Abstract
Polycystic ovary syndrome (PCOS) is a very frequent endocrine disorder in women since it occurs in as many as 8–10% of women of reproductive age [1, 2]. Due to the multiple heterogeneity of the syndrome [3], there has been no agreement on the criteria on which to base the diagnosis of PCOS.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
6.1 Introduction
Polycystic ovary syndrome (PCOS) is a very frequent endocrine disorder in women since it occurs in as many as 8–10% of women of reproductive age [1, 2]. Due to the multiple heterogeneity of the syndrome [3], there has been no agreement on the criteria on which to base the diagnosis of PCOS.
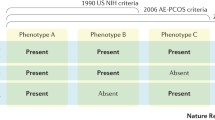
At the beginning, diagnostic criteria proposed by the NIH for PCOS were the presence of hyperandrogenism and chronic anovulation with clear exclusion of related ovulatory or other androgen excess disorders (i.e., hyperprolactinemia, thyroid diseases, androgen-secreting tumors, and adrenal dysfunction/hyperplasia) [4]. These criteria did not include the presence of polycystic ovaries at ultrasound examination because it was observed that polycystic ovaries could also be present in healthy eumenorrheic women [5]. A few years later the diagnostic criteria were expanded, and PCOS was considered as present when at least two of three features were diagnosed: oligo- or anovulation, clinical/biochemical hyperandrogenism, and polycystic ovaries as assessed by ultrasound examination [6]. This evolution was relevant because it permitted the inclusion of women with PCOS who were excluded by previous criteria: those with polycystic ovaries affected by hyperandrogenism and ovulatory cycles, or chronic anovulation and normal androgen levels. After assessing this, we have then to clarify that PCOS is completely different from PCO. PCO means polycystic ovary and refers only to the morphological aspect of the ovary at ultrasound examination, that’s it. Indeed, PCOS can be found in many other disendocrinopathies such as hyperprolactinemia, thyroid dysfunction, and stress-induced amenorrhea.
As a major feature, in this last decade, a new parameter has been introduced and taken into account to better approach not only the diagnosis but mainly the therapeutic choice, that is, insulin resistance (IR).
6.2 Endocrine Profile of PCOS
PCOS is characterized by higher plasma concentrations of ovarian and adrenal androgens, increased luteinizing hormone (LH) levels, high estrogen levels (especially estrone) due to extra-glandular conversion from androgens, lower levels of sex hormone-binding globulin (SHBG), and higher levels of prolactin and insulin, the latter often in presence of overweight or obesity.
Although the pathogenesis of PCOS is still controversial [7,8,9], PCOS typically shows elevated LH and normal or relatively low FSH secretion so that almost 50–60% of PCOS patients show a high LH/FSH ratio (>2.5) [7, 8], an exaggerated LH response to gonadotropin-releasing hormone (GnRH) stimulation test [7, 8], and a higher frequency of LH pulsatile release from pituitary [4, 7, 8, 10] that induces a higher stimulation on theca cells and an excess of androgen secretion as well as impaired follicular development [4].
Excess of androgens is classical of the syndrome, although it is not constant [7] and it is in great part of ovarian production with an adrenal contribution, since a certain percentage of PCOS patients might show a mild steroidogenetic defect in adrenal glands (such as for 21-hydroxylase) or just a higher adrenal hyper-activation due to stress [11]. Androstenedione and testosterone are the best markers of ovarian androgen secretion, while dehydroepiandrosterone sulfate (DHEAS) is the best marker of adrenal secretion. Most testosterone is derived from peripheral conversion of androstenedione and from direct ovarian production. In addition, adrenal glands contribute in part to testosterone although in hyperandrogenic PCOS the main source of androgens usually comes from the ovaries. Since cytochrome p450c17 is the androgen-forming enzyme in both the adrenal glands and the ovaries, whatever changes or increases its activity triggers the pathogenic mechanism underlying hyperandrogenism in PCOS [4]. In addition, in the presence of 5α-reductase, testosterone is converted within the cell to the more biologically potent androgen, namely, dihydrotestosterone. Excess or normal 5α-reductase activity in the skin determines the presence or absence of hirsutism [12]. Additionally, plasma levels of estrone, a weak estrogen with biological activity 100-times less than estradiol, are increased as a result of peripheral conversion of androstenedione by aromatase activity – more active in PCOS than in healthy controls – while estradiol levels are normal or low because of the frequent anovulatory cycles. All this results in a chronic hyperestrogenic state with the reversal of the estrone: estradiol ratio that might predispose to endometrial proliferation and to a possible increased risk for endometrial cancer [13, 14]. Another relevant aspect is the fact that normally less than 3% of testosterone circulates as unbound in the serum. In fact, most circulating androgens are bound to SHBG, thus being biologically inactive. Any condition that decreases the levels of SHBG (such as excess of circulating androgens) inducing a reduced hepatic synthesis induces a relative excess of free circulating androgens. In PCOS, hirsutism usually occurs with decreased SHBG levels and obesity [4].
6.3 Insulin Resistance (IR) and Compensatory Hyperinsulinism
The presence of increased insulin plasma level is a very frequent feature in PCOS patients, especially in those that show overweight or obesity. Indeed, overweight/obesity, depending on the geographical location, might be present in 50–70% of patients with PCOS. And this is not all. Another relevant feature is the presence of familial diabetes. It has been demonstrated that the presence of familial diabetes in first grade relatives (parents and/or grandparents) is a risk factor not only for the occurrence of IR but mainly for the high percentage of risk of occurrence of gestational diabetes and diabetes in late adulthood [15].
Such familial factors have always been evaluated through a quite detailed anamnestic investigation. In fact a risk factor of IR occurrence is not the presence of a familial diabetes only, but also the fact that the PCOS patients might be born as small for gestational age (SGA) and/or as after a IUGR (Intrauterine Growth Retardation) or may be born after a pregnancy during which a gestational diabetes occurred [16, 17].
Such kind of background(s) might predispose, at a higher grade, to the occurrence of insulin resistance due to specific genetic factors related to the familial predisposition to diabetes and also due to specific epigenetic factors that might be able to trigger the onset of a compensatory hyperinsulinemia [17].
It is clear that the presence of a familial diabetes predisposes to a less efficient post-receptor signalling driven by inositols not only for the insulin signal but also for FSH (on granulosa cells) and for TSH (on thyroid cells) [15, 18]. Also alpha-lipoic acid (ALA), a potent insulin sensitizer produced by mitochondrion, is impaired in case of diabetes or simply of predisposition to diabetes [19, 20].
In addition, androgen excess may both directly and indirectly induce alterations in glucose metabolism, ultimately being an additional cause of abnormal insulin sensitivity. Androgens may directly inhibit peripheral and hepatic insulin action. In fact, testosterone could induce insulin resistance in women with PCOS acting on the post-binding signal, in particular by reducing the number and efficiency of glucose transport proteins, such as the type 4 glucose transporter (GLUT-4), especially in muscle and fat tissues [21]. In addition, it has also been reported that women with central obesity, typical of obese PCOS, have higher free androgen levels and exhibit significantly higher levels of insulin insensitivity compared to weight-matched controls and show increased free fatty acids [4].
6.4 How to Manage and What to Do in PCOS?
The real target in PCOS patients is to teach them be aware of the great risk they have with such a disease. The real risk is not the anovulation or hyperandrogenism or hyperinsulinemia but the maintenance of such combination for a long time (quite often years!) so that that their biology is epigenetically induced to try to find “alternatives” to such a functional discomfort. The compensatory hyperinsulinemia is one of such biological solutions and is for sure a quite risky one since it is well known that it is a predisposition factor for metabolic syndrome in young as well as in adult or aged women.
The main solution is to take care of feeding, the choice of food, exercise, and, in case pregnancy is not an actual desire, a good choice of estro-progestin pill to overcome the hyperandrogenism that most of PCOS patients have. So, the putative question to a PCOS patient is: are you trying to be pregnant? If the answer is NO, all the solutions can be proposed, mainly a contraceptive pill; if the answer is YES, then contraception is absolutely skipped, and all integrative/anti-hyperinsulinemic treatment might be proposed together with a drastic lifestyle change especially overweight/obesity is present!
What is relevant to say is the fact that whatever is the biological situation that triggers PCOS and mainly the IR, the real risk is to maintain such abnormal condition up to the perimenopausal period when a lot of biological changes will occur, first of all a physiological increase of the insulin resistance. It is quite clear that a PCOS patient has to improve her metabolic health years before the occurrence of the perimenopausal transition. If not doing so, an increased risk of metabolic syndrome and of all cardiovascular risks up to death will take place.
6.5 Estrogen-Progestin Preparations and PCOS
Generally speaking, we can say that all combined estrogen-progestogen preparations are able to solve more or less the clinical complaints of any PCOS patient. This is due to the fact that such preparations block the ovary and suppress androgen production and improve SHBG synthesis thus reducing the circulating free androgens that are biologically effective on the target tissues such as skin, sebaceous glands, and hair follicles [22, 23].
Since it is well known that the estrogenic compound of the contraceptive pill (i.e., ethinyl estradiol) has only an ovario-static activity (no direct antiandrogenic effect), the antiandrogenic action has to be modulated by the progestogen compound. At present there are four progestogens with specific antiandrogenic activity: cyproterone acetate, dienogest, drospirenone, and chlormadinone acetate [22]. Cyproterone acetate is the progestogen with the highest antiandrogenic activity though being able to induce a relative higher rate of side effects such as cephalea, but all the others are able to induce similar positive effects [23]. The contraceptive pill administration is able not only to improve the clinical signs of the androgenization but also to normalize the ovarian size and morphology, typically impaired in PCOS patients [24]. As additional effect, estrogen-progestogen preparations protect for both follicular and corpus luteum cysts occurrence [23].
The efficacy of contraceptive preparations on the signs of hyperandrogenism (i.e., acne, hirsutism, seborrhea, and alopecia) is determined as function of time since the biological evolution of the skin and of all its annexes is more or less 110–120 days. This means that the youngest cells of the epithelium of the skin become old and superficial in more or less 4 months. Whatever is the contraceptive pill administered, the minimum treatment interval has to be 4–5 months, eventually up to 12 months, at least. Better results are obtained when such pills are administered for longer interval and/or coupled with anti-androgen compounds such as flutamide [25] or finasteride.
Most of the clinicians agree on the fact that treatment of dysendocrinopathy of PCOS supports greatly the psycho-emotional recovery of almost all the PCOS patients. Moreover, the use of the contraceptive pill, also for a long time, protects the patient from being victim of the recrudescence of the hyperandrogenism and of its induced diseases, mainly chronic anovulation and infertility. In fact, the use of estrogen-progestogen preparation has been reported to improve the chance of conception [26], and there is no difference in this kind of beneficial protective effect on ovarian function between progestin-only pill and combined oral contraceptives. After 12 months of discontinuation of the treatment to conceive, the conception rate was 95–99% in those using the pill versus 70–81% conception rate for those patients using depot medroxyprogesterone acetate (DMPA) injections or Norplant (levonorgestrel implants) [26].
If the rationale is correct and all the data we have in regards PCOS are true [27], environmental and genetic factors are able to induce the starting of the PCOS disease and will mark as “affected” that patient up to the menopause. This means that predisposition to all the clinical problems will be quiescent up to the moment the patient undergoes a treatment and will appear aging (more or less evident) soon after the discontinuation.
6.6 No Contraception but Let’s Overcome Dysmetabolism!
One of the main complaints of PCOS patients is the lack of ovulation and thus a consistent reduction of fertility. Obviously, the therapeutic use of the contraceptive pill is usually discarded but not so often. Indeed it might be proposed to use the pill for a certain amount of months during which correct lifestyle, i.e., diet and physical activity, is applied together with specific insulin sensitizers, such as metformin [2] and/or inositols and alpha-lipoic acid [19, 28,29,30,31,32]. The reduction of body weight is an essential feature for a good chance not only to recover a normal ovulatory function but also, if pregnancy starts, to have a controlled body mass that does not trigger a greater pregnant-induced insulin resistance that can trigger gestational diabetes.
Lots of studies have demonstrated that a correct lifestyle together with a correct treatment based on metformin and/or inositols and ALA greatly improves the chance of pregnancy, also while undergoing fertility programs [33, 34]. The clinical relevance of all these treatments is that they are all able to positively modulate the impaired and frequent compensatory hyperinsulinemia of PCOS patients, in particular in those that show a normal BMI [31], but the application of a correct lifestyle is the substrate for the best achievement of the desired result [35].
6.7 Long-Term Consideration for PCOS!
Since during the perimenopausal and postmenopausal transition there is a relevant modification of the endocrine profile in all women, those who have POCS during fertile life are more predisposed to have severe symptoms such as those related to behavior, mood, sleep, anxiety, as well as those related to metabolism, in particular insulin resistance and compensatory hyperinsulinemia. Menopausal transition induces, as a natural event, an insulin resistance that together with the hypoestrogenism and the lack of progesterone causes a greater tendency to gain body weight. There are convincing data that this metabolic link has to be considered as relevant when discussing about menopause with our ex-PCOS perimenopausal patients [36].
Substantially the menopausal transition might worsen a previously not perfect metabolic condition. Since both estrogens and progesterone are able to modulate the glucose metabolism, as soon as the perimenopausal modifications of the ovarian function take place and within few months/years menopause begins [37, 38], abnormalities of the metabolic pathways may be more relevant than expected if during fertile life abnormal metabolic function(s) were present, such as insulin resistance with overweight or obesity.
Though it cannot be generalized, the use of hormone replacement therapy is crucial and important for 1000 aims at the moment of the menopausal transition, being clear that the patient has no contraindications to it. It is relevant to maintain an adequate steroidal milieu so that biological pathways and in particular the metabolic ones are not crushed by the overlapping phenomena of menopause plus aging [39].
In conclusion lifestyle, good and healthy feeding, and the right amount of physical exercise are relevant in PCOS patients during fertile life, with or without the use of oral contraceptives, but when fertile life finishes and menopausal transition takes place all of the above need to be coupled with an adequate hormone replacement therapy to counteract the higher risk for PCOS-menopausal women to face higher rate diseases mainly cardiovascular diseases and dismetabolic/diabetes risks.
References
Carmina E, Lobo RA. Polycystic ovary syndrome: arguably the most common endocrinopathy is associated with significant morbidity in women. J Clin Endocrinol Metab. 1999;84(1897–1899):4.
Genazzani AD, Ricchieri F, Lanzoni C. Use of metformin in the treatment of polycystic ovary syndrome. Women's Health (Lond Engl). 2010;6:577–93.
Carmina E. Genetic and environmental aspects of polycystic ovary syndrome. J Endocrinol Investig. 2003;26:1151–9.
Zawadzki JK, Dunaif A: Diagnostic criteria for polycystic ovary syndrome: towards a rational approach. In: Polycystic Dunaif A, Givens JR, Haseltine FP, Merriam GR (Eds). Ovary Syndrome. Blackwell, MA, USA, 337–384 (1992).
Polson DW, Adams J, Wadsworth J, Franks S. Polycystic ovaries-a common finding in normal women. Lancet. 1988;1:870–2.
The Rotterdam ESHRE/ASRM-Sponsored PCOS Consensus Workshop Group. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome (PCOS). Hum Reprod. 2004;19:41–7.
Hirschberg AL. Polycystic ovary syndrome, obesity and reproductive implications. Womens Health. 2009;5:529–40.
Doi SA. Neuroendocrine dysfunction in PCOS: a critique of recent reviews. Clin Med Res. 2008;6:47–53.
Vrbikova J, Hainer V. Obesity and polycystic ovary syndrome. Obes Facts. 2009;2:26–35.
Kalro BN, Loucks TL, Berga SL. Neuromodulation in polycystic ovary syndrome. Obstet Gynecol Clin N Am. 2001;28:35–62.
Genazzani AD, Petraglia F, Pianazzi F, Volpogni C, Genazzani AR. The concomitant release of androstenedione with cortisol and luteinizing hormone pulsatile releases distinguishes adrenal from ovarian hyperandrogenism. Gynecol Endocrinol. 1993;7:33–41.
Plouffe L Jr. Disorders of excessive hair growth in the adolescent. Obstet Gynecol Clin N Am. 2000;27:79–99.
Vrbikova J, Cibula D. Combined oral contraceptives in the treatment of polycycstic ovary syndrome. Hum Reprod Update. 2005;11:277–91.
Cibula D, Gompel A, Mueck AO, La Vecchia C, Hannaford PC, Skouby SO, Zikan M, Dusek L. Hormonal contraception and risk of cancer. Hum Reprod Update. 2010;16:631–50.
Genazzani AD. Inositol as putative integrative treatment for PCOS. Reprod Biomed Online. 2016;33:770–80. https://doi.org/10.1016/j.rbmo.2016.08.024.
de Melo AS, Dias SV, Cavalli Rde C, Cardoso VC, Bettiol H, Barbieri MA, Ferriani RA, Vieira CS. Pathogenesis of polycystic ovary syndrome: multifactorial assessment from the foetal stage to menopause. Reproduction. 2015 Jul;150(1):R11–24.
Ibáñez L, Potau N, Francois I, de Zegher F. Precocious pubarche, hyperinsulinism, and ovarian hyperandrogenism in girls: relation to reduced fetal growth. J Clin Endocrinol Metab. 1998;83:3558–62.
Berridge MJ, Irvine RF. Inositol trisphosphate, a novel second messenger in cellular signal transduction. Nature. 1984;312(5992):315–21.
Genazzani AD, Shefer K, Della Casa D, Prati A, Napolitano A, Manzo A, Despini G, Simoncini T. Modulatory effects of alpha-lipoic acid (ALA) administration on insulin sensitivity in obese PCOS patients. J Endocrinol Invest. 2018 May;41(5):583–90.
Padmalayam I, Hasham S, Saxena U, Pillarisetti S. Lipoic acid synthase (LASY): a novel role in inflammation, mitochondrial function, and insulin resistance. Diabetes. 2009;58:600–8.
Ciaraldi TP, El-Roeiy A, Madar Z, et al. Cellular mechanisms of insulin resistance in polycystic ovarian syndrome. J Clin Endocrinol Metab. 2002;75:577–83.
Schindler AE. Non-contraceptive use of hormonal contraceptives for women with various medical problems. J Pediat Obstet Gynecol. 2008;34:183–200.
Schindler AE. Non-contraceptive benefits of oral hormonal contraceptives. In J Endocrinol Metab. 2013;11:41–7.
Falsetti L, Gambera A, Tisi G. Efficacy of the combination ethinyl oestradiol and cyproterone acetate on endocrine, clinical and ultrasonographic profile in polycystic ovarian syndrome. Hum Reprod. 2001;16(1):36–42.
Paradisi R, Fabbri R, Battaglia C, Venturoli S. Ovulatory effects of flutamide in the polycystic ovary syndrome. Gynecol Endocrinol. 2013;29:391–5.
Barnhart KT, Schreiber CA. Return to fertility following discontinuation of oral contraceptives. Fertil Steril. 2009;91:659–63.
Franks S, Berga SL. Does PCOS have developmental origins? Fertil Steril. 2012;97:2–6.
Genazzani AD, Prati A, Simoncini T, Napolitano A. Modulatory role of D-chiro-inositol and alpha lipoic acid combination on hormonal and metabolic arameters of overweight/obese PCOS patients. European Gynecology and Obstetrics. 2019;1(1):29–33.
Genazzani AD, Despini G, Santagni S, Prati A, Rattighieri E, Chierchia E, Simoncini T. Effects of a combination of alpha lipoic acid and myo-inositol on insulin dynamics in overweight/obese patients with PCOS. Endocrinol Metab Synd. 2014;3:3. https://doi.org/10.4172/2161-1017.1000140.
Genazzani AD, Santagni S, Rattighieri E, Chierchia E, Despini G, Marini G, Prati A, Simoncini T. Modulatory role of D-chiro-inositol (DCI) on LH and insulin secretion in obese PCOS patients. Gynecol Endocrinol. 2014;30(6):438–43.
Genazzani AD, Santagni S, Ricchieri F, Campedelli A, Rattighieri E, Chierchia E, Marini G, Despini G, Prati A, Simoncini T. Myo-inositol modulates insulin and luteinizing hormone secretion in normal weight patients with polycystic ovary syndrome. J Obstet Gynaecol Res. 2014;40(5):1353–60.
Genazzani AD, Prati A, Santagni S, Ricchieri F, Chierchia E, Rattighieri E, Campedelli A, Simoncini T, Artini PG. Differential insulin response to myo-inositol administration in obese polycystic ovary syndrome patients. Gynecol Endocrinol. 2012;28:969–73.
Artini PG, Di Berardino OM, Papini F, et al. Endocrine and clinical effects of myo-inositol administration in polycystic ovary syndrome. A randomized study. Gynecol Endocrinol. 2013;29:375–9.
Zheng X, Lin D, Zhang Y, Lin Y, Song J, Li S, Sun Y. Inositol supplement improves clinical pregnancy rate in infertile women undergoing ovulation induction for ICSI or IVF-ET. Medicine 2017;96:49(e8842).
Tieu J, Shepherd E, Middleton P, Crowther CA. Dietary advice interventions in pregnancy for preventing gestational diabetes mellitus. Cochrane Database of Systematic Reviews 2017, Issue 1. Art. No.: CD006674.
Puurunen J, Piltonen T, Morin-Papunen L, Perheentupa A, Jarvela I, Ruokonen A, Tapanainen JS. Unfavorable hormonal, metabolic, and inflammatory alterations persist after menopause in women with PCOS. J Clin Endocrinol Metab. 2011;96:1827–34.
Dos Reis CM, de Melo NR, Meirelles ES, Vezozzo DP, Halpern A. Body composition, visceral fat distribution and fat oxidation in postmenopausal women using oral or transdermal oestrogen. Maturitas. 2003 Sep 25;46(1):59–68.
Davis SR, Castelo-Branco C, Chedraui P, Lumsden MA, Nappi RE, Shah D, Villaseca P; Writing Group of the International Menopause Society for World Menopause Day 2012. Understanding weight gain at menopause. Climacteric 2012;15(5):419–29.
Cagnacci A, Zanin R, Cannoletta M, Generali M, Caretto S, Volpe A. Menopause, estrogens, progestins, or their combination on body weight and anthropometric measures. Fertil Steril. 2007 Dec;88(6):1603–8.
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 International Society of Gynecological Endocrinology
About this chapter
Cite this chapter
Genazzani, A.D. et al. (2021). Polycystic Ovary Syndrome: Considerations About Therapeutic Strategies Choices from Fertile Life to Menopause. In: Genazzani, A.R., Ibáñez, L., Milewicz, A., Shah, D. (eds) Impact of Polycystic Ovary, Metabolic Syndrome and Obesity on Women Health. ISGE Series. Springer, Cham. https://doi.org/10.1007/978-3-030-63650-0_6
Download citation
DOI: https://doi.org/10.1007/978-3-030-63650-0_6
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-63649-4
Online ISBN: 978-3-030-63650-0
eBook Packages: MedicineMedicine (R0)