Abstract
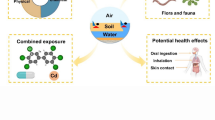
Microplastics are small plastic fragments, flakes, or beads of size less than 5 mm diameter. Due to the anthropogenic activities, these persist ubiquitously in the environment. The high surface area and functionalized surface due to aging and weathering persuade the sorption of toxic contaminants to the microplastics. The microplastics with the adsorbed chemicals are ingested and accumulated by the terrestrial and aquatic organisms. The ingested microplastics and the adsorbed chemicals cause endocrine disruption, reproductive failure, and other developmental disorders. When humans are exposed to these microplastics through food chain, inhalation, and dermal contact, this exposure can lead to an array of health impacts, including inflammation, genotoxicity, oxidative stress, apoptosis, and necrosis. This chapter highlights the interaction mechanisms of the sorption process between the microplastics and the toxic chemicals, factors influencing the sorption process, and the combined toxic effects of microplastics and their adsorbed chemicals on ecosystem.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
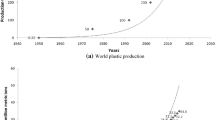
The global production of plastics is about 381million tons every year, and it will get doubled by 2034 (Plastic in the Ocean Statistics, 2020). Over 10 million tons of plastic wastes is dumped into our oceans every year and 150 million tons into the soil and in internal waters (Axel Barrett, 2020). Low-density polyethylene (LDPE), high-density polyethylene (HDPE), polypropylene (PP), polystyrene (PS), polyethylene terephthalate (PET), and polyvinyl chloride (PVC), nylon, acrylic, polyurethanes, polylactic acid, polycarbonate, and other biodegradable plastics are the generally used polymers across the world (Ajith et al., 2020). These plastic wastes persist in the environment for decades because of its durability and become the ubiquitous pollutant even in the most isolated areas of the world causing a global threat. The fragmentation of larger plastic particles due to physical, chemical, and biological weathering process when discharged into the environment leads to the formation of microparticles (Andrady, 2011).
Microplastics (MPs) are fine plastic particles of size lesser than 5 mm in diameter (GESAMP, 2015, 2016). Depending on their source, they are classified as primary and secondary microplastics. Primary microparticles are designed intently in microscale for commercial use. The microbeads, microfibers, capsules, and nurdles used in the personal care products, textiles, pharmaceuticals, and plastic manufacturing industries became the main source of primary microplastic. The secondary microplastics enter the environment through the breakdown of larger plastic materials such as water bottles, fishing nets, and household items. Due to the anthropogenic activities, these microparticles are flushed into the terrestrial, aquatic, and atmospheric environment.
When microplastics are discarded into the environment, it detrimentally affects the humans and animals through the consumption of seafood and drinking water, contact with food packaging, or inhalation of particles (Hwang et al., 2019). The living organisms that ingest microplastics are at a high health risk such as reduced growth rates, cytotoxicity, hypersensitivity, increased mortality, decreased reproductive ability, unwanted immune response, ulcers, abrasions, and oxidative stress and acute response like hemolysis (Ajith et al., 2020).
Microplastics absorb and accumulate toxic chemicals and carry harmful bacteria due to their strong sorption capability. Microplastics can store more pollutants than macroplastics because of their larger specific surface area (Lee et al., 2014). These plastics when ingested by the living organisms exert ecological effects, either from the plastic particle, the pollutants trapped to them, or both, and many studies have confirmed this (Wang et al., 2018). The high surface area of microplastics not only sorbs organic and inorganic contaminants but also supports chemical transport of plasticizers, other plastic additives, and constitutional monomers to the ecosystem through leaching (Teuten et al., 2009).
Studies have reported the sorption of various contaminants to microplastics, such as polycyclic aromatic hydrocarbons (PAHs), polychlorinated biphenyls (PCBs), dioxin-like chemicals, polybrominated diphenyl ethers (PBDEs), toxic metals, hydrophilic organic compounds (ciprofloxacin), and pharmaceuticals (antibiotics and anti-depressants) (Fred-Ahmadu et al., 2020). The most common plastic additives include phthalates, bisphenol A (BPA), polybrominated diphenyl ethers (PBDE), nonylphenols (NP), and antioxidants (Hermabessiere et al., 2017). Table 1 stretches some common toxic pollutants sorbed to microplastics and their toxic effects. Several studies imply the interaction between toxic pollutants and microplastics and their potential toxicological impact on the ecosystem.
2 Interaction Mechanisms
Microplastics act as a vector for the hazardous pollutants; therefore, the interaction mechanisms are necessary for the toxicity perspective of these pollutants in living organisms.
2.1 Adsorption of Hydrophobic Organic Pollutants onto Microplastics
2.1.1 Hydrophobic Attraction
Microplastics have low polarity on their surface due to electrostatic interactions, and this enables higher sorption capacity for the hydrophobic pollutant’s adsorption of chemicals onto their surfaces (Liu et al., 2018). Hydrophobic chemicals such as polychlorinated biphenyls (PCBs), polycyclic aromatic hydrocarbons (PAHs), organochlorine pesticides, and other benzene-ring derivatives adsorb and concentrate on the surface of the microplastics and form a micelle shape-like structure (Verla et al., 2019). The octanol-water partition coefficient (Kow or log Kow) is commonly used as a hydrophobicity parameter . Substances with high log Kow values tend to absorb more readily to organic matter due to their low affinity for water (Mei et al., 2020).
2.1.2 Hydrogen Bonding
Hydrogen bonds are relatively weak electrostatic interactions, involving hydrogen ion H+, and can affect the sorption of polymers when proton donor and proton acceptor groups are involved (Tourinho et al., 2019). PA and PU have a highest sorption capacity for BPA. It is attributed by the formation of hydrogen bonding between hydrogen donating BPA and hydrogen accepting groups in PA and PU (Liu et al., 2019).
2.1.3 π–π Interaction
π–π interactions are attraction forces also called non-covalent interaction between conjugated structure. Aromatic polymers, such as PS, can undergo π–π interactions. Rochman et al. (2013a) showed polystyrene plastics sorbed higher concentration of PAHs than PET, HDPE, PVC, LDPE, and PP because of the contribution of π − π and strong hydrophobic interactions.
2.1.4 Electrostatic Interactions
Electrostatic interaction occurs when microplastics and the pollutants are oppositely charged and repulsion occurs when they are of same charge. The charge of the microparticles is affected by pH and point of zero charge (pHpzc). When the pH of point of zero charge being lower than most environmental pHs, MP becomes negatively charged otherwise it is positively charged. The negatively charged MP attracts the positively charged pollutants. Wu et al. (2019) showed that bisphenols undergo partial ionization. Bisphenol F shows higher ionization due to low pKa value. The low pHpzc of PVC indicates the surface is negatively charged; therefore, the adsorption is electrostatic repulsion between PVC MPs and anionic fraction ionized from bisphenols.
2.1.5 Van der Waals Force
Van der Waals forces are weak interactions occurring between molecules not involving covalent or ionic bonding. Hüffer and Hofmann (2016) observed that the aliphatic PE microplastics without any specific functional group can only undergo non-specific van der Waals interaction.
2.1.6 Pore Filling
The microparticles consist of a number of pores of different sizes, and the hydrophobic organic pollutant enters the polymer matrix and is trapped inside. It depends on the pore diameter of a polymer structure and the molecular size of the pollutant. Pollutants with lower molecular weights will easily travel into a polymer matrix with larger pores (Lambert & Wagner, 2017).
2.1.7 Halogen Bonding
Wu et al. (2019) studied the sorption of bisphenols on PVC microplastics. The halogen bond promotes adsorption of bisphenol to PVC. The –Cl on the branch of PVC which act as an electron acceptor and the benzene rings with hydroxyl group act as electron donor generate the halogen bond.
2.2 Adsorption of Hydrophilic Organic Pollutants onto Microplastics
Hydrophilic organic pollutants are polar, ionizable compounds used as pesticides, plasticizers, flame retardants, etc. Due to anthropogenic activities, these pollutants are washed off and enter water streams. Hydrophilic organic pollutants act as endocrine disruption at trace concentrations, which poses immense threat to the ecosystem and human health.
When microplastics enter the environment, their properties get changed, and photo-induced surface oxidation takes place and oxygen-containing functional groups are formed. Liu et al. (2019) investigated the adsorption of hydrophilic organic pollutant ciprofloxacin on pristine and aged PS and PVC microplastics. The adsorption in the aged microplastics was mainly through hydrogen bonding because of the presence of oxygen-containing functional groups. The pristine microplastics were negatively charged and CIP was cationic in lower pH and the adsorption was due to the electrostatic attractions. The pristine PS sorbed through π–π bonds, because the π–π bond strengthened the interactions between microplastics and CIP.
Xu et al. (2018b) observed Van der Waals interaction between sulfamethoxazole and PE microplastics. Sulfamethoxazole is a hydrophilic compound and exists as anion under pH 6.8. PE microplastics carry a negative charge; hence, there exist electrostatic repulsion than attraction. So, the sorption process would have more interactions such as electrostatic interaction and chemical bonds interaction. Guo et al. (2019) also reported the electrostatic interaction of sulfamethoxazole onto microplastics. In acidic environment, sorption of sulfamethoxazole to microplastic increases because microplastics tend to protonate with decreasing pH. Similar trends were observed in the sorption of perfluorooctanesulfonate (PFOS) on PE, PS, and PVC (Wang et al., 2015) and tylosin on PS and PVC (Guo et al., 2018).
Xu et al. (2018a) studied the interaction of tetracycline with three types of microplastics (polyethylene (PE), polypropylene (PP), and polystyrene (PS)) in batch sorption experiments. The interaction depends on the pH, at neutral medium hydrophobic, and at lower or higher pH, electrostatic interactions takes place . The sorption of tetracycline on PS microplastics was due to the presence of polar interactions and π–π interactions.
2.3 Adsorption of Metals onto Microplastics
Microplastics are negatively charged due to low polarity on their surface and metal ions are positively charged. The negatively charged microplastics bind to the positively charged metal by electrostatic attraction and neutralize their charge by forming a micelle shape-like structure (Verla et al., 2019).
Zou et al. (2020) observed electrostatic interaction in the sorption of Pb2+ to MPs and electrostatic interaction and complexation in the sorption of Cd2+ and Cu2+. When the metal ions enter the solution, they exist in hydrated form. Pb2+ has a minimum hydrated ionic radius and therefore has the largest electrostatic interactions with the MPs. While in Cd2+ and Cu2+, the electrostatic interaction, together with surface complexation, takes place onto the plastic surface. The sorption of Cd2+ and Cu2+ could also affected by the pH and ionic strength of the solution.
3 Factors Affecting the Interaction of Microplastics and Toxic Chemicals
3.1 The Materials of Microplastics
The structural properties of the polymer influence the process of sorption between the microplastics and contaminants. Polymers consist of crystalline and amorphous regions. The molecular segments of crystalline region are regularly packed, whereas in the amorphous region it is randomly packed. The crystalline region needs a high amount of energy for the chemical absorption. The amorphous region has a larger degree of free volume because of the distance among polymeric chains which allows the contaminants to diffuse readily through the polymer. The glass transition temperature (Tg) is related with amorphous domain of the polymer. Below the Tg, polymers are in glassy state so the molecules can only vibrate, and above Tg, polymers are in rubbery state so they have higher freedom of movement and hence absorption of pollutants is enhanced. The properties of polymers such as surface charge, surface area, molecular chain arrangement, functional group, and acid-base character also affect the sorption of chemicals (Fred-Ahmadu et al., 2020).
In the sorption studies of microplastics, PE has the highest affinity for sorption of pyrene followed by PS and PVC. The nonpolar nature and rubber-like character could be reason for maximum adsorption (Wang & Wang, 2018). When compared to PS and PVC, PE has greater segmental mobility and free volume in its molecular structure which enhances its sorption efficiency, whereas in PVC the chlorine atoms in the polymeric structure cause a reduction in free volume and make difficult for the migration of adsorbate diffuse into the polymer. The presence of benzene backbone in PS restricts the segmental mobility thus lower the transport of chemicals into the matrix (Wang et al., 2018).
Planar molecules can be easily sorbed to the polymer surface than the non-polar molecules because planar molecules can easily travel to the plastic surface than bulkier non-planar molecules (Velzeboer et al., 2014). The pore size affects the sorption process and small pores leads to the formation of monolayer while large pore size forms mono and multilayer adsorption (Tourinho et al., 2019).
3.2 The Size of the Microplastics
The sorption capacity depends on the MP’s particle size, and a decreased particle size increases the surface area to volume ratio. Wang et al. (2019b) studied the size effect of polystyrene microplastics in the sorption of phenanthrene and nitrobenzene. The log Kd values increase with decreasing particle size of MPs and hence adsorption increases. The log Kd value of 50 nm polystyrene was significantly lower because its aggregation greatly reduced the effective surface area available for sorption. Teuten et al. (2007) showed PE, PP, and PVC of same size (200–250 μm) have different sorption capacities due to varying BET surface area. Since PE possess larger log Kd value and surface area than those of PP and PVC, it has higher sorption capacity.
3.3 The Aging and Weathering of Microplastics
The environmental conditions enhanced fragments and cracking of aged or weathered plastic particles into smaller particles due to the degradation process, namely, thermal, mechanical, biological, radiative, oxidative breakdown, or hydrolysis. The change in the property of the plastic surface enhanced the sorption of contaminants. The weathering increases polymer crystallinity and decreases hydrophobicity due to surface oxidation which affects the sorption of contaminants. Aging of polymer decreases its molecular weight which affects the sorption properties (Pѐrez et al., 2010). The formation of biofilm on aged MPs due to biological effects also affects the sorption of contaminants.
The concentration of pollutants was higher on yellow plastic than the white. The yellowing of plastic is due to photooxidative weathering, the phenolic antioxidant added as additive results in the formation of quinonoidal structures. Black pellets have polyurethane as a constituent which increase the sorption of chemicals (Veerasingam et al., 2016).
3.4 The Chemical Properties of Contaminants
The organic pollutants present in the environment are mostly hydrophobic in nature. The sorption of organic pollutants mainly depends on hydrophobicity, molecular weight, and molar volume. The sorption of different organic pollutants onto polymers is related to their octanol-water coefficient (Kow) values. The sorption of different contaminants has different octanol-water coefficient (Kow) values for the same type of polymer. The molecular weight of the contaminant is more significant than Kow if diffusion is the rate-limiting process (Tourinho et al., 2019).
3.5 The Environmental Factors
The environmental factors such as temperature, pH, salinity, and ionic strength affect the sorption of pollutants on microplastics. The sorption increases with increase in temperature. Xu et al. (2019) showed the Kd value increases with increasing the temperature from 5 °C to 15 °C indicating that the sorption of PBDEs on MPs was affected by temperature. Above 20 °C, Qiu et al. (2019) showed a decrease in sorption which could be due to an increase in ionic activity, which may compete with polyhalogenated carbazoles for sorption on MPs’ surface in the simulated seawater.
When the pH of aqueous system increases, electrostatic repulsion between the contaminant and the microplastic decreases. A high pH can also increase the π–π interaction between the sorbate and the sorbent. Wang et al. (2019a) showed that increase in the pH of Cd solution led to an increase in the adsorption of Cd to PE microplastics. At PH >8, Cd(OH)2 precipitates out which attributes to the adsorption of Cd.
Salinity also plays an important role in sorption of pollutant in aqueous system. Salinity is based on the degree of electrostatic interaction or ion-exchange mechanism. Zhang et al. (2020) in their study showed high salinity decreased the surface zeta-potential of the MPs and enhanced PHE sorption to MPs due to the salting-out effect. Ionic strength is the total ion concentration which is due to the dissolved salts in the water bodies. Zhang et al. (2018) showed a decreased sorption of oxytetracycline to PS MPs with increase in ionic strength. This is because of the competition for sorption between the ions in solution and toxic chemicals. The presence of DOM could also affect the sorption processes of chemicals by plastics as DOM competes with other chemicals for adsorption sites on the surface of the plastics by entering the pores or cover the surface of the polymer matrix (Mei et al., 2020).
4 Toxic Effects of Chemicals Adsorbed on Microplastics
4.1 Toxicity of Organic Pollutants
4.1.1 Polychlorinated Biphenyls (PCBs)
Polychlorinated biphenyls are chlorinated organic compounds that vary in the number of attached chlorine atoms, with the general formula C12H10-xClx. There are about 209 congeners of PCBs with different physical and chemical properties (Pascal et al., 2005). The international treaty on persistent organic pollutants, in the Stockholm Convention of 2001, listed PCBs in one of the “dirty dozen” chemicals. Environmental Protection Agency classifies PCBs as a “probable carcinogen.” It also affects immune system, reproductive system, nervous system, and endocrine system in humans and animals (EPA, 2000). Although PCBs were banned in the 1970s, their residues are still present in the environment through biomagnifications due to their stability, lipophilicity, and high toxicity. Microplastics have greater affinity for PCBs due to their relatively high surface area to volume ratio (Velzeboer et al., 2014).
The concentrations of PCBs have been reported in many field studies. Frias et al. (2010) collected samples from two Portuguese beaches and found PCB concentration in the range from 0.02 to 15.56 ng g−1 on microplastics. Mendoza and Jones (2015) collected microscale plastic particles in September 2007 in the North Pacific Central Gyre and identified PCBs in the range of 1–223 ng g−1. The main fragments of plastics collected in North Pacific Central Gyre were polypropylene (PP), polyethylene (PE), nylon, and polyurethane.
Gassel and Rochman (2019) investigated the chemical contaminants and microplastics in lanternfish (Myctophidae) in the North Pacific Gyre. Fish samples were collected from the North Pacific Subtropical Gyre and the California Current and the tissue concentrations of various chemicals sorbed from microplastics were examined. Lower chlorinated PCB congener’s concentrations were higher in lanternfish, and it was about 11 ng g−1lw compared to the other fish whose concentration is 0.87–4.04 ng g−1lw.
In the beaches around San Diego, California, plastic debris were analyzed, and PCBs range was from 3.8–42 ng g−1 (Van et al., 2012). Total PCB concentrations were in the range 0.04–124 ng g−1 in the Tokyo bay, and it was higher in the urban areas (Yeo et al., 2019). Lo et al. (2019) examined PCB samples in Hong Kong and the total PCB concentration was in the range 13.0 and 1083 ng g−1. Bouhroum et al. (2019) investigated the PCB concentrations in the open ocean and coastal debris and weres found to be 12.2 ng g−1 and 1.4 × 104 ng g−1, respectively. Fraser et al. (2020) studied the distribution of PCBs and microplastics in sediments from the Qiantang River and Hangzhou Bay, China. The sediment in the microplastics mainly contains PET, PS, and PET. These sediments contain PCB concentrations in the range from 1.13 to 1.65 ng g−1.
Besseling et al. (2013) studied the exposure effect of Arenicola marina for 28 days to natively PCB contaminated sediment pre-equilibrated with PS. The effects of polystyrene (PS) microplastic on survival activity, and bodyweight, as well as the transfer of 19 polychlorinated biphenyls (PCBs), were assessed in bioassays with A. marina (L.). The uptake of plastic particles showed the weight loss of A. marina and also reduces the feeding activity. A low PS dose of 0.074% increased bioaccumulation of PCBs in tissues by a factor of 1.1–3.6. Norway lobster (Nephrops norvegicus) when ingested with PCB-spiked polyethylene shows limited bioaccumulation of PCB in Nephrops tail tissue, whereas PCB-spiked polystyrene shows no bioaccumulation (Devriese et al., 2017).
Grigorakis and Drouillard (2018) evaluated the diet assimilation efficiencies of goldfish (Carassius auratus) when exposed to microplastics spiked with PCBs and fish pellets spiked with PCBs. When these were fed to the fish, the microplastic-associated PCBs showed lower diet assimilation efficiencies compared to food matrix-associated PCBs. Jiang et al. (2018) examined the bioaccumulation of PCBs on Daphnia magna when incorporated in polystyrene nanoparticle. The PCBs accumulated 1.4–2.6 times more in D. magna than PCBs in the absence of polystyrene particles during the 24-h exposure. Asian clams (Corbicula fluminea) were exposed to PE, PET, PVC, and PS microplastics with and without PCBs and their predator’s white sturgeon (Acipenser transmontanus) exposed to the calms. Tubular dilation occurs to the clam by the microplastic, and it has been enhanced by the presence of PCBs. The predators when fed with clams exposed to PCB spiked MP shows less vitellogenin proteins and glycogen depletion (Rochman et al., 2017).
Van der Hal et al. (2019) studied the viability for the transfer of PCBs when incorporated with polypropylene microplastic to herbivorous rabbitfish (Siganus rivulatus). The incorporated microplastics were mixed with dough and fed to the fish in two concentrations 500 ng g−1 and 5000 ng g−1. PCB congener 194 was found in muscle tissue in the concentration of 200 ng g−1 after 2 weeks of exposure to 5000 ng g−1 PCB concentration. Lin et al. (2019) studied the toxicity of PCB-18 and 100 nm PS on Daphnia magna in water. The combined toxicity to D. magna depended on the relative concentration of nano-sized PS and PCB. At high PS concentration, lethality increased due to PS instead of PCB. The ingestion of PCB-spiked microplastics by the marine organism even in small amount led to bioaccumulation harm in them in long run.
4.1.2 Polyaromatic Hydrocarbon (PAHs)
Polyaromatic hydrocarbons (PAHs) are a group of toxic organic pollutants composed of two or more benzene rings bonded in linear, cluster, or angular arrangements (Abdel-Shafy & Mansour, 2016). About 16 PAHs have been listed as priority pollutants in the environment, due to their high toxicity for living organisms (USEPA, 1980). The main source of PAHs is from petroleum industry. PAHs are well-known carcinogens and their toxicity includes genotoxicity, immunotoxicity, oxidative stress, and endocrine disruption (Honda & Suzuki, 2020). Its hydrophobic nature allows it to sorb easily to the hydrophobic surfaces of most MPs.
Lo et al. (2019) identified 19 PAH species in microplastics collected from the sandy beaches in Hong Kong. Total PAH concentration ranged from 70.8 ng g−1 to 1509 ng g−1. Among the 16 PAHs in microplastics, phenanthrene has the highest average concentration of 121 ng g−1. In Bandar Abbas, Iran, three urban intertidal regions, Khor-e-Yekshabeh, Gorsozan, and Suru were selected to study the relationship between microplastic and toxic chemicals accumulated in the sediment. The MPs ranged from 3542 to 33,561 items per m2 in different regions with variety of shapes and colors. Among the different PAHs found, naphthalene was the predominant component. The total PAH concentration was in the range from 75.80 to 116.10 ppb (Yazdani Foshtomi et al., 2019).
Chen et al. (2020) collected the MPs from the surface waters of estuary in the southwestern coast of Taiwan and analyzed the PAHs accumulated with the MPs. The average PAH concentration was 818 ± 874 ng g−1. Tan et al. (2019) analyzed the spatial distribution of microplastics, as well as microplastics and their adsorbed PAHs in the surface water of the Feilaixia Reservoir. Among the 16 identified PAHs, chrysene, benzo [ghi] perylene, and phenanthrene were the predominant. The total concentration of the 16 PAHs was ranged from 282.4 to 427.3 ng g−1.
Sharma et al. (2020) reported the existence of carcinogens in microplastics which act as vector of PAHs originated from e-waste. The cancer risk in terms of lifetime of microplastic ingestion was 10−5 which is higher than the recommended value of 10−6. The smaller microplastics of size smaller than 5 mm show a higher adsorption capacity of about 110 mg g−1 which is 40% higher than big size particle.
4.1.2.1 Phenanthrene
Phenanthrene is a polycyclic aromatic hydrocarbon that occurs in fossil fuels, and due to incomplete combustion, it is present ubiquitous in the environment. Phenanthrene is one of the PAHs in EPA’s priority pollutant list. Karami et al. (2016) analyzed the biomarker responses of African catfish (Clarias gariepinus) when exposed to LDPE microplastics loaded with phenanthrene for 96 h. The degree of tissue change, the plasmaglobulin, total protein levels, and the content of glycogen-positive material in the liver were increased, while the transcription levels of forkheadboxL2 (foxl2) and tryptophanhydroxylase2 (tph2) in the brain were decreased when exposed to Phe-loaded MPs.
Ma et al. (2016) studied combined toxicity of phenanthrene with five different sizes (from 50 nm to 10 mm), of polystyrene microparticles. The combined toxicity shows an additive effect on the D. magna species. The presence of NPs significantly enhanced bioaccumulation of phenanthrene-derived residues in daphnid body and inhibited the dissipation and transformation of phenanthrene in the medium, while 10-mm MPs did not show significant effects on the bioaccumulation, dissipation, and transformation of phenanthrene. The differences may be attributed to higher adsorption of phenanthrene on 50-nm NPs than 10-mm MPs. The MPs aggravate the toxicity of organic pollutants, whereas in some cases it contradicts. Li et al. (2019) studied the toxicity of phenanthrene-induced polystyrene microplastics to fertilized eggs of marine medaka (Oryzias melastigma). The combined exposure increased the hatchability, decreased malformation and mortality rates, and restored Phe-induced abnormal expressions of cardiac development-related genes.
4.1.2.2 Pyrene
Pyrene is a polyaromatic hydrocarbon that consists of four fused benzene rings. It is ubiquitously present in the environment due to the incomplete combustion of fossil fuels. It is also used in the manufacturing of dyes, plastics, and pesticides. Animal studies in mice shown that it is toxic to kidneys and liver. Oliveira et al. (2013) studied the combined toxicity effects of pyrene – microplastics on juveniles (0+ age group) of the common goby (Pomatoschistus microps). The exposure resulted in lethargic swimming behavior which is due to the decrease in AChE activity. The reduced isocitrate dehydrogenase (IDH) activity decreases the antioxidant defense and reduces the organism fitness in the environment.
The study of Avio et al. (2015) reveals that PS and PE adsorb pyrene , and when it is exposed to mussels (Mytilus galloprovincialis), accumulation of pyrene was observed in hemolymph, gills, and digestive tissues. The adsorption of chemical to the PS and PE does not differ much. The cellular response hazard is severe for those exposed to pyrene-contaminated PE or PS, respectively. Guven et al. (2018) revealed that tropical fish juveniles (Lates calcarifer) when exposed to PS-divinylbenzene MP spiked with pyrene for 24 h showed no effect of feeding, but the swimming speed decreased.
4.1.2.3 Benzopyrene Toxicity
Benzopyrene comes under PAHs. International Agency for Research in Cancer (IARC, 2011) classified benzo [a] pyrene in group 1 human carcinogen. The benzopyrene metabolite produced by cytochrome P450 enzymes (CYP) covalently binds to DNA and causes mutation. The lipophilic nature of benzopyrene makes it adsorbs onto sediments and particulate matter which then undergoes slow degradation.
Batel et al. (2016) developed an artificial food chain with Artemia sp. nauplii and zebrafish (Danio rerio) to study the transport of microplastic particles and associated persistent organic pollutants (POPs). The transfer and retention time of microplastic-associated benzopyrene via nauplii to zebrafish was analyzed by fluorescence tracking technique. The fluorescence tracking of benzo[a]pyrene indicates that the POP is transferred to the intestinal epithelium and liver from the intestine of fish. O’Donovan et al. (2018) found out after 14 days exposure of LDPE+BaP to calms, a considerable increase in BaP concentration was observed in the whole tissues, reaching a concentration of about 7.3 ± 2.0 ng g−1 dw. An increase in superoxide dismutase (SOD) activity was noticed after exposing the calms to spiked microplastics for 7 days which results in increased oxidative stress and deficiency of antioxidant.
Dietary exposure of different sized polystyrene microplastics sorbed with benzo[a]pyrene to mussels Mytilus galloprovincialis (González-Soto et al., 2019) shows smaller sized MPs (SMPB) with higher toxicity than the larger MPs. After 7 days exposure to SMPB, the transfer of BP to M. galloprovincialis shows 66-fold increase and after 26 days there was a 150-fold increase in mussel’s tissues when compared to the control . The smaller size microplastics caused higher toxicity to DNA damage and cell type composition of digestive tubules than larger size microplastics.
Cousin et al. (2020) studied the transfer of MPs and associated contaminants to the larvae of zebrafish and the marine medaka via Paramecium and Artemia plankton. The larvae incubated with waterborne MPs did not feed directly on MPs. Ingestion of Paramecium and Artemia nauplii containing MPs was recorded inside the gastrointestinal tract of both the fishes using Cyp1a induction and fluorescent analysis. Pittura et al. (2018) in their study on combined chemical and physical hazards to mussels M. galloprovincialis found a potential transfer of BaP from MPs to hemolymph, gills, and especially digestive tissues which resulted in alterations on the immune system, on the oxidative status, neurotoxicity, and genotoxicity.
4.1.2.4 Fluoranthene (FLU)
Fluoranthene is one of the US Environmental Protection Agency’s 16 priority polycyclic aromatic hydrocarbons. Fluoranthene is released into the environment by burning of fossil fuels and wood. It is used as an ingredient in dyes, pharmaceuticals, and insulating oils. Long-term exposure to fluoranthene causes nephropathy (kidney disease), increased liver weight, and increases in liver enzymes. Paul-pont et al. (2016) evidenced that micro-PS exhibited higher sorption capacity for fluoranthene than marine algae Chaetoceros muelleri as indicated by the partition coefficient log Kd values, and this confirmed a strong affinity of fluoranthene for polystyrene. After 14 days exposure of combined exposure to marine mussels Mytilus spp. shows highest histopathological damages and levels of antioxidant markers.
Magara et al. (2018) examined the effect of polyethylene MP on the accumulation and related oxidative stress response to fluoranthene in blue mussel, Mytilus edulis. Individual exposure of fluoranthene or MP shows antioxidant response more in gills than in digestive gland but co-exposure did not yield additive or synergistic effects. Magara et al. (2019) investigated the possible effects of PHB microplastics as a single contaminant and in combination with fluoranthene on the oxidative stress system of blue mussel M. edulis. The study revealed the inhibition of CAT, SeGPx, and GST activity and also modified the antioxidant responses in both gills and digestive glands. The combined effects of microplastics with fluoranthene were not significant compared with those exerted by MPs alone.
4.1.3 Nonylphenol (NP)
Nonylphenol is used in the manufacturing of antioxidants, lubricating oil additives, laundry and dish detergents, emulsifiers, and solubilizers. It persists in the environment, and it is extremely toxic to aquatic organisms. NP has been detected in human breast milk, blood, and urine due to its ability to mimic estrogen and in turn disrupt the natural balance of hormones in affected organisms. Beiras et al. (2019) exposed polyethylene MP to 4-n-Nonylphenol and the 4-Methylbenzylidene-camphor to marine zooplankton organisms, meroplanktonic larvae, and holoplanktonic larvae of the copepod Acartia clausi. Microplastics did not increase the bioaccumulation and toxicity of 4-n-NP or 4-MBC to zooplankton and sea-urchin larvae compared to the water-borne exposure.
Beiras and Tato (2019) studied the vector effect of PE MP in transferring NP to marine planktonic organism sea-urchin larvae and their toxicity. The ingestion of MPs by larvae does not increase the toxicity of NP, but in the absence of MPs NP was toxic. The effective concentration of NP reduces the larvae growth. The combined toxicity of PE, PS, PA, PE1000, and PA1000 microplastics and nonylphenol on microalgae Chlorella pyrenoidosa was investigated. The microplastics alleviate the nonylphenol toxicity to microalgae. The smaller size MPs has higher adsorption and led to oxidative damage . Antagonistic effect on C. pyrenoidosa growth was influenced by microplastics and NP (Yang et al., 2020).
4.1.4 Polybrominated Diphenyl Ethers (PBDEs)
PBDEs are organobromine compounds used as a flame retardant which is added as an additive during the manufacturing of plastics. On aging, PBDEs tend to leach out of the plastics. PBDEs are endocrine-disrupting chemicals, since they interfere with the endocrine system function of animals. Tanaka et al. (2013) examined oceanic seabirds (short-tailed shearwaters, Puffinus tenuirostris) collected in northern North Pacific Ocean. All the examined sea birds contain plastic in their stomach. PBDEs were measured in the abdominal adipose of the 12 birds and in the ingested plastics, and in the whole tissues of 6 lanternfishes and one squid. The lower-brominated congeners such as BDE47, BDE99, and BDE154 were dominant. These lower-brominated congeners were accumulated in the body of the seabird through the food web.
Granby et al. (2018) investigated PCB- and PBDE-associated microplastic contaminants can alter toxicokinetics in European seabass (Dicentrarchus labrax). The microplastics increased the accumulation of the sorbed contaminants in the seabass. The contaminants altered the gene expression and increased the toxic effects.
4.1.5 Pthalates
Li et al. (2020a) studied the combined toxicity of polystyrene microplastics and dibutyl phthalate (DBT) on the microalgae Chlorella pyrenoidosa . The mixtures of MPs and DBP intensify the morphological damage of the cell. The distorted and deformed thylakoids show that photosynthesis was inhibited and the Antagonistic effect is caused by the combination of hetero- and homo-aggregation of microalgal cells.
Deng et al. (2020) examined that MPs adsorb phthalate esters and desorb into mouse gut and cause intestinal accumulation. The Di(2-ethylhexyl) phthlate (DEHP)-contaminated MPs cause intestinal inflammation and metabolic disorders due to accumulation of DEHP in the intestine. Schrank et al. (2019) examined morphology, reproductivity, and mortality of Daphnia magna when exposed to rigid PVC and PVC with Diisononyl phthalate. The leached DiNP from flexible PVC significantly affects the number of offspring as well as the growth of D. magna. The marine copepod Tigriopus japonicus exhibits antagonistic effect for acute and chronic reproduction tests when exposed to DBP and polystyrene microplastics (Li et al., 2020b).
4.1.6 Bisphenol (BPA)
Chen et al. (2017) studied the uptake and distribution of BPA in the tissues of zebra fish and neurotoxicity in the presence of PE nanoplastic. The neurotoxic effect in CNS and dopaminergic systems increased in the co-exposure of NPs and BPA. The co-exposure shows no significant reduction of AChE activity because the associated BPA molecules on NPPs cannot interact with AChE directly.
Daphnia magna a fresh water zooplankton was exposed to polyacrylamide and BPA. The daphinds ingested PA MPs loaded with BPA and cause lower BPA body burden (Rehse et al., 2018). Yu et al. (2020) studied the sorption behavior of tetrabromobisphenol a (TBBPA) and PE microbeads and their effect on zebrafish. The co-exposure induced alteration in antioxidant level indicating higher antioxidative stress in liver when compared to either PE or TBBPA alone. The methane production from the anaerobic digestion of waste-activated sludge (WAS) was influenced by the presence of PVC microplastics. The BPA leached from the microplastics ruptured microbial cell walls and extracellular polymeric substances and decreased the methane production by inhibiting the hydrolysis–acidification process (Wei et al., 2019).
4.1.7 Chlorpyrifos (CPF)
Chlorpyrifos is an organophosphate pesticide, and its toxicity leads to neurological dysfunctions, endocrine disruption, and cardiovascular diseases. Bellas and Gil (2020) investigated the toxicity of polyethylene MP and CPF to Acartia tonsa, a calanoid copepod. The survival rates, fecundity, feeding, and egg viability were studied. The combination of CPF and MP caused a 70% decrease in egg production than alone. CPF-loaded MP shows decrease in survival and feeding rate.
Garrido et al. (2019) assessed the toxicity of MPs and the pesticide chlorpyrifos (CPF) to the microalgae, Isochrysis galbana, clone t-ISO. The microalgae growth rate was monitored. The exposure to MPs alone does not affect the microalgae growth. However, CPF affects the microalgae growth. When microalgae exposed to CPF sorbed to MPs, lower percent on inhibition of growth rate was observed. MPs reduced the toxicity of CPF because MPs were too big to penetrate into the microalgal cell wall. Bour et al. (2020) exposed Gasterosteus aculeatus to polyethylene MPs spiked with CPF via prey ingestion. The fish exposed to MP-CPF shows AChE inhibition and hyperactivity.
4.1.8 Triphenyltin Chloride
Triphenyltin chloride is an organotin compound used as a fungicide and antifoulant. Yi et al. (2019a) studied the combined toxicity of polystyrene MPs and triphenyltin chloride to the green algae Chlorella pyrenoidosa. The exposure to 0.55 μm PS led to the structural damage of algal cell which facilitates the uptake of TPTCl and increased its toxicity.
Yi et al. (2019b) investigated the effect of polystyrene (PS) particles on the toxicity of triphenyltin (TPT) to the marine diatom Skeletonema costatum. MPs with smaller size of 0.1 μm have larger surface area for adsorption of TPT than with larger size PS particles. The PS microplastics decreased the toxicity of TPT to the diatom as the IC50 value increased in the presence of PS.
Rainieri et al. (2018) studied the organ toxicity of zebrafish (Danio rerio) when exposed to the mixture of PCBs, BFRs, PFCs , and methylmercury combined with LD-PE 125–250 μm of diameter for a period of 3 weeks. The differential gene expression of selected biomarker genes and quantification of perfluorinated compounds in liver, brain, muscular tissue, and intestine of some selected samples were analyzed. The combined toxicity affects their organ’s homeostasis to higher extent than the contaminants alone. The liver is the most affected organ, and it also has highest concentration of perfluorinated compounds followed by brain and then muscles.
Rochman et al. (2014) showed early-warning signs of endocrine disruption in Japanese medaka (Oryzias latipes) fish exposed to a mixture of contaminants such as PAHs, PCBs, and PBDEs sorbed on polyethylene deployed in San Diego Bay. In a similar study (Rochman et al., 2013b), PBT exposure inhibits AhR activity and decreases expression of CYP1A. Since the fish exposed to the sorbed chemicals in the PE causes bioaccumulation of chemicals leading to liver toxicity.
4.2 Pharmaceutical Toxicity
The European Environmental Agency identified pharmaceutical toxicity as emerging environmental problem. The widespread use of pharmaceutical products increased their discharge into the waterbodies and its toxicity begins to increase. The discharged waste into the environment interacts with the MPs, and when these mixtures are ingested by the organisms, their toxicity increases.
4.2.1 Cefalexin
P. microps juveniles at 25 °C when exposed to cefalexin and microplastics mixtures induced mortality rate (Fonte et al., 2016). The mortality rate of the mixture manifests when the temperature increases. The MPs containing higher concentration of cefalexin indued the predatory performance up to 66%. The presence of microplastics in the water influences the toxicity of cefalexin to P. microps juveniles, decreasing it to a relatively small extent. The temperature rise increased the toxicity of microplastics and of cefalexin , alone and in mixture with microplastics, to P. microps juveniles.
4.2.2 Roxithromycin (ROX)
Interactive effects of microplastics and roxithromycin on red tilapia (Oreochromis niloticus) fish were studied by Zhang et al. (2019a). Compared to roxithromycin exposure, the co-exposure shows bioaccumulation of roxithromycin in gut, liver, brain, and gills. The co-exposure induced the oxidative damage and AChE activity.
Zhang et al. (2019b) studied the toxicity impacts of ROX combined PS microplastics on Daphnia magna. The mortality rate and oxidative stress using SOD, CAT, GST, and GPx were assessed. The smaller size (1-μm) PS might immobilize to daphnids and cause physical damage than 10-μm PS. Hence the co-exposure to 1-μm PS and ROX was more toxic and led to the strongest biological responses in D. magna. Further it induces oxidative damage to cells in combination or microplastics and ROX alone.
4.2.3 Florfenicol (FLO)
Guilhermino et al. (2018) exposed fresh water Corbicula fluminea to the individual and mixtures of the antimicrobial florfenicol and MPs. The toxicology studies show that the mixture of MPs and florfenicol were more toxic than their components separately. MPs-florfenicol mixture caused significant feeding inhibition, neurotoxicity through cholinesterase enzyme (ChE) inhibition, isocitrate dehydrogenase IDH inhibition, and increased oxidative stress and lipid peroxidation levels.
Zhou et al. (2020) investigated the toxicity of oxytetracycline (OTC) and florfenicol with or without the presence of MPs in the blood clam. MPs are easily ingested by blood clam so they easily carry antibiotics into the body and increase the concentration of OTC and FLO. The MPs suppress the GST activity and gene expression by detoxification. The consumption of antibiotic accumulated clams by humans develops antibiotic resistance in the human gut microbiota.
4.2.4 Sertraline
The immune response of bivalve mollusk Tegillarca granosa was studied when exposed to polystyrene MPs (diameters 500 nm and 30 μm) and sertraline antidepressants alone and in combination (Shi et al., 2020). A synergistic immune-toxic effect was observed for Ser and nanoscale MPs, which may be due to the size-dependent interactions.
4.2.5 Sulfamethoxazole (SMX)
Huang et al. (2021) studied the interactive effect of aged and virgin microplastics (MPs) with the antibiotic sulfamethoxazole on red tilapia (Oreochromis niloticus). The co-exposure causes changes in enzyme activities and gene expressions. The red tilapia exposed to aged MPs and SMX causes severe stress lower neurotoxicity, reduced lipid peroxidation damages, and higher inhibitions of cytochrome P450 enzyme activities.
4.2.6 Triclosan (TCS)
Zhu et al. (2018) studied the toxicity of triclosan with four kinds of microplastics, namely, polyethylene, polystyrene, polyvinyl chloride, and PVC on microalgae Skeletonema costatum. The growth inhibition and oxidative stress including superoxide dismutase (SOD) and malondialdehyde (MDA) were determined. The TCS had obvious inhibition effect on microalgae growth within the test concentrations , and single microplastics also had significant inhibition effect which followed the order of PVC800 > PVC > PS > PE. However, the joint toxicity of PVC and PVC800 in combination with TCS decreased more than that of PE and PS. Browne et al. (2013) showed when lugworms are exposed to contaminated chemicals with PVC, they transfer chemicals from microplastics to gut. The results showed that survivorship and feeding were diminished by triclosan associated with PVC.
Syberg et al. (2017) showed that the mixture of PE microbeads and TCS has more toxic effect than TCS alone in marine copepod Acartia tonsa. The presence of MP in the mixture potentiates the toxicity of TCS. Nobre et al. (2020) studied the combined effect of TCS-spiked PE microbeads on oyster Crassostrea brasiliana. The gills, digestive glands, and adductor muscles were dissected and biomarkers responses were studied. Glutathione S-transferases were inhibited in the gills when exposed to PE and TCS-spiked PE. The increase in dibenzylfluorescein dealkylase (DBF) activity was observed in the tissues of oyster.
4.2.7 Venlafaxine
Venlafaxine is a chiral antidepressant. Qu et al. (2019) examined the interactions and ecological impact of PVC MP and venlafaxine and its metabolite O-desmethylvenlafaxine on aquatic ecosystem. MPs may help to transport pollutants to liver subcellular structure and decrease the contaminant’s metabolism. The MPs combined with chemicals enhance the oxidative stress in loach (Misgurnus anguillicaudatus). Enantioselective effects of the co-exposure might cause more adverse effects to organisms.
4.2.8 Procainamide Doxycycline
Microplastics-procainamide and microplastics-doxycycline mixtures were more toxic to marine microalga (Tetraselmis chuii). The mixture enhanced the toxicity significantly than each of the substances tested alone. Interaction of MPs with cell wall facilitates the uptake of chemicals in higher amount. The mixtures significantly affect the average growth and chlorophyll concentration (Prata et al., 2018).
4.3 Toxicity of Heavy Metals
4.3.1 Copper
Copper significantly decreased the average specific growth rate of Tetraselmis chuii. In the presence of MP, no significant differences among the toxicity curves were found. These results indicate that MP did not influence the copper-induced toxicity on T. chuii. The copper interacts with environmental aged microplastics (plastic particles) significantly than virgin ones (Davarpanah & Guilhermino, 2015).
Fu et al. (2019) investigated the interaction of copper with PVC microplastics and cell growth, physiological and biochemical responses of microalga Chlorella vulgaris. Mixture exposure enhances the cell growth, whereas single treatments significantly inhibit the growth of C. vulgaris and cause oxidative stress.
4.3.2 Chromium
Toxicology interaction between chromium and polyethylene was examined by exposing early juveniles of Pomatoschistus microps (Luís et al., 2015). The simultaneous exposure shows decreased predatory performance and inhibition of AChE activity. AChE inhibition was lower in the test with Cr(VI) alone and MP alone. From this study, it is evident that joint exposure increases the AChE inhibition.
4.3.3 Mercury
Mercury is very toxic at even very low concentration. Barboza et al. (2018a) studied the short-term toxic effects of microplastics and mercury exposures, individually and in binary mixtures, on juveniles of the European seabass Dicentrarchus labrax (Linnaeus, 1758). Microplastics and mercury when exposed individually caused neurotoxicity through acetylcholinesterase (AChE) inhibition (62–74%), increased lipid oxidation (LPO) in brain and muscle, and changed the activities of the energy-related enzymes, namely, lactate dehydrogenase (LDH) and isocitrate dehydrogenase (IDH). The binary mixtures caused significant inhibition of brain AChE activity (64–76%), and significant increase of LPO levels in brain (2.9–3.4 fold) and muscle (2.2–2.9 fold).
In another study, the swimming velocity and resistance time of juveniles of the European seabass Dicentrarchus labrax and Dicentrarchus labrax were studied. A significant reduction in swimming velocity was observed in fish exposed to the highest concentration of microplastics (64%), and mercury (53–76%) individually, and in the mixtures (80–87%). A considerable decrease in resistance time was observed in animals exposed to microplastics alone (5–28%), mercury alone (45–53%), and in the mixtures (52–64%). These results may be due to negative effects induced on metabolic, endocrine, and nervous systems that are known to be targets of microplastics and mercury. Since these substances are ubiquitous pollutants, their combined effects may adversely affect wild populations, ecosystem functions, and human health (Barboza et al., 2018b).
4.3.4 Silver
Khan et al. (2015) reported an increased proportion of intestinal silver and significantly lower silver in the body tissue when exposed to Ag-incubated PE microbeads in zebrafish (Danio rerio). Further, Khan et al. (2017) reported that the co-exposure of particulate plastics and Ag, and Ag-incubated particulate plastics, had no effect on the uptake of Ag in the intestine of rainbow trout fish.
Biofouling affects the properties and toxicity of microplastics. The changes in properties and the microplastic toxicity to daphnids Daphnia magna and duckweed Lemna minor were evaluated by Kalčíková et al. (2020). Higher concentration of silver was adsorbed on MPBIO than MP and more silver was leached from MPBIO which was probably due to the weak adsorption of Ag on the algal and bacterial biomass . A decrease in root length and root cell viability of duckweed was observed with MPAg and MPBIOAg at higher concentration when compared to MP and MPBIO.
4.3.5 Zinc
The microplastics generated from the HDPE bags act as a vector for heavy metal adsorption. Hodson et al. (2017) established that microplastics generated from HDPE plastic carrier bags can accumulate Zn and act as a source of exposure to soil fauna. Lumbricus terrestris earthworms when exposed to Zn adsorbed microplastics, but there was no evidence of Zn accumulation, mortality, or weight change. This study signifies that the microplastics act as a vector and increase the metal exposure of the earthworm.
4.3.6 Cadmium
Wen et al. (2018) studied the exposure of MPs and Cd to juvenile S. aequifasciatus. This study shows the reduced bioaccumulation of Cd in the body of the fish which might be due to size, nature, and concentrations of MPs and metal. However, the mixture of MPs and Cd induced severe oxidative damage and stimulate the innate immune responses.
4.3.7 Gold Nanoparticle
The microplastics increase the toxicity of gold nanoparticles to the marine microalgae Tetraselmis chuii (Davarpanah & Guilhermino, 2019). The cultures were exposed to the mixture of microplastic gold nanoparticle for 96 h. AuNP alone and MP alone did not cause significant decrease of average specific growth rate of T. chuii. The mixture significantly reduced the average specific growth rate of the microalgae. The increased toxicity of the mixture relative to the effects caused by the substances alone may have been due to some damage in the cell wall induced by microplastics that facilitated the entrance of AuNP into the cells, resulting in increased toxicity.
A decreased predatory performance which results in delayed growth and reproduction was reported in fish Pomatoschistus microps when exposed to Au-NP and MP (Ferreira et al., 2016). Pacheco et al. (2018) studied the chronic effects of mixtures of citrate-stabilized gold nanoparticles (AuNP) and microplastics on Daphnia magna. The mortality caused by the mixture was higher than the AuNP and MP alone. The higher mortality rate indicates toxicological interactions between AuNP and MP in D. magna.
4.3.8 Lead Chromate
Lead chromate is added as an additive in the plastic manufacturing. Lead chromate toxicity arises due to the leaching of Pb and Cr from the aged microplastics because of surface cracks and fragmentation. Higher concentration of leachate inhibited the cell growth and photosynthesis in Microcystis aeruginosa (Luo et al., 2019).
4.3.9 Arsenic
The US Environmental Protection Agency recognized arsenic as one of the five most harmful soil pollutants. Dong et al. (2020) conducted a study to investigate the effects of microplastic particles of polystyrene and polytetrafluoroethylene and arsenic on leaves and roots of rice seedlings. Microplastic particles and As(III) inhibited the biomass accumulation, photosynthetic rate, chlorophyll fluorescence, and the chlorophyll-a content of rice. Further it induced an oxidative burst in rice tissues through mechanical damage and destruction of the tertiary structure of antioxidant enzymes.
4.4 Health Effects on Humans
Humans are exposed to microplastics and the adsorbed chemicals through ingestion, inhalation, and dermal contact in products, foodstuff, and air. The major source of MP ingestion is through the sea food. Humans could also ingest microplastics by consuming contaminated fruits and vegetable through the uptake from polluted soil (Galloway, 2015). During inhalation, airborne microplastics enter the lungs causing localized biological response, and the main source of airborne microplastics is textile fibers. The dermal exposure occurs through skin pores by the interaction of MPs with contaminated water or soil contaminated or from contact with particulate MPs (Enyoh et al., 2020).
Currently, there are no literatures related to the health effect on the direct or indirect consumption of MPs contaminated with endocrine disruptors. The toxicokinetics of absorption and distribution are only available, and no information is available on metabolism. The MPs and their contaminant chemicals are primarily ingested by lower trophic levels and bioaccumulate. The monomers and additives that leach from the surface of microplastics even in lower concentration act as endocrine disruptors (Cole et al., 2011). The transfer of these chemicals to higher trophic levels including humans raises the possibility of biomagnification. Bakir et al. (2014) showed that desorption of POPs under gut condition could be 30 times greater than in sea water. The gut microbiome is linked with the immune health function and lead to adverse effects such as the proliferation of harmful species, increase in intestinal permeability, and endotoxemia. However, contribution of microplastics to the exposure to POPs seems negligible when compared to the intake from food and water (Bakir et al., 2016). The release of chemicals or microorganisms adsorbed to microplastics will be highly dependent on the types associated with ingested particles, the clearance time and translocation of vector microplastics, the release rate and extent of the contaminant, and its translocation and noxious effects in human tissues. Greim et al. (2001) showed that stable and unstable lesions may arise after metabolism of microplastics associated with PAHs.
The exposure to PS and polyethylene in cerebral and epithelial human cells was not able to induce cytolysis, but increased reactive oxygen species (ROS) to high concentrations, contributing to cytotoxicity (Schirinzi et al., 2017). Furthermore, exposure of macrophage and lung epithelial cell cultures to PS (60 μm) caused ROS and endoplasmic reticulum stress leading to autophagic cell death (Chiu et al., 2015). The medical literature on impact of micro- and nanoplastics originating from inhalation or released from wear debris from plastic prosthetic implants shows diverse effects varying from DNA damage, changes in gene and protein expression, cell clotting, necrosis, apoptosis, and proliferation. However, human health impacts on monomers, additives, and degradation products migrating from plastics and microplastics should be further explored.
5 Conclusion
Microplastics are considered as a harmful potential threat to the aquatic and terrestrial environment as well as to the human health. The sorption of organic chemicals, trace elements on microplastics, and its exposure to the ecosystem led to the accumulation in food chains through agricultural soils, terrestrial, aquatic food chains, and water supply and augment its toxic effects. Several researchers assessed the synergistic effects and the potential transfer of microplastics and associated toxic chemicals to the living organisms. However, the harmful human health effects when exposed to the microplastics and the adsorbed toxic chemicals were mostly unknown which should be further examined.
References
Abdel-Shafy, H. I., & Mansour, M. S. M. (2016). A review on polycyclic aromatic hydrocarbons: Source, environmental impact, effect on human health and remediation. Egyptian Journal of Petroleum, 25, 107–123.
Ajith, N., Arumugam, S., Parthasarathy, S., Manupoori, S., & Janakiraman, S. (2020). Global distribution of microplastics and its impact on marine environment-a review. Environmental Science and Pollution Research International, 27(21), 25970–25986.
Andrady, A. L. (2011). Microplastics in the marine environment. Marine Pollution Bulletin, 62(8), 1596–1605.
Avio, C. G., Gorb,i S., Milan, M., Benedetti, M., Fattorin,i D., d’Erric,o G., Pauletto, M., Bargellon,i L., & Regoli, F. (2015). Pollutants bioavailability and toxicological risk from microplastics to marine mussels. Environmental Pollution, 198, 211–222.
Axel Barrett. (2020). Plastic facts and figures. https://bioplasticsnews.com/2020/03/06/plastic-facts-and-figures
Bakir, A., Rowland, S. J., & Thompson, R. C. (2014). Enhanced desorption of persistent organic pollutants from microplastics under simulated physiological conditions. Environmental Pollution, 185, 16–23.
Bakir, A., O’Connor, I. A., Rowland, S. J., Hendriks, A. J., & Thompson, R. C. (2016). Relative importance of microplastics as a pathway for the transfer of hydrophobic organic chemicals to marine life. Environmental pollution, 219, 56–65.
Barboza, L. G. A., Vieira, L. R., Branco, V., Figueiredo, N., Carvalho, F., Carvalho, C., & Guilhermino, L. (2018a). Microplastics cause neurotoxicity, oxidative damage and energy-related changes and interact with the bioaccumulation of mercury in the European seabass, Dicentrarchus labrax (Linnaeus, 1758). Aquatic Toxicology, 195, 49–57.
Barboza, L. G. A., Vieira, L. R., & Guilhermino, L. (2018b). Single and combined effects of microplastics and mercury on juveniles of the European seabass (Dicentrarchus labrax): Changes in behavioural responses and reduction of swimming velocity and resistance time. Environmental Pollution, 236, 1014–1019.
Batel, A., Linti, F., Scherer, M., Erdinger, L., & Braunbeck, T. (2016). Transfer of benzo[a]pyrene from microplastics toArtemianauplii and further to zebrafish via a trophic food web experiment: CYP1A induction and visual tracking of persistent organic pollutants. Environmental Toxicology and Chemistry, 35, 1656–1666.
Beiras, R., & Tato, T. (2019). Microplastics do not increase toxicity of a hydrophobic organic chemical to marine plankton. Marine Pollution Bulletin, 138, 58–62.
Beiras, R., Muniategui-Lorenzo, S., Rodil, R., et al. (2019). Polyethylene microplastics do not increase bioaccumulation or toxicity of nonylphenol and 4-MBC to marine zooplankton. Science of the Total Environment, 692, 1–9.
Bellas, J., & Gil, I. (2020). Polyethylene microplastics increase the toxicity of chlorpyrifos to the marine copepod Acartia tonsa. Environmental Pollution, 260, 114059.
Besseling, E., Wegner, A., Foekema, E. M., van den Heuvel-Greve, M. J., & Koelmans, A. A. (2013). Effects of microplastic on fitness and PCB bioaccumulation by the lugworm Arenicola marina (L.). Environmental Science & Technology, 47(1), 593–600.
Bouhroum, R., Boulkamh, A., Asia, L., Lebarillier, S., Halle, A. T., Syakti, A. D., Doumenq, P., Malleret, L., & Wong-Wah-chung, P. (2019). Concentrations and fingerprints of PAHs and PCBs adsorbed onto marine plastic debris from the Indonesian Cilacap coast and the North Atlantic gyre. Regional Studies in Marine Science, 29, 100611.
Bour, A., Sturve, J., Höjesjö, J., & Almroth, B. C. (2020). Microplastic vector effects: are fish at risk when exposed via the trophic chain? Frontiers in Environmental Science, 8, 90.
Browne, M. A., Niven, S. J., Galloway,T. S., Rowland, S.J., & Thompson, R. C. (2013). Microplastic moves pollutants and additives to worms, reducing functions linked to health and biodiversity. Current Biology, 23(23), 2388–2392.
Campanale, C., Massarelli, C., Savino, I., Locaputo, V., & Uricchio, V. F. (2020). A detailed review study on potential effects of microplastics and additives of concern on human health. International Journal of Environmental Research and Public Health, 17(4), 1212.
Chen, Q., Yin, D., Jia, Y., Schiwy, S., Legradi, J., Yang, S., & Hollert, H. (2017). Enhanced uptake of BPA in the presence of nanoplastics can lead to neurotoxic effects in adult zebrafish. Science of the Total Environment, 609, 1312–1321.
Chen, C. F., Ju, Y. R., Lim, Y. C., Hsu, N. H., Lu, K. T., Hsieh, S. L., & Chen, C. W. (2020). Microplastics and their affiliated PAHs in the sea surface connected to the southwest coast of Taiwan. Chemosphere, 254, 126818.
Chiu, H. W., Xia, T., Lee, Y. H., Chen, C. W., Tsai, J. C., & Wang, Y. J. (2015). Cationic polystyrene nanospheres induce autophagic cell death through the induction of endoplasmic reticulum stress. Nanoscale, 7(2), 736–746.
Cole, M., Lindeque, P., Halsband, C., & Galloway, T. S. (2011). Microplastics as contaminants in the marine environment: A review. Marine Pollution Bulletin, 62(12), 2588–2597.
Cousin, X., Batel, A., Bringer, A., Hess, S., Bégout, M. L., & Braunbeck, T. (2020). Microplastics and sorbed contaminants – Trophic exposure in fish sensitive early life stages. Marine Environmental Research, 161, 105126.
Davarpanah, E., & Guilhermino, L. (2015). Single and combined effects of microplastics and copper on the population growth of the marine microalgae Tetraselmis chuii. Estuarine, Coastal and Shelf Science, 167, 269–275.
Davarpanah, E., & Guilhermino, L. (2019). Are gold nanoparticles and microplastics mixtures more toxic to the marine microalgae Tetraselmis chuii than the substances individually? Ecotoxicology and Environmental Safety, 181, 60–68.
Deng, Y., Yan, Z., Shen, R., Wang, M., Huang, Y., Ren, H., Zhang, Y., & Lemos, B. (2020). Microplastics release phthalate esters and cause aggravated adverse effects in the mouse gut. Environment International, 143, 105916.
Devriese, L. I., De Witte, B., Vethaak, A. D., Hostens, K., & Leslie, H. A. (2017). Bioaccumulation of PCBs from microplastics in Norway lobster (Nephrops norvegicus): An experimental study. Chemosphere, 186, 10–16.
Dong, Y., Gao, M., Song, Z., & Qiu, W. (2020). Microplastic particles increase arsenic toxicity to rice seedlings. Environmental Pollution, 259, 113892.
Enyoh, C. E., Shafea, L., Verla, A. W., Verla, E. N., Qingyue, W., Chowdhury, T., & Paredes, M. (2020). Microplastics exposure routes and toxicity studies to ecosystems: An overview. Environmental Analysis, Health and Toxicology, 35(1), e2020004.
EPA. (2000). Hudson river PCBs reassessment RI/FS phase 3 report: Feasibility study. U.S. environmental protection agency, and U.S. army corps of engineers.
Ferreira, P., Fonte, E., Soares, M. E., Carvalho, F., & Guilhermino, L. (2016). Effects of multi-stressors on juveniles of the marine fish Pomatoschistus microps : Gold nanoparticles, microplastics and temperature. Aquatic Toxicology, 170, 89–103.
Fonte, E., Ferreira, P., & Guilhermino, L. (2016). Temperature rise and microplastics interact with the toxicity of the antibiotic cefalexin to juveniles of the common goby (Pomatoschistus microps): Post-exposure predatory behavior, acetylcholinesterase activity and lipid peroxidation. Aquatic Toxicology, 180, 173–185.
Fraser, M. A., Chen, L., Ashar, M., Huang, W., Zeng, J., Zhang, C., & Zhang, D. (2020). Occurrence and distribution of microplastics and polychlorinated biphenyls in sediments from the Qiantang River and Hangzhou Bay, China. Ecotoxicology and Environmental Safety, 196, 110536.
Fred-Ahmadu, O. H., Bhagwat, G., Oluyoye, I., Benson, N. U., Ayejuyo, O. O., & Palanisami, T. (2020). Interaction of chemical contaminants with microplastics: Principles and perspectives. Science of The Total Environment, 706, 135978.
Frias, J. P. G. L., Sobral, P., & Ferreira, A. M. (2010). Organic pollutants in microplastics from two beaches of the Portuguese coast. Marine Pollution Bulletin, 60, 1988–1992.
Fu, D., Zhang, Q., Fan, Z., Qi, H., Wang, Z., & Peng, L. (2019). Aged microplastics polyvinyl chloride interact with copper and cause oxidative stress towards microalgae Chlorella vulgaris. Aquatic Toxicology, 216, 105319.
Galloway, T. S. (2015). Micro- and nano-plastics and human health. In M. Bergmann, L. Gutow, & M. Klages (Eds.), Marine anthropogenic litter (pp. 343–366). Cham: Springer.
Garrido, S., Linares, M., Campillo, J. A., & Albentosa, M. (2019). Effect of microplastics on the toxicity of chlorpyrifos to the microalgae Isochrysis galbana, clone t-ISO. Ecotoxicology and Environmental Safety, 173, 103–109.
Gassel, M., & Rochman, C. M. (2019). The complex issue of chemicals and microplastic pollution: A case study in North Pacific lanternfish. Environmental Pollution, 248, 1000–1009.
GESAMP. (2015). Sources, fate and effects of microplastics in the marine environment (part 1). http://www.gesamp.org/publications/reports-and-studies-no-90
GESAMP. (2016). Sources, fate and effects of microplastics in the marine environment: Part two of a global assessment. In: Kershaw PJ, Rochmann CM (Eds.), Joint group of experts on the scientific aspects of marine environmental protection. Rep Stud GESAMP No. 93, 220.
González-Soto, N., Hatfield, J., Katsumiti, A., Duroudier, N., Lacave, J. M., Bilbao, E., Orbea, A., Navarro, E., & Cajaraville, M. P. (2019). Impacts of dietary exposure to different sized polystyrene microplastics alone and with sorbed benzo[a]pyrene on biomarkers and whole organism responses in mussels Mytilus galloprovincialis. Science of the Total Environment, 684, 548–566.
Granby, K., Rainieri, S., Rasmussen, R. R., Kotterman, M. J. J., Sloth, J. J., Cederberg, T. L., Barranco, A., Marques, A., & Larsen, B. K. (2018). The influence of microplastics and halogenated contaminants in feed on toxicokinetics and gene expression in European seabass (Dicentrarchus labrax). Environmental Research, 164, 430–443.
Greim, H., Borm, P., Schins, R., Donaldson, K., Driscoll, K., Hartwig, A., Kuempel, E., Oberdorster, G., & Speit, G. (2001). Toxicity of fibers and particles report of the workshop held in Munich, Germany, October 26–27, 2000. Inhalation Toxicology, 13, 737–754.
Grigorakis, S., & Drouillard, K. G. (2018). Effect of microplastic amendment to food on diet assimilation efficiencies of PCBs by fish. Environmental Science & Technology, 52(18), 10796-10802.
Guilhermino, L., Vieira, L. R., Ribeiro, D., Tavares, A. S., Cardoso, V., Alves, A., & Almeida, J. M. (2018). Uptake and effects of the antimicrobial florfenicol, microplastics and their mixtures on freshwater exotic invasive bivalve Corbicula fluminea. Science of the Total Environment, 622–623, 1131–1142.
Guo, X., Pang, J., Chen, S., & Jia, H. (2018). Sorption properties of tylosin on four different microplastics. Chemosphere, 209, 240–245.
Guo, X., Chen, C., & Wang, J. (2019). Sorption of sulfamethoxazole onto six types of microplastics. Chemosphere, 228, 300–308.
Guven, O., Bach, L., Munk, P., Dinh, K. V., Mariani, P., & Nielsen, T. G. (2018). Microplastic does not magnify the acute effect of PAH pyrene on predatory performance of a tropical fish (Lates calcarifer). Aquatic Toxicology, 198, 287–293.
Hermabessiere, L., Dehaut, A., Paul-Pont, I., Lacroix, C., Jezequel, R., Soudant, P., & Duflos, G. (2017). Occurrence and effects of plastic additives on marine environments and organisms: A review. Chemosphere, 182, 781–793.
Hodson, M. E., Duffus-Hodson, C. A., Clark, A., Prendergast-Miller, M. T., & Thorpe, K. L. (2017). Plastic bag derived-microplastics as a vector for metal exposure in terrestrial invertebrates. Environmental Science & Technology, 51, 4714–4721.
Honda, M., & Suzuki, N. (2020). Toxicities of polycyclic aromatic hydrocarbons for aquatic animals. International Journal of Environmental Research and Public Health, 17, 1363.
Huang, Y., Ding, J., Zhang, G., Liu, S., Zou, H., Wang, Z., Zhu, W., & Geng, J. (2021). Interactive effects of microplastics and selected pharmaceuticals on red tilapia: Role of microplastic aging. Science of the Total Environment, 752, 142256.
Hüffer, T., & Hofmann, T. (2016). Sorption of non-polar organic compounds by micro-sized plastic particles in aqueous solution. Environmental Pollution, 214, 194–201.
Hwang, J., Choi, D., Han, S., Choi, J., & Hong, J. (2019). An assessment of the toxicity of polypropylene microplastics in human derived cells. Science of the Total Environment, 684, 657–669.
IARC. (2011). Monographs on the evaluation of carcinogenic risks to humans, a review of human carcinogens. Lyon: International Agency for Research on Cancer.
Jiang, R., Lin, W., Wu, J., Xiong, Y., Zhu, F., Bao, L., & Zeng, E. Y. (2018). Quantifying nanoplastic-bound chemicals accumulated in Daphnia magna with a passive dosing method. Environmental Science: Nano, 5, 776–781.
Kalčíková, G., Skalar, T., Marolt, G., & Kokalj, A. J. (2020). An environmental concentration of aged microplastics with adsorbed silver significantly affects aquatic organisms. Water Research, 175, 115644.
Karami, A., Romano, N., Galloway, T., & Hamzah, H. (2016). Virgin microplastics cause toxicity and modulate the impacts of phenanthrene on biomarker responses in African catfish (Clarias gariepinus). Environmental Research, 151, 58–70.
Khan, F. R., Syberg, K., Shashoua, Y., & Bury, N. R. (2015). Influence of polyethylene microplastic beads on the uptake and localization of silver in zebrafish (Danio rerio). Environmental Pollution, 206, 73–79.
Khan, F. R., Boyle, D., Chang, E., & Bury, N. R. (2017). Do polyethylene microplastic beads alter the intestinal uptake of Ag in rainbow trout (Oncorhynchus mykiss)? Analysis of the MP vector effect using in vitro gut sacs. Environmental Pollution, 231, 200–206.
Lambert, S., & Wagner, M. (2017). Microplastics are contaminants of emerging concern in freshwater environments: An overview. Freshwater Microplastics, 1–23.
Lee, H., Shim, W. J., & Kwon, J. H. (2014). Sorption capacity of plastic debris for hydrophobic organic chemicals. Science of the Total Environment, 470–471, 1545–1552.
Li, Y., Wang, J., Yang, G., Lu, L., Zheng, Y., Zhang, Q., et al. (2019). Low level of polystyrene microplastics decreases early developmental toxicity of phenanthrene on marine medaka (Oryzias melastigma). Journal of Hazardous Materials, 385, 121586.
Li, Z., Yi, X., Zhou, H., Chi, T., Li, W., & Yang, K. (2020a). Combined effect of polystyrene microplastics and dibutyl phthalate on the microalgae Chlorella pyrenoidosa. Environmental Pollution, 257, 113604.
Li, Z., Zhou, H., Liu, Y., Zhan, J., Li, W., Yang, K., & Yi, X. (2020b). Acute and chronic combined effect of polystyrene microplastics and dibutyl phthalate on the marine copepod Tigriopus japonicus. Chemosphere, 261, 127711.
Lin, W., Jiang, R., Xiong, Y., Wu, J., Xu, J., Zheng, J., & Ouyang, G. (2019). Quantification of the combined toxic effect of polychlorinated biphenyls and nano-sized polystyrene on Daphnia magna. Journal of Hazardous Materials, 364, 531–536.
Liu, G., Zhu, Z., Yang, Y., Sun, Y., Yu, F., & Ma, J. (2018). Sorption behavior and mechanism of hydrophilic organic chemicals to virgin and aged microplastics in freshwater and seawater. Environmental Pollution, 246, 26–33.
Liu, G., Zhu, Z., Yang, Y., Sun, Y., Yu, F., & Ma, J. (2019). Sorption behavior and mechanism of hydrophilic organic chemicals to virgin and aged microplastics in freshwater and seawater. Environmental Pollution, 246, 26–33.
Lo, H. S., Wong, C. Y., Tam, N. F., & Cheung, S. G. (2019). Spatial distribution and source identification of hydrophobic organic compounds (HOCs) on sedimentary microplastic in Hong Kong. Chemosphere, 219, 418–426.
Luís, L. G., Ferreira, P., Fonte, E., Oliveira, M., & Guilhermino, L. (2015). Does the presence of microplastics influence the acute toxicity of chromium(vi) to early juveniles of the common goby (Pomatoschistus microps)? A study with juveniles from two wild estuarine populations. Aquatic Toxicology, 164, 163–174.
Luo, H., Li, Y., Zhao, Y., Xiang, Y., He, D., & Pan, X. (2019). Effects of accelerated aging on characteristics, leaching, and toxicity of commercial lead chromate pigmented microplastics. Environmental Pollution, 257, 113475.
Ma, Y. N., Huang, A. N., Cao, S. Q., Sun, F., Wang, L., Guo, H., & Ji, R. (2016). Effects of nanoplastics and microplastics on toxicity, bioaccumulation, and environmental fate of phenanthrene in fresh water. Environmental Pollution, 219, 166–173.
Magara, G., Elia, A. C., Syberg, K., & Khan, F. R. (2018). Single contaminant and combined exposures of polyethylene microplastics and fluoranthene: Accumulation and oxidative stress response in the blue mussel, Mytilus edulis. Journal of Toxicology and Environmental Health, Part A, 81, 761–773.
Magara, G., Khan, F. R., Pinti, M., Syberg, K., Inzirillo, A., & Elia, A. C. (2019). Effects of combined exposures of fluoranthene and polyethylene or polyhydroxybutyrate microplastics on oxidative stress biomarkers in the blue mussel (Mytilus edulis). Journal of Toxicology & Environmental Health Part A: Current Issues, 82, 616–625.
Mei, W., Chen, G., Bao, J., Song, M., Li, Y., & Luo, C. (2020). Interactions between microplastics and organic compounds in aquatic environments: A mini review. Science of the Total Environment, 736, 139472.
Mendoza, L. M. R., & Jones, P. R. (2015). Characterisation of microplastics and toxic chemicals extracted from microplastic samples from the North Pacific Gyre. Environmental Chemistry, 12, 611–617.
Michałowicz, J. (2014). Bisphenol A – Sources, toxicity and biotransformation. Environmental Toxicology and Pharmacology, 37(2), 738–758.
Miyagawa, S., Sato, T., & Iguchi, T. (2016). Octylphenol. In Handbook of hormones (pp. 575–576). Academic Press.
Nobre, C. R., Moreno, B. B., Alves, A. V., de Lima, R. J., da Rosa, F. H., Abessa, D. M., et al. (2020). Effects of microplastics associated with triclosan on the oyster Crassostrea brasiliana: An integrated biomarker approach. Archives of Environmental Contamination and Toxicology, 79(1), 101–110.
O’Donovan, S., Mestre, N. C., Abel, S., Fonseca, T. G., Carteny, C. C., Cormier, B., & Bebianno, M. J. (2018). Ecotoxicological effects of chemical contaminants adsorbed to microplastics in the clam Scrobicularia plana. Frontiers in Marine Science, 5, 143.
Oliveira, M., Ribeiro, A., Hylland, K., & Guilhermino, L. (2013). Single and combined effects of microplastics and pyrene on juveniles (0+group) of the common goby Pomatoschistusmicrops (Teleostei, Gobiidae). Ecological Indicators, 34, 641–647.
Pacheco, A., Martins, A., & Guilhermino, L. (2018). Toxicological interactions induced by chronic exposure to gold nanoparticles and microplastics mixtures in Daphnia magna. Science of the Total Environment, 628–629, 474–483.
Pascal, M. A., Zabik, M. E., Zabik, M. J., & Hernandez, R. J. (2005). Uptake of polychlorinated biphenyls (PCBs) from an aqueous medium by polyethylene, polyvinyl chloride, and polystyrene films. Journal of Agricultural and Food Chemistry, 53, 164–169.
Paul-Pont, I., Lacroix, C., González Fernández, C., Hégaret, H., Lambert, C., Le Goïc, N., et al. (2016). Exposure of marine mussels Mytilus spp. to polystyrene microplastics: Toxicity and influence on fluoranthene bioaccumulation. Environmental Pollution, 216, 724–737.
Pittura, L., Avio, C. G., Giuliani, M. E., d’Errico, G., Keiter, S. H., Cormier, B., Gorbi, S., & Regoli, F. (2018). Microplastics as vehicles of environmental PAHs to marine organisms: Combined chemical and physical hazards to the mediterranean mussels, Mytilus galloprovincialis. Frontiers in Marine Science, 5, 103.
Plastic in the Ocean Statistics and Facts. (2020). Shocking ocean plastic statistics: The threat to marine life, the ocean & humanity. https://www.condorferries.co.uk/plastic-in-the-ocean-statistics
Prata, J. C., Lavorante, B. R. B. O., Montenegro MdaC, B. S. M., & Guilhermino, L. (2018). Influence of microplastics on the toxicity of the pharmaceuticals procainamide and doxycycline on the marine microalgae Tetraselmis chuii. Aquatic Toxicology, 197, 143–152.
Pѐrez, J. M., Vilas, J. L., Laza, J. M., Arnáiz, S., Mijangos, F., Bilbao, E., Rodríguez, M., & Leon, L. M. (2010). Effect of reprocessing and accelerated ageing on thermal and mechanical polycarbonate properties. Journal of Materials Processing Technology, 210, 727–733.
Qiu, Y., Zheng, M., Wang, L., Zhao, Q., Lou, Y., Shi, L., & Qu, L. (2019). Sorption of polyhalogenated carbazoles (PHCs) to microplastics. Marine Pollution Bulletin, 146, 718–728.
Qu, H., Ma, R., Wang, B., Yang, J., Duan, L., & Yu, G. (2019). Enantiospecific toxicity, distribution and bioaccumulation of chiral antidepressant venlafaxine and its metabolite in loach (Misgurnus anguillicaudatus) co-exposed to microplastic and the drugs. Journal of Hazardous Materials, 370, 203–211.
Rainieri, S., Conlledo, N., Larsen, B. K., Granby, K., & Barranco, A. (2018). Combined effects of microplastics and chemical contaminants on the organ toxicity of zebrafish (Danio rerio). Environmental Research, 162, 135–143.
Rehse, S., Kloas, W., & Zarfl, C. (2018). Microplastics reduce short-term effects of environmental contaminants. Part I: Effects of bisphenol a on freshwater zooplankton are lower in presence of polyamide particles. International Journal of Environmental Research and Public Health, 15(2), 280.
Rochman, C. M., Manzano, C., Hentschel, B. T., Simonich, S. L. M., & Hoh, E. (2013a). Polystyrene plastic: A source and sink for polycyclic aromatic hydrocarbons in the marine environment. Environmental Science & Technology, 47(24), 13976–13984.
Rochman, C. M., Hoh, E., Kurobe, T., & Teh, S. J. (2013b). Ingested plastic transfers hazardous chemicals to fish and induces hepatic stress. Scientific Reports, 3, 1–7.
Rochman, C. M., Kurobe, T., Flores, I., & Teh, S. J. (2014). Early warning signs of endocrine disruption in adult fish from the ingestion of polyethylene with and without sorbed chemical pollutants from the marine environment. Science of the Total Environment, 493, 656–661.
Rochman, C. M., Parnis, J. M., Browne, M. A., Serrato, S., Reiner, E. J., Robson, M., & Teh, S. J. (2017). Direct and indirect effects of different types of microplastics on freshwater prey (Corbicula fluminea) and their predator (Acipenser transmontanus). PLoS One, 12(11), e0187664.
Sánchez-Bayo, F. (2011). Sources and toxicity of pollutants. In Ecological impacts of toxic chemicals (pp. 3–12). Bentham Science Publishers Ltd.
Sarma, B., & Joshi, S. R. (2015). Environmental toxicity and the role of pseudomonads in biodegradation of xenobiotics. In Biology, biotechnology and sustainable development (pp. 41–61). Research India Publications.
Schirinzi, G. F., Pérez-Pomeda, I., Sanchís, J., Rossini, C., Farré, M., & Barceló, D. (2017). Cytotoxic effects of commonly used nanomaterials and microplastics on cerebral and epithelial human cells. Environmental Research, 159, 579–587.
Schrank, I., Trotter, B., Dummert, J., Scholz-Böttcher, B. M., Löder, M. G. J., & Laforsch, C. (2019). Effects of microplastic particles and leaching additive on the life history and morphology of Daphnia magna. Environmental Pollution, 255(Pt 2), 113233.
Sharma, M. D., Elangickal, A. I., Mankar, J. S., & Krupadam, R. J. (2020). Assessment of cancer risk of microplastics enriched with polycyclic aromatic hydrocarbons. Journal of Hazardous Materials, 398, 122994.
Shi, W., Han, Y., Sun, S., Tang, Y., Zhou, W., Du, X., & Liu, G. (2020). Immunotoxicities of microplastics and sertraline, alone and in combination, to a bivalve species: Size-dependent interaction and potential toxication mechanism. Journal of Hazardous Materials, 396, 122603.
Shukla, S. K., Mangwani, N., Rao, T. S., & Das, S. (2014). Biofilm-mediated bioremediation of polycyclic aromatic hydrocarbons. In Microbial biodegradation and bioremediation (pp. 203–232). Elsevier.
Syberg, K., Nielsen, A., Khan, F. R., Banta, G. T., Palmqvist, A., & Jepsen, P. M. (2017). Microplastic potentiates triclosan toxicity to the marine copepod Acartia tonsa (Dana). Journal of Toxicology and Environmental Health. Part A, 80(23–24), 1369–1371.
Tan, X., XubiaoYu, C. L., Wang, J., & Peng, J. (2019). Occurrence and organic contaminant-carrier effect of microplastics in surface water from the Feilaixia Reservoir in the Beijiang River, China. Chemosphere, 221, 834–840.
Tanaka, K., Takada, H., Yamashita, R., Mizukawa, K., Fukuwaka, M. A., & Watanuki, Y. (2013). Accumulation of plastic-derived chemicals in tissues of seabirds ingesting marine plastics. Marine Pollution Bulletin, 69(1–2), 219–222.
Teuten, E. L., Rowland, S. J., Galloway, T. S., & Thompson, R. C. (2007). Potential for plastics to transport hydrophobic contaminants. Environmental Science & Technology, 41(22), 7759–7764.
Teuten, E. L., Saquing, J. M., Knappe, D. R. U., et al. (2009). Transport and release of chemicals from plastics to the environment and to wildlife. Philosophical Transactions of the Royal Society B: Biological Sciences, 364, 2027–2045.
Tourinho, P. S., Koˇcí, V., Loureiro, S., & van Gestel, C. A. (2019). Partitioning of chemical contaminants to microplastics: Sorption mechanisms, environmental distribution and effects on toxicity and bioaccumulation. Environmental Pollution, 252, 1246–1256.
USEPA. (1980). Ambient water quality criteria for polynuclear aromatic hydrocarbons.
Van der Hal, N., Yeruhamb, E., Shukisa, D., Rilovb, G., Astrahan, P., & Angel, D. L. (2019). Uptake and incorporation of PCBs by eastern Mediterranean rabbitfish that consumed microplastics. Marine Pollution Bulletin, 150, 110697.
Van, A., Rochman, C. M., Flores, E. M., Hill, K. L., Vargas, E., Vargas, S. A., & Hoh, E. (2012). Persistent organic pollutants in plastic marine debris found on beaches in San Diego, California. Chemosphere, 86, 258–263.
Veerasingam, S., Saha, M., Suneel, V., Vethamony, P., Rodrigues, A. C., Bhattacharyya, S., & Naik, B. G. (2016). Characteristics, seasonal distribution and surface degradation features of microplastic pellets along the Goa coast, India. Chemosphere, 159, 496–505.
Velzeboer, I., Kwadijk, C., & Koelmans, A. (2014). Strong sorption of PCBs to nanoplastics, microplastics, carbon nanotubes and fullerenes. Environmental Science & Technology, 48, 4869–4876.
Verla, A. W., Enyoh, C. E., Verla, E. N., & Nwarnorh, K. O. (2019). Microplastic–toxic chemical interaction: A review study on quantified levels, mechanism and implication. SN Applied Sciences, 1, 1400.
Wang, W., & Wang, J. (2018). Comparative evaluation of sorption kinetics and isotherms of pyrene onto microplastics. Chemosphere, 193, 567–573.
Wang, F., Shih, K. M., & Li, X. Y. (2015). The partition behavior of perfluorooctanesulfonate (PFOS) and perfluorooctanesulfonamide (FOSA) on microplastics. Chemosphere, 119, 841–847.
Wang, F., Wong, C. S., Da Chen, L. X., Wang, F., & Zeng, E. Y. (2018). Interaction of toxic chemicals with microplastics: A critical review. Water Research, 139, 208–219.
Wang, F., Yang, W., Cheng, P., Zhang, S., Zhang, S., Jiao, W., & Sun, Y. (2019a). Adsorption characteristics of cadmium onto microplastics from aqueous solutions. Chemosphere, 235, 1073–1080.
Wang, J., Liu, X., Liu, G., Zhang, Z., Wu, H., Cui, B., Bai, J., & Zhang, W. (2019b). Size effect of polystyrene microplastics on sorption of phenanthrene and nitrobenzene. Ecotoxicology and Environmental Safety, 173, 331–338.
Wei, W., Huang, Q. S., Sun, J., Wang, J. Y., Wu, S. L., & Ni, B. J. (2019). Polyvinyl chloride microplastics affect methane production from the anaerobic digestion of waste activated sludge through leaching toxic bisphenol-A. Environmental Science & Technology, 53, 2509–2517.
Wen, B., Jin, S. R., Chen, Z. Z., Gao, J. Z., Liu, Y. N., Liu, J. H., & Fen, X. S. (2018). Single and combined effects of microplastics and cadmium on the cadmium accumulation, antioxidant defence and innate immunity of the discus fish (Symphysodon aequifasciatus). Environmental Pollution, 243, 462–471.
Wu, P., Cai, Z., Jin, H., & Tang, Y. (2019). Adsorption mechanisms of five bisphenol analogues on PVC microplastics. Science of the Total Environment, 650(Pt 1), 671–678.
Xu, B., Liu, F., Brookes, P. C., & Xu, J. (2018a). Microplastics play a minor role in tetracycline sorption in the presence of dissolved organic matter. Environmental Pollution, 240, 87–94.
Xu, B., Liu, F., Brookes, P. C., & Xu, J. (2018b). The sorption kinetics and isotherms of sulfamethoxazole with polyethylene microplastics. Marine Pollution Bulletin, 131, 191–196.
Xu, P., Ge, W., Chai, C., Zhang, Y., Jiang, T., & Xia, B. (2019). Sorption of polybrominated diphenyl ethers by microplastics. Marine Pollution Bulletin, 145, 260–269.
Yang, W., Gao, X., Wu, Y., Wan, L., Tan, L., Yuan, S., Ding, H., & Zhang, W. (2020). The combined toxicity influence of microplastics and nonylphenol on microalgae Chlorella pyrenoidosa. Ecotoxicology and Environmental Safety, 195, 110484.
Yazdani Foshtomi, M., Oryan, S., Taheri, M., Darvish Bastami, K., & Zahed, M. A. (2019). Composition and abundance of microplastics in surface sediments and their interaction with sedimentary heavy metals, PAHs and TPH (total petroleum hydrocarbons). Marine Pollution Bulletin, 149, 110655.
Yeo, B. G., Takada, H., Yamashita, R., Okazaki, Y., Uchida, K., Tokai, T., & Trenholm, N. (2019). PCBs and PBDEs in microplastic particles and zooplankton in open water in the Pacific Ocean and around the coast of Japan. Marine Pollution Bulletin, 151, 110806.
Yi, X., Chi, T., Li, Z., Wang, J., Yu, M., Wu, M., & Zhou, H. (2019a). Combined effect of polystyrene plastics and triphenyltin chloride on the green algae Chlorella pyrenoidosa. Environmental Science and Pollution Research, 26, 15011–15018.
Yi, X., Wang, J., Li, Z., Zhang, Z., Chi, T., Guo, M., Li, H., & Zhou, H. (2019b). The effect of polystyrene plastics on the toxicity of triphenyltin to the marine diatom Skeletonema costatum—influence of plastic particle size. Environmental Science and Pollution Research International, 26(25), 25445–25451.
Yu, Y., Ma, R., Qu, H., Zuo, Y., Yu, Z., Hu, G., Li, Z., Chen, H., Lin, B., Wang, B., & Yu, G. (2020). Enhanced adsorption of tetrabromobisphenol a (TBBPA) on cosmetic-derived plastic microbeads and combined effects on zebrafish. Chemosphere, 248, 126067.
Zhang, H., Wang, J., Zhou, B., Zhou, Y., Dai, Z., Zhou, Q., Chriestie, P., & Luo, Y. (2018). Enhanced adsorption of oxytetracycline to weathered microplastic polystyrene: Kinetics, isotherms and influencing factors. Environmental Pollution, 243(Pt B), 1550–1557.
Zhang, S., Ding, J., Razanajatovo, R. M., Jiang, H., Zou, H., & Zhu, W. (2019a). Interactive effects of polystyrene microplastics and roxithromycin on bioaccumulation and biochemical status in the freshwater fish red tilapia (Oreochromis niloticus). Science of the Total Environment, 648, 1431–1439.
Zhang, P., Yan, Z., Lu, G., & Ji, Y. (2019b). Single and combined effects of microplastics and roxithromycin on Daphnia magna. Environmental Science and Pollution Research, 26, 17010–17020.
Zhang, P., Huang, P., Sun, H., Ma, J., & Li, B. (2020). The structure of agricultural microplastics (PT, PU and UF) and their sorption capacities for PAHs and PHE derivates under various salinity and oxidation treatments. Environmental Pollution, 257, 113525.
Zhou, W., Han, Y., Tang, Y., Shi, W., Du, X., Sun, S., & Liu, G. (2020). Microplastics aggravate the bioaccumulation of two waterborne veterinary antibiotics in an edible bivalve species: Potential mechanisms and implications for human health. Environmental Science & Technology, 54(13), 8115–8122.
Zhu, Z., Wang, S., Zhao, F., Wang, S., Liu, F., & Liu, G. (2018). Joint toxicity of microplastics with triclosan to marine microalgae Skeletonema costatum. Environmental Pollution, 246, 509–517.
Zou, J., Liu, X., Zhang, D., & Yuan, X. (2020). Adsorption of three bivalent metals by four chemical distinct microplastics. Chemosphere, 248, 126064.
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Sunitha, T.G., Sivasankar, V., Prabhakaran, M., Omine, K. (2022). Microplastics – Pollutants’ Interactions, Mechanisms, and Potential Toxicity. In: Vasanthy, M., Sivasankar, V., Sunitha, T.G. (eds) Organic Pollutants. Emerging Contaminants and Associated Treatment Technologies. Springer, Cham. https://doi.org/10.1007/978-3-030-72441-2_22
Download citation
DOI: https://doi.org/10.1007/978-3-030-72441-2_22
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-72440-5
Online ISBN: 978-3-030-72441-2
eBook Packages: Earth and Environmental ScienceEarth and Environmental Science (R0)