Abstract
The diversity of glycan presentation in a cell, tissue and organism is enormous, which reflects the huge amount of important biological information encoded by the glycome which has not been fully understood. A compelling body of evidence has been highlighting the fundamental role of glycans in immunity, such as in development, and in major inflammatory processes such as inflammatory bowel disease, systemic lupus erythematosus and other autoimmune disorders. Glycans play an instrumental role in the immune response, integrating the canonical circuits that regulate innate and adaptive immune responses. The relevance of glycosylation in immunity is demonstrated by the role of glycans as important danger-associated molecular patterns and pathogen-associated molecular patterns associated with the discrimination between self and non-self; also as important regulators of the threshold of T cell activation, modulating receptors signalling and the activity of both T and other immune cells. In addition, glycans are important determinants that regulate the dynamic crosstalk between the microbiome and immune response. In this chapter, the essential role of glycans in the immunopathogenesis of inflammatory disorders will be presented and its potential clinical applications (diagnosis, prognosis and therapeutics) will be highlighted.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Role of Glycans in Adaptive Immune Development
Protein glycosylation has the potential to take part in the majority of cellular processes and to regulate cell-fate decisions, activity, function, for instance. Adaptive immune cells play a central role in the orchestration of an inflammatory response, as well as in its resolution. One of the factors contributing to inflammation is the recognition of non-self or altered biological material. T and B cells acquire the potential to recognize such signals during their early stages of development and glycans have been shown to play an essential role in these processes. The next section discusses the work on the influence of glycans in developmental checkpoints.
1.1 Glycans in Early T Cell Development
T cell development is one of the major physiological processes that occur in complex organisms, which ensures the formation of a proper repertoire of T cell receptors (TCRs), fundamental in immune responses (Koch and Radtke 2011). T lymphocytes develop in the thymus from a multistep program characterized by the sequential rearrangement of the Tcrb and Tcra loci in combination with lineage and control steps (Takaba and Takayanagi 2017). As the several events of development are dependent on both cell-intrinsic and -extrinsic factors, the study of glycans constitutes a major developmental feature of knowledge (Marth and Grewal 2008; Pereira et al. 2018a, b).
The initial step of T cell development occurs in the bone marrow, where lymphoid progenitors exit to the bloodstream, then to enter into the corticomedullar tissue of the thymus, in which they start to expand and develop. The trafficking of thymus seeding progenitors (TSPs) requires the expression of P-selectin glycoprotein ligand-1 (PSGL-1) by those progenitor cells and its partner, P-selectin, by the thymic epithelium (Rossi et al. 2005). The glycosylation profile of PSGL-1, namely its α1,3 fucosylation, was shown to be required for its binding to P-selectin, and its absence, by the genetic deletion of the Fut4 and Fut7 fucosyltransferases, led to the impairment of TSPs homing into the thymus (Sultana et al. 2012). In the sialyltransferase St8Sia-IV−/− mouse model, a deficient thymic seeding was also observed, caused by inefficient progenitor bone marrow egression (Drake et al. 2009). Once TSPs enter the thymus, they develop into early thymocyte progenitors (ETPs), a subset of the CD4−CD8−double negative 1 (DN1) population, which can give rise to multiple lymphoid lineages (Takaba and Takayanagi 2017). The major determinant responsible for the commitment of DN1 thymocytes to the T cell lineage is the presence of Notch signalling (Shah and Zúñiga-Pflücker 2014). The glycoprofile of Notch receptors (and ligands) was shown to regulate Notch-dependent intracellular signal transduction. These proteins are modified by the lunatic, manic and radical Fringe glycosyltransferases, which transfer N-acetylglucosamine (GlcNAc) to O-linked fucose glycans, in the repeats of the consensus epidermal growth factor-like (EGF-like) sequences present in the extracellular domain of Notch (Rampal et al. 2005). The presence of these glycans regulates the trafficking of the Notch receptors (Takeuchi et al. 2017) and its differential affinity for Notch-ligands (Rampal et al. 2005). It was shown that the mouse model of triple fringe deficiency had reduced the binding of Notch to Delta-like ligands (DLL), namely DLL4, altering the frequencies of several T cell subsets in the thymus (Song et al. 2016). The decisive commitment to the T cell lineage occurs at the DN3 stage, where a recombination-activating genes (RAG)-mediated productive rearrangement of the Tcrb leads to the expression of the ß chain of the TCR (TCRß) and the formation of a pre-TCR signalling complex (Takaba and Takayanagi 2017). Together with Notch and IL-7, the pre-TCR signalling initiates ß-selection, by inducing the transition to DN4 cells. These cells then begin a round of multiple divisions, giving rise to the most represented thymocyte population, the CD4+CD8+double-positive (DP) cells.
The major function of the thymus as an organ is to generate an environment where randomly generated TCRs are probed for their reactivity and selected according to their self-reactive potential (Miller 2020). These biological processes occur in the DP stage, after the Tcra locus suffers rearrangements by the RAG complex, leading to the expression of a mature TCR (Shih et al. 2011). The new mature TCRs are then screened by thymic epithelial cells (TECs) by the specificity and binding strength for the presented MHC ligands. The initial process is named positive selection, where the DP population is enriched for cells that express an immunocompetent TCR. Afterwards, cells that display high levels of activation, which indicates self-reactive potential, are targeted for apoptosis, a process called negative selection. Finally, cells that go through both selections develop into CD4+CD8− or CD4−CD8+single positive (SP) cells. One of the major contributions of glycans is represented by the ones of the CD4 and CD8 co-receptors. It was shown that chemical desialylation of CD8 mature cells increases CD8/MHC-I interactions (Daniels et al. 2001), and in fact, ST3Gal1−/− mice show a significantly altered TCR repertoire, indicating a role for sialylation in thymocyte selection (Moody et al. 2001). Furthermore, it was demonstrated that branching N-glycosylation expands the range of TCR signalling of positive selection by differentially controlling both the lower and upper limits of positively selected TCR–MHC–antigen interactions. This was pointed out to be due to decreased surface expression of CD4 and CD8 receptors, in Mgat1 and Mgat2 DP-conditional knockout models (Zhou et al. 2014).
Surface glycans also modulate galectin (gal-) binding, which in turn influence cellular functions (Rabinovich and Toscano 2009). Histological analysis of thymus from mice revealed differential galectin spatial distribution, suggesting functions in distinct developmental stages (Nio-Kobayashi 2018). Perillo et al. showed that gal-1 was able to induce apoptosis of human thymocytes in vitro, with high effect in combination with anti-CD3 stimulation, suggesting a role in negative selection (Perillo et al. 1997). Later on, Galvan et al. demonstrated that the downregulation of galectin-binding glycans occurs in positively selected DP thymocytes, which are resistant to gal-1-induced apoptosis (Galvan et al. 2000). Gal-3 was shown to regulate thymocyte-epithelial cell interaction, influencing developmental transitions. In fact, Lgals3−/− mice show decreased absolute numbers of thymocytes, with lower levels of proliferation (Oliveira-de-Abreu et al. 2018).
1.2 Glycans in Early B Cell Development
B cell lymphocytes are key players in the adaptive immune response, being essential regulators of immunity through the secretion of antibodies, soluble proteins with antigen specificity. B cell development is a highly regulated process that takes place in the bone marrow and the spleen (Hardy and Hayakawa 2001). Much like T cells, B cells undergo somatic gene rearrangement in the immunoglobulin loci, and are selected to ensure a self-tolerant repertoire of antibodies. Its development is crucial to ensure proper immune function throughout the life of the organism.
B cells are generated from common lymphocyte progenitors (CLPs) that remained in the bone marrow. As in DN1 thymocytes T cell lineage commitment, the CLP commitment to the B cell lineage is influenced by Notch signalling. CLPs require absent Notch signalling to develop into progenitor B cells (pro-B). Unlike the role of this pathway in T cell development (promoting effect), Notch has to be absent for B cell development to occur (Stanley and Guidos 2009). Glycosylation profiles of the Notch receptor and ligands regulate affinity and surface expression. Interestingly, conditional knockout of Lfng in thymocytes led to B cell development in the thymus (Koch et al. 2001).
When pro-B cells successfully rearrange the Igh locus, mediated by the RAG complex, they develop into precursor B cells (pre-B). The cells in this developmental stage express a pre-BCR complex, with no antigen specificity, which triggers a signalling cascade, driving proliferation. The pre-BCR receptor is composed by the rearranged immunoglobulin heavy chain that assembles with a surrogate chain, to ensure surface expression (Burrows et al. 2002). It was seen that the N-glycans of the rearranged immunoglobulin heavy chain influence this assembly and are specifically required for pre-BCR function (Übelhart et al. 2010). Moreover, core fucosylation was also demonstrated to play a role in pre-BCR formation (Li et al. 2012). Stromal gal-1 was shown to be a ligand of the pre-BCR, triggering its signalling pathway and enabling further B cell development (Gauthier et al. 2002). In fact, B cells in this stage are found in the stromal niches where gal-1 is enriched (Mourcin et al. 2011) and their development is compromised in the absence of gal-1(Espeli et al. 2009). The signalling activation of the pre-BCR cascade leads to the rearrangement of the immunoglobulin light chain, which results in the expression of a mature BCR, and development into the immature B cell stage.
Immature B cells are then screened for their autoreactive potential in the bone marrow. Cells reacting with low or high affinity suffer receptor editing, with a secondary rearrangement of their immunoglobulin light chain allele (Schatz and Ji 2011). Afterwards, BCR with affinity for self-peptides are negatively selected, and cells that express a tolerant BCR are positively selected, by which central B-cell tolerance is achieved (Nemazee 2017). Recently, it was shown that branched N-glycans are required for B cell selection (Mortales et al. 2020). By the conditional knockout of Mgat1 in the B cell lineage, it was observed that branched N-glycan deficiency decreased the surface expression of the BCR co-receptor CD19, which inhibited positive selection. Moreover, the nerve growth factor IB (Nur77) was shown to be upregulated in immature B cells in the absence of branched N-glycans, indicating a role of threshold establishment, similarly to T cells (Mortales et al. 2020).
B cells that go through central selection migrate to the spleen and commit to either the marginal zone (MZ) or follicular cell fates, according to the strength of their BCR signal (Pillai and Cariappa 2009). Interestingly, mice deficient for CD22, a B cell siglec that binds α2,6-sialic acids, which inhibits BCR signalling, show decreased cellularity on the MZ B cell compartment (Samardzic et al. 2002). B cell homing was shown to be compromised in a Cosmc−/− genetic background, demonstrating the role of elongated O-glycans in this process (Zeng et al. 2020).
When resting B cells encounter an antigen, one of the major features of the humoral response takes place: the antibody diversification and maturation. This process occurs in germinal centres (GC) where the selection of B cells according to their antigen affinity. Cell clones which display high antigenic affinity, proliferate and differentiate into antibody-secreting plasma cells and memory B cells (Mesin et al. 2016). Galectins have been implicated in the regulation of BCR signalling, influencing therefore B cell germinal centre selection. Interestingly, loss of gal-3 resulted in increased B cell activation and spontaneous GC formation (Beccaria et al. 2018). It was also shown that both gal-1 and gal-8 promote plasma cell differentiation (Tsai et al. 2011). In fact, it was demonstrated that binding of gal-8 endorses antigen recognition by B cells, promoting the formation of the immunological synapse (Obino et al. 2018).
2 Role of Glycans in the Inflammatory Process
2.1 Glycans in Innate Immune Responses
Innate immune cells are the first line of defence against pathogens and play a major role in the inflammatory response. This immune cellular group comprises mast cells, phagocytes (dendritic cells (DCs), macrophages and neutrophils), NK cells and innate lymphoid cells (ILC). Their glycosylation profile should be taken into consideration to understand their functions in an inflammatory environment or during an immune response. Trafficking and recruitment of innate cells to the sites of tissue injury are controlled through glycosylation-mediated recognition between leukocytes and endothelial cells. Moreover, the migration of these cells to the inflammation sites allows the accumulation of specific cytokines and chemokine cocktails which are also responsible for altering the glycosylation of surrounding cells. Moreover, this interplay between innate immune cells and the inflammatory environment is also mediated by specific glycan-recognizing receptors. It is clear that protein glycosylation plays a fundamental role in each major step of the inflammatory process—initiation, propagation and abrogation of inflammation—and the next section details the contribution of glycans in each one.
2.1.1 Glycans in Immune Cell Trafficking and Recruitment
Upon infection or tissue damage, endothelial cells exhibit cell surface changes in order to promote the migration and extravasation of immune cells to the site of injury. Roll, arrest and adherence steps are controlled through the interaction between endothelial selectins (E-Selectin and P-Selectin) and ligands present in leukocytes (Zarbock et al. 2011). Selectins are C-type lectins (calcium-dependent glycan-binding proteins) characterized by their ability to recognize and bind specific carbohydrates structures, such as Sialyl Lewis X (sLeX) (Schnaar 2016). Changes in cellular glycosylation affect selectin-binding, such as PSGL-1 (P-Selectin) or ESL-1 (E-Selectin), modulating the process of recruitment and homing of immune cells (Sperandio 2006). In fact, it was described that mice lacking ST6Gal1 gene, which codes the sialyltransferase responsible for the addition of terminal α2,6-sialic acids, showed an impaired migration of immune cells toward draining lymph nodes (Zarbock et al. 2011). The hyaluronic acid receptor CD44 is also an important leukocyte ligand for endothelial E-Selection. CD44-E-Selectin interaction is highly dependent on the surface sialylation and fucosylation of its N-glycans (Kansas 1996). Thus, the activity of glycosyltransferases during this process of recruitment is vital to mount a correct in situ inflammatory response.
2.1.2 Glycans as Recognition Moieties (PAMPs and DAMPs)
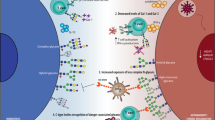
After cellular migration to the injury site, the recognition of pathogens and/or injured host cells is an important step in the inflammatory process. Innate immune cells express a variety of cell surface receptors with the specific capacity of recognize ‘danger’ structures, known as pathogen-associated molecular pattern (PAMP) or damage-associated molecular pattern (DAMP). PAMPs and DAMPs are often glycosylated biomolecules present in the surface of the pathogens or damaged cells, or even released to the extracellular space (Ablasser and Chen 2019). One of the mechanisms by which these molecules are recognized by innate immune cells is through carbohydrate-recognizing receptors, such as C-type lectins (CTLs). Antigen-presenting cells, such as macrophages and DCs, bear a diverse and robust collection of several C-type lectins that enable them to recognize several danger glycosignals (Brown et al. 2018). The Dectin-1 CTL recognizes β-linked glucose polymers, a major constituent of pathogen cell walls, and is able to trigger cellular activation with its intracellular domain, through the NF-κB signalling cascade (Ferwerda et al. 2009). Dectin-2 recognizes mannose residues displayed by pathogens and host cells and is able to interact with FcγRs through its extracellular domain, inducing cellular activation (Hollmig et al. 2009). Interestingly, Dectin-2−/−mice have shown a decreased susceptibility to house dust mite-induced lung inflammation, highlighting the importance of this first mechanism of defence (Parsons et al. 2014). Another well-known CTL, mostly studied in the context of cancer , is dendritic cell-specific ICAM-grabbing non-integrin (DC-SIGN). This CTL is mainly expressed in immature DCs, monocytes and macrophages, and recognizes high-mannose and fucose residues. DC-SIGN assists also in the antigen recognition leading to the maturation of DCs (RodrÍguez et al. 2018;Brown et al. 2018).
2.1.3 Glycans in Innate Immune Cell Function
During an inflammatory process, APCs and monocytes migrate to the tissue where they encounter the inflammatory agent (tumour cell, pathogen, for instance). After recognition, in which specific receptors bind to the surface molecules of the agent, DCs suffer a shift on its phenotype, characterized by increased expression of MHC-II, costimulatory molecules (CD80, CD86) upregulation, as well as increased cytokine and chemokine secretion. This protein shift is accompanied with changes in surface glycosylation that distinguish mature (mDCs) and immature (iDCs) DCs. Maturation of DCs is associated with an increase of elongated poly-N-acetyllactosamine chains with terminal α2,3-sialic acid and fucose (Bax et al. 2007). These glycan expression alterations represent an important mechanism that regulates DC functions, such as antigen presentation to T and B cells in the lymph nodes. Higher abundance of terminal sialic acid was shown to enable the trans binding of sialoadhesins (CD22), expressed by B cells, supporting a potential DC-B cell interaction during antigen presentation (Varki and Gagneux 2012). Moreover, the increased presence of elongated poly-N-acetyllactosamine chains favours galectins binding, which in turn have been shown to regulate cell adhesion, cell activation, chemoattraction, cell growth and apoptosis in DCs (Videira et al. 2008).
Macrophages, characterized by the spectrum between classical M1 and M2 phenotype, also bear glycan-binding receptors, such as mannose receptor (MR) and DC-SIGN. Interestingly, the role of these CTLs in macrophages is still very broad, strongly relying on the context of inflammation. For instance, in the context of germinal centre formation, mannosylated IgM B cell receptor seems to promote the activation of B cell receptor (Amin et al. 2015). On the other hand, in a context of Mycobacterium tuberculosis infection , DC-SIGN-mediated recognition of bacterial glycans seems to induce an anti-inflammatory polarization in macrophages (Lugo-Villarino et al. 2018).
2.2 Role of Glycans in Adaptive Immune Cells Functions
Initially, the adaptive immune response relies on the ability to differentiate self-antigens derived from pathogens/damage host cells. This process is orchestrated by antigen-presenting cells (APCs), which display antigens attached to major histocompatibility complexes (MHCs) that are presented to T cells (den Haan et al. 2014; Lee et al. 2020). The MHC is a family of structurally and genetically related glycoproteins that are able to control immune response through T cell activation (Neefjes et al. 2011). It encompasses two major classes of MHCs: the MHC class I (MHC-I), which can be expressed by all nucleated cells and reacts to intracellular bacteria, viral infections and cellular transformation (Hewitt 2003; Comber and Philip 2014); and the MHC class II (MHC-II), which responds to exogenous proteins (Storni and Bachmann 2004). MHC-II expression is limited to professional APCs, such as DCs, macrophages and B cells (Roche and Furuta 2015).
The glycosylation of proteins and receptors that participate in adaptive immune response, such as MHC molecules and TCRs, is essential for correct protein folding (Trombetta and Helenius 1998) to protect protein backbone from proteolysis (Wang et al. 2001) and to ensure a suitable distance between receptors and other molecules at the cell surface, in order to facilitate interactions (Grigorian et al. 2009). Indeed, it was already described that the blockade of MHC1a N-glycosylation leads to a severe increase in intracellular misfolded protein content and an impairment in cell surface expression and peptide presentation (Barbosa et al. 1987).
MHC-II molecules are constituted by two α and two β chains, each of which contains a transmembrane domain, contrasting with MHC-I molecules, which display only one β chain. The glycan composition of both chains within MHC-II also differs, with the α chain displaying predominantly N-linked high-mannose and complex N-glycans and the β chain being composed by complex N-glycans (Unanue et al. 2016). MHC-I exhibits one single conserved site for N-glycosylation at Asn86, whereas MHC-II has three highly conserved N-glycosylation sites, two on the α chain (Asn78 and Asn118) and one on the β chain (Asn19) (Gauthier et al. 1998; Ryan and Cobb 2012). The N-glycan site on MHC-I is important for antigen binding to occur due to its role in recruiting chaperones that are involved in peptide loading (68). Glycoprotein modifications of MHC molecules are able to mediate immune responses. Ostankovitch and colleagues have described that the presentation of an MHC-I restricted epitope, derived from the membrane protein tyrosinase, requires retrotranslocation of glycosylated molecules from the endoplasmic reticulum to the cytosol. In particular, they have shown that proteasomes degrade tyrosinase molecules that are glycosylated and generate intermediate molecules that are not found in degradation of non-glycosylated molecules. In this context, the authors suggest that the glycosylation of these intermediate molecules influences their processing by the proteasome, stating a relevant role for glycosylation for the presentation of an MHC-I-restricted epitope derived from tyrosinase (Ostankovitch et al. 2009).
Besides presentation of glycan antigens to T cells, the glycosylation of MHC-II is intricately associated with T cell response also by modulating antigen binding. Accordingly, in APCs deficient for Mgat2 glycogene it was demonstrated that the lack of complex N-glycans was detrimental for glycan antigen presentation by MHC-II and led to loss of T-cell activity (Ryan et al. 2011).
As mentioned above, T cell development, growth and differentiation are regulated by N-glycans and O-glycans (see Sect.13.1.1). The role of glycans in these cells has been demonstrated in several studies on different autoimmune disorders and also murine models of inflammation-associated diseases (Mkhikian et al. 2011; Dias et al. 2018a; Verhelst et al. 2020). Foremost, over the past 20 years, we have witnessed how critical are N-glycosylation alterations in regulating adaptive immunity, namely by mediating the T cell function, not only through the regulation of its primordial receptor, the TCR, but also its partner receptors (namely CTLA-4, CD45, CD25, CD28) (Pereira et al. 2018a, b).
An altered glycosylation of the TCR, namely reduced expression of complex branched N-glycans, has been described to lower the threshold for T cell activation, further modulating the surface expression of important growth inhibitory receptors such as cytotoxic T lymphocyte antigen-4 (CTLA-4) (Demetriou et al. 2001; Morgan et al. 2004; Grigorian et al. 2007). In fact, one decade ago it was demonstrated that TCR N-glycosylation is regulated by TCR signalling. More precisely, it upregulates N-acetylglucosaminyltransferase V (GnT-V) and Golgi α-mannosidase enzymes at the mRNA level, in a synchronous manner, to enhance complex branching N-glycans branching in activated T cells, to avoid hyperactivation (Chen et al. 2009a).
Similarly, an increase of branched N-glycans on CD25 has been associated with increased cell surface retention with impact in the regulation of T cell differentiation and immune tolerance. It was demonstrated that by reducing UDP-GlcNAc (substrate for the initiation of branching in N-glycans) intracellular availability or the expression of branching glycosyltransferase , there is a decrease of CD25 surface retention and IL-2signalling, which promotes T helper-17 (Th17) over induced regulatory T cell (iTreg) differentiation (Araujo et al. 2017). Additionally, the function of CD28 co-stimulatory receptor has been shown to be mediated by N-glycosylation which can negatively regulate the interaction between CD28/CD80. Different approaches inhibiting N-glycosylation (in vitro by site mutagenesis on the five N-glycosylation sites in the extracellular domain and by enzymatic inhibitors of biosynthesis of N-linked oligosaccharide structures) resulted in a defective CD28 glycosylation and enhancement of the binding to CD80 expressed on APCs (Ma et al. 2004). Later, other study demonstrates that the high levels of polylactosamine in CD8 are on N-glycans, further suggesting polylactosamine structures on CD28 as critical players in regulating T cell activation (Togayachi et al. 2007). Moreover, in 2011, another study has also shown that IL-2 and IL-7 have distinct effects in resting and activate T cells. Early by lowering branching N-glycans and TCR activation thresholds, these cytokines enhance T cell growth. Later, they promote self-tolerance by enhancing branching and CTLA-4 surface retention (Mkhikian et al. 2011).
Additionally, galectins are at the crossroad of tolerance and inflammation (Modenutti et al. 2019). Different members of the galectin family exhibit a ‘double-edge sword’ effect, acting either as negative or positive mediators of T cell homeostasis. Galectin-1, -2, -3 act as inhibitors of inflammation and T cell activity. One of the major regulations of T cell function by galectins is related to Gal-1’s ability to negatively regulate Th1 and Th17 effector cells by inducing cell death. Interestingly, it was also shown that Th2 cells upregulate the expression of terminal α2,6-sialic acids, which inhibit Gal-1 binding, render a Gal-1-induced-apoptosis resistance by these cells (Toscano et al. 2007). Gal-3 also plays a pivotal role in the regulation of T cell activity, as it can constrict TCR clustering, by the binding to complex branched N-glycans of these receptors, generating lattice formation, controlling the threshold of T cell activation (Demetriou et al. 2001; Chen et al. 2009b). Moreover, Gal-2 also exhibits a suppressive effect by inducing apoptosis of lamina propria T lymphocytes attenuating acute and chronic mouse colitis (Paclik et al. 2008). In contrast, Gal-8 and -4 act as promoters of T cell activation, as it was described that Gal-8 binds to T cells through unique interactions with CD45 and promotes T-cell proliferation (Tribulatti et al. 2009), and that Gal-4 mediates CD4+T cell stimulation, through IL-6 production, leading to exacerbation of T cell-mediated chronic colitis (Hokama et al. 2004).
The dual impact of Gal-9 on inflammation is still controversial since most studies have been conducted only using exogenous Gal-9 and the endogenous counterpart has been overlooked. In fact, exogenous Gal-9-based approaches have suggested it as a immunoregulator of T cell function and a suppressor of immune disease in vivo (Zhu et al. 2005; Madireddi et al. 2014; Wu et al. 2014). However, a very recent study has elucidated the role of endogenous Gal-9, being crucial for Th17 differentiation and T cell proliferation. Moreover, the same work described that high levels of Gal-9 in CD4+ T cells isolated from PBMCs of multiple sclerosis patients are positively correlated with disease severity, highlighting its potential value as biomarker for autoimmune diseases (Chen et al. 2020).
Overall, galectins have a master role in the regulation of inflammation but one of the main questions remains to be elucidated: What are the precise mechanisms involved in the anti-inflammatory and immunoregulatory effects of different members of the galectin family?
3 Glycans in Chronic Inflammatory Diseases
3.1 Role of Glycans in Inflammatory Bowel Disease
Inflammatory bowel disease (IBD ) is a chronic debilitating disorder from the gastrointestinal tract comprising Crohn’s disease (CD) and ulcerative colitis (UC). The etiopathogenesis of IBD is influenced by a complex interaction between environmental and genetics factors, microbiome and/or host immune response (Kaplan and Ng 2017). Its incidence has been increasing worldwide, including in paediatric populations (Ruel et al. 2014; Kaplan and Ng 2017).
Over the past decade, several studies on IBD pathogenesis have unveiled the fundamental role of glycans in the regulation of innate and adaptive immune responses associated with IBD development and progression (as reviewed in detail in (Dias et al. 2018a, b)).
3.1.1 Role of Glycans in the Regulation of Innate Immune Response and Gut Microbiome in IBD
The intestinal epithelial barrier represents the largest interface between the internal organs and the environment . Placed at the cell surface of enterocytes, heavily glycosylated membrane mucins constitute the intestinal glycocalyx, which is the first line of defence against microbial translocation. This dense microbial community exerts a significant impact on intestinal physiology due to their ability to modulate immune development and to inhibit pathogen colonization (Hooper and Gordon 2001). Indeed, it poses an enormous challenge to the immune system, particularly for innate cells, since it needs to properly respond to pathogens without mounting an inflammatory response that may be detrimental to commensal microbes and may trigger the development of spontaneous inflammation.
Although innate immune cells are essential regulators of a healthy intestinal environment, not much is known about how glycosylation alterations dictate innate cell functions. Intestinal macrophages are one of the most represented populations of leukocytes in the intestine, which makes them first-aid players to maintain intestinal homeostasis (Bain and Mowat 2014). As it was discussed above for general inflammatory processes, alterations in glycosylation pathways were described to influence this innate cell population in the context of IBD . Shinzaki et al. have demonstrated, using a transgenic mouse model of GnT-V overexpression, that increased expression of complex branched N-glycans leads to increased colitis severity by inducing macrophage dysfunction and subsequently enhancing colorectal tumorigenesis (Shinzaki et al. 2016). Moreover, intestinal epithelial cell–specific deficiency of core 1–derived O-glycans in mice is associated with development of spontaneous colitis, inducing exacerbated infiltration by TNF-producing myeloid cells in colon mucosa (Fu et al. 2011; Nakayama et al. 2019).
Innate lymphoid cells (ILCs) display a relevant role in the establishment of intestinal homeostasis, since they are highly responsive to microbial stimulation (Vivier et al. 2018). In particular, group 3 ILCs (ILC3) are considerably abundant in mucosal tissues, being particularly involved in epithelial barrier integrity through the production of IL-17, IL-22 and GM-CSF (Neill and Flynn 2018). ILC3 are also able to modulate the intestinal glycocalyx via production of IL-22, which induces the upregulation of fucosyltransferase 2 (FUT2) mRNA on intestinal epithelial cells that in turn lead a protective effect against pathogens through the stabilization of commensal gut microbiota (Goto et al. 2014).
Glycans can act as major sources of energy for the microbiota (Koropatkin et al. 2012). Besides being able to utilize glycans derived from the diet, several bacteria can degrade O-linked glycans present in the epithelial mucus layer or N-linked glycans shed by epithelial cells to use them as nutrient source (Tailford et al. 2015; Ravcheev and Thiele 2017). Specific deletion of core 1–derived O-glycans on gut epithelial cells, using an IEC conditional mouse model lacking C1galt1 specifically in intestinal epithelial cells, was shown to induce spontaneous colitis in mice (Fu et al. 2011). To clearly demonstrate that the loss of intestinal epithelial core 1–derived O-glycans is at the basis of colitis development in adult mice, the authors crossed C1galt1f/f mice with VillinCre-ERT2 transgenic mice, creating an inducible model of deficiency of intestinal epithelial O-glycans that developed colitis 5 days after induction with tamoxifen (Shinzaki et al. 2016). Indeed, alterations in the O-glycosylation profile of mucin-2 are described in UC patients with active disease, with an increment of smaller glycans and a decrease of complex glycans, and it associates with increased intestinal inflammation (Larsson et al. 2011), stating the crucial role of glycosylation alterations in intestinal epithelial barrier function.
3.1.2 Role of Glycans in the Regulation of Adaptive Immune Response in IBD
The disruption of gut mucosal barrier leads to a cascade of events starting with innate immune response, which initiates and drives a subsequent adaptive immune response within the colon lamina propria. This second line of defence involves mainly the activation of Th1, Th2, Th17 cells and suppression of the activity of Treg cells (Iwasaki and Medzhitov 2010). Nevertheless, in inflamed intestinal mucosa (in CD but not in UC or healthy controls), there is a unique subset of FoxP3+ T cells that produce IL17 (the so-called Treg/Th17 axis) (Hovhannisyan et al. 2011). The origin of this distinct cell population is not fully understood, but it is postulated to be shaped by gut microbiome, despite the precise mechanism(s) underlying it remains unclear (Omenetti and Pizarro 2015).
Several studies have been shown that the T cell differentiation can be influenced by N-glycosylation alterations, both in mouse and human cells (Araujo et al. 2017; Dias et al. 2018a, b). Interestingly, in UC patients with active disease and also murine models of IBD (DSS-induced colitis), characterized by highly activated Th1 and Th17 immune response, it was demonstrated that T lymphocytes of lamina propria are deficient in complex branched N- glycans (codified by MGAT5) on the TCR (Dias et al. 2014). However, when this deficient mechanism is repaired by in vitro glycan supplementation of patient-derived colonic T cells, both Th1 and Th17 responses are diminished through reduction of respective pro-inflammatory cytokines production (TNF-α, IFN-γ and IL17A) and transcription factors at mRNA level (T-bet and RORγT). Moreover, in vivo studies showed that disease severity and progression were attenuated upon the restore of TCR branched N-glycosylation (Dias et al. 2018a, b). Similar impact was observed in the absence of core fucose N-glycans (codified by FUT8) which are highly expressed in T cells from patients with active IBD. Regarding the impact of glycans in Treg population, there are no significant alterations in IBD models (Dias et al. 2018a, b). Accordingly, in mouse and human cells, it was demonstrated that Treg suppressive function correlates with glycan expression levels, as Tregs with high expression of tri/tetra-antennary complex N-glycans present an enhanced ability to suppress CD4+ and CD8+ T cell proliferation (Cabral et al. 2017). However, these findings still impose more comprehensive studies about the glycophenotype of Treg subset, using realistic in vivo disease models and in clinical settings.
More recently, in two independent European cohorts of UC patients, specific genetic variants of MGAT5 were shown to have a functional impact in the modulation of T cell glycosylation and plasma IgG glycome, being associated with worse disease course (Pereira et al. 2020). Briefly, UC patients display MGAT5 single-nucleotide polymorphisms (SNPs) that are functionally associated with low transcription levels of MGAT5 glycogene in colonic and circulating T cells and with agalactosylation of IgGs (a pro-inflammatory glycophenotype of IgG, observed in other autoimmune disorders (Ercan et al. 2010; Vučković et al. 2015; Momozawa et al. 2018)). Importantly, despite these evidences suggesting MGAT5 gene as a common driver that simultaneously regulates the function and activity of both humoral and adaptive components involved in IBD development, it is not clarified yet whether the alterations on IgG glycoprofile are or not a T-cell-dependent mechanism.
Overall, glycans are master regulators of intestinal homeostasis and inflammation as they play a role on driving a fine-tuned dynamic between intestinal epithelial barrier function, the immune system and the gut microbiome. Nevertheless, further studies focused on the glycobiome are needed to fully understand the regulatory mechanisms associated with IBD .
3.2 Role of Glycans in Glomerulonephritis
Glomerulonephritis is a general inflammation of a specific portion of the kidney—glomeruli. Glomeruli represent an essential functional unit of the kidney, composed by podocytes organized together to build a filtration barrier. This barrier is highly regulated by the ability of podocytes of contracting and stretching, therefore controlling the passage of water or proteins/compounds (Petrosyan et al. 2019).
Inflammation of glomeruli can occur as an acute event which can be resolved within days, or be prolonged through months/years, precluding in a chronic inflammation with severe damage to the kidney function. The etiopathogenesis of glomerulonephritis is still to be elucidated , however it can be associated with autoimmune disorders (such as SLE or IgA nephropathy) as well as with an aggravation and extension of a primary acute inflammation, such as drug- or infection-induced (Webster and Pusey 2017). In both scenarios, it is important to account for two different compartments which can be dysregulated: both immune infiltration, which seems to be over-activated and instructing a cytotoxic immune response against podocytes and mesangial cells in the glomeruli, as well as the non-immune compartment, which could be triggering an aberrant immune recruitment. Glycosylation plays a crucial role in both compartment perspectives, giving rise to new hypotheses in the etiopathogenesis of glomerulonephritis.
GnT-V (as mentioned before) is the enzyme responsible for the addition of β1,6-branched GlcNAc, allowing the following extension of poly-N-acetyl-lactosamine (Gal-β1,4-GlcNAc) group. This group is a binding motif for Gal-3 lattice, allowing a spatial distance between TCRs, preventing TCR clustering and activation. Hereupon, Demetriou et al. showed that at 12–20 months of age, Mgat5 null mice appear to exhibit signs of glomerulonephritis (Demetriou et al. 2001). This glomerulonephritis was characterized by immune infiltration in the glomeruli and mesangial area, as well as severe glomeruli destruction; phenotypically most severe cases of glomerulonephritis (32%) showed haematuria, proteinuria and a characteristic crescentic glomerulonephritis with fibrosis in the Bowman capsule. The hyperactivation observed in Mgat5−/− could be instructing a stronger immune response in the kidney, as it does in the colon (Demetriou et al. 2001; Dias et al. 2018a, b).
Interestingly, Chui et al. have observed that murine kidney glycoproteins are highly dependent on α-mannosidase II for the correct glycan repertoire, a special feature which was not observed in other tissues, since an alternative form of α-mannosidase II takes its role. This observation reveals the importance of α-mannosidase II enzyme kidney-associated inflammation. Accordingly, these mice develop a severe autoimmune-associated glomerulonephritis at 12 months of age, displaying high levels of autoantibodies, with IgG, IgM, IgA and C3 complement deposition on glomeruli, as well as plasma cells and neutrophil infiltration (Chui et al. 2001).
In both mice models, it is possible to assume that non-immune compartment is also playing a role, since MGAT5 or MAN2A1 are absent in all organism cells/tissues. Given that, an interesting library of C-type lectins has been studied in the scope of immune cell recognition, not only in infection models, but also in inflammatory diseases, since aberrant glycans are being exposed at the surface of supposedly healthy cells. It was interesting to observe that glomerulonephritis was attenuated in mice after treatment with anti-DC-SIGN antibody (Cai et al. 2016). Moreover, mannose-binding lectin (MBL), a soluble c-type lectin which recognizes agalactosylated glycoproteins, was detected in the glomerulonephritis kidney biopsies of lupus patients, with no changes in the serum levels (Lhotta et al. 1999). Altogether, there is a body of evidence that argues a possible role in the aberrant glycoprofile of non-immune compartment for the immune response triggering.
Despite the lack of knowledge regarding the role of glycosylation in SLE, recent findings have been describing a deficiency in complex N-glycans at the surface of kidney epithelial compartment (Alves et al. 2020). This altered glycoprofile appears to be associated with the promotion of a chronic inflammatory response , however more studies are needed to validate these observations.
3.3 Role of Glycans in Myopathies
Idiopathic inflammatory myopathies (IIM) are a group of rare diseases of autoimmune nature, whose etiopathogenesis is far from being totally understood. Factors implicated in autoimmune diseases in general, namely immune innate and adaptive dysregulations associated with non-immune mechanisms, have been implicated, but how they interplay to give rise to the diverse pathogenic phenotypes remains elusive (Miller et al. 2018). Muscle cells surface is enriched in glycoproteins, either alone or in glycoprotein complexes, and several lines of evidence provide support for a fundamental role of glycosylation in muscle homeostasis and function (Broccolini et al. 2009; Townsend 2014; McMorran et al. 2016). Hereditary inclusion-body myositis (hIBM) bears a muscle phenotype that resembles in most aspects sporadic inclusion-body myositis (sIBM) (one of the IIM subgroups) and is associated with mutations in the UDP-N-acetylglucosamine 2-epimerase/N-acetylmannosamine kinase (GNE) gene. GNE codes for an enzyme expressed in several tissues and it is critical for the biosynthesis of sialic acid. GNE mutations result in glycosylation changes, namely hyposialylation of muscle glycoproteins and prophylactic supplementation of a sialic acid precursor (N-acetylmannosamine (ManNAc) was shown to prevent the muscle phenotype in mice with gene mutations that cause hIBM (Malicdan et al. 2009). Changes in muscle glycosylation in human disease have focused in muscular dystrophies and congenital glycosylation disorders, but recent studies have shown that muscle cell surface glycosylation is finely regulated and subjected to alterations under inflammatory condition (Wiendl et al. 2005), pointing to an interaction between muscle glycocalyx and the extracellular milieu, which is particularly enriched in immune cells and antibodies in IIM patients (Afzali et al. 2018).
Changes in the signature of healthy muscle cells could be indeed associated with immune infiltration and development of a dysregulated response in a tissue-target manner. Understanding the role of N-glycans, either at the immune compartment as well as in the epithelial component may contribute to a better knowledge of the etiopathogenesis of inflammatory myopathies.
4 Glycans as Potential Targets for Diagnosis, Prognosis and Therapy in Chronic Inflammatory Diseases
Chronic Inflammatory Diseases including IBD , SLE and IIM present a big challenge for the correct diagnosis and are characterized by a large variation in response to treatment and issues in predicting outcomes to conventional therapies (Podolsky 2002; Dias et al. 2018a, b; Verhelst et al. 2020). Early diagnosis and prognosis of these types of diseases are critical to decrease morbidity, improve quality of life and decrease disability of patients, since the delayed initiation of appropriate therapy may contribute to unsuccessful outcomes. Therefore, there is a clinical need to develop better diagnostics, more effective and preventive approaches. The identification of novel biomarkers of disease whose exploitation may represent a promising novel therapeutic strategy for inflammatory diseases will allow the selection of patients according to their proneness to develop aggressive/complicated disease course and consequently an adequate redirection of therapy (Pereira et al. 2018a, b).
Due to their role in cell functions, glycans have recently been appreciated as a crucial factor regulated in pathologic events leading to development of immune-mediated diseases. In fact, the relationship between glycosylation alterations and its functional impact in the etiopathogenesis of many autoimmune diseases is a new and promising field for the development of novel therapies directed to improve individualized therapy and to develop better diagnostic and prognostic approaches (Dias et al. 2018a, b; Hanić et al. 2019; Verhelst et al. 2020). Lower levels of branched N-glycans in colon biopsies diagnosis predict patients that do not respond to standard therapy with 75% specificity, whereas patients presenting high levels of branched N-glycans display a favourable therapeutic and disease outcome. Interestingly, this glyco-biomarker combined with the analysis of C-reactive protein (CRP), a clinical biomarker used as a predictor of inflammation, constitutes a powerful tool with improved prognostic capacity. IBD patients with low branched N-glycans and high CRP levels at diagnosis early require an aggressive and non-conventional therapy (Pereira et al. 2018a, b).
Additionally, recent discoveries on the role of plasma glycoproteins have pushed forward the expanding area of GlycoMedicine to the forefront for many clinical applications (Theodoratou et al. 2014; Hanić et al. 2019; Reily et al. 2019). Highly inflammatory glycosylation signature of plasma immunoglobulin G (IgG) has been associated as clinical features of IBD and SLE (Arnold et al. 2007; Šimurina et al. 2018). In fact, a pronounced decreased galactosylation of IgG-Fc is observed in SLE and IBD patients, representing in turn a great indicator of chronic inflammation with significant diagnostic value. Moreover, a decrease of di-galactosylated IgG N-glycans in IBD patients compared with healthy controls was already reported (Shinzaki et al. 2008). The role of glycans as diagnostic and prognostic IBD biomarkers was further illustrated by N-glycan analysis of total plasma proteins where an increase in glycan branching, a decreased abundance of hybrid and high-mannose structures, higher total sialylation, and lower fucosylation were observed in IBD patients compared with healthy individuals (Clerc et al. 2018). Likewise, some reports have shown that the glycosignature of IgG changes between patients with UC and CD, revealing differences on level of fucosylation, galactosylation, and bisection (Trbojević Akmačić et al. 2015; Šimurina et al. 2018). A higher α2,3-linked sialylation and higher bisection of plasma glycoproteins were detected in CD compared with UC (Clerc et al. 2018). Interestingly, increased agalactosylation (loss of a terminal galactose) of IgGs serum levels displayed by patients with CD is correlated with levels of CRP and associated as well with more extensive and progressive disease (Dubé et al. 1990). Thus, IgG Fc-galactosylation seems to be a relevant biomarker for the prognosis of IBD .
Another glycobiomarker for IBD is glycoprotein acetylation (GlycA). Higher complex N-glycans expression in acute-phase glycoproteins (such as haptoglobin, α-1-acid glycoprotein , transferrin and α-1-antichymotrypsin) has been associated with worse disease severity in SLE (Connelly et al. 2017; Dierckx et al. 2019).
The exploration of pathogenic role of glycans variation in IBD is also extended to the expression of glycan receptors. Studies demonstrated that differential expression of glycan receptors, such as galectins, play a major influence on the IBD development and the serum galectins can be used as potential biomarkers of IBD and disease activity (Yu et al. 2020). As already mentioned along the chapter, circulating galectins are usually altered in disease context. L-selectin, a cell adhesion molecule of lymphocytes that recognizes endothelial ligands, was found to be increased in serum samples of UC patients compared with healthy controls (Seidelin et al. 1998). Patients with active IBD showed higher serum levels of galectin-1 and -3 comparatively with healthy individuals (Frol’ová et al. 2009). Galectin-1 discriminated IBD from healthy individuals with 71% sensitivity and 87% specificity (Yu et al. 2020); in turn galectin-3 discriminated IBD from healthy controls with 53% sensitivity and 87% specificity (Yu et al. 2020). Hence, galectins might be useful as a powerful biomarker for the diagnosis of IBD .
It has been reported the contribution of genetic variants of key glycoenzymes has an important role in regulating susceptibility and etiopathogenesis in chronic inflammatory diseases (Podolsky 2002; Dias et al. 2018a, b). Recently, a novel genetic risk locus was identified including intronic SNPs in the glycogene MGAT5 that are functionally correlated with glycosylation alterations on T cells and on plasma IgGs and that presented strong association with clinical severity/complication of the UC disease (Dias et al. 2018a, b; Pereira et al. 2020). The rs3814022 and rs4953911 were found to be significantly correlated with lower levels of Fc domain monogalactosylation of IgG2 and IgG3. The rs4953911 was also found to be associated with agalactosylation of IgG1 of which it is associated with induction of a proinflammatory effector functions. Individuals with genetic variations on FUT2 are known to present increased susceptibility to develop IBD (Rausch et al. 2011; Lewis et al. 2015).
Additional biomarkers used into diagnostic of autoimmune and inflammatory disorders are the serum antibodies against glycans (Zhou et al. 2016). Anti-glycoprotein 2, anti-mannobioside carbohydrate IgG (Li et al. 2008; Bogdanos et al. 2011) and anti-Saccharomyces cerevisiae are some of the anti-glycan antibodies (Annese et al. 2004) used to perform the diagnosis of IBD and differentiate UC from CD with a prognostic value (prediction of aggressive disease course). These observations pinpointed the role of glycosylation patterns, such as serum glycome, as a potential diagnostic and prognostic tool among different inflammatory disorders.
Recent discoveries on the role of glycans as a powerful translational therapy have pushed forward the expanding area of glycotherapy to the forefront of in clinical context.
The application of carbohydrate-recognizing receptor inhibitors as a pharmacological tool to block target-pathogenic processes is an example of new generation of therapeutics. Pharmacological blockade of selectins by anti-selectin monoclonal antibodies has gained special interest as a therapy to IBD . Natalizumab (an α4-integrin antagonist) (Gordon et al. 2001, 2002) and vedolizumab (which selectively blocks trafficking of α4β7-positive lymphocytes to the gut) (Feagan et al. 2013; Sandborn et al. 2013) have been clinically applicated to treat IBD . Moreover, the administration of glycoengineered therapeutic monoclonal antibodies, as deglycosylated antibodies (treated with endoglycosidase S), were found to hamper the formation of immune complexes, reducing pathology of disease in case of SLE (Lood et al. 2012).
Interestingly, the potential of glycans supplementation has been described as a promising adjuvant therapy, namely GlcNAc for patients with UC. The metabolic GlcNAc supplementation of mucosal T cells isolated from patients with active UC improved branched N-glycosylation on the T cell receptor, consequently controlling T cell activation and function (Dias et al. 2018a, b). Remarkably, a pilot study of oral supplementation with GlcNAc in paediatric IBD reveals a potential role of GlcNAc as a powerful therapeutic agent. More than half of children under GlcNAc treatment exhibited clinical remission with evidence of histological improvement. Moreover, the properties of GlcNAc as a therapy were already verified in a pilot study of paediatric patients with IBD (Salvatore et al. 2000).
5 Concluding Remarks/Conclusion
The relevance of glycans in the study of inflammatory diseases extends from the intrinsic effects on immune cells as well as the correct recognition of danger glycosylation patterns. From immune cells’ correct development through the migration and extravasation to the inflamed tissues, glycosylation plays an important role. Moreover, specific glycan switches seem to contribute to the regulation and/or dysregulation of the immune cell response.
Various advances on the field of glycosylation have been contributing to a paradigm shift in the patients’ stratification, enabling a personalized medicine through optimized preventive and improving prognostic accuracy in the clinical management of patients. Moreover, the identification of glycosylation unbalance into inflammatory diseases’ pathophysiology constitutes an opportunity to improve the target-specific therapy of patients without side effects and with a low cost.
Compliance with Ethical Standards
Funding
The Institute of Molecular Pathology and Immunology of the University of Porto integrates the i3S research unit, which is partially supported by the Portuguese Foundation for Science and Technology. This article is a result of the project NORTE-01-0145-FEDER-000029, supported by the Norte Portugal Regional Programme (NORTE 2020) under the PORTUGAL 2020 Partnership Agreement through the European Regional Development Fund. This work was also funded by Fundo Europeu de Desenvolvimento Regional (FEDER) funds through the COMPETE 2020—Operacional Programme for Competitiveness and Internationalization (POCI), Portugal 2020, and by Portuguese funds through the Portuguese Foundation for Science and Technology (FCT) in the framework of the projects (POCI-01/0145-FEDER-016601/ PTDC/DTP-PIC/0560/2014 and POCI-01-0145-FEDER-028772). Inês Alves [SFRH/BD/128874/2017] and Manuel Vicente [PD/BD/135452/2017] received funding from the FCT. Ana Campar acknowledges Group of Studies for Autoimmune diseases from Portuguese Society of Internal Medicine (NEDAI) for funding. Salomé S. Pinho acknowledges the Broad Medical Research Program at the Crohn’s and Colitis Foundation of America; the International Organization for the study of Inflammatory Bowel Disease; and the Portuguese Group of Study in IBD (GEDII) for funding. Salomé S. Pinho also acknowledges the US Department of Defense, US Army Medical Research Acquisition Activity, FY18 Peer Reviewed Medical Research Program Investigator-Initiated Research Award.
Disclosure of Interests
All authors declare they have no conflict of interest.
Ethical Approval
This article does not contain any studies with human participants performed by any of the authors. This article does not contain any studies with animals performed by any of the authors.
References
Ablasser A, Chen ZJ (2019) cGAS in action: expanding roles in immunity and inflammation. Science 363:eaat8657
Afzali AM, Müntefering T, Wiendl H et al (2018) Skeletal muscle cells actively shape (auto)immune responses. Autoimmun Rev 17:518–529
Alves I, Pinto V, Santos-Pereira B et al (2020) AB0123 changes in cellular glycosylation as a key factor in the immunopathogenesis of systemic lupus erythematosus. Ann Rheum Dis 79BMJ Publishing Group Ltd:1361–1362
Amin R, Mourcin F, Uhel F et al (2015) DC-SIGN-expressing macrophages trigger activation of mannosylated IgM B-cell receptor in follicular lymphoma. Blood 126:1911–1920
Annese V, Piepoli A, Perri F et al (2004) Anti-Saccharomyces cerevisiae mannan antibodies in inflammatory bowel disease: comparison of different assays and correlation with clinical features. Aliment Pharmacol Ther 20:1143–1152
Araujo L, Khim P, Mkhikian H et al (2017) Glycolysis and glutaminolysis cooperatively control T cell function by limiting metabolite supply to N-glycosylation. Elife 6
Arnold JN, Wormald MR, Sim RB et al (2007) The impact of glycosylation on the biological function and structure of human immunoglobulins. Annu Rev Immunol 25:21–50
Bain CC, Mowat AM (2014) Macrophages in intestinal homeostasis and inflammation. Immunol Rev 260:102–117. https://doi.org/10.1111/imr.12192
Barbosa JA, Santos-Aguado J, Mentzer SJ et al (1987) Site-directed mutagenesis of class I HLA genes. Role of glycosylation in surface expression and functional recognition. J Exp Med 166:1329–1350. https://doi.org/10.1084/jem.166.5.1329
Bax M, García-Vallejo JJ, Jang-Lee J et al (2007) Dendritic cell maturation results in pronounced changes in glycan expression affecting recognition by siglecs and galectins. J Immunol 179:8216–8224
Beccaria CG, Amezcua Vesely MC, Fiocca Vernengo F et al (2018) Galectin-3 deficiency drives lupus-like disease by promoting spontaneous germinal centers formation via IFN-γ. Nat Commun 9:1628
Bogdanos DP, Rigopoulou EI, Smyk DS et al (2011) Diagnostic value, clinical utility and pathogenic significance of reactivity to the molecular targets of Crohn’s disease specific-pancreatic autoantibodies. Autoimmun Rev 11:143–148
Broccolini A, Gidaro T, Morosetti R, Mirabella M (2009) Hereditary inclusion-body myopathy: clues on pathogenesis and possible therapy. Muscle Nerve 40:340–349. https://doi.org/10.1002/mus.21385
Brown GD, Willment JA, Whitehead L (2018) C-type lectins in immunity and homeostasis. Nat Rev Immunol 18:374–389
Burrows PD, Stephan RP, Wang Y-H et al (2002) The transient expression of pre-B cell receptors governs B cell development. Semin Immunol 14:343–349
Cabral J, Hanley SA, Gerlach JQ et al (2017) Distinctive surface glycosylation patterns associated with mouse and human CD4+ regulatory T cells and their suppressive function. Front Immunol 8:987
Cai M, Zhou T, Wang X et al (2016) DC-SIGN expression on podocytes and its role in inflammatory immune response of lupus nephritis. Clin Exp Immunol 183:317–325
Chen H-L, Li CF, Grigorian A et al (2009a) T cell receptor signaling co-regulates multiple Golgi genes to enhance N-glycan branching. J Biol Chem 284:32454–32461. https://doi.org/10.1074/jbc.M109.023630
Chen H-Y, Fermin A, Vardhana S et al (2009b) Galectin-3 negatively regulates TCR-mediated CD4+ T-cell activation at the immunological synapse. Proc Natl Acad Sci U S A 106:14496–14501
Chen H-Y, Wu Y-F, Chou F-C et al (2020) Intracellular Galectin-9enhances proximal TCR signaling and potentiates autoimmune diseases. J Immunol 204:1158–1172
Chui D, Sellakumar G, Green R et al (2001) Genetic remodeling of protein glycosylation in vivo induces autoimmune disease. Proc Natl Acad Sci U S A 98:1142–1147
Clerc F, Novokmet M, Dotz V et al (2018) Plasma N-glycan signatures are associated with features of inflammatory bowel diseases. Gastroenterology 155:829–843
Comber JD, Philip R (2014) MHC class I antigen presentation and implications for developing a new generation of therapeutic vaccines. Ther Adv Vaccine 2:77–89
Connelly MA, Otvos JD, Shalaurova I et al (2017) GlycA, a novel biomarker of systemic inflammation and cardiovascular disease risk. J Transl Med 15:219
Daniels MA, Devine L, Miller JD et al (2001) CD8 binding to MHC class I molecules is influenced by T cell maturation and glycosylation. Immunity 15:1051–1061
Demetriou M, Granovsky M, Quaggin S, Dennis JW (2001) Negative regulation of T-cell activation and autoimmunity by Mgat5 N-glycosylation. Nature 409:733–739
den Haan JMM, Arens R, van Zelm MC (2014) The activation of the adaptive immune system: cross-talk between antigen-presenting cells, T cells and B cells. Immunol Lett 162:103–112
Dias AM, Dourado J, Lago P et al (2014) Dysregulation of T cell receptor N-glycosylation: a molecular mechanism involved in ulcerative colitis. Hum Mol Genet 23:2416–2427
Dias AM, Correia A, Pereira MS et al (2018a) Metabolic control of T cell immune response through glycans in inflammatory bowel disease. Proc Natl Acad Sci U S A 115:E4651–E4660
Dias AM, Pereira MS, Padrão NA et al (2018b) Glycans as critical regulators of gut immunity in homeostasis and disease. Cell Immunol 333:9–18
Dierckx T, Verstockt B, Vermeire S, van Weyenbergh J (2019) GlycA, a nuclear magnetic resonance spectroscopy measure for protein glycosylation, is a viable biomarker for disease activity in IBD. J Crohns Colitis 13:389–394
Drake PM, Stock CM, Nathan JK et al (2009) Polysialic acid governs T-cell development by regulating progenitor access to the thymus. Proc Natl Acad Sci U S A 106:11995–12000
Dubé R, Rook GA, Steele J et al (1990) Agalactosyl IgG in inflammatory bowel disease: correlation with C-reactive protein. Gut 31:431–434
Ercan A, Cui J, Chatterton DEW et al (2010) Aberrant IgG galactosylation precedes disease onset, correlates with disease activity, and is prevalent in autoantibodies in rheumatoid arthritis. Arthritis Rheum 62:2239–2248
Espeli M, Mancini SJC, Breton C et al (2009) Impaired B-cell development at the pre-BII-cell stage in galectin-1–deficient mice due to inefficient pre-BII/stromal cell interactions. Blood 113:5878–5886
Feagan BG, Rutgeerts P, Sands BE et al (2013) Vedolizumab as induction and maintenance therapy for ulcerative colitis. N Engl J Med 369:699–710
Ferwerda B, Ferwerda G, Plantinga TS et al (2009) Human dectin-1deficiency and mucocutaneous fungal infections. N Engl J Med 361:1760. https://doi.org/10.1056/NEJMOA0901053
Frol’ová L, Smetana K, Borovská D et al (2009) Detection of galectin-3 in patients with inflammatory bowel diseases: new serum marker of active forms of IBD? Inflamm Res 58:503–512. https://doi.org/10.1007/s00011-009-0016-8
Fu J, Wei B, Wen T et al (2011) Loss of intestinal core 1-derived O-glycans causes spontaneous colitis in mice. J Clin Invest 121:1657–1666
Galvan M, Tsuboi S, Fukuda M, Baum LG (2000) Expression of a specific glycosyltransferase enzyme regulates T cell death mediated by galectin-1. J Biol Chem 275:16730–16737
Gauthier L, Smith KJ, Pyrdol J et al (1998) Expression and crystallization of the complex of HLA-DR2 (DRA, DRB1*1501) and an immunodominant peptide of human myelin basic protein. Proc Natl Acad Sci U S A 95:11828–11833
Gauthier L, Rossi B, Roux F et al (2002) Galectin-1 is a stromal cell ligand of the pre-B cell receptor (BCR) implicated in synapse formation between pre-B and stromal cells and in pre-BCR triggering. Proc Natl Acad Sci U S A 99:13014–13019
Gordon FH, Lai CW, Hamilton MI et al (2001) A randomized placebo-controlled trial of a humanized monoclonal antibody to alpha4 integrin in active Crohn’s disease. Gastroenterology 121:268–274
Gordon FH, Hamilton MI, Donoghue S et al (2002) A pilot study of treatment of active ulcerative colitis with natalizumab, a humanized monoclonal antibody to alpha-4 integrin. Aliment Pharmacol Ther 16:699–705
Goto Y, Obata T, Kunisawa J et al (2014) Innate lymphoid cells regulate intestinal epithelial cell glycosylation. Science 345:1254009
Grigorian A, Lee S-U, Tian W et al (2007) Control of T cell-mediated autoimmunity by metabolite flux to N-glycan biosynthesis. J Biol Chem 282:20027–20035
Grigorian A, Torossian S, Demetriou M (2009) T-cell growth, cell surface organization, and the galectin-glycoprotein lattice. Immunol Rev 230:232–246
Hanić M, Trbojević-Akmačić I, Lauc G (2019) Inflammatory bowel disease – glycomics perspective. Biochim Biophys Acta Gen Subj 1863:1595–1601
Hardy RR, Hayakawa K (2001) B cell development pathways. Annu Rev Immunol 19:595–621
Hewitt EW (2003) The MHC class I antigen presentation pathway: strategies for viral immune evasion. Immunology 110:163–169
Hokama A, Mizoguchi E, Sugimoto K et al (2004) Induced reactivity of intestinal CD4(+) T cells with an epithelial cell lectin, galectin-4, contributes to exacerbation of intestinal inflammation. Immunity 20:681–693
Hollmig ST, Ariizumi K, Cruz PD (2009) Recognition of non-self-polysaccharides by C-type lectin receptors dectin-1 and dectin-2. Glycobiology 19:568–575
Hooper LV, Gordon JI (2001) Commensal host-bacterial relationships in the gut. Science 292:1115–1118
Hovhannisyan Z, Treatman J, Littman DR, Mayer L (2011) Characterization of interleukin-17-producing regulatory T cells in inflamed intestinal mucosa from patients with inflammatory bowel diseases. Gastroenterology 140:957–965
Iwasaki A, Medzhitov R (2010) Regulation of adaptive immunity by the innate immune system. Science 327:291–295
Kansas G (1996) Selectins and their ligands: current concepts and controversies. Blood 88:3259–3287
Kaplan GG, Ng SC (2017) Understanding and preventing the global increase of inflammatory bowel disease. Gastroenterology 152:313–321.e2
Koch U, Radtke F (2011) Mechanisms of T cell development and transformation. Annu Rev Cell Dev Biol 27:539–562
Koch U, Lacombe TA, Holland D et al (2001) Subversion of the T/B lineage decision in the thymus by lunatic fringe-mediated inhibition of Notch-1. Immunity 15:225–236
Koropatkin NM, Cameron EA, Martens EC (2012) How glycan metabolism shapes the human gut microbiota. Nat Rev Microbiol 10:323–356
Larsson JMH, Karlsson H, Crespo JG et al (2011) Altered O-glycosylation profile of MUC2 mucin occurs in active ulcerative colitis and is associated with increased inflammation. Inflamm Bowel Dis 17:2299–2307
Lee MY, Jeon JW, Sievers C, Allen CT (2020) Antigen processing and presentation in cancer immunotherapy. J Immunother Cancer 8:e001111
Lewis ZT, Totten SM, Smilowitz JT et al (2015) Maternal fucosyltransferase 2 status affects the gut bifidobacterial communities of breastfed infants. Microbiome 3:13
Lhotta K, Würzner R, König P (1999) Glomerular deposition of mannose-binding lectin in human glomerulonephritis. Nephrol Dial Transplant 14:881–886
Li X, Conklin L, Alex P (2008) New serological biomarkers of inflammatory bowel disease. World J Gastroenterol 14:5115–5124
Li W, Liu Q, Pang Y et al (2012) Core fucosylation of μ heavy chains regulates assembly and intracellular signaling of precursor B cell receptors. J Biol Chem 287:2500–2508
Lood C, Allhorn M, Lood R et al (2012) IgG glycan hydrolysis by endoglycosidase S diminishes the proinflammatory properties of immune complexes from patients with systemic lupus erythematosus: a possible new treatment? Arthritis Rheum 64:2698–2706
Lugo-Villarino G, Troegeler A, Balboa L et al (2018) The C-type lectin receptor DC-SIGN has an anti-inflammatory role in human M(IL-4) macrophages in response to Mycobacterium tuberculosis. Front Immunol 9:1123
Ma BY, Mikolajczak SA, Yoshida T et al (2004) CD28 T cell costimulatory receptor function is negatively regulated by N-linked carbohydrates. Biochem Biophys Res Commun 317:60–67
Madireddi S, Eun S-Y, Lee S-W et al (2014) Galectin-9 controls the therapeutic activity of 4-1BB-targeting antibodies. J Exp Med 211:1433–1448
Malicdan MCV, Noguchi S, Hayashi YK et al (2009) Prophylactic treatment with sialic acid metabolites precludes the development of the myopathic phenotype in the DMRV-hIBM mouse model. Nat Med 15:690–695
Marth JD, Grewal PK (2008) Mammalian glycosylation in immunity. Nat Rev Immunol 8:874–887
McMorran BJ, McCarthy FE, Gibbs EM et al (2016) Differentiation-related glycan epitopes identify discrete domains of the muscle glycocalyx. Glycobiology 26:1120–1132
Mesin L, Ersching J, Victora GD (2016) Germinal center B cell dynamics. Immunity 45:471–482
Miller JFAP (2020) The function of the thymus and its impact on modern medicine. Science (80-) 369:eaba2429
Miller FW, Lamb JA, Schmidt J, Nagaraju K (2018) Risk factors and disease mechanisms in myositis. Nat Rev Rheumatol 14:255–268
Mkhikian H, Grigorian A, Li CF et al (2011) Genetics and the environment converge to dysregulate N-glycosylation in multiple sclerosis. Nat Commun 2:334
Modenutti CP, Capurro JIB, Di Lella S, Martí MA (2019) The structural biology of galectin-ligand recognition: current advances in modeling tools, protein engineering, and inhibitor design. Front Chem 7:823
Momozawa Y, Dmitrieva J, Théâtre E et al (2018) IBD risk loci are enriched in multigenic regulatory modules encompassing putative causative genes. Nat Commun 9:2427
Moody AM, Chui D, Reche PA et al (2001) Developmentally regulated glycosylation of the CD8alphabeta coreceptor stalk modulates ligand binding. Cell 107:501–512
Morgan R, Gao G, Pawling J et al (2004) N-acetylglucosaminyltransferase V (Mgat5)-mediated N-glycosylation negatively regulates Th1 cytokine production by T cells. J Immunol 173:7200–7208
Mortales C-L, Lee S-U, Demetriou M (2020) N-glycan branching is required for development of mature B cells. J Immunol 205:630–636
Mourcin F, Breton C, Tellier J et al (2011) Galectin-1–expressing stromal cells constitute a specific niche for pre-BII cell development in mouse bone marrow. Blood 117:6552–6561
Nakayama K, Wakamatsu K, Fujii H et al (2019) Core fucose is essential glycosylation for CD14-dependent Toll-like receptor 4 and Toll-like receptor 2 signalling in macrophages. J Biochem 165:227–237
Neefjes J, Jongsma MLM, Paul P, Bakke O (2011) Towards a systems understanding of MHC class I and MHC class II antigen presentation. Nat Rev Immunol 11:823–836
Neill DR, Flynn RJ (2018) Origins and evolution of innate lymphoid cells: Wardens of barrier immunity. Parasite Immunol 40:e12436
Nemazee D (2017) Mechanisms of central tolerance for B cells. Nat Rev Immunol 17:281–294
Nio-Kobayashi J (2018) Histological mapping and subtype-specific functions of galectins in health and disease. Trend Glycosci Glycotechnol 30:SJ47–SJ53
Obino D, Fetler L, Soza A et al (2018) Galectin-8favors the presentation of surface-tethered antigens by stabilizing the B cell immune synapse. Cell Rep 25:3110–3122.e6
Oliveira-de-Abreu E, Silva-dos-Santos D, Lepletier A et al (2018) Lack of galectin-3disrupts thymus homeostasis in association to increase of local and systemic glucocorticoid levels and steroidogenic machinery. Front Endocrinol (Lausanne) 9:365
Omenetti S, Pizarro TT (2015) The Treg/Th17 axis: adynamic balance regulated by the gut microbiome. Front Immunol 6:639
Ostankovitch M, Altrich-Vanlith M, Robila V, Engelhard VH (2009) N-glycosylation enhances presentation of a MHC class I-restricted epitope from tyrosinase. J Immunol 182:4830–4835
Paclik D, Berndt U, Guzy C et al (2008) Galectin-2 induces apoptosis of lamina propria T lymphocytes and ameliorates acute and chronic experimental colitis in mice. J Mol Med (Berl) 86:1395–1406
Parsons MW, Li L, Wallace AM et al (2014) Dectin-2regulates the effector phase of house dust mite–elicited pulmonary inflammation independently from its role in sensitization. J Immunol 192:1361–1371
Pereira MS, Alves I, Vicente M et al (2018a) Glycans as key checkpoints of T cell activity and function. Front Immunol 9:2754
Pereira MS, Maia L, Azevedo LF et al (2018b) A [Glyco]biomarker that predicts failure to standard therapy in ulcerative colitis patients. J Crohns Colitis 13:39–49
Pereira MS, Durães C, Catarino TA et al (2020) Genetic variants of the MGAT5gene are functionally implicated in the modulation of T cells glycosylation and plasma IgG glycome composition in ulcerative colitis. Clin Transl Gastroenterol 11:e00166
Perillo NL, Uittenbogaart CH, Nguyen JT, Baum LG (1997) Galectin-1, an endogenous lectin produced by thymic epithelial cells, induces apoptosis of human thymocytes. J Exp Med 185:1851–1858
Petrosyan A, Cravedi P, Villani V et al (2019) A glomerulus-on-a-chip to recapitulate the human glomerular filtration barrier. Nat Commun 10:3656
Pillai S, Cariappa A (2009) The follicular versus marginal zone B lymphocyte cell fate decision. Nat Rev Immunol 9:767–777
Podolsky DK (2002) Inflammatory bowel disease. N Engl J Med 347:417–429
Rabinovich GA, Toscano MA (2009) Turning “sweet” on immunity: galectin–glycan interactions in immune tolerance and inflammation. Nat Rev Immunol 9:338–352
Rampal R, Li ASY, Moloney DJ et al (2005) Lunatic fringe, manic fringe, and radical fringe recognize similar specificity determinants in O-fucosylated epidermal growth factor-like repeats. J Biol Chem 280:42454–42463
Rausch P, Rehman A, Künzel S et al (2011) Colonic mucosa-associated microbiota is influenced by an interaction of Crohn disease and FUT2 (Secretor) genotype. Proc Natl Acad Sci U S A 108:19030–19035
Ravcheev DA, Thiele I (2017) Comparative genomic analysis of the human gut microbiome reveals a broad distribution of metabolic pathways for the degradation of host-synthetized mucin glycans and utilization of mucin-derived monosaccharides. Front Genet 8:111
Reily C, Stewart TJ, Renfrow MB, Novak J (2019) Glycosylation in health and disease. Nat Rev Nephrol 15:346–366
Roche PA, Furuta K (2015) The ins and outs of MHC class II-mediated antigen processing and presentation. Nat Rev Immunol 15:203–216
RodrÍguez E, Schetters STT, van Kooyk Y (2018) The tumour glyco-code as a novel immune checkpoint for immunotherapy. Nat Rev Immunol 18:204–211
Rossi FMV, Corbel SY, Merzaban JS et al (2005) Recruitment of adult thymic progenitors is regulated by P-selectin and its ligand PSGL-1. Nat Immunol 6:626–634
Ruel J, Ruane D, Mehandru S et al (2014) IBD across the age spectrum: is it the same disease? Nat Rev Gastroenterol Hepatol 11:88–98
Ryan SO, Cobb BA (2012) Host glycans and antigen presentation. Microbes Infect 14:894–903
Ryan SO, Bonomo JA, Zhao F, Cobb BA (2011) MHCII glycosylation modulates Bacteroides Fragiliscarbohydrate antigen presentation. J Exp Med 208:1041–1053
Salvatore S, Heuschkel R, Tomlin S et al (2000) A pilot study of N-acetyl glucosamine, a nutritional substrate for glycosaminoglycan synthesis, in paediatric chronic inflammatory bowel disease. Aliment Pharmacol Ther 14:1567–1579
Samardzic T, Marinkovic D, Danzer C-P et al (2002) Reduction of marginal zone B cells in CD22-deficient mice. Eur J Immunol 32:561–567
Sandborn WJ, Feagan BG, Rutgeerts P et al (2013) Vedolizumab as induction and maintenance therapy for Crohn’s disease. N Engl J Med 369:711–721
Schatz DG, Ji Y (2011) Recombination centres and the orchestration of V(D)J recombination. Nat Rev Immunol 11:251–263
Schnaar RL (2016) Glycobiology simplified: diverse roles of glycan recognition in inflammation. J Leukoc Biol 99:825–838
Seidelin JB, Vainer B, Horn T, Nielsen OH (1998) Circulating L-selectin levels and endothelial CD34 expression in inflammatory bowel disease. Am J Gastroenterol 93:1854–1859
Shah DK, Zúñiga-Pflücker JC (2014) An overview of the intrathymic intricacies of T cell development. J Immunol 192:4017–4023
Shih H-Y, Hao B, Krangel MS (2011) Orchestrating T-cell receptor α gene assembly through changes in chromatin structure and organization. Immunol Res 49:192–201
Shinzaki S, Iijima H, Nakagawa T et al (2008) IgG oligosaccharide alterations are a novel diagnostic marker for disease activity and the clinical course of inflammatory bowel disease. Am J Gastroenterol 103:1173–1181
Shinzaki S, Ishii M, Fujii H et al (2016) N-Acetylglucosaminyltransferase V exacerbates murine colitis with macrophage dysfunction and enhances colitic tumorigenesis. J Gastroenterol 51:357–369
Šimurina M, de Haan N, Vučković F et al (2018) Glycosylation of immunoglobulin G associates with clinical features of inflammatory bowel diseases. Gastroenterology 154:1320–1333.e10
Song Y, Kumar V, Wei H-X et al (2016) Lunatic, manic, and radical fringe each promote T and B cell development. J Immunol 196:232–243
Sperandio M (2006) Selectins and glycosyltransferases in leukocyte rolling in vivo. FEBS J 273:4377–4389
Stanley P, Guidos CJ (2009) Regulation of Notch signaling during T- and B-cell development by O -fucose glycans. Immunol Rev 230:201–215
Storni T, Bachmann MF (2004) Loading of MHC class I and II presentation pathways by exogenous antigens: a quantitative in vivo comparison. J Immunol 172:6129–6135
Sultana DA, Zhang SL, Todd SP, Bhandoola A (2012) Expression of functional P-selectin glycoprotein ligand 1 on hematopoietic progenitors is developmentally regulated. J Immunol 188:4385–4393
Tailford LE, Crost EH, Kavanaugh D, Juge N (2015) Mucin glycan foraging in the human gut microbiome. Front Genet 6:81
Takaba H, Takayanagi H (2017) The mechanisms of T cell selection in the thymus. Trends Immunol 38:805–816
Takeuchi H, Yu H, Hao H et al (2017) O-glycosylation modulates the stability of epidermal growth factor-like repeats and thereby regulates Notch trafficking. J Biol Chem 292:15964–15973
Theodoratou E, Campbell H, Ventham NT et al (2014) The role of glycosylation in IBD. Nat Rev Gastroenterol Hepatol 11:588–600
Togayachi A, Kozono Y, Ishida H et al (2007) Polylactosamine on glycoproteins influences basal levels of lymphocyte and macrophage activation. Proc Natl Acad Sci U S A 104:15829–15834
Toscano MA, Bianco GA, Ilarregui JM et al (2007) Differential glycosylation of TH1, TH2 and TH-17 effector cells selectively regulates susceptibility to cell death. Nat Immunol 8:825–834
Townsend D (2014) Finding the sweet spot: assembly and glycosylation of the dystrophin-associated glycoprotein complex. Anat Rec 297:1694–1705
Trbojević Akmačić I, Ventham NT, Theodoratou E et al (2015) Inflammatory bowel disease associates with proinflammatory potential of the immunoglobulin G glycome. Inflamm Bowel Dis 21:1237–1247
Tribulatti MV, Cattaneo V, Hellman U et al (2009) Galectin-8 provides costimulatory and proliferative signals to T lymphocytes. J Leukoc Biol 86:371–380
Trombetta ES, Helenius A (1998) Lectins as chaperones in glycoprotein folding. Curr Opin Struct Biol 8:587–592
Tsai C-M, Guan C-H, Hsieh H-W et al (2011) Galectin-1 and galectin-8 have redundant roles in promoting plasma cell formation. J Immunol 187:1643–1652
Übelhart R, Bach MP, Eschbach C et al (2010) N-linked glycosylation selectively regulates autonomous precursor BCR function. Nat Immunol 11:759–765
Unanue ER, Turk V, Neefjes J (2016) Variations in MHC class II antigen processing and presentation in health and disease. Annu Rev Immunol 34:265–297. https://doi.org/10.1146/annurev-immunol-041015-055420
Varki A, Gagneux P (2012) Multifarious roles of sialic acids in immunity. Ann N Y Acad Sci 1253:16–36
Verhelst X, Dias AM, Colombel J-F et al (2020) Protein glycosylation as a diagnostic and prognostic marker of chronic inflammatory gastrointestinal and liver diseases. Gastroenterology 158:95–110
Videira PA, Amado IF, Crespo HJ et al (2008) Surface alpha 2-3- and alpha 2-6-sialylation of human monocytes and derived dendritic cells and its influence on endocytosis. Glycoconj J 25:259–268. https://doi.org/10.1007/s10719-007-9092-6
Vivier E, Artis D, Colonna M et al (2018) Innate lymphoid cells: 10years on. Cell 174:1054–1066
Vučković F, Krištić J, Gudelj I et al (2015) Association of systemic lupus erythematosus with decreased immunosuppressive potential of the IgG glycome. Arthritis Rheumatol (Hoboken, NJ) 67:2978–2989
Wang Y, Tan J, Sutton-Smith M et al (2001) Modeling human congenital disorder of glycosylation type IIa in the mouse: conservation of asparagine-linked glycan-dependent functions in mammalian physiology and insights into disease pathogenesis. Glycobiology 11:1051–1070
Webster P, Pusey C (2017) Crescentic glomerulonephritis: beyond the immune system. Nat Rev Nephrol 13:198–200
Wiendl H, Hohlfeld R, Kieseier BC (2005) Immunobiology of muscle: advances in understanding an immunological microenvironment. Trends Immunol 26:373–380
Wu C, Thalhamer T, Franca RF et al (2014) Galectin-9-CD44 interaction enhances stability and function of adaptive regulatory T cells. Immunity 41:270–282
Yu TB, Dodd S, Yu L-G, Subramanian S (2020) Serum galectins as potential biomarkers of inflammatory bowel diseases. PLoS One 15:e0227306
Zarbock A, Ley K, McEver RP, Hidalgo A (2011) Leukocyte ligands for endothelial selectins: specialized glycoconjugates that mediate rolling and signaling under flow. Blood 118:6743–6751
Zeng J, Eljalby M, Aryal RP et al (2020) Cosmc controls B cell homing. Nat Commun 11:3990
Zhou RW, Mkhikian H, Grigorian A et al (2014) N-glycosylation bidirectionally extends the boundaries of thymocyte positive selection by decoupling Lck from Ca2+ signaling. Nat Immunol 15:1038–1045
Zhou G, Song Y, Yang W et al (2016) ASCA, ANCA, ALCA and many more: are they useful in the diagnosis of inflammatory bowel disease? Dig Dis 34:90–97
Zhu C, Anderson AC, Schubart A et al (2005) The Tim-3 ligand galectin-9 negatively regulates T helper type 1 immunity. Nat Immunol 6:1245–1252
Author information
Authors and Affiliations
Corresponding authors
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Alves, I. et al. (2021). The Role of Glycosylation in Inflammatory Diseases. In: Lauc, G., Trbojević-Akmačić, I. (eds) The Role of Glycosylation in Health and Disease. Advances in Experimental Medicine and Biology, vol 1325. Springer, Cham. https://doi.org/10.1007/978-3-030-70115-4_13
Download citation
DOI: https://doi.org/10.1007/978-3-030-70115-4_13
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-70114-7
Online ISBN: 978-3-030-70115-4
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)