Abstract
Even though Campylobacter spp. are known to be fastidious organisms, they can survive within the natural environment. One mechanism to withstand unfavourable conditions is the formation of biofilms, a multicellular structure composed of different bacterial and other microbial species which are embedded in an extracellular matrix. High oxygen levels, low substrate concentrations and the presence of external DNA stimulate the biofilm formation by C. jejuni. These external factors trigger internal adaptation processes, e.g. via regulating the expression of genes encoding proteins required for surface structure formation, as well as motility, stress response and antimicrobial resistance. Known genes impacting biofilm formation will be summarized in this review. The formation of biofilms as well as the expression of virulence genes is often regulated in a cell density depending manner by quorum sensing, which is mediated via small signalling molecules termed autoinducers. Even though quorum sensing mechanisms of other bacteria are well understood, knowledge on the role of these mechanisms in C. jejuni biofilm formation is still scarce. The LuxS enzyme involved in generation of autoinducer-2 is present in C. jejuni, but autoinducer receptors have not been identified so far. Phenotypes of C. jejuni strains lacking a functional luxS like reduced growth, motility, oxygen stress tolerance, biofilm formation, adhesion, invasion and colonization are also summarized within this chapter. However, these phenotypes are highly variable in distinct C. jejuni strains and depend on the culture conditions applied.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
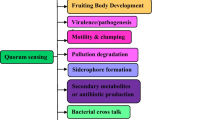
Compared to other food-borne pathogenic bacteria, Campylobacter spp. are susceptible to various stressors including elevated ambient oxygen concentrations, dehydration and UV-light, which are present in the natural environments and in food processing plants. Nevertheless, Campylobacter spp. are widespread in the environment and persist in the food production chain indicating that these bacteria are capable to survive these unfavourable conditions (Boronowsky et al. 2014; Golz et al. 2018; Hansson et al. 2018; Tram et al. 2020a). However, how they regulate their stress responses and environmental adaptation is still not fully understood as campylobacters are lacking several classical regulatory factors. One microbial strategy to survive within hostile surroundings is the formation of biofilms. Biofilms are organized aggregates of microorganisms encased by an extracellular matrix. This extracellular matrix structures the biofilm and also protects microorganisms from stressful conditions present outside of the biofilm (Kostakiotis et al. 2013). The process of biofilm formation as well as the expression of virulence factors is often coordinated at a multicellular stage, which depends on the detection of the cell density via quorum sensing (QS) systems which are present in many bacteria, fungi and parasites (Mukherjee and Bassler 2019). Within this article, we summarize information on external factors and genes involved in biofilm formation and QS of C. jejuni.
2 Microbial Biofilm Formation
Bacteria can switch from a planktonic single-cell lifestyle to a multicellular lifestyle, e.g. in biofilms, and back to planktonic style. In biofilms, bacterial species live in close contact with communities which can also contain fungi, algae, protists and archaea (Flemming et al. 2016). These biofilms can be found either attached to a surface or as free-floating aggregates, which are both surrounded by a matrix of extracellular polymeric substances (EPS) (Joshua et al. 2006; Roy et al. 2018). Depending on the microorganisms within the biofilm, the EPS consists of proteins, nucleic acids, polysaccharides, lipids and other compounds which form part of highly viscous watery solutions (Flemming et al. 2016). Within these biofilms, microorganisms are protected from several external stressors—such as dehydration, and exposure to oxygen radicals, disinfectants or antimicrobial substances—and grow much more slowly compared to planktonic cells, thereby facilitating survival under unfavourable conditions in diverse environmental niches. Furthermore, microorganisms within biofilms can support each other by exchanging of substrates or by degradation of toxigenic substances (Flemming et al. 2016). The ability to form biofilms and to colonize preformed biofilms as well as the specific architecture of biofilms depends on the microbial composition, the genetic background of the individual strains involved and the environmental conditions.
2.1 Building and Dispersion of Microbial Biofilms
Biofilm formation takes place in three major steps: In the first two steps, the microorganisms build up microcolonies by attachment to surfaces and/or to each other, and the production of EPS establishes the biofilm structure, which matures the microcolonies into a three-dimensional architecture. In the third phase, the microorganisms actively or passively detach from the biofilm and are released back to the planktonic lifestyle. In bacterial biofilms, surface or cell-to-cell attachment is mediated by extracellular adhesive appendages, like flagella, pili or outer membrane proteins, secreted adhesins as well as by the molecular structure and adhesive properties of the abiotic surfaces (Kostakioti et al. 2013). Once the microcolonies are built, multiple regulatory networks translate signals to concerted gene expression changes, which lead to building of the extracellular matrix and mediate the spatial and temporal reorganization of the microbial cells within the final biofilm (Petrova and Sauer 2016). The biofilm matures into a well-organized architecture, with intervening water channels for nutrient and waste exchange which is embedded in a viscous EPS matrix (Coughlan et al. 2016). In the final state, biofilms represent highly dynamic structures, in which the bacteria could disperse passively or actively. Passive dispersal is due to external shear forces or abrasion when the biofilm structure grows (Kaplan 2010). Active dispersion of biofilms is triggered by beneficial conditions outside the biofilm or detrimental conditions inside the biofilm. These include scarcity of substrates including carbon and energy sources, accumulation of signalling molecules and in case of Campylobacter also elevated oxygen levels (Kostakioti et al. 2013, Petrova and Sauer 2016). The release of microorganisms from biofilms is supported by increased motility. Active dispersion of biofilms can be mediated by bacterial secretion of EPS-degrading enzymes including glycosidases, lipases, proteases and deoxyribonuleases, as well as by production of surfactants (Kaplan 2010).
2.2 Methods to Analyse Biofilms
Analysis of biofilms is focussed on the quantification and successful measurement of several multiple parameters including the biomass and architecture of biofilms, the bacterial viability, attachment and motility within biofilms and the composition of the EPS (reviewed by Azeredo et al. 2017). Briefly, the total amount of the biofilm-mass is commonly quantified by indirect staining methods, e.g. by the Crystal Violet Assay, while the viable cell count can be determined by, e.g. direct plating, flow cytometry or live/dead staining combined with confocal laser scanning microscopy (CLSM). CLSM is further applied to study the spatial structure of biofilms. The metabolic activity of bacteria in biofilms can be measured by colorimetric determination of the conversion of tetrazolium salts to formazan by a spectrophotometer. The amount of initially attached bacteria can be quantified by direct plating or microscopic methods.
2.3 Environmental Conditions Influencing Campylobacter jejuni Biofilm Formation
Investigations focused on the biofilm formation capacity of C. jejuni were mostly conducted under laboratory conditions with well-defined reference strains of the pathogen as monospecies biofilms, which do not reflect the situation outside the laboratory (Lamas et al. 2018; Teh et al. 2014). The resulting data demonstrate that C. jejuni bacteria are able to form biofilms on glass, polystyrene and stainless steel surfaces (Joshua et al. 2006; Li et al. 2017; Oh et al. 2016; Teh et al. 2016; Wagle et al. 2019). However, the whole biofilm formation process of C. jejuni is modulated by many extrinsic and intrinsic factors which will be discussed in more detail.
2.3.1 Substrate Availability and Oxygen
Distinct external stress conditions which all depend on the specific metabolic properties of C. jejuni have been found to regulate the biofilm formation and lifestyle of the pathogen (Fig. 1). Corresponding results confirmed that biofilm formation enables C. jejuni to survive hostile environmental conditions. Nutrient availability is a key factor in the regulation of biofilm formation by C. jejuni. Starvation induces biofilm formation by C. jejuni which was indicated by significantly higher biofilm production by bacteria grown in less nutrient-rich Mueller–Hinton medium as compared to bacteria grown in Brucella or Bolton broth (Reeser et al. 2007). Similarly, addition of fucose inhibited biofilm formation of C. jejuni strains encoding enzymes required for fucose utilization (Dwivedi et al. 2016). In contrast, meat exudate significantly enhanced biofilm formation of C. jejuni grown on surfaces or in liquid media. However, this might be rather due to enhanced attachment than to active biofilm formation (Brown et al. 2014; Li et al. 2017; Wagle et al. 2019). In further support of the role of nutrients in biofilm formation, a recent study demonstrated that addition of energy sources such as fumarate and formate enhanced biofilm formation in a microaerobic atmosphere, but reduced biofilm formation under aerobic conditions (Kassem et al. 2017). Supplementation of growth media with formate additionally enhanced biofilm formation under anaerobic conditions (Kassem et al. 2017). In most studies, aerobic atmosphere enhanced the biofilm formation of several C. jejuni strains (Feng et al. 2018; Pascoe et al. 2015; Reuter et al. 2010; Stetsenko et al. 2019; Turonova et al. 2015; Zhong et al. 2020). Results from a recent study revealed that extracellular DNA (eDNA) enhances biofilm formation by C. jejuni (Feng et al. 2018). Interestingly, release of eDNA was induced by exposure of C. jejuni to aerobic conditions. In other studies, however, biofilm formation was similar or even lower if C. jejuni were incubated under aerobic conditions (Kassem et al. 2017; Reeser et al. 2007; Teh et al. 2017). These conflicting results might be explained by the different strains and methods used (see also Section Genetic Background and Genes Impacting Biofilm Formation of C. jejuni). Besides that, also small genomic variations within clones of one strain might influence investigated phenotypes, as recently shown for several clones of the reference strain NCTC11168 by Pascoe and co-workers (2019). Furthermore, the application of the bile salt deoxycholate in sublethal concentrations enhanced the biofilm formation of C. jejuni, while no differences in biofilm formation have been observed by the addition of sublethal concentrations of other detergents, such as Triton X-100, Tween-20 or sodium dodecyl sulphate (Svensson et al. 2009). In contrast, osmotic stress generated by NaCl, glucose or sucrose inhibited biofilm formation of C. jejuni (Reeser et al. 2007). The knowledge about the influence of temperature on C. jejuni biofilm production is still scarce. In two studies, biofilm production was higher if C. jejuni was incubated at 37 °C as compared to 25 °C or 20 °C, respectively (Reeser et al. 2007; Wagle et al. 2019). Taken together, biofilm formation of C. jejuni is influenced by multiple factors. Under laboratory conditions, biofilm formation was induced by nutrient starvation and oxygen stress, while osmotic stress rather reduced the biofilm formation. However, as the results obtained by the studies described above were generated in artificial systems, the transferability of these results to the real world is only limited. The multitude of conflicting results obtained in this highly innovative field of research underlines the urgent need for standardization and better control of future studies on factors influencing C. jejuni biofilm formation as a major mechanism to survive outside the vertebrate hosts.
Impact of environmental conditions relevant to formation and survival of C. jejuni biofilms. The biofilm formation by C. jejuni is enhanced by starvation, aerobic stress, extracellular DNA (eDNA), sublethal bile salt (desoxycholate, DOC sub) concentrations, formate at microaerobic (micro) and anaerobic (ana) conditions, as well as surface coating with meat exudates. Osmotic stress (induced by NaCl, glucose and sucrose), fumarate and formate at aerobic conditions and fucose decreased biofilm formation
2.3.2 Other Bacterial Species in Multispecies Biofilms
Even though C. jejuni forms biofilms in monocultures, the biomass of these monospecies biofilms is much lower as compared to biofilms formed by monocultures of Pseudomonas aeruginosa or Escherichia coli. If C. jejuni were co-cultivated in biofilms with E. coli, P. aeruginosa, Enterococcus faecalis, Salmonella enterica or Staphylococcus simulans, the survival of C. jejuni was prolonged as compared to monocultured cells, and the biofilm-mass was increased to levels produced by the co-cultured species (Feng et al. 2016; Indikova et al. 2015; Teh et al. 2019, 2010; Zhong et al. 2020). Furthermore, it has been demonstrated that in C. jejuni-Salmonella dual-species biofilms C. jejuni is located at the bottom of the biofilms in areas with high eDNA concentrations, while Salmonella is located at the top of the biofilm and in areas where less eDNA is present (Feng et al. 2018). It was assumed that other bacteria in co-cultures establish a more favourable environment, e.g. by lowering the oxygen level, providing CO2 and alteration of metabolite concentrations (Zhong et al. 2020). Taken together, these results indicate that C. jejuni is able to colonize multispecies biofilms but the use of multispecies biofilms as a target for pathogen control via biosafety measures awaits further investigations.
2.3.3 Antimicrobial Substances
Within biofilms, microorganisms are protected against the antimicrobial activities of various substances including well-established antibiotics (Sharma et al. 2019). The molecular mechanisms by which biofilms protect bacteria from antimicrobial activity are multifactorial. The EPS structure hampers penetration of distinct antibiotics and can contain enzymes which actively inactivate antibiotics by molecular modifications (Hall and Mah 2017). In addition, the dormant state of bacteria in biofilms may passively enhance the tolerance to antimicrobial substances (Petrova and Sauer 2016). On the other hand, the close cell proximity within biofilms and the eDNA in the EPS structure support horizontal gene transfer. In accordance, C. jejuni transfers chromosomally encoded antibiotic resistance genes more frequently in biofilms as compared to bacteria in the planktonic lifestyle (Bae et al. 2014). Furthermore, antibiotic resistance is also influencing the biofilm formation ability of C. jejuni strains. Of 206 C. jejuni and C. coli strains isolated from poultry products, biofilm-producing strains possessed a significantly higher resistance to ampicillin, neomycin, sulfamethoxazole, amikacin, clindamycin and erythromycin as compared to strains unable to form biofilms (Zhang et al. 2017). Another study reported that fluoroquinolone resistance of C. jejuni is associated with an increased ability to form biofilms in oxygen-rich environments (Whelan et al. 2019). These aspects of enhanced antimicrobial resistance gene transfer within biofilms and higher biofilm formation in antibiotic resistant strains indicate the necessity to control and reduce C. jejuni biofilms.
2.4 Genetic Background and Genes Impacting Biofilm Formation of C. jejuni
The transition from planktonic lifestyle to the embedding of bacterial cells in the biofilm matrices goes along with substantial alterations in gene expression, which result in the production of adhesive surface molecules and in a comprehensive metabolic reprogramming (Kostakiotis et al. 2013). Recently, it has been reported that the expression of approx. 600 genes was differentially regulated during the biofilm formation of C. jejuni, with increased expression of genes involved in iron metabolism and acquisition, cell division, glycan production and attachment and reduced expression of genes involved in energy metabolism, amino acid catabolism and chemotaxis (Tram et al. 2020b). However, which of these changes are responsible for biofilm formation itself or which are going along with altered lifestyle in the established biofilm have to be determined. Nevertheless, several genes, impacting the biofilm formation capacity of C. jejuni, are summarized in Table 1, and their putative involvement in the biofilm formation process is described in more detail below.
2.4.1 Genetic Background of Individual C. jejuni Strains
The composition of genes differentially regulated during biofilm formation and genes directly involved in the synthesis of biofilm matrix molecules is highly variable in genomes of individual C. jejuni strains. These differences are suspected to be responsible for the fact that some strains of the pathogen form only weak or nearly no biofilm-mass and others produce biofilm-mass in large amounts (Bronnec et al. 2016; Feng et al. 2018; Joshua et al. 2006; Melo et al. 2017). For example, C. jejuni strains encoding for extracellular DNases, mostly located on the mobile elements CJIE1, CJIE2 and CJIE4, are unable or only poor biofilm producer and are further able to remove pre-established biofilms of other C. jejuni strains (Brown et al. 2015).
Moreover, biofilm formation capacities of individual C. jejuni isolates are significantly associated with distinct multilocus sequence types (MLST) and with several clonal complexes, which display specific features concerning host adaptation, termed host-generalists and host-specialists, respectively (see also Pascoe et al. 2015 and Chapters “Population Biology and Comparative Genomics of Campylobacter Species” and “Emission Sources of Campylobacter from Agricultural Farms, Impact on Environmental Contamination and Intervention Strategies” in this book). It is of note that a strong biofilm formation capacity of C. jejuni isolates is correlated with the absence of specific host adaptation, leading to the fact that the host-generalist group of C. jejuni isolates displays an enhanced capacity for biofilm formation. Furthermore, nearly 2/3 of the C. jejuni isolates belonging to the chicken-specialists belonged to the group of weak biofilm producers (Pascoe et al. 2015). Even though genes with a robust association to biofilm formation differed between the isolates of the host-generalist group, most of these genes are involved in adhesion, motility, glycosylation, capsular polysaccharides and oxidative stress response (Pascoe et al. 2015). Taken together, these findings provide evidence that the genomic repertoire necessary for biofilm formation is highly variable within C. jejuni isolates and that biofilm formation is more important for isolates that are not adapted to specific vertebrate hosts.
2.4.2 Flagella-Associated Genes and Motility
Besides the involvement in motility and chemotaxis, the flagella of C. jejuni is also crucial for secretion of proteins, autoagglutination, microcolony formation and avoidance of the innate immune response (Guerry 2007), indicating that mutation of the flagella might have multifactorial effects. Generally, motility mediated by flagella is essential for the biofilm formation capacity of C. jejuni. Loss of motility caused by targeted mutation of flagella-associated C. jejuni genes flhA, fliA, flaA, flaB, flaC, flaG, flgA and fliS, resulted in impaired biofilm formation (Feng et al. 2018; Joshua et al. 2006; Kalmokoff et al. 2006; Kim et al. 2015; Li et al. 2017; Reuter et al, 2010; Turonova et al. 2015). Besides the fact that flagella-associated motility is essential to reach substrates where biofilms can be formed, also flagella-associated attachment seems to impact C. jejuni biofilm formation. This was supported by the observation that aflagellated C. jejuni mutants (mutation of flhA) formed less biofilm-mass as compared to pflA mutants with paralyzed flagella only (Svensson et al. 2014). Furthermore, the biofilm formation capacity of C. jejuni depends on flagellar O-linked glycan modifications. This was shown by targeted deletion of the cj1324 gene, which resulted in the loss of flagellar sugar modifications and reduced biofilm formation but does not alter the motility (Howard et al. 2009). Additionally, the reduced biofilm-mass formation of a flaA/flaB mutant could be restored by addition of chicken meat exudate (Li et al. 2017). Taken together, these findings indicate that surface attachment mediated by the flagella is essential for C. jejuni biofilm formation.
2.4.3 Chemotaxis-Associated Genes
Directed movement of bacteria is interactively controlled and directed by the sensing of attractants or repellents by transducer-like proteins (Tlp). The activation of Tlp results in a signalling cascade mediated by the Che proteins, which modulate flagellar rotation (Tram et al. 2020b). Deletion of cheY and cheW genes in C. jejuni enhanced the formation of biofilm-mass, even though motility of both mutants was significantly reduced in the planktonic state (Tram et al. 2020b). The authors suggested that the enhanced biofilm-mass production could be due to the higher autoagglutination displayed by these mutants. In contrast, defects in robust biofilm formation at the air-media interface were reported for C. jejuni mutants lacking functional cheA, cheY, cheW or cheV genes (Reuter et al. 2020). The authors concluded that the chemotaxis signalling system is rather necessary for organized biofilm formation at the air-media interface than for biofilm formation per se. The contradicting findings described in these studies might also be due to differences in the experimental conditions or biofilm detection assays applied. Moreover, deletion of the chemoreceptor Tlp3 resulted in enhanced biofilm formation, while deletion of Tlp8 resulted in reduced biofilm formation rates by respective C. jejuni mutants (Chandrashekhar et al. 2015; Rahman et al. 2014). These data indicate that distinct chemotactic compounds as well as chemotaxis signalling pathway are essentially involved in biofilm formation by C. jejuni.
2.4.4 Stress Response-Associated Genes
The influence of oxidative stress on the biofilm formation capacity of C. jejuni has been intensively investigated at the molecular level. Deletion of alkyl hydroperoxide reductase (ahpC) and catalase A (katA) genes increased biofilm formation by the respective mutant strains (Oh and Jeon 2014). Results from confocal laser scanning microscopy support the assumption that AhpC is involved in the development of C. jejuni microcolonies at the early stages of biofilm formation. This role of ahpC was further confirmed elegantly by genetic manipulation of perR and cosR genes encoding positive and negative regulators of ahpC, respectively (Oh and Jeon 2014; Turonova et al. 2015). The important role of oxygen stress responses in biofilm formation of C. jejuni was further confirmed by the finding that deletion of the sensor for the Campylobacter planktonic growth regulation system (cprS) reduced oxidative stress resistance, but enhanced biofilm formation in respective mutants (Svensson et al. 2009). However, deletion of the gene encoding the major carbon-starvation regulator csrA also rendered C. jejuni more prone to aerobic stress but reduced the biofilm formation capacity, which is in contrast to many other bacteria in which csrA represses the biofilm formation (Fields and Thompson 2008). However, given that the translation of more than 100 genes is dysregulated in a csrA mutant, it is difficult to determine which of them are responsible for the observed phenotype (Fields et al. 2016; El Abbar et al. 2019). The role of csrA in biofilm formation of C. jejuni is further supported by the fact that deletion of the gene encoding polyphosphate acetyltransferase Pta (Cj0688), also under post-transcriptional control of csrA, resulted in reduced biofilm formation (Joshua et al. 2006). Additionally, a C. jejuni mutant lacking the gene for the iron-binding protein Dps displayed increased susceptibility to H2O2 but reduced biofilm formation (Theoret et al. 2012). Deletion of spoT (involved in the stringent stress response) and recombinase A (recA) enhanced biofilm formation especially at aerobic conditions (Feng et al. 2018; Svensson et al. 2009). In addition, results from both studies demonstrated that the lack of spoT and recA enhanced lysis of the bacteria thereby releasing high molecular DNA, which is one of the prerequisites for bacterial biofilm production.
Finally, the C. jejuni biofilm production is linked to intracellular levels of inorganic polyphosphates, which play crucial roles in stress tolerance and virulence of the pathogen (Kumar et al. 2016). Deletion of genes coding for both polyphosphate kinases Pkk 1 and Pkk 2 as well as for the alkaline phosphatase PhoX (Cj0145) resulted in enhanced C. jejuni biofilm production and surface attachment. (Drozd et al. 2014; Gangaiah et al. 2009, 2010). Taken together these data demonstrate that various stressors induce biofilm formation of C. jejuni via activation of the major stress response regulons known to date.
2.4.5 Surface Structure-Associated Genes
The production of the peptidyl prolyl cis–trans isomerase Peb4, involved in folding of integral outer membrane proteins, is increased in C. jejuni cells living in biofilms (Kalmokoff et al. 2006). Mutational analysis of the corresponding gene revealed that Peb4 is required for both adhesion and attachment of C. jejuni to host cells in vitro and for biofilm-mass formation (Asakura et al. 2007). In contrast, deletion of this gene in another C. jejuni strain resulted in enhanced biofilm-mass formation (Rathbun et al. 2009). These conflicting results might be due to strain-specific variations in the genetic background or by polar effects of the mutation strategy, but this awaits further evaluation. In addition, protein glycosylation is essentially involved in C. jejuni biofilm formation. Mutational analysis of the pglB gene by targeted deletion revealed that N-linked protein glycosylation reduces the biofilm formation capacity of C. jejuni, is required for resistance to heat and salt but decreases the resistance to peroxide (Cain et al. 2019). In contrast, N-linked protein glycosylation mediated by EptC enhances biofilm formation, indicating that the modulation of biofilm formation by N-linked glycosylation is highly dependent on the glycosylated proteins involved (Cullen et al. 2013; Lim and Kim 2017; Scott et al. 2012). Finally, C. jejuni lipooligosaccharide (LOS) structures influence the biofilm formation capacity as indicated by enhanced biofilm formation in C. jejuni waaF or lgtF deletion mutants with truncated LOS. However, mutational analysis by targeted deletion revealed that LOS modifications by GalT or CstII enzymes did not influence the biofilm-mass, which was comparable in deletion mutants and the wild-type strain (Naito et al. 2010). Besides the LOS surface structure, C. jejuni has the ability to coat its surface with a polysaccharide capsule (CPS), being the major serodeterminant of the Penner scheme (Karlyshev et al. 2000). Given that polysaccharides are a common component in the EPS, the knowledge about the impact of CPS on the biofilm formation capacity of C. jejuni is still scarce. Deletion of the gene kpsM, involved in the transport of capsular polysaccharides across the inner membrane, resulted in enhanced biofilm formation of this uncapsulated C. jejuni mutant (Joshua et al. 2006). However, the mechanisms responsible for this phenotype have to be elucidated in future studies. In conclusion, these observations indicate that glycosylation state of surface molecules is essentially involved in C. jejuni biofilm formation.
2.5 Control Strategies Targeting C. jejuni Biofilms
Given that the EPS structure of biofilms protects the microorganisms from physical, chemical and environmental stresses, disruption of the EPS structure is a favoured strategy to combat bacterial pathogens in biofilms (Devaraj et al. 2019). Since eDNA is an essential component of the EPS produced by many bacteria, DNase treatment is a promising measure for inhibition of biofilm formation and for the degradation of established biofilms which has been also successfully proven for C. jejuni biofilms (Brown et al. 2015; Feng et al. 2018; Sharma and Pagedar Singh 2018; Svensson et al. 2014). In addition, treatment of C. jejuni with the phytochemicals trans-cinnamonaldehyde, eugenol and carvacrol before and after biofilm formation reduced the biofilm-mass (Wagle et al. 2019). Application of all three substances at bactericidal concentrations killed the majority of bacterial cells also in mature biofilms within 10 min (Wagle et al. 2019). Notably, sublethal concentrations of these phytochemicals downregulated periplasmic nitrate reductase NapA involved in energy generation and the chaperon DnaK involved in stress responses by C. jejuni cells in the biofilms (Wagle et al. 2019). While the mechanisms by which phytochemicals reduce C. jejuni biofilm formation capacity await further investigation, it seems noteworthy that citrus extracts reduced the biofilm-mass of C. jejuni (Castillo et al. 2014), most likely by reduction of AI-2 activity (as described in Section Phenotypes of C. jejuni luxS Mutants). Finally, biofilm-mass formation by C. jejuni in mono- and multispecies cultures was significantly inhibited by zinc oxide nanoparticles, which are small and have a high oxidative potential (Melo et al. 2017; Zhong et al. 2020). In summary, even though several strategies to inhibit C. jejuni biofilm formation or to eliminate C. jejuni in mature biofilms have been developed, their efficacy as hygiene measures under practical conditions still needs to be investigated in detail.
3 Quorum Sensing
Bacteria adapt their metabolism according to the surrounding environment not only within single cells but also at a multicellular level (Miller et al. 2002). Several processes such as biofilm formation, expression of virulence factors, competence for DNA-uptake or bioluminescence are of particular benefit in multicellular communities (Mukherjee and Bassler 2019). To collectively regulate these processes, bacteria use a cell-to-cell communication system known as quorum sensing (QS). QS is mediated by small signalling molecules, termed autoinducers (AIs), which accumulate in the environment in a cell density dependent manner. The AIs bind to specific bacterial receptors and induce the expression of distinct target genes. Depending on the signalling molecule produced and the presence of appropriate receptors, bacteria can communicate on intra-species, inter-species, inter-genera as well as inter-kingdom levels. The regulation by QS is assumed to be a highly complex process since many QS processes involve more than one signal-receptor combination, exerting their functions in a hierarchical cascade (Abisado et al. 2018). For example, four different QS-pathways are known in P. aeruginosa, namely the Las-, Rhl-, Pqs- and IQS-systems. Expression of virulence genes is regulated by AI-RhlR complex, and for the induction of RhlR-system, one of the other three QS-pathways is required (Papenfort and Bassler 2016). Furthermore, it has been described that some bacteria might only sense an AI without the ability to produce it. This is also true for P. aeruginosa, which does not produce AI-2, whereas AI-2 molecules generated by other bacteria alter the gene expression in this pathogen (Duan et al. 2003).
3.1 Quorum Sensing Signalling Mechanisms
Three major categories of signalling molecules, namely AI-1, AI oligopeptides (AIP) and AI-2, have been described. AI-1 are used by Gram-negative bacteria, while AIP serve as signalling molecules in Gram-positive bacteria. Both Gram-positive and Gram-negative bacteria utilize AI-2 (a furanone) as signalling molecules. To date, additional AI molecules were identified such as the Pseudomonas quinolone signal, diffusible signal factors and AI-3. It is reasonable to postulate that additional AI molecules exist (LaSarre and Federle 2013; Papenfort and Bassler 2016).
AI-1 molecules are acylated homoserine lactones (AHL) composed of an invariant homoserine lactone ring attached to an acyl chain, which can vary in the length of carbon atoms, in saturation and in the oxidation state (LaSarre and Federle 2013). These AHLs are synthesized from S-adenosylmethionine (SAM) by concerted action of the LuxI enzyme family members and acylated acyl carrier proteins. Notably, AI-2 is a by-product of the activated methyl cycle (AMC). Within the AMC, LuxS catalyzes the cleavage of S-ribosylhomocysteine (SRH) to homocysteine and 4,5-dihydroxyl-2,3-pentanedion (DPD), which spontaneously cyclize into AI-2 (Winzer et al. 2002). While Vibrio harveyi recognizes the borated form of AI-2, E. coli and other Enterobacteriaceae sense the borate-free form of AI-2 (Chen et al. 2002; Miller et al. 2002). Even though the knowledge about QS mechanisms in other bacterial species is constantly growing, information regarding QS in bacteria of the genus Campylobacter is rather limited. In 2002, the presence of a luxS gene homolog and active production of AI-2 by C. jejuni was reported for the first time (Elvers and Park 2002). Whereas several other Campylobacter species also produce AI-2, no AI-2 production could be determined in C. lari, C. insulanigrae and C. peloridis (Golz et al. 2012; Tazumi et al. 2011). So far, no AI-1 synthase has been identified in the C. jejuni genome. Only one publication described the production of a putative AI-1 molecule (cjA) by C. jejuni (Moorhead and Griffiths 2011). The structure of cjA could not be determined, but it was demonstrated that addition of exogenous AI-1 compounds induced the expression of the C. jejuni virulence genes cadF, ciaB, cdtB and flaA and supported the transition of the pathogen to the dormant—so-called viable but not culturable (VBNC)—state. To date, no additional C. jejuni QS signalling molecules have been identified. While most AI-1 molecules can diffuse freely across bacterial membrane, several AI-1 as well as hydrophilic AI-2 molecules might require active transport across the cell membrane (LaSarre and Federle 2013; Pereira et al. 2013). In E. coli, AI-2 export is mediated by YdgG, a transmembrane protein belonging to the large group of the so-called AI-2 exporter superfamily (Herzberg et al. 2006; Rettner and Saier 2010). So far, no further AI export systems have been described. However, AI-2 export in C. jejuni is modulated by a small non-coding RNA (CjNC110). Mutational analysis by targeted deletion of the CjNC110 sequence revealed decreased extracellular AI-2 levels but increased intracellular levels of AI-2, suggesting that CjNC110 is required for modulation of the AI-2 transport to the extracellular space (Kreuder et al. 2020).
Gram-negative bacteria commonly sense AI-1 molecules by cytoplasmic LuxR-Type receptors, which act as transcription factors or by two-component membrane-bound histidine kinases (Papenfort and Bassler 2016). For detection of AI-2, different receptor types have been described so far. Vibrionaceae sense AI-2 by a transmembrane receptor, thereby inducing an intracellular signalling cascade. In contrast, AI-2 is imported and phosphorylated via ABC-transporters by several Enterobacteriaceae, Bacillaceae and Rhizobiaceae (Pereira et al. 2013). The phosphorylated AI-2 stabilizes transcription factors, which in turn enable the regulation of target gene expression. For E. coli and Helicobacter pylori, chemoreceptors have been identified sensing AI-2 as chemoattractant and chemorepellent, respectively (Hegde et al. 2011; Rader et al. 2011). However, the low sequence homologies of the AI-2 receptors led to the postulation that additional receptor types may exist (Papenfort and Bassler 2016; Pereira et al. 2013). No AI-2 receptor homolog has been identified in Campylobacter yet. However, the results obtained from an AI-2 uptake assay prompted us to speculate that C. jejuni might perceive AI-2 by a two-component regulatory system rather than by an ABC-transporter system (Adler et al. 2015).
3.2 Phenotypes of C. jejuni luxS Mutants
It is still under debate whether C. jejuni is using AI-2 to regulate their behaviour as mostly conflicting results were reported. Whether these conflicting results depend on strain variation, culture conditions, methods and/or mutation strategies applied has to be elucidated in the future. Nevertheless, we tried to summarize the findings on putative QS-related C. jejuni phenotypes published so far. Since no specific AI-2 receptor of Campylobacter is known so far, AI-2-dependent phenotypes have primarily been investigated using luxS mutants of various C. jejuni strains. Given that LuxS is required for the AMC, it is necessary to complement all experimental assays including a luxS mutant by the addition of exogenous AI-2 to determine whether the phenotypes observed are due to interrupted metabolism or lack of AI-2. Recent investigations confirmed that homocysteine and SHR concentrations were significantly reduced or enhanced in a C. jejuni luxS mutant compared to the parental strain, respectively (Mou and Plummer 2016). However, reduction of the methionine and SAM concentrations as a result of the luxS deletion was less pronounced as expected. Furthermore, the methylome profile of this luxS mutant was comparable to that of the wild-type (Mou et al. 2014), indicating that the observed phenotypes of luxS mutants are not due to a complete lack of methionine or SAM metabolites (Mou and Plummer 2016). Furthermore, no morphological changes in cell shape or flagella morphology have been determined for luxS mutants of C. jejuni strains 81116, NCTC11168 or IA3902 (Jeon et al. 2003; Mou and Plummer 2016). The phenotypes of C. jejuni luxS mutants are summarized in Fig. 2.
Overview of C. jejuni luxS mutant phenotypes. Besides enhanced chemotaxis towards amino acids, reduced colonization, adhesion, invasion, biofilm formation and swarming abilities as well as reduced oxidative stress tolerance and growth kinetics have been described for C. jejuni luxS mutants. However, several phenotypes were only observed for different C. jejuni luxS mutants or under specific culture conditions (for details see text)
Despite that fact that besides AI-2, also the disruption of the AMC may influence bacterial growth, the multiplication of C. jejuni luxS mutants has been extensively investigated. Remarkably, reduced growth rates were reported for the C. jejuni strain 81–176 with inactivated luxS gene, but not for the strains NCTC11168, 81116 and M129 (Elvers and Park 2002; He et al. 2008; Holmes et al. 2009; Jeon et al. 2003; Mou and Plummer 2016; Plummer 2012; Quinones et al. 2009; Reeser et al. 2007). Strain-specific differences in growth-related phenotypes of C. jejuni luxS mutants were confirmed by a detailed analysis of various strains grown under different conditions (Adler et al. 2014). These results indicated that the NCTC11168ΔluxS mutant in which the luxS gene is replaced by an antibiotic resistance gene showed reduced growth in comparison with the wild-type strain both under substrate limited and nutrient-rich conditions. In contrast, two different luxS mutants of C. jejuni strain 81–176 exhibited growth defects under substrate limited conditions only. Genetic complementation restored the growth kinetics of both mutants of strain 81–176, while the chemical complementation by AI-2 only partially restored growth of the ΔluxS mutant of the C. jejuni NCTC11168 strain. These data indicate that C. jejuni growth might be influenced by luxS and AI-2 but in a strain-dependent manner and under certain nutritional conditions only.
Results from a majority of studies showed that motility of C. jejuni luxS mutants on swarming plates is strongly reduced, which was independent of strain background or culture conditions (Adler et al. 2014; Elvers and Park 2002; Holmes et al. 2009; Jeon et al. 2003; Plummer et al. 2011; Quinones et al. 2009; Simunovic et al. 2020). However, for the 81–176ΔluxS mutant constructed by He and colleagues (2008), reduced motility was only detected if bacteria were incubated on Mueller–Hinton medium-based swarming plates at 37 °C. In Brucella broth, however, the motility of this mutant was neither reduced at 37 °C nor at 42 °C (Adler et al. 2014; He et al. 2008). In contrast, the motility of the 81–176::luxS mutant (insertion of antibiotic resistance cassette within the luxS gene) constructed by Quinones and co-workers (2009) was reduced in both media and at both temperatures (Adler et al. 2014; Quinones et al. 2009). These results suggest that differences in some strain-specific phenotypic properties of C. jejuni luxS mutants are indeed caused by polar effects generated by the genetic manipulations applied. Even though the motility of the NCTC11168ΔluxS mutant was not restored by the addition of exogenous AI-2 in the study of Holmes and colleagues (2009), the motility of other C. jejuni luxS mutants was at least partially restored by genetic complementation or upon the addition of exogenous AI-2 (Adler et al. 2014; Plummer et al. 2011; Quinones et al. 2009). The latter studies revealed that AI-2 influences the motility of C. jejuni on swarming agar. So far, the mechanisms of AI-2-dependent regulation are not understood. Several studies investigating gene expression patterns of luxS mutants revealed conflicting results. While reduced flaA gene expression was reported for a 81116luxS mutant, no difference on protein level nor in flagellar morphology was observed (Jeon et al. 2003). Several flagellar assembly/regulation genes were differentially expressed in 81–176ΔluxS cultivated at 42 °C, even though under these conditions the swarming ability of the mutant was comparable to the wild-type (He et al. 2008). Furthermore, Holmes and colleagues (2009) determined the downregulation of several flagellar-associated genes and subsequently reduced swarming capabilities, but the authors could neither restore the gene expression pattern nor the phenotype by adding exogenous AI-2. Therefore, the confusing and in part contradicting results obtained by mutational analysis of the C. jejuni luxS locus indicate that further work under standardized and better controlled experimental conditions is essential for the investigation of the complex mechanisms underlying the interactions of C. jejuni LuxS and/or AI-2 with motility.
Whether AI-2 exhibits a direct chemotactic function in C. jejuni has not been determined yet. Nevertheless, when compared to the wild-type strain, a ΔluxS mutant of the 81–176 strain displayed enhanced chemotactic behaviour towards amino acids (Quinones et al. 2009). Holmes and co-workers (2009) reported reduced mRNA-levels of the genes encoding cheA and the chemoreceptors Tlp1, -2 and -4 (Cj1506, Cj0144, Cj0262) in a NCTC11168ΔluxS mutant. However, no significantly different regulation in expression of the cheA, cheB, cheR, cheV and cheW genes has been observed for the ΔluxS mutant of C. jejuni strain 81–176 (He et al. 2008).
Molecular mechanisms related to host-interactions like adhesion, invasion, cytotoxicity and intestinal colonization are basic to C. jejuni pathogenicity (see Chapter “Campylobacter Virulence Factors and Molecular Host–pathogen Interactions” of this book). Expression of Pseudomonas, Vibrio cholerae and E. coli virulence factors is regulated by QS (Furniss and Clements 2018; Jiang et al. 2019; Papenfort and Bassler 2016). However, studies investigating the AI-2-dependent regulation of pathogenicity in Campylobacter are still scarce. C. jejuni LuxS was essential for adhesion of C. jejuni 81–176 as demonstrated with a luxS mutant of this strain and cultured LMH cells in vitro (Quinones et al. 2009). In contrast, deletion of luxS did not alter adhesion of C. jejuni strain NCTC11168 on INT-407 cells, while the invasion rate of the ΔluxS mutant used in this study was reduced (Simunovic et al. 2020). Interestingly, the invasion rate of a NCTC11168ΔluxS mutant was only slightly reduced in Caco-2 cells (Elvers and Park 2002). Whether these highly varying and confusing observations were due to different properties of luxS mutants or of the different cell lines remains open. However, complementation with exogenous AI-2 is needed to prove that all these phenotypes were caused by the lack of AI-2 and did not result from disruption of the AMC.
Additional contradicting results concerning the influence of luxS on C. jejuni colonization capacity were obtained by the analysis of C. jejuni luxS mutant strains in animal models in vivo. While the luxS mutant of C. jejuni strain IA3902 displayed a loss of chicken colonization, this ability was only reduced in a luxS mutant of C. jejuni strain 81–176, whereas the NCTC11168 luxS mutant colonized chickens with similar rates compared to the wild-type strain (Plummer et al. 2012; Quinones et al. 2009). It is not clear yet if these contradicting findings are the result of real strain-dependent differences or are caused by the mutational strategy applied. No general conclusion regarding the impact of AI-2 on the C. jejuni colonization capabilities could be drawn. Given that AI-2 produced by commensal microbiota could also have an impact on the phenotype of C. jejuni luxS mutants, and despite the difficulties in determining whether altered phenotypes were due to lack of AI-2 or disrupted AMC, the results summarized here should be interpreted with caution.
The NCTC11168 luxS mutant constructed by Elvers and Park (2002) did not show altered hydrogen peroxide or paraquat susceptibility, while the 81–176 luxS mutant was less resistant to cumene hydroperoxide and hydrogen peroxide as compared to the wild-type (He et al. 2008). Gene expression analysis revealed that expression of the peroxide stress defence-related genes ahpC (encoding an alkyl hydroxide reductase) and tpx (encoding a thiol peroxidase) was reduced in the 81–176 luxS mutant after oxidative stress treatment as compared to the wild-type strain (He et al. 2008). The important role of LuxS in the C. jejuni oxidative stress response is further underlined by the fact that the Campylobacter oxidative stress regulator (CosR) negatively regulates the expression of luxS (Hwang et al. 2011). Furthermore, a C. jejuni NCTC11168 luxS mutant displayed lower survival rates as compared to the wild-type strain at cold stress (Ligowska et al. 2011). However, whether these stress responses are directly modulated via AI-2-dependant QS remains to be elucidated.
The biofilm-mass developed by a ΔluxS mutant of C. jejuni strain M129 was significantly reduced compared to the wild-type and could be partially restored by the addition of cell free supernatants of the wild-type strain (Reeser et al. 2007). Furthermore, reduced adhesion on polystyrene surfaces was reported for a NCTC11168 luxS mutant (Simunovic et al. 2020). In contrast, the attachment on stainless steel coupons was comparable for both the NCTC11168 luxS mutant and the wild-type strain (Bezek et al. 2016). In addition, biofilm formation of the C. jejuni strain 81–176 was reduced by the application of AI-1 molecule cjA (Moorhead and Griffiths 2011). Taken together, these data suggest that the process of C. jejuni biofilm formation is regulated by concerted action of several QS systems like in other bacteria (Paluch et al. 2020; Papenfort and Bassler 2016).
3.3 Quorum Quenching
The inhibition of QS, also termed quorum quenching (QQ), has raised much attention in recent years (Paluch et al. 2020). QQ could be implemented as a preventive or therapeutic approach to combat pathogenic bacteria and could be achieved at different stages, e.g. by inhibition of signalling molecule production, degradation of signalling molecules or blockage of the receptor. This is underlined by increasing numbers of patents and applications for QQ compounds and their functions (reviewed by Chen et al. 2018). Even though the exact role of AI-2-mediated QS has not been elucidated for C. jejuni, several authors investigated putative agents that can interrupt QS mechanisms. For example, the application of citrus extract nearly eliminated AI-2 activity in cell-free supernatant of several C. jejuni strains and further reduced their motility, biofilm formation, adhesion and invasion of HeLa cells as well as the expression of cadF and ciaB virulence genes (Castillo et al. 2014, 2015). In support, nearly all the 20 natural plant extracts investigated by Simunovic and co-workers (2020) altered several phenotypes of C. jejuni. The ethanolic extract of Rhodiola rosea (roseroot) had the greatest potential to inhibit AI-2 production, motility, adhesion to polystyrene surface and invasion into INT-407 cells by strain NCTC11168, which were comparably to phenotypes observed for the C. jejuni ΔluxS mutant. Furthermore, none of the tested compounds exerted a synergistic effect on the phenotype of the C. jejuni ΔluxS mutant. These data implicate that these compounds could inhibit AI-2 QS circuit and thereby alter the behaviour of C. jejuni. Furthermore, quinolinone alkaloid mixture extracts from the tree Euodia ruticarpa reduced AI-2 production and number of attached bacteria on a polystyrene surface by C. jejuni NCTC11168 as well as by the ΔluxS and ΔcmeB mutants (Bezek et al. 2016). However, as attachment of the parental strain and the ΔluxS mutant were comparable, the authors concluded that the effects of the extract are not related to AI-2-dependent QQ.
4 Concluding Remarks
The comprehensive review of the literature documented in this book chapter indicates that some aspects of QS and biofilm formation by C. jejuni have been investigated to date, but both processes are still not well understood at the molecular level. Obvious shortcomings in these important fields of research are caused by lack of precise genetic analysis of the biological systems involved and by the extensive genetic variation of C. jejuni at the isolate level. These limitations should be overcome in the future by standardized and complete genetic analysis including the mutation strategies applied and by whole genome analysis of C. jejuni at the strain level. The facts that C. jejuni produces AI-2 and that some phenotypes of luxS mutants could be partially restored by exogenous AI-2 point towards regulatory functions of AI-2 in C. jejuni. Therefore, regarding the QS system, it seems highly recommended that future research should focus on the identification and biochemical characterization of a possible AI-2 receptor including complementation of manipulated pathways by exogenous AI molecules to finally prove the QS-dependent phenotypes of artificially generated C. jejuni mutants. The promising results obtained by AI-2-dependent QS-signalling should strengthen intensive research on potential additional AI molecules and their regulatory functions in C. jejuni and other Campylobacter species.
The manifold environmental and intrinsic conditions affecting C. jejuni biofilm formation provide strong evidence that C. jejuni actively produces biofilms to survive unfavourable conditions outside vertebrate hosts. Therefore, a deeper understanding of C. jejuni biofilm formation is a key to direct future research for improvement of biosafety and hygiene in slaughter and food processing lines. Altogether, biochemical properties of C. jejuni QS and biofilms will guide the development of innovative and novel strategies to diminish the entry or cross-contamination of Campylobacter in livestock and the food processing chain.
References
Abisado RG, Benomar S, Klaus JR, Dandekar AA, Chandler JR (2018) Bacterial quorum sensing and microbial community interactions. mBio 9. https://doi.org/10.1128/mBio.02331-17
Adler L, Alter T, Sharbati S, Golz G (2014) Phenotypes of Campylobacter jejuni luxS mutants are depending on strain background, kind of mutation and experimental conditions. PLoS ONE 9:e104399. https://doi.org/10.1371/journal.pone.0104399
Adler L, Alter T, Sharbati S, Gölz G (2015) The signalling molecule autoinducer-2 is not internalised in Campylobacter jejuni. Berl Munch Tierarztl 128:6
Asakura H, Yamasaki M, Yamamoto S, Igimi S (2007) Deletion of peb4 gene impairs cell adhesion and biofilm formation in Campylobacter jejuni. FEMS Microbiol Lett 275:278–285. https://doi.org/10.1111/j.1574-6968.2007.00893.x
Azeredo J, Azevedo NF, Briandet R, Cerca N, Coenye T, Costa AR, Desvaux M, Di Bonaventura G, Hébraud M, Jaglic Z, Kačániová M, Knøchel S, Lourenço A, Mergulhão F, Meyer RL, Nychas G, Simões M, Tresse O, Sternberg C (2017) Critical review on biofilm methods. Crit Rev Microbiol 43(3):313–351. https://doi.org/10.1080/1040841X.2016.1208146
Bae J, Oh E, Jeon B (2014) Enhanced transmission of antibiotic resistance in Campylobacter jejuni biofilms by natural transformation. Antimicrob Agents Chemother 58(12):7573–5. https://doi.org/10.1128/AAC.04066-14
Bezek K, Kurincic M, Knauder E, Klancnik A, Raspor P, Bucar F, Smole Mozina S (2016) Attenuation of adhesion, biofilm formation and quorum sensing of Campylobacter jejuni by Euodia ruticarpa. Phytother Res 30:1527–1532. https://doi.org/10.1002/ptr.5658
Bronowski C, James CE, Winstanley C (2014) Role of environmental survival in transmission of Campylobacter jejuni. FEMS Microbiol Lett 356:8–19. https://doi.org/10.1111/1574-6968.12488
Bronnec V, Turonova H, Bouju A, Cruveiller S, Rodrigues R, Demnerova K, Tresse O, Haddad N, Zagorec M (2016) Adhesion, biofilm formation, and genomic features of Campylobacter jejuni Bf, an atypical strain able to grow under aerobic conditions. Front Microbiol 7:1002. https://doi.org/10.3389/fmicb.2016.01002
Brown HL, Reuter M, Hanman K, Betts RP, van Vliet AH (2015) Prevention of biofilm formation and removal of existing biofilms by extracellular DNases of Campylobacter jejuni. PLoS ONE 10:e0121680. https://doi.org/10.1371/journal.pone.0121680
Brown HL, Reuter M, Salt LJ, Cross KL, Betts RP, van Vliet AH (2014) Chicken juice enhances surface attachment and biofilm formation of Campylobacter jejuni. Appl Environ Microbiol 80:7053–7060. https://doi.org/10.1128/AEM.02614-14
Cain JA, Dale AL, Niewold P, Klare WP, Man L, White MY, Scott NE, Cordwell SJ (2019) Proteomics reveals multiple phenotypes associated with n-linked glycosylation in Campylobacter jejuni. Mol Cell Proteomics 18:715–734. https://doi.org/10.1074/mcp.RA118.001199
Castillo S, Heredia N, Arechiga-Carvajal E, Garcia S (2014) Citrus extracts as inhibitors of quorum sensing, biofilm formation and motility of Campylobacter jejuni. Food Biotechnol 28:106–122. https://doi.org/10.1080/08905436.2014.895947
Castillo S, Heredia N, Garcia S (2015) 2(5H)-Furanone, epigallocatechin gallate, and a citric-based disinfectant disturb quorum-sensing activity and reduce motility and biofilm formation of Campylobacter jejuni. Folia Microbiol (Praha) 60:89–95. https://doi.org/10.1007/s12223-014-0344-0
Chandrashekhar K, Gangaiah D, Pina-Mimbela R, Kassem II, Jeon BH, Rajashekara G (2015) Transducer like proteins of Campylobacter jejuni 81–176: role in chemotaxis and colonization of the chicken gastrointestinal tract. Front Cell Infect Microbiol 5:46. https://doi.org/10.3389/fcimb.2015.00046
Chen X, Schauder S, Potier N, Van Dorsselaer A, Pelczer I, Bassler BL, Hughson FM (2002) Structural identification of a bacterial quorum-sensing signal containing boron. Nature 415:545–549. https://doi.org/10.1038/415545a
Chen X, Zhang L, Zhang M, Liu H, Lu P, Lin K (2018) Quorum sensing inhibitors: a patent review (2014–2018). Expert Opin Ther Pat 28:849–865. https://doi.org/10.1080/13543776.2018.1541174
Coughlan LM, Cotter PD, Hill C, Alvarez-Ordonez A (2016) New weapons to fight old enemies: novel strategies for the (bio)control of bacterial biofilms in the food industry. Front Microbiol 7:1641. https://doi.org/10.3389/fmicb.2016.01641
Cullen TW, O’Brien JP, Hendrixson DR, Giles DK, Hobb RI, Thompson SA, Brodbelt JS, Trent MS (2013) EptC of Campylobacter jejuni mediates phenotypes involved in host interactions and virulence. Infect Immun 81:430–440. https://doi.org/10.1128/IAI.01046-12
Devaraj A et al (2019) The extracellular DNA lattice of bacterial biofilms is structurally related to Holliday junction recombination intermediates. Proc Natl Acad Sci USA 116(50):25068–25077. https://doi.org/10.1073/pnas.1909017116
Drozd M, Chandrashekhar K, Rajashekara G (2014) Polyphosphate-mediated modulation of Campylobacter jejuni biofilm growth and stability. Virulence 5:680–690. https://doi.org/10.4161/viru.34348
Duan K, Dammel C, Stein J, Rabin H, Surette MG (2003) Modulation of Pseudomonas aeruginosa gene expression by host microflora through interspecies communication. Mol Microbiol 50:1477–1491. https://doi.org/10.1046/j.1365-2958.2003.03803.x
Dwivedi R, Nothaft H, Garber J, Xin Kin L, Stahl M, Flint A, van Vliet AH, Stintzi A, Szymanski CM (2016) L-fucose influences chemotaxis and biofilm formation in Campylobacter jejuni. Mol Microbiol 101:575–589. https://doi.org/10.1111/mmi.13409
El Abbar FM, Li J, Owen HC, Daugherty CL, Fulmer CA, Bogacz M, Thompson SA (2019) RNA binding by the Campylobacter jejuni post-transcriptional regulator csrA. Front Microbiol 10:1776. https://doi.org/10.3389/fmicb.2019.01776
Elvers KT, Park SF (2002) Quorum sensing in Campylobacter jejuni: detection of a luxS encoded signalling molecule. Microbiology 148:1475–1481. https://doi.org/10.1099/00221287-148-5-1475
Feng J, Lamour G, Xue R, Mirvakliki MN, Hatzikiriakos SG, Xu J, Li H, Wang S, Lu X (2016) Chemical, physical and morphological properties of bacterial biofilms affect survival of encased Campylobacter jejuni F38011 under aerobic stress. Int J Food Microbiol 238:172–182. https://doi.org/10.1016/j.ijfoodmicro.2016.09.008
Feng J, Ma L, Nie J, Konkel ME, Lu X (2018) Environmental stress-induced bacterial lysis and extracellular DNA release contribute to Campylobacter jejuni biofilm formation. Appl Environ Microbiol 84. https://doi.org/10.1128/AEM.02068-17
Fields JA, Thompson SA (2008) Campylobacter jejuni csrA mediates oxidative stress responses, biofilm formation, and host cell invasion. J Bacteriol 190:3411–3416. https://doi.org/10.1128/JB.01928-07
Fields JA, Li J, Gulbronson CJ, Hendrixson DR, Thompson SA (2016) Campylobacter jejuni csrA regulates metabolic and virulence associated proteins and is necessary for mouse colonization. PLoS ONE 11:e0156932. https://doi.org/10.1371/journal.pone.0156932
Flemming HC, Wingender J, Szewzyk U, Steinberg P, Rice SA, Kjelleberg S (2016) Biofilms: an emergent form of bacterial life. Nat Rev Microbiol 14:563–575. https://doi.org/10.1038/nrmicro.2016.94
Furniss RCD, Clements A (2018) Regulation of the locus of enterocyte effacement in attaching and effacing pathogens. J Bacteriol 200. https://doi.org/10.1128/JB.00336-17
Gangaiah D, Kassem II, Liu Z, Rajashekara G (2009) Importance of polyphosphate kinase 1 for Campylobacter jejuni viable-but-nonculturable cell formation, natural transformation, and antimicrobial resistance. Appl Environ Microbiol 75:7838–7849. https://doi.org/10.1128/AEM.01603-09
Gangaiah D, Liu Z, Arcos J, Kassem II, Sanad Y, Torrelles JB, Rajashekara G (2010) Polyphosphate kinase 2: a novel determinant of stress responses and pathogenesis in Campylobacter jejuni. PLoS ONE 5:e12142. https://doi.org/10.1371/journal.pone.0012142
Golz G, Adler L, Huehn S, Alter T (2012) LuxS distribution and AI-2 activity of Campylobacter spp. J Appl Microbiol 112:571–578. https://doi.org/10.1111/j.1365-2672.2011.05221.x
Golz G, Kittler S, Malakauskas M, Alter T (2018) Survival of Campylobacter in the food chain and the environment. Curr Clin Microbiol 5:126–134. https://doi.org/10.1007/s40588-018-0092-z
Guerry P (2007) Campylobacter flagella: not just for motility. Trends Microbiol 15:456–461. https://doi.org/10.1016/j.tim.2007.09.006
Hall CW, Mah TF (2017) Molecular mechanisms of biofilm-based antibiotic resistance and tolerance in pathogenic bacteria. FEMS Microbiol Rev 41(3):276–301. https://doi.org/10.1093/femsre/fux010
Hansson I, Sandberg M, Habib I, Lowman R, Engvall EO (2018) Knowledge gaps in control of Campylobacter for prevention of campylobacteriosis. Transbound Emerg Dis. 65:30–48. https://doi.org/10.1111/tbed.12870
He Y, Frye JG, Strobaugh TP, Chen CY (2008) Analysis of AI-2/luxS-dependent transcription in Campylobacter jejuni strain 81–176. Foodborne Pathog Dis 5:399–415. https://doi.org/10.1089/fpd.2008.0106
Hegde M, Englert DL, Schrock S, Cohn WB, Vogt C, Wood TK, Manson MD, Jayaraman A (2011) Chemotaxis to the quorum-sensing signal AI-2 requires the Tsr chemoreceptor and the periplasmic LsrB AI-2-binding protein. J Bacteriol 193:768–773. https://doi.org/10.1128/JB.01196-10
Herzberg M, Kaye IK, Peti W, Wood TK (2006) YdgG (TqsA) controls biofilm formation in Escherichia coli K-12 through autoinducer 2 transport. J Bacteriol 188:587–598. https://doi.org/10.1128/JB.188.2.587-598.2006
Holmes K, Tavender TJ, Winzer K, Wells JM, Hardie KR (2009) AI-2 does not function as a quorum sensing molecule in Campylobacter jejuni during exponential growth in vitro. BMC Microbiol 9:214. https://doi.org/10.1186/1471-2180-9-214
Howard SL, Jagannathan A, Soo EC, Hui JP, Aubry AJ, Ahmed I, Karlyshev A, Kelly JF, Jones MA, Stevens MP, Logan SM, Wren BW (2009) Campylobacter jejuni glycosylation island important in cell charge, legionaminic acid biosynthesis, and colonization of chickens. Infect Immun 77:2544–2556. https://doi.org/10.1128/IAI.01425-08
Hwang S, Kim M, Ryu S, Jeon B (2011) Regulation of oxidative stress response by CosR, an essential response regulator in Campylobacter jejuni. PLoS ONE 6:e22300. https://doi.org/10.1371/journal.pone.0022300
Indikova I, Humphrey TJ, Hilbert F (2015) Survival with a helping hand: Campylobacter and microbiota. Front Microbiol 6:1266. https://doi.org/10.3389/fmicb.2015.01266
Jeon B, Itoh K, Misawa N, Ryu S (2003) Effects of quorum sensing on flaA transcription and autoagglutination in Campylobacter jejuni. Microbiol Immunol 47:833–839. https://doi.org/10.1111/j.1348-0421.2003.tb03449.x
Jiang Q, Chen J, Yang C, Yin Y, Yao K (2019) Quorum sensing: a prospective therapeutic target for bacterial diseases. Biomed Res Int 2019:2015978. https://doi.org/10.1155/2019/2015978
Joshua GW, Guthrie-Irons C, Karlyshev AV, Wren BW (2006) Biofilm formation in Campylobacter jejuni. Microbiology 152:387–396. https://doi.org/10.1099/mic.0.28358-0
Kalmokoff M, Lanthier P, Tremblay TL, Foss M, Lau PC, Sanders G, Austin J, Kelly J, Szymanski CM (2006) Proteomic analysis of Campylobacter jejuni 11168 biofilms reveals a role for the motility complex in biofilm formation. J Bacteriol 188:4312–4320. https://doi.org/10.1128/JB.01975-05
Kaplan JB (2010) Biofilm dispersal: mechanisms, clinical implications, and potential therapeutic uses. J Dent Res 89(3):205–18. https://doi.org/10.1177/0022034509359403
Karlyshev AV, Linton D, Gregson NA, Lastovica AJ, Wren BW (2000) Genetic and biochemical evidence of a Campylobacter jejuni capsular polysaccharide that accounts for Penner serotype specificity. Mol Microbiol 35(3):529–541. https://doi.org/10.1046/j.1365-2958.2000.01717.x
Kassem II, Candelero-Rueda RA, Esseili KA, Rajashekara G (2017) Formate simultaneously reduces oxidase activity and enhances respiration in Campylobacter jejuni. Sci Rep 7:40117. https://doi.org/10.1038/srep40117
Kim JS, Park C, Kim YJ (2015) Role of flgA for flagellar biosynthesis and biofilm formation of Campylobacter jejuni NCTC11168. J Microbiol Biotechn 25:1871–1879. https://doi.org/10.4014/jmb.1504.04080
Kostakioti M, Hadjifrangiskou M, Hultgren SJ (2013) Bacterial biofilms: development, dispersal, and therapeutic strategies in the dawn of the postantibiotic era. Cold Spring Harb Perspect Med 3:a010306. https://doi.org/10.1101/cshperspect.a010306
Kreuder AJ, Ruddell B, Mou K, Hassall A, Zhang Q, Plummer PJ (2020) Small non-coding RNA CjNC110 influences motility, autoagglutination, AI-2 localization, hydrogen peroxide sensitivity and chicken colonization in Campylobacter jejuni. Infect Immun. https://doi.org/10.1128/IAI.00245-20
Kumar A, Gangaiah D, Torrelles JB, Rajashekara G (2016) Polyphosphate and associated enzymes as global regulators of stress response and virulence in Campylobacter jejuni. World J Gastroenterol 22:7402–7414. https://doi.org/10.3748/wjg.v22.i33.7402
Lamas A, Regal P, Vazquez B, Miranda JM, Cepeda A, Franco CM (2018) Salmonella and Campylobacter biofilm formation: a comparative assessment from farm to fork. J Sci Food Agr 98:4014–4032. https://doi.org/10.1002/jsfa.8945
LaSarre B, Federle MJ (2013) Exploiting quorum sensing to confuse bacterial pathogens. Microbiol Mol Biol Rev 77:73–111. https://doi.org/10.1128/MMBR.00046-12
Li J, Feng J, Ma L, de la Fuente NC, Golz G, Lu X (2017) Effects of meat juice on biofilm formation of Campylobacter and Salmonella. Int J Food Microbiol 253:20–28. https://doi.org/10.1016/j.ijfoodmicro.2017.04.013
Ligowska M, Cohn MT, Stabler RA, Wren BW, Brondsted L (2011) Effect of chicken meat environment on gene expression of Campylobacter jejuni and its relevance to survival in food. Int J Food Microbiol 145(Suppl 1):S111–S115. https://doi.org/10.1016/j.ijfoodmicro.2010.08.027
Lim ES, Kim JS (2017) Role of eptC in Biofilm Formation by Campylobacter jejuni NCTC11168 on Polystyrene and Glass Surfaces. J Microbiol Biotechn 27:1609–1616. https://doi.org/10.4014/jmb.1610.10046
Melo RT, Mendonca EP, Monteiro GP, Siqueira MC, Pereira CB, Peres PABM, Fernandez H, Rossi DA (2017) Intrinsic and extrinsic aspects on Campylobacter jejuni biofilms. Front Microbiol 8:1332. https://doi.org/10.3389/fmicb.2017.01332
Miller MB, Skorupski K, Lenz DH, Taylor RK, Bassler BL (2002) Parallel quorum sensing systems converge to regulate virulence in Vibrio cholerae. Cell 110:303–314. https://doi.org/10.1016/s0092-8674(02)00829-2
Moorhead SM, Griffiths MW (2011) Expression and characterization of cell-signalling molecules in Campylobacter jejuni. J Appl Microbiol 110:786–800. https://doi.org/10.1111/j.1365-2672.2010.04934.x
Mou KT, Muppirala UK, Severin AJ, Clark TA, Boitano M, Plummer PJ (2014) A comparative analysis of methylome profiles of Campylobacter jejuni sheep abortion isolate and gastroenteric strains using PacBio data. Front Microbiol 5:782. https://doi.org/10.3389/fmicb.2014.00782
Mou KT, Plummer PJ (2016) The impact of the luxS mutation on phenotypic expression of factors critical for Campylobacter jejuni colonization. Vet Microbiol 192:43–51. https://doi.org/10.1016/j.vetmic.2016.06.011
Mukherjee S, Bassler BL (2019) Bacterial quorum sensing in complex and dynamically changing environments. Nat Rev Microbiol 17:371–382. https://doi.org/10.1038/s41579-019-0186-5
Naito M, Frirdich E, Fields JA, Pryjma M, Li J, Cameron A, Gilbert M, Thompson SA, Gaynor EC (2010) Effects of sequential Campylobacter jejuni 81–176 lipooligosaccharide core truncations on biofilm formation, stress survival, and pathogenesis. J Bacteriol 192:2182–2192. https://doi.org/10.1128/JB.01222-09
Oh E, Jeon B (2014) Role of alkyl hydroperoxide reductase (AhpC) in the biofilm formation of Campylobacter jejuni. PLoS ONE 9:e87312. https://doi.org/10.1371/journal.pone.0087312
Oh E, Kim JC, Jeon B (2016) Stimulation of biofilm formation by oxidative stress in Campylobacter jejuni under aerobic conditions. Virulence 7:846–851. https://doi.org/10.1080/21505594.2016.1197471
Paluch E, Rewak-Soroczynska J, Jedrusik I, Mazurkiewicz E, Jermakow K (2020) Prevention of biofilm formation by quorum quenching. Appl Microbiol Biotechnol 104:1871–1881. https://doi.org/10.1007/s00253-020-10349-w
Papenfort K, Bassler BL (2016) Quorum sensing signal-response systems in Gram-negative bacteria. Nat Rev Microbiol 14:576–588. https://doi.org/10.1038/nrmicro.2016.89
Pascoe B, Meric G, Murray S, Yahara K, Mageiros L, Bowen R, Jones NH, Jeeves RE, Lappin-Scott HM, Asakura H, Sheppard SK (2015) Enhanced biofilm formation and multi-host transmission evolve from divergent genetic backgrounds in Campylobacter jejuni. Environ Microbiol 17:4779–4789. https://doi.org/10.1111/1462-2920.13051
Pascoe B, Williams LK, Calland JK, Meric G, Hitchings MD, Dyer M, Ryder J, Shaw S, Lopes BS, Chintoan-Uta C, Allan E, Vidal A, Fearnley C, Everest P, Pachebat JA, Cogan TA, Stevens MP, Humphrey TJ, Wilkinson TS, Cody AJ, Colles FM, Jolley KA, Maiden MCJ, Strachan N, Pearson BM, Linton D, Wren BW, Parkhill J, Kelly DJ, van Vliet AHM, Forbes KJ, Sheppard SK (2019) Domestication of Campylobacter jejuni NCTC11168. Microb Genom 5. https://doi.org/10.1099/mgen.0.000279
Pereira CS, Thompson JA, Xavier KB (2013) AI-2-mediated signalling in bacteria. FEMS Microbiol Rev 37:156–181. https://doi.org/10.1111/j.1574-6976.2012.00345.x
Petrova OE, Sauer K (2016) Escaping the biofilm in more than one way: desorption, detachment or dispersion. Curr Opin Microbiol 30:67–78. https://doi.org/10.1016/j.mib.2016.01.004.
Plummer P, Sahin O, Burrough E, Sippy R, Mou K, Rabenold J, Yaeger M, Zhang Q (2012) Critical role of luxS in the virulence of Campylobacter jejuni in a guinea pig model of abortion. Infect Immun 80:585–593. https://doi.org/10.1128/IAI.05766-11
Plummer P, Zhu J, Akiba M, Pei D, Zhang Q (2011) Identification of a key amino acid of luxS involved in AI-2 production in Campylobacter jejuni. PLoS ONE 6:e15876. https://doi.org/10.1371/journal.pone.0015876
Plummer PJ (2012) LuxS and quorum-sensing in Campylobacter. Front Cell Infect Microbiol 2:22. https://doi.org/10.3389/fcimb.2012.00022
Quinones B, Miller WG, Bates AH, Mandrell RE (2009) Autoinducer-2 production in Campylobacter jejuni contributes to chicken colonization. Appl Environ Microbiol 75:281–285. https://doi.org/10.1128/AEM.01803-08
Rader BA, Wreden C, Hicks KG, Sweeney EG, Ottemann KM, Guillemin K (2011) Helicobacter pylori perceives the quorum-sensing molecule AI-2 as a chemorepellent via the chemoreceptor TlpB. Microbiology 157:2445–2455. https://doi.org/10.1099/mic.0.049353-0
Rahman H, King RM, Shewell LK, Semchenko EA, Hartley-Tassell LE, Wilson JC, Day CJ, Korolik V (2014) Characterisation of a multi-ligand binding chemoreceptor CcmL (Tlp3) of Campylobacter jejuni. PLoS Pathog 10:e1003822. https://doi.org/10.1371/journal.ppat.1003822
Rathbun KM, Hall JE, Thompson SA (2009) Cj0596 is a periplasmic peptidyl prolyl cis-trans isomerase involved in Campylobacter jejuni motility, invasion, and colonization. BMC Microbiol 9:160. https://doi.org/10.1186/1471-2180-9-160
Reeser RJ, Medler RT, Billington SJ, Jost BH, Joens LA (2007) Characterization of Campylobacter jejuni biofilms under defined growth conditions. Appl Environ Microbiol 73:1908–1913. https://doi.org/10.1128/AEM.00740-06
Rettner RE, Saier MH Jr (2010) The autoinducer-2 exporter superfamily. J Mol Microbiol Biotechnol 18:195–205. https://doi.org/10.1159/000316420
Reuter M, Mallett A, Pearson BM, van Vliet AH (2010) Biofilm formation by Campylobacter jejuni is increased under aerobic conditions. Appl Environ Microbiol 76:2122–2128. https://doi.org/10.1128/AEM.01878-09
Reuter M, Ultee E, Toseafa Y, Tan A, van Vliet AHM (2020) Inactivation of the core cheVAWY chemotaxis genes disrupts chemotactic motility and organised biofilm formation in Campylobacter jejuni. FEMS Microbiol Lett https://doi.org/10.1093/femsle/fnaa198
Roy R, Tiwari M, Donelli G, Tiwari V (2018) Strategies for combating bacterial biofilms: A focus on anti-biofilm agents and their mechanisms of action. Virulence 9:522–554. https://doi.org/10.1080/21505594.2017.1313372
Scott NE, Nothaft H, Edwards AV, Labbate M, Djordjevic SP, Larsen MR, Szymanski CM, Cordwell SJ (2012) Modification of the Campylobacter jejuni N-linked glycan by EptC protein-mediated addition of phosphoethanolamine. J Biol Chem 287:29384–29396. https://doi.org/10.1074/jbc.M112.380212
Sharma K, Pagedar Singh A (2018) Antibiofilm effect of DNase against single and mixed species biofilm. Foods 7(3):42. https://doi.org/10.3390/foods7030042
Sharma D, Misba L, Khan AU (2019) Antibiotics versus biofilm: an emerging battleground in microbial communities. Antimicrob Resist Infect Control 8:76. https://doi.org/10.1186/s13756-019-0533-3
Simunovic K, Ramic D, Xu C, Smole Mozina S (2020) Modulation of Campylobacter jejuni motility, adhesion to polystyrene surfaces, and invasion of INT407 cells by quorum-sensing inhibition. Microorganisms 8. https://doi.org/10.3390/microorganisms8010104
Stetsenko VV, Efimochkina NR, Pichugina TV (2019) Growth and persistence of Campylobacter jejuni in foodstuffs. Bull Exp Biol Med 166:759–765. https://doi.org/10.1007/s10517-019-04435-x
Svensson SL, Davis LM, MacKichan JK, Allan BJ, Pajaniappan M, Thompson SA, Gaynor EC (2009) The CprS sensor kinase of the zoonotic pathogen Campylobacter jejuni influences biofilm formation and is required for optimal chick colonization. Mol Microbiol 71:253–272. https://doi.org/10.1111/j.1365-2958.2008.06534.x
Svensson SL, Pryjma M, Gaynor EC (2014) Flagella-mediated adhesion and extracellular DNA release contribute to biofilm formation and stress tolerance of Campylobacter jejuni. PLoS ONE 9:e106063. https://doi.org/10.1371/journal.pone.0106063
Tazumi A, Negoro M, Tomiyama Y, Misawa N, Itoh K, Moore JE, Millar BC, Matsuda M (2011) Uneven distribution of the luxS gene within the genus Campylobacter. Br J Biomed Sci 68:19–22. https://doi.org/10.1080/09674845.2011.11732836
Teh AH, Lee SM, Dykes GA (2014) Does Campylobacter jejuni form biofilms in food-related environments? Appl Environ Microbiol 80(17):5154–5160. https://doi.org/10.1128/AEM.01493-14
Teh AH, Lee SM, Dykes GA (2016) The influence of prior modes of growth, temperature, medium, and substrate surface on biofilm formation by antibiotic-resistant Campylobacter jejuni. Curr Microbiol 73:859–866. https://doi.org/10.1007/s00284-016-1134-5
Teh AHT, Lee SM, Dykes GA (2017) Identification of potential Campylobacter jejuni genes involved in biofilm formation by EZ-Tn5 Transposome mutagenesis. BMC Res Notes 10:182. https://doi.org/10.1186/s13104-017-2504-1
Teh AHT, Lee SM, Dykes GA (2019) Association of some Campylobacter jejuni with Pseudomonas aeruginosa biofilms increases attachment under conditions mimicking those in the environment. PLoS ONE 14:e0215275. https://doi.org/10.1371/journal.pone.0215275
Teh KH, Flint S, French N (2010) Biofilm formation by Campylobacter jejuni in controlled mixed-microbial populations. Int J Food Microbiol 143:118–124. https://doi.org/10.1016/j.ijfoodmicro.2010.07.037
Theoret JR, Cooper KK, Zekarias B, Roland KL, Law BF, Curtiss R, Joens LA (2012) The Campylobacter jejuni Dps homologue is important for in vitro biofilm formation and cecal colonization of poultry and may serve as a protective antigen for vaccination. Clin Vaccine Immunol 19:1426–1431. https://doi.org/10.1128/CVI.00151-12
Tram G, Day CJ, Korolik V (2020a) Bridging the Gap: A Role for Campylobacter jejuni Biofilms. Microorganisms 8(3):452. https://doi.org/10.3390/microorganisms8030452
Tram G, Klare WP, Cain JA, Mourad B, Cordwell SJ, Day CJ, Korolik V (2020b) Assigning a role for chemosensory signal transduction in Campylobacter jejuni biofilms using a combined omics approach. Sci Rep 10:6829. https://doi.org/10.1038/s41598-020-63569-5
Turonova H, Briandet R, Rodrigues R, Hernould M, Hayek N, Stintzi A, Pazlarova J, Tresse O (2015) Biofilm spatial organization by the emerging pathogen Campylobacter jejuni: comparison between NCTC11168 and 81–176 strains under microaerobic and oxygen-enriched conditions. Front Microbiol 6:709. https://doi.org/10.3389/fmicb.2015.00709
Wagle BR, Upadhyay A, Upadhyaya I, Shrestha S, Arsi K, Liyanage R, Venkitanarayanan K, Donoghue DJ, Donoghue AM (2019) Trans-cinnamaldehyde, eugenol and carvacrol reduce Campylobacter jejuni biofilms and modulate expression of select genes and proteins. Front Microbiol 10:1837. https://doi.org/10.3389/fmicb.2019.01837
Whelan MVX, Ardill L, Koide K, Nakajima C, Suzuki Y, Simpson JC, Cróinín TÓ (2019) Acquisition of fluoroquinolone resistance leads to increased biofilm formation and pathogenicity in Campylobacter jejuni. Sci Rep 9:18216. https://doi.org/10.1038/s41598-019-54620-1
Winzer K, Hardie KR, Burgess N, Doherty N, Kirke D, Holden MT, Linforth R, Cornell KA, Taylor AJ, Hill PJ, Williams P (2002) LuxS: its role in central metabolism and the in vitro synthesis of 4-hydroxy-5-methyl-3(2H)-furanone. Microbiology 148:909–922. https://doi.org/10.1099/00221287-148-4-909
Zhang T, Dong J, Cheng Y, Lu Q, Luo Q, Wen G, Liu G, Shao H (2017) Genotypic diversity, antimicrobial resistance and biofilm-forming abilities of Campylobacter isolated from chicken in Central China. Gut Pathog 9:62. https://doi.org/10.1186/s13099-017-0209-6
Zhong X, Wu Q, Zhang J, Ma Z, Wang J, Nie X, Ding Y, Xue L, Chen M, Wu S, Wei X, Zhang Y (2020) Campylobacter jejuni biofilm formation under aerobic conditions and inhibition by ZnO nanoparticles. Front Microbiol 11:207. https://doi.org/10.3389/fmicb.2020.00207
Acknowledgements
This work is supported by the German Federal Ministry of Education and Research (BMBF) in frame of the zoonoses research consortium PAC-Campylobacter to C.P. and G.G. (project IP2/01KI1725A).
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Püning, C., Su, Y., Lu, X., Gölz, G. (2021). Molecular Mechanisms of Campylobacter Biofilm Formation and Quorum Sensing. In: Backert, S. (eds) Fighting Campylobacter Infections. Current Topics in Microbiology and Immunology, vol 431. Springer, Cham. https://doi.org/10.1007/978-3-030-65481-8_11
Download citation
DOI: https://doi.org/10.1007/978-3-030-65481-8_11
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-65480-1
Online ISBN: 978-3-030-65481-8
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)