Abstract
In the last years, new systemic and locoregional therapeutic schemes have been developed for the management of liver secondaries due to neuroendocrine character tumors. Among the novel therapeutic procedures, intra-arterial infusions of high doses of 111In-Octreotide were leading to encouraging results covering the whole spectrum of liver metastases both in early and in advanced cases. The transhepatic radiopeptide infusions to confront liver metastases could be in fact an increasingly used form of “brachytherapy” which consists of the intra-arterially injection of octreotide tagged with 111In, a gamma, Auger, and internal conversion electron emitter with a 2.8-day half-life and an average 0.02–550μm tissue penetration, acting as a source of endovascular radiation purposes.
In this chapter, it is aimed to evaluate the efficiency of the outcome and toxicity of peptide-receptor-radionuclide therapy (PRRT) in patients with liver-metastasized neuroendocrine tumor positive for somatostatin receptors due to surgically excised colorectal carcinoma, using 111In-Octreotide, in high doses, discussing and analyzing the recent ergography developments on the field.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
15.1 Introduction
The rate of detection of colorectal neuroendocrine neoplasms (NENs) is increasing, due to the widespread use of colonoscopy, including an established screening tool, and the followed biopsy of the removed lesions [1]. Colorectal cancer is a hindgut tumor which according to the statement of the authors of the recent ENETS consensus has to be treated not individually but as two different malignancies, i.e., the NEN of the rectum and the NEN of the colon [2].
Rectal NENs are usually small in size lesions (most <1 cm), and their histological malignancy is low to moderate (G1, G2), whereas NENs of the colon are often of larger dimension, aggressive, poorly differentiated, and more malignant (G3). Colonic NENs account for 7.8% and rectal NENs for 13.7% of all neuroendocrine neoplasms [3]. The most common site for colonic tumors is the caecum, and this location is more frequent in females [3]. The mean age at disease onset is 70 years [4, 5]. Rectal tumors are the third largest group of gastrointestinal NENs, accounting 1% of all rectal tumors. According to Japanese and Korean data, rectal NENs are more common in the male population [6] with highest incidence in Asian and African patients [6, 7]. The mean age of patients with rectal NENs is 56 years. Statistically, rectal NENs record 4.2 cases per 1,000,000 citizens [6, 7]. Rectal NENs are usually single lesions. If neuroendocrine lesions are found in the rectum [8], complete colonoscopy is recommended.
According to the WHO classification of 2010, rectal NETs can be separated into three categories based on both Ki-67 index and mitotic count, as follows: low grade (G1) = <2 mitoses/10 high-power fields (HPFs) and ≤2% Ki-67 index; intermediate grade (G2) = 2–20 mitoses/10 HPFs or 3–20% Ki-67 index; or high grade (G3) = >20 mitoses/10 HPFs or >20% Ki-67 index [8, 9] (Table 15.1).
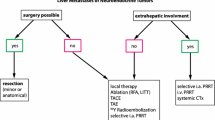
Surgery consists the only curative option for rectal NETs, but as in the bronchopulmonary NENs, there is a lack of precision-consensus between for both colonic and rectal NET management and guidelines regarding optimal treatment approaches in the unresectable/metastatic setting on account of the limited availability of high-level clinical evidence. As a result, a multidisciplinary approach for the management of colorectal NETs is required to ensure a consistent and optimal level of care (Table 15.2) [10]. Lesions ≤10 mm without invasion of the muscularis propria and without depression or ulceration seen macroscopically could be locally resected safely [11]. Rectal NETs between 10 and 20 mm have sparked more controversy and can be managed with endoscopy or surgery depending on the stage. Alternatively, larger lesions >20 mm should be managed like rectal adenocarcinoma with low anterior resection or, in rare cases, abdominoperineal resection [8].
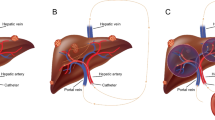
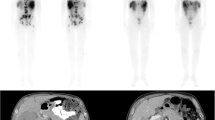
We report on the effectiveness of PRRT in 11 colorectal, liver metastasized patients [3 males, 8 females (Table 15.3)] discussing and analyzing the recent ergography developments. Indication for PRRT was decided in an interdisciplinary tumor board. Planar and SPECT scans were performed for all therapy cycles to calculate tumor and critical organ doses, followed by quantitative dosimetry. Accordingly, absorbed doses delivered to liver metastases, kidneys, and red marrow were calculated according to OLINDA 1.1 program, and response assessment was classified, based on RECIST 1.1 criteria. Response to salvage PRRT was assessed by CT/MRI scans performed before, during, and after the end of treatment and monthly ultrasound images for liver follow-up measurements. Toxicity (WHO criteria) was measured using blood and urine tests of renal, hepatic, and bone marrow function. Progression-free survival (PFS) analysis was performed with the Kaplan-Meier survival plot. High doses [4070–5920 MBq (110–160 mCi)] of 111In-Octreotide were intra-arterially infused, after catheterization of the hepatic artery of these 11 patients with surgically removed ascendant colonic (2 cases), descendant colonic (4 cases), sigmoid (3 cases), cecum (1 case), and rectal (1 case) NENs, associated with liver metastases, positive for somatostatin receptors [verified by OctreoScan® (Figs. 15.1 and 15.2) and confirmed by biopsy].
15.2 111In-Octreotide Treatment Results
Liver metastatic load: None of the 11 treated patients resulted in complete response, and partial response was assessed in 8 cases. Table 15.3: One out of the aforementioned 11 cases resulted in disease stabilization, whereas 2 did not respond at all and died within about 1 year after the initialization of the therapeutic scheme (patients 2 and 7) due to disease aggravation (Fig. 15.3). The 36-month PFS ratio was 6/11 (54.54%), and the ratio for OS was 8/11 (72.72%).
In both U/S and CT, liver-metastasized tumor lesions showed a mean target diameter shrinkage ranging from 33 to 45%. A Grade II to III erythro-, leuko-, and thrombocytopenia occurred in all PD cases. Dosimetric calculations (Table 15.4) were found as follows: (a) liver tumor 15.2 mGy/MBq, (b) liver 0.14 mGy/MBq, (c) kidneys 0.41 mGy/MBq, (d) spleen 1.4 mGy/MBq, and (e) bone marrow 0.0035 mGy/MBq. Remarkably, a threefold tumor-/liver-absorbed dose difference is observed between intra-arterial (i.a.) and intravenous (i. v.) one.
15.3 Discussion
In the limited cohort of the present study of 11 patients suffering from liver-metastasized colorectal NENs treated with 111In-Octreotide with surgically removed primaries, an objective response was observed in 8 (72.72%) patients with a median PFS of 36 months. This single-center analysis provides efficacy results including a clearly limited progression-free survival and overall survival as well. Despite the small patient number (n = 11), these findings can act as a first class forwarding the feature of the persistent antiproliferative activity of 111In-Octrerotide Auger and internal conversion electron emission in this specific NET entity at an advanced stage.
Few data are available internationally concerning the effectiveness of targeted therapy with radioisotope-labeled somatostatin analogues in patients with colorectal NENs (Table 15.5), and according to Kwekkeboom et al., the observed survival following PRRT is shorter than in midgut tumors [12]. The relevant international references report on 90Y- and 177Lu-labeled peptides but not with 111In.
In a retrospective study of Lung et al., in 2017, 6 out of 26 patients with metastatic G3 advanced NETs, originated from the rectum, were treated with 177Lu-DOTATE [13]. The authors reported an OS of 18 months and a median time to death (TNTD) of 12 months with a poor prognosis and limited treatment options (usually based on chemotherapy). In a recent prospective study of Garske-Román et al. on 11 rectal NETs, median PFS and OS were 34 (17–35) and 50 months, respectively, in 8 out of 11 patients, in whom the absorbed dose to the kidneys reached 23 Gy; in the rest three, in whom it did not, PFS and OS were 12 (3–12) and 12 (11–33) months, accordingly [14]. In another similar retrospective review study of Kong et al. on 27 patients with rectal NETs, treated with 177Lu-DOTATATE, 10 patients died with a median OS of 81 months and a median PFS of 29 months. The authors reported a PR in 19 (70%) patients and a SD in 7 (26%) [15].
15.4 Conclusion
In unresectable metastatic liver lesions positive for somatostatin receptors, colorectal originated, repeated, intra-arterially infused, high doses of 111In-Octreotide resulted in an 11 NET patient cohort an overall response rate of 72.72%. The Auger and internal conversion electron emission of 111In-Octreotide seems to be a promising therapeutic tool to confront liver-metastasized hindgut tumors, especially in those secondaries not exceeding the 20 mm in diameter, having the same successful outcome observed in GEP-NET cases.
References
Bogacka B, Marlicz W, Białek A, et al. Trends in colorectal neuroendocrine tumors: a 10 years review. Gut. 2009;58:A296.
Ramage JK, De Herder WW, Delle Fave G, et al. Vienna Consensus Conference participants. ENETS consensus guidelines update for colorectal neuroendocrine neoplasms. Neuroendocrinology. 2016;103(2):139–43.
Modlin IM, Lye KD, Kidd M. A 5-decade analysis of 13,715 carcinoid tumors. Cancer. 2003;97:934–59.
Modlin I, Kidd M, Latich I, et al. Current status of gastrointestinal carcinoids. Gastroenterology. 2005;128:1717–51.
Rydzewska G, Cichocki A, Ćwikła JB, et al. Gastroduodenal neuro-endocrine neoplasms including gastrinoma—management guidelines (recommended by the Polish Network of Neuroendocrine Tumours). Endokrynol Pol. 2013;64(6):444–58.
Maggard MA, O’Connell JB, Ko CY. Updated population-based review of carcinoid tumours. Ann Surg. 2004;240:117–22.
Matsui K, Iwase T, Kitagawa M. Small, polypoid-appearing carcinoid tumors of the rectum: clinicopathologic study of 16 cases and effectiveness of endoscopic treatment. Am J Gastroenterol. 1993;88:1949–53.
Caplin M, Sundin A, Nillson O, et al. ENETS Consensus guidelines for the management of patients with digestive neuroendocrine neoplasms: colorectal neuroendocrine neoplasms. Neuroendocrinology. 2012;95:88–97.
Anthony LB, Strosberg JR, Klimstra DS, et al. The NANETS consensus guidelines for the diagnosis and management of gastrointestinal neuroendocrine tumors (nets): well-differentiated nets of the distal colon and rectum. Pancreas. 2010;39:767–74.
Limouris GS, Chatziioannou A, Kontogeorgakos D, et al. Selective hepatic arterial infusion of In-111-DTPA-Phe1-octreotide in neuro-endocrine liver metastases. Eur J Nucl Med Mol Imaging. 2008;35:1827–37.
Kobayashi K, Katsumata T, Yoshizawa S, et al. Indications of endoscopic polypectomy for rectal carcinoid tumors and clinical usefulness of endoscopic ultrasonography. Dis Colon Rectum. 2005;48:285–91.
Kwekkeboom DJ, de Herder WW, van Eijck CHJ, et al. Peptide receptor radionuclide therapy in patients with gastroenteropancreatic neuroendocrine tumors. Semin Nucl Med. 2010;40(2):78–88.
Lung MS, Hofman M, Kong G. Outcomes of peptide receptor radionuclide therapy (PRRT) in metastatic grade 3 neuroendocrine tumors (NETs). J Clin Oncol. 2017;2017:35. (15 suppl. Abstract e15694).
Garske-Román U, Sandström M, Fröss-Baron K, et al. Prospective observational study of 177Lu-DOTA-octreotate therapy in 200 patients with advanced metastasized neuroendocrine tumours (NETs): feasibility and impact of a dosimetry-guided study protocol on outcome and toxicity. Eur J Nucl Med Mol Imaging. 2018;45:970–88.
Kong G, Grozinsky-Glasberg S, Hofman MS, et al. Highly favorable outcomes with peptide receptor radionuclide therapy (PRRT) for metastatic rectal neuroendocrine neoplasia (NEN). Eur J Nucl Med Mol Imaging. 2019;46(3):718–27.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Limouris, G.S., Zafeirakis, A.G. (2021). 111In-Octreotide Infusions for the Treatment of Colorectal Carcinoma. In: Limouris, G.S. (eds) Liver Intra-arterial PRRT with 111In-Octreotide. Springer, Cham. https://doi.org/10.1007/978-3-030-70773-6_15
Download citation
DOI: https://doi.org/10.1007/978-3-030-70773-6_15
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-70772-9
Online ISBN: 978-3-030-70773-6
eBook Packages: MedicineMedicine (R0)