Abstract
Pulmonary surfactant is a complex mixture of phospholipids and four surfactant-associated proteins (SP-A, SP-B, SP-C and SP-D). The biological functions of SP-A and SP-D are primarily twofold, namely surfactant homeostasis and host defense. The hydrophobic surfactant proteins, SP-B and SP-C, are required for achieving the optimal surface tension reducing properties of surfactant by promoting the rapid adsorption of surfactant phospholipids along the alveolar surface. Despite the promising findings, only little is known about the extrapulmonary distribution of these proteins. Therefore, in this study, the presence of SP-A, SP-B, SP-C and SP-D in early human placenta has been investigated. First-trimester placental tissues (22–56 days) were obtained from women undergoing curettage during normal pregnancies. In parallel tissue sections, vimentin, cytokeratin-7 and CD-68 immunostainings were used for the identification of mesenchymal cells, trophoblast cells and Hofbauer cells, respectively. According to immunohistochemistry (IHC) results, SP-A, SP-B, SP-C and SP-D immunoreactivities with different staining intensities were observed in trophoblastic layers of chorionic villous tree, trophoblastic cell columns, stromal cells, Hofbauer cells, angiogenic cell cords and vascular endothelium. Fetal hematopoietic cells showed a variable staining pattern for all four surfactant proteins ranging from none to strong intensity. Western blotting of tissue extracts confirmed our IHC results. The presence of surfactant glycoproteins in early human placenta may yield a very important feature of surfactants during first trimester and enables further studies of the role of surfactants in various pregnancy complications.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
During fetal development, the type II alveolar cells of fetal lung eventually synthesize and release surfactant into pulmonary secretions (Van Golde et al. 1988), which is intermittently discharged into the amniotic fluid (Pryhuber et al. 1991). Lung surfactant is a mixture of lipids, mostly phospholipids, and proteins, which is essential for normal breathing as it can reduce alveolar surface tension to extremely low values; therefore, it prevents alveolar collapse during expiration and reducing the work of breathing (Van Golde et al. 1988).
Surfactant also contains four unique proteins: surfactant protein A (SP-A), SP-B, SP-C and SP-D (Johansson and Curstedt 1997; Kuroki and Voelker 1994). These proteins can be divided into two groups: SP-A and SP-D are large hydrophilic proteins, while SP-B and SP-C are two small hydrophobic proteins.
The water-soluble surfactant proteins-A and -D are members of the collectin subgroup of the C-type lectin superfamily that play a major role within the immune system, participating in the initiation and having further roles in non-antibody-mediated immune responses in lung (Crouch 1998; McCormack 1998; Wright 2005). In addition, SP-A and SP-D interact with various cell surface ligands on inflammatory cells, and activate or inactivate cellular functions involving phagocytosis and production of reactive oxygens or cytokines (Sano and Kuroki 2005).
For years, SPs have been considered to be lung-specific and related to air breathing. Although SP-A and SP-D are synthesized in alveolar epithelium and influence the homeostasis of the surfactant system (Sano and Kuroki 2005), they are expressed in other tissues as well (Akiyama et al. 2002; Leth-Larsen et al. 2004; Madsen et al. 2003; Miyamura et al. 1994; Pritchard 2008). For instance, SP-D is present in human coronary arteries (Snyder et al. 2008), female reproductive tract (Leth-Larsen et al. 2004). On the other hand, SP-A mRNA was clearly seen in the trachea, prostate gland and pancreas (Madsen et al. 2003). Both SP-A and SP-D were also found to be present in mare genital tract (Kankavi et al. 2007), amniotic fluid (Miyamura et al. 1994) suggesting a role in intrauterine inflammatory responses.
In contrast to the collectin-like surfactant-associated proteins A and D, SP-B and SP-C seem to be essential components during the formation of surface active monolayers, and they are required for rapid adsorption of phospholipids to an air/liquid interface (Curstedt et al. 1987). SP-B especially has been shown to be essential for normal surfactant function, lowering surface tension within the alveolus and maintaining lung volume at end expiration (Curstedt et al. 1987), as the absence of SP-B at birth leads to death caused by respiratory insufficiency (Tokieda et al. 1997), and conditional knockout of SP-B in adult animals leads to respiratory failure (Melton et al. 2003). However, one study describes the detection of SP-B in the gastrointestinal tract (as well as SP-A and SP-D) which also argues for a more extended function of SP-B than just surface activity related ones (Eliakim et al. 1989).
Although absence of SP-C at birth is not lethal like SP-B, experiments performed in specific SP-C knockout mice demonstrated that despite the fact that surfactant pool sizes and lung morphology were similar in wild-type and SP-C-knockout mice, the absence of SP-C leads to a decreased stability of the surfactant at low volumes (Glasser et al. 2001) and mutations in the gene encoding SP-C may cause interstitial lung disease and increase susceptibility to infection (Bridges et al. 2006).
Currently, only little is known about the extrapulmonary or extrarespiratory distribution of these small surfactant proteins. The various aspects are presented in the literature together with the question of the possible biological significance of surfactants in non-respiratory organs. However, the detailed expression patterns of surfactant proteins during early pregnancy have not been investigated in humans. Therefore, we aimed to investigate the presence and cellular localization of surfactant proteins, SP-A, SP-B, SP-C and SP-D, in the early human placenta by immunohistochemistry and Western blot methods.
Materials and methods
Tissue collection
A total of 26 samples of human placental tissue [(6 samples at 22–28 days (fourth week p.c.); 8 samples at 29–35 days (fifth week p.c.); 7 samples at 36–42 days (sixth week p.c.); 5 samples at 43–49 days (seventh week p.c.)] in the first trimester of pregnancy were obtained after legal termination of pregnancy by curettage for medical or psychosocial reasons, which were unlikely to affect placental structure and function (Demir et al. 1989). None of the normal pregnancies were receiving hormone treatment. Histological analysis of placental samples further confirmed the normal placental structure on tissue samples studied (data not shown). Tissues were obtained from the Department of Obstetrics and Gynecology, Faculty of Medicine, Akdeniz University, and Clinic of Obstetrics and Gynecology, Government Hospital, Antalya. Informed consent forms and protocols to use the tissue were approved by the Ethical Committee of the Faculty of Medicine in Akdeniz University.
Immediately after vacuumed aspiration of the conceptus (no application of prostaglandins), placental samples (a) were embedded in paraffin for further immunohistochemical analysis, (b) were snap frozen in liquid nitrogen for Western blot analysis. For light microscopic analysis, tissue samples were fixed with 10% neutral formaldehyde for 12 h and were dehydrated in ethanol, cleared in xylene and embedded in paraffin. Methodological details were previously described elsewhere (Sati et al. 2007, 2008).
Immunohistochemistry
Serial sections at 5 μm thickness were collected on poly-l-lysine coated slides (Sigma, St. Louis, MO, USA) and incubated overnight at 56°C. Deparaffinized sections were heated in citrate–phosphate buffer, pH 6 by treating the samples once in a microwave oven at 650 W for 6 min. After cooling for 20 min at room temperature, the sections were washed in PBS and then kept in 3% H2O2 for 30 min to remove endogenous peroxidase activity, followed by three washes with PBS. After blocking with Ultra V blocking reagent (Lab Vision, Fremont, CA) for 7 min at room temperature to reduce non-specific binding, sections were incubated with 1:700 dilution of goat polyclonal SP-A (sc-7699; Santa Cruz Biotechnology Inc.), 1:150 dilution of rabbit polyclonal SP-B (sc-13978; Santa Cruz Biotechnology Inc.), 1:300 dilution of rabbit polyclonal SP-C (sc-13979; Santa Cruz Biotechnology Inc.) and 1:150 dilution of rabbit polyclonal SP-D (sc-13980; Santa Cruz Biotechnology Inc.) antibodies and incubated overnight at +4°C in a humidified chamber. As cytokeratin has been regarded as a highly reliable marker for cells of the trophoblast lineage, characterization of placental trophoblasts cells was performed by the presence of cytokeratin 7 (MS-1352-P1; NeoMarkers, Fremont, CA; 1:200 dilution). Mouse monoclonal anti-human CD68 (M0814; Clone KP1; dilution 1:800; Dako, Glostrup, Denmark) for the identification of placental macrophages and mouse monoclonal anti-vimentin (V9; sc-6260; Santa Cruz Biotechnology Inc.; 1:400 dilution) to localize the cells of mesenchymal origin in human placental tissues were used by incubating 1.5 h at room temperature. Sections were washed three times in PBS and incubated with the biotinylated anti-goat (BA-9500; 1:400 Dilution; Vector Laboratories, Burlingame, CA), biotinylated anti-rabbit (BA-1000; 1:400 Dilution; Vector Laboratories) and biotinylated anti-mouse (BA-9200; 1:400 Dilution; Vector Laboratories) secondary antibodies for 45 min at room temperature. After three washes with PBS, the antigen–antibody complexes were detected using a streptavidin–peroxidase complex (TP-060-HL; LabVision, Fremont, CA, USA) for 30 min followed by three rinses in PBS. The resulting signal was developed with diaminobenzidine (DAB) tablets (D-4293; Sigma) and sections were counterstained with Mayer’s Hematoxylin (S3309, Dako) and mounted with Permount (Fisher Chemicals, Springfield, NJ, USA) on glass slides. The lung tissue samples from normal mice were used as positive controls. For negative controls, sections were treated with either appropriate normal goat serum, normal rabbit IgG or mouse IgG depending on the primary antibody used and diluted to the same final protein concentration as the primary antibody.
Photomicrographs were taken with an Axioplan microscope (Zeiss, Oberkochen, Germany) using Spot Imaging software version 4.6 (Diagnostic Instruments, Inc). All samples for each individual antibody were exposed to the same protocol at the same time and were stained using the same incubation periods.
Semi-quantitative analysis of staining intensities
The intensities of SP-A, SP-B, SP-C and SP-D immunoreactivity were evaluated semi-quantitatively as follows. Positively stained cells were grouped according to the following categories: 0 (no staining), + (weak but detectable), ++ (moderate or distinct), +++ (intense). In addition, for each slide, an HSCORE value was calculated by summing the percentages of cells grouped in one intensity category and multiplying this number with the weighted intensity of the staining, using the formula [HSCORE = Pi (i + 1)], where i represents the intensity scores and Pi is the corresponding percentage of the cells. For each placental sample, five parallel tissue sections were randomly selected and five randomly selected areas were evaluated for each tissue section under a microscope using 200× original magnification. The percentage of cells for each intensity within these areas was determined by two investigators in “blind” (coded) studies, and the average score was used. The HSCORE values were graphed.
SDS-PAGE and Western blotting
Total protein from first-trimester human placentas was extracted with cell extraction buffer [BioSource International; Camarillo, CA] containing 3 mM phenylmethylsulfonyl fluoride (PMSF) and protease inhibitor cocktail (Sigma-Aldrich). The protein concentration was determined by a detergent compatible protein assay (Bio-Rad Laboratories; Hercules, CA). Samples (50 μg) were loaded on 15% Tris–HCl Gels, electrophoretically separated and electroblotted onto PVDF membrane (Bio-Rad Laboratories). The membrane was blocked with 5% non-fat dry milk in TBS containing 0.1% Tween 20 (TBS-T) for 1 h to reduce non-specific binding. Subsequently, the membrane was incubated for overnight at +4°C with primary antibodies against SP-A (1:200 dilution), SP-B (1:300 dilution), SP-C (1:200 dilution) and SP-D (1:200 dilution) in TBS-T. The membrane was washed with TBS-T for 1 h and incubated with horseradish peroxidase conjugated anti-goat (1:4,000 dilution), anti-rabbit (1:4,000 dilution) and anti-mouse (1:10,000 dilution) secondary antibodies (Vector Laboratories) diluted in 2.5% non-fat dry milk in TBS-T for 2 h at room temperature. The protein was visualized by light emission on film (Amersham Biosciences; Buckinghamshire, England) and signal was detected using SuperSignal chemiluminescent kit (Pierce Biotechnology) and quantified. The mouse lung tissue extracts were prepared and used as positive control. As the internal control to confirm the equal loading of the samples, a β-actin antibody (sc-47778, C4; Santa Cruz Biotechnology Inc., 1:5,000 in 5% non-fat dry milk) was used for 2 h at room temperature to probe each immunoblot simultaneous to the probe for antibody probes. Immunoblot bands for surfactant antibodies and β-actin were quantified using IMAGE J Version 1.38 (NIH). The intensity of β-actin bands was quantified and their value was used to establish a ratio of the SP/β-actin.
Statistical analysis
For the data obtained from HSCORE analysis, all pairwise multiple comparison procedures (Holm-Sidak method) were performed for SP-A, SP-B, SP-C and SP-D immunostaining as the data passed normality (P > 0.050) and equal variance tests. Statistical calculations were performed using SigmaStat for Windows, version 3.0 (Jandel Scientific Corp. San Rafael, CA). Statistical significance was defined as P < 0.05.
Results
Immunohistochemistry
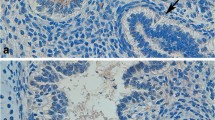
Cytokeratin-7 (Fig. 1a), vimentin (Fig. 1b) and CD-68 (Fig. 1c) immunostainings on parallel tissue sections were used to identify trophoblastic layers of chorionic villous tree, cells of mesencyhmal origin and Hofbauer cells in the early human placental tissues, respectively.
a Cytokeratin-7, b vimentin and c CD-68 immunostainings in early human placental tissue samples (4th week). Arrows indicate positively stained cells (a, b). Please note negative staining of trophoblastic cell columns (CC) with vimentin in mesenchymal villi (MV). Hofbauer cells (H) were positively stained with CD68. d–f The distribution of SP-A in the early human placenta (4th week). CC showed a moderate to strong immunoreactions with SP-A. e SP-A showed a strong immunostaining in both syncytiotrophoblast (SN) and cytotrophoblasts (CT). Arrows indicate moderately stained stromal cells. Besides, Hofbauer cells, angiogenic cell cords (ACC) and vascular endothelium (double arrow heads; inset) showed a moderate staining with SP-A. f Some of the hematopoietic cells were found to be immunopositive (arrows) but some were not (arrow heads). f The lung control slide showed positive staining with SP-A antibody (inset). g–i The distribution of SP-D in the early human placental villi (4th week). g A moderate SP-D immunolabeling in CC was presented. h ACC was weakly immunopositive with SP-D even though vascular endothelium were moderately stained (double arrows). Please observe positively (arrows) and negatively stained (arrow heads) hematopoietic cells in immature intermediate villi (IMIV). i A strong membranous immunostaining (arrow heads) in CT compared to that of observed for SN, and a moderate staining of Hofbauer cells with SP-D were seen. No staining was observed in the negative controls (d, g; inset). i Please observe positively stained lung tissue with SP-D (inset). Scale bars represent 50 μm (a–g); 100 μm (f, i; insets)
Expression of SP-A in early human placental tissues
Immunohistochemical analysis revealed that the trophoblastic layers of chorionic villous tree were strongly immunopositive with SP-A in both mesenchymal (MV) and immature intermediate villi (IMIV) (Fig. 1d–f). On the other hand, trophoblastic cell columns (CC) showed moderate to strong immunoreactions (Fig. 1d). SP-A was also observed with a moderate immunoreactivity in stromal cells, Hofbauer cells, angiogenic cell cords (ACC) and vascular endothelium (Fig. 1e, f). Interestingly, some of the fetal hematopoietic cells were strongly or moderately immunopositive for SP-A even though there were still some negatively stained hematopoietic cells (Fig. 1f).
SP-A HSCORE values showed significant differences between 4th week versus 5th, 6th, 7th and 8th weeks of pregnancy (overall significance level = 0.05) and 7th week versus 6th and 8th weeks (P < 0.05) (Fig. 3a).
Expression of SP-D in early human placental tissues
Cytotrophoblast cells (CT) showed a cytoplasmic staining pattern in addition to a membranous immunostaining with SP-D compared to that of observed for syncytiotrophoblast cells (SN) (Fig. 1g–i). However, SP-D immunolabeling was variable in CC depending on tissue sample ranging from moderate to strong immunoreactions based on overall analysis of tissue sections (Fig. 1g). Stromal cells and ACC were weakly immunopositive with SP-D (Fig. 1h, i). In addition, Hofbauer cells and vascular endothelium were moderately stained (Fig. 1h, i). As observed with SP-A, some of the fetal hematopoietic cells were negatively stained whereas there were still strongly or moderately stained hematopoietic cells (Fig. 1h, i).
According to the HSCORE analysis, SP-D showed a significant difference from the 4th week to the 5th, 6th and 8th weeks (P < 0.05), and a significant increase toward the 7th week compared to 4th, 5th, 6th and 8th weeks (P < 0.05). The HSCORE values were also statistically significant between 8th and 5th weeks as pregnancy progressed (P < 0.05) (Fig. 3b).
Expression of SP-B in early human placental tissues
SP-B immunostaining was mainly localized in trophoblastic layers of chorionic villous tree (Fig. 2a–c). In some of the tissue sections, the localization in the CT was particularly membranous with a similar pattern that observed for SP-D (Fig. 2b). The stromal cells showed a weak immunostaining with SP-B. However, CC, Hofbauer cells, vascular endothelium and ACC were moderately stained (Fig. 2a, b). A similar staining pattern was also present for fetal hematopoietic cells with none to strong staining intensities in the vessels (Fig. 2a–c).
a–c SP-B expression in first-trimester placental tissues. a A strong immunostaining in trophoblastic layers of chorionic villous tree with SP-B was observed. However, trophoblastic cell columns (CC) showed a moderate staining (8th week). b In addition, Hofbauer cells (H), vascular endothelium (double arrows) were moderately stained (4th week). c A none (arrow heads) to strong (arrows) staining intensities of fetal hematopoietic cells within the vessels was presented (8th week). c Please note the immunoreaction in lung tissue section as a positive control (inset). d–f SP-C expression in very early human placental tissues. d The trophoblastic layers of chorionic villous tree and ACC showed a strong immunostaining pattern with SP-C. However, a moderate immunolabeling in CC was observed (4th week). e Please note a strong staining in vascular endothelium (double arrows) (4th week). f Hofbauer cells showed a moderate staining. Both positively (arrows) and negatively stained (arrow heads) hematopoietic cells were seen. Vascular endothelium (7th week) (double arrows). There were no immunoreactions in negative control slides (a, inset). Mesenchymal villous (MV), immature intermediate villi (IMIV). Positive control of lung tissue immunostained for SP-C protein (f, inset). Scale bars represent 50 μm (a–f); 100 μm (c, f; insets)
An HSCORE value was also calculated for SP-B immunostaining. The statistical analysis of the data with overall significance level = 0.05 showed that there was a statistical difference between 4th week versus 5th, 6th, 7th and 8th weeks of pregnancy (P < 0.05) and also 7th week versus 5th and 8th weeks during early pregnancy (Fig. 3c).
a The HSCORE of SP-A immunostaining intensities in the early human placental villi. The data are represented as mean ± SEM: P < 0.05, 4th week versus 5th, 6th, 7th and 8th weeks (a); P < 0.05, 7th week versus 6th and 8th weeks (b). b The HSCORE analysis of SP-D immunostaining intensities in the early human placental tissues. The data are represented as mean ± SEM: P < 0.05, 4th week versus 5th, 6th and 8th weeks (a); P < 0.05, 7th week versus 4th, 5th, 6th and 8th weeks (b); P < 0.05, 8th week versus 5th week of pregnancy (c). c HSCORE analysis for SP-B immunostaining: P < 0.05, 4th week versus 5th, 6th, 7th and 8th weeks (a); P < 0.05, 7th week versus 5th, and 8th weeks of pregnancy (b). d HSCORE analysis for SP-C immunostaining: P < 0.05, 4th week versus 5th, 6th and 8th weeks (a); P < 0.05, 7th week versus 5th, 6th and 8th weeks of early pregnancy (b)
Expression of SP-C in early human placental tissues
The trophoblastic layers of chorionic villous tree, ACC and vascular endothelium were strongly immunostained with SP-C (Fig. 2d–f). In contrast, stromal cells showed a weak immunolabeling. However, a moderate immunolabeling in CC and Hofbauer cells, and none to strong staining pattern in fetal hematopoietic cells were the other prominent observations of SP-C immunostaining in early human placental tissue samples (Fig. 2d–f).
In addition, HSCORE analysis of the SP-C immunolabeling revealed that the HSCORE values were statistically different for 4th week versus 5th, 6th and 8th weeks of pregnancy. In addition, the HSCORE values obtained from 7th week of early pregnancy were significantly higher than that of 5th, 6th and 8th weeks (P < 0.05) (Fig. 3d).
There were no immunoreactions in negative control slides which were treated with normal goat serum substituting the SP-A antibody or with normal rabbit IgG substituting the SP-B, SP-C or SP-D antibodies or with mouse IgG for vimentin, cytokeratin 7 or CD68 at the same final protein concentration (Figs. 1d, g, 2a; insets). The control sections from mouse lung tissues showed positive staining (Figs. 1f, i, insets; 2c, f, insets).
Protein expression levels of surfactant proteins in early human placenta
Western blot analysis on tissue extracts from early human placentas was also evaluated. Our western blot results showed the expected bands for SP-A, SP-B, SP-C and SP-D at ~34, 43, ~11 and 43 kDa, respectively (Fig. 4a). The intensity of β-actin on the bands was quantified and their value was used to establish a ratio of the SP-A/β-actin, SP-B/β-actin, SP-C/β-actin and SP-D/β-actin (Fig. 4b). Altogether, these Western blot results confirmed the presence of the four surfactant proteins in the early placenta and supported the data obtained from immunohistochemistry.
a Western blot analysis of SP-A, SP-B, SP-C and SP-D proteins in the early days of human pregnancy. The bands for SP-A (approximately 34 kDa), SP-B (43 kDa), SP-C (approximately 11 kDa) and SP-D (43 kDa) were detected by Western blot. The expression of β-actin (43 kDa) was used to confirm equivalent amounts of total proteins loaded per lane. Lung (L) tissue extracts displayed positive control bands with each antibody. b The optical density (OD) values of surfactant bands were normalized to the OD values of β-actin bands and then graphed
Discussion
Herein, we have shown the novel finding about the presence of four surfactant glycoproteins in the early human placenta in detail. Our results indicated that all four surfactants were localized to the trophoblastic layers of chorionic villous tree, CC, Hofbauer cells, angiogenic cell cords, vascular endothelium and fetal hematopoietic cells with different staining intensities and expression patterns.
It is known that the human feto-placental unit is alloantigenic to the mother but it does not undergo recognizable allograft rejection during normal pregnancy despite there being extensive contact between the placenta, the maternal blood and the endometrium. Therefore, the survival of the allogeneic conceptus has long been an immunological paradox. Trophoblast cells (which form the interface of fetal tissue with the mother) have specialized immunological features which may confer unique transplantation protection for the fetus throughout pregnancy (Johnson et al. 1980). On the other hand, animal models of collectin deficiency have clarified the crucial roles of SP-A and SP-D in innate immune systems (Haagsman et al. 2008; Sano and Kuroki 2005; Wright 2005). Therefore, the presence of SP-A and SP-D in the early human placenta, particularly in trophoblast cells, may further extend our knowledge about placental immunobiology and also might suggest different approaches.
As observed in our study, Hofbauer cells which are the fetal placental antigen presenting cells, placental macrophages that share a number of features with macrophages but also have some distinct properties (Demir et al. 2004; Seval et al. 2007), were positively stained with SP-A, SP-B, SP-C and SP-D as well. It is supposed that SP-A and SP-D interact with multiple components on the cell surface. 210 kDa cell surface protein (SPR-210) has been purified as an SP-A receptor on macrophage-like cell line U937 cells (Chroneos et al. 1996) and are also found on type II cells and alveolar macrophages. In addition, SP-C is also known to associate very rapidly with lung tissue and alveolar macrophages (Baritussio et al. 1994). Therefore, our study would potentially provide the first evidence of surfactants in Hofbauer cells of early human placenta and may support the idea about the relationship between macrophages and surfactant proteins.
In line with the presence of surfactants in macrophage-like cells, lung collectins also play a role in the uptake of damaged cells. SP-A and SP-D bind to apoptotic cells and enhance the uptake of these cells by alveolar macrophages limiting inflammation (Schagat et al. 2001). According to our HSCORE analysis, there were statistical differences throughout 4th–8th weeks of early pregnancy for all surfactant proteins with a similar decrease at 8th week. Thus, it would be interesting to investigate whether the expressions of surfactant proteins would significantly differ as pregnancy progressed as a part of normal physiological development during the second and third trimesters of pregnancy.
Placenta and fetus exist in a hypoxic environment during early pregnancy. Pregnancy per se is a state of oxidative stress (Wisdom et al. 1991). Both SP-A and SP-D prevent lipid peroxidation in vitro and have been identified as having antioxidative potential (Bridges et al. 2000). In order to clarify how SP-A and SP-D modulate the cellular functions in human placental tissues, further investigations are required to identify and characterize the collectins’ potential receptors and the receptor-mediated intracellular signaling and show whether these proteins involve in oxidative stress events in early human placenta.
Both SP-A and SP-D show specific interactions with various microorganisms and leukocytes in vitro (Borron et al. 1996). This might be in accordance with our surfactant protein immunoreactions observed in fetal hematopoietic cells that some of the hematopoietic cells were found to be immunopositive but some were not. However, little is known about physiological regulation of surfactant secretion.
Madsen et al. (2003) using RT-PCR showed that there is no expression of SP-A mRNA in human placental tissue. However, Sun et al. found that both SP-A protein and mRNA were present in amniotic epithelial cells, fibroblasts and chorionic trophoblasts, suggesting that local synthesis of SP-A exists in all these three cell types of the fetal membranes (Sun et al. 2006). In addition, Han et al. (2007) indicated that extravillous trophoblasts but not villous trophoblasts express SP-A mRNA but interstitial trophoblasts at the placental bed express SP-A mRNA by in situ hybridization in mice. Therefore, the data available on the localization of SP-A in placental tissues are still contradictory.
Moreover, Leth-Larsen et al. (2004) have shown that the SP-D positive staining in the cytoplasm of villous and extravillous trophoblast subpopulations in early and late gestations in the placenta speculating amniotic fluid SP-D may originate from the fetal lung as well as from trophoblastic sources. Our results regarding trophoblast SP-D expression are in accordance with their data. However, none of the studies have so far investigated the expression of all surfactant proteins in placental samples belonging to very early stages, as early as the 4th week of pregnancy in detail.
In addition, glucocorticoids have complex effects on the production of pulmonary surfactant (Bolt et al. 2001). However, relatively little is known regarding the significance of glucocorticoids in early pregnancy. These steroids may also be implicated in obstetric complications, including intrauterine growth restriction, pre-term labor, pre-eclampsia and chorio-amnionitis (Michael and Papageorghiou 2008). In combination with this, the presence of surfactant protein in human placenta might be important in these complex interactions.
Poelma et al. examined the effect of SP-B and SP-C on the uptake of lipids by both alveolar type II cells and alveolar macrophages in vivo in ventilated rats as well as in vitro (Poelma et al. 2004). On the other hand, human pregnancy is characterized by a major invasion of the uterine wall by trophoblastic cells of extravillous origin and major alterations in lipid metabolism (Loke and King 1995). Therefore, it is of interest whether or not SP-B and SP-C might involve in these processes in early human placental tissues. This is very important in our understanding of preeclampsia, a condition in which lipid peroxidation is increased, and trophoblast invasion is defective (Patil et al. 2008; Sattar et al. 1997). Although their actual functions in placenta still remains speculative.
According to our results, SP-B and SP-C showed a moderate to strong immunoreactivity in the vascular endothelium and ACC. The absence of isoforms of vascular endothelial growth factor (VEGF) has shown to impair lung microvascular development and delay airspace maturation in mice fetuses reflecting their essential role for normal lung development as a potent trigger for surfactant synthesis (Galambos et al. 2002). Type II pneumocytes respond to VEGF by increasing their expression of SP-B and SP-C (Compernolle et al. 2002). Therefore, one can speculate that surfactant proteins might also involve in angiogenesis and vasculogenesis processes during early pregnancy.
Although this is a pure descriptive study, these basic findings are of relevance with regard to the presence of surfactant glycoproteins in early human placenta. Although various effects of surfactants have been suggested, we have to know more precise mechanisms. Therefore, it is important to investigate whether they are related to the immunological processes as pregnancy progressed or other critical cellular functions such as invasion, differentiation or migration in the human placenta. Therefore, the presence of surfactant proteins described here may also yield a very important feature of these proteins and leads further studies investigating the roles of surfactants in various pregnancy complications.
References
Akiyama J, Hoffman A, Brown C, Allen L, Edmondson J, Poulain F, Hawgood S (2002) Tissue distribution of surfactant proteins A and D in the mouse. J Histochem Cytochem 50:993–996
Baritussio A, Alberti A, Quaglino D, Pettenazzo A, Dalzoppo D, Sartori L, Pasquali-Ronchetti I (1994) SP-A, SP-B, and SP-C in surfactant subtypes around birth: reexamination of alveolar life cycle of surfactant. Am J Physiol 266:L436–L447
Bolt RJ, van Weissenbruch MM, Lafeber HN, Delemarre-van de Waal HA (2001) Glucocorticoids and lung development in the fetus and preterm infant. Pediatr Pulmonol 32:76–91
Borron P, Veldhuizen RA, Lewis JF, Possmayer F, Caveney A, Inchley K, McFadden RG, Fraher LJ (1996) Surfactant associated protein-A inhibits human lymphocyte proliferation and IL-2 production. Am J Respir Cell Mol Biol 15:115–121
Bridges JP, Davis HW, Damodarasamy M, Kuroki Y, Howles G, Hui DY, McCormack FX (2000) Pulmonary surfactant proteins A and D are potent endogenous inhibitors of lipid peroxidation and oxidative cellular injury. J Biol Chem 275:38848–38855
Bridges JP, Xu Y, Na CL, Wong HR, Weaver TE (2006) Adaptation and increased susceptibility to infection associated with constitutive expression of misfolded SP-C. J Cell Biol 172:395–407
Chroneos ZC, Abdolrasulnia R, Whitsett JA, Rice WR, Shepherd VL (1996) Purification of a cell-surface receptor for surfactant protein A. J Biol Chem 271:16375–16383
Compernolle V, Brusselmans K, Acker T, Hoet P, Tjwa M, Beck H, Plaisance S, Dor Y, Keshet E, Lupu F, Nemery B, Dewerchin M, Van Veldhoven P, Plate K, Moons L, Collen D, Carmeliet P (2002) Loss of HIF-2alpha and inhibition of VEGF impair fetal lung maturation, whereas treatment with VEGF prevents fatal respiratory distress in premature mice. Nat Med 8:702–710
Crouch EC (1998) Collectins and pulmonary host defense. Am J Respir Cell Mol Biol 19:177–201
Curstedt T, Jornvall H, Robertson B, Bergman T, Berggren P (1987) Two hydrophobic low-molecular-mass protein fractions of pulmonary surfactant. Characterization and biophysical activity. Eur J Biochem 168:255–262
Demir R, Kaufmann P, Castellucci M, Erbengi T, Kotowski A (1989) Fetal vasculogenesis and angiogenesis in human placental villi. Acta Anat (Basel) 136:190–203
Demir R, Kayisli UA, Seval Y, Celik-Ozenci C, Korgun ET, Demir-Weusten AY, Huppertz B (2004) Sequential expression of VEGF and its receptors in human placental villi during very early pregnancy: differences between placental vasculogenesis and angiogenesis. Placenta 25:560–572
Eliakim R, DeSchryver-Kecskemeti K, Nogee L, Stenson WF, Alpers DH (1989) Isolation and characterization of a small intestinal surfactant-like particle containing alkaline phosphatase and other digestive enzymes. J Biol Chem 264:20614–20619
Galambos C, Ng YS, Ali A, Noguchi A, Lovejoy S, D’Amore PA, DeMello DE (2002) Defective pulmonary development in the absence of heparin-binding vascular endothelial growth factor isoforms. Am J Respir Cell Mol Biol 27:194–203
Glasser SW, Burhans MS, Korfhagen TR, Na CL, Sly PD, Ross GF, Ikegami M, Whitsett JA (2001) Altered stability of pulmonary surfactant in SP-C-deficient mice. Proc Natl Acad Sci USA 98:6366–6371
Haagsman HP, Hogenkamp A, van Eijk M, Veldhuizen EJ (2008) Surfactant collectins and innate immunity. Neonatology 93:288–294
Han YM, Romero R, Kim YM, Kim JS, Richani K, Friel LA, Kusanovic JP, Jeanty C, Vitale S, Nien JK, Espinoza J, Kim CJ (2007) Surfactant protein-A mRNA expression by human fetal membranes is increased in histological chorioamnionitis but not in spontaneous labour at term. J Pathol 211:489–496
Johansson J, Curstedt T (1997) Molecular structures and interactions of pulmonary surfactant components. Eur J Biochem 244:675–693
Johnson P, Brown P, Faulk W (1980) Immunobiological aspects of the human placenta. In: Finn CA (ed) Oxford reviews of reproductive biology, vol 2. Oxford University Press, Oxford, p 1
Kankavi O, Ata A, Gungor O (2007) Surfactant proteins A and D in the genital tract of mares. Anim Reprod Sci 98:259–270
Kuroki Y, Voelker DR (1994) Pulmonary surfactant proteins. J Biol Chem 269:25943–25946
Leth-Larsen R, Floridon C, Nielsen O, Holmskov U (2004) Surfactant protein D in the female genital tract. Mol Hum Reprod 10:149–154
Loke Y, King A (1995) Human trophoblast development. In: Loke Y, King A (eds) Human implantation: cell biology and immunology. Cambridge University Press, Cambridge, pp 32–62
Madsen J, Tornoe I, Nielsen O, Koch C, Steinhilber W, Holmskov U (2003) Expression and localization of lung surfactant protein A in human tissues. Am J Respir Cell Mol Biol 29:591–597
McCormack FX (1998) Structure, processing and properties of surfactant protein A. Biochim Biophys Acta 1408:109–131
Melton KR, Nesslein LL, Ikegami M, Tichelaar JW, Clark JC, Whitsett JA, Weaver TE (2003) SP-B deficiency causes respiratory failure in adult mice. Am J Physiol Lung Cell Mol Physiol 285:L543–L549
Michael AE, Papageorghiou AT (2008) Potential significance of physiological and pharmacological glucocorticoids in early pregnancy. Hum Reprod Update 14:497–517
Miyamura K, Malhotra R, Hoppe HJ, Reid KB, Phizackerley PJ, Macpherson P, Lopez Bernal A (1994) Surfactant proteins A (SP-A) and D (SP-D): levels in human amniotic fluid and localization in the fetal membranes. Biochim Biophys Acta 1210:303–307
Patil SB, Kodliwadmath MV, Kodliwadmath SM (2008) Lipid peroxidation and antioxidant status in hypertensive pregnancies. Clin Exp Obstet Gynecol 35:272–274
Poelma DL, Zimmermann LJ, van Cappellen WA, Haitsma JJ, Lachmann B, van Iwaarden JF (2004) Distinct effects of SP-B and SP-C on the uptake of surfactant-like liposomes by alveolar cells in vivo and in vitro. Am J Physiol Lung Cell Mol Physiol 287:L1056–1065
Pritchard KA Jr (2008) Surfactant protein D: not just for the lung anymore. Am J Physiol Heart Circ Physiol 294:H1994
Pryhuber GS, Hull WM, Fink I, McMahan MJ, Whitsett JA (1991) Ontogeny of surfactant proteins A and B in human amniotic fluid as indices of fetal lung maturity. Pediatr Res 30:597–605
Sano H, Kuroki Y (2005) The lung collectins, SP-A and SP-D, modulate pulmonary innate immunity. Mol Immunol 42:279–287
Sati L, Seval Y, Demir AY, Kosanke G, Kohnen G, Demir R (2007) Cellular diversity of human placental stem villi: an ultrastructural and immunohistochemical study. Acta Histochem 109:468–479
Sati L, Demir AY, Sarikcioglu L, Demir R (2008) Arrangement of collagen fibers in human placental stem villi. Acta Histochem 110:371–379
Sattar N, Bendomir A, Berry C, Shepherd J, Greer IA, Packard CJ (1997) Lipoprotein subfraction concentrations in preeclampsia: pathogenic parallels to atherosclerosis. Obstet Gynecol 89:403–408
Schagat TL, Wofford JA, Wright JR (2001) Surfactant protein A enhances alveolar macrophage phagocytosis of apoptotic neutrophils. J Immunol 166:2727–2733
Seval Y, Korgun ET, Demir R (2007) Hofbauer cells in early human placenta: possible implications in vasculogenesis and angiogenesis. Placenta 28:841–845
Snyder GD, Oberley-Deegan RE, Goss KL, Romig-Martin SA, Stoll LL, Snyder JM, Weintraub NL (2008) Surfactant protein D is expressed and modulates inflammatory responses in human coronary artery smooth muscle cells. Am J Physiol Heart Circ Physiol 294:H2053–H2059
Sun K, Brockman D, Campos B, Pitzer B, Myatt L (2006) Induction of surfactant protein A expression by cortisol facilitates prostaglandin synthesis in human chorionic trophoblasts. J Clin Endocrinol Metab 91:4988–4994
Tokieda K, Whitsett JA, Clark JC, Weaver TE, Ikeda K, McConnell KB, Jobe AH, Ikegami M, Iwamoto HS (1997) Pulmonary dysfunction in neonatal SP-B-deficient mice. Am J Physiol 273:L875–L882
Van Golde LM, Batenburg JJ, Robertson B (1988) The pulmonary surfactant system: biochemical aspects and functional significance. Physiol Rev 68:374–455
Wisdom SJ, Wilson R, McKillop JH, Walker JJ (1991) Antioxidant systems in normal pregnancy and in pregnancy-induced hypertension. Am J Obstet Gynecol 165:1701–1704
Wright JR (2005) Immunoregulatory functions of surfactant proteins. Nat Rev Immunol 5:58–68
Acknowledgments
We would like to thank Sibel Ozer for her excellent technical assistance. This study was partly supported by the Scientific and Technological Research Council of Turkey (TUBITAK)-Project# SBAG-3267 (105-S460) and by Akdeniz University The Scientific Research Projects Coordination Unit (Project No: 2007.03.0122.004).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sati, L., Seval-Celik, Y. & Demir, R. Lung surfactant proteins in the early human placenta. Histochem Cell Biol 133, 85–93 (2010). https://doi.org/10.1007/s00418-009-0642-9
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00418-009-0642-9