Abstract
Catalase (CAT) is an important enzyme in the antioxidant defense system in marine bivalves. This present work consists first of all in studying the effect, of an acute exposure (bioassays) of the mussels Mytilus galloprovincialis to three types of real effluents [industrial wastewater (IW), desalination station effluent (DSE) and harbor effluents (HE)], on the enzymatic activities CAT. In situ, the transplantation of mussels into natural environments was carried out in the same context. The medium size class of the Mytilus mussel has proven to be the most resistant to contamination by the IW. Notwithstanding, this same size class showed hypersensitivity to contamination by the SDE, thus translating the high toxicity of the latter which led to very high CAT inductions whatever the concentration tested. Furthermore, under controlled laboratory conditions, the HE demonstrated a certain toxicity by inducing the CAT enzyme. On the other hand, the Mytilus mussels transplanted to the khemesti port site are found all dead after ten days of immersion. The deoxygenation of the medium probably had an additional and aggressive effect on our specimens of mussels. This study makes it possible to qualify catalase as a relevant, sensitive, rapid and effective defense biomarker in the evaluation of the health state of the surrounding environment. However, more studies must be made on metabolic activities and energy reserves while adopting the multibiomarker approach.
Access provided by Autonomous University of Puebla. Download conference paper PDF
Similar content being viewed by others
Keywords
1 Introduction
Surface and coastal waters are often contaminated by many chemicals released by industry, agriculture and urban communities. The estuarine and coastal zones, under strong continental influence, are the most affected by this type of contamination. The problems posed by the dispersion of pollutants into the environment have attracted the interest of the scientific community for many decades now. Awareness of the need to preserve aquatic ecosystems has led to the emergence of certain issues, including the fate of these pollutants in the environment and their effects on animal and plant communities (Bouzahouane et al., 2018; Cappello et al., 2013; Flammarion et al., 2001; Jing et al., 2019; Sillero-Rios et al., 2018; Vlahogianni et al., 2007).
In order to know and monitor the evolution of chemical contamination of aquatic ecosystems, research and monitoring programs based on the measurement of contaminants concentration in water and sediments have been implemented. However, the chemical analysis of the pollutants present in the various compartments of the aquatic ecosystems is not always possible because of the multiplicity of the molecules present, and this often at concentrations lower than the limits of analytical detection, which makes sampling and measurement techniques quite complex. The risks of contamination at the time of sampling and analysis are numerous, making measurements difficult. These problems have been overcome by the use of “ultra-clean” techniques in sampling. However, the direct measurement of contaminants in water uses sophisticated analytical techniques that are difficult to apply in a perennial network for the routine measurement of the quality and health of aquatic ecosystems. Moreover, the temporal variability of the littoral and limnic environment confers little representativity to a point measurement in the water column. Finally, such an approach does not provide information on the risks faced by animal or plant populations exposed to pollutants, and cannot, on its own, predict the biological effects of contaminant mixtures (synergies, etc.) or simply quantify the bioavailability of contaminants for living organisms. As a result, the manager lacks information on the urgency of taking action to improve the health status of these ecosystems, or to protect biodiversity and the integrity of these ecosystems (Aouini et al., 2018; Barillet et al., 2011; Boukadida et al., 2017; Brooks et al., 2015; Casas and Bacher, 2006; Mejdoub et al., 2017).
With this in mind, Goldberg (1975) proposed monitoring the concentrations of contaminants in living organisms to monitor the surrounding environment. This is the principle of “quantitative bioindicators” based on the fact that aquatic organisms concentrate contaminants at concentrations higher than those present in the environment (Aouini et al., 2018; Arienzo et al., 2019; Benali et al., 2017; Casas and Bacher, 2006).
Current surveillance strategies are diverse, and the use of fixed molluscs and relatively sedentary fish are most commonly developed in environmental monitoring programs. Mytilus galloprovincialis mussels and other marine bivalves have characteristics that make them good bioindicators of coastal water contamination (Akaishi et al., 2007; Arienzo et al., 2019; Aslan et al., 2018; Cappello et al., 2013; Casas and Bacher, 2006; Forbes et al., 2006; Sparks et al., 2019).
Other tools, the biomarkers (reflecting an interaction between a biological system and a potential hazard) can be used to study the bioavailability of contaminants. They are then studied more for their contribution to the mechanistic understanding of the action of pollutants (Al-Fanharawi et al., 2018; Aslan et al., 2018; Beiras, 2018; Peric et al., 2017; Sparks et al., 2019).
In this context, the National Center for Research and Development of Fisheries and Aquaculture CNRDPA-BouIsmail is currently developing a program of management of aquatic ecosystems and environmental monitoring contributing to the research of pollution indicators. Within this framework, we carried out our research on the Mediterranean mussel “Mytilus galloprovincialis” as a biological model in order to better define the ecotoxicology research topics of real effluents, in laboratory and in situ, whose main objective is the contribution looking for biomarkers like an early response that can provide integrated information on the state of the aquatic ecosystem as well as on bioaccumulation phenomena.
2 Materials and Methods
2.1 Experimental Procedure
The different experiments were conducted and carried out at the CNRDPA-BouIsmail pilot shellfish breeding center (Fig. 1).
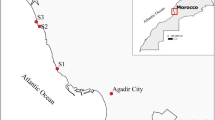
On the map below are indicated the sampling points of the different liquid discharges used in this study, as well as the collection points of the Mytilus mussels and the transplant sites of artificial cages (mussels caging).
2.1.1 First Series of Ecotoxicity Tests
Mytilus galloprovincialis mussels, which are used as a biological model, are collected from the sea buoyage signals of the Fouka marine seawater desalination plant (Fig. 1). Sampling targeted the three size classes (22 mm; 28 mm), (43 mm; 52 mm) and (71 mm; 86 mm). After the collection of the mussels, the latter are sorted, cleaned, cleared of their epibionts and measured using a Vernier caliper before their adaptation. The set of mussels is maintained under identical environmental conditions, and the use of air pumps ensures the aeration of the water tanks. A daily renewal of the rearing water is carried out during the adaptation period and throughout the entire experimental cycle.
In order to minimize other factors that may constitute a source of disturbance or interference of ours results, a measurement of the physicochemical parameters (temperature, salinity, pH and dissolved oxygen) is carried out each day during the whole experimental cycle before and after the change of water using a YSI 556 multiparameter.
The strategy of the ecotoxicity tests is to contaminate the Mytilus mussels, of different size classes (large, medium and small), by the waters of an industrial wastewater (IW), discharging directly at sea in BouIsmail Bay (Fig. 1), and this at different concentrations by dilution in tanks of 70 L.
Thus, the mussels are exposed for four days at four increasing concentrations (C1 = 0.01%; C2 = 0.15%; C3 = 0.3% and C4 = 0.5% (v/v)) of the IW. The manipulation is automatically compared to a control aquarium (0 mg/L pollutant).
The four selected concentrations were tested to establish dose–response relationships between the chemical pressure represented by the pollutants of the release and the biological responses observed in the mussels.
2.1.2 Second Series of Experience
In the second series of experiments, we chose to work on the medium-sized class (juvenile mussels) judged to be the most resistant. The experimental conditions are the same as the first series of tests. But this time, the mussel specimens are exposed to two types of effluents, namely the water discharge from the BouIsmail seawater desalination plant (DSE) and the water from the site port of Khemisti (HE) (Fig. 1). The concentrations chosen are the following: C2 = 0.15%; C3 = 0.3% and C4 = 0.5% (v/v), and the exposure time is always 96 h.
2.1.3 In Situ Study: Artificial Cage Transplantation Technique
The mussels this time from an aquaculture farm (AF) located at Ain-Tagouraite (Fig. 1) (Tipasa, Algeria) are put in plastic nets (40 cm × 30 cm and mesh 20 mm) and divided into four lots of 15 individuals in wool pockets. The nets are stiffened by a 30 mm diameter PVC tube split in their width and threaded on the upper part.
The artificial stations (mussels caging) were transplanted at two sites: Khemisti port site (PSK Fig. 1) (contaminated environment) and in an open environment (low contamination site) near the shellfish center (SCS) (see map). The immersion time of the stations was planned for one month with a periodic sampling of mussels every ten days. However, we were limited to ten days following the total mortality of transplanted individuals in the port site.
2.2 Analytical Methods and Biochemical Assays
Total proteins are assayed by Lowry method using bovine serum albumin (BSA) as standard. Catalase activity is determined according to the method of Lartilot et al. 1988 described by Atli et al. (2006).
In ice-cold, tissues are homogenized (1/10 w/v) in Tris (tris (hydroxylmethyl) aminomethane) buffer (20 mM, pH 7.8) for three minutes using a mixer. Centrifugation of the homogenate is done at 10,000 g for 30 min at 4 °C. The supernatant thus obtained (Fraction S9) is used to assay the proteins and catalase.
In our practice, 2.5 ml of substrate solution (25 mM H2O2 in 75 mM phosphate buffer at pH7) are placed in a cuvette of the spectrophotometer already set in the kinetic mode. 50 μl of the S9 fraction (enzyme source) are added to the mixture; thus, the kinetic mode of the spectrophotometer is triggered and the decomposition of hydrogen peroxide is monitored in a time interval of 60 s.
2.3 Statistical Analyzes
Data are tested for normality and homoscedasticity using Shapiro–Wilk and Levene tests, respectively. A statistical analysis is then performed using a bilateral analysis of variance (ANOVA 2), followed by a post hoc analysis of Tukey. The significance was set at p < 0.05. The results of the in situ study are tested using ANOVA one way (STATISTICA 6.0).
3 Results
3.1 First Series of Experiments
The results relating to the determination of catalase (CAT) in mussels (Mytilus galloprovincialis) contaminated by industrial rejection are shown in Fig. 2.
Concerning the medium-sized Mytilus mussel, the statistical analyzes did not show any significant effect of the contamination, by industrial effluent, on the responses of the defense enzyme catalase, and this compared to the controls and to the gradient concentration examined. The four concentrations of pollutants tested did not seem to have a remarkable effect on the induction of the antioxidant defense mechanism where no significant effect was measured between concentrations.
A significant effect of contamination by industrial discharge is noted in Mytilus mussels of the large size class exposed to concentrations C2 and C3. Also, compared to the control individuals (CAT: 307.89 ± 23.86 U/mg Proteins/min), a proportional increase in catalase activity is measured in mussels exposed to the effluent xenobiotics for concentrations C1, C2 and C3. The activities achieved, respectively, are 545.89 ± 106.65, 623.71 ± 81.48 and 688.09 ± 35.33 U/mg proteins/min. The C4 concentration (CAT: 551.08 ± 90.46 U/mg Proteins/min) had an insignificant and identical effect as that of the C1 concentration on the induction of the antioxidant enzyme CAT.
In mussels of the small size class, individuals of the latter have shown a hyperactivity in the induction of the antioxidant mechanism translated by the CAT. Thus, compared to the controls, the highest induction of catalase (253.25%) is measured under the effect of the C4 concentration. Also, the trend was almost proportional with the four concentrations tested. This time, no significant effect was noted between the concentration C1 (CAT: 737.59 ± 4.33 U/mg Protein/min) and C3 (CAT: 760.33 ± 64.27 U/mg Proteins/min), while a very significant effect of the concentration is marked compared to controls (416.13 ± 65.25 U/mg Protein/min).
The study of the independent effect of each concentration on the CAT response in the three size classes reveals significant and non-significant effects depending on the case considered. Thus, the measured values of the catalase activity in the control individuals (no contaminant) did not show any significant difference between the three classes studied. Under the effect of the C2 concentration, the statistical difference is significant whatever the size class studied. Also, compared to the small size class, the difference is always significant between the compared size classes. However, the statistical analysis reveals an insignificant effect between the medium and large size class and this under the effect of concentrations C1, C3 and C4.
In light of the results obtained, the statistical analysis of the effects of the contaminant concentration and the interaction with the size class revealed that the Mytilus mussels of the medium size class (juveniles) are the most resistant to contamination. Mytilus individuals of the large size class showed medium sensitivity, while small Mytilus appeared the most sensitive to concentration.
3.2 Second Series of Experiments
-
Comparative study between the different types of effluents (Mytillus mussel, medium size)
The results relating to the determination of catalase (CAT) in the mussel (Mytilus galloprovincialis of medium size) contaminated by the different types of liquid discharges are represented by Fig. 3.
Whatever the concentration tested for industrial liquid discharge (IW), ANOVA-2 (P ˂ 0.05) did not highlight any significant difference between the CAT activities measured (Fig. 3). Also, for the harbor effluent (HE), compared to the effect of the concentration C2, the statistical analysis did not show any significant difference in the CAT activities. However, the difference in the activity of the antioxidant enzyme being significant (P ˂ 0.01) between the effect of concentration C3 and that of concentration C4.
In addition, the discharge from the seawater desalination station (DSE) led to very large CAT inductions proportional to the concentrations tested. The highest induction (573.22%) of the antioxidant enzyme is noted under the effect of the C4 concentration. The differences in CAT activities were very significant (P ˂ 0.0001) regardless of the concentration used for the rejection (DSE).
The study of the independent effect of the concentration C2 (ANOVA-2; P ˂ 0.05) on the CAT responses did not show any significant difference (P ˂ 0.05) between the port discharge and that of the desalination plant. Notwithstanding, the difference being significant between IW and HE but still very significant between IW and DSE.
For the concentration C3, there was no significant difference of the effect of the nature of effluents between IW and HE. However, the differences are clearly significant between IW-DSE and HE-DSE.
Whatever the type of liquid effluent tested, the differences in CAT activity measured under the effect of the concentration C4 are significant (P ˂ 0.05) between IW-HE and very significant (P ˂ 0.0001) between IW-DSE and HE-DSE.
3.3 Mussels Caging
The results relating to the determination of catalase (CAT) activity in the mussel (Mytilus galloprovincialis) transplanted in the port of Khemisti (PSK) and in an open environment (SCS) near the shellfish farming center of CNRDPA-BouIsmail are represented in Fig. 4.
According to the graph in Fig. 4, it emerges that the catalase activity in mussels transplanted in an open medium (SCS) like that of origin of provenance (AF) is identical examining ANOVA one way (P ˂ 0.05).
The conditions of the open transplant medium (for ten days) were within the tolerance range for Mytilus individuals, which did not lead to a variation in the activity of the antioxidant enzyme CAT. It is also likely that the two media are not really affected by a source of contamination by the different types of xenobiotic.
Furthermore, the mussels transplanted to the Khmesti port site (PSK) were unable to survive, when they showed a total mortality on the tenth day. It is likely that the environmental conditions were not favorable for the mussel specimens to be able to exercise their physiological activity of basic metabolism (respiration, nutrition, etc.) in an optimal manner without any external aggression due to environmental conditions.
4 Discussion
4.1 First and Second Series of Experiment
Since the development of the “Mussel Watch” program by Goldberg in 1975, the use of bivalve molluscs has been widespread in order to monitor the improvement or degradation of the environment and to study its biological and physiological impacts (Arienzo et al., 2019; Benali et al., 2017; Dong et al., 2019; Pellerin and Amiard, 2009; Zorita et al., 2008). By their filtration capacity of sea water, in order to feed on suspended matter, bivalves can ingest a large quantity of contaminants and therefore concentrate the trace elements (chemical or biological) at concentrations higher than those encountered in its environment (Aouini et al., 2018; Arienzo et al., 2019; Box et al., 2007; Gorbi et al., 2013; Maanan, 2008; Pellerin and Amiard, 2009; Perosevic et al., 2018; Silva Dos Santos et al., 2018). In bivalves, the accumulation of contaminants is mainly by passive diffusion via gill respiration and by active transport resulting from the filtration of water and the ingestion of the particles present (Aasen et al., 2006; Casas and Bacher, 2006). Tissues directly exposed to xenobiotics accumulate these according to their bioavailability (e Silva et al., 2006). The accumulation of chemicals in organisms is the cause of oxidative stress (Aouini et al., 2018; Vlahogianni et al., 2007).
Indeed, by their redox properties, many xenobiotics such as hydrocarbons, metals, quinones or even certain pesticides are known to exert their deleterious effects through the formation of reactivated oxygen species (ROS) (Akcha et al., 2000; Boukadida et al., 2017; Mejdoub et al., 2017; Peric et al., 2017; Verlecar et al., 2008; Vlahogianni et al., 2007). The reactivity of ROS can be the cause of harmful biological effects (oxidation of components such as DNA, proteins, lipids and general disturbance of the redox state) in invertebrates (Barillet et al., 2011; Bouzahouane et al., 2018; Sparks et al., 2019; Vernon and Jha, 2019; Yavasoglu et al., 2016).
In fact, to cope with the production of ROS, aquatic organisms have developed antioxidant defense systems made up of enzymes (superoxide dismutase, catalase CAT, glutathione peroxidase) and molecules which trap radical species at the level of membranes (vitamin E, β-carotene) or the aqueous phase (ascorbic acid, uric acid and glutathione). The antioxidant defense enzyme CAT is known to be induced to deal with oxidative stress (Al-Fanharawi et al., 2018; Bao et al., 2018; Barillet et al., 2011; Boukadida et al., 2017; Cappello et al., 2013; Damiens et al., 2004). Measured in the Mytilus galloprovincialis mussel, the catalase activity can tell us about the levels of pollution (Box et al., 2007; Vlahogianni et al., 2007) and the degree of cell damage (Akcha et al., 2000; da Silva Barreto et al., 2018; Di Giulio et al., 1993; Teixeira et al., 2017).
In fact, the CAT activities noted in this study, under the effect of the various liquid discharges, can clearly tell us about the degree of toxicity of each effluent. Effluent from the desalination plant is the most toxic regardless of the concentration tested.
In situations of severe stress, defense enzymes are induced very quickly and amply to cope with the endured effects. Sometimes, the end result of such a situation is the inhibition of the activity of the various defense mechanisms (Aouini et al., 2018; Benali et al., 2017). In our study, we noted remarkable mortalities at the end of the exposure of the mussels to the different concentrations of rejection from the desalination station, thus reflecting the total impairment of the different physiological mechanisms of the Mytilus mussels as a bioindicator of the level of contamination. Under the effect of industrial discharge and that of port effluent, no mortality was noted. Thus, it is easy to conclude on the high toxicity of the discharge from the desalination plant.
The bioaccumulation of xenobiotics varies according to their chemical properties giving a good indication of their bioavailability in the environment. Acquiring knowledge on the type and rate of contamination will make it possible to analyze the risks they represent at different physiological levels (e Silva et al., 2006; Lemaire et al., 2006; Pellerin and Amiard, 2009).
The introduction of different concentrations of industrial (IW) and port effluents (HE) did not lead to any variation in the physicochemical parameters of the water in aquariums compared to the control groups. So, the deleterious effect on our specimens can be attributed to the xenobiotics present in the two effluents. On the other hand, the liquid discharge of the desalination station (SDE) led to an increase in the salinity and the conductivity of the water in the aquariums. The effect of the xenobiotics present in the effluent therefore combines and probably increases with the increase in salinity leading to a multiplication of the stress signal (CAT).
As was the case in our study, industrial effluents are generally discharged directly into receiving environments (in our case coastal waters) without any pretreatment and are often very loaded with suspended matter, dissolved salts, trace chemical elements organic and/or inorganic. Reaching the receiving environment, liquid effluents can have very harmful effects on the biological component of the environment as they can modify the physicochemical properties of the water column and even sedimentary funds following the accumulation and precipitation of xenobiotics transported by the continuous flow of liquid discharges.
Certain contaminants have the particularity of not being eliminated by living organisms, thus generating a bioaccumulation throughout the food chain with remarkable harmful effects in particular: neurotoxicity, immunotoxicity, stress and oxidative damage, behavior modification, growth inhibition and impaired reproduction (Coppola et al., 2017; Oliveira et al., 2018). The effects are very worrying if we consider the direct short and long-term impact on the receiving environment.
Also, the continuous flow rates of industrial liquid discharges will certainly bring a significant load in xenobiotics on the one hand, but also on the other hand, it can lead to a slight reduction in the salinity of the coastal waters which receive them. The sensitivity of aquatic organisms to the decrease in salinity has been reported by several scientists.
(Hamer et al., 2008) showed an increased mortality of mussels Mytilus galloprovincialis under lowest salinities during two acclimation experiments at high/low seawater temperature (27/13 °C), especially during the summer period. Their results revealed that the oxygen consumption rate of mussels increase inversely with the salinity. Furthermore, the DNA integrity from mussels sampled in the winter period showed a significantly lower DNA integrity status than those sampled during the summer season.
Under stressful environmental conditions, homeostasis of organisms is compromised. The hypothesis is that this will in turn increase the susceptibility of mussels to other stresses. The hypothesis is that this will in turn increase the susceptibility of mussels to other stresses.
According to Bussell et al. (2008), the reducing seawater salinity to half that of normal caused a significant reduction in several measures of immune function, including the concentration of hemocytes, percentage of eosinophilic hemocytes and phagocytosis. Wang et al (2011) found that clearance rate, absorption efficiency, respiration rate and scope for growth of the mussel Perna viridis decreased with decreasing salinity and dissolved oxygen concentration. (Bakhmet et al., 2005) report that, when exposed to moderate hyposalinity (15 g/L), the mussel Mytilus edulis showed a significant decrease in the heart rate with respect to the control salinity (25 g/L). The heart beat quickly accelerated in all organisms when they were returned to the control salinity medium. In the mussel Mytilus galloprovincialis, Freitas et al. (2017) notice that, at low salinity, despite an increase of antioxidant enzymes activity, lipids peroxidation (LPO) increased, probably as a result of ROS overproduction from higher electron transport system activity.
On the other hand, discharges from desalination plants can increase the salinity of the areas close to the outfalls. The dispersion of brine can vary considerably depending on site-specific characteristics, the volume of effluent, the method of discharge and the existing hydrographic conditions. However, salinity and temperature are higher than the reference standards for discharge sites (Fernández-Torquemada et al., 2009).
The southern shore of the Mediterranean is considered to be one of the world's poorest regions of water. As a result, desalination efforts around the Mediterranean are concentrated mainly on its southern and eastern shores, as well as in Spain. In 2013, more than 1532 seawater desalination plants were set up around the Mediterranean Sea with a total cumulative capacity of around 12 Mm3/day. Spain was the main producer (31% of total capacity), while in third place comes Algeria followed by Libya with 20 and 11%, respectively (POL, 2015). Given the desalination effort thus mentioned, the biodiversity of our coastal waters is therefore compromised.
Salinity and temperature have long been perceived as environmental factors that inhibit the survival and growth of marine biota (Murray and Wingard, 2006; Wiltshire et al., 2010).
Laboratory and mesocosm experiments have shown the sensitivity of certain seagrass beds to hypersalinity with tolerances which vary from one species to another. Generally, physiology, leaf growth and survival rate are affected (Fernández-Torquemada and Sánchez-Lizaso, 2011; Fernández-Torquemada et al., 2005; Koch et al., 2007; Marín-Guirao et al., 2013; Ruíz et al., 2009; Sandoval-Gil et al., 2012).
In the field, it has been shown that a shallow Posidonia oceanica meadow was affected after six years of exposure to brine (Sanchez-Lizaso et al., 2008), while the benthic community was changed (de-la-Ossa-Carretero et al., 2016; Del-Pilar-Ruso et al., 2008; Ruso et al., 2007) with disappearance of certain echinoderm.
In the distant vicinity of the outlets of the liquid discharges of the present study, we observed a disappearance of the herbarium Posidonia oceanica, of the sea urchin Paracentrotus lividus, while the spat of Mytilus mussels installed on hard substrates did not find the opportunity to grow.
Several authors agree on the importance of salinity, and their results show its influence as much as an abiotic factor on metabolic activity and above all on enzyme responses (Hamer et al., 2008).
Results found by Carregosa et al. (2014) showed that clams under salinity associated stress can alter their biochemical mechanisms, such as increasing their antioxidant defenses, to cope with the higher oxidative stress resulting from hypo and hypersaline conditions. Among the physiological and biochemical parameters that they analyzed: glycogen and protein content, lipid peroxidation levels, antioxidant enzymes (Catalase and especially superoxide dismutase), the latter proved to be sensitive biomarkers to assess the impact of salinity in clams. Among the physiological and biochemical parameters that they analyzed: glycogen and protein content, lipid peroxidation levels, antioxidant enzymes (Catalase and especially superoxide dismutase), the latter proved to be sensitive biomarkers to assess the impact of salinity in clams.
4.2 Mussels Caging Discussion
Several authors describe the transplantation of mussels from a reference site that is not/or little polluted to more polluted sites, as a very promising and very relevant technique and strategy in order to assess the degree of contamination of marine ecosystems (Box et al., 2007; Cappello et al., 2017, 2013; Gherras Touahri et al., 2016; Risso-de Faverney et al., 2010; Schintu et al., 2008; Verlecar et al., 2008).
According to Box et al. (2007) and Sillero-Rios et al. (2018), measuring the same biomarker simultaneously in different localities provides information on the degree of pollution of the latter and provides a better understanding of the mechanisms of the mode of action of pollutants in the environment on transplanted organisms. In fact, Box et al. (2007) found that the degree of induction of CAT activity was greater in mussels (Mytilus galloprovincialis) transplanted in estuaries most affected by urban discharges than in specimens transplanted in estuaries little affected by anthropogenic activity for which they measured a lesser induction of the antioxidant enzyme CAT.
In agreement with Cappello et al. (2013), significantly greater CAT activity was recorded in mussels caged at the site influenced by anthropogenic activities compared to the reference site. By applying passive biomonitoring of pollution in coastal areas from the Saronikos Gulf of Greece, Vlahogianni et al. (2007) found that CAT activity was increased 2–3 times at the polluted sites, with high activity in the winter and spring time, compared to the control site. Data for increases in CAT activity demonstrate a disturbance from pollutants in the Elefsis Bay with seasonal variations reflecting the intensity of pollution in the area.
In fact, it is easy to attribute the induction levels of the antioxidant enzyme CAT, from the present study, to the overall quality of the different mussels caging sites. In addition to chemical analyzes of bioaccumulation, the measurement of biomarkers provides information on the nature and level of chemical contamination but also on the health of living organisms and populations of aquatic ecosystems (Aouini et al., 2018; Benali et al., 2017; Gorbi et al., 2008; Kopecka et al., 2006; Verlecar et al., 2008; Vlahogianni et al., 2007).
It is recognized that the biological responses of organisms (growth, bioaccumulation and detoxification of chemical pollutants, enzymatic responses of biomarkers, etc.) can be affected not only by chemical pollutants but also by a certain number of natural stressors such as temperature, salinity, dissolved oxygen, food availability and the reproductive state (Balbi et al., 2017; Blanco-Rayon et al., 2019; Cappello et al., 2017; Chatel et al., 2010; Gonzalez-Fernandez et al., 2017; Nardi et al., 2018; Richir and Gobert, 2014). Thus, to exclude the effect of fluctuating temperatures and the reproductive cycle on the signals of the CAT biomarker, the immersion of artificial stations was carried out during the period of sexual rest in the months of June and July.
In terms of volume, the port of Khemisti is small. The discharges reaching the latter are of domestic, industrial origin (two activities are located nearby) and those linked to fishing activities, which leads to a significant concentration of pollution in situ.
The dissolved oxygen contents, which are the result of physical, chemical and biological factors (photosynthesis, respiration, redox reaction, etc.) and which govern the majority of chemical and biological processes, oscillate between a minimum of 3.93 mg/L and a maximum of 4.41 mg/L in the port of Khemisti (PSK), while in SCS and AF sites, the values often exceed 7 mg/L. In assessing the degree of a given pollution, direct measurement of the dissolved oxygen level can tell us about the eutrophication of aquatic ecosystems. Eutrophication can lead to water suffocation (Mucci, 1997). Dissolved oxygen is therefore a sensitive and practical indicator in a first assessment of an environmental diagnosis. In fact, the lower content of dissolved oxygen in the port of Khemisti is the likely result of the latter's eutrophication. Adding to this, the presence of xenobiotics of different forms, the defense system of mussels transplanted in the port site did not have the possibility of coping with the stresses endured, ultimately leading to total mortality of the transplanted individuals. Dellali et al. (2001) reported that a decrease in the oxygen content is at the origin of an increase in the activity of catalase in the mussels of the lagoon of Bizerte (Tunisia).
Toxic molecules interact with biological molecules. Consequently, the exposed organisms develop various defense mechanisms: avoidance and/or isolation, active elimination, neutralization by complexation with proteins, etc. (Jean-Claude and Claude, 2008). Studies of metabolism and biomass production in organisms exposed to toxins (Ducrot, 2005) have shown that these defense mechanisms are costly for the organism in terms of energy. These defense costs are added to maintenance costs: there is therefore a quantitative correlation between the organism's defense capacity (survival) and its biomass production capacity (growth and reproduction). In addition, energy allocation for the defense, repair and regeneration of cells is favored over growth and reproduction. In extreme situations, the survival of the living is uncertain. According to Jean-Claude and Claude (2008), defense biomarkers allow organisms to fight and survive in the presence of pollutants at reasonable levels, but this has an energy cost for the individual.
In mussels, since the avoidance or isolation strategy is not possible, the use of antioxidant enzymes seems to be the best strategy for dealing with xenobiotics. The excretion and sequestration of xenobiotics in the tissues can also intervene. However, it has been shown that the first responses to the presence of contaminants are those linked to the antioxidant system (Aouini et al., 2018; Box et al., 2007; Damiens et al., 2004; Verlecar et al., 2008; Vlahogianni et al., 2007).
In addition, valve closure is a known behavior in bivalves in response to environmental stress (Freitas et al., 2017; Geffard et al., 2001). As a result, the decrease in the volume of water filtered by the mussels will lead to a decrease in food capture which will have a direct effect on energy intake. Consequence: bad physiological activities. On the other hand, damage to the digestive enzyme by port pollutants either by trophic route or via water will probably have an effect on the efficiency of food energy conversion. The digestive gland is the main organ involved in food processing, digestion and nutrient delivery, and these processes could interfere with the processes of accumulation, detoxification and elimination of pollutants (Arrighetti et al., 2019; Blanco-Rayon et al., 2019; Faggio et al., 2018).
The results obtained in this study have shown that the Mytilus mussel has a number of characteristics which make it an excellent bioindicator of the quality of the marine environment, the importance of which has already been emphasized in numerous research studies (Arienzo et al., 2019; Benali et al., 2017; Boukadida et al., 2017; Chatel et al., 2010). Also, the CAT as a stress biomarker demonstrates its sensitivity, its precocity and its effectiveness in predicting the deleterious effects of the real effluents of the present study. Also, the major interest of catalase as a biomarker resides in the non-specific character of its response, which constitutes an advantage in the context of multiple contamination of aquatic ecosystems. According to several authors, the biomarkers of defense like CAT help maintain the organism’s homeostasis. The latter depends on the dose, duration/effect relationship (Al-Fanharawi et al., 2018; Aouini et al., 2018; Bao et al., 2018; Benali et al., 2017).
Catalase can be influenced by intrinsic or extrinsic factors beyond the control of the researcher. Thus, to compensate for the variations in biomarker responses due to these factors, the multimarker approach seems to be a promising key for a better interpretation of the results and thus a more precise evaluation of the health state of the aquatic environment.
5 Conclusion
In conclusion, the practice of biomarkers, in our present study, has proven to be very interesting from the point of view of precocity of rest of the measured signals and their correlations with the levels of pollution. They can indeed constitute an obvious complement to chemical monitoring programs by translating a risk for organisms in aquatic ecosystems. As a result, biomarkers can be used and are still of great ecological relevance, particularly in the case of a long-term monitoring program for the coastal and estuarine environments.
Furthermore, the integration of these biological variables into a long-term monitoring and measurement network will provide managers of aquatic environments with better information on the evolutionary trend of the monitored environments (sign of good management or degradation) and will allow scientists to better understand the functioning of the systems studied.
References
Aasen, J. A., Hardstaff, W., Aune, T., & Quilliam, M. A. (2006). Discovery of fatty acid ester metabolites of spirolide toxins in mussels from Norway using liquid chromatography/tandem mass spectrometry. Rapid Communications in Mass Spectrometry: An International Journal Devoted to the Rapid Dissemination of Up-To-The-Minute Research in Mass Spectrometry, 20, 1531–1537.
Akaishi, F. M., St-Jean, S. D., Bishay, F., Clarke, J., da, S. R. I., & de Oliveira Ribeiro, C. A. (2007). Immunological responses, histopathological finding and disease resistance of blue mussel (Mytilus edulis) exposed to treated and untreated municipal wastewater. Aquatic Toxicology, 82, 1–14.
Akcha, F., Izuel, C., Venier, P., Budzinski, H., Burgeot, T., & Narbonne, J. (2000). Enzymatic biomarker measurement and study of DNA adduct formation in benzo [a] pyrene-contaminated mussels, Mytilus galloprovincialis. Aquatic Toxicology, 49, 269–287.
Al-Fanharawi, A. A., Rabee, A. M., & Al-Mamoori, A. M. J. (2018). Biochemical and molecular alterations in freshwater mollusks as biomarkers for petroleum product, domestic heating oil. Ecotoxicology and Environmental Safety, 158, 69–77.
Aouini, F., Trombini, C., Volland, M., Elcafsi, M., & Blasco, J. (2018). Assessing lead toxicity in the clam Ruditapes philippinarum: Bioaccumulation and biochemical responses. Ecotoxicology and Environmental Safety, 158, 193–203.
Arienzo, M., Toscanesi, M., Trifuoggi, M., Ferrara, L., Stanislao, C., Donadio, C., et al. (2019). Contaminants bioaccumulation and pathological assessment in Mytilus galloprovincialis in coastal waters facing the brownfield site of Bagnoli, Italy. Marine Pollution Bulletin, 140, 341–352.
Arrighetti, F., Landro, S. M., Lambre, M. E., Penchaszadeh, P. E., & Teso, V. (2019). Multiple-biomarker approach in the assessment of the health status of a novel sentinel mussel Brachidontes rodriguezii in a harbor area. Marine Pollution Bulletin, 140, 451–461.
Aslan, E., Ugur Gorgun, A., Katalay, S., Filizok, I., Becerik, S., & Aydemir, T. (2018). An investigation on the seasonal variations of the biomarkers of oxidative stress response and their correlations to Polonium-210 in mussel (Mytilus galloprovincialis) and common sole (Solea solea) from Izmir Bay, Turkey. Journal of Environmental Radioactivity, 189, 103–108.
Atli, G., Alptekin, Ö., Tükel, S., & Canli, M. (2006). Response of catalase activity to Ag+, Cd2+, Cr6+, Cu2+ and Zn2+ in five tissues of freshwater fish Oreochromis niloticus. Comparative Biochemistry and Physiology Part C: Toxicology & Pharmacology, 143, 218–224.
Bakhmet, I., Berger, V. J., & Khalaman, V. (2005). The effect of salinity change on the heart rate of Mytilus edulis specimens from different ecological zones. Journal of Experimental Marine Biology and Ecology, 318, 121–126.
Balbi, T., Fabbri, R., Montagna, M., Camisassi, G., & Canesi, L. (2017). Seasonal variability of different biomarkers in mussels (Mytilus galloprovincialis) farmed at different sites of the Gulf of La Spezia, Ligurian sea, Italy. Marine Pollution Bulletin, 116, 348–356.
Bao, M., Huo, L., Wu, J., Ge, D., Lv, Z., Chi, C., et al. (2018). A novel biomarker for marine environmental pollution of CAT from Mytilus coruscus. Marine Pollution Bulletin, 127, 717–725.
Barillet, S., Adam-Guillermin, C., Palluel, O., Porcher, J. M., & Devaux, A. (2011). Uranium bioaccumulation and biological disorders induced in zebrafish (Danio rerio) after a depleted uranium waterborne exposure. Environmental Pollution, 159, 495–502.
Beiras, R. (2018). Biological Tools for Monitoring (pp. 265–291)
Benali, I., Boutiba, Z., Grandjean, D., de Alencastro, L. F., Rouane-Hacene, O., & Chevre, N. (2017). Spatial distribution and biological effects of trace metals (Cu, Zn, Pb, Cd) and organic micropollutants (PCBs, PAHs) in mussels Mytilus galloprovincialis along the Algerian west coast. Marine Pollut. Bull., 115, 539–550.
Blanco-Rayon, E., Guilhermino, L., Irazola, M., Ivanina, A. V., Sokolova, I. M., Izagirre, U., & Marigomez, I. (2019). The influence of short-term experimental fasting on biomarker responsiveness in oil WAF exposed mussels. Aquatic Toxicol., 206, 164–175.
Boukadida, K., Cachot, J., Clerandeaux, C., Gourves, P. Y., & Banni, M. (2017). Early and efficient induction of antioxidant defense system in Mytilus galloprovincialis embryos exposed to metals and heat stress. Ecotoxicology and Environmental Safety, 138, 105–112.
Bouzahouane, H., Barour, C., Sleimi, N., & Ouali, K. (2018). Multi-biomarkers approach to the assessment of the southeastern Mediterranean Sea health status: Preliminary study on Stramonita haemastoma used as a bioindicator for metal contamination. Chemosphere, 207, 725–741.
Box, A., Sureda, A., Galgani, F., Pons, A., & Deudero, S. (2007). Assessment of environmental pollution at Balearic Islands applying oxidative stress biomarkers in the mussel Mytilus galloprovincialis. Comparative Biochemistry and Physiology Part C: Toxicology & Pharmacology, 146, 531–539.
Brooks, S. J., Farmen, E., Heier, L. S., Blanco-Rayon, E., & Izagirre, U. (2015). Differences in copper bioaccumulation and biological responses in three Mytilus species. Aquatic Toxicology, 160, 1–12.
Bussell, J. A., Gidman, E. A., Causton, D. R., Gwynn-Jones, D., Malham, S. K., Jones, M. L. M., et al. (2008). Changes in the immune response and metabolic fingerprint of the mussel, Mytilus edulis (Linnaeus) in response to lowered salinity and physical stress. Journal of Experimental Marine Biology and Ecology, 358, 78–85.
Cappello, T., Maisano, M., Mauceri, A., & Fasulo, S. (2017). (1)H NMR-based metabolomics investigation on the effects of petrochemical contamination in posterior adductor muscles of caged mussel Mytilus galloprovincialis. Ecotoxicology and Environmental Safety, 142, 417–422.
Cappello, T., Mauceri, A., Corsaro, C., Maisano, M., Parrino, V., Lo Paro, G., et al. (2013). Impact of environmental pollution on caged mussels Mytilus galloprovincialis using NMR-based metabolomics. Marine Pollution Bulletin, 77, 132–139.
Carregosa, V., Velez, C., Soares, A. M., Figueira, E., & Freitas, R. (2014). Physiological and biochemical responses of three Veneridae clams exposed to salinity changes. Comparative Biochemistry and Physiology Part B: Biochemistry and Molecular Biology, 177, 1–9.
Casas, S., & Bacher, C. (2006). Modelling trace metal (Hg and Pb) bioaccumulation in the Mediterranean mussel, Mytilus galloprovincialis, applied to environmental monitoring. Journal of Sea Research, 56, 168–181.
Chatel, A., Hamer, B., Talarmin, H., Dorange, G., Schroder, H. C., & Muller, W. E. (2010). Activation of MAP kinase signaling pathway in the mussel Mytilus galloprovincialis as biomarker of environmental pollution. Aquatic Toxicology, 96, 247–255.
Coppola, F., Almeida, A., Henriques, B., Soares, A., Figueira, E., Pereira, E., & Freitas, R. (2017). Biochemical impacts of Hg in Mytilus galloprovincialis under present and predicted warming scenarios. Science of the Total Environment, 601–602, 1129–1138.
da Silva Barreto, J., de Melo Tarouco, F., de Godoi, F. G. A., Geihs, M. A., Abreu, F. E. L., Fillmann, G., et al. (2018). Induction of oxidative stress by chlorothalonil in the estuarine polychaete Laeonereis acuta. Aquatic Toxicology, 196, 1–8.
Damiens, G., His, E., Gnassia-Barelli, M., Quiniou, F., & Roméo, M. (2004). Evaluation of biomarkers in oyster larvae in natural and polluted conditions. Comparative Biochemistry and Physiology Part C: Toxicology & Pharmacology, 138, 121–128.
de-la-Ossa-Carretero, J. A., Del-Pilar-Ruso, Y., Loya-Fernández, A., Ferrero-Vicente, L. M., Marco-Méndez, C., Martinez-Garcia, E., and Sánchez-Lizaso, J. L. (2016). Response of amphipod assemblages to desalination brine discharge: Impact and recovery. Estuarine, Coastal and Shelf Science 172, 13-23.
Del-Pilar-Ruso, Y., De-la-Ossa-Carretero, J. A., Giménez-Casalduero, F., & Sánchez-Lizaso, J. L. (2008). Effects of a brine discharge over soft bottom Polychaeta assemblage. Environmental Pollution, 156, 240–250.
Dellali, M., Romeo, M., & Aissa, P. (2001). Suivi annuel de l’activité catalase chez des moules et des palourdes originaires de la lagune de Bizerte. Oceanologica Acta, 24, 263–271.
Di Giulio, R. T., Habig, C., & Gallagher, E. P. (1993). Effects of Black Rock Harbor sediments on indices of biotransformation, oxidative stress, and DNA integrity in channel catfish. Aquatic Toxicology, 26, 1–22.
Dong, S., Yang, Y., Cheng, B., Ren, C., Zhang, H., Xu, H., et al. (2019). Responses of antioxidant defenses in the clam Mactra veneriformis to 2,2’,4,4’tetrabromodiphenyl ether exposure. Comparative Biochemistry and Physiology Part C: Toxicology & Pharmacology, 217, 98–105.
Ducrot, V. (2005). Compréhension et modélisation des relations entre les caractéristiques biologiques et écologiques et la sensibilité aux contaminants des communautés d'invertébrés benthiques: perspectives pour l'évaluation des effets des substances chimiques. Université Paul Verlaine-Metz.
e Silva, C. A. R., Smith, B. D., & Rainbow, P. S. (2006). Comparative biomonitors of coastal trace metal contamination in tropical South America (N. Brazil). Marine Environmental Research, 61, 439–455.
Faggio, C., Tsarpali, V., & Dailianis, S. (2018). Mussel digestive gland as a model tissue for assessing xenobiotics: An overview. Science of the Total Environment, 636, 220–229.
Fernández-Torquemada, Y., Gónzalez-Correa, J. M., Loya, A., Ferrero, L. M., Díaz-Valdés, M., & Sánchez-Lizaso, J. L. (2009). Dispersion of brine discharge from seawater reverse osmosis desalination plants. Desalination and Water Treatment, 5, 137–145.
Fernández-Torquemada, Y., & Sánchez-Lizaso, J. L. (2011). Responses of two Mediterranean seagrasses to experimental changes in salinity. Hydrobiologia, 669, 21.
Fernández-Torquemada, Y., Sánchez-Lizaso, J. L., & González-Correa, J. M. (2005). Preliminary results of the monitoring of the brine discharge produced by the SWRO desalination plant of Alicante (SE Spain). Desalination, 182, 395–402.
Flammarion, P., Devaux, A., & Garric, J. (2001). Marqueurs biochimiques de pollution dans les écosystèmes aquatiques continentaux. Exemples d'utilisation et perspectives pour le gestionnaire. Bulletin Français de la Pêche et de la Pisciculture, 209–226.
Forbes, V. E., Palmqvist, A., & Bach, L. (2006). The use and misuse of biomarkers in ecotoxicology. Environmental Toxicology and Chemistry, 25, 272–280.
Freitas, R., De Marchi, L., Bastos, M., Moreira, A., Velez, C., Chiesa, S., et al. (2017). Effects of seawater acidification and salinity alterations on metabolic, osmoregulation and oxidative stress markers in Mytilus galloprovincialis. Ecological Indicators, 79, 54–62.
Geffard, A., Amiard-Triquet, C., Amiard, J. C., & Mouneyrac, C. (2001). Temporal variations of metallothionein and metal concentrations in the digestive gland of oysters (Crassostrea gigas) from a clean and a metal-rich site. Biomarkers, 6, 91–107.
Gherras Touahri, H., Boutiba, Z., Benguedda, W., & Shaposhnikov, S. (2016). Active biomonitoring of mussels Mytilus galloprovincialis with integrated use of micronucleus assay and physiological indices to assess harbor pollution. Marine Pollution Bulletin, 110, 52–64.
Gonzalez-Fernandez, C., Albentosa, M., & Sokolova, I. (2017). Interactive effects of nutrition, reproductive state and pollution on molecular stress responses of mussels, Mytilus galloprovincialis Lamarck, 1819. Marine Environment Research, 131, 103–115.
Gorbi, S., Avio, G. C., Benedetti, M., Totti, C., Accoroni, S., Pichierri, S., et al. (2013). Effects of harmful dinoflagellate Ostreopsis cf. ovata exposure on immunological, histological and oxidative responses of mussels Mytilus galloprovincialis. Fish & Shellfish Immunology, 35, 941–950.
Gorbi, S., Lamberti, C. V., Notti, A., Benedetti, M., Fattorini, D., Moltedo, G., & Regoli, F. (2008). An ecotoxicological protocol with caged mussels, Mytilus galloprovincialis, for monitoring the impact of an offshore platform in the Adriatic sea. Marine Environment Research, 65, 34–49.
Hamer, B., Jakšić, Ž, Pavičić-Hamer, D., Perić, L., Medaković, D., Ivanković, D., et al. (2008). Effect of hypoosmotic stress by low salinity acclimation of Mediterranean mussels Mytilus galloprovincialis on biological parameters used for pollution assessment. Aquatic Toxicology, 89, 137–151.
Jean-Claude, A., & Claude, A.-T. (2008). Les biomarqueurs dans l'évaluation de l'état écologique des milieux aquatiques. Lavoisier.
Jing, W., Lang, L., Lin, Z., Liu, N., & Wang, L. (2019). Cadmium bioaccumulation and elimination in tissues of the freshwater mussel Anodonta woodiana. Chemosphere, 219, 321–327.
Koch, M., Schopmeyer, S., Kyhn-Hansen, C., Madden, C., & Peters, J. (2007). Tropical seagrass species tolerance to hypersalinity stress. Aquatic Botany, 86, 14–24.
Kopecka, J., Lehtonen, K. K., Barsiene, J., Broeg, K., Vuorinen, P. J., Gercken, J., & Pempkowiak, J. (2006). Measurements of biomarker levels in flounder (Platichthys flesus) and blue mussel (Mytilus trossulus) from the Gulf of Gdansk (southern Baltic). Marine Pollution Bulletin, 53, 406–421.
Lemaire, N., Pellerin, J., Fournier, M., Girault, L., Tamigneaux, E., Cartier, S., & Pelletier, E. (2006). Seasonal variations of physiological parameters in the blue mussel Mytilus spp. from farm sites of eastern Quebec. Aquaculture, 261, 729–751.
Maanan, M. (2008). Heavy metal concentrations in marine molluscs from the Moroccan coastal region. Environmental Pollution, 153, 176–183.
Marín-Guirao, L., Sandoval-Gil, J. M., Bernardeau-Esteller, J., Ruíz, J. M., & Sánchez-Lizaso, J. L. (2013). Responses of the Mediterranean seagrass Posidonia oceanica to hypersaline stress duration and recovery. Marine Environment Research, 84, 60–75.
Mejdoub, Z., Fahde, A., Loutfi, M., & Kabine, M. (2017). Oxidative stress responses of the mussel Mytilus galloprovincialis exposed to emissary’s pollution in coastal areas of Casablanca. Ocean & Coastal Management, 136, 95–103.
Mucci, A. (1997). Chimie des Milieux Aquatiques. Chimie des Eaux Naturelles et des Interfaces dans l’Environnement. Geochimica Et Cosmochimica Acta, 61, 2158–2159.
Murray, J. B., & Wingard, G. L. (2006). Salinity and temperature tolerance experiments on selected Florida Bay mollusks. Rep. No. 2331–1258.
Nardi, A., Benedetti, M., d’Errico, G., Fattorini, D., & Regoli, F. (2018). Effects of ocean warming and acidification on accumulation and cellular responsiveness to cadmium in mussels Mytilus galloprovincialis: Importance of the seasonal status. Aquatic Toxicology, 204, 171–179.
Oliveira, P., Lírio, A. V., Canhoto, C., & Guilhermino, L. (2018). Toxicity of mercury and post-exposure recovery in Corbicula fluminea: Neurotoxicity, oxidative stress and oxygen consumption. Ecological Indicators, 91, 503–510.
Pellerin, J., & Amiard, J.-C. (2009). Comparison of bioaccumulation of metals and induction of metallothioneins in two marine bivalves (Mytilus edulis and Mya arenaria). Comparative Biochemistry and Physiology Part C: Toxicology & Pharmacology, 150, 186–195.
Peric, L., Nerlovic, V., Zurga, P., Zilic, L., & Ramsak, A. (2017). Variations of biomarkers response in mussels Mytilus galloprovincialis to low, moderate and high concentrations of organic chemicals and metals. Chemosphere, 174, 554–562.
Perosevic, A., Joksimovic, D., Durovic, D., Milasevic, I., Radomirovic, M., & Stankovic, S. (2018). Human exposure to trace elements via consumption of mussels Mytilus galloprovincialis from Boka Kotorska Bay, Montenegro. Journal of Trace Elements in Medicine and Biology, 50, 554–559.
Pol, J. S. M. (2015). Environment programme mediterranean action plan.
Richir, J., & Gobert, S. (2014). The effect of size, weight, body compartment, sex and reproductive status on the bioaccumulation of 19 trace elements in rope-grown Mytilus galloprovincialis. Ecological Indicators, 36, 33–47.
Risso-de Faverney, C., Guibbolini-Sabatier, M. E., & Francour, P. (2010). An ecotoxicological approach with transplanted mussels (Mytilus galloprovincialis) for assessing the impact of tyre reefs immersed along the NW Mediterranean Sea. Marine Environment Research, 70, 87–94.
Ruíz, J. M., Marín-Guirao, L., & Sandoval-Gil, J. M. (2009). Responses of the Mediterranean seagrass Posidonia oceanica to in situ simulated salinity increase. Botanica Marina, 52, 459–470.
Ruso, Y. D. P., De la Ossa Carretero, J. A., Casalduero, F. G., & Lizaso, J. S. (2007). Spatial and temporal changes in infaunal communities inhabiting soft-bottoms affected by brine discharge. Marine Environment Research, 64, 492–503.
Sanchez-Lizaso, J. L., Romero, J., Ruiz, J., Gacia, E., Buceta, J. L., Invers, O., et al. (2008). Salinity tolerance of the Mediterranean seagrass Posidonia oceanica: Recommendations to minimize the impact of brine discharges from desalination plants. Desalination, 221, 602–607.
Sandoval-Gil, J. M., Marín-Guirao, L., & Ruiz, J. M. (2012). Tolerance of Mediterranean seagrasses (Posidonia oceanica and Cymodocea nodosa) to hypersaline stress: Water relations and osmolyte concentrations. Marine Biology, 159, 1129–1141.
Schintu, M., Durante, L., Maccioni, A., Meloni, P., Degetto, S., & Contu, A. (2008). Measurement of environmental trace-metal levels in Mediterranean coastal areas with transplanted mussels and DGT techniques. Marine Pollution Bulletin, 57, 832–837.
Sillero-Rios, J., Sureda, A., Capo, X., Oliver-Codorniu, M., & Arechavala-Lopez, P. (2018). Biomarkers of physiological responses of Octopus vulgaris to different coastal environments in the western Mediterranean Sea. Marine Pollution Bulletin, 128, 240–247.
Silva Dos Santos, F., Neves, R. A. F., Carvalho, W. F., Krepsky, N., & Crapez, M. A. C. (2018). Evaluation of the immune responses of the brown mussel Perna perna as indicators of fecal pollution. Fish & Shellfish Immunology, 80, 115–123.
Sparks, C., Marnewick, J., Toefy, R., Snyman, R., & Odendaal, J. (2019). Baseline levels of antioxidant activities in Mytilus galloprovincialis along the coast of Cape Town, South Africa. Marine Pollution Bulletin, 140, 287–293.
Teixeira, M., Almeida, A., Calisto, V., Esteves, V. I., Schneider, R. J., Wrona, F. J., et al. (2017). Toxic effects of the antihistamine cetirizine in mussel Mytilus galloprovincialis. Water Research, 114, 316–326.
Verlecar, X., Jena, K., & Chainy, G. (2008). Seasonal variation of oxidative biomarkers in gills and digestive gland of green-lipped mussel Perna viridis from Arabian Sea. Estuarine, Coastal and Shelf Science, 76, 745–752.
Vernon, E. L., & Jha, A. N. (2019). Assessing relative sensitivity of marine and freshwater bivalves following exposure to copper: Application of classical and novel genotoxicological biomarkers. Mutation Research, 842, 60–71.
Vlahogianni, T., Dassenakis, M., Scoullos, M. J., & Valavanidis, A. (2007). Integrated use of biomarkers (superoxide dismutase, catalase and lipid peroxidation) in mussels Mytilus galloprovincialis for assessing heavy metals’ pollution in coastal areas from the Saronikos Gulf of Greece. Marine Pollution Bulletin, 54, 1361–1371.
Wang, Y., Hu, M., Wong, W. H., Shin, P. K., & Cheung, S. G. (2011). The combined effects of oxygen availability and salinity on physiological responses and scope for growth in the green-lipped mussel Perna viridis. Marine Pollution Bulletin, 63, 255–261.
Wiltshire, K. H., Kraberg, A., Bartsch, I., Boersma, M., Franke, H.-D., Freund, J., et al. (2010). Helgoland roads, North Sea: 45 years of change. Estuaries and Coasts, 33, 295–310.
Yavasoglu, A., Ozkan, D., Guner, A., Katalay, S., Oltulu, F., & Yavasoglu, N. U. K. (2016). Histopathological and apoptotic changes on marine mussels Mytilus galloprovincialis (Lamark, 1819) following exposure to environmental pollutants. Marine Pollution Bulletin, 109, 184–191.
Zorita, I., Ortiz-Zarragoitia, M., Apraiz, I., Cancio, I., Orbea, A., Soto, M., et al. (2008). Assessment of biological effects of environmental pollution along the NW Mediterranean Sea using red mullets as sentinel organisms. Environmental Pollution, 153, 157–168.
Acknowledgements
This work could not have happened without the support of some people. I particularly want to thank Mr Djamal Eddine ABASSI for his contribution to the English translation. Also, I am very grateful to Mr Amar DILMI who counted enormously in the outcome of this work. I am also very grateful to the intern Mr Nabil KRITLI.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this paper
Cite this paper
Meknachi, A., Djellali, M., Badis, A. (2021). Ecotoxicological Assessment of Three Types of Wastewater Effluents: Catalase as a Biomarker of Oxidative Stress in Marine Bivalves. In: Al-Maktoumi, A., et al. Water Resources in Arid Lands: Management and Sustainability. Advances in Science, Technology & Innovation. Springer, Cham. https://doi.org/10.1007/978-3-030-67028-3_23
Download citation
DOI: https://doi.org/10.1007/978-3-030-67028-3_23
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-67027-6
Online ISBN: 978-3-030-67028-3
eBook Packages: Earth and Environmental ScienceEarth and Environmental Science (R0)