very high proliferation rates (Ki-67 proliferative index >20%) and CgA weakly stains the tumor cells
LU-NENs WHO classification considered Ki-67 LI (%) (expressed as the percentual of positive tumor cells) only as additional parameter, in contrast European Neuroendocrine Tumor Society (ENETS) required Ki-67 for adequate diagnostic and prognostic LU-NENs assessment [31]. Deeply according to WHO, the main diagnostic Ki-67 role is still limited to: (1) distinguish the TC and AC from the high-grade LCNEC and SCLC [32], with a practical cutoff point of 25% to operate this distinction [29, 32]; (2) distinguish TC and AC from poorly differentiated NECs, in particular SCLC, in limited diagnostic material (cytology and biopsies) [33].
Even if Ki-67 role in LU-NENs prognostic stratification remains controversial, an intriguing proposal for LU-NENs “classification” has recently advanced. It has been based on the integration of three parameters: (1) Ki-67 labeling index evaluation, (2) necrosis assessment, and (3) MI, each of these categorized by three different “cutoffs” leading to the identification of three LU-NENs prognostic categories (G1, G2, and G3). These levels are indicated as follows: level 1 (G1) (2 mitoses, Ki-67 <4%, tumor necrosis absent), level 2 (G2) (>2–47 mitoses, Ki-67 4–25, tumor necrosis <10%), and level 3 (G3) (>47 mitoses, Ki-67 >25, tumor necrosis >10%) [34, 35]. In depth, G1 (well-differentiated, low-grade) tumors are those that show at least two of three parameters at level 1, G2 (well-differentiated, intermediate-grade) tumors are identified by two of three parameters at level 2, and finally G3 (poorly differentiated, high-grade) tumors are characterized by at least two out of three parameters at level 3. Thus, tumors G1 include all TC and a fraction of AC, G2 tumors include most of AC but also some SCLC and LCNEC, and G3 tumors add up most of SCLC and LCNEC but even a small fraction of AC. This subdivision is in line with the literature data that see AC and LCNEC as somewhat heterogeneous categories of tumors from the behavioral point of view and a fraction of SCLC characterized by long survival. However, this proposal must be confirmed by independent and prospective validation studies [35, 36].
In conclusion even if the WHO includes only MI and assessment of necrosis [30], the ENETS in consensus statement on best practices for pulmonary neuroendocrine tumors noted that tumor grading based on a combination of Ki-67, mitotic rate, and necrosis may be of clinical importance but lacks validation [31]. According to recent evidences, a major role for Ki-67 also in LU-NENs classifications is requested [29, 37].
1.3 TNM in GEP- and LU-NENs
NENs are malignant tumors and consequently should be staged according to a site-specific staging system (TNM). NENs spread equally via blood or lymphatic system, and metastasis represents the most important prognostic determinant after grading. Lymph nodes represent the main GEP-NENs metastatic site followed by liver, lung, peritoneum, and pancreas.
At present, two different TNM systems work as NENs staging systems in more details:
-
1.
The Union for International Cancer Control and the American Joint Committee on Cancer (UICC/AJCC) have published TNM classification (eighth edition) that have been applied to well-differentiated NETs (Grade 1 and 2) of the gastrointestinal tract, including the pancreas. Whereas, poorly differentiated (Grade 3) NECs are excluded and should be classified according to criteria for classifying carcinomas at the respective site [20, 38].
-
2.
Based on clinical, surgical, and imaging data, European Neuroendocrine Tumor Society (ENETS) has published TNM staging recommendations that have been applied to all grades of NENs [39, 40]. Information on the presence or absence of metastasis has to be available as a minimum requirement for ENETS staging [39, 40].
ENETS staging system introduced different staging rules in comparison to UICC/AJCC (eighth edition) in gastric, appendicular, and pancreatic locations [40, 41] staging of pancreatic NENs (PanNENs) according to ENETS staging stratify better than UICC/AJCC into prognostically significant groups, whereas UICC/AJCC has been proved better in appendix NEN’s patients clinical outcome prediction [42]. It is advisable to apply both schemes.
ENETS system applies to gastrointestinal NENs of foregut, midgut, and hindgut. Foregut NETs are further divided into three groups: (a) gastric NENs; (b) duodenum, ampulla, and proximal jejunum NENs; and (c) PanNENs. Midgut and hindgut NENs category include lower jejunum/ileum, appendix, and colon/rectum. ENETS staging criteria use stage 0 only for the stomach because this is the only anatomic site where T is defined. Stage I includes the T1 NENs with limited growth. Stage II applies to the tumors larger in size or more invasive, either T2 or T3, but always in the absence of metastasis. Stage III includes tumors with invasion into surrounding structures but without lymph node metastasis (stage IIIA) and tumors of any AJCC T stages (T1, T2, T3, and T4) in the presence of regional node metastasis (stage IIIB). Stage IV includes tumors of any AJCC T stage and of any AJCC N stage (N0 or N1) with the presence of distal metastasis (M1) at the time of initial diagnosis [39, 40].
LU-NENs staging currently is represented by the TNM UICC/AJCC system, eighth edition, and should be classified according to criteria for carcinoma of the lung [38]. For carcinoids, however, the descriptive categories and the impact of multicentricity will have to be better defined, to better adhere to the biological reality of these neoplasms. For example, many multiple carcinoids, TC or AC, are multicentric synchronous primitive neoplasms rather than intrapulmonary metastases, especially if born in diffuse idiopathic pulmonary neuroendocrine cell hyperplasia. For SCLCs, the use of terms such as “extended disease” and “limited disease” is discouraged, the latter being, in turn, diversifiable into subgroups with different prognosis according to the TNM system [43, 44].
2 Moving to 2017/2019 GEP Classifications
2.1 NET G3 History (Fig. 3.5)
The term well-differentiated neuroendocrine tumor (NET) G3 is a neologism used for the first time by a French group [13], and then later also by other authors [14, 45, 46], that means GEP-NEN with a well-differentiated (WD) morphology and a Ki-67 higher than 20%. NETs G3, until 2017 WHO classification was enclosed in NEC G3 covered 10–20% of all GEP NECs [12, 14, 46]. Compared to the latter, NET G3 patients were younger, the primary tumor site was especially the pancreas, and the overall survival was significantly better than the NECs [14, 47]. When gastrointestinal (GI) NETs G3 were analyzed separately, as it happened for pancreatic (Pan) NETs G3, a great difference was detected with the respective NECs (both GI and Pan); it came to light that colorectal locations, such as the pancreas, have a poor prognosis [12, 14,15,16,17].
Classification of high-grade gastro-entero-pancreatic neuroendocrine neoplasms (H-NENs) according to scientific literature. The prognostic categories neuroendocrine tumor (NET) G3 and neuroendocrine carcinoma (NEC) <55% and NEC ≥55% are defined by the contextual application of the morphological characterization and the quantitative evaluation of the proliferation, mainly through Ki-67, of the neoplastic cells. Therefore, the three categories of H-NENs show important differences between them both in prognostic terms and in terms of therapeutic approach
Mitotic index (MI) was considered inferior to Ki-67 LI to define NET G3 [12]. A possible explanation is that mitoses are related to a shorter phase (M phase) of the cell cycle than the Ki-67 antigen, present in the nucleus in all phases of the cell cycle, while it is absent in phase G0 (growth).
Although all authors agree that NET G3 Ki-67 should be above 20%, a precise upper limit was not defined, with variable reported values: 55% in Italian study, 60% in French, and 70% in the European study [12]. In the studies that compare GEP-NET G3 to NEC, median Ki-67 (range) was 30 [13,14,15,16,17, 26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70] in NET G3 compared to 80 (25–100) in NEC [14, 46, 48]. A possible explanation could be related to the absence of reproducibility of study method: only Italian group selected case after a centralized pathologist revision, only the Nordic Group and Italian studies performing a specific analysis to find a threshold for Ki-67, showed that the 55% threshold works well from the prognostic point of view [14, 48], otherwise other groups reported descriptive statistics to evaluate of Ki-67 identified WD NETs with Ki-67 > 55%. Probably, in these rare conditions, WD NETs could have a component mixed of poorly differentiated (PD) NECs, and the Ki-67 could be evaluated as the median of the whole neoplasm, resulting in a Ki-67 >55% [49]. Therefore, in these particular cases, it would be more appropriate to evaluate Ki-67 separately in WD and PD areas, assigning two different values [12].
Over the years, given the previous WHO 2010 classification, NET G3 patients have been treated similarly to NEC even in the absence of sufficient information that these are clinically very different malignancies. Several retrospective studies showed that NETs G3 are less sensitive to platinum-based chemotherapy, cisplatin or carboplatin plus etoposide, than NEC. This is probably due to the fact that prognosis NET G3 is more related to the WD morphology of the tumor rather than the Ki-67 value, considering NETs G3 closer to NETs G2 than to NECs. In addition, positive responses to chemotherapy have been reported in patients NET G3 undergoing a combination of oxaliplatin with fluoropyrimidines, based on clinical evidence that oxaliplatin shows synergy with chemotherapeutic treatments potentially active both in G2 and in G3 NENs [50,51,52,53]. However, even if much rare, NETs G3 with Ki-67 index above 55% should be evaluated with particular consideration in terms of progression, prognosis, and behavior versus the traditional protocol, [54] and cisplatin or carboplatin plus etoposide regimen may be considered.
In clinical practice, identifying a PanNET G3 is perhaps more important of a GI NET G3, since molecular targeted agents, sunitinib and everolimus, are approved in well/moderately differentiated PanNET regardless of Ki-67 value.
Regarding the use of treatment with immune checkpoint inhibitors targeting PD-L1 or PD-1, NETs G3 have a cold immune microenvironment with few tumor infiltrating lymphocytes and the lower expression of PD-L1 compared to NECs. Likewise, NET G3s have a lower mutation burden than NECs, not making so them a potential target for immune checkpoint inhibitors [26, 55,56,57].
As concluding remark, it is important for clinicians that pathologists report both information on tumor morphology and the value of Ki-67.
2.2 Ki-67 New CutOff (Fig. 3.5)
Only about 5% of all GEP-NENs belongs to the G3 category [42], anyhow the distinction between NET G3 and NEC G3 is clinically and prognostically significant, and it is associated with adverse prognosis, good response to platinum-based chemotherapy (PBC), and insufficient response to temozolomide chemotherapy [12,13,14,15, 28, 45,46,47, 58,59,60]. Since 2011, several studies specifically investigating large series of GEP-NENs G3 have been published, including about 800 patients [12].
Category G3 with Ki-67 >20% is extremely heterogeneous and the broad interval (Ki-67 between 21% and 100%) include a spectrum of different neoplasms, with several responses to therapy [12,13,14,15, 28, 45,46,47, 58,59,60]. Median Ki-67 in poorly differentiated (PD) was 80% (range 25–100) and in well-differentiated (WD) 30% (range 21–70). Hence the ENETS, in the context of G3 category, proposed that the division of high-grade NENs (H-NENs) into three categories (NET G3, NEC <55%, and NEC ≥55%) in addition to a prognostic value also has therapeutic implications [42]. On the one hand, the prognostic role of the 55% threshold (Ki-67 value) into NECs has been already validated in different studies, and it has also included into ENETS guidelines since 2016. From these evidences, we can consider the spectrum of high-grade NENs (H-NENs) in three prognostic categories NET G3, NEC <55%, and NEC ≥55%, respectively, with good, intermediate, and worst prognosis [14, 24, 28, 42, 48, 49, 61]. In fact, from the study of several cohorts of patients with high-grade and PD lesions (PD NECs), a remarkable heterogeneity emerged from a molecular, prognostic, and therapeutic response point of view. For example, an Italian study has shown that for these lesions (PD NECs), the 55% threshold for the fraction of Ki-67-positive tumor cells (Ki-67 value or Ki-67 labeling index) allows a better stratification of the prognosis estimate; in particular, NECs with Ki-67 value <55% (NEC <55%) were associated with a median overall survival (mOS) of 12.9 months, whereas NECs with Ki-67 value ≥55% (NEC ≥55%) an mOS of 5.3 months [14]. On the other hand, for NET G3, a therapeutic approach similar to that used for category G2 is often considered, debating biological therapies, chemotherapy, peptide receptor radionuclide therapy, and liver-directed treatments within a multidisciplinary team. In GEP-NENs G3, chemotherapy represents the most common therapeutic approach. Although these neoplasms appear relatively chemosensitive, their prognosis is poor. The most frequently proposed therapy is PBC possibly combined with etoposide. It has been seen that an alkylating-based or oxaliplatin-based chemotherapy can be considered in NECs <55%, whereas therapies represented by PBC combined with etoposide in NECs ≥55% [13, 14, 46, 48, 61, 62].
From a molecular point of view, it is impressive observing that gene mutations were significantly enhanced in NECs ≥55%, involving TP53, KRAS, and BRAF genes [28]. In the context of H-NENs, the distinction between NET and NEC is also important for the study of the tumor microenvironment and the possible use of immune checkpoint inhibitors. We know that expression of PD-L1, tumor-infiltrating lymphocytes (TILs), tumor mutation burden (TBM), and neoantigen load can predict response to immune checkpoint blockade. Having said that, WD NETs do not seem like good candidates for immunotherapy, at least theoretically. Indeed, NETs G1/G2 have a cold immune microenvironment with few TILs and heterogeneous expression of PD-L1, whereas NECs have hot immune microenvironment with abundant TILs, a greater expression of PD-L1 and a high TBM. Moreover, applying the 55% threshold (Ki-67 value) into PD H-NENs, NECs ≥55% present an extensive mutational load, a dense immune infiltration and a higher expression of PD-L1 than NECs <55%. Therefore, due to their immune background, NECs ≥55% seem to represent excellent candidates for immunotherapy [26, 28, 55,56,57, 63].
In conclusion, based on growing evidence, the Ki-67 proliferative index should be used together with morphology, to define the following five categories: NET G1 (Ki-67 <3%, WD), NET G2 (Ki-67 3–20%, WD), NET G3 (Ki-67 21–54%, WD), NEC G3 (Ki-67 21–54%, PD), and NEC G4 (Ki-67 ≥55%, PD) [12].
However, part of the scientific community does not fully agree on the 55% threshold [64]. The latter consider Ki-67 thresholds intrinsically arbitrary and not necessarily generalizable between tumor types (i.e., NET versus NEC, various sites of origin) and outcomes (as prognosis or prediction). In particular, they claim that an absolute Ki-67 threshold is not applicable to the distinction between NET G3 and NEC. Therefore, further studies are needed to validate this threshold.
2.3 From MANEC to MiNEN (Figs. 3.6 and 3.7)
Mixed neuroendocrine–non-neuroendocrine neoplasms (MiNENs) are defined by the coexistence in the same neoplasm of two different oncotypes: the neuroendocrine type plus the non-neuroendocrine type, variously associated from a qualitative and quantitative point of view [3, 4, 6, 8].
Analogous view for mixed (neuroendocrine non-neuroendocrine) neuroendocrine neoplasms (MiNENs). The classification of gastro-entero-pancreatic neuroendocrine neoplasms (GEP-NENs) according to WHO 2019 includes all mixed neuroendocrine and non-neuroendocrine neoplasms in the new “mixed neuroendocrine non-neuroendocrine neoplasm” (MiNEN) category. MiNEN combines the category of mixed adeno-neuroendocrine carcinoma (MANEC) and the categories of the very rare mixed adeno-neuroendocrine tumor (MANET). MiNEN always consists of invasive neoplasms; on the contrary, the association of “adenoma” and “neuroendocrine carcinoma” (NEC) will not produce either MiNEN or MANEC but simply NEC and adenoma
Quantitative Association
The 2019 WHO classification has maintained the “magic” number of 30%, that it is an arbitrarily defined percentage but on which there is wide agreement in the scientific world [3]. A neoplasm is considered mixed if at least one of the two “neuroendocrine and non-neuroendocrine” components is represented in at least 30% of the entire tumor [2].
Given the importance of the number 30%, when the presence of a mixed neoplasm on a histological section is suspected, it is necessary to ensure that the whole neoplasm has been sampled in such a way that this assessment is effectively applied to the entire neoplastic extension, the partial evaluation of the neoplasm risks underestimating and/or overestimating the real extension of the two components [4, 9].
There are mixed neoplasms from a morphological and biological point of view which however do not respond to the 30% cutoff on which currently there are no classification criteria, and they are generally described as adenocarcinomas with neuroendocrine differentiation (NED) or neuroendocrine carcinomas with focal glandular differentiation [65,66,67,68,69]. The standard of >2% cut is according to the 1% neuroendocrine cell in normal mucous [66, 70]. These latter conditions are rare, or perhaps not yet studied and understood, and there are no targeted studies so there is no scientifically validated information on the prognostic weight of the presence of the mixed component in these cases compared to the same pure tumor.
The presence of neuroendocrine cells within colorectal adenocarcinomas (CR-ADCs) has been documented and therefore it is not an unexpected data [71, 72]. In fact, up to 41% of CR-ADCs can have immunohistochemically detectable neuroendocrine cells and the frequency of these neuroendocrine cells appears to be dependent on the method of determination [73,74,75,76,77]. It has also been seen that poorly differentiated CR-ADC appeared to have more frequent NED [7, 78, 79]. In these poorly differentiated ADCs, neuroendocrine cells are poorly differentiated (presenting as an oval, round, or irregular shape without polarizations) and similar to the adjacent non-neuroendocrine tumor cells; by contrast well-differentiated ADCs include well-differentiated neuroendocrine cells (presenting as a pyramid or bar shaped with the apex pointing to the cavity of the gland) [74].
Qualitative Association
This represents the real revolution or novelty of the 2019 WHO classification, which has determined the new diagnostic category (MiNEN), previously adopted in 2017 for mixed neuroendocrine neoplasm of the pancreas [3, 4, 7].
MiNEN overcomes a problem of clinical and nosological practice represented by the previous definition of mixed adeno-neuroendocrine carcinoma (MANEC), which is a neoplasm that showed both glandular and neuroendocrine differentiation, thus overcoming the problem of neoplastic differentiation [2, 8,9,10].
With the term “MANEC,” it was assumed that the two components were both carcinomatous and in particular glandular (adenocarcinoma) and neuroendocrine (neuroendocrine carcinoma) [8,9,10, 24].
If the non-neuroendocrine component is restricted to a precursor lesion such as a noninvasive carcinoma (i.e., adenoma) the neoplasm has to be considered pure NEC [24].
Neoplasm previously treated with neoadjuvant therapy should not be considered MiNEN even if the mixed nature of the neoplasm was defined on different specimen obtained before the aforementioned treatment [24, 80, 81].
The new definition “MiNEN” goes further and widens the definition of the two components: neuroendocrine and non-neuroendocrine. On the one hand, it expands the spectrum of non-neuroendocrine lesions, including non-glandular histotypes (for example, squamous cell carcinomas or acinar cell carcinomas for pancreas) and also non-carcinomatous lesions (for example, adenomas). On the other hand, the term MiNEN includes also well-differentiated neuroendocrine neoplasms. Therefore, regardless of the type of non-neuroendocrine lesion, there will be two categories of mixed neoplasms: mixed neoplasms with well-differentiated neuroendocrine components (mixed adeno-neuroendocrine tumor, MANET) and mixed neoplasms with neuroendocrine carcinoma (mixed adeno-neuroendocrine carcinoma, MANEC). In conclusion, the sum of MANET plus MANEC defines the great MiNEN galaxy [2,3,4, 6,7,8,9,10, 24].
References
Dasari A, Shen C, Halperin D, Zhao B, Zhou S, Xu Y, et al. Trends in the incidence, prevalence, and survival outcomes in patients with neuroendocrine tumors in the United States. JAMA Oncol. 2017;3(10):1335–42.
Bosman F, Hruban R, Theise N, et al. WHO classification of tumours of the digestive system. Lyon: IARC Press; 2010.
Klimstra DS, Kloppell G, La Rosa S, Rindi G. Classification of neuroendocrine neoplasms of the digestive system. Digestive system tumours - WHO classification of tumours. 5th ed. Lyon: International Agency for Research on Cancer; 2019.
Lloyd RV, Osamura RY, Klöppel G, Rosai J, World Health Organization, International Agency for Research on Cancer. WHO classification of tumours of endocrine organs. 4th ed. Lyon: International Agency for Research on Cancer (IARC); 2017. 355 p
Ohike NA, La Rosa S, Volante M, Zamboni G. Mixed neuroendocrine-non neuroendocrine neoplasms. Lion: IARC; 2017.
La Rosa S, Sessa F, Uccella S. Mixed neuroendocrine-nonneuroendocrine neoplasms (MiNENs): unifying the concept of a heterogeneous group of neoplasms. Endocr Pathol. 2016;27(4):284–311.
de Mestier L, Cros J, Neuzillet C, Hentic O, Egal A, Muller N, et al. Digestive system mixed neuroendocrine-non-neuroendocrine neoplasms. Neuroendocrinology. 2017;105(4):412–25.
La Rosa S, Uccella S, Molinari F, Savio A, Mete O, Vanoli A, et al. Mixed adenoma well-differentiated neuroendocrine tumor (MANET) of the digestive system: an indolent subtype of mixed neuroendocrine-nonneuroendocrine neoplasm (MiNEN). Am J Surg Pathol. 2018;42(11):1503–12.
Volante M, Rindi G, Papotti M. The grey zone between pure (neuro)endocrine and non-(neuro)endocrine tumours: a comment on concepts and classification of mixed exocrine-endocrine neoplasms. Virchows Arch. 2006;449(5):499–506.
Scardoni M, Vittoria E, Volante M, Rusev B, Bersani S, Mafficini A, et al. Mixed adenoneuroendocrine carcinomas of the gastrointestinal tract: targeted next-generation sequencing suggests a monoclonal origin of the two components. Neuroendocrinology. 2014;100(4):310–6.
Volante M, Gatti G, Papotti M. Classification of lung neuroendocrine tumors: lights and shadows. Endocrine. 2015;50(2):315–9.
Fazio N, Milione M. Heterogeneity of grade 3 gastroenteropancreatic neuroendocrine carcinomas: new insights and treatment implications. Cancer Treat Rev. 2016;50:61–7.
Velayoudom-Cephise FL, Duvillard P, Foucan L, Hadoux J, Chougnet CN, Leboulleux S, et al. Are G3 ENETS neuroendocrine neoplasms heterogeneous? Endocr Relat Cancer. 2013;20(5):649–57.
Milione M, Maisonneuve P, Spada F, Pellegrinelli A, Spaggiari P, Albarello L, et al. The clinicopathologic heterogeneity of grade 3 gastroenteropancreatic neuroendocrine neoplasms: morphological differentiation and proliferation identify different prognostic categories. Neuroendocrinology. 2017;104(1):85–93.
La Rosa S, Inzani F, Vanoli A, Klersy C, Dainese L, Rindi G, et al. Histologic characterization and improved prognostic evaluation of 209 gastric neuroendocrine neoplasms. Hum Pathol. 2011;42(10):1373–84.
La Rosa S, Klersy C, Uccella S, Dainese L, Albarello L, Sonzogni A, et al. Improved histologic and clinicopathologic criteria for prognostic evaluation of pancreatic endocrine tumors. Hum Pathol. 2009;40(1):30–40.
La Rosa S, Sessa F, Capella C, Riva C, Leone BE, Klersy C, et al. Prognostic criteria in nonfunctioning pancreatic endocrine tumours. Virchows Arch. 1996;429(6):323–33.
Capella C, Heitz PU, Hofler H, Solcia E, Kloppel G. Revised classification of neuroendocrine tumours of the lung, pancreas and gut. Virchows Arch. 1995;425(6):547–60.
Rindi G, Kloppel G. Endocrine tumors of the gut and pancreas tumor biology and classification. Neuroendocrinology. 2004;80(Suppl 1):12–5.
Kloppel G, Rindi G, Perren A, Komminoth P, Klimstra DS. The ENETS and AJCC/UICC TNM classifications of the neuroendocrine tumors of the gastrointestinal tract and the pancreas: a statement. Virchows Arch. 2010;456(6):595–7.
Bonert M, Tate AJ. Mitotic counts in breast cancer should be standardized with a uniform sample area. Biomed Eng Online. 2017;16(1):28.
Milione M. Prognostic factors for gastroenteropancreatic neuroendocrine neoplasms (GEP-NENs): what’s better? Endocrine. 2018;59(1):1–3.
Milione M, Fazio N. G3 GEP NENs category: are basic and clinical investigations well integrated? Endocrine. 2018;60(1):28–30.
Milione M, Maisonneuve P, Pellegrinelli A, Grillo F, Albarello L, Spaggiari P, et al. Ki67 proliferative index of the neuroendocrine component drives MANEC prognosis. Endocr Relat Cancer. 2018;25(5):583–93.
Milione M, Maisonneuve P, Pellegrinelli A, Spaggiari P, Centonze G, Coppa J, et al. Ki-67 and presence of liver metastases identify different progression-risk classes in pancreatic neuroendocrine neoplasms (pNEN) undergoing resection. Eur J Surg Oncol. 2019;45(5):755–60.
Milione M, Miceli R, Barretta F, Pellegrinelli A, Spaggiari P, Tagliabue G, et al. Microenvironment and tumor inflammatory features improve prognostic prediction in gastro-entero-pancreatic neuroendocrine neoplasms. J Pathol Clin Res. 2019;5(4):217–26.
Rigaud G, Moore PS, Zamboni G, Orlandini S, Taruscio D, Paradisi S, et al. Allelotype of pancreatic acinar cell carcinoma. Int J Cancer. 2000;88(5):772–7.
Busico A, Maisonneuve P, Prinzi N, Pusceddu S, Centonze G, Garzone G, et al. Gastroenteropancreatic High-Grade Neuroendocrine Neoplasms (H-NENs): histology and molecular analysis, two sides of the same coin. Neuroendocrinology. 2020;110(7–8):616–29.
Rindi G, Klimstra DS, Abedi-Ardekani B, Asa SL, Bosman FT, Brambilla E, et al. A common classification framework for neuroendocrine neoplasms: an International Agency for Research on Cancer (IARC) and World Health Organization (WHO) expert consensus proposal. Mod Pathol. 2018;31(12):1770–86.
Travis WD, Brambilla E, Burke A, Marx A, Nicholson AG. International Agency for Research on Cancer. WHO classification of tumours of the lung, pleura, thymus and heart. Lyon: International Agency for Research on Cancer; 2015. 412 p
Caplin ME, Baudin E, Ferolla P, Filosso P, Garcia-Yuste M, Lim E, et al. Pulmonary neuroendocrine (carcinoid) tumors: European Neuroendocrine Tumor Society expert consensus and recommendations for best practice for typical and atypical pulmonary carcinoids. Ann Oncol. 2015;26(8):1604–20.
Aslan DL, Gulbahce HE, Pambuccian SE, Manivel JC, Jessurun J. Ki-67 immunoreactivity in the differential diagnosis of pulmonary neuroendocrine neoplasms in specimens with extensive crush artifact. Am J Clin Pathol. 2005;123(6):874–8.
Pelosi G, Rodriguez J, Viale G, Rosai J. Typical and atypical pulmonary carcinoid tumor overdiagnosed as small-cell carcinoma on biopsy specimens: a major pitfall in the management of lung cancer patients. Am J Surg Pathol. 2005;29(2):179–87.
Pelosi G, Fabbri A, Cossa M, Sonzogni A, Valeri B, Righi L, et al. What clinicians are asking pathologists when dealing with lung neuroendocrine neoplasms? Semin Diagn Pathol. 2015;32(6):469–79.
Rindi G, Klersy C, Inzani F, Fellegara G, Ampollini L, Ardizzoni A, et al. Grading the neuroendocrine tumors of the lung: an evidence-based proposal. Endocr Relat Cancer. 2014;21(1):1–16.
Righi L, Gatti G, Volante M, Papotti M. Lung neuroendocrine tumors: pathological characteristics. J Thorac Dis. 2017;9(Suppl 15):S1442–S7.
Oka N, Kasajima A, Konukiewitz B, Sakurada A, Okada Y, Kameya T, et al. Classification and prognostic stratification of bronchopulmonary neuroendocrine neoplasms. Neuroendocrinology. 2019;110(5):393–403.
Brierley J, Gospodarowicz MK, Wittekind C. TNM classification of malignant tumours. 8th ed. Chichester, West Sussex, UK; Hoboken, NJ: Wiley; 2017. xviii, 253 p
Rindi G, Kloppel G, Couvelard A, Komminoth P, Korner M, Lopes JM, et al. TNM staging of midgut and hindgut (neuro) endocrine tumors: a consensus proposal including a grading system. Virchows Arch. 2007;451(4):757–62.
Rindi G, Kloppel G, Alhman H, Caplin M, Couvelard A, de Herder WW, et al. TNM staging of foregut (neuro)endocrine tumors: a consensus proposal including a grading system. Virchows Arch. 2006;449(4):395–401.
Pavel M, Baudin E, Couvelard A, Krenning E, Oberg K, Steinmuller T, et al. ENETS Consensus Guidelines for the management of patients with liver and other distant metastases from neuroendocrine neoplasms of foregut, midgut, hindgut, and unknown primary. Neuroendocrinology. 2012;95(2):157–76.
Garcia-Carbonero R, Sorbye H, Baudin E, Raymond E, Wiedenmann B, Niederle B, et al. ENETS consensus guidelines for high-grade gastroenteropancreatic neuroendocrine tumors and neuroendocrine carcinomas. Neuroendocrinology. 2016;103(2):186–94.
Phan AT, Oberg K, Choi J, Harrison LH Jr, Hassan MM, Strosberg JR, et al. NANETS consensus guideline for the diagnosis and management of neuroendocrine tumors: well-differentiated neuroendocrine tumors of the thorax (includes lung and thymus). Pancreas. 2010;39(6):784–98.
Eberhardt WE, Mitchell A, Crowley J, Kondo H, Kim YT, Turrisi A 3rd, et al. The IASLC lung cancer staging project: proposals for the revision of the M descriptors in the forthcoming eighth edition of the TNM classification of lung cancer. J Thorac Oncol. 2015;10(11):1515–22.
Basturk O, Yang Z, Tang LH, Hruban RH, Adsay V, McCall CM, et al. The high-grade (WHO G3) pancreatic neuroendocrine tumor category is morphologically and biologically heterogenous and includes both well differentiated and poorly differentiated neoplasms. Am J Surg Pathol. 2015;39(5):683–90.
Heetfeld M, Chougnet CN, Olsen IH, Rinke A, Borbath I, Crespo G, et al. Characteristics and treatment of patients with G3 gastroenteropancreatic neuroendocrine neoplasms. Endocr Relat Cancer. 2015;22(4):657–64.
Sonbol MB, Halfdanarson TR. Management of well-differentiated high-grade (G3) neuroendocrine tumors. Curr Treat Options in Oncol. 2019;20(9):74.
Sorbye H, Welin S, Langer SW, Vestermark LW, Holt N, Osterlund P, et al. Predictive and prognostic factors for treatment and survival in 305 patients with advanced gastrointestinal neuroendocrine carcinoma (WHO G3): the NORDIC NEC study. Ann Oncol. 2013;24(1):152–60.
Tang LH, Untch BR, Reidy DL, O’Reilly E, Dhall D, Jih L, et al. Well-differentiated neuroendocrine tumors with a morphologically apparent high-grade component: a pathway distinct from poorly differentiated neuroendocrine carcinomas. Clin Cancer Res. 2016;22(4):1011–7.
Spada F, Antonuzzo L, Marconcini R, Radice D, Antonuzzo A, Ricci S, et al. Oxaliplatin-based chemotherapy in advanced neuroendocrine tumors: clinical outcomes and preliminary correlation with biological factors. Neuroendocrinology. 2016;103(6):806–14.
Bajetta E, Catena L, Biondani P, Pusceddu S, Valente M, Bianco N, et al. Activity of a three-drug combination including cisplatin (CLOVER regimen) for poorly differentiated neuroendocrine carcinoma. Anticancer Res. 2014;34(10):5657–60.
Bajetta E, Catena L, Procopio G, De Dosso S, Bichisao E, Ferrari L, et al. Are capecitabine and oxaliplatin (XELOX) suitable treatments for progressing low-grade and high-grade neuroendocrine tumours? Cancer Chemother Pharmacol. 2007;59(5):637–42.
Kunz PL, Balise RR, Fehrenbacher L, Pan M, Venook AP, Fisher GA, et al. Oxaliplatin-fluoropyrimidine chemotherapy plus bevacizumab in advanced neuroendocrine tumors: an analysis of 2 phase II trials. Pancreas. 2016;45(10):1394–400.
Tang LH. Hystopathology Net G3 versus NEC G3. In: Spain tAEcftdattoNTD-MB, editor. 2016.
Ferrata M, Schad A, Zimmer S, Musholt TJ, Bahr K, Kuenzel J, et al. PD-L1 expression and immune cell infiltration in gastroenteropancreatic (GEP) and non-GEP neuroendocrine neoplasms with high proliferative activity. Front Oncol. 2019;9:343.
Weber MM, Fottner C. Immune checkpoint inhibitors in the treatment of patients with neuroendocrine neoplasia. Oncol Res Treat. 2018;41(5):306–12.
Takahashi D, Kojima M, Suzuki T, Sugimoto M, Kobayashi S, Takahashi S, et al. Profiling the tumour immune microenvironment in pancreatic neuroendocrine neoplasms with multispectral imaging indicates distinct subpopulation characteristics concordant with WHO 2017 classification. Sci Rep. 2018;8(1):13166.
Hijioka S, Hosoda W, Mizuno N, Hara K, Imaoka H, Bhatia V, et al. Does the WHO 2010 classification of pancreatic neuroendocrine neoplasms accurately characterize pancreatic neuroendocrine carcinomas? J Gastroenterol. 2015;50(5):564–72.
Agaimy A, Erlenbach-Wunsch K, Konukiewitz B, Schmitt AM, Rieker RJ, Vieth M, et al. ISL1 expression is not restricted to pancreatic well-differentiated neuroendocrine neoplasms, but is also commonly found in well and poorly differentiated neuroendocrine neoplasms of extrapancreatic origin. Mod Pathol. 2013;26(7):995–1003.
Rinke A, Gress TM. Neuroendocrine cancer, therapeutic strategies in G3 cancers. Digestion. 2017;95(2):109–14.
Sorbye H, Baudin E, Perren A. The problem of high-grade gastroenteropancreatic neuroendocrine neoplasms: well-differentiated neuroendocrine tumors, neuroendocrine carcinomas, and beyond. Endocrinol Metab Clin N Am. 2018;47(3):683–98.
Sorbye H, Baudin E, Borbath I, Caplin M, Chen J, Cwikla JB, et al. Unmet needs in high-grade gastroenteropancreatic neuroendocrine neoplasms (WHO G3). Neuroendocrinology. 2019;108(1):54–62.
Cives M, Pelle E, Quaresmini D, Rizzo FM, Tucci M, Silvestris F. The tumor microenvironment in neuroendocrine tumors: biology and therapeutic implications. Neuroendocrinology. 2019;109(2):83–99.
Bellizzi AM. Immunohistochemistry in the diagnosis and classification of neuroendocrine neoplasms: what can brown do for you? Hum Pathol. 2020;96:8–33.
Kleist B, Poetsch M. Neuroendocrine differentiation: the mysterious fellow of colorectal cancer. World J Gastroenterol. 2015;21(41):11740–7.
Grabowski P, Schonfelder J, Ahnert-Hilger G, Foss HD, Heine B, Schindler I, et al. Expression of neuroendocrine markers: a signature of human undifferentiated carcinoma of the colon and rectum. Virchows Arch. 2002;441(3):256–63.
Lloyd RV, Schroeder G, Bauman MD, Krook JE, Jin L, Goldberg RM, et al. Prevalence and prognostic significance of neuroendocrine differentiation in colorectal carcinomas. Endocr Pathol. 1998;9(1):35–42.
Hamada Y, Oishi A, Shoji T, Takada H, Yamamura M, Hioki K, et al. Endocrine cells and prognosis in patients with colorectal carcinoma. Cancer. 1992;69(11):2641–6.
Jansson D, Gould VE, Gooch GT, Rittenhouse HG, Shin SS, Manderino GL, et al. Immunohistochemical analysis of colon carcinomas applying exocrine and neuroendocrine markers. APMIS. 1988;96(12):1129–39.
Rehfeld JF. A centenary of gastrointestinal endocrinology. Horm Metab Res. 2004;36(11–12):735–41.
Cho YB, Yang SS, Lee WY, Song SY, Kim SH, Shin HJ, et al. The clinical significance of neuroendocrine differentiation in T3-T4 node-negative colorectal cancer. Int J Surg Pathol. 2010;18(3):201–6.
Smith DM Jr, Haggitt RC. The prevalence and prognostic significance of argyrophil cells in colorectal carcinomas. Am J Surg Pathol. 1984;8(2):123–8.
Sun MH. Neuroendocrine differentiation in sporadic CRC and hereditary nonpolyosis colorectal cancer. Dis Markers. 2004;20(4–5):283–8.
Yao GY, Zhou JL, Lai MD, Chen XQ, Chen PH. Neuroendocrine markers in adenocarcinomas: an investigation of 356 cases. World J Gastroenterol. 2003;9(4):858–61.
Indinnimeo M, Cicchini C, Memeo L, Stazi A, Provenza C, Ricci F, et al. Correlation between chromogranin-a expression and pathological variables in human colon carcinoma. Anticancer Res. 2002;22(1A):395–8.
Foley EF, Gaffey MJ, Frierson HF Jr. The frequency and clinical significance of neuroendocrine cells within stage III adenocarcinomas of the colon. Arch Pathol Lab Med. 1998;122(10):912–4.
Pagani A, Papotti M, Abbona GC, Bussolati G. Chromogranin gene expressions in colorectal adenocarcinomas. Mod Pathol. 1995;8(6):626–32.
Shinji S, Naito Z, Ishiwata T, Tanaka N, Furukawa K, Suzuki H, et al. Neuroendocrine cell differentiation of poorly differentiated colorectal adenocarcinoma correlates with liver metastasis. Int J Oncol. 2006;29(2):357–64.
Staren ED, Gould VE, Jansson DS, Hyser M, Gooch GT, Economou SG. Neuroendocrine differentiation in “poorly differentiated” colon carcinomas. Am Surg. 1990;56(7):412–9.
Shia J, Guillem JG, Moore HG, Tickoo SK, Qin J, Ruo L, et al. Patterns of morphologic alteration in residual rectal carcinoma following preoperative chemoradiation and their association with long-term outcome. Am J Surg Pathol. 2004;28(2):215–23.
Shia J, Tickoo SK, Guillem JG, Qin J, Nissan A, Hoos A, et al. Increased endocrine cells in treated rectal adenocarcinomas: a possible reflection of endocrine differentiation in tumor cells induced by chemotherapy and radiotherapy. Am J Surg Pathol. 2002;26(7):863–72.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Milione, M., Cattaneo, L., Mangogna, A. (2021). New Concepts in Pathology. In: Beretta, G., Berruti, A., Bombardieri, E., Fazio, N., Goletti, O. (eds) Neuroendocrine Neoplasia Management. Springer, Cham. https://doi.org/10.1007/978-3-030-72830-4_3
Download citation
DOI: https://doi.org/10.1007/978-3-030-72830-4_3
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-72829-8
Online ISBN: 978-3-030-72830-4
eBook Packages: MedicineMedicine (R0)