Abstract
Non-communicable diseases (NCDs) are among the most significant health challenges of the twenty-first century, causing 7 out of 10 deaths worldwide. Despite recent technological and medical advances, NCDs mortality and morbidity rates are increasing, and it is expected that by 2030 they will have caused 52 million deaths. In 2017, 41 million people died due to NCDs, and 80% of these deaths could have been prevented. Cardiovascular disease, cancer, diabetes, and chronic lung disease are the primary causes of mortality among NCDs-related deaths. Autoimmune diseases (ADs) affect 5–10% of the globe and have detrimental effects on patients’ quality of life, life expectancy, and healthcare costs. Apart from the genetic background, 80% of the risk factors of NCDs are modifiable, including diet, hidden hunger, smoking, alcohol, air pollution, and physical activity, all discussed in this chapter. Accumulating evidence shows that changes in diet, lifestyle, and socioeconomic status have resulted in a substantial metabolic shift associated with the rapid increase of ADs. However, current approaches do not fully capture the individual variability on genes and lifestyle or consider the impact of modifiable factors on health. As such, there is growing pressure from patients’ increasing demand and substantial healthcare costs for prevention, prediction, early diagnosis, and effective treatment of NCDs. With the advent of precision medicine, there have been efforts made to deliver tailor-made solutions for NCDs. Metabolomics, an emerging field that gives a detailed analysis of the phenotype, is currently being investigated as a potential precision medicine tool for screening, patient stratification, and treatment personalization. In this chapter, we present up-to-date data on the mitigating epigenetic and lifestyle risk factors for NCDs and ADs and review the current methodology for their assessment.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Non-communicable diseases
- Autoimmune diseases
- Risk factors
- Metabolomics
- Hidden hunger
- Vitamin D
- Metabolic shift
- Insulin resistance
- Inflammation
1 Introduction
Non-communicable diseases (NCDs) are chronic diseases that occur from various genetic and non-genetic factors. The genetic background contributes by 20% to the NCDs risk, whereas 80% of the risk factors are epigenetic , thus, modifiable. These factors include but are not limited to dietary habits, smoking, physical activity, toxic load, alcohol, and others, which are going to be discussed in this chapter. NCDs are responsible for 7 out of 10 deaths worldwide. In 2017, they were responsible for 73.5% (41.1 million) of the deaths which occurred globally and are considered one of the most significant health challenges of the twenty-first century (Martinez et al. 2020). Morbidity rates are also increasing dramatically from 43% in 1990 to more than 60% in 2017 globally. In contrast, in high-income countries where infectious diseases are reduced due to improved life quality standards, the NCDs rate reaches 80% of the global disease burden and over 90% of deaths.
The increasing morbidity and mortality rates indicate that by 2030, NCDs will account for 52 million deaths globally. The main types of NCDs include cardiovascular diseases (CVD) , cancers, chronic respiratory diseases, diabetes, and autoimmune diseases. The primary mortality causes among NCD-related deaths are due to CVD, cancer, diabetes, and chronic lung disease. Autoimmune diseases (ADs) are a subgroup of NCDs and refer to conditions characterized by the malfunction of the immune system , which attacks self-tissues and organs in various parts of the body, causing inflammation. Nearly 5–10% of the global population is affected by ADs and suffers detrimental effects on their quality of life, life expectancy, and healthcare costs. More than 150 types of autoimmune diseases have been reported up to date, with the most common being Hashimoto’s thyroiditis , rheumatoid arthritis , inflammatory bowel disease (IBD) , multiple sclerosis , and lupus . Accumulating evidence shows that the rapid increase of ADs is associated with a metabolic shift caused by changes in lifestyle, diet, and socioeconomic status. Twin studies have indicated that there are significant genetic determinants for ADs, such as the high concordance of Major Histocompatibility Complex (MHC) haplotypes in monozygotic twins. However, the association mentioned above mainly affects early-onset diseases suggesting that other factors apart from genes may influence the development of ADs (Theofilopoulos et al. 2017).
Medicine advancements have made substantial progress in the diagnosis of NCDs, but preventive strategies, tools of prediction, and active therapeutic agents are not yet established. Regarding prevention, despite the pivotal role of diet and the other environmental factors on disease onset and progression, physicians lack proper knowledge and tools to assess and improve them (Strong et al. 2006; Tinetti et al. 2012; Devries 2019). Besides, current treatment approaches have proved beneficial for only 30–60% of the patients, and an additional 30% experience severe adverse effects indicating a large gap that needs to be addressed. The “one size fits all” model that is being applied at the moment does not take into consideration the modifiable risk factors or capture the genetic variability and lifestyle of the individual (Balashova et al. 2018). As a result, the demand for effective prevention, prediction, early diagnosis, and treatment of NCDs by patients is continuously increasing. Precision medicine is an emerging approach for the individualized treatment, forecasting, and early diagnosis of disease, taking into account the individual gene variability, lifestyle, and nutrition. Metabolomics , the study of metabolites and their interactions within the organism, gives a detailed analysis of the phenotype and has vast applications in medicine. It can capture the interrelationships of a biological system, including humans, under the influence of epigenetic factors. Specifically, it has been suggested that metabolomics can provide insight on a systemic dysfunction before the appearance of the symptoms, thus it is a valuable tool for the prevention and prediction of disease. At an advanced stage of the disease, metabolomics can be used to monitor the side effects of drug treatment allowing the treatment type or dose optimization and detect and assess the nutritional deficiencies that should be replenished to improve the life quality (Tsoukalas et al. 2019b).
This chapter includes recent data on the modifiable epigenetic and environmental risk factors for NCDs and ADs and reviews the available methodologies for their assessment. Specifically, we have included essential factors that shape an unhealthy diet and an unhealthy environment. We also discuss the challenges that precision medicine faces regarding its application in clinical practice while focusing on the potential future opportunities for personalized disease treatment.
2 Dietary and Lifestyle Factors
Nutritional epidemiology has linked the consumption of specific foods, nutrients, or dietary patterns with different types of cancer, cardiovascular disease, diabetes, increased blood pressure, insulin resistance, and hyperglycemia. According to recent data, the suboptimal diet is a more critical factor than smoking for global mortality. An insightful study published in Lancet last year demonstrated that more than 20% of global deaths of adults were linked to poor diet, and the most important cause of death was cardiovascular disease, cancer, and diabetes (Afshin et al. 2019). The poor diet included inadequate intake of healthy foods, mostly nuts, seeds, whole grains, and fruit and overconsumption of unhealthy food, mainly sweetener-rich beverages, sodium, and processed meat. Specifically, more than 50% of deaths related to diet were associated with high sodium intake (global mean 6 g), low whole-grain diet (global mean 29 g), and low fruit intake (global mean was less than 100 g). Here we discuss the role of hidden hunger (micronutrient deficiency), lack of vitamin D, excessive sodium and free sugar intake, alcohol consumption, smoking, and physical activity as critical factors of an unhealthy diet and lifestyle that increase the risk of NCDs morbidity and mortality.
2.1 Hidden Hunger
Hidden hunger (or micronutrient deficiency ) is defined as the lack of vitamins and minerals of an organism , and in contrast to micronutrient deficiency diseases, hidden hunger is asymptomatic. Micronutrients , including vitamins, minerals, amino acids, fatty acids, probiotics, enzymes, and antioxidants, are essential for the normal function of cells and tissues (Bailey et al. 2015). Vitamins are organic compounds that act as coenzymes of metabolic pathways, and most of them are essential and are obtained through diet. Vitamin B7 and vitamin K, though, are normally synthesized through the gut and vitamin D is synthesized with sun exposure. Minerals are also essential nutrients obtained through diet, and act as cofactors of enzymatic reactions. The inadequate intake or absorption of these nutrients due to poor diet or disease state can lead to severe cellular malfunction with complications in health. According to the World Health Organization (WHO), 2 billion people suffer from nutrient deficiencies globally (Bailey et al. 2015). The primary nutrient deficiencies that contribute to the development of hidden hunger are vitamins A, D, E, and C, and choline, calcium, magnesium, iron (for specific age/gender groups), potassium, and fiber. The Dietary Guidelines Advisory Committee, though, has recommended the public to increase the intake of only some of these nutrients because they have been linked to adverse health issues (Blumberg et al. 2017). Indeed, many of these deficiencies have been linked with the prevalence of NCDs , and their correction has been related to beneficial effects on the management of certain NCDs (Kivity et al. 2011; Moss and Ramji 2017; Wessels and Rink 2020a; Winther and Rayman 2020). Although there is a lack of enough studies showing a correlation between the deficiency of the rest of the nutrients and NCDs, there is growing evidence supporting that nutrients act synergistically (Faggi et al. 2019).
Nutritional deficiencies have been associated with ADs. In a study of patients with IBD, several nutritional deficiencies were observed (Vagianos et al. 2007). Biochemical measurements indicated a high prevalence of nutrient deficiencies of vitamin E (63%), vitamin D (36%), vitamin A (26%), calcium (23%), folate (19%), iron (13%), and vitamin C (11%) in these patients. Deficiencies were further demonstrated through insufficient blood serum levels of hemoglobin (40%), ferritin (39.2%), vitamin B6 (29%), carotene (23.4%), vitamin B12 (18.4%), vitamin D (17.6%), albumin (17.6%), and zinc (15.2%). Even though these deficiencies were not correlated to diet, the authors suggested that other factors may influence the low nutritional levels and that supplementation should be considered in the IBD patients. In a review by Manzel A. et al., the relation of autoimmune diseases to the Western Diet was discussed focusing on the importance of T cells, concluding that nutrition affects the gut mucosal immune system and the metabolic state of the body, which are both risk factors for autoimmunity (Manzel et al. 2014). Moreover, recent evidence indicate a pivotal role for vitamin D and zinc deficiencies in most common ADs suggesting potential effective and cost-effective strategies of prevention and treatment (Wessels and Rink 2020b).
2.2 Intermediate Metabolic Risk Factors
In affluent countries, hidden hunger as a result of malnutrition often co-exists with obesity or overweight and the rise of the NCDs. Specifically, studies indicate that micronutrient deficiencies under nutritional patterns of high-fat, high-protein, high-sugar, and excess salt intake that are commonly consumed in affluent countries (Western Diet) have been linked to an increased risk for obesity, high BMI, metabolic syndrome, and cardiovascular disease (Ames 2006; Manzel et al. 2014). Significant progress has been made in the description of the molecular pathways that associate diet, obesity, insulin resistance, low-grade inflammation, disrupted biochemical parameters, and NCDs. Obesity , defined as Body Mass Index over 30, has been classified as a risk factor for many NCDs, including type 2 diabetes, CVD, and cancers, and there is a common view that obesity triggers inflammation and insulin resistance being a subsequent effect (Johnson and Milner 2012). However, growing evidence suggests that insulin resistance is the primary disturbance and precedes inflammation (Giles et al. 2015). Briefly, the proposed mechanism for the diet-related onset of inflammation is that excessive intake of calorie-dense and nutrient-empty foods lead to the disruption of the physiological mechanism of the organisms to produce energy in a controlled manner. Insulin and leptin, the central hormones that regulate energy metabolism but also affect immune responses, are increased continuously to balance the excessive calorie intake. Continuous secretion of insulin and leptin leads to metabolic shift in the peripheral cells and the cells of the immune system, triggering inflammation. At the same time, hyperinsulinemia directly leads to insulin resistance, where cells require more insulin to receive the signal to uptake glucose and use it as energy. In turn, in conditions with established chronic low-grade inflammation, the inflammatory microenvironment further fuels insulin resistance, storage of fat and obesity, and related metabolic syndrome (Fig. 13.1). As for obesity , a series of interventional studies indicate that changes in insulin resistance or inflammation markers precede and can predict weight changes suggesting that obesity is a consequence and rather a cause of insulin resistance (Kong et al. 2013). Validation of this theory will be very significant for research but also for clinical practice since strategies targeting weight loss are far different from those targeting insulin resistance (Noakes 2018).
Vitamin B12 pathways of absorption and metabolism. Digestion of cobalamin (vitamin B12) bound to animal protein takes place in the stomach followed by the duodenum, facilitated by the transporters haptocorrin and intrinsic factor (IF). The complex cobalamin-IF enters the enterocyte where cobalamin is then bound to transcobalamin and depending on the type of transcobalamin, the complex follows a different path: (i) Cobalamin-Transcobalamin I is transported to the liver where cobalamin is stored (75% of cob), (ii) Cobalamin-Transcobalamin III is transported to the bile where it is excreted with the urine, and (iii) Cobalamin-Transcobalamin II enters the systemic blood circulation to reach and enter the cells. Upon entering the cell, cobalamin is released from transcobalamin and acts as a cofactor for intracellular metabolic pathways. Methyl-B12 is a cofactor of Methionine Synthase (MS) for the conversion of Homocysteine (HC) to Methionine (MTH), which in turn is metabolized to s-adenosyl methionine (SAM), a precursor of HC. Methylmalonyl-CoA mutase (MCM) catalyzes the conversion of methylmalonyl-CoA (MMA-CoA) to succinyl-CoA, with the presence of adenosyl-B12, which feeds the TCA cycle for energy production. Methylmalonic acid (MMA) is a downstream metabolite of MMA-CoA, and upon malfunction or inactivation of the adenosyl-B12 dependent pathway, MMA increases
Micronutrient deficiencies are mostly caused by poor dietary choices, although socioeconomic factors and the presence of underlying disease are also important (Bailey et al. 2015). Also, even if intake is sufficient, a deficiency may occur at a later stage due to disturbed absorption of nutrients for various reasons (e.g., metabolic disease or excessive toxic load from medication) (National Academies of Sciences, Engineering et al. 2017). For example, vitamin B12, which is obtained through diet and specifically animal sources, is released in blood circulation through a complicated journey of absorption from the stomach, the duodenum, and the enterocytes (Fig. 13.2). B12 released by enterocytes is bound to transcobalamin and can be either stored in the liver (75% of B12), excreted via the bile or enter the cells and participate in intracellular metabolism. After entering the cells, B12 is released and free to act as cofactor either for methionine synthase (MS), in the form of methyl B12, or for methylmalonyl-CoA mutase (MCM) in the form of adenosyl B12. Therefore, as discussed elsewhere, measuring serum vitamin B12 levels alone has many limitations and does not reflect vitamin B12 bioavailability or cellular levels of B12 (Hannibal et al. 2016). Current factors for the identification of vitamin B12 deficiency include markers participating in the metabolic pathway of vitamin B12 including methylmalonic acid, homocysteine, and total holotranscobalamin, which is the vitamin B12 bound to transcobalamin complex.
The assessment of nutritional intake is mostly done via food frequency questionnaires (FFQs) both in clinical practice and epidemiological studies. FFQs provide an overview of the macronutrients and the micronutrients obtained through diet, via a series of questions regarding the type and portion of ingested food. Also, a crude estimation on the toxicants obtained through diet can be obtained. Dietary patterns can be recognized and translated into a nutritional score in a computer-based system that matches the food choices with their nutritional composition. FFQs, although they are easy to use, economical, and high throughput, they have low sensitivity and accuracy as discussed elsewhere (Margină et al. 2020).
Biochemical and laboratory testing is another widely used method to assess the status of nutrients and is a valuable tool to diagnose severe micronutrient deficiencies. However, micronutrient deficiencies in NCDs can be multiple and to a smaller extent compared to a nutritional deficiency disease. Also, cells and tissues are very well-regulated systems that have mechanisms to adjust the nutrient requirements depending the nutrient availability or recycle to meet increased demands on a specific tissue (Pietrzik 1991; Nualart et al. 2014). Therefore, blood values of the micronutrient do not provide sufficient information on the physician (Bier and Mann 2015). Emerging technologies for the identification of biomarkers of the nutritional status that will allow grouping of individuals according to their nutrient requirements are at the center of attention.
Nutriomics is a novel field that focuses on the comprehensive study of the effect of ingested food on human health and disease risk. Nutrigenomics, studies the effect of diet on the expression of genes and risk for NCDs. This technology allows the determination of nutrient requirements and foods based on the genetic background and has provided valuable insights on the nutrition-genome interaction. However, downstream at the end of the gene expression and the environment-related post-translational modifications lies the metabolome which is the metabolites that take part in the metabolic pathways. Nutrient-regulated enzymes are catalyzing the metabolic pathways of an organism, thus making metabolites promising markers for nutrient adequacy, storage, and use. In addition to that, metabolites can give information regarding the load from heavy metals since the latter are antagonizing with some nutrients for the same enzymes (Zeisel 2007). Therefore, analysis of multiple metabolic pathways that use different combinations of nutrients and are affected by different heavy metals can give a detailed map of the missing nutrients and excess of heavy metals.
Overall, tuned research efforts are currently being made to uncover the complex interrelationships between diet, genes, metabolites, and health. Novel tools are under development to help physicians, clinical nutritionists, and every healthcare professional to detect nutritional deficiencies early and provide personalized recommendations for their replenishment.
2.3 Vitamin D
Vitamin D is a member of the steroid hormones participating in various functions of the human body and can be obtained from food and supplements, or can be endogenously sythesized through sunlight exposure (Wang et al. 2017). The leading roles of vitamin D include modulating cell growth and inducing the function of the immune, nervous, and muscular systems. On a molecular level, it accentuates the expression of genes that control cellular proliferation, differentiation, and apoptosis. According to a study by Ramagopalan et al., the nuclear vitamin D receptor (VDR) occupies 2776 positions on the human DNA, and 229 genes show changes in expression after treatment with vitamin D (Ramagopalan et al. 2010). After it has been absorbed by the intestine or synthesized by the skin due to sunlight exposure, vitamin D in the form of cholecalciferol is transported to the liver and converted to calcidiol, 25-hydroxycholecalciferol (25 (OH) D) which binds to specific proteins that transport calcidiol to the kidney through blood circulation. There, it is converted to the active vitamin D form, calcitriol 1,25-dihydroxycholecalciferol (1,25(OH)2D3). 25 (OH) D is a biomarker used in the measurement and assessment of vitamin D levels and the detection of deficiencies because it reflects the levels of vitamin D derived from both the diet and the skin and is more stable than 1,25(OH)2D3 (Wang et al. 2017).
Accumulating evidence from recent studies indicate that vitamin D deficiency can be directly associated with the incidence of NCDs and ADs. According to a recent review by Amrein K. et al., a deficiency of vitamin D is recognized as a concentration of 25 (OH) D lower than 20 ng/ml (Amrein et al. 2020). Even though most authors consider a range of below 30 ng/ml 25 (OH) D as vitamin D deficient, studies have shown that levels lower than 10 or 12 ng/ml increase the risk of osteomalacia and crickets (Holick et al. 2011a; Institute of Medicine of the National Academies 2011; Braegger et al. 2013; EFSA NDA Panel 2016). Therefore, these levels define severe vitamin D deficiency. The clinical practice guidelines of the Endocrine Society Task Force on vitamin D refer to 20 ng/ml as a cutoff level for vitamin deficiency, 21–29 ng/ml as vitamin insufficiency and 30–100 ng/ml as sufficient vitamin D levels (Holick et al. 2011a).
The levels mentioned above refer to bone health and reflect the minimum concentration of vitamin D, under which diseases have been reported to arise. However, vitamin D cutoff levels associated with the risk of NCDs have been shown to be higher. A study by Wang T. et al. examined vitamin D deficiency and its relation to the risk of developing cardiovascular disease in individuals without prior cardiovascular disease. A total of 1739 individuals with a mean age of 59 years participated in the study, and the amount of 25 (OH) D in the blood was used to evaluate the status of their vitamin D levels. The findings of this study indicated that vitamin D deficiency is positively associated with the risk of developing cardiovascular disease. More specifically, individuals with hypertension whose 25 (OH) D levels were less than 50 nmol/L had a twofold risk of cardiovascular incidence (Wang et al. 2008). The first case-control study examining the correlation between the development of Insulin Dependent Diabetes Mellitus (IDDM) and vitamin D administration during the first year of life by EURODIAB Substudy 2 study group showed a decreased risk of developing type 1 diabetes for children that received vitamin D supplements for at least 1 year during early childhood (Miettinen et al. 2020). The data of the study were collected by interviewing mothers of 3.155 children regarding their children’s supplementation of vitamin D during their first years of life. However, lack of consistent dosage of vitamin D and the inconsistent validity of the answers given by mothers constitute limitations of this study. Type I diabetes (IDDM) is an autoimmune disease caused by the destruction of beta pancreatic cells whose role is to produce insulin. As a result, insulin deficiency occurs, leading to hyperglycemia and having further health complications in other tissues and organs. Insulin injections are administered daily to promote the absorption of glucose by cells and maintain glucose levels within the optimal range (Kahanovitz et al. 2017). Further studies need to be taken into consideration to establish a causality linkage between IDDM and vitamin D. A systematic review and meta-analysis of this study by EURODIAB Substudy 2 study group indicated that the establishment of causality requires randomized controlled trials with long periods of follow-up (Zipitis and Akobeng 2008). A study conducted by Miettinen M. E. et al. investigated the association of serum 25-hydroxyvitamin D levels in childhood on the risk of developing islet autoimmunity and IDDM (Miettinen et al. 2020). A total of 732 infants participated in the observational study, and serum concentrations of 25(OH) were measured repeatedly for 10 years. The serum concentrations were then compared according to age at the first seroconversion. The results suggested that prenatal vitamin D supplementation can assist in the prevention of IDDM.
Clinical and preclinical evidence suggests that vitamin D deficiency plays a vital role in the management of inflammatory bowel disease (IBD) (Hlavaty et al. 2015). IBDs , referring to ulcerative colitis and Crohn’s disease, are NCDs characterized by extensive inflammation of the intestine. Specifically, ulcerative colitis affects the large intestine, while Crohn’s disease can affect any part of the digestive system. In a study by Levin A. D. aiming to associate vitamin D status with IBD location and severity, the importance of monitoring vitamin D status was emphasized for the management of the disease (Levin et al. 2011). Seventy-eight children with IBD participated in the study (45 males, 33 females), and their 25 (OH) D levels were measured for the period during 2006–2007. Vitamin D deficiency was defined as 25 (OH) D< 51 nmol/l (moderate) and 25 (OH) D< 30 nmol/l (severe), while insufficiency was for 25 (OH) D levels between 51 and 75 nmol/l. The results yielded that 15 children (19%) had a vitamin D deficiency, and 30 children (38%) had an insufficiency, and therefore a positive correlation was established. Further randomized trials are required to establish a causational relationship between vitamin D deficiency and IBD.
According to evidence from review articles and studies, vitamin D can also aid in the treatment of psoriasis (Morimoto et al. 1986; Fu and Vender 2011; Mattozzi et al. 2016; Kechichian and Ezzedine 2018). Psoriasis is an immune-mediated disease that affects the skin causing red patches to appear. A study by Morimoto S et al. indicated that oral administration and topical application of vitamin D derivatives were beneficial in the management of psoriasis and improvement of psoriatic skin lesions (Morimoto et al. 1986). A total of 40 patients were enrolled in the study, and active forms of vitamin D3 were either orally administered or topically applied. Vitamin D3 (cholecalciferol) is an active analog of vitamin D that is synthesized by the skin or obtained via supplements or diet. The results of the study suggested that psoriasis may respond to the active forms of vitamin D3 and that unresponsiveness of skin cells to the vitamin might be implicated in the pathogenesis of psoriasis. Another study by Finamor D. C. et al. investigated the effect, efficacy, and safety of administration of high daily doses of vitamin D on the clinical course of vitiligo and psoriasis (Finamor et al. 2013). A total of 25 patients (9 with psoriasis and 16 with vitiligo) received 35.000 IU of vitamin D per day for 6 months. The results showed that the treatment reduced disease activity for 9/9 patients with psoriasis and 14/16 patients with vitiligo.
Evidence from studies has shown that the supplementation of vitamin D can be beneficial for NCDs, including cancer and autoimmune diseases, as well as infections. Specifically, a review conducted by Garland F. C. et al. investigated the prospects of vitamin D3 supplementation in global cancer prevention, and the results were encouraging (Garland et al. 2007). According to the review, the intake of 2.000 IU per day of vitamin D3 would lead to a 25% decrease of annual cases of breast cancer and 27% of annual cases of colorectal cancer. Overall, vitamin D can aid in the prevention of NCDs (Gorham et al. 2005; Garland et al. 2009). Nevertheless, it was recently proposed that maintaining concentrations above 40–60 ng/mL can decrease the risk of infections (Grant et al. 2020).
Moreover, vitamin D levels have been associated with other ADs including autoimmune thyroiditis and rheumatoid arthritis while attention is being given at the recommended dose (Kivity et al. 2011). In populations with higher nutrient demands, as in the case of an established disease, the administered dose may exceed the Recommended Dietary Allowance (RDA), which need to be considered in clinical trials as well as in everyday clinical practice (Tsoukalas and Sarandi 2020). There is growing evidence that cells are insensitive to vitamin D in ADs suggesting that higher doses are required to exert the immunomodulatory effect of vitamin D (Jeffery et al. 2018). In addition, corticosteroids treatment commonly used in ADs has been shown to affect the catabolism of vitamin D, thus requiring higher doses to maintain optimum levels in the blood circulation (Singh and Kamen 2012; Kamen 2013). According to the American Endocrinology Society, the upper level for safe vitamin D intake is 10.000 IU daily for adults and 4000 IU for children over 8 years old. Higher dose recommendations require monitoring of the vitamin D blood levels (Holick et al. 2011b). Based on evidence from recent studies, vitamin D supplementation has not been associated with toxicity risks even at 700 ng/ml or the intake of 30,000 IU/day and the steady-state levels of vitamin D at 200 ng/ml for a long time (Hathcock et al. 2007).
Overall, randomized controlled trials are needed to specify the ideal supplementation dose as a preventive and treatment strategy considering the various involved factors. However, vitamin D supplementation is an established general recommendation for the reduction of risk for NCDs and ADs and as an adjunct tool in their management.
2.4 Sodium
High intake of sodium was the driving cause of mortality among diet-related deaths in China, among other countries, and mostly due to cardiovascular disease, according to the Lancet observational study, in line with others (Ezzati et al. 2014). Several salt alternatives have been proposed starting several years ago when the salt reduction program in Finland showed beneficial effects of low-sodium, high-potassium, and magnesium salt substitutes on hypertension (Katz et al. 1999),(Karpanen et al. 1984). Notably, Finland was one of the first countries that adopted a low salt routine in the late 1970s, and 20–30 years later, the mortality caused by stroke and coronary heart diseases decreased dramatically by 75–80% (Karppanen and Mervaala 2006). Later, the UK demonstrated that a 15% decrease in salt intake is linked to a significant reduction of blood pressure and mortality from stroke and ischemic episodes (Watroba and Szukiewicz 2016). Since then, 30% of sodium reduction has been included in the strategies of the World Health Organization to combat NCDs, and according to a 2015 review, 75 countries have adopted strategies to achieve this goal (Trieu et al. 2015).
Apart from the causal relationship of high sodium intake with blood pressure and CVD, some studies have shown possible associations with damage in several organs such as the kidney, stomach, and bones, malfunction in the immune system, hormonal and oxidation mechanism, and the gut microbiome balance (He et al. 2020). However, as many researchers discuss, salt reduction strategy is usually combined with a healthier lifestyle in observational studies, and the synergistic effect cannot be fully discriminated from the alt reduction alone. According to WHO and CDC recommendations, less than 5 g/day salt and 2 g of sodium with more than 3.5 g of potassium should be consumed daily by adults (World Health Organization 2013; CDC 2017). As with every nutrient, optimum intakes are required for the normal function at a cellular and organism level. A meta-analysis of sodium intake and CVD showed that not only excessive (>12.5 g) but also extremely low levels of salt (<5.6 g) were related to poor health outcomes. In addition, bio individuality stemming from our genes and other risk factors shapes different salt sensitivity levels that needs to be considered (Graudal et al. 2014). Overall, there is mounting evidence that high sodium intake has many adverse effects and especially when it is combined with the ingestion of processed foods, but at low levels sodium is crucial for the maintenance of fluid and blood volume and the normal function of nerve cells.
2.5 Free Sugars
Free sugars which are defined as “all monosaccharides and disaccharides added to foods by the manufacturer, cook, or consumer, plus sugars naturally present in honey, syrups, and fruit juices” according to the WHO and the FAO have been the subject of intense debate concerning health effects. As stated elsewhere, this term includes all sugars but the lactose found in milk and the naturally occurring sugars found in the outside structure of food such as the fruit skin (Ludwig et al. 2018). Sugar consumption has been known for its detrimental effects on oral health. Indeed, according to a systematic review that was later used by WHO experts, a 10% free sugar reduction is positively associated with lower caries. In comparison, 5% was associated with a better outcome, a strategy adopted by the Scientific Advisory Nutrition Committee (Moynihan and Kelly 2014; SACN 2015). Although there are some inconsistencies in the field, sugar intake has been associated with several disease outcomes, including CVD (Te Morenga et al. 2014; Dinicolantonio and Okeefe 2017), diabetes, and autoimmune diseases (Zhang et al. 2019; Correa-Rodríguez et al. 2020). Moreover, data from animals study show that a Western diet rather than a high-fat diet can lead to a psoriasis-like phenotype which occurs earlier than obesity, suggesting that sugars’ effect on health is independent of obesity (Shi et al. 2020).
Although studies linking sugar intake with skin diseases like psoriasis and atopic dermatitis are scarce, and the exact mechanism is not fully understood, it is suggested that sugar is a crucial contributor to chronic inflammation of autoimmune diseases and NCDs in general (Manzel et al. 2014; Nosrati et al. 2017). Specifically, positive associations have been demonstrated between intake of sugar and established cardiovascular markers , namely, blood pressure, and levels of triglycerides, LDL, and total cholesterol in meta-analyses of randomized controlled trials. The authors suggest that fructose commonly found in sugar-sweetened beverages, honey, sucrose syrup, and fruit is more likely to be the cause for the sugar-related increase of cardiometabolic indicators. Also, excessive fructose intake has been implicated in weight gain and as a critical contributor to the obesity epidemic. Because the sweet taste of fructose-rich products causes satiety, some suggest that weight gain stems from excessive food consumption caused by satiety. Recent data indicate that under physiological conditions, fructose is metabolized in the intestine and the liver, increasing blood glucose and insulin. However, when fructose is consumed excessively, it reaches the colon and liver where it is metabolized leading to de novo lipogenesis through several pathways, including the feeding of TCA cycle with the fructose-derived pathway with pyruvate which in turn is metabolized to citrate and then to acetyl-CoA by the enzyme ATP citrate lyase. However, a study published in Nature, March this year, showed for the first time that de novo lipogenesis can occur even in the absence of ACLY through a distinct pathway that involved the gut microbiome (Postic 2020; Zhao et al. 2020b). Briefly, using isotope-tracer methodology and metabolomics, it was demonstrated that fructose could be metabolized to acetate by the gut microbiome in the liver resulting in hepatocyte-related lipogenesis. Also, fructose even though its metabolism is not insulin-dependent, when is ingested excessively, it has been shown to augment hyperinsulinemia and insulin resistance via direct and indirect pathways in the liver, independently from weight increase and total calories, while promoting liver inflammation through mitochondrial fatty acids oxidation impairment and stress of the endoplasmic reticulum (Softic et al. 2020).
Interestingly, glucose, which is also a monosaccharide-like fructose with the same molecular formula but a different structure, has not been shown to act similarly with fructose when used as a sweetener in terms of de novo lipogenesis. The WHO guidelines include the reduction of sugar to 10% of energy intake while highlighting the beneficial effects of further reduction to 5%, based on existing literature (e-Library of Evidence for Nutrition Actions (eLENA) 2019). In other words, 25 g of free sugar per day or 2 oranges is recommended for a healthy individual. Sugar consumption today ranges from 13% to 17%, of which 50% is in the form of fructose (Merino et al. 2020). In the UK, children 4–10 years old 13.5% of energy intake is in the form of free sugars, according to the UK National Diet and Nutrition Survey (NDNS), and similar are the findings in the USA. Overall, there is accumulating evidence that excessive free sugar intake is involved in the onset of metabolic changes that promote the development of ADs and NCDs.
2.6 Alcohol
Alcohol consumption is one of the leading risk factors of NCDs. Some suggest a beneficial effect when consumed moderately, but recent comparative reviews question this relationship. On the contrary, a series of epidemiologic studies have indicated that heavy alcohol consumption increases the risk of cardiovascular disease and liver disease and has been associated with more than 50 diseases (WHO 2018b; Millwood et al. 2019). Specifically, more than 5% of the global burden of disease can be attributed to alcohol , and some of the major contributors are cancers, chronic liver disease, and cardiovascular diseases. Through the increase of blood pressure and the disturbance of lipid profile, excessive drinking is linked with overall CVD posing a major challenge of modern societies (Chiva-blanch and Badimon 2020). In addition, it has a detrimental effect on the gut microbiome and immunotolerance and has been regarded as an associating factor with the presence of ADs (Wang et al. 2010; Sarkar et al. 2015).
2.7 Physical Activity
Another important risk factor for NCDs is physical activity , the movement of the body that requires energy such as walking or cycling, which ranks among the top causes of early mortality (WHO 2018a). Globally, 30% of the population is not taking adequate physical activity according to the global recommendations on physical activity for health. Insufficient physical activity refers to less than 150 min/week of moderate-intensity aerobic exercise or less than 75 min/week of intense exercise for adults. According to a large epidemiological study on nearly two million people around the world, published in Lancet, it showed that high-income countries are twice more prevalent in physical inactivity than low income mostly due to the different means of transport and nature of work (Guthold et al. 2018; Lear et al. 2017).
Several studies have demonstrated the positive effects of physical activity not only in prevention but also for the improvement of disease progression and the quality of life of patients. Indeed, in a 130.000 people observational study from different countries of every income category, moderate physical activity was associated with a more than 20% reduction in risk for major CVD and risk for all-cause mortality. The negative association between physical activity and the risk was dose-dependent, suggesting that more exercise than the 150 min/week has additional benefits (Lear et al. 2017).
Moreover, physical activity has been shown to regulate the immune responses, thus benefiting patients with autoimmune diseases, including multiple sclerosis, rheumatoid arthritis, and inflammatory bowel diseases. Importantly, patients with autoimmune diseases experience musculoskeletal complications that significantly deteriorate their quality of life. Exercise can contribute to the maintenance of mobility function through enhanced muscle strength, coordination, and weight balance (Sharif et al. 2018). More importantly, it has been shown that regular moderate exercise can increase glucose uptake and reduce insulin resistance, which are determinant factors for the onset and progression of NCDs (DeFronzo et al. 1987). Finally, in a case-control study, it was shown that exercise was a very important factor for the development of a model predicting the presence of autoimmune diseases based on the levels of fatty acids and lifestyle factors (Tsoukalas et al. 2019c).
2.8 Cigarette Smoking
Tobacco use is the most prevalent modifiable risk factor of the main NCDs, including CVD, cancer, respiratory disease, and diabetes, as well as neurological disorders. It is estimated to cause around 71% of all lung cancer deaths, 42% of the chronic respiratory disease, and almost 10% of CVD. It is estimated that around six million people each year are killed by tobacco, approximately one person every 6 seconds, from whom more than five million are due to direct tobacco use and 600,000 due to their exposure to second-hand smoke. Moreover, tobacco is responsible for 14% of the global NCDs deaths of adults age for more than 30 years. In 2020, the number of deaths attributed to tobacco use increased to eight million people annually, with seven million of those deaths due to direct tobacco use and around 1.2 million due to non-smokers being exposed to second-hand smoke. Still, almost 80% of them, corresponding to 1.3 billion tobacco users, come from low- and middle-income countries, with tobacco use, greatly contributing to poverty and replacing basic needs, such as food. Due to the very high rates of tobacco use morbidity and mortality, the healthcare costs for treating the diseases caused by tobacco are significantly high in several countries (World Health Organization 2020).
The molecular pathways involved in the effect of cigarette smoking on NCDs, include metabolic shift and oxidative stress contributing to the development and progression of cardiovascular damage (Leone 2005). More specifically, metabolic changes mediated by cigarette smoking substances lead to the development of atherosclerotic lesions and atherosclerotic plaque through narrowing of the vascular lumen and induction of a hypercoagulable state that in turn increases the risk of acute thrombosis. Briefly, cigarette smoking leads to endothelium dysfunction by directly affecting the endothelial cells triggering the formation of atherosclerotic plaques, which with the combination of other inflammation mechanisms will develop into vulnerable plaques prone to rupture (Csordas and Bernhard 2013). Cigarette smoking also affects other risk factors, such as low levels of HDL cholesterol and glucose intolerance (CDC 2008). Moreover, hematological changes are also triggered by tobacco exposure, including increased white blood cells, platelet aggregation, changes in serum lipids, and fibrinogen levels. The most important specific markers used for the determination of exposure to tobacco include nicotine and its metabolites, such as carbon monoxide, cotinine, and thiocyanate with cotinine being the most potent urine marker.
Nevertheless, carboxyhemoglobin levels seem to be more a qualitative rather than a quantitative factor for the level of exposure, or the amount of cardiovascular damage (Leone 2005). Also, hair analysis is used to determine the levels of cotinine, which accumulates in the hair during hair growth allowing the long-term monitoring of the accumulative effects of tobacco exposure (Florescu et al. 2009). It should be noted that all forms of tobacco are harmful, including cigarette smoking, waterpipe tobacco, and other various smokeless tobacco products, cigars, pipe tobacco, etc. More specifically, the use of waterpipe tobacco and other smokeless tobacco products are harmful, similar to cigarette smoking. It has been suggested that waterpipe tobacco is highly addictive due to containing nicotine and significantly damaging for human health.
Moreover, heated tobacco products that are promoted the last years as being less harmful produce aerosols with nicotine and other toxic products upon tobacco heating that lead to increased risk of cancers of the head, neck, throat, esophagus, and oral cavity, as well as several dental diseases (Davis et al. 2019). Similarly, e-cigarettes that are electronic systems delivering nicotine or not produce an aerosol upon heating a liquid that is inhaled by the user. They can also be highly addictive and harmful, especially when used by children or adolescents whose brain is still under development, as well as pregnant women, as it can be damaging for the fetus . Finally, it has been shown to increase the risk of CVD and lung disease, but its long-term effects remain to be studied the following years (CDC 2020).
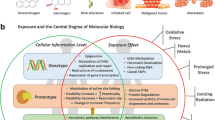
3 Environmental Factors
Environmental factors are very important contributors to disease, and recently they have been acknowledged as risk factors for NCDs. However, in countries like Southeast Asia, air pollution is the leading cause of NCDs. It is estimated that environment-related deaths from NCDs account for 2/3 (8.2 M) of total deaths (12.6 M) caused by the environment. Apart from air pollution, environmental factors include radiation, second-hand smoke, noise, unhealthy drinking water, smoking, exposure to carcinogens and other harmful toxic agents, heavy metals, and mostly lead and mercury (World Health Organization 2017). Health complications to these factors include mostly cardiovascular disease, where 1/3 of CVD is attributed mostly to air pollution and at a lower level to other environmental factors.
3.1 Air Pollution
According to data from the Global Health Observatory for mortality from all or specific causes, air pollution was responsible for 22% of CVD deaths, 26% of ischemic heart and 25% of stroke deaths, 53% of COPD deaths, and 40% of deaths from cancer in the lungs (Wang et al. 2016). In line with NCDs incidence and related death rise, ambient air pollution has risen by 9% for the period 2010–2016, raising the awareness of international health organizations to address this challenge. The third United Nations high-level meeting on NCDs recognized air pollution (ambient and household) as a risk factor for NCDs in 2018. Since then, several interventional strategies have been proposed towards a more sustainable environment (Prüss-Ustün et al. 2019). An important factor for the NCDs incidence caused by environmental risks is early-life exposure. More than 25% of deaths among children below 5 years old are associated with the environment, and exposure to polluted air has been linked with premature and low-weight birth for a pregnant mother and NCDs onset for the children and adolescents. Asthma, the most common NCD among children, has been studied extensively concerning the role of air pollution on its development. In contrast, a recent study showed that improvement in air quality could prevent almost 50% of asthma cases (Pierangeli et al. 2020).
Air pollution , referring to the polluting substances or particulate matters in the air that can have a harmful effect on living organisms, can have direct and immediate or indirect, and at a later stage, effects on health. Particulate matters normally are formed in the air through the interaction between chemical substances and are categorized based on their diameter. It has been shown that the smaller their diameter, the greater the risk for human health because of their increased penetration to the body. Immediate impact can be caused via the binding compounds present in gases or aerosols such as CO2 and NO2 to hemoglobin competing with oxygen, leading to hypoxia and toxicity (Schraufnagel et al. 2019). Studies investigating the short-term exposure effect of air pollutants showed increased hospitalization and admission at the emergency department for patients with respiratory issues such as asthma and COPD. In China, a longitudinal analysis of 84 patients with COPD showed that exposure to ambient air pollution and specifically NO2, CO, and SO2 was linked to reducing lung function measured by Forced Vital Capacity percentage (FVC%) and reduced the anti-inflammatory and increased pro-inflammatory markers (Gao et al. 2020). These findings are in line with previous studies with COPD patients, and notably, the correlation is stronger in patients that smoke suggesting a synergistic effect between pollutants and smoke agents (Dadvand et al. 2014).
Additionally, a large global study in 652 cities of 24 countries published in NEJM highlighted the positive association between CVD, respiratory disease and all-cause mortality, and short exposure to ambient air pollution , even below the allowed threshold of pollutants concentrations. Concerning CVD, several studies have reported significant associations between short-term exposure to particulate matters with blood pressure and out-of-hospital cardiac arrest (Zhao et al. 2020a).
In a more long-term manner, pollutants of the air promote oxidative stress and systemic inflammation and have been implicated in dysfunction of distinct organs reviewed by Schraufnagel D. et al. (Schraufnagel et al. 2019). Global health organizations have developed tools for the risk assessment of air pollution for long-term and short-term exposure. AirQ+ is a software developed by the World Health Organization (WHO) Regional Office for Europe enabling users to quantify and assess the magnitude of air population with specific characteristics used as input on health including morbidity and mortality incidence projections for acute and chronic diseases. A comparative review and discussion of the collected data from AirQ models by Conti G O et al. identified the limitation of not including a large variety of pollutants as input to the software, thus providing only a part of the picture (Oliveri Conti et al. 2017).
3.2 Heavy Metals
Another type of environmental pollution with a significant effect on human health is heavy metals . Although some are essential for life such as iron, zinc, and manganese at small doses, some others, including cadmium (Cd), mercury (Hg), and lead (Pb), have no known beneficial effect and can be rather dangerous. Heavy metals have increased dramatically due to the anthropogenic activity and can be found in the atmosphere, the water, the soil, and thus the living organisms. Through the food chain, the accumulation of these heavy metals to humans can be such that it will be dangerous. The absorption of heavy metals from vegetables through the soil resulting in the chronic-low grade exposure to them to humans has been well studied for years. Briefly, heavy metals can either directly affect organs such as the brain, the kidney, and the heart or displace essential nutrients leading to significant disruption of metabolic pathways and oxidative stress (Jaishankar et al. 2014). However, new evidence indicates an additional pathway through which heavy metals affect health, which is through alteration of the microbiome (Chiu et al. 2020).
3.3 Concluding Remarks
NCDs morbidity and mortality upward trends represent a major challenge for the healthcare sector. Based on epidemiological data and observational studies, global health agencies have defined the key environmental factors and the intermediate mechanisms that shape the unhealthy environment and trigger or aggravate NCDs (Fig. 13.2). Diet is the primary factor that is associated with NCDs mortality, suggesting that through an intervention to people’s daily dietary choices, 20% or 11 million of global deaths could be prevented. However, as studies suggest, the relationship between nutrients and health is complex and dynamic, which requires sophisticated tools to identify and monitor their metabolism.
Metabolomics is a promising tool that can be valuable to several healthcare positions and specialties. As a scanning tool , metabolomics can demonstrate nutritional deficiencies or hidden hunger that underlie an NCD, allowing primary care professionals to replenish these deficiencies under a balanced diet with a personalized dietary intervention (Tsoukalas et al. 2017). As shown in the explanatory figure of vitamin B12 pathway, blood levels of nutrients are not sufficient and reliable markers to reflect the bioavailability of nutrients, whereas intermediate metabolites participating in the pathways fueled by these nutrients are more robust (Fig. 13.1). Moreover, dietary interventions can be monitored for their efficacy in an individual with metabolomics, based on the genetic profile, underlying disease and drug treatment. These factors may affect nutrients absorption or metabolism. Dietary compounds can increase or reduce the risk of NCDs through their interaction with gene expression and post-translational modifications. Metabolomics can capture the effect of selected dietary compounds on metabolism, allowing the healthcare professional to personalize the intervention. Finally, specific metabolic biomarkers that are related to diet-related complications such as insulin resistance and pro-inflammatory context can be valuable predictive tools for individuals at risk of developing NCDs or ADs. For example, dihomogamma-linolenic acid is related to insulin resistance, inflammation, and the presence of autoimmune diseases suggesting the potency as a predictive biomarker (Tsoukalas et al. 2019a; Tsoukalas et al. 2019c).
An additional burden to health, apart from dietary and lifestyle factors, is toxicants from cigarette smoking, dietary heavy metals, and air pollution. The molecular mechanism by which these factors negatively affect human health is not fully described but accumulating data show their causal relationship with NCDs onset. Oxidative stress and inflammation are central mechanisms that have been shown to be significantly induced under the exposure to PM or cigarette smoke, also affecting the human metabolome and promoting insulin resistance (Fig. 13.2). Also, heavy metals obtained through diet have been shown to affect the normal function of the metabolic pathways through their interaction with the enzymes. As such, an association between exposure to toxicants with metabolic phenotypic changes can provide valuable information to health and governmental bodies towards sustainable environmental solutions. In a more patient-centered view, metabolomics can identify the specific metabolic pathways that are disturbed and the enzymes and metabolites that are involved indicating the points of the metabolism that require attention through dietary or medication interventions.
Overall, metabolomics, as a tool of precision medicine , presents an opportunity to move from evidence-based medicine that focuses on diseases and symptoms management of NCDs, towards medical approaches that combine effective screening, prevention, and health promotion strategies, while offering personalized intervention targeting the risk factors in line with the standard treatment.
References
Afshin, A. et al. (2019) ‘Health effects of dietary risks in 195 countries, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017’, The Lancet, 393(10184), pp. 1958–1972. doi: https://doi.org/10.1016/S0140-6736(19)30041-8.
Ames, B. N. (2006) ‘Low micronutrient intake may accelerate the degenerative diseases of aging through allocation of scarce micronutrients by triage’, PNAS, 103(47), pp. 17589–17594.
Amrein, K. et al. (2020) ‘Vitamin D deficiency 2.0: an update on the current status worldwide’, European Journal of Clinical Nutrition. Springer US. doi: https://doi.org/10.1038/s41430-020-0558-y.
Bailey, R. L., West, K. P. and Black, R. E. (2015) ‘The epidemiology of global micronutrient deficiencies’, Annals of Nutrition and Metabolism, 66(suppl 2), pp. 22–33. doi: https://doi.org/10.1159/000371618.
Balashova, E. E., Maslov, D. L. and Lokhov, P. G. (2018) ‘A metabolomics approach to pharmacotherapy personalization’, Journal of Personalized Medicine, 8(3). doi: https://doi.org/10.3390/jpm8030028.
Bier, D. M. and Mann, J. (2015) Nutrition for the Primary Care Provider. Edited by W. R. of N. and Dietetics. Karger. doi: https://doi.org/10.1159/isbn.978-3-318-02667-2.
Blumberg, J. B. et al. (2017) ‘Impact of frequency of multi-vitamin/multi-mineral supplement intake on nutritional adequacy and nutrient deficiencies in U.S. adults’, Nutrients, 9(8). doi: https://doi.org/10.3390/nu9080849.
Braegger, C. et al. (2013) ‘Vitamin d in the healthy European paediatric population’, Journal of Pediatric Gastroenterology and Nutrition, 56(6), pp. 692–701. doi: https://doi.org/10.1097/MPG.0b013e31828f3c05.
CDC (2008) ‘Smoking-attributable mortality, years of potential life lost, and productivity losses--United States, 2000-2004.’, MMWR. Morbidity and mortality weekly report. United States, 57(45), pp. 1226–1228.
CDC (2017) Get the facts: Sodium and the Dietary Guidelines.
CDC (2020) About Electronic Cigarettes (E-Cigarettes). Available at: https://www.cdc.gov/tobacco/basic_information/e-cigarettes/about-e-cigarettes.html (Accessed: 2 July 2020).
Chiu, K. et al. (2020) ‘The Impact of Environmental Chemicals on the Gut Microbiome’. Toxicol Sci., 176(2), pp. 253–284. doi: https://doi.org/10.1093/toxsci/kfaa065/5835885.
Chiva-blanch, G. and Badimon, L. (2020) ‘Benefits and Risks of Moderate Alcohol Consumption on Cardiovascular Disease: Current Findings’, Nutrients, 12(108), pp. 1–19. doi: https://doi.org/10.3390/nu12010108.
Correa-Rodríguez, M. et al. (2020) ‘Dietary intake of free sugars is associated with disease activity and dyslipidemia in systemic lupus erythematosus patients’, Nutrients, 12(4). doi: https://doi.org/10.3390/nu12041094.
Csordas, A. and Bernhard, D. (2013) ‘The biology behind the atherothrombotic effects of cigarette smoke’, Nature Reviews Cardiology. Nature Publishing Group, 10(4), pp. 219–230. doi: https://doi.org/10.1038/nrcardio.2013.8.
Dadvand, P. et al. (2014) ‘Air pollution and biomarkers of systemic inflammation and tissue repair in COPD patients’, European Respiratory Journal. European Respiratory Society, 44(3), pp. 603–613. doi: https://doi.org/10.1183/09031936.00168813.
Davis, B., Williams, M. and Talbot, P. (2019) ‘iQOS: evidence of pyrolysis and release of a toxicant from plastic’, Tobacco Control, 28(1), pp. 34 LP – 41. doi: https://doi.org/10.1136/tobaccocontrol-2017-054104.
DeFronzo, R. A., Sherwin, R. S. and Kraemer, N. (1987) ‘Effect of physical training on insulin action in obesity’, Diabetes, 36(12), pp. 1379–1385. doi: https://doi.org/10.2337/diab.36.12.1379.
Devries, S. (2019) ‘A global deficiency of nutrition education in physician training: the low hanging fruit in medicine remains on the vine’, The Lancet Planetary Health. The Authors(s). Published by Elsevier Ltd. This is an Open Access article under the CC BY-NC-ND 4.0 license, 3(9), pp. e371–e372. doi: https://doi.org/10.1016/S2542-5196(19)30173-1.
Dinicolantonio, J. J. and Okeefe, J. H. (2017) ‘Added sugars drive coronary heart disease via insulin resistance and hyperinsulinaemia: a new paradigm’, Open Heart, 4(e000729), pp. 1–4. doi: https://doi.org/10.1136/openhrt-2017-000729.
EFSA NDA Panel (EFSA Panel on Dietetic Products, Nutrition and Allergies). (2016) Scientific opinion on dietary reference values for vitamin D. EFSA Journal 14(10):4547, 145 pp. doi: https://doi.org/10.2903/j.efsa.2016.4547.
e-Library of Evidence for Nutrition Actions (eLENA) (2019) Reducing free sugars intake in adults to reduce the risk of noncommunicable diseases, WHO. Available at: https://www.who.int/elena/titles/free-sugars-adults-ncds/en/ (Accessed: 2 July 2020).
Ezzati, M. et al. (2014) ‘Global Sodium Consumption and Death from Cardiovascular Causes’, NEJM, 371:624–634. doi: https://doi.org/10.1056/NEJMoa1304127.
Faggi, L. et al. (2019) ‘A Polyphenol-Enriched Supplement Exerts Potent Epigenetic-Protective Activity in a Cell-Based Model of Brain Ischemia’, Nutrients, pp. 1–15. doi: https://doi.org/10.3390/nu11020345.
Finamor, D. C. et al. (2013) ‘A pilot study assessing the effect of prolonged administration of high daily doses of vitamin D on the clinical course of vitiligo and psoriasis’, Dermato-Endocrinology, 5(1), pp. 222–234. doi: https://doi.org/10.4161/derm.24808.
Florescu, A. et al. (2009) ‘Methods for quantification of exposure to cigarette smoking and environmental tobacco smoke: focus on developmental toxicology’, Therapeutic drug monitoring, 31(1), pp. 14–30. doi: https://doi.org/10.1097/FTD.0b013e3181957a3b.
Fu, L. W. and Vender, R. (2011) ‘Systemic role for vitamin D in the treatment of psoriasis and metabolic syndrome’, Dermatology Research and Practice, 2011. doi: https://doi.org/10.1155/2011/276079.
Gao, N. et al. (2020) ‘Lung function and systemic inflammation associated with short-term air pollution exposure in chronic obstructive pulmonary disease patients in Beijing, China’, Environmental Health, 19(1), p. 12. doi: https://doi.org/10.1186/s12940-020-0568-1.
Garland, C. F. et al. (2007) ‘Vitamin D and prevention of breast cancer: Pooled analysis’, Journal of Steroid Biochemistry and Molecular Biology, 103(3–5), pp. 708–711. doi: https://doi.org/10.1016/j.jsbmb.2006.12.007.
Garland, C. F. et al. (2009) ‘Vitamin D for Cancer Prevention: Global Perspective’, Annals of Epidemiology, 19(7), pp. 468–483. doi: https://doi.org/10.1016/j.annepidem.2009.03.021.
Giles, J. T. et al. (2015) ‘Insulin Resistance in Rheumatoid Arthritis. Disease-Related Indicators and Associations With the Presence and Progression of Subclinical Atherosclerosis’, Arthritis & Rheumatology, 67(3), pp. 626–636. doi: https://doi.org/10.1002/art.38986.
Gorham, E. D. et al. (2005) ‘Vitamin D and prevention of colorectal cancer’, Journal of Steroid Biochemistry and Molecular Biology, 97(1–2), pp. 179–194. doi: https://doi.org/10.1016/j.jsbmb.2005.06.018.
Grant, W. B. et al. (2020) ‘Evidence that vitamin d supplementation could reduce risk of influenza and covid-19 infections and deaths’, Nutrients, 12(4), pp. 1–19. doi: https://doi.org/10.3390/nu12040988.
Graudal, N. et al. (2014) ‘Compared With Usual Sodium Intake, Low- and Excessive- Sodium Diets Are Associated With Increased Mortality: A Meta-Analysis’, American Journal of Hypertension, 27(9), pp. 1129–1137. doi: https://doi.org/10.1093/ajh/hpu028.
Guthold, R. et al. (2018) ‘Worldwide trends in insufficient physical activity from 2001 to 2016: a pooled analysis of 358 population-based surveys with 1·9 million participants’, The Lancet Global Health. World Health Organization, 6(10), pp. e1077–e1086. doi: https://doi.org/10.1016/S2214-109X(18)30357-7.
Hannibal L, et al. (2016) Biomarkers and Algorithms for the Diagnosis of Vitamin B12 Deficiency. Front Mol Biosci, 3:27. doi: https://doi.org/10.3389/fmolb.2016.00027.
Hathcock, J. N. et al. (2007) ‘Risk assessment for vitamin D 1, 2’, Am J Clin Nutr, 85(6), pp. 6–18.
He, F. J. et al. (2020) ‘Salt Reduction to Prevent Hypertension and Cardiovascular Disease: JACC State-of-the-Art Review’, Journal of the American College of Cardiology, 75(6), pp. 632–647. doi: https://doi.org/10.1016/j.jacc.2019.11.055.
Hlavaty, T., Krajcovicova, A. and Payer, J. (2015) ‘Vitamin D therapy in inflammatory bowel diseases: Who, in what form, and how much?’, Journal of Crohn’s and Colitis, 9(2), pp. 198–209. doi: https://doi.org/10.1093/ecco-jcc/jju004.
Holick, M. F. et al. (2011a) ‘Evaluation, treatment, and prevention of vitamin D deficiency: An endocrine society clinical practice guideline’, Journal of Clinical Endocrinology and Metabolism, 96(7), pp. 1911–1930. doi: https://doi.org/10.1210/jc.2011-0385.
Holick, M. F. et al. (2011b) ‘Evaluation, treatment, and prevention of vitamin D deficiency: An endocrine society clinical practice guideline’, Journal of Clinical Endocrinology and Metabolism, pp. 1911–1930. doi: https://doi.org/10.1210/jc.2011-0385.
Institute of Medicine of the National Academies (2011) Dietary Reference Intakes for Calcium and Vitamin D. Edited by C. Ross et al. Washington DC: The National Academy Press.
Jaishankar, M. et al. (2014) ‘Toxicity, mechanism and health effects of some heavy metals’, Interdisciplinary Toxicology. Slovak Toxicology Society, 7(2), pp. 60–72. doi: https://doi.org/10.2478/intox-2014-0009.
Jeffery, L. E. et al. (2018) ‘Decreased sensitivity to 1,25-dihydroxyvitamin D3 in T cells from the rheumatoid joint’, Journal of Autoimmunity, 88, pp. 50–60. doi: https://doi.org/10.1016/j.jaut.2017.10.001.
Johnson, A. R. and Milner, J. J. (2012) ‘The inflammation highway: metabolism accelerates inflammatory traffic in obesity’, Immunological Reviews, 249, pp. 218–238.
Kahanovitz, L. et al. (2017) “Type 1 Diabetes - A Clinical Perspective.” Point of Care, 16(1), pp. 37-40. doi: https://doi.org/10.1097/POC.0000000000000125.
Kamen, D. L. (2013) ‘Vitamin D in Lupus’, National Institutes of Health, 71(2), pp. 1–8. doi: https://doi.org/10.1038/mp.2011.182.doi.
Karpanen, H. et al. (1984) ‘Safety and effects of potassium- and magnesium- containing low sodium salt mixtures.pdf’, Journal of Cardiovascular Pharmacology, 6, pp. 236–243.
Karppanen, H. and Mervaala, E. (2006) ‘Sodium Intake and Hypertension’, Progress in Cardiovascular disease, 49(2), pp. 59–75. doi: https://doi.org/10.1016/j.pcad.2006.07.001.
Katz, A. et al. (1999) ‘Effect of a mineral salt diet on 24-h blood pressure monitoring in elderly hypertensive patients’, Journal of Human Hypertension, 13(11), pp. 777–780. doi: https://doi.org/10.1038/sj.jhh.1000837.
Kechichian, E. and Ezzedine, K. (2018) ‘Vitamin D and the Skin: An Update for Dermatologists’, American Journal of Clinical Dermatology. Springer International Publishing, 19(2), pp. 223–235. doi: https://doi.org/10.1007/s40257-017-0323-8.
Kivity, S., Agmon-Levin, N., et al. (2011) ‘Vitamin D and autoimmune thyroid diseases’, Cellular and Molecular Immunology, 8(3), pp. 243–247. doi: https://doi.org/10.1038/cmi.2010.73.
Kong, L. C. et al. (2013) ‘Insulin resistance and inflammation predict kinetic body weight changes in response to dietary weight loss and maintenance in overweight and obese subjects by using a Bayesian’, Am J Clin Nutr, 98(1), pp. 1385–1394. doi: https://doi.org/10.3945/ajcn.113.058099.
Lear, S. A. et al. (2017) ‘The effect of physical activity on mortality and cardiovascular disease in 130 000 people from 17 high-income, middle-income, and low-income countries: the PURE study’, The Lancet. Elsevier Ltd, 390(10113), pp. 2643–2654. doi: https://doi.org/10.1016/S0140-6736(17)31634-3.
Leone, A. (2005) ‘Biochemical markers of cardiovascular damage from tobacco smoke.’, Current pharmaceutical design. United Arab Emirates, 11(17), pp. 2199–2208. doi: https://doi.org/10.2174/1381612054367391.
Levin, A. D. et al. (2011) ‘Vitamin D deficiency in children with inflammatory bowel disease’, Digestive Diseases and Sciences, 56(3), pp. 830–836. doi: https://doi.org/10.1007/s10620-010-1544-3.
Ludwig, D. S. et al. (2018) ‘Dietary carbohydrates: Role of quality and quantity in chronic disease’, BMJ (Online), 361. doi: https://doi.org/10.1136/bmj.k2340.
Manzel, A. et al. (2014) ‘Role of “western diet” in inflammatory autoimmune diseases’, Current Allergy and Asthma Reports, 14(1). doi: https://doi.org/10.1007/s11882-013-0404-6.
Margină, D. et al. (2020) ‘Chronic Inflammation in the Context of Everyday Life: Dietary Changes as Mitigating Factors’, pp. 1–30. doi: https://doi.org/10.3390/ijerph17114135.
Martinez, R. et al. (2020) ‘Trends in premature avertable mortality from non-communicable diseases for 195 countries and territories, 1990–2017: a population-based study’, The Lancet Global Health. Pan American Health Organization. 8(4), pp. e511–e523. doi: https://doi.org/10.1016/S2214-109X(20)30035-8.
Mattozzi, C. et al. (2016) ‘Psoriasis, Vitamin D and the importance of the cutaneous barrier’s integrity: An update’, Journal of Dermatology, 43(5), pp. 507–514. doi: https://doi.org/10.1111/1346-8138.13305.
Merino, B. et al. (2020) ‘Intestinal fructose and glucose metabolism in health and disease’, Nutrients, 12(1), pp. 1–35. doi: https://doi.org/10.3390/nu12010094.
Miettinen, M. E. et al. (2020) ‘Serum 25-hydroxyvitamin D concentration in childhood and risk of islet autoimmunity and type 1 diabetes: the TRIGR nested case–control ancillary study’, Diabetologia, 63(4), pp. 780–787. doi: https://doi.org/10.1007/s00125-019-05077-4.
Millwood, I. Y. et al. (2019) ‘Conventional and genetic evidence on alcohol and vascular disease aetiology: a prospective study of 500 000 men and women in China’, The Lancet, 393(10183), pp. 1831–1842. doi: https://doi.org/10.1016/S0140-6736(18)31772-0.
Morenga, L. A. Te et al. (2014) ‘Dietary sugars and cardiometabolic risk: systematic review and meta-analyses of randomized controlled trials of the effects on blood pressure and lipids 1 – 3’, Am J Clin Nutr, 100, pp. 65–79. doi: https://doi.org/10.3945/ajcn.113.081521.1.
MORIMOTO, S. et al. (1986) ‘An open study of vitamin D3 treatment in psoriasis vulgaris’, British Journal of Dermatology, 115(4), pp. 421–429. doi: https://doi.org/10.1111/j.1365-2133.1986.tb06236.x.
Moss, J. W. E. and Ramji, D. P. (2017) ‘Nutraceutical therapies for atherosclerosis’, Nat Rev Cardiol., 13(9), pp. 513–532. doi: https://doi.org/10.1038/nrcardio.2016.103.Nutraceutical.
Moynihan, P. J. and Kelly, S. A. M. (2014) ‘Effect on caries of restricting sugars intake: Systematic review to inform WHO guidelines’, Journal of Dental Research, 93(1), pp. 8–18. doi: https://doi.org/10.1177/0022034513508954.
National Academies of Sciences, Engineering, and M. et al. (2017) ‘Guiding Principles for Developing Dietary Reference Intakes Based on Chronic Disease’. National Academies Press (US), Washington (DC) (The National Academies Collection: Reports funded by National Institutes of Health). Available at: http://europepmc.org/books/NBK465024.
Noakes, T. D. (2018) ‘So What Comes First: The Obesity or the Insulin Resistance ? And Which Is More Important ?’, Clinical Chemistry, 64(1), pp. 7–9. doi: https://doi.org/10.1373/clinchem.2017.282962.
Nosrati, A. et al. (2017) ‘Dietary modifications in atopic dermatitis: patient-reported outcomes’, J Dermatolog Treat, 28(6), pp. 523–538. doi: https://doi.org/10.1080/09546634.2016.1278071.
Nualart, F. et al. (2014). Vitamin C Transporters, Recycling and the Bystander Effect in the Nervous System: SVCT2 versus Gluts. Journal of Stem Cell Research & Therapy, 4(5), 209. https://doi.org/10.4172/2157-7633.1000209.
Oliveri Conti, G. et al. (2017) ‘A review of AirQ Models and their applications for forecasting the air pollution health outcomes’, Environmental Science and Pollution Research. Springer Verlag, 24(7), pp. 6426–6445. doi: https://doi.org/10.1007/s11356-016-8180-1.
Pierangeli, I. et al. (2020) ‘Health equity and burden of childhood asthma - related to air pollution in Barcelona’, Environmental Research. Elsevier Inc., (May 2019), p. 109067. doi: https://doi.org/10.1016/j.envres.2019.109067.
Pietrzik, K. (1991) Modern Lifestyles, Lower Energy Intake and Micronutrient Status. Springer.
Postic, C. (2020) ‘Conversion of a dietary fructose: new clues from the gut microbiome’, Nature Metabolism. Springer US, 2(3), pp. 217–218. doi: https://doi.org/10.1038/s42255-020-0185-x.
Prüss-Ustün, A. et al. (2019) ‘Environmental risks and non-communicable diseases’, BMJ, 364(1265).
Ramagopalan, S. V. et al. (2010) ‘A ChIP-seq defined genome-wide map of vitamin D receptor binding: Associations with disease and evolution’, Genome Research, 20(10), pp. 1352–1360. doi: https://doi.org/10.1101/gr.107920.110.
Sarkar, D., Jung, K. M. and Wang, J. (2015) ‘Alcohol and the immune system.’, Alcohol Research, 37(2), pp. 153–155. doi: https://doi.org/10.1136/bmj.298.6673.543.
Schraufnagel, D. E. et al. (2019) ‘Air Pollution and Noncommunicable Diseases: A Review by the Forum of International Respiratory Societies’ Environmental Committee, Part 2: Air Pollution and Organ Systems’, Chest. Elsevier Inc, 155(2), pp. 417–426. doi: https://doi.org/10.1016/j.chest.2018.10.041.
Scientific Advisory Committee on Nutrition Report (2015) Carbohydrates and Health. London.
Sharif, K. et al. (2018) ‘Physical activity and autoimmune diseases: Get moving and manage the disease’, Autoimmunity Reviews. Elsevier B.V., 17(1), pp. 53–72. doi: https://doi.org/10.1016/j.autrev.2017.11.010.
Shi, Z. et al. (2020) Short-Term Exposure to a Western Diet Induces Psoriasiform Dermatitis by Promoting Accumulation of IL-17A–Producing γδ T Cells, Journal of Investigative Dermatology. Society for Investigative Dermatology. doi: https://doi.org/10.1016/j.jid.2020.01.020.
Singh, A. and Kamen, D. L. (2012) ‘Potential benefits of vitamin D for patients with systemic lupus erythematosus’, Dermato-Endocrinology, 4(2), pp. 146–151. doi: https://doi.org/10.4161/derm.20443.
Singh, S., Young, P. and Armstrong, A. W. (2017) ‘An update on psoriasis and metabolic syndrome: A meta-analysis of observational studies’, PLoS ONE, 12(7), pp. 1–13. doi: https://doi.org/10.1371/journal.pone.0181039.
Softic, S. et al. (2020) ‘Fructose and hepatic insulin resistance’, Critical Reviews in Clinical Laboratory Sciences. Taylor & Francis, pp. 1–15. doi: https://doi.org/10.1080/10408363.2019.1711360.
Strong, K. et al. (2006) ‘Preventing chronic disease: a priority for global health’, International Journal of Epidemiology, 35(2), pp. 491–492. doi: https://doi.org/10.1093/ije/dyi314.
Theofilopoulos, A. N., Kono, D. H. and Baccala, R. (2017) ‘The Multiple Pathways to Autoimmunity’, Nat. Immunol., 18(7), pp. 716–724. doi: https://doi.org/10.1016/j.cogdev.2010.08.003.Personal.
Tinetti, M. E., Fried, T. R. and Boyd, C. M. (2012) ‘Designing health care for the most common chronic condition—multimorbidity’, JAMA, 307(23), pp. 2493–2494. doi: https://doi.org/10.1002/nbm.3066.Non-invasive.
Trieu, K. et al. (2015) ‘Salt reduction initiatives around the world-A systematic review of progress towards the global target’, PLoS ONE, 10(7). doi: https://doi.org/10.1371/journal.pone.0130247.
Tsoukalas, D., and Sarandi, E. (2020) Micronutrient deficiencies in patients with COVID-19: how metabolomics can contribute to their prevention and replenishment, BMJ Nutrition, Prevention & Health, 3. https://doi.org/10.1136/bmjnph-2020-000169.
Tsoukalas, D. et al. (2017) ‘Application of metabolomics: Focus on the quantification of organic acids in healthy adults’, International Journal of Molecular Medicine, 40(1), pp. 112–120. https://doi.org/10.3892/ijmm.2017.2983.
Tsoukalas, D et al. (2019a) ‘Application of metabolomics part II: Focus on fatty acids and their metabolites in healthy adults’, International Journal of Molecular Medicine, 43(1), pp. 233–242. doi: https://doi.org/10.3892/ijmm.2018.3989.
Tsoukalas, D, et al. (2019b) ‘Metabolic fingerprint of chronic obstructive lung diseases: A new diagnostic perspective’, Metabolites, 9(12). doi: https://doi.org/10.3390/metabo9120290.
Tsoukalas, D et al. (2019c) ‘Targeted Metabolomic Analysis of Serum Fatty Acids for the Prediction of Autoimmune Diseases’, Frontiers in Molecular Biosciences, 6(November), pp. 1–14. doi: https://doi.org/10.3389/fmolb.2019.00120.
Vagianos, K. et al. (2007) ‘Nutrition assessment of patients with inflammatory bowel disease’, Journal of Parenteral and Enteral Nutrition, 31(4), pp. 311–319. doi: https://doi.org/10.1177/0148607107031004311.
Wang, H. et al. (2016) ‘Global, regional, and national life expectancy, all-cause mortality, and cause-specific mortality for 249 causes of death, 1980-2015: a systematic analysis for the Global Burden of Disease Study 2015’, The Lancet. Elsevier, 388(10053), pp. 1459–1544. doi: https://doi.org/10.1016/S0140-6736(16)31012-1.
Wang, H. et al. (2017) ‘Vitamin D and chronic diseases’, Vitamin D: Oxidative Stress, Immunity, and Aging, 8(3), pp. 346–353. doi: https://doi.org/10.14336/AD.2016.1021.
Wang, H. J., Zakhari, S. and Jung, M. K. (2010) ‘Alcohol, inflammation, and gut-liver-brain interactions in tissue damage and disease development’, World Journal of Gastroenterology, 16(11), pp. 1304–1313. doi: https://doi.org/10.3748/wjg.v16.i11.1304.
Wang, T. J. et al. (2008) ‘Vitamin D deficiency and risk of cardiovascular disease’, Circulation, 117(4), pp. 503–511. doi: https://doi.org/10.1161/CIRCULATIONAHA.107.706127.
Watroba, M. and Szukiewicz, D. (2016) ‘The role of sirtuins in aging and age-related diseases’, Advances in Medical Sciences, 61(1), pp. 52–62.
Wessels, I. and Rink, L. (2020a) ‘Micronutrients in autoimmune diseases: possible therapeutic benefits of zinc and vitamin D’, The Journal of Nutritional Biochemistry. Elsevier Inc., 77, p. 108240. doi: https://doi.org/10.1016/j.jnutbio.2019.108240.
Wessels, I. and Rink, L. (2020b) ‘Micronutrients in autoimmune diseases: possible therapeutic benefits of zinc and vitamin D’, Journal of Nutritional Biochemistry. Elsevier Inc., 77, p. 108240. doi: https://doi.org/10.1016/j.jnutbio.2019.108240.
WHO (2018a) Fact sheets-Physical activity. Available at: https://www.who.int/news-room/fact-sheets/detail/physical-activity (Accessed: 2 July 2020).
WHO (2018b) Global status report on alcohol and health 2018.
Winther, K. H. and Rayman, M. P. (2020) ‘Selenium in thyroid disorders — essential knowledge for clinicians Kristian’, Nat Rev Endocrinol., 16, pp. 165–175. doi: https://doi.org/10.1038/s41574-019-0311-6.
World Health Organization (2013) WHO issues new guidance on dietary salt and potassium. Cent Eur J Public Health, 21(1):16. PMID: 23741892.
World Health Organization. (2017). Preventing noncommunicable diseases (NCDs) by reducing environmental risk factors. World Health Organization. https://apps.who.int/iris/handle/10665/258796. License: CC BY-NC-SA 3.0 IGO
World Health Organization (2020) Fact Sheets, Tobacco.
Zeisel, S. H. (2007) ‘Nutrigenomics and metabolomics will change clinical nutrition and public health practice: insights from studies on dietary requirements for choline’, Am J Clin Nutr, 86(3), pp. 542–548. doi: https://doi.org/10.1038/jid.2014.371.
Zhang, D. et al. (2019) ‘High Glucose Intake Exacerbates Autoimmunity through Reactive-Oxygen-Species-Mediated TGF-β Cytokine Activation’, Immunity. Elsevier Inc., 51(4), pp. 671-681.e5. doi: https://doi.org/10.1016/j.immuni.2019.08.001.
Zhao, B. et al. (2020a) ‘Short-term exposure to ambient fine particulate matter and out-of-hospital cardiac arrest: a nationwide case-crossover study in Japan’, The Lancet Planetary Health, pp. e15–e23. doi: https://doi.org/10.1016/S2542-5196(19)30262-1.
Zhao, S. et al. (2020b) ‘Dietary fructose feeds hepatic lipogenesis via microbiota-derived acetate’, Nature. Springer US, 579(7800), pp. 586–591. doi: https://doi.org/10.1038/s41586-020-2101-7.
Zipitis, C. S. and Akobeng, A. K. (2008) ‘Vitamin D supplementation in early childhood and risk of type 1 diabetes: a systematic review and meta-analysis’, Archives of Disease in Childhood, 93(6), pp. 512–517. doi: https://doi.org/10.1136/adc.2007.128579.
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Tsoukalas, D., Sarandi, E., Thanasoula, M. (2021). Non-communicable Diseases in the Era of Precision Medicine: An Overview of the Causing Factors and Prospects. In: Koukios, E., Sacio-Szymańska, A. (eds) Bio#Futures. Springer, Cham. https://doi.org/10.1007/978-3-030-64969-2_13
Download citation
DOI: https://doi.org/10.1007/978-3-030-64969-2_13
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-64968-5
Online ISBN: 978-3-030-64969-2
eBook Packages: Earth and Environmental ScienceEarth and Environmental Science (R0)