Abstract
Polycystic ovary syndrome (PCOS) is a common disorder characterized by oligo-anovulation, clinical and/or biochemical signs of hyperandrogenism, and polycystic ovaries. Its pathophysiology is complex and commonly includes functional ovarian hyperandrogenism (FOH) and in some instances functional adrenal hyperandrogenism (FAH). It is occasionally associated with elevation of insulin, anti-mullerian hormone (AMH), and/or luteinizing hormone (LH). PCOS may be associated with obesity, hyperlipidemia, diabetes mellitus type 2, infertility, and depression. Therefore, it is imperative that primary care providers can identify patients that should be evaluated for PCOS and can initiate the diagnostic evaluation and treatment plan for PCOS and its complications. Treatment strategies are based on concomitant patient comorbidities and patient preferences.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- PCOS
- Hyperandrogenism
- Ovulatory dysfunction
- Irregular menses
- Functional ovarian hyperandrogenism
- Polycystic ovaries
- Hirsutism
- Infertility
- Metabolic syndrome
- Endometrial cancer
-
1.
List the diagnostic criteria for PCOS.
-
2.
Discuss the diagnostic work-up for PCOS.
-
3.
Describe the underlying pathophysiology of PCOS.
-
4.
Demonstrate knowledge of metabolic abnormalities in patients with PCOS such as insulin resistance, diabetes, and hyperlipidemia.
-
5.
Describe short-term and long-term sequela associated with PCOS, including symptoms of androgen excess, endometrial cancer risk, and depression.
-
6.
Formulate a treatment plan for a patient with PCOS that addresses weight management, symptoms of androgen excess such as acne/hirsutism, endometrial cancer prevention, and metabolic abnormalities, if present.
-
7.
Discuss how to address infertility issues in women with PCOS.
Shazia is a 32-year-old woman here for a new annual visit who notes irregular menses. For 6 years, she has had fewer than six menses per year and her last menstrual period was 5 months ago. The irregularities worsened after she gained weight in the past few years. She is sexually active and is not using any form of contraception since she does not believe she can become pregnant. You highly suspect PCOS.
Epidemiology
Polycystic ovary syndrome (PCOS) is the most common endocrine disorder seen in women of childbearing age [1,2,3] with an estimated prevalence between 5% and 16% depending on the population studied and the criteria applied [1, 4]. PCOS has been identified as a complex and heterogenous disorder that results from the interaction of diverse genetic and environmental factors and can lead to adverse reproductive and metabolic complications in affected women [5]. The syndrome was first described by Stein and Leventhal in 1935 and encompasses three cardinal features, oligo-anovulation, polycystic ovaries, and hyperandrogenism and/or hyperandrogenemia [4, 6]; further discussion of each of these features will follow below. PCOS is a common cause of infertility in women due to oligo-anovulation [2, 3] and can be associated with a wide range of metabolic abnormalities such as insulin resistance, diabetes mellitus type 2, hyperlipidemia, and increased risk of cardiovascular disease [2, 3, 7]. It can be accompanied by symptoms of androgen excess such as acne and hirsutism, increased risk of endometrial cancer, and depression [5, 7, 8]. Therefore, primary care providers must be familiar with the diagnostic criteria and basic steps in management for PCOS to identify and treat this disorder in their patients.
Diagnostic Criteria and Phenotypes
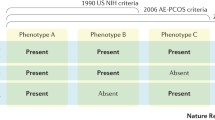
Over the past three decades, significant efforts have been made to classify PCOS. The first formal attempt was made at the National Institutes of Health (NIH) conference, April 1990 [9]; the NIH criteria served as a standard for researchers and clinicians for more than a decade. Based on NIH criteria, clinical or biochemical hyperandrogenism (HA) and chronic oligo-anovulation (OA) were considered key diagnostic features of PCOS, after exclusion of related disorders [7]. In 2003, a consensus workshop in Rotterdam, Netherlands, developed new diagnostic criteria, the Rotterdam criteria, which added ultrasound characteristics for polycystic ovary morphology (PCOM) to the NIH criteria definition. The 2003 Rotterdam criteria required the presence of two of the following three findings: signs of clinical or biochemical hyperandrogenism; chronic ovulatory dysfunction (OD); and the presence of polycystic ovary morphology, after exclusion of secondary causes [7, 10, 11] (Table 6.1). As a growing body of evidence supported the presence of hyperandrogenism as a key factor in the pathophysiology of PCOS and a strong predictor of the associated metabolic dysfunctions [12], a task force assembled in 2006 by the Androgen Excess and PCOS Society proposed the AE-PCOS criteria. These criteria require the diagnosis of PCOS to be based on the presence of clinical or biochemical hyperandrogenism in combination with ovarian dysfunction (i.e., OD or PCOM), excluding other causes [13]. Given the multiplicity of criteria that could cause confusion in clinical practice, the NIH sponsored an Evidence-Based Methodology PCOS Workshop in 2012 that addressed the benefits and drawbacks of existing diagnostic criteria [11]. As a result, the panel recommended the use of the broader Rotterdam 2003 criteria, while also providing detailed description of the different PCOS phenotypes defined by above criteria [7, 11].
Based on the 2012 NIH criteria, four clinical phenotypes can be defined for PCOS (Table 6.2). Phenotypes A and B are defined as “classic PCOS.” This group of patients may experience more pronounced menstrual irregularities [14, 15] and is at a higher risk for metabolic dysfunction such as insulin resistance, atherogenic dyslipidemia, and obesity [7, 14, 16, 17] when compared with women diagnosed with nonclassic or non-hyperandrogenic PCOS phenotypes (phenotypes C and D). Phenotype C, “ovulatory PCOS,” generally includes women with preserved ovulation who show an intermediate level of symptoms [18, 19] compared with patients with other subtypes. Phenotype D, also defined as “non-hyperandrogenic PCOS” [7], has the mildest degree of metabolic dysfunction and the lowest prevalence of metabolic syndrome of all subtypes [14, 19, 20].
Pathophysiology
The most consistent biochemical abnormality in women with PCOS is an overproduction of androgens [8]. There are two main sources of androgen production in women: the ovaries and the adrenal glands. It has been hypothesized that in most PCOS cases, intrinsic dysregulation in ovarian steroidogenesis results in functional ovarian hyperandrogenism (FOH) (Fig. 6.1). This inherent abnormality is further influenced by other hypothalamic-pituitary axis factors, including higher baseline gonadotropin-releasing hormone (GnRH) pulse frequency and reduced hypothalamic feedback response to circulating sex steroids [6, 8]. This in turn leads to hypersecretion of luteinizing hormone (LH) and subsequent enhanced ovarian androgen synthesis and folliculogenesis. Insulin-resistant hyperinsulinemia also plays an important role in PCOS. Insulin has been shown to enhance the response of androgen-producing theca cells in the ovaries to LH stimuli. In a smaller number of PCOS cases, dysregulation at the adrenal zona reticularis causes hyperandrogenism by increased production of dehydroepiandrosterone (DHEA) [1].
This figure depicts the main processes involved in the pathophysiology of PCOS. Functional ovarian hyperandrogenism (FOH) is the cardinal proposed feature, and it can explain the different clinical manifestations of PCOS. LH acts on theca cells of ovarian follicles to start the process of follicle development and stimulates androgen production. Theca cells in women with PCOS have an exaggerated response to LH that leads to increase in androgens. Meanwhile, granulosa cells of antral follicles respond to FSH for further development. Inhibin-B produced by granulosa cells has an inhibitory feedback on FSH. It also increases androgen production in theca cells. Patients with PCOS have elevated levels of inhibin-B. Anti-mullerian hormone (AMH) is another key player in control of follicular growth. Its levels are increased in PCOS due to excessive number of growing follicles. It can also have a suppressing effect on FSH. Androgens accelerate luteinization of ovarian follicles (a part of normal follicular development). However, when in excess, they can cause premature luteinization and follicular growth arrest. This leads potentially to anovulation and development of polycystic ovaries. The picture also depicts contribution of the hypothalamus-pituitary axis. Patients with PCOS can have increase in pulse frequency of GnRH. This can lead to a higher LH production (compared to FSH), which at the end can contribute to enhanced androgen production by ovaries. Sex steroids produced by ovaries have negative regulatory feedback on gonadotropins. It is hypothesized that the hypothalamus-pituitary axis is less responsive to this feedback in PCOS patients as well. Insulin-resistant hyperinsulinemia can have an independent contributory role in pathophysiology of PCOS. Insulin synergizes with LH to stimulate theca cells’ androgen production. Insulin also, similar to androgens, enhances luteinization of ovarian follicles. Hyperinsulinemia can trigger peripheral adiposity, and adiposity can in turn worsen insulin resistance. The figure does not depict the contribution of the adrenal glands to androgen production (functional adrenal hyperandrogenism), which is seen either alone (in a smaller number of patients) or as an adjunct to FOH
Functional Ovarian Hyperandrogenism (FOH)
Normal ovarian function:
Ovulation takes place due to synchronized activity between the hypothalamus, pituitary, and ovarian follicles (see Chap. 5 on Menstruation and Secondary Amenorrhea). In the follicular phase, theca cells express LH receptors; LH stimulates the production of androstenedione from its precursor cholesterol [6]. Androstenedione, an androgen, is required for ovarian estrogen biosynthesis. A delicate balance exists between adequate and overproduction of androgens (Fig. 6.2).
Ovarian function in PCOS:
There are several proposed mechanisms to explain anovulatory cycles in PCOS.
Theca cell dysfunction:
Women with PCOS are suspected to have intrinsic abnormalities in the ovarian theca cells’ steroidogenesis which leads to hyperandrogenemia. In vivo and in vitro studies have shown overexpression of P450 enzymes along with LH receptors in ovaries of women with PCOS (21). This hyper-responsiveness can increase androgen production. When produced in excess, androgens cause an arrest in follicular maturation, cause follicular atresia , and hinder ovulation [5].
Granulosa cell dysfunction:
Granulosa cells convert androgens coming from the thecal cells to estradiol by aromatase. In women with PCOS, there seems to be a relative aromatase deficiency likely due to inhibition by anti-mullerian hormone (AMH). Therefore, there is limited conversion of androgens to estrogens, leading to hyperandrogenemia [22]. In addition to AMH, inhibin-B is a peptide that is produced in granulosa cells and is in a reciprocal negative regulatory feedback loop with FSH. It is essential and permissive for thecal androgen production. Women with PCOS tend to have elevated serum inhibin-B as well as AMH [23].
Polycystic ovary morphology (PCOM) and role of anti-mullerian hormone (AMH):
The ovaries of women with PCOS often show an excessive number of follicles. AMH is an important intrafollicular regulator of follicle growth, and women with PCOS tend to have higher baseline AMH levels due to a higher number of growing follicles. Initially, insulin and androgen promote the primordial to primary follicle transition until FSH becomes the primary regulator at the early antral follicle stage [1]. However, FSH is decreased in PCOS due to elevated levels of AMH, which is in a negative regulatory feedback loop with FSH as stated above. Therefore, follicle maturation arrest occurs.
Hypothalamic-pituitary axis:
Gonadotropin-releasing hormone (GnRH) is secreted in a pulsatile manner to stimulate FSH and LH secretion from the pituitary. Changes in amplitude and frequency of the GnRH pulse determine the amplitude and frequency of LH and FSH production throughout the menstrual cycle. At higher pulses, GnRH promotes the production of LH, while lower pulsation frequencies enhance the production of FSH. In women with PCOS, accelerated GnRH-LH pulsatile activity as well as decreased sensitivity of the hypothalamus to negative feedback from ovarian steroids leads to higher LH production [1, 6]. Higher LH pulses generally lead to higher production of ovarian androgens.
Hyperinsulinemia:
Insulin resistance is common in both obese and lean women with PCOS. Insulin has a direct role on ovaries and enhances androgen production from theca cells in response to an LH stimulus (Figs. 6.1 and 6.2). It is proposed that with higher insulin levels, both ovarian theca cells and adrenal zona reticularis cells have enhanced androgen production in response to LH and ACTH, respectively.
Genetics
Familial clustering of PCOS suggests a genetic basis. Heritable traits that have been identified as PCOS risk factors are maternal PCOS, polycystic ovary morphology, hyperandrogenemia, and metabolic syndrome [1]. Phenotypic features associated with PCOS such as hyperandrogenism and metabolic abnormalities can be seen in aggregate in certain families, suggesting a genetic cause. An example is sisters with hyperandrogenism and metabolic derangements, with or without menstrual irregularities. Several susceptibility genes have been implicated, especially in the region of insulin receptor genes [21]. Defects in androgen steroidogenesis as well as beta cell function have been observed in brothers of women with PCOS, manifesting itself as elevated levels of dehydroepiandrosterone-sulfate (DHEA-S) and increased risk for type 2 diabetes [21]. Furthermore, it is thought that PCOS evolved to preserve anabolism and reproductive capacity via increased androgen and insulin production in times of nutritional deprivation [1].
Clinical Manifestations
Shazia noted that she had her upper lip waxed regularly due to bothersome facial hair. She had moderate acne across her upper back as well. Based on these features and her oligomenorrhea, PCOS is highest on your differential. You discuss the diagnosis and work-up with her.
Ovulatory dysfunction with or without menstrual abnormalities:
Ovulatory dysfunction typically presents with obvious disruption in menstrual flow but can present subclinically without obvious menstrual irregularity [13].
Overt dysfunction:
Overt dysfunction occurs for the majority of the patients with PCOS [9, 12, 24,25,26] in the form of oligomenorrhea, defined as vaginal bleeding episodes occurring at greater than 35-day intervals or less than ten bleeds per year. A much smaller percentage of patients present with polymenorrhea, defined as bleeding episodes occurring frequently with less than 25 days between cycles [13, 27].
Subclinical ovulatory dysfunction:
Roughly 15–40% of oligo-ovulatory patients with PCOS present with eumenorrhea (cycles every 25–35 days in length) [13, 26, 28]. In eumenorrheic patients for whom there is a high suspicion of PCOS, day 18–24 progesterone levels can clarify the diagnosis. Levels below 3 to 4 ng/mL may suggest an anovulatory cycle but should be checked on at least two different occasions as the presence of one anovulatory cycle may not indicate chronic anovulation [13].
Hyperandrogenemia or hyperandrogenism:
Hyperandrogenemia refers to higher than normal levels of circulating endogenous androgens, including testosterone (T), androstenedione (A4), and DHEA-S [13]. Clinical features of elevated androgens (known as hyperandrogenism) include hirsutism, acne, and androgenic alopecia [8, 13].
Hirsutism:
Hirsutism refers to the presence of course, pigmented hair on the face and/or body in a male pattern distribution, including the upper lip, chin, chest, upper back and shoulders, lower back, abdomen, upper arms, and thighs. While the degree of hirsutism can vary based on race and ethnicity [13], hirsutism affects approximately 65–75% of patients with PCOS [16], including women of White, Black, and Southeast Asian backgrounds. If a clinician is uncertain regarding the presence of hirsutism, the Ferriman-Gallwey score can be used to further quantify the degree of hirsutism present [29, 30]. This score, originally introduced in 1961, assesses terminal hair growth in nine body areas. The degree of hirsutism can be assessed by assigning a score of 0–4 based on the density of terminal hairs [13, 21, 29, 30]. A total score of 8 or greater based on the 95th percentile of the data originally collected by Ferriman and Gallwey may suggest hirsutism. Race and ethnicity specific normative ranges are not well established. Figure 6.3 depicts the visual scoring method used for assessing hirsutism .
Facial and body terminal hair growth scored according to the modified Ferriman-Gallwey method. All were taken on women who had not used laser or electrolysis for at least 3 months, not depilated or waxed for at least 4 weeks, and not shaved or plucked for at least 5 days before the photograph. The photographs depict scores of 1 through 4 for the upper lip (a), chin (b), chest (c), arm (d), upper abdomen (e), lower abdomen (f), upper back (g), lower back (h), and thighs (i). The areas were photographed with a standard single-lens reflex camera (Nikon N50, Nikon Corp, Melville, NY, USA) equipped with a macro lens (Vivitar 50 or 100 mm Auto Focus Macro, Vivitar Corp, Newbury Park, Calif) and ring flash (Vivitar Macroflash 5000, Vivitar Corp). For film, Kodacolor VR 200 ISO film (Eastman Kodak Co, Rochester, NY, USA) was used. Representative areas were selected. All photographs of hair were anonymized and all identifying information removed, meeting current Institutional Review Board for Human Use and Health Insurance Portability and Accountability Act of 1996
A score of 0–4 based on the density of hair is given in each region; scores >8 are suggestive of hirsutism (Reprinted from Yildiz et al. [31], by permission of Oxford University Press)
Acne:
The prevalence of acne varies by ethnicity but is estimated to affect 15–25% of patients with PCOS [10]. There is no consistent scoring for assessment, and it is unclear how much PCOS raises the prevalence of acne over that in the general population given a general prevalence of 5–20% [13].
Androgenic alopecia:
Women with PCOS who experience androgenic alopecia tend to lose hair in the anterior midvertex area extending to the crown. The anterior hairline remains intact in women with PCOS, and significant bitemporal scalp hair recession is unusual except in virilizing syndromes [32, 33]. The prevalence of androgenic alopecia is reported to be as high as 22% in some studies [34].
Polycystic ovaries:
Polycystic ovaries are defined by three features: ovarian size and volume, stromal volume, and follicle size and number. Based on the Rotterdam criteria, polycystic ovaries contain 12 or more follicles measuring 2–9 mm in diameter and/or increased ovarian volume >10 mL in at least 1 ovary [35]. It should be noted that this definition cannot be used for women on oral contraceptives. The prevalence of polycystic ovaries in patients with PCOS is high: in one study, 60% of women met size criteria, while another 35% met follicular criteria [36,37,38].
Other Features Associated with PCOS
While the clinical criteria for diagnosing PCOS include ovulatory dysfunction, hyperandrogenemia or its clinical findings, or polycystic ovary features on ultrasound, a number of other clinical features may accompany this syndrome (Fig. 6.4).
Clinical components of PCOS. This figure illustrates the clinical features of PCOS that need to be carefully assessed and addressed. NAFLD refers to nonalcoholic fatty liver disease (Reprinted from Trikudanathan [21], with permission from Elsevier)
Insulin resistance, hyperinsulinemia, and the metabolic syndrome:
Impaired glucose tolerance or diabetes mellitus type 2 develops in about 40% of women with 1990 NIH-defined PCOS by the fourth decade of life. Glycemic control worsens with age and weight gain [8, 39]. Women with PCOS can also have dyslipidemia, which manifests as lower levels of high-density lipoprotein (HDL) cholesterol, increased levels of triglycerides, and increased low-density lipoprotein (LDL) cholesterol [40,41,42]. Metabolic syndrome is also highly prevalent in patients with PCOS compared to BMI-matched controls [25, 43]. Despite the higher prevalence of obesity among patients with PCOS, not all of the metabolic abnormalities can be explained by BMI. Studies suggest that even lean PCOS women exhibit a higher prevalence of insulin resistance and dyslipidemia compared to weight- and age-matched controls.
Newer studies have suggested links between PCOS and surrogate markers of cardiovascular disease (CVD) such as increased left ventricular mass, endothelial dysfunction, and subclinical vascular disease [8]. Nevertheless, there is limited data to suggest that women with PCOS are experiencing higher CVD events. This is likely explained by the later onset of clinical CVD in women and paucity of studies in older women with history of PCOS [8].
Increased risk of endometrial cancer:
Women with PCOS have risk factors for endometrial cancer including obesity, metabolic abnormalities, and chronic oligo-anovulation resulting in prolonged exposure of the endometrium to unopposed estrogen. Therefore, it has been shown that women with PCOS that have oligomenorrhea can have a 2.7-fold increase in risk of developing endometrial cancer compared to the general population [8, 44] (see also Chap. 15 on Gynecologic Malignancies). Endometrial protection is a key focus of PCOS treatment (see below).
Infertility:
Women with PCOS are at increased risk for infertility due to anovulatory cycles. They also have higher risk of preterm delivery, gestational diabetes, and preeclampsia [8].
Psychosocial issues:
The prevalence of depression and anxiety is higher in women with PCOS than in the general population [45]. These symptoms may be even more pronounced in young adult women concerned with fertility but can affect women of all ages with respect to weight and body habitus and clinical signs of androgen excess [46, 47]. Figure 6.5 displays the effect of PCOS on women, at different stages of life.
Main clinical and metabolic manifestations of polycystic ovary syndrome according to women’s stage of life (Reprinted by permission from Springer Nature, [47]). IUGR= Intrauterine Growth Restriction, SGA= Small for Gestational Age
Diagnostic Evaluation
It is essential to start with a detailed history and physical exam when evaluating a patient for PCOS. The history should include questions about (1) onset of menses and menstrual patterns; (2) hirsutism, specifically on the chin, jawline, chest, back, breasts, and stomach; (3) acne; (4) weight gain or the inability to lose weight; (5) the presence of galactorrhea; (6) behaviors and practices to offset symptoms such as plucking/waxing hair, acne treatments, excessive exercise/dieting, and taking medications that may mask or induce symptoms like hormones or steroids; and (7) family history, specifically regarding the presence of endocrinopathies, PCOS, cardiovascular disease, lipid disorders, and diabetes. The exam should focus on the skin looking for acne, alopecia, striae, and hirsutism; the presence of a goiter or thyroid nodule; adiposity; virilization which may result from an androgen-producing tumor; and the genitourinary tract, evaluating for clitoromegaly and uterine and ovarian abnormalities.
It is important to consider other possible diagnoses that can mimic PCOS and to evaluate for these conditions. For example, in women presenting with hyperandrogenism, consideration should be given to nonclassic congenital adrenal hyperplasia (NCCAH) and/or androgen-secreting tumors. For a woman with oligomenorrhea as her presenting feature, pregnancy, hypothyroidism, hyperprolactinemia, primary ovarian insufficiency, and Cushing syndrome should be considered (see Chap. 5 on Menstruation and Secondary Amenorrhea).
According to the 2003 Rotterdam criteria, a diagnosis of PCOS is made when two of the following are present: signs of hyperandrogenism, including clinical features like hirsutism or acne, or biochemical features such as elevated testosterone or DHEA-S; chronic ovulatory dysfunction; and/or the presence of polycystic ovaries on imaging, preferably on transvaginal ultrasound. Once the diagnosis of PCOS is secured, patients should be screened for diabetes, hyperlipidemia, and metabolic syndrome. Diabetes screening options include fasting blood glucose, hemoglobin A1c and 2-hour oral glucose tolerance test (OGTT). Hemoglobin A1c is often used instead of OGTT for patient convenience, though A1c alone may miss patients with isolated postprandial hyperglycemia. A fasting blood glucose or A1c may also underestimate the degree of insulin resistance for these patients. Screening every 3 years for those with normal results and annual screening for those with impaired results is recommended.
It is also important to note that assessments of free testosterone levels are more sensitive than the measurement of total testosterone for the diagnosis of hyperandrogenic disorders [1, 13, 21]. Table 6.3 provides an outline of the recommended evaluation for different features associated with PCOS.
Shazia had mild elevation of her testosterone and normal thyroid function tests, prolactin, and FSH. Her ultrasound did not have features of polycystic ovaries. She asks how best to manage her symptoms and if she is at risk of any other conditions—she read that PCOS can lead to infertility and endometrial cancer and is worried.
PCOS Treatment
The goals of treatment for PCOS are to restore menses and/or ovulation, reduce hyperandrogenism, and/or reduce the risk of developing associated complications such as diabetes (Table 6.4). Treatment of PCOS should target the patient’s most bothersome symptoms (i.e., hirsutism) and/or complications that can cause harm (i.e., anovulatory cycles, metabolic syndrome). Not all treatments recommended for PCOS will treat all complications of PCOS; therefore, patient and provider together must outline a management plan based on patient preferences, clinical manifestations, and medical comorbidities.
One key goal of PCOS treatment includes reducing insulin resistance. Weight loss, medications, and bariatric surgery are all employed to achieve this goal with improvement in ovulation and hyperandrogenemia as the final outcome [48]. A weight reduction of as little as 5% can restore ovulation in up to 60% of patients with PCOS [49]. The Endocrine Society Clinical Practice Guideline suggests the use of exercise therapy along with diet modification as first-line treatment to manage obesity in women with PCOS [50]. Based on studies on rodent models, it is suggested that early intervention with dietary restrictions and exercise in young adolescents with PCOS as well as in prepubertal children at risk of PCOS may improve metabolic, reproductive, and endocrine parameters. This improvement is caused by regulation of the neuropeptides in the hypothalamic-pituitary-gonadal axis [50, 51]. Pretreatment weight loss has also been studied as infertility treatment in PCOS. Pretreatment lifestyle modification and weight loss for 16 weeks, with or without concurrent oral contraceptive therapy, is associated with a significant improvement in the ovulation rate and an even greater increase in live birth rates as compared to immediate fertility treatment without lifestyle modification [49, 52].
The following sections will be organized by treatment modalities that target specific symptoms and manifestations of PCOS.
Menstrual Regulation and Endometrial Protection
Oral contraceptive pills (OCPs):
OCPs are useful for women with oligomenorrhea, hirsutism, and acne and/or those who desire contraceptive benefit [53]. The most commonly used OCPs contain both estrogen and progestin, also known as combined oral contraceptive pills (COCs) (Table 6.4). The estrogen component increases sex hormone-binding globulin (SHBG), which binds testosterone and helps reduce hirsutism. One specific combination shown to have benefit in patients with PCOS includes both ethinyl estradiol and a low-androgenic progestin such as norgestimate; in general, any COC is fine. Patients need to be counseled that there is an increased risk of venous thromboembolism, along with potential increase in blood pressure, triglycerides, and HDL cholesterol levels [53] (see Chap. 4 on Patient-Centered Contraceptive Counseling).
Progestin therapy:
Women may choose to take progestin-only therapy for endometrial protection. Examples include medroxyprogesterone acetate 5–10 mg or micronized progesterone 200 mg for 10–14 days monthly or continuous therapy with norethindrone 0.35 mg daily. The latter also provides contraception. Unlike COCs, progestin-only therapy will not reduce symptoms of acne or hirsutism as estrogen is required for SHBG to increase and subsequently bind testosterone.
Intrauterine device (IUD):
COCs are recommended as first-line therapy given the multiple potential benefits described above. For women who cannot or choose not to take COCs, IUDs can provide endometrial protection and contraception for a woman with oligomenorrhea. While IUDs can be either hormonal (levonorgestrel-releasing) or nonhormonal, only the levonorgestrel-releasing hormone has endometrial protective effect. For primary care providers who do not place IUDs, referral to a gynecologist can facilitate IUD placement, particularly for patients who desire highly effective contraception.
Metformin:
Metformin can be useful for women who do not want to take OCPs but have oligomenorrhea. Recall that insulin resistance and hyperinsulinemia are part of the pathophysiology of PCOS. By acting to improve insulin resistance, metformin can both impact patients’ glucose metabolism and oligomenorrhea. A recent study found that metformin, at a dose of at least 1000 mg daily, restored menses in at least 42% of women within 6 months of treatment [58].
Metformin has been available for use for many years and has a mostly tolerable adverse effect profile. The most common adverse effect is GI distress such as bloating and diarrhea, which occasionally resolves after a few weeks of use. Metformin does not seem to have a significant effect on hirsutism, and it may increase pregnancy risk given its effect to restore menses and ovulation; patients who do not desire pregnancy require effective contraception.
Hyperandrogenism
Spironolactone:
If a patient is bothered by hirsutism and acne, spironolactone, an anti-androgen, is an option. Spironolactone works by competing with dihydrotestosterone (DHT) for binding to the androgen receptor and inhibits enzymes involved in androgen biosynthesis. In general, it is recommended to start patients on a COC for 6 months, and if desired reduction in hirsutism is not attained, spironolactone can be started [50]. There is danger that a male fetus could be feminized by spironolactone therapy, so women desiring treatment with spironolactone also require adequate contraception. The typical effective dose is 50–100 mg twice daily and the clinical effect is dose-dependent. Despite these high doses, patients with normal blood pressure tend to tolerate spironolactone quite well. It is also important to be mindful that spironolactone could cause adverse effects including, but not limited to, hypotension, hyperkalemia, kidney injury, GI discomfort , and headache.
Other treatment options:
In addition to COCs and spironolactone, topical agents can be used to treat bothersome acne. Commonly used topical therapies include, but are not limited to, benzoyl peroxide, retinoids, sulfone agents, and salicylic acid. Both oral and topical antibiotics are often used in conjunction with these therapies. Benzoyl peroxide is an antibacterial agent that kills P. acnes and is mildly comedolytic. Strengths available for acne treatment range from 2.5% to 10%. Topical retinoids are vitamin A derivatives and are both comedolytic and anti-inflammatory. Examples are tretinoin (0.025–0.1% in cream or gel), adapalene (0.1%, 0.3% cream or 0.1% lotion), and tazarotene (0.05%, 0.1% cream, gel, foam). The sulfone agent, dapsone 5% gel, is available as a twice-daily agent. It works primarily for inflammatory lesions. Combination with topical retinoids may be indicated if comedones are also present. Finally, salicylic acid is a comedolytic agent that is available over the counter in 0.5% to 2% strengths. If acne is treatment-resistant, scarring, or causing severe distress, oral isotretinoin may be appropriate, and referral to a dermatologist should be made [54].
Metabolic Complications
Weight loss and exercise can help to improve the metabolic profile in patients with PCOS. Metformin can be used for treatment of prediabetes and diabetes. Newer treatments such as liraglutide, a GLP-1 agonist, can help treat diabetes mellitus type 2 in women with PCOS while also mediating weight loss.
Infertility
It is important for women with PCOS to know that they are indeed fertile but that it may be more challenging to conceive due to anovulation. Some women with PCOS conceive naturally; when they do not, ovulation induction is possible with medical management and/or assisted reproduction techniques. As stated earlier, metformin can restore ovulation in some patients, but referral to a reproductive endocrinologist is encouraged if patients do not conceive after 6 months to 1 year of unprotected and frequent intercourse.
Clomiphene , a selective estrogen receptor modulator (SERM), and letrozole, an aromatase inhibitor (AI), have been studied for use in ovulation induction (Fig. 6.6). Both inhibit the negative feedback of estrogen at the hypothalamus with a consequent increase in ovarian stimulation by endogenous gonadotropin [55]. A randomized trial of ovulation induction involving women with PCOS and infertility showed a higher live-birth rate among women who received clomiphene than among women who received metformin alone (22.5% vs. 7.2%). There was an even greater live-birth rate in the combination therapy group (26.8%) [56]. However, a subsequent randomized trial from 2014 compared clomiphene 50 mg daily to letrozole 2.5 mg daily and found more live births in the group that took letrozole (27.5% to 19.1%). There was also significantly more ovulation, conception, and pregnancy. This effect was most significantly seen in women with a BMI >30.3 to <=39.4 kg/m2 [57].
Mental Health
As noted above, depression is more common in patients with PCOS than in those without. Patients with PCOS should be screened using common primary care depression screening tools like the PHQ-2 and PHQ-9. If positive, standard treatments should be offered (see Chap. 33 on Depressive and Anxiety Disorders).
Addressing patients’ concerns in regard to their signs and symptoms of hirsutism, as well as providing appropriate counseling and timely referral to specialists when it comes to their concerns about fertility , can be important in managing patients with PCOS.
Summary Points
-
1.
PCOS is a common disorder in women of childbearing age. It is important to ask about each woman’s menstrual pattern and consider PCOS in women who report oligomenorrhea or other menstrual changes.
-
2.
The main clinical features of PCOS are oligo-anovulation, androgen excess, and polycystic ovaries. Two of these three features are necessary to diagnose PCOS according to the currently used Rotterdam criteria.
-
3.
PCOS can be associated with a spectrum of metabolic abnormalities such as impaired glucose tolerance and diabetes. Guidelines often recommend screening every 3 years. Those with abnormal test results should be screened annually.
-
4.
While a diagnosis of PCOS can be made clinically based on history and physical examination, lab tests and ultrasound are often necessary to exclude other causes of oligo-anovulation and to assess hyperandrogenism.
-
5.
Treatments focus on reducing the risk of endometrial cancer, improving insulin sensitivity, and reducing hirsutism. Cornerstones of treatment include weight loss and hormonal contraceptives; metformin is also frequently used.
-
6.
Infertility and depression are common in patients with PCOS. Patients should be screened and treated for depression, while patients who do not conceive within 6–12 months should be promptly referred to a reproductive endocrinologist.
References
Rosenfield R, Ehrmann D. The pathogenesis of polycystic ovary syndrome (PCOS): the hypothesis of PCOS as functional ovarian hyperandrogenism revisited. Endocr Rev. 2016;37(5):467–520.
Carvalho L, Ferreira C, Sóter M, Sales M, Rodrigues K, Martins S, et al. Microparticles: inflammatory and haemostatic biomarkers in polycystic ovary syndrome. Mol Cell Endocrinol. 2017;443:155–62.
Palomba S, Santagni S, Falbo A, La Sala G. Complications and challenges associated with polycystic ovary syndrome: current perspectives. Int J Women's Health. 2015;7:745–63.
Lauritsen M, Bentzen J, Pinborg A, Loft A, Forman J, Thuesen L, et al. The prevalence of polycystic ovary syndrome in a normal population according to the Rotterdam criteria versus revised criteria including anti-Mullerian hormone. Hum Reprod. 2014;29(4):791–801.
Baskind N, Balen A. Hypothalamic–pituitary, ovarian and adrenal contributions to polycystic ovary syndrome. Best Pract Res Clin Obstet Gynaecol. 2016;37:80–97.
Stein IF, Leventhal ML. Amenorrhea associated with bilateral polycystic ovaries. Am J Obstet Gynecol. 1935;29(2):181–91.
Lizneva D, Suturina L, Walker W, Brakta S, Gavrilova-Jordan L, Azziz R. Criteria, prevalence, and phenotypes of polycystic ovary syndrome. Fertil Steril. 2016;106(1):6–15.
Dumesic D, Oberfield S, Stener-Victorin E, Marshall J, Laven J, Legro R. Scientific statement on the diagnostic criteria, epidemiology, pathophysiology, and molecular genetics of polycystic ovary syndrome. Endocr Rev. 2015;36(5):487–525.
Zawadzki JK, Dunaif A. Diagnostic criteria for polycystic ovary syndrome; towards a rational approach. In: Dunaif A, Givens JR, Haseltine F, Merriam G, editors. Polycystic ovary syndrome. Boston: Blackwell Scientific; 1992.
Rotterdam ESHRE/ASRM-Sponsored PCOS Consensus Workshop Group. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome. Fertil Steril. 2004;81(1):19–25.
National Institutes of Health. Evidence-based methodology workshop on polycystic ovary syndrome, December 3–5, 2012. Executive summary. Available at: https://prevention.nih.gov/docs/programs/pcos/FinalReport.pdf. Accessed March 1, 2016.
Georgopoulos NA, Papadakis E, Armeni AK, Katsikis I, Roupas ND, Panidis D. Elevated serum androstenedione is associated with a more severe phenotype in women with polycystic ovary syndrome (PCOS). Hormones. 2014;13(2):213–21.
Azziz R, Carmina E, Dewailly D, Diamanti-Kandarakis E, Escobar-Morreale HF, Futterweit W, et al. The Androgen Excess and PCOS Society criteria for the polycystic ovary syndrome: the complete task force report. Fertil Steril. 2009;91(2):456–88.
Kim J, Hwang K, Choi Y, Moon S, Chae S, Park C, et al. Complete phenotypic and metabolic profiles of a large consecutive cohort of untreated Korean women with polycystic ovary syndrome. Fertil Steril. 2014;101(5):1424–30.e3.
Panidis D, Tziomalos K, Papadakis E, Chatzis P, Kandaraki E, Tsourdi E, et al. Associations of menstrual cycle irregularities with age, obesity and phenotype in patients with polycystic ovary syndrome. Hormones. 2015;14:431–7.
Diamanti-Kandarakis E, Panidis D. Unravelling the phenotypic map of polycystic ovary syndrome (PCOS): a prospective study of 634 women with PCOS. Clin Endocrinol. 2007;67(5):735–42.
Moran L, Teede H. Metabolic features of the reproductive phenotypes of polycystic ovary syndrome. Hum Reprod Update. 2009;15(4):477–88.
Rizzo M, Berneis K, Hersberger M, Pepe I, Di Fede G, Rini G, et al. Milder forms of atherogenic dyslipidemia in ovulatory versus anovulatory polycystic ovary syndrome phenotype. Hum Reprod. 2009;24(9):2286–92.
Guastella E, Longo R, Carmina E. Clinical and endocrine characteristics of the main polycystic ovary syndrome phenotypes. Fertil Steril. 2010;94(6):2197–201.
Goverde A, van Koert A, Eijkemans M, Knauff E, Westerveld H, Fauser B, et al. Indicators for metabolic disturbances in anovulatory women with polycystic ovary syndrome diagnosed according to the Rotterdam consensus criteria. Hum Reprod. 2008;24(3):710–7.
Trikudanathan S. Polycystic ovarian syndrome. Med Clin North Am. 2015;99(1):221–35.
Jakimiuk A. Aromatase mRNA expression in individual follicles from polycystic ovaries. Mol Hum Reprod. 1998;4(1):1–8.
Balen A. The pathophysiology of polycystic ovary syndrome: trying to understand PCOS and its endocrinology. Best Pract Res Clin Obstet Gynaecol. 2004;18(5):685–706.
Williamson K, Gunn AJ, Johnson N, Milsom SR. The impact of ethnicity on the presentation of polycystic ovarian syndrome. Aust N Z J Obstet Gynaecol. 2001;41(2):202–6.
Glueck CJ, Papanna R, Wang P, Goldenberg N, Sieve-Smith L. Incidence and treatment of metabolic syndrome in newly referred women with confirmed polycystic ovarian syndrome. Metab Clin Exp. 2003;52(7):908–15.
Chang WY, Knochenhauer ES, Bartolucci AA, Azziz R. Phenotypic spectrum of polycystic ovary syndrome: clinical and biochemical characterization of the three major clinical subgroups. Fertil Steril. 2005;83(6):1717–23.
Azziz R, Sanchez LA, Knochenhauer ES, Moran C, Lazenby J, Stephens KC, et al. Androgen excess in women: experience with over 1000 consecutive patients. J Clin Endocrinol Metab. 2004;89(2):453–62.
Carmina E, Lobo RA. Do hyperandrogenic women with normal menses have polycystic ovary syndrome? Fertil Steril. 1999;71(2):319–22.
Ferriman D, Gallwey JD. Clinical assessment of body hair growth in women. J Clin Endocrinol Metab. 1961;21(11):1440–7.149.
Hatch R, Rosenfield RL, Kim MH, Tredway D. Hirsutism: implications, etiology, and management. Am J Obstet Gynecol. 1981;140:815–30.
Yildiz B, Bolour S, Woods K, Moore A, Azziz R. Visually scoring hirsutism. Hum Reprod Update. 2009;16(1):51–64.
O’Driscoll JB, Mamtora H, Higginson J, Pollock A, Kane J, Anderson DC. A prospective study of the prevalence of clear-cut endocrine disorders and polycystic ovaries in 350 patients presenting with hirsutism or androgenic alopecia. Clin Endocrinol. 1994;41(2):231–6.
Ludwig E. Classification of the types of androgenetic alopecia (common baldness) occurring in the female sex. Br J Dermatol. 1977;97(3):247–54.
Quinn M, Shinkai K, Pasch L, Kuzmich L, Cedars M, Huddleston H. Prevalence of androgenic alopecia in patients with polycystic ovary syndrome and characterization of associated clinical and biochemical features. Fertil Steril. 2014;101(4):1129–34.
Jonard S, Robert Y, Cortet-Rudelli C, Pigny P, Decanter C, Dewailly D. Ultrasound examination of polycystic ovaries: is it worth counting the follicles? Hum Reprod. 2003;18(3):598–603.
Adams J, Polson DW, Franks S. Prevalence of polycystic ovaries in women with anovulation and idiopathic hirsutism. Br Med J. 1986;293(6543):355–9.
Carmina E, Orio F, Palomba S, Longo RA, Lombardi G, Lobo RA. Ovarian size and blood flow in women with polycystic ovary syndrome and their correlations with endocrine parameters. Fertil Steril. 2005;84(2):413–9.
Tehrani F, Simbar M, Tohidi M, Hosseinpanah F, Azizi F. The prevalence of polycystic ovary syndrome in a community sample of Iranian population: Iranian PCOS prevalence study. Reprod Biol Endocrinol. 2011;9(1):39.
Boudreaux MY, Talbott EO, Kip KE, Brooks MM, Witchel SF. Risk of T2DM and impaired fasting glucose among PCOS subjects: results of an 8-year follow-up. Curr Diab Rep. 2006;6(1):77–83.
Valkenburg O, Steegers-Theunissen RP, Smedts HP, et al. A more atherogenic serum lipoprotein profile is present in women with polycystic ovary syndrome: a case-control study. J Clin Endocrinol Metab. 2008;93(2):470–6.
Wild RA, Carmina E, Diamanti-Kandarakis E, et al. Assessment of cardiovascular risk and prevention of cardiovascular disease in women with the polycystic ovary syndrome: a consensus statement by the Androgen Excess and Polycystic Ovary Syndrome (AE-PCOS) Society. J Clin Endocrinol Metab. 2010;95(5):2038–49.
Valkenburg O, Steegers-Theunissen R, Smedts H, Dallinga-Thie G, Fauser B, Westerveld E, et al. A more atherogenic serum lipoprotein profile is present in women with polycystic ovary syndrome: a case-control study. J Clin Endocrinol Metab. 2008;93(2):470–6.
Dokras A, Bochner M, Hollinrake E, Markham S, Vanvoorhis B, Jagasia DH. Screening women with polycystic ovary syndrome for metabolic syndrome. Obstet Gynecol. 2005;106(1):131–7.
Haoula Z, Salman M, Atiomo W. Evaluating the association between endometrial cancer and polycystic ovary syndrome. Hum Reprod. 2012;27(5):1327–31.
Cooney L, Lee I, Sammel M, Dokras A. High prevalence of moderate and severe depressive and anxiety symptoms in polycystic ovary syndrome: a systematic review and meta-analysis. Hum Reprod. 2017;32(5):1075–91.
Elsenbruch S, Hahn S, Kowalsky D, Öffner A, Schedlowski M, Mann K, et al. Quality of life, psychosocial well-being, and sexual satisfaction in women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2003;88(12):5801–7.
Bellver J, Rodríguez-Tabernero L, Robles A, Muñoz E, Martínez F, Landeras J, et al. Polycystic ovary syndrome throughout a woman’s life. J Assist Reprod Genet. 2018;35(1):25–39.
McCartney CR, Marshall JC. Clinical practice. Polycystic ovary syndrome. N Engl J Med. 2016;375(1):54–64.
Palomba S. Infertility in women with polycystic ovary syndrome: pathogenesis and management. Cham: Springer; 2018.
Legro R, Arslanian S, Ehrmann D, Hoeger K, Murad M, Pasquali R, et al. Diagnosis and treatment of polycystic ovary syndrome: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2013;98(12):4565–92.
Diane A, Kupreeva M, Borthwick F, Proctor S, Pierce W, Vine D. Cardiometabolic and reproductive benefits of early dietary energy restriction and voluntary exercise in an obese PCOS-prone rodent model. J Endocrinol. 2015;226(3):193–206.
Legro R, Dodson W, Kunselman A, Stetter C, Kris-Etherton P, Williams N, et al. Benefit of delayed fertility therapy with preconception weight loss over immediate therapy in obese women with PCOS. J Clin Endocrinol Metab. 2016;101(7):2658–66.
Dokras A. Noncontraceptive use of oral combined hormonal contraceptives in polycystic ovary syndrome—risks versus benefits. Fertil Steril. 2016;106(7):1572–9.
Descamps V. Clinical guidelines for management of acne vulgaris. JAMA. 2017;317(2):213.
Legro R. Ovulation induction in polycystic ovary syndrome: current options. Best Pract Res Clin Obstet Gynaecol. 2016;37:152–9.
Legro RS, et al. “Clomiphene, metformin, or both for infertility in the polycystic ovary syndrome.” N Engl J Med. 2007;356(6):551–6.
Legro RS, et al. “Letrozole versus clomiphene for infertility in the polycystic ovary syndrome.” N Engl J Med. 2014;371(2):119–29.
Yang P-K, Hsu C-Y, Chen M-J, et al. The efficacy of 24-month metformin for improving menses, hormones, and metabolic profiles in polycystic ovary syndrome. J Clin Endocrinol Metab. 2018;103(3):890–9.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Review Questions
Review Questions
-
1.
Jane is a 30-year-old woman who visits in your office for her annual exam. She has recently gained 15 pounds. Her BMI is now 29. One of her friends who had a recent weight gain was diagnosed with a condition called “polycystic ovarian syndrome” or (PCOS). She is worried that she also has this condition. Which of the following would be needed so that you can make the same diagnosis for her?
-
A.
Menstrual cycles between 24 and 28 days, mild acne, mildly elevated insulin levels
-
B.
Prolonged episodes of amenorrhea, A1c in 6 range, low normal FSH
-
C.
Menstrual cycles generally >35 days apart, increased hair growth under chin area, normal range A1c
-
D.
Transvaginal ultrasound showing enlarged ovaries with multiple cysts, normal testosterone level, and impaired glucose tolerance test
The correct answer is C. Although most of the features described in the answer choices above can be associated with PCOS, the criteria for diagnosis require generally two out of the three characteristics of chronic oligo-anovulation, signs and symptoms of hyperandrogenism or hyperandrogenemia, and polycystic ovarian morphology on ultrasound [4, 6]. This is based on the most recent and agreed-upon criteria for diagnosis of PCOS (2012 extension of Rotterdam criteria) [7, 10]. Oligo-anovulatory cycles are generally defined as vaginal bleeding episodes occurring at greater than 35-day intervals or less than ten bleeds per year. A much smaller percentage of patients present with polymenorrhea, defined as bleeding episodes occurring frequently with less than 25 days between cycles [13, 27]. Hyperandrogenemia refers to higher than normal levels of circulating endogenous androgens, including testosterone (T), androstenedione (A4), and DHEA-S [13]. Clinical features of elevated androgens (known as hyperandrogenism) include hirsutism, acne, and androgenic alopecia [8, 13].
Although PCOS can be associated with hyperinsulinemia and increased risk of diabetes (manifesting itself as elevation in A1C or abnormalities in the glucose tolerance test) [8, 39], these endocrine abnormalities are not a part of criteria for diagnosis of PCOS. FSH and other hormone levels are only used to rule out other endocrine abnormalities causing oligo-anovulatory pictures (i.e., primary ovarian insufficiency). They are not a part of criteria for diagnosis of PCOS.
-
A.
-
2.
Tina is 35 years old. She is in your office to discuss her new onset of amenorrhea for almost 6 months. She used to have cycles every 26–28 days. She has noticed new hair growth under her chin area; she is embarrassed to go out with her friends. What are the best next steps in her care?
-
A.
You explain to her that she is a typical case of a condition called “polycystic ovarian syndrome or PCOS” and you think since she makes two out of three Rotterdam criteria, no further work-up is necessary.
-
B.
You explain to her that she is a typical case of a condition called PCOS, but to further confirm the diagnosis, you order a transvaginal ultrasound to assess her ovaries as well.
-
C.
You explain to her that although you suspect that her symptoms can be suggestive of PCOS, however given new prolonged amenorrhea and new onset of hirsutism, you think further work-up including TSH, prolactin, FSH, and testosterone levels are necessary to rule out secondary causes of amenorrhea and testosterone-secreting ovarian tumors.
-
D.
You explain to her that although you suspect that her symptoms can be suggestive of PCOS, however to further confirm the diagnosis, you need to order an insulin level and a glucose tolerance test.
The correct answer is C. Your patient seems to have two out of three criteria for PCOS based on the Rotterdam criteria (chronic anovulatory cycles based on her prolonged amenorrhea episode and hyperandrogenism manifesting itself as increased hair growth under her chin area) [7]. Meanwhile, generally when making the diagnosis of PCOS, one needs to rule out other causes of amenorrhea (especially a new onset of amenorrhea in this case). These can include but are not limited to hypothyroidism, hyperprolactinemia, primary ovarian insufficiency, and Cushing syndrome (please see Table 6.3 in the text). Also, given the new onset of signs of hyperandrogenism, androgen levels need to be checked. Mild elevation is expected in PCOS.
-
A.
-
3.
You make the diagnosis of PCOS for one of your patients Leslie, who had come to you with new onset of hair growth in her upper lip area, and finding of polycystic ovaries on her pelvic ultrasound for evaluation of intermittent heavy cycles. She informs you that she had been a biology major while she was in college and is particularly interested to understand the underlying mechanism for her syndrome. What are the cardinal features that contribute to the pathophysiology of polycystic ovary syndrome that are responsible for her symptoms?
-
A.
Functional ovarian hyperandrogenism is the cardinal feature in most patients and can be worsened by insulin-resistant hyperinsulinemia.
-
B.
Functional ovarian hyperandrogenism is the cardinal feature in most patients but is only seen in obese patients.
-
C.
Overproduction of androgens from the ovaries is mainly due to abnormalities of LH and FSH production from the pituitary.
-
D.
Overproduction of testosterone from ovaries is always the primary cause.
The correct answer is A. Functional ovarian hyperandrogenism is considered the cardinal feature leading to symptoms of PCOS [1]. Women with PCOS are suspected to have intrinsic abnormalities in the ovarian theca cells’ steroidogenesis which leads to hyperandrogenemia [21]. This hyper-responsiveness can increase androgen production and excess androgens can ultimately hinder ovulation [5]. Hyperinsulinemia plays a direct role on ovaries and enhances androgen production from theca cells in response to LH stimulus. Insulin resistance is common in both obese and lean women with PCOS.
LH and FSH production are mediated by GnRH secreted from the hypothalamus. GnRH is generally secreted in a pulsatile manner. At higher pulses, GnRH promotes the production of LH in the pituitary gland, while lower pulsation frequencies enhance the production of FSH. In women with PCOS, accelerated GnRH-LH pulsatile activity as well as decreased sensitivity of the hypothalamus to negative feedback from ovarian steroids leads to higher LH production [1, 48]. Higher LH pulses generally lead to higher production of ovarian androgens. That being said, the ovarian androgen production abnormalities are considered largely an inherent characteristic of the ovaries in patients with PCOS. In a smaller number of PCOS cases, dysregulation at the adrenal zona reticularis causes hyperandrogenism by increased production of DHEA [1].
-
A.
-
4.
Eleanor is a 35-year-old female patient presenting with a 6-month history of amenorrhea. You have confirmed she is not pregnant. Her TSH, prolactin, and FSH levels are normal. She has mild hirsutism and her testosterone levels are in the 40 range. At this point you make the diagnosis of PCOS for her. What are some of the concerns that you make sure you should address with her?
-
A.
You counsel her for weight loss, but given she has no family history of diabetes, you don’t feel it is necessary to check her for metabolic abnormalities.
-
B.
You assess her family planning goals, and if she is not planning to get pregnant, you start her only on a norgestimate containing birth control.
-
C.
Given her hirsutism is mild, there is no indication for her to get started on any specific treatment.
-
D.
You screen her for depressive symptoms and you refer her to specialist as indicated.
The correct answer is D. The cornerstones of treatment in patients with PCOS are weight loss, protection of the endometrium against prolonged unopposed exposure to estrogen (which can increase the risk of future endometrial cancer), screening and treatment for metabolic abnormalities such as diabetes and hypercholesterolemia, treating hirsutism or other signs of hyperandrogenism (based on patient’s preferences), treatments to address infertility and/or to increase chance of ovulation, and screening and providing appropriate treatments for depression and other psychosocial conditions associated with PCOS [49, 50, 53,54,55]. When addressing endometrial protection, all the different contraceptive options are potentially acceptable treatments. The choice depends on the patients’ comorbid conditions, as well as their personal preferences. Treatment for signs of hyperandrogenism largely depends on patients’ preferences and can be offered at any stage of hirsutism. Screening patients for depression and other psychosocial consequences is an important part of evaluation and treatment of patients with PCOS and can also include referral to behavioral health specialists.
-
A.
-
5.
Rose, a patient that you recently diagnosed with PCOS, returns to your office 6 months after her initial visit and states that she is interested in getting pregnant. What available treatments for ovulation induction could you recommend?
-
A.
Metformin only
-
B.
Metformin and clomiphene
-
C.
Clomiphene and letrozole
-
D.
Metformin, clomiphene, and letrozole
The correct answer is D. Metformin, clomiphene, and letrozole are all acceptable treatments for ovulation induction and treatment of infertility in PCOS patients. The choice of medication can depend on how long the couple has been trying to get pregnant, patient’s age, BMI, and personal preferences. Trials have suggested higher rates of pregnancy and live births with addition of clomiphene compared to metformin alone [55, 56]. Another study found that letrozole was associated with higher live birth and ovulation rates compared with clomiphene [55, 57]. In general, if the patient is not able to conceive after 6–12 months of unprotected and frequent intercourse and other treatments directed toward increased chance of ovulation (weight loss, metformin), referral to an infertility specialist is recommended.
-
A.
Rights and permissions
Copyright information
© 2020 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Nasseh, A., Sarvaideo, J. (2020). Polycystic Ovary Syndrome. In: Tilstra, S.A., Kwolek, D., Mitchell, J.L., Dolan, B.M., Carson, M.P. (eds) Sex- and Gender-Based Women's Health. Springer, Cham. https://doi.org/10.1007/978-3-030-50695-7_6
Download citation
DOI: https://doi.org/10.1007/978-3-030-50695-7_6
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-50694-0
Online ISBN: 978-3-030-50695-7
eBook Packages: MedicineMedicine (R0)