Abstract
Bleeding from the hepatobiliary-pancreatic tract is an uncommon source of nonvariceal occult upper gastrointestinal bleeding and a high index of suspicion is needed for early diagnosis. This includes ampullary lesions, hemobilia, hemosuccus pancreaticus, etc. However, most cases are often related to an iatrogenic cause from either bile duct or liver manipulation [1]. The key to recognition involves focused history-taking and awareness of the possibility in the correct clinical setting. Of note, standard upper endoscopy can miss lesions on the duodenal sweep and ampulla. In this chapter, we will cover the anatomy and relevant vasculature of the hepatobiliary-pancreatic tract; etiologies, clinical presentation, diagnosis, and management of occult bleeding related to the hepatobiliary-pancreatic tract.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
Anatomy and Relevant Vasculature
The right and left hepatic ducts, shortly after leaving the porta hepatis, unite to form the 2- to 3-cm long hepatic duct, which then joins the cystic duct to form the common bile duct (CBD), 10 to 15 cm in length. The CBD is partially or fully covered by pancreatic tissue posteriorly as it descends into the duodenum. In more than 66% of cases, the CBD and the major pancreatic duct share a common channel, 2 to 7 mm in length. The two ducts unite to form the hepatopancreatic ampulla (ampulla of Vater), where the distal end of the ampulla opens into the duodenum through the major duodenal papilla [2]. See Fig. 9.1 for illustration.
The biliary tree [4]. https://springerlink.bibliotecabuap.elogim.com/chapter/10.1007/978-1-4939-1884-3_5
Generally, the arterial supply to the pancreas, common bile duct, and adjacent portions of the duodenum comes from branches of the gastroduodenal, superior mesenteric, and splenic arteries. The arteries supplying the bile duct include the posterior superior pancreaticoduodenal artery and gastroduodenal artery, supplying the retroduodenal part of the duct; cystic artery, supplying the proximal part of the duct; and right hepatic artery, supplying the middle part of the duct [3].
Etiologies
Hemobilia arises where there is a fistula between the surrounding vascular structure and the biliary tree. Bleeding may result from obstruction or injury of any of the organs or structures associated with the hepatobiliary-pancreatic tract. The arterial system is usually the source of the bleeding due to relatively greater intravascular pressure creating unidirectional flow into the biliary tree. However, cases of hemobilia associated with higher venous pressure have been reported in the presence portal hypertension [5]. Most cases of hemobilia result from an iatrogenic injury, through inflammation, trauma, or vascular anomalies and can also occur spontaneously in setting of coagulopathy [6,7,8,9,10,11,12,13,14,15]. Malignancy in the hepatobiliary-pancreatic tract can also contribute to obscure nonvariceal upper GI bleeding as it has been demonstrated in cases of ampullary and periampullary tumors [16, 17].
Percutaneous Liver Procedures
Percutaneous liver interventions are of widespread use due to their minimal invasive approach and they can often be performed in the outpatient setting. Some of those procedures include liver biopsy, percutaneous transhepatic cholangiography (PTC), and percutaneous biliary therapies, including radiofrequency ablation. The insertion of a needle through the liver for tissue sampling or intervention can potentially cause inadvertent injury to interior blood vessels which are anatomically near the biliary tree. Formation of fistula and communication between hepatic vasculature and biliary system can ensue. The true incidence of hemobilia due to liver biopsies is unclear. However, a large multicenter retrospective study on 68,267 biopsies only revealed 4 reported cases of hemobilia but no deaths [18]. It has been reported that there is a higher incidence of hemobilia with percutaneous transhepatic biliary drainage (PTBD) in comparison to percutaneous transhepatic cholangiography (PTC) [19]. This is likely due to the increased instrumentation and manipulation prior to drain insertion in PTBD [19].
Hepatobiliary Surgery
Surgery of the hepatobiliary system carries potential risk for hemobilia. Cystic artery and right hepatic artery pseudoaneurysms leading to hemobilia have been reported as complications of laparoscopic and open cholecystectomy [20,21,22]. Though the exact pathogenesis of the pseudoaneurysm formation has yet to be determined, it is thought to be related to bile leakage causing blood vessels irritation and associated peritoneal infection; hence delaying proper healing of damaged vasculature. Cases of hemobilia have also been reported with liver transplantation and pancreaticoduodenectomy (Whipple procedure) [23, 24].
Endoscopic Hepatobiliary Procedures
Bleeding during endoscopic sphincterotomy or post-sphincterotomy is a common complication, typically from an arterial source associated with the margin of cut sphincter. This is manifested as bright red blood flowing outward from the lesion to the duodenum. Anomalous location of the ampulla creates a set of challenges and difficulties for endoscopic retrograde cholangiopancreatography (ERCP) and is a risk factor for sphincterotomy associated hemobilia [25]. Other clinical settings where ERCP associated hemobilia have been observed include portal biliopathy, metal biliary stenting, and intrahepatic vascular anomalies associated with hereditary hemorrhagic telangiectasia [26,27,28]. Portal biliopathy refers to abnormalities of the biliary system seen as a late complication of portal hypertension in patients with extrahepatic portal venous obstruction. It gives rise to cavernous transformation of the blood vessels nearing the biliary tree. In essence, this creates choledochal varices that can easily damage or rupture especially in setting intraductal manipulations. Spontaneous hemobilia has also been reported in portal biliopathy without endoscopic intervention. Of note, ERCP-associated hemobilia increases in coagulopathy and biliary stenosis [5].
Accidental Trauma
Liver injury frequently occurs in blunt abdominal trauma victims and the mortality rate related to the liver injury is estimated to range from 4.1% to 11.7% [29]. When the liver sustains a blunt trauma, a shearing injury of the hepatic artery may develop resulting in a hepatic pseudoaneurysm [30]. Intraperitoneal hemorrhage can result if these pseudoaneurysms rupture. They can also drain into the biliary system leading to hemobilia. Cases of hemobilia are seen in less than 3% of liver trauma. Hemobilia related abdominal trauma is more common to the pediatric population [31].
Cholelithiasis
Cholelithiasis can cause minor trauma to the bile duct, hence mild intraductal bleeding. However, significant hemobilia can occur if the stone erodes through the hepatoduodenal ligament or cystic artery, potentially resulting in cystic artery aneurysm [32]. In severe cases, choledocholithiasis can provoke necrotic erosion through the ductal wall and into surrounding blood vessels, leading to significant hemorrhage [33].
Inflammation and Infection
Hemobilia can arise from known complications of chronic pancreatitis; namely pseudoaneurysm of the pancreaticoduodenal, splenic, hepatic, and gastroduodenal arteries. Similarly, chronic cholecystitis may be complicated by cystic artery pseudoaneurysm resulting in hemobilia [34].
Infection of the biliary tree and liver is also a risk factor for hemobilia. Ascaris lumbricoides, Clonorchis sinensis, and Fasciola hepaticum are known parasites that are associated with infection and obstruction in the biliary tree and liver. Some of the clinical manifestations are ascending cholangitis, acute cholecystitis, pancreatitis, hepatic abscess, and hemobilia [34]. The mechanism of hemobilia in that setting is inflammation of perivascular tissue, weakening of vessel walls, and pseudoaneurysm formation [34].
Malignancy
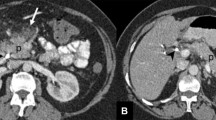
Hepatobiliary tumors (primary or metastatic) are potential causes of hemobilia. Tumor tissue is often friable and hypervascular, which creates the likelihood of spontaneous hemorrhage. Furthermore, the biliary tree can be invaded by those tumors [35]. Minimal invasive procedures, such as radiofrequency ablation, are effective and relatively safe at managing unresectable hepatic tumors, however a range of complication have been reported. Hemobilia is a known potential complication of percutaneous intervention and has been related to radiofrequency ablation. Hepatocellular carcinoma (HCC), cholangiocarcinoma (Fig. 9.2), pancreatic adenocarcinoma, gallbladder cancer, and liver metastases have reportedly caused hemobilia [36,37,38].
Missed Lesions
Ampullary (Fig. 9.3) and periampullary lesions are rare entities, however they are often missed on routine upper endoscopies. They have also been reported as etiologies of occult GI bleeding, likely related to ulceration and slow blood loss due to the hypervascular and friable nature of those lesions [16, 17]. Radiographic imaging techniques such as CT angiography and radionucleotide tagged red blood cell scan, are usually useful only during active bleeding. Side-viewing endoscope should be strongly considered while evaluating the duodenal papilla and the ampullary complex, especially in cases of suspected hemobilia or nonvariceal upper gastrointestinal bleeding.
Hemosuccus Pancreaticus (HP)
Hemosuccus pancreaticus (HP) is a rare entity of gastrointestinal bleeding. It is clinically distinct from hemobilia as bleeding originates from the pancreatic duct instead of the common bile duct. It is often associated with pancreatic diseases. Hemosuccus pancreaticus can be due to chronic pancreatitis, pancreatic pseudocysts, and pancreatic neoplasms where bleeding occurs from an erosion into a blood vessel forming a fistula with the pancreatic duct [39]. Therapeutic endoscopic intervention of the pancreas, such as pancreatic sphincterotomy or stone removal, can contribute to hemosuccus pancreaticus [39].
Clinical Presentation
The classic triad of hemobilia is right upper quadrant pain, jaundice, and bleeding, however all three findings are only present in 22–35% of cases [38]. Most cases of hemobilia are characterized by minor hemorrhage that resolve spontaneously, though profuse bleeding presenting with hematemesis, melena or hematochezia can also occur. Slow oozing in the biliary tract can lead to blood clot, which can then cause obstructive jaundice and is associated with biliary sepsis. The presentation of hemobilia and its timing vary based on the etiology. For instance, hemobilia due to ERCP usually occurs immediately or within the next few days [40, 41]. Presentation can also be delayed in cases pseudoaneurysm formation or following a trauma. In patients with a percutaneous transhepatic biliary drain (PTBD), blood output from the biliary drain is often noted.
Diagnosis
Hemobilia should be suspected in patients presenting with GI bleeding, history of right upper quadrant abdominal pain, jaundice, recent biliary instrumentation and abnormal liver function tests (LFTs). Diagnosis of hemobilia, however, can be delayed or missed due to lack awareness from clinicians. Moreover, given its rare occurrence and sometimes insidious presentation, diagnosis of hemobilia can be challenging. Imaging and endoscopic studies play a crucial role in establishing an initial diagnosis, assessing possible etiology and extent of bleeding, and guiding treatment modalities. Figure 9.4 provides a flow diagram for management of suspected hemobilia.
Diagnostic Imaging
Computed Tomography Angiography (CTA)
CT angiography of the abdomen has been recognized as the first-line investigative modality in suspected hemobilia due to its noninvasive nature, rapid results, and diagnostic yield and characteristics. CTA can not only help confirm hemobilia but also provide details about potential etiology [42]. Some the pathologies identified on CTA include extravasation into the parenchyma, clots in the gallbladder or biliary system, biliary dilatation, pseudoaneurysms, and other vascular malformations (e.g., aneurysms, angiodysplasia, arteriovenous malformations and hemangiomas).
Angiography
Angiography remains the gold standard for diagnosis and treatment of hemobilia as it can help localize the actual bleeding source and provide detailed visualization of the vasculature and potential anomalies (arteriobiliary fistula, vascular malformations such as aneurysms, pseudoaneurysms, hemangiomas etc.). It does, however, involve higher radiation exposure and endovascular access unlike the CTA. It has been reported that angiography yields the correct etiology of massive hemobilia in more than 90% of cases [43].
Magnetic Resonance Cholangiopancreatography (MRCP)
MRCP is not often used for the diagnosis of hemobilia though it has been reported in cases involving biliary obstruction [8]. It is a noninvasive alternative to endoscopic retrograde cholangiopancreatography (ERCP) that shows imaging of the pancreaticobiliary system [8].
Ultrasound
Abdominal ultrasound is dependent on operator’s skill and experience and body habitus can certainly be a detrimental factor in preventing proper visualization of the biliary tree. Moreover, blood clots within the bile ducts lose echogenicity over time and can be missed during ultrasound examination [43]. Therefore, the diagnostic effectiveness of abdominal ultrasound in identifying hemobilia is limited.
Endoscopy
Upper GI endoscopy (esophagogastroduodenoscopy) is commonly used in the evaluation of patients presenting with upper GI bleeding. It is very effective at ruling out common etiologies of upper GI bleeding and can, sometimes incidentally, diagnose cases of hemobilia. When hemobilia is suspected, a duodenoscope (i.e., side-viewing scope) should be used to directly visualize the ampulla for the presence of blood and/or clots or ampullary lesions (Fig. 9.5a).
(a) Blood clot at the papilla in a case of hemobilia seen with side-viewing duodenoscopy. (b) ERCP demonstrating radiolucent filling defects in the extrahepatic bile duct in a patient found to have hemobilia [34]. https://springerlink.bibliotecabuap.elogim.com/article/10.1007/s11894-010-0092-5
ERCP helps to visualize the bile ducts, gallbladder and can even play a therapeutic role in cases of hemobilia and/or associated with biliary obstruction. ERCP findings of blood clots to suggest hemobilia are characterized by amorphous, tubular, or cast-like filling defects in the biliary tree or gallbladder (Fig. 9.5b) [43].
Endoscopic ultrasound (EUS) has been used to successfully diagnosed hemobilia from hepatic artery pseudoaneurysm [44]. It can also be helpful at identifying blood within the biliary tree, common bile duct, which would be characterized by mobile, hyperechoic material [45].
Cholangioscopy is an advanced endoscopic technique that has been reported to determine the etiology of hemobilia in rare cases [46]. It provides direct visualization of the biliary tree however endoscopic therapy is limited due to small accessory channel.
Management
After adequate resuscitation, management of hemobilia comprises of 2 objectives: achieve hemostasis and maintain biliary patency and adequate bile flow. The modalities used in the management of hemobilia depend on the etiology of the bleed, however they mostly encompass conservative therapy, percutaneous radiologic intervention, endoscopic treatment and surgery.
Conservative management is usually reserved for minor hemobilia and interventions usually include correction of coagulopathy if needed, and IV fluid hydration. Minor hemobilia can often be seen in injury related to PTBD catheters where catheter exchange and upsize and position adjustment can help tamponade blood and resolve hemobilia [42].
Angiography
Radiologic interventions for management of hemobilia can serve both diagnostic and therapeutic modalities. Angiography has emerged as the gold standard for management of hemobilia and has a success rate of 80% to 100% [47]. Of note, iatrogenic hemobilia is commonly related to hepatic artery injury. Angiography with transcatheter arterial embolization (TAE) is often considered when hemobilia is secondary to large arterial aneurysms or pseudoaneurysms, arteriobiliary fistulae, and/or intrahepatic or extrahepatic vascular lesions.
After selecting the injured artery, embolization is performed both proximal and distal to the injury to ensure that no back bleeding occurs via intrahepatic arterial collaterals [48]. As the liver receives dual blood supply (portal vein and hepatic artery), TAE is contraindicated when portal vein thrombosis or obstruction is present in order to prevent potential significant hepatic ischemia. TAE should be avoided in patients with liver transplant, cirrhosis with concurrent shock, as more extensive ischemic liver injury can result due to the already compromised collateral blood flow [49]. An alternative to TAE is arterial stenting which works as a tamponade measure. It may be beneficial in cases where preserving blood flow is critical and when TAE is contraindicated. Complications of TAE include hepatic ischemia leading to necrosis, hepatic abscesses, hyperaminotransaminemia, and gallbladder infarction [50].
Percutaneous thrombin injection is a salvage technique used to management pseudoaneurysms when TAE fails [51,52,53].
ERCP
There is a role for the use of ERCP in management of hemobilia especially in hemodynamically stable patients. Moreover, it can help maintain biliary patency by removing blood clots within the biliary tree; hence preventing obstructive jaundice, acute cholangitis, acute cholecystitis, and acute pancreatitis [43]. Post-sphincterotomy hemobilia arises from injury of the posterior branch of the superior pancreaticoduodenal artery and many endoscopic techniques can be used in the management of this postprocedural bleeding. Balloon tamponade, monopolar or bipolar coagulation, epinephrine injection, hemoclipping, and biliary stenting have been reported to be successful at post-sphincterotomy hemobilia or in cases where the site of bleeding is distal (i.e., at the level of the papilla or ampulla) [54,55,56].
Biliary stents have been reported to help control bleeding located at the extrahepatic bile duct. They work by providing a tamponade effect on the bile duct wall while maintaining ductal patency to allow bile flow. Metallic and plastic stents have been used successfully in managing hemobilia related to therapeutic maneuvers of sphincterotomy, stent removal, papillary balloon dilation, bile duct biopsy, pancreatic fine-needle aspiration, and malignancy with bile duct invasion [35, 57,58,59,60,61,62].
Surgery
Surgical intervention is indicated in cases of failed, endoscopic, endovascular, and/or percutaneous therapies. Surgery is also needed when hemobilia is complicated by cholecystitis. When pseudoaneurysms are infected or are compromising surrounding vasculature, surgery is also preferred [43, 44]. When the lesion or injury is located, the damaged vessel is ligated, or the infected pseudoaneurysm is excised. Cholecystectomy can be performed concurrently if indicated. Of note, in setting of uncontrolled intrahepatic bleeding, partial hepatectomy is an option.
References
Yoshida J, Donahue PE, Nyhus LM. Hemobilia: review of recent experience with a worldwide problem. Am J Gastroenterol. 1987;82:448–53.
Floch MH, et al. Netter’s gastroenterology. Philadelphia: Saunders/Elsevier; 2010.
Moore KL, Agur AMR, Dalley AF. Essential clinical anatomy. Philadelphia: Lippincott Williams & Wilkins; 2002.
Wagner-Bartak NA, Prabhakar AM, Menias CO, Prabhakar HB, Elsayes KM. The biliary tree. In: Elsayes KM, editor. Cross-sectional imaging of the abdomen and pelvis. New York: Springer; 2015. https://doi.org/10.1007/978-1-4939-1884-3_5.
Schlansky B, Kaufman JA, Bakis G, et al. Portal biliopathy causing recurrent biliary obstruction and hemobilia. ACG Case Rep J. 2013;1:44–6.
Ahn J, Trost DW, Mitty HA, Sos TA. Pseudoaneurysm formation after catheter dissection of the common hepatic artery: report of two cases. Am J Gastroenterol. 1997;92:696–9.
Albuquerque W, Arantes V, de Paula FK, Lambertucci JR. Acute pancreatitis and acute cholecystitis caused by hemobilia after percutaneous ultrasound-guided liver biopsy. Endoscopy. 2005;37:1159–60.
Asselah T, Condat B, Sibert A, et al. Haemobilia causing acute pancreatitis after percutaneous liver biopsy: diagnosis by magnetic resonance cholangiopancreatography. Eur J Gastroenterol Hepatol. 2001;13:877–9.
Attiyeh FF, McSweeney J, Fortner JG. Hemobilia complicating needle liver biopsy: a case report with arteriographic demonstration. Radiology. 1976;118:559–60.
Bichile LS, Malkan G, Patel YS, et al. Recurrent haemobilia in a patient with polyarteritis nodosa. J Assoc Physicians India. 1996;44:49–50.
de Quinta FR, Moles ML, Docobo DF, et al. Hemobilia secondary to chronic cholecystitis. Rev Esp Enferm Dig. 2004;96:221–5.
de Sio I, Castellano L, Calandra M. Hemobilia following percutaneous ethanol injection for hepatocellular carcinoma in a cirrhotic patient. J Clin Ultrasound. 1992;20:621–3.
Edden Y, St Hilaire H, Benkov K, Harris MT. Percutaneous liver biopsy complicated by hemobilia-associated acute cholecystitis. World J Gastroenterol. 2006;12:4435–6.
Enne M, Pacheco-Moreira LF, Cerqueira A, et al. Fatal hemobilia after radiofrequency thermal ablation for hepatocellular carcinoma. Surgery. 2004;135:460–1.
Forlee MV, Krige JE, Welman CJ, Beningfield SJ. Haemobilia after penetrating and blunt liver injury: treatment with selective hepatic artery embolization. Injury. 2004;35:23–8.
Janzen RM, Ramj AS, Flint JDA, Scudamore CH, Yoshida EM. Obscure gastrointestinal bleeding from an ampullary tumour in a patient with a remote history of renal cell carcinoma: a diagnostic conundrum. Can J Gastroenterol. 1998;12(1):75–8.
Kashani A, Nissen NN, Guindi M, Jamil LH. Metastatic periampullary tumor from hepatocellular carcinoma presenting as gastrointestinal bleeding. Case Rep Gastrointest Med. 2015;2015:732140.
Piccinino F, Sagnelli E, Pasquale G, et al. Complications following percutaneous liver biopsy. A multicentre retrospective study on 68,267 biopsies. J Hepatol. 1986;2:165–73.
Fidelman N, Bloom AI, Kerlan RK Jr, et al. Hepatic arterial injuries after percutaneous biliary interventions in the era of laparoscopic surgery and liver transplantation: experience with 930 patients. Radiology. 2008;247:880–6.
Park JY, Ryu H, Bang S, et al. Hepatic artery pseudoaneurysm associated with plastic biliary stent. Yonsei Med J. 2007;48:546–8.
Ribeiro A, Williams H, May G, et al. Hemobilia due to hepatic artery pseudoaneurysm thirteen months after laparoscopic cholecystectomy. J Clin Gastroenterol. 1998;26:50–3.
Wen F, Dong Y, Lu ZM, et al. Hemobilia after laparoscopic cholecystectomy: imaging features and management of an unusual complication. Surg Laparosc Endosc Percutan Tech. 2016;26:e18–24.
Mori K, Murata S, Yoshioka H, et al. Transcatheter embolization of celiac artery pseudoaneurysm following pancreaticoduodenectomy for pancreatic cancer. A case report. Acta Radiol. 1998;39:690–2.
Londono MC, Balderramo D, Cardenas A. Management of biliary complications after orthotopic liver transplantation: the role of endoscopy. World J Gastroenterol. 2008;14:493–7.
Tsou YK, Liu NJ, Jan YY. Biliary obstruction caused by hemobilia after endoscopic sphincterotomy in a patient with an anomalous location of papilla of Vater. Gastrointest Endosc. 2008;68:1232–4.
Dhiman RK, Behera A, Chawla YK, et al. Portal hypertensive biliopathy. Gut. 2007;56:1001–8.
Costa MT, Maldonado R, Valente A, et al. Hemobilia in hereditary hemorrhagic telangiectasia: an unusual complication of endoscopic retrograde cholangiopancreatography. Endoscopy. 2003;35:531–3.
Rai R, Rose J, Manas D. Potentially fatal haemobilia due to inappropriate use of an expanding biliary stent. World J Gastroenterol. 2003;9:2377–8.
Matthes G, Stengel D, Seifert J, et al. Blunt liver injuries in polytrauma: results from a cohort study with the regular use of whole-body helical computed tomography. World J Surg. 2003;27:1124–30.
Croce MA, Fabian TC, Spiers JP, Kudsk KA. Traumatic hepatic artery pseudoaneurysm with hemobilia. Am J Surg. 1994;168:235–8.
Jones PM, Subbarao G. Hemobilia caused by hepatic artery pseudoaneurysm following abdominal trauma. J Pediatr Gastroenterol Nutr. 2015;60:e40.
Yu YH, Sohn JH, Kim TY, et al. Hepatic artery pseudoaneurysm caused by acute idiopathic pancreatitis. World J Gastroenterol. 2012;18:2291–4.
Jee SL, Lim KF, Krishnan R. A rare case of fulminant hemobilia resulting from gallstone erosion of the right hepatic artery. Med J Malaysia. 2014;69:191–2.
Chin MW, Enns R. Hemobilia. Curr Gastroenterol Rep. 2010;12:121–9.
Kawaguchi Y, Ogawa M, Maruno A, et al. A case of successful placement of a fully covered metallic stent for hemobilia secondary to hepatocellular carcinoma with bile duct invasion. Case Rep Oncol. 2012;5:682–6.
Ahmad SS, Basheer FT, Idris SF, et al. Cholangiocarcinoma presenting as hemobilia and recurrent iron-deficiency anemia: a case report. J Med Case Rep. 2010;4:133.
Coulier B, Maldague P, Ramboux A, et al. Mass-forming intrahepatic cholangiocarcinoma presenting with painful obstructive hemobilia. JBR-BTR. 2014;97:366–9.
Ooishi T, Saeki I, Yamasaki T, et al. Hepatocellular carcinoma-induced hemobilia. Intern Med. 2014;53:1579.
Etienne S, Pessaux P, Tuech JJ, et al. Hemosuccus pancreaticus: a rare cause of gastro-intestinal bleeding. Gastroenterol Clin Biol. 2005;29:237–47.
Green MHA, Duell RM, Johnson CD, Jamieson NV. Haemobilia. Br J Surg. 2001;88(6):773–86.
Goffette PP, Laterre PF. Traumatic injuries: imaging and intervention in post-traumatic complications (delayed intervention). Eur Radiol. 2002;12(5):994–1021.
Cathcart S, Birk JW, Tadros M, Schuster M. Hemobilia an uncommon but notable cause of upper gastrointestinal bleeding. J Clin Gastroenterol. 2017;51:796–804.
Berry R, Han J, Girotra M, Tabibian JH. Hemobilia: perspective and role of the advanced endoscopist. Gastroenterology research and practice. 2018; vol. 2018, Article ID 3670739, 12 pages.
Konerman MA, Zhang Z, Piraka C. Endoscopic ultrasound as a diagnostic tool in a case of obscure hemobilia. ACG Case Rep J. 2016;3:e170.
Cattan P, Cuillerier E, Cellier C, et al. Hemobilia caused by a pseudoaneurysm of the hepatic artery diagnosed by EUS. Gastrointest Endosc. 1999;49:252–5.
Foong KS, Lee A, Kudakachira S, Ramberan H. Hemobilia from biliary angiodysplasia diagnosed with cholangioscopy. ACG Case Rep J. 2016;3(4):e132.
Marynissen T, Maleux G, Heye S, et al. Transcatheter arterial embolization for iatrogenic hemobilia is a safe and effective procedure: case series and review of the literature. Eur J Gastroenterol Hepatol. 2012;24:905–9.
Saad WEA, Davies MG, Darcy MD. Management of bleeding after percutaneous transhepatic cholangiography or transhepatic biliary drain placement. Tech Vasc Interv Radiol. 2008;11(1):60–71.
Zaydfudim VM, Angle J, Adams R. Current management of hemobilia. Curr Surg Rep. 2014;2:54.
Srivastava DN, Sharma S, Pal S, et al. Transcatheter arterial embolization in the management of hemobilia. Abdom Imaging. 2006;31:439–48.
Kumar A, Sheikh A, Partyka L, et al. Cystic artery pseudoaneurysm presenting as a complication of laparoscopic cholecystectomy treated with percutaneous thrombin injection. Clin Imaging. 2014;38:522–5.
Robert Y, Dubrulle F, Chambon JP, et al. Iatrogenic aneurysm of the hepatic artery. Treatment by percutaneous injection of thrombin and selective arterial embolization. Ann Chir. 1994;48:210–3.
Ghassemi A, Javit D, Dillon EH. Thrombin injection of a pancreaticoduodenal artery pseudoaneurysm after failed attempts at transcatheter embolization. J Vasc Surg. 2006;43:618–22.
Bagla P, Erim T, Berzin TM, et al. Massive hemobilia during endoscopic retrograde cholangiopancreatography in a patient with cholangiocarcinoma: a case report. Endoscopy. 2012;44(suppl 2 UCTN):E1.
Leung JW, Chan FK, Sung JJ, et al. Endoscopic sphincterotomy-induced hemorrhage: a study of risk factorsand the role of epinephrine injection. Gastrointest Endosc. 1995;42:550–4.
Katsinelos P, Paroutoglou G, Beltsis A, et al. Endoscopic hemoclip placement for postsphincterotomy bleeding refractory to injection therapy: report of two cases. Surg Laparosc Endosc Percutan Tech. 2005;15:238–40.
Itoi T, Yasuda I, Doi S, et al. Endoscopic hemostasis using covered metallic stent placement for uncontrolled postendoscopic sphincterotomy bleeding. Endoscopy. 2011;43:369–72.
Valats JC, Funakoshi N, Bauret P, et al. Covered selfexpandable biliary stents for the treatment of bleeding after ERCP. Gastrointest Endosc. 2013;78:183–7.
Aslinia F, Hawkins L, Darwin P, et al. Temporary placement of a fully covered metal stent to tamponade bleeding from endoscopic papillary balloon dilation. Gastrointest Endosc. 2012;76:911–3.
Song JY, Moon JH, Choi HJ, et al. Massive hemobilia following transpapillary bile duct biopsy treated by using a covered self-expandable metal stent. Endoscopy. 2014;46(suppl 1 UCTN):E161–2.
Barresi L, Tarantino I, Ligresti D, et al. Fully covered selfexpandable metal stent treatment of spurting bleeding into the biliary tract after endoscopic ultrasound-guided fine-needle aspiration of a solid lesion of the pancreatic head. Endoscopy. 2015;47(suppl 1 UCTN):E87–8.
Shinjo K, Matsubayashi H, Matsui T, et al. Biliary hemostasis using an endoscopic plastic stent placement for uncontrolled hemobilia caused by transpapillary forceps biopsy (with video). Clin J Gastroenterol. 2016;9:86–8.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Alexandre, C., Ells, P., Lee, H. (2021). Biliary- Pancreatic System. In: Tadros, M., Wu, G.Y. (eds) Management of Occult GI Bleeding. Clinical Gastroenterology. Humana, Cham. https://doi.org/10.1007/978-3-030-71468-0_9
Download citation
DOI: https://doi.org/10.1007/978-3-030-71468-0_9
Published:
Publisher Name: Humana, Cham
Print ISBN: 978-3-030-71467-3
Online ISBN: 978-3-030-71468-0
eBook Packages: MedicineMedicine (R0)