Abstract
The basic mechanical, physical, esthetic, and bonding properties of dental resin-based materials have greatly improved with technological developments, and the current materials used for restorative, prosthetic, and preventive treatments show excellent clinical performance. The target of their continued development is therefore shifting towards bioactive functionality to prevent primary/secondary diseases or promote tissue regeneration. Among the several bioactive properties proposed to further enhance dental materials, antibacterial effects that contribute to controlling bacterial infection are one of the most popular. Two approaches are available for conferring antibacterial activity to dental resin-based materials. One is immobilization of antimicrobial components in/on the materials that demonstrate the so-called contact inhibition—inhibition of bacteria that come into contact with the surfaces without any active components being released. Such technology involves utilization of a polymerizable bactericide such as quaternary ammonium compound (QAC)-based resin monomers or QAC-functionalized nanoparticles. In the other approach, the ability to liberate antibacterial components through controlled release is introduced using a carrier, e.g., nonbiodegradable polymer particles loaded with antimicrobials, silver nanoparticles, and ion-releasing glass fillers. In this chapter, two different approaches to providing dental resins with antibacterial properties are summarized. In addition, the future perspectives for each material are addressed based on the continued development of each approach. Both technologies for achieving antibacterial materials described here have great potential to contribute to successful dental treatments.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
10.1 Introduction
The need for new-generation dental materials with bioactive functions is a topic of great interest that is discussed not only by researchers but also by dental practitioners. Among the several bioactive properties proposed as beneficial enhancements for dental materials, antibacterial effects that contribute to controlling bacterial infection are some of the most popular.
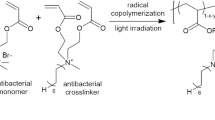
Two major approaches are available for introducing antibacterial activity into dental resins. One is immobilization of antimicrobial components in/on the materials (Fig. 10.1a). This type of material demonstrates the so-called contact inhibition, which means inhibition of bacteria that come into contact with its surface without any active components being released. The technology that has been intensively investigated to achieve immobilization of antibacterial components in dental resins is the use of polymerizable bactericides such as quaternary ammonium compound (QAC)-based resin monomers. Imazato et al. invented the world’s first antibacterial QAC monomer, 12-methacryloyloxydodecylpyridinium bromide (MDPB) [1, 2], and many QAC monomers have subsequently been developed [3]. Dental resins with QAC-functionalized nanofillers [4, 5] have also been reported as another mode of QAC immobilization.
The other major approach is introducing into resins the ability to liberate antibacterial components by controlled release. The application of various filler particles as drug carriers has been investigated for this purpose [6] (Fig. 10.1b). It is not possible to achieve controlled-release kinetics using the classical method of mixing antimicrobial agents directly into materials, and the time span in which such materials exhibit antimicrobial effects is short. The use of drug carriers is an effective approach for overcoming such limitations and achieving long-lasting antimicrobial effects.
In this chapter, two different approaches for introducing antibacterial activity into dental resin-based materials are summarized along with the future perspectives for each material.
10.2 Contact Inhibition of Microorganisms by Immobilized Antimicrobials
10.2.1 Polymerizable Bactericide: QAC-Based Antibacterial Monomer MDPB
In 1994, Imazato et al. reported the first polymerizable bactericide for dental resins: antibacterial resin monomer MDPB. MDPB was synthesized by combining alkylpyridinium, a type of QAC, with a methacryloyl group [1] (Fig. 10.2). The QAC in the MDPB molecule is responsible for antibacterial activity, while the methacryloyl group allows for copolymerization with other conventional monomers. The antibacterial component is immobilized in the resin matrix after polymerization of MDPB (Fig. 10.3). The immobilized antimicrobial is not hydrolyzed and the antimicrobial components do not leach out in a wet environment. Therefore, cured resins containing MDPB exhibit contact inhibition at their surfaces without detriment to their mechanical properties after function in the mouth.
10.2.2 Application of MDPB to Restorative Materials
Dental caries has been recognized as an infectious disease induced by cariogenic bacteria. Attempts to create restorative materials with antibacterial effects have provided an important topic in dental materials science. The control of bacteria around/beneath restorations could be advantageous for eliminating the risk of further demineralization and cavitation, contributing to the prevention of secondary caries. To address the growing need for antibacterial restorative materials, efforts have been made to apply the antibacterial monomer MDPB to several different materials.
The representative example of MDPB use is for adhesive systems. Experimental self-etching primer was prepared by incorporating MDPB, and its antibacterial activity and cavity-disinfecting effects were confirmed by a number of in vitro and in vivo studies. Based on these findings, Clearfil Protect Bond (present product name: Clearfil SE Protect, Kuraray Noritake Dental Inc., Japan; Fig. 10.4), which employs a 5% MDPB-containing self-etching primer, was commercialized as the world’s first antibacterial adhesive system.
MDPB-containing primer can kill bacteria rapidly based on the mechanism of action of unpolymerized MDPB—as a QAC—on bacteria (Fig. 10.5). When the primer containing MDPB is kept in direct contact with planktonic bacteria, effective bacterial killing can be attained within 30 s. It is noteworthy that the Clearfil Protect Bond primer can penetrate a 500-μm-thick dentin block and eradicate caries-related species inside the dentin [2]. In vivo studies using beagle dogs revealed that the MDPB-containing primer inactivated bacteria in the cavity [7]. Since residual bacteria are one of the primary causes of secondary caries, the cavity-disinfecting effects of the MDPB-containing primer are expected to improve the outcomes of restorative treatments of caries lesions. In addition, Brambilla et al. found from in vitro studies that biofilm formation around the margin of restorations placed using Clearfil Protect Bond was reduced owing to “contact inhibition” shown by immobilized MDPB [8].
The original aim of developing antibacterial monomer MDPB was for development of anti-plaque composites. MDPB was first immobilized in the resin matrix of composites [1], and to increase the concentration of immobilized bactericide, the prepolymerized resin filler containing MDPB was fabricated [9]. The composites incorporating MDPB-immobilized filler were reported to exhibit inhibitory effects against the growth of Streptococcus mutans on its surface and reduce plaque accumulation in vitro.
Recently, applications of MDPB have been expanded to other resinous materials such as a root canal sealer [10] and cavity disinfectant [11]. The experimental root canal sealer, which consisted of HEMA-based chemically cured primer containing MDPB at 5% and Bis-GMA-based sealing resin, was effective for killing the bacteria inside the dentinal tubules of root dentin and demonstrated excellent sealing ability. The experimental cavity disinfectant, intended for use for various direct and indirect restorations and prepared by adding 5% MDPB to 80% ethanol, was more effective in eradicating bacterial infection in dentin than the commercially available chlorhexidine-based cavity disinfectant. These findings confirmed that unpolymerized MDPB can penetrate deeply into dentinal tubules and exhibit strong bactericidal activity, which is expected to contribute to better clinical results in endodontic and restorative treatments.
10.2.3 Various QAC Monomers
Based on the concept of MDPB, new QAC monomers have subsequently been developed (Fig. 10.6). Huang et al. [12] synthesized 2-methacryloxylethyl dodecyl methyl ammonium bromide (MAE-DB) as a QAC with dimethacrylate groups. The cured experimental Bis-GMA/TEGDMA resin containing MAE-DB at 10 (wt)% demonstrated antibacterial effects against Streptococcus mutans even after immersion in water for 180 days. The low-viscosity ionic dimethacrylate monomer, bis(2-methacryloyloxy ethyl)dimethylammonium bromide (IDMA-1), was synthesized by the Menshutkin reaction [13]. Incorporation of 10 (wt)% IDMA-1 into Bis-GMA/TEGDMA resin reduced bacterial colonization without affecting the viability or metabolic activity of mammalian cells.
Li et al. [14] investigated the structure-property relationship of QAC monomers. A series of QAC monomers with alkyl chain lengths of 3, 6, 12, 16, and 18 were synthesized and their antimicrobial activities were evaluated. For short-chained quaternary ammonium compounds, the antimicrobial activity appeared to rely on positively charged ammonium groups coupling with negatively charged bacterial membranes to disrupt membrane functions, alter the balance of essential ions, interrupt protein activity, and damage bacterial DNA. Increasing chain length reduced the metabolic activity and acid production of saliva-derived microcosm biofilms. Among the monomers with different alkyl chain lengths, the molecules with a chain length of 12 or 16, such as dimethylaminohexadecyl methacrylate (DMAHDM, Fig. 10.6c), were found to possess stronger antimicrobial effects.
Currently, many different QAC monomers are available including methacryloxylethyl cetyl dimethyl ammonium chloride (DMAE-CB), dimethylaminododecyl methacrylate (DMADDM), dimethylammoniumethyl dimethacrylate (DMAEDM), 2-dimethylamino ethyl methacrylate (DMAEMA), 2-methacryloxylethyl hexadecyl methyl ammonium bromide (MAE-HB), IDMA-2, methacryloyloxyundecylpyridinium bromide (MUPB), N-benzyl-11-(methacryloyloxy)-N,N-dimethylundecan-1-aminium fluoride (monomer II), [2-(methacryloyloxy)ethyl] trimethylammonium chloride (MADQUAT), urethane dimethacrylate quaternary ammonium methacrylate (UDMQA), and quaternary ammonium bisphenol A glycerolate dimethacrylate (QABGMA) [3, 6]. The effectiveness against oral microorganisms of the incorporation of each monomer, without impeding the physical properties, was demonstrated using in vitro assessments.
10.2.4 QAC-Functionalized Nanoparticles
Based on the development of various QAC monomers, antibacterial solid nanoparticles with QAC functionality have also been fabricated. Quaternary ammonium polyethyleneimine (QPEI) nanoparticles exhibit strong antibacterial effects against gram-positive and gram-negative bacteria [15], with their antimicrobial potency being attributed to the abundance of quaternary ammonium groups along the polymer backbone. Owing to their small size and large surface area, incorporation of a small amount of the QAC nanoparticles is sufficient to confer antibacterial effects and addition of 1–2 (wt)% provides contact killing effects to various resin-based materials [16,17,18]. Several recent studies have focused on surface modification of quaternary ammonium silica-based nanoparticles (QASi) to achieve QAC-functionalized nanoparticles [5]. These nanoparticles can be distributed more homogenously in polymers with less aggregation compared with QPEI nanoparticles.
10.2.5 Advantages and Disadvantages
Bactericide immobilized by polymerization of the antibacterial monomer is covalently bound to the base resins and does not leach out. Therefore, dental resins containing the immobilized bactericide demonstrate long-lasting inhibitory effects against microorganisms on their surfaces. MDPB-containing composites were confirmed to exhibit the same level of inhibition of biofilm formation when tested as cured and after 1 year of immersion in water.
As the immobilized component has limited molecular movement and disrupts the bacterial surface structure through contact action, its effect is essentially bacteriostatic rather than bactericidal. To obtain killing effects, the density of immobilized component exposed on the outer surface must be high, as shown by QPEI nanoparticles. A further disadvantage of the strategy of immobilizing bactericide in resins is that antibacterial effects are greatly reduced when the surface is covered by salivary protein. This is a critical problem for restorative materials used in the oral environment, and different key technologies need to be combined to achieve clinically effective anti-plaque activity.
10.3 Controlled Release of Antibacterial Components
10.3.1 Nonbiodegradable Polymer Particles Loaded with Antimicrobials
Simple approaches for adding antimicrobial components that get released in a wet environment to resinous materials have been reported for many years. However, the release behavior of the antimicrobial agents cannot be controlled using these methods and continuous delivery of the agents is challenging.
Imazato et al. [19] developed nonbiodegradable polymer particles made from the hydrophilic monomer 2-hydroxyethyl methacrylate (HEMA) and a cross-linking monomer trimethylolpropane trimethacrylate (TMPT), and reported their utility as antimicrobial reservoirs. By modifying the ratio of HEMA and TMPT, the hydrophilicity and polymer network density can be controlled [20]. Kitagawa et al. investigated loading of cetylpyridinium chloride (CPC) into these polymer particles using two different methods [20]. One method was to immerse the particles in a CPC aqueous solution to take up CPC. While it was found that the polymer particles consisting of 50% HEMA/50% TMPT were useful for loading and inhibition of bacteria, the duration of release of CPC from the particles loaded using the immersion method was short. Another method was to add CPC powder to the HEMA/TMPT monomer mixture and cure it to produce polymers (Fig. 10.7). With this method, a marked extension of the release period to over 120 days was achieved. The experimental resin-based endodontic sealer containing CPC-premixed particles was shown to exhibit antibacterial effects for a long period.
Moreover, the recharging of CPC into HEMA/TMPT particles by exposure to CPC solution was possible, and persistent antimicrobial effects with sustained release of CPC were achieved (Fig. 10.8). Such protocols are useful for maintaining clean surfaces for resinous materials such as denture bases.
10.3.2 Silver Nanoparticles
Silver is known to have antibacterial, antifungal, and antiviral activity. Silver ions strongly interact with thiol groups of vital enzymes and inactivate them, causing DNA to lose the ability to replicate and leading to cell death. Therefore, throughout the long history of dentistry, mixing silver into restorative materials has often been tried in attempts to provide antimicrobial effects. However, this method is not effective for providing continuous delivery of silver ions. In addition, silver incorporation results in blackening of the materials and is not suitable for esthetic restorations.
To overcome these problems, the addition of silver (Ag) nanoparticles to resinous materials has been investigated. Ag nanoparticles work as a kind of reservoir of Ag+ that can be released in a controlled manner at a steady rate, allowing for long-term antibacterial effects. However, Ag nanoparticles are difficult to be dispersed in a resin matrix since nano-sized particles tend to aggregate and agglomerate. Therefore, to prevent aggregation, Ag nanoparticles are stabilized by various functional groups on their surface using coating agents or stabilizers such as polymers, polysaccharides, or citrates [6].
Another approach for avoiding the aggregation of Ag nanoparticles in resinous materials is a technique for fabricating polymers with evenly dispersed Ag nanoparticles using coupling photo-initiated free radical polymerization of dimethacrylates with in situ silver ion reduction [21, 22]. Experimental resins prepared with this technology exhibit good Ag dispersion (Fig. 10.9), and strong reduction in bacteria coverage on their surface was observed in vitro for low-concentration addition of Ag nanoparticles [21].
10.3.3 Ion-Releasing Glass Fillers
10.3.3.1 Bioglass
Bioglass 45S5 (commercially known as Bioglass®), composed of SiO2, Na2O, CaO, and P2O5, is a potential candidate for use as antibacterial filler particles in restorative materials because it exerts antimicrobial effects as well as enhances hard tissue regeneration by releasing ions [6]. Its antimicrobial effects are attributed to the release of ions such as Ca2+, which leads to a local increase in pH that is not well tolerated by bacteria. Resin composites containing Bioglass 65S, another type of bioglass composed of SiO2, CaO, and P2O5, reduced bacterial penetration into the marginal gaps of simulated tooth restorations [23].
Davis et al. [24] developed glass fillers containing calcium and fluoride, prepared using the sol-gel method. It was shown that resin composites incorporating these fillers acted as a single source of both Ca2+ and F− in aqueous solutions, and that the composites could be readily recharged with F−. However, because the effective concentrations of Ca2+ and F− against microorganisms are so high, the antimicrobial effects of a local increase in pH due to Ca2+ from bioglass, or the release of F− from fluoride-containing glass fillers, are limited. As a result, additional components are needed to obtain apparent effects against oral microorganisms.
10.3.3.2 S-PRG Filler
Surface-pre-reacted glass-ionomer (S-PRG) filler is a material that releases multiple ions [2, 6], and many products containing S-PRG filler (SHOFU Inc., Japan) are already on the market (Fig. 10.10). This filler is prepared via an acid–base reaction between fluoro-boro-aluminosilicate glass and a polyacrylic acid. The pre-reacted glass-ionomer phase on the surface of the glass core allows S-PRG filler to release and recharge F−. Moreover, S-PRG filler releases Al3+, BO33−, Na+, SiO32−, and Sr2+ ions (Fig. 10.11). Several studies have clearly demonstrated the antibacterial effects of resin composites containing S-PRG fillers against oral bacteria including Streptococcus mutans, and bacterial growth inhibition was obtained by release of BO33− and F− from the material. It was also reported that the eluate from the S-PRG filler suppressed the adherence of Streptococcus mutans, and thus biofilm formation on the surface of resin composites containing S-PRG fillers could be inhibited [6].
Recent investigations have revealed that Streptococcus mutans glucose metabolism and acid production were inhibited by low concentrations of BO33− or F− that did not affect bacterial growth [25]. In addition, the eluate from S-PRG fillers effectively inhibited Streptococcus mutans growth through downregulation of genes related to sugar metabolism, resulting in attenuation of the cariogenicity of Streptococcus mutans [26]. Indeed, a coating resin containing S-PRG fillers, which produces a coating layer with a thickness of approximately 200 μm, inhibited the reduction in pH induced by glucose consumption of Streptococcus mutans on the material surface [27].
Ion release from S-PRG fillers is effective in suppressing the activity of periodontal pathogens. The eluate of S-PRG fillers shows inhibitory effects on the protease and gelatinase activity of Porphyromonas gingivalis. It has been demonstrated using animal studies that the eluate from S-PRG fillers exhibited preventive effects against tissue destruction in periodontal disease [28]. It was also found that the coaggregation of P. gingivalis and Fusobacterium nucleatum could be prevented in the presence of S-PRG filler eluate [29], indicating that the release of ions may contribute to the prevention of periodontitis.
10.3.3.3 BioUnion Filler
BioUnion filler is a glass powder composed of SiO2, ZnO, CaO, and F, and can be categorized as a bio-functional multi-ion-releasing filler [6]. It has a silicon-based glass structure and is capable of releasing Zn2+, Ca2+, and F− (Fig. 10.12). Zn2+ is known to exhibit antibacterial effects against oral bacteria, and its MIC/MBC values against Streptococcus mutans are lower than those of fluoride. Liu et al. [30] reported unique characteristics of BioUnion filler; that is, release of Zn2+ was accelerated under acidic conditions. Such technology enables on-demand release of antimicrobial components from the materials. Once dental plaque is formed on the surface and acidogenic bacteria produce acids, a greater amount of Zn2+ is released and effectively attacks the cariogenic bacteria in the plaque (Fig. 10.13). While an inorganic cement for root surface restoration (Caredyne-Restore, GC Corporation, Japan) is on the market at present (Fig. 10.14), this technology is also of interest for use in resin-based materials.
10.3.4 Advantages and Disadvantages
Technologies for controlling release kinetics, such as the application of filler particles as drug reservoirs, are an effective way of enabling the sustained release of antibacterial components and achieving long-lasting antimicrobial effects. Particles capable of recharging the antibacterial component could be particularly practical for sustained release in clinical applications. In addition, the antibacterial effects of immobilized bactericide depend on direct contact with bacteria, while antimicrobial release systems are effective at inhibiting bacteria not only on the surface but also in some areas distant from the material. Therefore, the effectiveness in inhibiting biofilm formation for controlled antimicrobial release technology is essentially greater and reaches a much wider area.
The major disadvantage of the release of antimicrobials is the possibility of inducing microbial shift to disrupt homeostasis. Microbial shift in the oral cavity has been recognized as the help to cause infectious dental diseases. It is also important to assure biological safety regardless of the release of antimicrobials to attain clear, long-lasting antibacterial effects. This is not simply about toxicity to cells or organs, but also bacterial tolerance issues. It has been found that repeated exposure of Enterococcus faecalis and Streptococcus gordonii to chlorhexidine leads to resistance [31, 32], suggesting that sustained release of antimicrobials from materials may lead to drug resistance in oral bacteria.
10.4 Clinical Effectiveness
Despite the fact that many studies conducted in vitro or under clinically relevant experimental conditions have confirmed the benefits of both approaches—contact inhibition and controlled release—for providing antibacterial activity with dental resins, only a few clinical investigations are available so far.
For QAC-immobilized resins, the clinical effectiveness of MDPB-containing adhesive Clearfil Protect Bond (SE Protect) has been demonstrated by several studies. Uysal et al. [33] bonded orthodontic brackets to the premolars of 14 patients using Clearfil Protect Bond and examined demineralization of the enamel around the brackets after 30 days. They found that the hardness of the enamel around the brackets bonded with Clearfil Protect Bond was significantly greater than that around brackets bonded by other commercial orthodontic adhesives. The inhibitory effects of Clearfil Protect Bond against tooth demineralization in the case of composite restorations were also confirmed by in situ testing [34]. In this study, composite restoration was made in enamel blocks and kept in the human mouth for 14 days using an intraoral appliance. When Clearfil Protect Bond was used, no demineralization was found close to the restoration margin, while extensive demineralization was observed at the area away from the restoration. In a similar in situ study using a custom-made removable acrylic appliance, the effectiveness of experimental composites with incorporated QPEI nanoparticles in inhibiting biofilm formation was demonstrated [4].
Since there are many commercial products containing S-PRG filler on the market, their clinical effectiveness in terms of biofilm inhibition has been relatively well documented. For example, commercial composites containing S-PRG filler (Beautifil II; SHOFU Inc.) significantly inhibited plaque accumulation on their surface after intraoral exposure for 24 h compared with the control composites without S-PRG filler (Fig. 10.15) [35].
Although information obtained by in situ assessments reflects the significant effectiveness of antibacterial materials in clinical settings, such studies are labor and time consuming. In addition, it is not easy to collect a large amount of results. Ethical issues are also raised, particularly for testing experimental materials that release antimicrobial components. Therefore, an important future direction is to develop convenient in vitro evaluation systems that are specifically designed for antibacterial materials, with realistic simulations of the oral environment. More information on this topic is available in Chap. 4.
10.5 Future Perspectives
10.5.1 Further Improvement of the Immobilization Approach
10.5.1.1 Protein-Repellent Properties
Antibacterial component immobilized by polymerization of QAC monomers shows inhibition of bacteria and its effect can last for long periods as the immobilized component does not leach out. However, immobilized component exhibits antibacterial effects by contact inhibition; hence its effectiveness is reduced as a result of salivary protein coverage in the oral environment. One of the approaches for solving this problem is to introduce protein-repellent properties at the surface with immobilized bactericide. The application of molecules such as 2-methacryloyloxyethyl phosphorylcholine (MPC) polymer is promising. MPC polymer is hydrophilic and has an abundance of free water, but no bound water, when in the hydrated state. The large amount of free water around the phosphorylcholine groups is thought to detach proteins effectively, thereby repelling protein adsorption. The combination of MPC with QAC monomers has been reported to assist in impeding biofilm formation on dental materials [36, 37].
10.5.1.2 Grafting Approach
Resinous materials containing QAC monomer are able to exhibit bactericidal effects only when the density of immobilized QAC is high enough to disrupt bacterial cell structure. Grafting QAC monomers onto the surface has been shown to be effective for achieving restorative materials with strong effects [38]. Several techniques for grafting monomers on polymer surfaces are used in industry for surface modification and functionalization with polymers. However, most of these approaches require the addition of reactive functional groups or initiators to the grafted surfaces, and such complications make it difficult to apply the methods to dental materials for direct restoration. Simple methods for grafting QAC monomers on any type of resinous dental materials are therefore desirable.
10.5.2 Further Improvement of Controlled Release
Dental caries is a disease caused by acids from glucose metabolism of specific bacteria in the oral cavity. One of the aims of conferring antibacterial activities to restorative or preventive materials is to suppress caries-related bacteria and inhibit the pH decrease that occurs in dental plaque. The BioUnion fillers described above are capable of releasing Zn2+ when the environmental pH decreases. Similar responsive technology that produces antibacterial effects as a result of environmental (e.g., pH or microbiota) changes is of great interest. The future design of dental materials with agent release needs to involve “smart” behavior for the maintenance of the oral environment and to safeguard human health.
References
Imazato S, Torii M, Tsuchitani Y, et al. Incorporation of bacterial inhibitor into resin composite. J Dent Res. 1994;73:1437–43.
Imazato S, Ma S, Chen JH, et al. Therapeutic polymers for dental adhesives: loading resins with bio-active components. Dent Mater. 2014;30:97–104.
Jiao Y, Niu L-N, Ma S, et al. Quaternary ammonium-based biomedical materials: state-of-the-art, toxicological aspects and antimicrobial resistance. Prog Polym Sci. 2017;71:53–90.
Beyth N, Yudovin-Farber I, Perez-Davidi M, et al. Polyethyleneimine nanoparticles incorporated into resin composite cause cell death and trigger biofilm stress in vivo. Proc Natl Acad Sci. 2010;107:22038–43.
Zaltsman N, Ionescu AC, Weiss EI, et al. Surface-modified nanoparticles as anti-biofilm filler for dental polymers. PLoS One. 2017;12:1–18.
Imazato S, Kohno T, Tsuboi R, et al. Cutting-edge filler technologies to release bio-active components for restorative and preventive dentistry. Dent Mater J. 2020;39:69–79.
Imazato S, Kaneko T, Takahashi Y, et al. In vivo antibacterial effects of dentin primer incorporating MDPB. Oper Dent. 2004;29:369–75.
Brambilla E, Ionescu A, Fadini L, et al. Influence of MDPB-containing primer on Streptococcus mutans biofilm formation in simulated class I restorations. J Adhes Dent. 2013;15:431–8.
Imazato S, Ebi N, Takahashi Y, et al. Antibacterial activity of bactericide-immobilized filler for resin-based restoratives. Biomaterials. 2003;24:3605–9.
Kitagawa R, Kitagawa H, Izutani N, et al. Development of an antibacterial root canal filling system containing MDPB. J Dent Res. 2014;93:1277–82.
Hirose N, Kitagawa R, Kitagawa H, et al. Development of a cavity disinfectant containing antibacterial monomer MDPB. J Dent Res. 2016;95:1487–93.
Huang L, Sun X, Xiao YH, et al. Antibacterial effect of a resin incorporating a novel polymerizable quaternary ammonium salt MAE-DB against Streptococcus mutans. J Biomed Mater Res B Appl Biomater. 2012;100:1353–8.
Antonucci JM, Zeiger DN, Tang K, et al. Synthesis and characterization of dimethacrylates containing quaternary ammonium functionalities for dental applications. Dent Mater. 2012;28:219–28.
Li F, Weir MD, Xu HH. Effects of quaternary ammonium chain length on antibacterial bonding agents. J Dent Res. 2013;92:932–8.
Farah S, Aviv O, Laout N, et al. Quaternary ammoniumpolyethylenimine nanoparticles for treating bacterial contaminated water. Colloids Surf B. 2015;128:614–9.
Yudovin-Farber I, Beyth N, Nyska A, et al. Surface characterization and biocompatibility of restorative resin containing nanoparticles. Biomacromolecules. 2008;9:3044–50.
Shvero DK, Davidi MP, Weiss EI, et al. Antibacterial effect of polyethyleneimine nanoparticles incorporated in provisional cements against Streptococcus mutans. J Biomed Mater Res B Appl Biomater. 2010;94:367–71.
Shvero DK, Abramovitz I, Zaltsman N, et al. Towards antibacterial endodontic sealers using quaternary ammonium nanoparticles. Int Endod J. 2013;46:747–54.
Imazato S, Kitagawa H, Tsuboi R, et al. Non-biodegradable polymer particles for drug delivery: a new technology for “bio-active” restorative materials. Dent Mater J. 2017;36:524–32.
Kitagawa H, Takeda K, Kitagawa R, et al. Development of sustained antimicrobial-release systems using poly(2-hydroxyethyl methacrylate)/trimethylolpropane trimethacrylate hydrogels. Acta Biomater. 2014;10:4285–95.
Cheng Y-J, Zeiger DN, Howarter JA, et al. In situ formation of silver nanoparticles in photocrosslinking polymers. J Biomed Mater Res B Appl Biomater. 2011;97B:124–31.
Zhang K, Li F, Imazato S, et al. Dual antibacterial agents of nano-silver and 12-methacryloyloxydodecylpyridinium bromide in dental adhesive to inhibit caries. J Biomed Mater Res B Appl Biomater. 2013;101B:929–38.
Khvostenko D, Hilton TJ, Ferracane JL, et al. Bioactive glass fillers reduce bacterial penetration into marginal gaps for composite restorations. Dent Mater. 2016;32:73–81.
Davis HB, Gwinner F, Mitchell JC, et al. Ion release from, and fluoride recharge of a composite with a fluoride-containing bioactive glass. Dent Mater. 2014;30:1187–94.
Kitagawa H, Miki-Oka S, Mayanagi G, et al. Inhibitory effect of resin composite containing S-PRG filler on Streptococcus mutans glucose metabolism. J Dent. 2018;70:92–6.
Nomura R, Morita Y, Matayoshi S, et al. Inhibitory effect of surface pre-reacted glass-ionomer (S-PRG) eluate against adhesion and colonization by Streptococcus mutans. Sci Rep. 2018;8:5056.
Ma S, Imazato S, Chen J-H, et al. Effects of a coating resin containing S-PRG filler to prevent demineralization of root surfaces. Dent Mater J. 2012;31:909–15.
Iwamatsu-Kobayashi Y, Abe S, Fujieda Y, et al. Metal ions from S-PRG filler have the potential to prevent periodontal disease. Clin Exp Dent Res. 2017;3:126–33.
Yoneda M, Suzuki N, Masuo Y, et al. Effect of S-PRG eluate on biofilm formation and enzyme activity of oral bacteria. Int J Dent. 2012;2012:1–6.
Liu Y, Kohno T, Tsuboi R, et al. Acidity-induced release of zinc ion from BioUnion fillerTM and its inhibitory effects against Streptococcus mutans. Dent Mater J. 2020;39:547–53.
Kitagawa H, Izutani N, Kitagawa R, et al. Evolution of resistance to cationic biocides in Streptococcus mutans and Enterococcus faecalis. J Dent. 2016;47:18–22.
Wang S, Wang H, Ren B, et al. Drug resistance of oral bacteria to new antibacterial dental monomer dimethylaminohexadecyl methacrylate. Sci Rep. 2018;8:5509.
Uysal T, Amasyali M, Ozcan S, et al. Effect of antibacterial monomer-containing adhesive on enamel demineralization around orthodontic brackets: an in-vivo study. Am J Orthod Dentofac Orthop. 2011;139:650–6.
Pinto CF, Berger SB, Cavalli V, et al. In situ antimicrobial activity and inhibition of secondary caries of self-etching adhesives containing an antibacterial agent and/or fluoride. Am J Dent. 2015;28:167–73.
Saku S, Kotake H, Scougall-vilchis RJ, et al. Antibacterial activity of composite resin with glass-ionomer filler particles. Dent Mater J. 2010;29:193–8.
Zhang K, Zhang K, Xie X, et al. Nanostructured polymeric materials with protein-repellent and anti-caries properties for dental applications. Nanomaterials. 2018;8:E393.
Thongthai P, Kitagawa H, Kitagawa R, et al. Development of novel surface coating composed of MDPB and MPC with dual functionality of antibacterial activity and protein repellency. J Biomed Mater Res B Appl Biomater. 2020;108:3241–9.
Müller R, Eidt A, Hiller KA, et al. Influences of protein films on antibacterial or bacteria-repellent surface coatings in a model system using silicon wafers. Biomaterials. 2009;30:4921–9.
Acknowledgment
We thank Dr. Hockin H.K. Xu for providing Fig. 10.8.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Imazato, S., Kitagawa, H. (2021). Dental Resin-Based Materials with Antibacterial Properties: Contact Inhibition and Controlled Release. In: Ionescu, A.C., Hahnel, S. (eds) Oral Biofilms and Modern Dental Materials . Springer, Cham. https://doi.org/10.1007/978-3-030-67388-8_10
Download citation
DOI: https://doi.org/10.1007/978-3-030-67388-8_10
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-67387-1
Online ISBN: 978-3-030-67388-8
eBook Packages: MedicineMedicine (R0)