Abstract
Collectins, such as surfactant protein A (SP-A), surfactant protein D (SP-D) and mannose binding lectin (MBL), belong to evolutionarily conserved family of C-type lectins that are integral to innate immune system. These molecules can bind to a range of pathogens and endogenous danger-associated molecular patterns and bring about their clearance by several mechanisms such as phagocytosis via opsonization, aggregation, and complement activation (in the case of MBL). Collectins also interact with immune cell receptors, and thus, modulate immune cell properties in order to accomplish immune homeostasis. During pregnancy, collectins are expressed by gestational tissues as well as amniotic fluid indicating their relevance in feto-maternal cross-talk and pregnancy development. A number of studies have been undertaken to unravel their functional significance in implantation, placentation, pregnancy maintenance and parturition in normal and adverse pregnancies. Here, we discuss the expression profile, hormonal regulation and functional roles that have been attributed to SP-A, SP-D and MBL in pregnancy, parturition and pregnancy disorders such as spontaneous abortion, preterm birth, preeclampsia and gestational diabetes.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
Introduction
Implantation of embryo in the mother’s womb involves biological trade-offs, or even conflicts, between factors that improve the maternal health and those that support the fetal development and growth. This has led to evolutionary adaptations in mammals resulting in an effective cross-talk at the feto-maternal interface. A diverse range of hormonal and immunological factors of maternal and embryonic origin at the feto-maternal interface modify maternal immune system in order to protect the conceptus from attack by the mother’s immune system (Robinson and Klein 2012).
Pregnancy requires profound changes in the maternal immune system as the mother needs to harbor the semi-allogenic fetus. It has been suggested that a significant decline in the adaptive immune response during pregnancy enables the pregnant body to tolerate the fetal allograft. This may compromise maternal host-defense mechanisms making the mother more susceptible to infections (Mor and Cardenas 2010). During pregnancy, infections can gain access to gestational tissues and maternal endometrium via the maternal circulation, by ascending into the uterus from the lower reproductive tract, or by descending into the uterus from the peritoneal cavity (Espinoza et al. 2006). Intrauterine infections can significantly influence the pregnancy outcome. Bacterial and viral infections have been strongly associated with several pregnancy complications such as preterm labor (Goldenberg 2000; Lamont 2003; Espinoza et al. 2006), preeclampsia and intrauterine growth restriction (IUGR) (Arechavaleta-Velasco 2002; Hsu and Witter 1995; Adams Waldorf and McAdams 2013; von Dadelszen and Magee 2002; Mor and Cardenas 2010).
The innate immune system by means of its ability to distinguish ‘non-infectious self” (mother, placenta, and fetus) from ‘infectious non-self’ (bacteria, virus, etc.), represents the first line of immune defense against pathogens (Janeway and Medzhitov 2002). An evolutionary conserved system of pattern recognition is one of the ways by which innate immune system confers the host defense. Pattern recognition receptors (PRRs), an integral component of the innate immunity, can recognize and bind to highly conserved ‘pathogen-associated molecular patterns’ (PAMPs) and endogenous stress proteins or ‘damage-associated molecular patterns (DAMPs)’ (Takeuchi and Akira 2010). This interaction results in the activation of PRRs and generation of an inflammatory response. There are different PRRs, including the well-defined family of cell surface Toll-like receptors (TLRs), scavenger receptors and C-type lectins, humoral mannose binding lectin (MBL), surfactant proteins, and cytoplasmic nucleotide-binding oligomerization domain (NOD)-like receptors (NLRs).
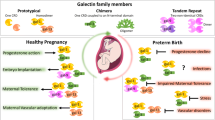
Collectins, calcium-dependent C-type lectins belonging to a family of evolutionarily conserved mammalian pattern recognition molecules, are integral to immunomodulation and host defense. Surfactant protein A (SP-A), surfactant protein D (SP-D) and mannose binding lectins (MBL) are the three well characterized members of the collectin family (Wright 2005; Stuart et al. 2005). Both SP-A and SP-D are secreted by the alveolar epithelium, non-ciliated bronchiolar cells and other mucosal surfaces exposed to the external environment (Wright 2005), whereas MBL is secreted by hepatocytes to serum (Howard et al. 2018). The primary structure of collectins mainly consists of four regions: (i) a cysteine-containing N-terminus (required for disulfide-dependent oligomerization of monomeric subunits); (ii) A triple-helical collagen region composed of repeating Gly-X-Y triplets (associated with maintaining the molecule’s shape, dimension, stability and oligomerization); (iii) α-helical coiled coil neck region (involved in protein trimerization); and (iv) a globular structure at the C-terminus comprising a C-type lectin or carbohydrate recognition domain (CRD) (which mediates calcium dependent ligand-binding to pathogens, carbohydrates and phospholipids etc.) (Kishore et al. 2006). SP-A and SP-D are large oligomeric structures. The monomeric subunits assemble into trimers at the neck region which further oligomerize to form large multimeric structures. Six such trimers oligomerize to form the octadecameric bouquet shaped structure of SP-A (650 kDa) and MBL (228–304 kDa), four trimers form a cruciform structure of 520 kDa for SP-D (Vieira et al. 2017; Holmskov et al. 2003; Turner 1996). SP-A and SP-D, as well as MBL, bind to a broad spectrum of pathogens including viruses, bacteria, fungi, allergens, apoptotic cells and enhance their uptake by innate immune cells such as macrophages, monocytes, neutrophils and dendritic cells (DCs). Clearance of pathogens by the collectins is achieved by multiple mechanisms, including opsonization and aggregation of the pathogens, in addition to regulating the cell-surface-receptor expression (Vieira et al. 2017; Holmskov and Jensenius 1993). MBL activates the lectin complement pathway, releases cytokines and coagulation factors during infection and tissue injury in co-operation with three MBL-associated serine proteases (MASPs 1, 2 and 3) (Matsushita and Fujita 1992). The evidences available so far clearly signify that these soluble PRRs not only recognize and eliminate the pathogens but they are at the junction of both innate and adaptive immunity. Collectins regulate inflammation, host-pathogen interactions, immune tolerance and thus, offer immune protection at multiple levels. Also, it is fascinating that the collectins bring about both pro-inflammatory and anti-inflammatory functions based on their interaction with candidate receptors, SIRP-α and calreticulin/CD91 (Gardai et al. 2003). Importantly, there are increasing evidences highlighting the role of these soluble proteins in novel functions related to maintenance of pregnancy (implantation, vascular remodeling, and placenta formation) and parturition (Fig. 1).
We discuss here the expression profile of collectins at the feto-maternal interface, hormonal regulation and functional relevance of these molecules at different stages of pregnancy. In addition, this chapter will reveal the role of collectins in various pregnancy complications and their potential to serve as early disease prediction markers in pregnancy.
Expression and Localization of Collectins at the Feto-Maternal Interface and in Gestational Tissues and Maternal Serum
The expression of SP-D at the feto-maternal interface was first reported by Leth-Larsen and colleagues. The authors demonstrated immunostaining for SP-D in the cytoplasm of villous and extravillous trophoblast, interstitial and vascular trophoblasts of both early and late gestational placenta (Leth-Larsen et al. 2004). Both SP-A and SP-D were shown to be present in the chorio-decidual layer of the late pregnant uterus (Miyamura et al. 1994), the human fetal membranes, amniotic epithelium and chorionic membrane (Han et al. 2007), trophoblast of the late normal placental villi, early human placenta (Sati et al. 2010). Cytotrophoblasts and mesenchymal cells have been shown to express MBL during early pregnancy (Kilpatrick et al. 1995; Yadav et al. 2016). Synthesis of SP-A, SP-D and MBL by the human first trimester and term placental and decidual tissues has also been demonstrated (Yadav et al. 2014; Yadav et al. 2016). Madhukaran et al. reported the expression of SP-A and SP-D in early human decidua specifically on stromal cells and extracellular trophoblast (EVT) (Madhukaran et al. 2016). Expression of collectins by the early and late gestational tissues suggests plausible role of these soluble PRRs in the cellular processes and other signaling pathways relevant for the placental development, immune tolerance and thereby establishment and maintenance of pregnancy.
Expression Profile of Collectins in the Amniotic Fluid
Amniotic fluid (AF), being a rich source of various nutritive factors and anti-microbial peptides, provides protection and support to the developing fetus (Underwood et al. 2005). In view of the presence of numerous innate immune factors, AF performs a significant role in the host defense (Underwood et al. 2005). Fetal lungs have been shown to contribute to the AF levels of surfactant proteins previously (Condon et al. 2004). SP-A levels were detected in the human amniotic fluid at mid-pregnancy (15 to 19th weeks of gestation) and its concentration increased significantly at term (Pryhuber et al. 1991; Chaiworapongsa et al. 2008). Human AF SP-A levels increased from <3 μg/mL at 30 weeks gestation to >24 μg/mL at the term (Snyder et al. 1988). Also, the amniotic fluid SP-A concentration in the women at term in labor was shown to be significantly lower than that in the women not in labor (1.2–10.1 μg/mL vs. 2.2–15.2 μg/mL) (Chaiworapongsa et al. 2008). In contrast to SP-A, SP-D levels were detected in human AF at 26 weeks of gestation and its concentration increased gradually, reaching up to 3 μg/mL at term (Miyamura et al. 1994). Similar to SP-A and SP-D, the AF levels of MBL increased with advancing gestational age (Malhotra et al. 1994). MBL levels before and after 35 weeks of gestation were 304 μg/mL and 1070 μg/mL, respectively (Malhotra et al. 1994). Levels of SP-A in the umbilical cord blood of newborn babies delivered by the spontaneous labor between 36 and 38 weeks (4.8–50.2 ng/mL) were two-fold higher compared to those delivered through cesarean section i.e., with no labor (2.7–21.7 ng/mL) (Cho et al. 1999). Increasing levels of collectins in AF with the advancing gestation implicate their potential to serve as potential biomarkers to evaluate fetal lung maturity.
Collectins in the Umbilical Cord
The concentrations of SP-D in the umbilical cord blood and capillary blood in premature infants were twice as high as in mature infants. This increase in SP-D levels has been attributed to the capillary leak and an increased release of SP-D from epithelial cells due to inflammatory response (Dahl et al. 2006). Low levels of MBL in the umbilical cord blood are associated with increased risk of respiratory infections whereas, the high levels are associated with increased risk of respiratory morbidity in infants of asthmatic patients (Schlapbach et al. 2009).
Levels of Collectins in the Maternal Serum
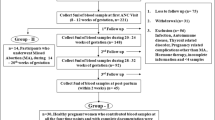
Although, there were adequate evidences to show AF levels of collectins, limited reports were available on maternal serum levels of the collectins in pregnancy. A modest increase in serum MBL levels was reported in the third trimester of normal pregnant women (Kilpatrick 2000). Serum levels of MBL increased in the pregnancy as early by 12th weeks of gestation and dropped significantly post-pregnancy (Van de Geijn et al. 2007). Recently, we determined the profile of collectins in the maternal serum during pregnancy and post-partu. We observed that the maternal serum levels of SP-A and SP-D declined significantly at 8–12 weeks of gestation and remained constant till 32 weeks of gestation compared to non-pregnant women. However, post-partum, a significant increase was observed in the SP-A levels, whereas SP-D levels increased gradually. On the contrary, maternal serum MBL levels increased significantly in pregnancy compared to post-partum (Kale et al. 2020a) (Fig. 2). The gestational age-dependent changes in the serum and AF levels of collectins suggest distinct roles of these proteins at different stages of pregnancy and parturition in addition to their role in the host-defense.
(a, b) Proposed functions of SP-A, SP-D and MBL in pregnancy. SP-A, SP-D and MBL expressed by feto-maternal interface and maternal serum have been associated with numerous functions in pregnancy. Among the functions sumarized in the figure the role of SP-A, SP-D and Fig. 1 (continued) MBL in parturition and modulation of immune response have been adequetly adressed while others xare proposed functions and need abound experimental investigation. Recently, in our study we reported significant inhibition of trophoblast migration/proliferation in the presence of recombinant human SP-D (Kale et al. 2020a). Aletred angiogenic [vascular endothelial growth factors, placental growth factors, matrix metalloproteinases, tissue inhiibitor of matrix metalloproteinases], hormone synthesizing genes [Aromatase, Catechol-o-methyltransferase] and inflammatory molecules [TNF-α, IL-10] at feto-maternal interface of SP-D gene deficient mice indicates importance of SP-D for regulation of immune and angiogenic response essential for immune tolerance and placental development (Data unpublished). MBL have been shown to inhibit C1q mediated trophoblast migration of extravillous trophoblast cells isolated from first trimseter placental tissue (Agostinis et al. 2012). Treatment of peripheral blood mononuclear cells (PBMCs) of severe early onset PE with recombinant human SP-D polarized the pro-inflammatory M1 monocytes to immunoregulatory M2 phenotype, decreased pro-inflammatory cytokine response and retained anti-inflmmatory response suggests their role in modulation of sysetemic immune response in pregnancy (Data unpublished)
Collectins in the Induction of Parturition (Term and Preterm Labor)
The cellular and molecular mechanisms that promote parturition at term are complex and multifactorial. Parturition is associated with an elevated local and systemic inflammatory response, induced by the infiltration of maternal and fetal tissues by neutrophils and macrophages (Thomson et al. 1999). This inflammatory signal activates pro-inflammatory transcription factors, nuclear factor-κB (NF-κB), which enhances the expression of contraction-associated protein (CAP), connexin-43 (CX43), the oxytocin receptor (OXTR), and cyclooxygenase 2 (COX-2) that transform the quiescent myometrium to a contractile state. Growing body of evidences suggests that the signal for inflammatory stimulus required for the initiation of parturition arises from fetus (Condon et al. 2004; Mitchell et al. 1984).
Role of SP-A and SP-D in Parturition
Condon et al. 2004 reported that the SP-A is detected at 17 dpc in murine AF and its levels increased markedly towards term. The AF SP-A further promoted the migration of fetal amniotic fluid macrophages in murine uterus and enhanced the production of pro-inflammatory cytokines (IL-1β), through stimulation of NF-κB and induced parturition. Intra-amniotic injection of SP-A at 15 dpc caused preterm parturition at 16–17 dpc, whereas the injection of SP-A antibody delayed parturition by 24 h, suggesting that the activation of fetal AF macrophages by SP-A and their subsequent migration into the maternal myometrium are key events for spontaneous parturition in mice (Condon et al. 2004). Consistent with the report by Condon et al., delayed parturition and lower levels of both pro-inflammatory and anti-inflammatory activation markers (IL-1β, IL-6, ARG1, YM1), CAP genes, connexin-43, and oxytocin receptor were observed at 18.5 dpc in the myometrium of TLR2, SP-A and SP-D gene deficient mice (Montalbano et al. 2013). Salminen et al. 2011 demonstrated that the administration of LPS to rSP-A mice (mice transgenically overexpressing rat SP-A) modified the inflammatory cytokine response and the expression of the pattern recognition receptors in fetal and gestational tissues. Furthermore, in rSP-A mice, the maternal LPS challenge increased TNF-α concentration and expression in the AF and fetal membranes, respectively. Similarly, mice overexpressing rat SP-D (rSP-D) showed significantly increased levels of TNF-α, IL-10 in the AF and fetal serum and the expression of IL-10 was noted in placenta after LPS challenge (Salminen et al. 2011; Salminen et al. 2012). These results suggested that both SP-A and SP-D in the fetal and gestational tissues contribute to inflammatory processes related to parturition and modulate the levels of intrauterine inflammatory mediators involved in the preterm birth.
While there are strong indications that SP-A and SP-D are involved in the parturition, Agrawal et al., suggested that SP-A suppressed the preterm labor and reduced inflammation in response to TLR2 ligands in mice (Agrawal et al. 2013). The discrepancy in these observations has been attributed to the different routes of administration of SP-A. Consistent with this finding, expression of SP-D transcripts was found to be reduced near parturition in mice and the presence of SP-A and SP-D significantly decreased TNF-α production by LPS challenged decidual macrophages (Madhukaran et al. 2016). The study suggested that the presence of SP-A and SP-D in decidua may be critical for protection of fetus against infection while the decreased SP-D transcripts near term at 19.5 dpc could be an indirect way to upregulate PGE2, cyclooxygenase enzyme-2 (COX2) and increased myometrial sensitivity to estrogen in association with parturition (Madhukaran et al. 2016).
The concentration of AF SP-A decreased during the spontaneous labor in humans; no fetal macrophages were found in the myometrium after labor (Chaiworapongsa et al. 2008; Lee et al. 2010; Kim et al. 2006). No significant difference was observed by Han et al. in the expression level of SP-A1 transcripts in chorioamniotic membranes between term with labor and term without labor (Han et al. 2007), suggesting that the mechanisms of labor in mice and humans are different. In contrast to these reports, Lee et al. 2010 investigated a potential role for SP-A in the human pregnancy and parturition by examining SP-A expression patterns in the AF and amnion. The results showed that the high molecular weight oligomeric SP-A was increased in AF with advancing gestation. Interestingly, these oligomers were more abundant in the placental amnion (amnion overlying the placental disc) before labor at term, while they increased primarily in reflected amnion during the labor (amnion of the extraplacental chorioamniotic membranes). SP-A significantly and selectively inhibited Prostaglandin F2α (PGF2α) production without affecting the production of other inflammatory mediators and angiogenic factors (including IL-6, IL-8, TNF-α, MMP-3, MCP-1, IL-1β, PGE2, sFlt-1 and VEGF) at the high dose of 100 μg/mL, suggesting its key role in the decidual activation and onset of labor (Snegovskikh et al. 2011).
Human myometrial cells expressed SP-A and the treatment of SP-A triggered cellular signaling events leading to protein kinase C zeta (PRKCZ), MAPK1/3, and RELA (NF-κB p65 subunit) activation, PTGS2 (cyclooxygenase 2) induction, and cytoskeleton reorganization in the cultured human myometrial cells (Verdugo et al. 2008). These signaling pathways are vital for the myometrium to develop contractile activity at the end of pregnancy (Verdugo et al. 2008). In line with this report, Sotiriadis et al. showed that the treatment of human myometrial cell line (ULTR) with recombinant SP-A and SP-D induced contractility-associated protein (CAP) genes and pro-inflammatory cytokines, thus, shifting the uterus from a quiescent to a contractile state (Sotiriadis et al. 2015). We reported a significant increase in the transcripts and protein of SP-A and SP-D in the term human placenta of women undergoing spontaneous labor (Yadav et al. 2014). Further, treatment of placental tissue explants with native and recombinant human SP-D showed a significant increase in the labor associated cytokines, IL-1α, IL-1β, IL-6, IL-8, IL-10, TNF-α and MCP-1, implicating that SP-D plausibly contributes to the pro-inflammatory immune milieu of feto-maternal tissues at term (Yadav et al. 2014). Increased maternal serum SP-D and SP-A levels were observed at the term and after 2 weeks post-partum in one of our recent study (Kale et al. 2020a), thus, supporting the relevance for SP-A and SP-D in the pathways regulating parturition.
Genetics plays an important role in the risk of preterm birth. In addition, incidence of preterm deliveries is higher among mothers with a history of previous such deliveries. Common polymorphism Met31Thr of the gene encoding SP-D influencing the concentration, oligomerization and binding properties of SP-D was associated with the spontaneous preterm birth (SPTB) in preterm infants of families with recurrent SPTBs in northern Finnish population (Karjalainen et al. 2012). Future investigations into association of polymorphisms in the SP-A and SP-D genes and the maternal serum levels of these proteins during parturition may be useful to predict the preterm birth.
Involvement of MBL in Parturition
Although the role of MBL in parturition is still far from clear, a significant increase in maternal serum MBL levels during pregnancy and its sharp decrease after parturition (Van de Geijn et al. 2007) implicates its plausible association with the parturition and onset of labor. In a recent study, low MBL levels have been associated with the preterm birth and low birth weight (Koucký et al. 2018). However, Wang et al. 2017 reported no significant difference in the second trimester MBL levels of Taiwanese mothers with preterm and term deliveries (Wang et al. 2017). Besides preterm birth, low MBL concentrations have been considered a significant risk factor for spontaneous abortion, idiopathic recurrent late pregnancy loss, unexplained recurrent miscarriages, chorioamnionitis, and preeclampsia (Christiansen et al. 1999, 2002, 2009; Kruse et al. 2002; Van de Geijn et al. 2007; Holmberg et al. 2008). MBL levels of ≤0.1 μg/mL are considered a clinically-significant risk factor for spontaneous abortion (Kilpatrick et al. 1999). In a recent study, Canda et al., reported decreased MBL immunoreactivity in the decidua and villous trophoblast of first time pathologic first trimester human miscarriage, suggesting decreased lectin pathway activity (Canda et al. 2018).
Serum/plasma levels of MBL are genetically determined. Bodamer et al. determined the five common MBL2 polymorphisms (codon 52, 54, 57; promoter −550, −221) in DNA samples isolated from blood of infants born prematurely and normally (Bodamer et al. 2006). The results of the study indicated that the frequency of the codon 52 polymorphism was significantly higher in the pre-term group (10.8%) compared to the term group (4.9%). Furthermore, the authors reported that carriers of genotypes (O/O) likely conferring deficient MBL plasma levels were more common in the group of premature birth (9.8% vs. 2.9%), while the promoter −550 C/C genotype was underrepresented in the pre-term birth group (24.5% vs. 39.2%). The study suggested that the fetal MBL2 genotype might be an additional genetic factor contributing to the risk of premature delivery (Bodamer et al. 2006). Hartz et al. reported that the infants with deficiency of MBL2 born between 32 and 36 weeks of gestation are at a higher risk of infection (Hartz et al. 2017). In contrast, no significant association was reported between MBL 2 promoter variants (−550 C > G and −221 G > C) and structural MBL variants with missense mutation (R52C, G54D, G57E) in exon 1 contributing to the low levels of MBL with unexplained recurrent pregnancy loss or miscarriage (Baxter et al. 2001; Berger et al. 2013). Single nucleotide polymorphisms (SNPs) in the promoter region of the MBL2 gene (H/L or X/Y) have been shown to modify basal levels of MBL in serum (Madsen et al. 1998). The individuals with high MBL genotype ((H/L) YA/ (H/L) YA and (H/L) YA/LXA) are associated with high MBL serum levels, the intermediate MBL genotype, (LXA/LXA and (H/L) YA/O); and the individuals with low MBL genotype (LXA/O and O/O) are associated with the intermediate and lowest MBL serum levels respectively (Frakking et al. 2006). Van de Geijn et al. determined the association of MBL genotype in 157 nulliparous women with parturition and gestational age. The authors reported that the maternal high MBL production genotype is associated with the premature birth (before 29 weeks of gestation) (~13%) compared to those with the intermediate and low MBL serum genotype groups (3%). The study postulated that during pregnancy the MBL-associated inflammation caused by higher MBL activity may contribute to an early delivery (Van de Geijn et al. 2008).
Hormonal Regulation of Collectins in Female Reproductive Tract and Pregnancy
During menstrual cycle, maternal endometrium undergoes cyclic changes in morphology and function under the influence of two primary steroid hormones, estradiol and progesterone. These changes are crucial for uterine receptivity, implantation and sustenance of the pregnancy (Su and Fazleabas, 2015). On the other hand, results of a number of clinical studies have suggested that the sex hormones influence the expression of innate immune proteins in the reproductive tract making it susceptible to infections by many pathogens (Sonnex 1998).
Transcript and protein expression of both SP-A and SP-D have been reported in the female vagina, uterus, ovary, cervix and oviduct (Akiyama et al. 2002; Oberley et al. 2004; Leth-Larsen et al. 2004). SP-A is reported to be expressed in the vaginal stratified squamous epithelium and cervico-vaginal lavage of both pre-and post-menopausal women (MacNeill et al. 2004). Similarly, MBL was shown to be expressed in endometrium and basal layer of the vaginal epithelium and cervico-vaginal lavage (Bulla et al. 2010). Importantly, expression of collectins in the female reproductive tract (FRT) has been shown to be regulated by ovarian steroid hormones.
Variations in the Expression of Collectins During Menstrual Cycle
Expression of SP-D in the epithelium of the endometrial glands is increased towards the secretory phase of regular menstrual cycle compared to the proliferative phase (Leth-Larsen et al. 2004). Expression of SP-D in the uterus of ovariectomized mice is positively regulated by estradiol, and negatively regulated by progesterone; expression of SP-D is increased nine-fold after administration of both estradiol and progesterone (Kay et al. 2015). In addition, in the cycling mouse uterus, SP-D has been shown to be hormonally regulated, with peak levels present in the estrogen dominated estrus phase, which then decreased in progesterone dominated diestrus phase (Kay et al. 2015). Indeed, Oberley et al. showed that administration of progesterone to mice in the diestrus phase makes them more susceptible to chlamydial infection, whereas it is difficult to infect the female reproductive tract with Chlamydia during the estrous stage of the reproductive cycle when estradiol levels are high (Oberley et al. 2007, Ito et al. 1984) suggestive of involvement of SP-D in innate defense against chlamydia. Using immunohistochemistry, SP-A expression within the glandular and stromal cells has been observed in the maternal endometrium during the early proliferative phase (day 6–7) of the menstrual cycle. The levels of MBL in the vaginal epithelial cells vary in a cycle-dependent manner with greater changes occurring in the secretory phase of the menstrual cycle. This change, which is associated with progesterone, implicates the protective role of MBL in the female genital tract (Bulla et al. 2010). These findings strongly suggest that cyclic variations in SP-A, SP-D and MBL expression are hormonally regulated and affect innate host defense against pathogens in the FRT.
Regulation of Collectin Expression During Pregnancy
Until now, there were no adequate evidences to show hormonal regulation of collectin expression during pregnancy. Kay et al. reported a decreased expression of uterine SP-D at early stages of pregnancy when progesterone levels were much higher and estradiol was at basal level (Kay et al. 2015). Recently, we reported that the systemic levels of SP-A and SP-D are decreased and consistently maintained till 32 weeks of gestation in normal pregnancy. Importantly, our study indicated that the ratio of progesterone to estradiol rather than hormones alone, influences the systemic levels of collectins during pregnancy (Kale et al. 2020a). The relevance of decreased SP-D levels in pregnancy was further substantiated with an in vitro assay showing significant inhibition in HTR-8/SVneo (first trimester trophoblastic cell line) cell migration and proliferation with the treatment of a recombinant fragment of human SP-D (rfhSP-D) composed of trimeric CRDs.
Collectins in Implantation and Spontaneous Abortion
Implantation, which is essential for pregnancy initiation, involves opposition and adherence of the blastocyst to uterine luminal epithelium, followed by migration and invasion of trophoblastic cells through maternal spiral arteries present into the endometrial decidua (Kim and Kim 2017). Both the arms of the immune system under the regulation of ovarian hormones are modulated to regulate this process and substantiate a protective environment in maternal decidua to prevent fetal demise. However, any impairment may lead to implantation failure which can cause infertility, abortions, placental insufficiency and other complication related to pregnancy (Kim and Kim 2017).
SP-D in Spontaneous Abortions in Humans and Mice
Spontaneous abortion (SA) is the most common complication in the first trimester affecting 15% pregnancy (Cohain et al. 2017). Dysregulated immunoregulatory mechanisms at the feto-maternal interface and in the maternal circulation have been associated with SA previously (Ticconi et al. 2019; Calleja-Agius et al. 2012). As mentioned above, collectins are expressed by the luminal and glandular epithelium as well as stromal cells of the maternal endometrium (Kay et al. 2015). We reported several novel findings using pregnant SP-D−/− knock-out female mice crossed with SP-D+/+ males, such as (i) fertility defects with smaller litter size and increased pre-implantation embryo loss; (ii) extended estrous cycles with alterations in ovarian hormone profile and uterine expression of hormone responsive genes; and (iii) skewed uterine immune milieu towards inflammation (Kay and Madan 2016). Consistent with our observation, reduced liter size was reported earlier also in SP-A and SP-D double knockout mice (Montalbano et al. 2013).
In another study, we analyzed the expression of collectins in the inflamed human gestational tissues of SA and in 13.5 dpc placental tissues from resorption survived embryos of murine model (CBA/J X DBA/2J) of SA. We reported a significant downregulation in SP-A transcripts and upregulation in the SP-D transcripts in placental and decidual tissues of SA group compared to healthy pregnant women. Significant upregulation was observed in SP-A and SP-D transcripts at 13.5 and 14.5 dpc in the placental tissues of viable embryos from CBA/J X DBA/2J mated females wherein the inflammation was regulated compared to control (CBA/J X Balb/c) (Yadav et al. 2016). Recently, we reported a significant downregulation in the SP-D levels and P4 (Progesterone)/E2(Estradiol) ratio at 8–12 weeks of gestation in the women with a subsequent missed abortion (MA)/asymptomatic SA compared to normal healthy pregnant women (Kale et al. 2020a), suggesting their potential to act as early screening biomarkers for identification and preventative management of MA.
MBL and Pregnancy Loss
Higher expression of MBL has been reported in the uterine lumen of patients with unexplained infertility (Bulla et al. 2009). In addition, expression of MBL-A was detected at implantation site as early as 3.5 d of pregnancy in abortion-prone mice mating, whereas its deficiency prevented the pregnancy loss (Petitbarat et al. 2015). Similarly, we reported increased transcripts of MBL in placental tissues at 14.5 dpc in viable embryos of abortion-prone mice. These reports suggest that activation of the lectin pathway is accompanied by implantation failure both in human and mice. In contrast to this, genetic polymorphisms contributing to low maternal serum MBL levels have been associated with recurrent pregnancy losses and poor pregnancy outcome (Cieslinski et al. 2017; Kruse et al. 2002; Christiansen et al. 2009). In a recent study, Canda et al. reported decreased lectin pathway activity in tissues from first-time pathologic human miscarriage (Canda et al. 2018).
Together, these evidences suggest that collectins may perform a balancing act to regulate both local and systemic immune response during pregnancy. Optimal levels of these proteins are essential for a healthy pregnancy outcome whereas any dysregulation may lead to an unfavorable pregnancy outcome.
Collectin profile in maternal serum. The figure is graphical representation of the SP-A, SP-D and MBL profile in maternal sera collected progressively from pregnant women (n = 30) in the first trim: (8–12 weeks), second trim: (20–24 weeks), third trim: (28–32 weeks), PP: Post-partum (within 2 weeks of Parturition) and NP: Non-pregnant women (Mid-Luteal Phase). During normal pregnancy SP-A and SP-D levels are downregulated in the first trimester compared to non-pregnant women and maintained consistently till 32 weeks of gestation and increased post-partum. Both, SP-A, and SP-D have been associated with anti-tumor function (Mitsuhashi et al. 2013; Mahajan et al. 2013). Similarity of the immune micro-environment at feto-maternal interface and tumor progression (Holtan et al. 2009), also significant inhibition of trophoblast cell migration/proliferation in presence of the recombinant human SP-D (Kale et al., 2020b) suggests relevance of decreased SP-A, SP-D levels for placental development. Increased levels of SP-A and SP-D post-partum implicates their important role in parturition induction. In contrast to SP-A and SP-D, levels of MBL show gradual decrease in the first trimester when compared to non-pregnant women and increase compared to post-partum. Consistently maintained collectins in pregnancy is essential to ensure balance between immune tolerance and host-defense function as their abnormal levels have been associated to pregnancy disorders
Preeclampsia and Gestational Hypertension: Role of Collectins in the Placental Development
Hypertensive disorders of pregnancies—preeclampsia (PE) and gestational hypertension, contribute inordinately to maternal and fetal morbidity and mortality. PE is characterized by hypertension, proteinuria, edema, hepatic and renal dysfunction after 20 weeks of pregnancy (Duhig et al. 2018). The disease has worldwide incidence of about 2–8% (Steegers et al. 2010). The WHO predicts the incidence of PE to be seven times higher in developing countries than developed (2.8% vs. 0.4% of live births) (WHO 2005). Although the precise etiology of the disease is still unknown, PE is considered as a disease with the placental origin. Impaired angiogenesis, increased oxidative stress, hormonal and immunological alterations in the early placental microenvironment may contribute to the placental defects in PE (Escudero et al. 2014; Hertig et al. 2010; Cornelius 2018). Intriguingly, most of these functions dysregulated in PE are governed by the collectins. While there are adequate reports showing association of SP-A, SP-D and MBL with SA and preterm birth, role of SP-A and SP-D in PE has been largely unexplored until recently whereas reports on MBL are scarce.
Involvement of SP-A and SP-D in Preeclampsia: Human and Murine Studies
Recently, we determined the systemic and placental profile of collectins before and after the disease onset and association of collectins with steroid hormones in pregnancy. It was observed that collectins are differentially expressed in the serum and placenta of PE women. SP-A and SP-D decreased significantly prior to onset (i.e. at 10–20 weeks) in women with severe early onset PE (EOPE), whereas both collectins increased significantly after the onset (i.e. at 28–32 weeks) in sera and term human placental tissue of these women. Moreover, the shift in SP-A and SP-D during the disease progression was found to be regulated by P4/E2 ratio. This transposition in SP-A, SP-D and P4/E2 levels in severe EOPE appears fascinating; however, the exact transition point is still not known. Weekly collection of serum samples between 20 and 32 week of gestation may facilitate determination of the transition point of SP-A and SP-D in severe EOPE. Most importantly, decreased SP-A, SP-D and P4/E2 ratio at 10–20 week of gestation were found to be potential risk factors for severe EOPE (Kale et al., 2020b). In corroboration, screening of SP-D gene deficient pregnant mice showed a significant defect in the placental development and elevation in mean arterial pressure. Additionally, we also observed significant decrease in the transcripts of several angiogenic markers, such as vascular endothelial growth factor (VEGF), placental growth factor (PLGF), Matrix metalloproteinases 2 (MMP2), catechol-o-methyltransferase (COMT) and increase in tissue inhibitor of matrix metalloproteinases 2 (TIMP2) at 15.5 dpc in the placenta of SP-D gene deficient mice (Data unpublished). With an in vitro study using a recombinant fragment of human SP-D (rfhSP-D), we provided a proof of concept that elevated levels of SP-D after the onset in severe EOPE women could be essential for polarization of monocytes to immunomodulatory M2 phenotype and restore cytokine balance in peripheral blood mononuclear cells of severe EOPE women (Data unpublished). Observations from our studies provided an important insight to the plausible roles of SP-A and SP-D in the placental development and regulation of immune response in PE for the first time. Figure 3 shows a hypothetical model depicting plausible roles of SP-D in pregnancy and PE.
Hypothetical model of role of SP-D in pregnancy and severe EOPE. During normal pregnancy SP-D levels are decreased in the first trimester and consistently maintained similar to that of P4/E2 ratio. As SP-D is potent anti-tumor molecule and shown to inhibit trophoblast migration/proliferation (Kale et al. 2020b), decreased SP-D may promote trophoblast migration/invasion during normal pregnancy. Decreased P4/E2 ratio below optimal prior to onset in severe early onset PE (EOPE) and/or missed abortion (MA) might affect trophoblast invasion directly or indirectly by decreasing the levels of SP-D. Further, loss of SP-D or levels of SP-D below optimal though would favor placental development but would also affect clearance of trophoblastic debris, a process critical to regulation of immune response in pregnancy. Impaired clearance of trophoblastic debris may lead to immune dysregulation which eventually affect placental development. Defective placental development might lead to elevated P4/E2 ratio, exaggerated inflammatory response and thereby, elevated SP-D after the onset to polarize monocyte to M2 phenotype and restore immune homeostasis
MBL Serum Levels in Pregnant Mothers
Significantly increased maternal serum MBL levels have been observed in the third trimester of women with PE (Kale et al. 2020b; Than et al. 2008; Celik and Ozan 2008; Agostinis et al. 2012). Furthermore, the increased MBL levels in PE patient sera have been shown to strongly inhibit the interaction of extravillous trophoblast (EVT) with C1q interfering with the process of EVT adhesion to and migration through decidual endothelial cells (DECs), suggesting that the increased level of MBL in PE may inhibit endovascular invasion of trophoblast cells (Agostinis et al. 2012). These observations warrant future studies defining the roles of collectins in placental development and associated hypertensive pregnancy disorders, such as gestational hypertension, eclampsia and HELLP (hemolysis, elevated liver enzymes, and a low platelet count) syndrome.
Gestational Diabetes: Metabolic Roles of Collectins?
Gestational diabetes mellitus (GDM), defined by glucose intolerance, is the most common metabolic disorder during pregnancy. GDM affects 3–17% of all pregnancies. Although the precise mechanisms underlying GDM are still elusive, a close immune–metabolic relationship have been documented (Wojcik et al. 2016). Zhao et al. performed genomic expression profiling of blood and placenta of GDM positive women of the Chinese ethnicity. The authors reported GDM-dependent alterations in the expression of numerous immune-related genes both in blood and placenta, supporting the notion of a link between immune system and GDM (Zhao et al. 2011).
Wojcik et al. reported increased SP-D mRNA levels in the blood leukocytes of hyperglycemic GDM patients (Wojcik et al. 2016). GDM is also associated with an increased risk of neonatal respiratory distress syndrome (RDS). Transcripts of SP-A mRNA were decreased in the fetal lungs of rat model of diabetic pregnancy, while insulin treatment led to a substantial increase in the SP-A mRNA levels in fetal lungs (Moglia and Phelps 1996). The study provided an important evidence that glucose metabolism dysfunction and/or inflammation during pregnancy can lead to alterations in the expression of surfactant proteins.
Several genetic factors have also been implicated in the pathogenesis of GDM. Megia et al. reported that women carrying low plasma levels of MBL and G54D mutant allele for MBL2 have a higher risk for developing GDM and having heavier infants. Thus, G54D mutation may lead to sustained release of inflammatory cytokines (TNF-α), known to down-regulate the insulin sensitivity and the development of GDM (Megia et al. 2004). Müller et al. suggested that the presence of MBL2 variants, leading to increased inflammatory response, may cause inflammatory damage to the pancreatic β-cell function, thereby affecting insulin secretion rather than insulin action (Müller et al. 2010).
Conclusions
The microenvironment at the feto-maternal interface creates a tolerogenic niche to facilitate the development of the semi-allogeneic fetus; perturbations in the feto-maternal immune cross-talk may give rise to various pregnancy complications. The presence of collectins in both early and late placental tissues can alter mother’s immune response to the allogenic fetus, and thus, is relevant in mediating feto-maternal interactions. Consistently maintained AF and maternal serum levels of collectins during the pregnancy, and their altered levels in the early first trimester and at term and/or at post-partum, signifies gestational stage-dependent role of these proteins in pregnancy. Inhibition of trophoblast cell migration and proliferation in the presence of rfhSP-D signifies the relevance of decreased maternal serum levels of SP-D during the first trimester in the establishment of immune tolerance and placental development. Most importantly, altered serum levels of SP-A, SP-D and P4/E2 ratio at early stages of pregnancy in women with subsequent MA and severe EOPE in our studies (Kale et al. 2020a, b) highlights their potential to act as predictive biomarkers. We think that the maternal serum levels of SP-A, SP-D and P4/E2 ratio along with current prediction model such as combination of serum biomarkers [PLGF, sFLT1] and maternal risk factors [e.g. Mean Arterial Pressure (MAP), and uterine-artery Doppler Pulsatility Index (PI)] may improve the prediction rate for women at risk of EOPE (O’Gorman et al. 2017). This would facilitate counseling of such high-risk pregnant women during early stages of pregnancy and also to administer potential preventive interventions such as low dose aspirin (Rolnik et al. 2017). However, future studies involving large multi-centric cohorts are needed to validate these speculations. As parental genotype may determine the circulatory levels of immune proteins in pregnancy, collectin polymorphisms along with their maternal serum levels need further investigations. In addition, studies with in vitro and in vivo model systems would provide better insight into the role of collectins in immune tolerance, implantation, angiogenesis, trophoblast invasion, tissue remodeling and removal of apoptotic cells, processes that critical for placental development.
References
Adams Waldorf KM, McAdams RM. Influence of infection during pregnancy on fetal development. Reproduction. 2013;146(5):R151–62.
Agostinis C, Bossi F, Masat E, et al. MBL interferes with endovascular trophoblast invasion in pre-eclampsia. Clin Dev Immunol. 2012;2012:484321.
Agrawal V, Smart K, Jilling T, Hirsch E. Surfactant protein (SP)-A suppresses preterm delivery and inflammation via TLR2. PLoS One. 2013;8(5):e63990.
Akiyama J, Hoffman A, Brown C, et al. Tissue distribution of surfactant proteins A and D in the mouse. J Histochem Cytochem. 2002;50(7):993–6.
Arechavaleta-Velasco F, Koi H, Strauss JF, et al. Viral infection of the trophoblast: time to take a serious look at its role in abnormal implantation and placentation? J Reprod Immunol. 2002; 55(1-2):113-21.
Baxter N, Sumiya M, Cheng S, Erlich H, Regan L, Simons A, Summerfield JA. Recurrent miscarriage and variant alleles of mannose binding lectin, tumour necrosis factor and lymphotoxin alpha genes. Clin Exp Immunol. 2001;126(3):529–34.
Berger DS, Merhi Z, Hogge WA, Ferrell RE. Mannose binding lectin genotypes are not associated with increased risk of unexplained recurrent pregnancy loss. J Assist Reprod Genet. 2013;30(5):723–7.
Bodamer OA, Mitterer G, Maurer W, et al. Evidence for an association between mannose-binding lectin 2 (MBL2) gene polymorphisms and pre-term birth. Genet Med. 2006;8(8):518–24.
Bulla R, Bossi F, Agostinis C, et al. Complement production by trophoblast cells at the feto-maternal interface. J Reprod Immunol. 2009;82:119–12510.
Bulla R, De Seta F, Radillo O, et al. Mannose-binding lectin is produced by vaginal epithelial cells and its level in the vaginal fluid is influenced by progesterone. Mol Immunol. 2010;48(1–3):281–6.
Calleja-Agius J, Jauniaux E, Muttukrishna S. Inflammatory cytokines in maternal circulation and placenta of chromosomally abnormal first trimester miscarriages. Clin Dev Immunol. 2012;2012:175041.
Canda MT, Caglayan LD, Demir N, Ortaç R. Increased C4d and Bb immunoreactivity and decreased MBL immunoreactivity characterise first-time pathologic first-trimester miscarriage: a case-control study. J Obstet Gynaecol. 2018;38(1):90–5.
Celik N, Ozan H. Maternal serum mannose-binding lectin in severe preeclampsia. Clin Exp Obstet Gynecol. 2008;35:179–82.
Chaiworapongsa T, Hong JS, Hull WM, et al. The concentration of surfactant protein-A in amniotic fluid decreases in spontaneous human parturition at term. J Maternal-Fetal Neonatal Med. 2008;21(9):652–9.
Cho K, Matsuda T, Okajima S, et al. Factors influencing pulmonary surfactant protein A levels in cord blood, maternal blood and amniotic fluid. Biol Neonate. 1999;75(2):104–10.
Christiansen OB, Kilpatrick DC, Souter V, et al. Mannan-binding lectin deficiency is associated with unexplained recurrent miscarriage. Scand J Immunol. 1999;49:193–6.
Christiansen OB, Nielsen HS, Lund M, Steffensen R, Varming K. Mannose-binding lectin-2 genotypes and recurrent late pregnancy losses. Hum Reprod. 2009;24(2):291–9.
Christiansen OB, Pedersen B, Rosgaard A, Husth M. A randomized, double-blind, placebo-control-led trial of intravenous immunoglobulin in the prevention of recurrent miscarriage: evidence for a therapeutic effect in women with secondary recurrent miscarriage. Hum Reprod. 2002;17(3):809–16.
Cieslinski JZ, Goeldner I, Skare TL, et al. Mannose-binding lectin deficiency and miscarriages in rheumatoid arthritis. Autoimmunity. 2017;50(7):409–13.
Cohain JS, Buxbaum RE, Mankuta D. Spontaneous first trimester miscarriage rates per woman among parous women with 1 or more pregnancies of 24 weeks or more. BMC Pregnancy Childbirth. 2017;17:437.
Condon JC, Jeyasuria P, Faust JM, Mendelson CR. Surfactant protein secreted by the maturing mouse fetal lung acts as a hormone that signals the initiation of parturition. Proc Natl Acad Sci U S A. 2004;101(14):4978–83.
Cornelius DC. Preeclamspsia: from inflammation to immunoregulation. Clin Med Insights Blood Disord. 2018;11:1179545X17752325.
Dahl M, Holmskov U, Husby S, Juvonen PO. Surfactant protein D levels in umbilical cord blood and capillary blood of premature infants. The influence of perinatal factors. Pediatr Res. 2006;59(6):806–10.
Duhig K, Vandermolen B, Shennan A. Recent advances in the diagnosis and management of pre-eclampsia. F1000Res. 2018;7:242.
Escudero C, Roberts JM, Myatt L, Feoktistov I. Impaired adenosine-mediated angiogenesis in preeclampsia: potential implications for fetal programming. Front Pharmacol. 2014;5:134.
Espinoza J, Erez O, Romero R. Preconceptional antibiotic treatment to prevent preterm birth in women with a previous preterm delivery. Am J Obstet Gynecol. 2006;194(3):630–7.
Frakking FNJ, Brouwer N, Zweers D, Merkus MP, Kuijpers TW, Offringa M, Dolman KM. High prevalence of mannose-binding lectin (MBL) deficiency in premature neonates. Clin Exp Immunol. 2006;145(1):5–12.
Gardai SJ, Xiao YQ, Dickinson M, et al. By binding SIRP or calreticulin/CD91, lung collectins act as dual function surveillance molecules to suppress or enhance inflammation. Cell. 2003;115(1):13–23.
Goldenberg RL, Hauth JC, Andrews WW. Intrauterine infection and preterm delivery. N Engl J Med. 2000;342(20):1500–7.
Han YM, Romero R, Kim YM, et al. Surfactant protein-A mRNA expression by human fetal membranes is increased in histological chorioamnionitis but not in spontaneous labour at term. J Pathol. 2007;211(4):489–96.
Hartz A, Pagel J, Humberg A, et al. German Neonatal Network (GNN). The association of mannose-binding lectin 2 polymorphisms with outcome in very low birth weight infants. PLoS One. 2017;12(5):e0178032.
Hertig A, Liere P, Buffet NC, et al. Steroid profiling in preeclamptic women: evidence for aromatase deficiency. Am J Obstet Gynecol. 2010;203(5):477.e1–9.
Holmberg V, Schuster F, Dietz E, Visconti JCS, Anemana SD, Bienzle U, Mockenhaupt FP. Mannose-binding lectin variant associated with severe malaria in young African children. Microbes Infect. 2008;10(4):342–8.
Holmskov U, Jensenius JC. Structure and function of collectins: humoral C-type lectins with collagenous regions. Behring Inst Mitt. 1993;93:224–35.
Holmskov U, Thiel S, Jensenius JC. Collections and ficolins: humoral lectins of the innate immune defense. Annu Rev Immunol. 2003;21:547–78.
Holtan SG, Creedon DJ, Haluska P, Markovic SN. Cancer and pregnancy: parallels in growth, invasion, and immune modulation and implications for cancer therapeutic agents. Mayo Clin Proc. 2009;84(11):985–1000.
Howard M, Farrar CA, Sacks SH. Structural and functional diversity of collectins and ficolins and their relationship to disease. Semin Immunopathol. 2018;40(1):75–85.
Hsu CD, Witter FR. Urogenital infection in preeclampsia. Int J Gynaecol Obstet. 1995;49(3):271–5.
Ito JI Jr, Harrison HR, Alexander ER, Billings LJ. Establishment of genital tract infection in the CF-1 mouse by intravaginal inoculation of a human oculogenital isolate of Chlamydia trachomatis. J Infect Dis. 1984;150:577–82.
Janeway CA, Medzhitov R. Innate immune recognition. Annu Rev Immunol. 2002;20:197–216.
Kale K, Vishwekar P, Balsarkar G, et al. Serum levels of collectins are sustained during pregnancy: surfactant protein D levels are dysregulated prior to missed abortion. Reprod Sci. 2020a; 27:1894–908.
Kale K, Vishwekar P, Balsarkar G, Jassawalla MJ, Sawant G, Madan T. Differential levels of surfactant protein A, surfactant protein D, and progesterone to estradiol ratio in maternal serum before and after the onset of severe early-onset preeclampsia. Am J Reprod Immunol. 2020b; 83(2):e13208.
Karjalainen MK, Huusko JM, Tuohimaa A, et al. A study of collectin genes in spontaneous preterm birth reveals an association with a common surfactant protein D gene polymorphism. Pediatr Res. 2012; 71(1):93–9.
Kay S, Madan T. Fertility defects in surfactant associated protein D knockout female mice: altered ovarian hormone profile. Mol Immunol. 2016;71:87–97.
Kay S, Metkari SM, Madan T. Ovarian hormones regulate SP-D expression in the mouse uterus during estrous cycle and early pregnancy. Am J Reprod Immunol. 2015;74(1):77–88.
Kilpatrick DC. Mannan-binding lectin concentration during normal human pregnancy. Hum Reprod. 2000;15(4):941–3.
Kilpatrick DC, Bevan BH, Liston WA. Association between mannan binding protein deficiency and recurrent miscarriage. Hum Reprod. 1995;10(9):2501–5.
Kilpatrick DC, Starrs L, Moore S, et al. Mannan binding lectin concentration and risk of miscarriage. Hum Reprod. 1999;14(9):2379–80.
Kim CJ, Kim JS, Kim YM, Cushenberry E, Richani K, Espinoza J, Romero R. Fetal macrophages are not present in the myometrium of women with labor at term. Am J Obstet Gynecol. 2006;195(3):829–33.
Kim SM, Kim JS. Review of mechanisms of implantation. Dev Reprod. 2017;21(4):351–9.
Kishore U, Greenhough TJ, Waters P, Shrive AK, Ghai R, Kamran MF, Bernal AL, Reid KB, Madan T, Chakraborty T. Surfactant proteins SP-A and SP-D: structure, function and receptors. Mol Immunol. 2006;43(9):1293–315.
Koucký M, Malíčková K, Kopřivová H, et al. Low maternal serum concentrations of mannose-binding lectin are associated with the risk of shorter duration of pregnancy and lower birthweight. Scand J Immunol. 2018;88(1):e12675.
Kruse C, Rosgaard A, Steffensen R, Varming K, Jensenius JC, Christiansen OB. Low serum level of mannan-binding lectin is a determinant for pregnancy outcome in women with recurrent spontaneous abortion. Am J Obstet Gynecol. 2002;187(5):1313–20.
Lamont RL. Infection in the prediction and antibiotics in the prevention of spontaneous preterm labour and preterm birth. BJOG. 2003;110(Suppl 20):71–5.
Lee DC, Romero R, Kim CJ, et al. Surfactant protein A as an anti-inflammatory component in the amnion: implications for human pregnancy. J Immunol. 2010;184(11):6479–691.
Leth-Larsen R, Floridon C, Nielsen O, Holmskov U. Surfactant protein D in the female genital tract. Mol Hum Reprod. 2004;10(3):149–54.
MacNeill C, Umstead TM, Phelps DS, et al. Surfactant protein A, an innate immune factor, is expressed in the vaginal mucosa and is present in vaginal lavage fluid. Immunology. 2004;111(1):91–9.
Madhukaran SP, Gopalakrishnan ARK, Pandit H et al. Expression of surfactant proteins SP-A and SP-D in murine decidua and immunomodulatory effects on decidual macrophages. Immunobiology. 2016; 221(2):377–86.
Madsen HO, Satz ML, Hogh B, et al. Different molecular events result in low protein levels of mannan-binding lectin in populations from Southeast Africa and South America. J Immunol. 1998; 161:3169–3175.
Mahajan L, Pandit H, Madan T. Human surfactant protein D alters oxidative stress and HMGA1 expression to induce p53 apoptotic pathway in eosinophil leukemic cell line. PLoS One. 2013;8(12):e85046.
Malhotra R, Willis AC, Lopez A, et al. Mannan-binding protein levels in human amniotic fluid during gestation and its interaction with collectin receptor from amnion cells. Immunology. 1994;82(3):439–44.
Matsushita M, Fujita T. Activation of the classical complement pathway by mannose-binding protein in association with a novel C1s-like serine protease. J Exp Med. 1992;176(6):1497–502.
Megia A, Gallart L, Real JMF, Vendrell J, Simón I, Gutierrez C, Richart C. Mannose-binding lectin gene polymorphisms are associated with gestational diabetes mellitus. J Clin Endocrinol Metab. 2004;89(10):5081–7.
Mitchell MD, MacDonald PC, Casey ML. Stimulation of prostaglandin E2 synthesis in human amnion cells maintained in monolayer culture by a substance(s) in amniotic fluid. Prostaglandins Leukot Med. 1984;15(3):399–407.
Mitsuhashi A, Goto H, Kuramoto T, et al. Surfactant protein A suppresses lung cancer progression by regulating the polarization of tumor-associated macrophages. Am J Pathol. 2013;182(5):1843–53.
Miyamura K, Malhotra R, Hoppe HJ, et al. Surfactant proteins A (SP-A) and D (SP-D): levels in human amniotic fluid and localization in the fetal membranes. Biochim Biophys Acta. 1994;1210(3):303–7.
Moglia BB, Phelps DS. Changes in surfactant protein A mRNA levels in a rat model of insulin-treated diabetic pregnancy. Pediatr Res. 1996;39(2):241–7.
Montalbano AP, Hawgood S, Mendelson CR. Mice deficient in surfactant protein A (SP-A) and SP-D or in TLR2 manifest delayed parturition and decreased expression of inflammatory and contractile genes. Endocrinology. 2013;154(1):483–98.
Mor G, Cardenas I. The immune system in pregnancy: a unique complexity. Am J Reprod Immunol. 2010;63(6):425–33.
Müller S, Schaffer T, Flogerzi B, Schmid BS, Schnider J, Takahashi K, et al. Mannan-binding lectin deficiency results in unusual antibody production and excessive experimental colitis in response to mannose-expressing mild gut pathogens. Gut. 2010;59(11):1493–500.
O’Gorman N, Wright D, Poon LC, et al. Accuracy of competing-risks model in screening for pre-eclampsia by maternal factors and biomarkers at 11-13 weeks’ gestation. Ultrasound Obstet Gynecol. 2017;49:751–5.
Oberley RE, Goss KL, Ault KA, et al. Surfactant protein D is present in the human female reproductive tract and inhibits Chlamydia trachomatis infection. Mol Hum Reprod. 2004;10(12):861–70.
Oberley RE, Goss KL, Hoffmann DS, et al. Regulation of surfactant protein D in the mouse female reproductive tract in vivo. Mol Hum Reprod. 2007;13(12):863–8.
Petitbarat M, Durigutto P, Macor P, Bulla R, Palmioli A, Bernardi A, et al. Critical role and therapeutic control of the lectin pathway of complement activation in an abortion-prone mouse mating. J Immunol. 2015;195(12):5602–7.
Pryhuber GS, Hull WM, Fink I, McMahan MJ, Whitsett JA. Ontogeny of surfactant proteins A and B in human amniotic fluid as indices of fetal lung maturity. Pediatr Res. 1991;30:597–605.
Robinson DP, Klein SL. Pregnancy and pregnancy-associated hormones alter immune responses and disease pathogenesis. Horm Behav. 2012;62(3):263–71.
Rolnik DL, Wright D, Poon LC, et al. Aspirin versus placebo in pregnancies at high risk for preterm preeclampsia. N Engl J Med. 2017;377:613–22.
Salminen A, Vuolteenaho R, Paananen R, Ojaniemi M, Hallman M. Surfactant protein A modulates the lipopolysaccharide-induced inflammatory response related to preterm birth. Cytokine. 2011;56(2):442–9.
Salminen A, Vuolteenaho R, Paananen R, Ojaniemi M, Hallman M. Surfactant protein D modulates levels of IL-10 and TNF—in intrauterine compartments during lipopolysaccharide-induced preterm birth. Cytokine. 2012;60(2):423–30.
Sati L, Seval-Celik Y, Demir R. Lung surfactant proteins in the early human placenta. Histochem Cell Biol. 2010;133(1):85–93.
Schlapbach LJ, Latzin P, Regamey N, et al. Mannose-binding lectin cord blood levels and respiratory symptoms during infancy: a prospective birth cohort study. Pediatr Allergy Immunol. 2009;20(3):219–26.
Snegovskikh VV, Bhandari V, Wright JR, et al. Surfactant protein-A (SP-A) selectively inhibits prostaglandin F2alpha (PGF2alpha) production in term decidua: implications for the onset of labor. J Clin Endocrinol Metab. 2011;96(4):E624–32.
Snyder JM, Kwun JE, O’Brien JA, Rosenfeld CR, Odom MJ. Changes in surfactant protein A mRNA levels in a rat model of insulin-treated diabetic pregnancy. Pediatr Res. 1988;24:728–34.
Sonnex C. Influence of ovarian hormones on urogenital infection. Sex Transm Infect. 1998;74(1):11–9.
Sotiriadis G, Dodagatta-Marri E, Kouser L, et al. Surfactant proteins SP-A and SP-D modulate uterine contractile events in ULTR myometrial cell line. PLoS One. 2015;10:e0143379.
Steegers EA, von Dadelszen P, Duvekot JJ, Pijnenborg R. Pre-eclampsia. Lancet. 2010;376(9741):631–44.
Stuart LM, Takahashi K, Shi L, Savill J, Ezekowitz RA. Mannose-binding lectin-deficient mice display defective apoptotic cell clearance but no autoimmune phenotype. J Immunol. 2005;174(6):3220–6.
Su RW, Fazleabas AT. Implantation and establishment of pregnancy in human and nonhuman primates. Adv Anat Embryol Cell Biol. 2015;216:189–213.
Takeuchi O, Akira S. Pattern recognition receptors and inflammation. Cell. 2010;140:805–20.
Than NG, Romero R, Erez O, et al. A role for mannose-binding lectin, a component of the innate immune system in pre-eclampsia. Am J Reprod Immunol. 2008;60(4):333–45.
Thomson AJ, Telfer JF, Young A, Campbell S, Stewart CJ, Cameron IT, Greer IA, Norman JE. Leukocytes infiltrate the myometrium during human parturition: further evidence that labour is an inflammatory process. Hum Reprod. 1999;14(1):229–36.
Ticconi C, Pietropolli A, Simone ND, Piccione E, Fazleabas A. Endometrial immune dysfunction in recurrent pregnancy loss. Int J Mol Sci. 2019;20(21):5332.
Turner MW. Mannose-binding lectin: the pluripotent molecule of the innate immune system. Immunol Today. 1996;17(11):532–40.
Underwood MA, Gilbert WM, Sherman MP. Amniotic fluid: not just fetal urine anymore. J Perinatol. 2005;25(5):341–8.
Van de Geijn FE, Dolhain RJEM, Rijs WV, Willemsen SP, Hazes JMW, de Groot CGM. Mannose-binding lectin genotypes are associated with shorter gestational age. An evolutionary advantage of low MBL production genotypes? Mol Immunol. 2008;45(5):1514–8.
Van de Geijn FE, Roos A, de Man YA, et al. Mannose-binding lectin levels during pregnancy: a longitudinal study. Hum Reprod. 2007;22(2):362–71.
Verdugo IG, Tanfin Z, Dallot E, Leroy MJ, Fouché MB. Surfactant protein A signaling pathways in human uterine smooth muscle cells. Biol Reprod. 2008;79(2):348–55.
Vieira F, Kung JW, Bhatti F. Structure, genetics and function of the pulmonary associated surfactant proteins A and D: the extra-pulmonary role of these C type lectins. Ann Anat. 2017;211:184–201.
von Dadelszen P, Magee LA. Could an infectious trigger explain the differential maternal response to the shared placental pathology of preeclampsia and normotensive intrauterine growth restriction? Acta Obstet Gynecol Scand. 2002;81(7):642–8.
Wang LK, Huang MC, Liu CC, et al. Second-trimester plasma mannose-binding lectin levels and risk of preterm birth. J Matern Fetal Neonatal Med. 2017;30(6):678–83.
WHO. Detecting pre-eclampsia: a practical guide. In: using and maintaining blood pressure equipment; 2005.
Wojcik M, Zieleniak A, Zurawska-Klis M, Cypryk K, Wozniak LA. Increased expression of immune-related genes in leukocytes of patients with diagnosed gestational diabetes mellitus (GDM). Exp Biol Med (Maywood). 2016;241(5):457–65.
Wright JR. Immunoregulatory functions of surfactant proteins. Nat Rev Immunol. 2005;5(1):58–68.
Yadav AK, Chaudhari H, Shah PK, Madan T. Expression and localization of collectins in feto-maternal tissues of human first trimester spontaneous abortion and abortion prone mouse model. Immunobiology. 2016;221(2):260–8.
Yadav AK, Chaudhari H, Warke H, et al. Differential expression of collectins in human placenta and role in inflammation during spontaneous labor. PLoS One. 2014;9(10):e108815.
Zhao C, Dong J, Jiang T, Shi Z, Yu B, Zhu Y, et al. Early second-trimester serum miRNA profiling predicts gestational diabetes mellitus. PLoS One. 2011;6(8):e23925.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Kale, K., Singh, I., Kishore, U., Madan, T. (2021). Collectins in Regulation of Feto-Maternal Cross-Talk. In: Kishore, U., Madan, T., Sim, R.B. (eds) The Collectin Protein Family and Its Multiple Biological Activities. Springer, Cham. https://doi.org/10.1007/978-3-030-67048-1_9
Download citation
DOI: https://doi.org/10.1007/978-3-030-67048-1_9
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-67047-4
Online ISBN: 978-3-030-67048-1
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)