Abstract
The impact of chromosome architecture in the formation of chromosome aberrations is a meanwhile well-established finding of interphase-directed molecular cytogenetic studies. Up to recent years, biomedical research of interphase chromosomes in their integrity was hindered by technical limitations. The introduction of three-dimensional suspension-based fluorescence in situ hybridization (S-FISH) in combination with microdissection-based engineered DNA probes and fluorescence multicolor chromosome banding (MCB) allowed studying interphase chromosome organization, numbers, and rearrangements in different kind of cells. Such studies already provided comprehensive information on the interphase architecture of normal human sperm, as well as first insights into the influence of chromosomal rearrangements on the 3D structure of the sperm nuclei. Also, the influence of additional chromosomal fragments present in a nucleus was successfully visualized by S-FISH. Finally, S-FISH supported the idea that disease-specific chromosomal translocations could be due to tissue specific genomic organization. Overall, S-FISH combined with MCB but also other DNA probes is a tool with high potential to resolve the influence of chromosomal imbalances and/or rearrangements on the interphase architecture, the latter being possibly a part of the epigenetic cell regulation, also being denominated as chromosomics.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Suspension-based fluorescence in situ hybridization (S-FISH)
- Chromosomics
- Interphase architecture
- Sperm
- Multicolor chromosome banding (MCB)
- Chromosome positioning
Introduction
In the interphase nucleus, chromosomes are located in specific regions, which are called “chromosome territories” (Cremer and Cremer 2001; Williams and Fisher 2003; Branco and Pombo 2006). Own multicolor banding (MCB)-based studies revealed that the chromosome shape itself is not lost in the interphase nucleus, and one can even identify “interphase chromosomes” instead of only chromosome territory, even irrespective of the cell cycle phase (Weise et al. 2002; Lemke et al. 2002).
Both chromosome size and gene density are discussed to have an important impact on the nuclear position of chromosomes. Small chromosomes preferentially locate close to the center of the nucleus, while large chromosomes can be found in the nuclear periphery (Sun et al. 2000; Bolzer et al. 2005). On the other hand, Croft et al. (1999) demonstrated a gene density-correlated radial arrangement of chromosomes in nuclei. Mainly gene-dense and early replicating chromatin can be found in the central part of the nucleus, while gene-poor and later replicating chromatin is located in nuclear periphery (Croft et al. 1999). Interestingly, this nuclear topological arrangement is conserved during primate evolution (Manvelyan et al. 2008a).
Here, we summarize the yet published applications of suspension-based fluorescence in situ hybridization (S-FISH) combined with FISH banding (Liehr et al. 2002, 2006), particularly the yet most used approach array-proven MCB (Weise et al. 2008). Besides, also other protocols were suggested for FISH studies in 3D-preserved nuclei (e.g., Walter et al. 2006). Also, recent studies showed that inter- and metaphase chromosomes preserve a genome-wide haploid order (Weise et al. 2016) and that this order is completely changed in senescent cells (Roediger et al. 2014). All these studies provide to the more and more emerging field of chromosomics, as predicted in 2005 by Prof. Uwe Claussen (Claussen 2005).
S-FISH, the Method
Performing of a FISH experiment on human meta- and interphase cells after air-drying method is a well-established approach; it is routinely done as one- to multicolor-FISH test (Liehr et al. 2004a). However, the air-drying procedure of chromosome preparation, leading to well-spread metaphases under appropriate conditions, leads at the same time to flattening of the originally spherical interphase nuclei. Thus, interphase architecture is hard to be studied reliably on such kind of preparation (Hunstig et al. 2009), even though some basic insights can also be gained using such material for FISH banding (Weise et al. 2002; Lemke et al. 2002).
Still, there is an easy way to do studies in three-dimensionally (3D) preserved interphase nuclei obtained from routinely prepared cytogenetic preparations stored in Carnoy’s fixative. One just needs to do the whole FISH procedure in cell suspension, and as a final step, the nuclei are placed on a polished concave slide before evaluation, immobilized in agarose. This approach for 3D-FISH analyses on totally spherical interphase nuclei, called suspension-based fluorescence in situ hybridization (S-FISH), was published first in 2002 (Steinhaeuser et al. 2002) and further developed and slightly modified later (Manvelyan et al. 2008a; Hunstig et al. 2009). Its principle is shown in Fig. 8.1.
Schematic drawing of the suspension-based fluorescence in situ hybridization (S-FISH) procedure. Overall, S-FISH avoids this flattening and artificial swelling of the interphase nuclei, and the whole experiment is performed in suspension. A certain loss of cells during the washing steps is normal, shown here by the reduction of cells/nuclei from step 1 to step 4. In principle, Carnoy’s fixative is replaced subsequently by solutions necessary for a FISH, and washing steps are included. Finally, the cells/nuclei are immobilized and counterstained in an agarose (AGAR) on a glass slide under a coverslip. The details of the protocol are described in Hunstig et al. (2009)
S-FISH: Which DNA Probes May Be Applied?
For S-FISH, all available chromosome or chromosome region-specific DNA are principally suited. However, for application in S-FISH, at least double amount of the probe is necessary than for “normal” FISH experiments (Hunstig et al. 2009). To resolve the chromosome structure as a whole, single chromosome-directed FISH banding based on partial chromosome painting probes like in MCB is suited best (Weise et al. 2008). Besides, centromeric and/or locus-specific probes can be used as well for special questions (e.g., Manvelyan et al. 2009; Hunstig et al. 2009).
Applications of S-FISH
Besides some studies done in comparative interphase cytogenetics of human and whitehanded gibbon and gorilla (Manvelyan et al. 2008a), S-FISH combined with MCB is mainly applied in the field of biomedical basic research of the human interphase nucleus. Here, still many questions are open and unanswered, mainly due to lack of suited methods, before introduction of S-FISH. Besides, more and more studies in other animals/species provide insights into the nuclear architecture (Karamysheva et al. 2017).
Human Sperm
For the first time , the distribution of all human chromosomes in sperm was resolved comprehensively by S-FISH−/MCB studies. Strikingly, for the majority of the 24 human chromosomes, the distribution of the territories was alike as in lymphocytes; only the acrocentric chromosomes showed another location as in sperm, no nucleolus is formed (Manvelyan et al. 2008b). Thus, this nonrandom positioning must have a biological meaning. In other words, each chromosome needs to have a special position in the nucleus in order that the cell can work properly. Sperm are translationally inactive cells; however, they need to have chromosomes at the right places as soon as a sperm enters an oocyte and needs to become active again.
The study of Manvelyan et al. (2008b) showed a direct correlation of chromosome positions and their sizes, apart from chromosomes 1, 2, 6, 14, 18, 20, 21, and Y, i.e., large chromosomes were in the periphery, small in the center. Exactly those eight chromosomes not fitting in the correlation before perfectly aligned with gene density theory, i.e., gene-dense chromosomes were in the nuclear center, and gene-poor in the periphery.
There are also already other one studies in sperm of male with a chromosomal aberration (Bhatt et al. 2009; Karamysheva et al. 2015). Three males with paracentric inversion were studied, and no gross changes in the interphase positioning of the affected chromosomes were found. Here for sure, more studies on the influence of inborn rearrangements on the nuclear architecture of sperm , but also other in tissues, are necessary.
Different Tissues with Additional Chromosomal Fragments
Additional chromosomal material present in the cell is suspected to alter or at least influence the chromosomal architecture. Besides complete trisomies as inborn or acquired aberrations, there is the possibility of partial trisomies induced either by derivative chromosomes or by the presence of a small supernumerary marker chromosome (sSMC). The latter condition may be seen in 0.043% of newborn infants, 0.077% of prenatal cases, 0.433% of mentally retarded patients, and 0.171% of subfertile people (Liehr and Weise 2007). sSMC are defined as structurally abnormal chromosomes that cannot be identified or characterized unambiguously by conventional banding cytogenetics alone and are generally equal in size or smaller than a chromosome 20 of the same metaphase spread. sSMC are mostly detected unexpectedly in routine cytogenetics (Liehr et al. 2004b). Also, they are not easy to correlate with a specific clinical outcome as besides induction of genomic imbalance, mosaicism and other most often epigenetic factors can influence the phenotype of an sSMC carrier: Uniparental disomy, heterochromatization, and even their influence on the interphase architecture may play a role here. Also, a pilot study revealed some potential influence of sSMC presence on nuclear architecture recently (Karamysheva et al. 2015).
In a recent study (Klein et al. 2012), S-FISH revealed that an extra piece of DNA like an sSMC leads to gross rearrangements within the interphase nucleus, mainly concerning the sSMCs’ normal sister chromosomes. Primarily, the position of the sSMC is influenced by and/or influencing the position of the homologous chromosomes. sSMC and one sister chromosome tend to colocalize; this seems to be driven mainly by the amount of euchromatin present in the sSMC. Also, the sSMC seems to take over the position of one normal sister chromosome. Thus, the remainder sister chromosome is displaced toward another location within the nucleus. These observations were made in B and T lymphocytes and/or skin fibroblasts .
Different Female Tissues and the Position of the X Chromosome
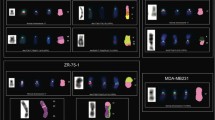
S-FISH/MCB studies in buccal mucosa, B and T lymphocytes, and skin fibroblasts for the positioning of normal and derivative X chromosomes in female cells also may lead to interesting, yet impossible insights into the nuclear architecture. Preliminary yet unpublished results (Fig. 8.2) firstly confirmed that active and inactive X chromosomes are located in the cell periphery and that the inactive X chromosome colocalizes to big parts, even though not perfectly, with the Barr body. Interestingly, a dicentric X chromosome, leading to an almost complete trisomy X, altered the positioning of the two X chromosomes to each other, inducing a larger distance between both normal and derivative X chromosome compared to the normal cells. Thus, new insights may be obtained also by studying well-known phenomenon like X inactivation by the S-FISH approach .
S-FISH results after application of X chromosome-specific DNA probe sets. (a) Active and inactive X chromosomes in a lymphocyte nucleus of a normal female labeled with an MCB-X probe set. (b) A normal (X) and derivative X chromosome (dic(X)) labeled with partial chromosome paints for Xp (green) and Xq (yellow) visualized in the fibroblast cell line GM15859 (Coriell). The female carrier had a constitutional karyotype 46,X,dic(X)(pter->q28::q28->pter)
Leukemia and the Positions of Chromosomes 8 and 21
Nonrandom positioning of chromosomes in interphase nuclei is known to be of importance for genomic stability and formation of chromosome aberrations. So tissue specificity of chromosomal translocations could be due to tissue-specific genome organization (Meaburn et al. 2007; Brianna Caddle et al. 2007), and a positive correlation between spatial proximity of chromosomes/genes in interphase nuclei and translocation frequencies was shown (Bickmore and Teague 2002; Roix et al. 2003; Branco and Pombo 2006; Meaburn et al. 2007; Brianna Caddle et al. 2007; Grasser et al. 2008).
Manvelyan et al. (2008a, b) provided evidence that there might be an effect of specific chromosome positioning in myeloid bone marrow cells, i.e., a colocalization of chromosomes 8 and 21 could promote a translocation providing selective advantage of t(8;21) cells in AML-M2. Additional S-FISH studies confirmed that this is specifically true for AML patients having a trisomy 8 (Othman et al. 2012). Overall, studies to enlighten the nuclear position of tumor-related oncogenes, which are known to be activated by specific translocations are promising targets of future S-FISH-studies, as supported by recent comparable findings in thyroid cancer (Gandhi et al. 2009).
S-FISH, Conclusions, and Perspectives
Overall, the combination of S-FISH and MCB for a three-dimensional analysis of chromosome position in interphase nucleus is a powerful tool, which can be accompanied by the use of locus-specific probes. The topological organization in interphase nucleus is nonrandom, and it becomes more and more obvious that there is a physiological reason behind that.
The already done and above summarized S-FISH studies in human show the potential of this approach for (i) genome-wide analysis of interphase architecture in yet not studied tissues (like done for sperm (Manvelyan et al. 2008b)), (ii) studies on architectural changes in nuclei with additional chromosomes or chromosomal material (like done for sSMC (Klein et al. 2012; Karamysheva et al. 2015) or the X chromosome), and (iii) analysis for the susceptibility of specific parts of the human genome for rearrangements due to colocalization (like done for the t(8;21) in AML (Manvelyan et al. 2009; Othman et al. 2012)). For sure, additional biomedical research aspect of interphase chromosomes may also be covered using the S-FISH/MCB approach, like recently the proof of interaction between distant chromosomal regions (Maass et al. 2018) and the description of nuclear architecture in hematopoietic stem cells (Grigoryan et al. 2018).
Overall, the approach discussed can be used not only based on human but also, if MCB probes are available for, based on probes from other species as already demonstrated by one example for murine mcb (Ktistaki et al. 2010). In conclusion, big advances in the field of chromosomics can be expected in the future from high-resolution FISH banding (MCB/mcb) in three-dimensionally preserved human interphase nuclei.
References
Bhatt S, Moradkhani K, Mrasek K, Puechberty J, Manvelyan M, Hunstig F, Lefort G, Weise A, Lespinasse J, Sarda P, Liehr T, Hamamah S, Pellestor F (2009) Breakpoint mapping and complete analysis of meiotic segregation patterns in three men heterozygous for paracentric inversions. Eur J Hum Genet 17:44–50
Bickmore WA, Teague P (2002) Influences of chromosome size, gene density and nuclear position on the frequency of constitutional translocations in the human population. Chromosom Res 10:707–715
Bolzer A, Kreth G, Solovei I, Koehler D, Saracoglu K, Fauth C, Muller S, Eils R, Cremer C, Speicher MR, Cremer T (2005) Three-dimensional maps of all chromosomes in human male fibroblast nuclei and prometaphase rosettes. PLoS Biol 3:e157
Branco MR, Pombo A (2006) Intermingling of chromosome territories in interphase suggests role in translocations and transcription-dependent associations. PLoS Biol 4:e138
Brianna Caddle L, Grant JL, Szatkiewicz J, van Hase J, Shirley BJ, Bewersdorf J, Cremer C, Arneodo A, Khalil A, Mills KD (2007) Chromosome neighborhood composition determines translocation outcomes after exposure to high-dose radiation in primary cells. Chromosom Res 15:1061–1073
Claussen U (2005) Chromosomics. Cytogenet Genome Res 111:101–106
Cremer T, Cremer C (2001) Chromosome territories, nuclear architecture and gene regulation in mammalian cells. Nat Rev Genet 2:292–301
Croft JA, Bridger JM, Boyle S, Perry P, Teague P, Bickmore WA (1999) Differences in the localization and morphology of chromosomes in the human nucleus. J Cell Biol 45:1119–1131
Gandhi MS, Stringer JR, Nikiforova M, Medvedovic M, Nikiforov YE (2009) Gene position within chromosome territories correlates with their involvement in distinct rearrangement types in thyroid cancer cells. Genes Chromosom Cancer 48:222–228
Grasser F, Neusser M, Fiegler H, Thormeyer T, Cremer M, Carter NP, Cremer T, Miller S (2008) Replication-timing correlated spatial chromatin arrangements in cancer and in primate interphase nuclei. J Cell Sci 121:1876–1886
Grigoryan A, Guidi N, Senger K, Liehr T, Soller K, Marka G, Vollmer A, Markaki Y, Leonhardt H, Buske C, Lipka DB, Plass C, Zheng Y, Mulaw MA, Geiger H, Florian MC (2018) LaminA/C regulates epigenetic and chromatin architecture changes upon aging of hematopoietic stem cells. Genome Biol 19:189
Hunstig F, Manvelyan M, Bhatt S, Steinhaeuser U, Liehr T (2009) Three-dimensional interphase analysis enabled by suspension FISH. In: Liehr T (ed) Fluorescence in situ hybridization (FISH) – application guide, 1st edn. Springer, Berlin
Karamysheva T, Kosyakova N, Guediche N, Liehr T (2015) Small supernumerary marker chromosomes and the nuclear architecture of sperm – a study in a fertile and an infertile brother. Syst Biol Reprod Med 61:32–36
Karamysheva TV, Torgasheva AA, Yefremov YR, Bogomolov AG, Liehr T, Borodin PM, Rubtsov NB (2017) Spatial organization of fibroblast and spermatocyte nuclei with different B-chromosome content in Korean field mouse, Apodemus peninsulae (Rodentia, Muridae). Genome 60:815–824
Klein E, Manvelyan M, Simonyan I, Hamid AB, Santos Guilherme R, Liehr T, Karamysheva T (2012) Centromeric association of small supernumerary marker chromosomes with their sister-chromosomes detected by three dimensional molecular cytogenetics. Mol Cytogenet 5:15
Ktistaki E, Garefalaki A, Williams A, Andrews SR, Bell DM, Foster KE, Spilianakis CG, Flavell RA, Kosyakova N, Trifonov V, Liehr T, Kioussis D (2010) CD8 locus nuclear dynamics during thymocyte development. J Immunol 184:5686–5695
Lemke J, Claussen J, Michel S, Chudoba I, Mühlig P, Westermann M, Sperling K, Rubtsov N, Grummt UW, Ullmann P, Kromeyer-Hauschild K, Liehr T, Claussen U (2002) The DNA-based structure of human chromosome 5 in interphase. Am J Hum Genet 71:1051–1059
Liehr T, Weise A (2007) Frequency of small supernumerary marker chromosomes in prenatal, newborn, developmentally retarded and infertility diagnostics. Int J Mol Med 19:719–731
Liehr T, Heller A, Starke H, Claussen U (2002) FISH banding methods: applications in research and diagnostics. Expert Rev Mol Diagn 2:217–225
Liehr T, Starke H, Weise A, Lehrer H, Claussen U (2004a) Multicolor FISH probe sets and their applications. Histol Histopathol 19:229–237
Liehr T, Claussen U, Starke H (2004b) Small supernumerary marker chromosomes (sSMC) in humans. Cytogenet Genome Res 107:55–67
Liehr T, Starke H, Heller A, Kosyakova N, Mrasek K, Gross M, Karst C, Steinhaeuser U, Hunstig F, Fickelscher I, Kuechler A, Trifonov V, Romanenko SA, Weise A (2006) Multicolor fluorescence in situ hybridization (FISH) applied to FISH-banding. Cytogenet Genome Res 114:240–244
Maass PG, Weise A, Rittscher K, Lichtenwald J, Barutcu AR, Liehr T, Aydin A, Wefeld-Neuenfeld Y, Pölsler L, Tinschert S, Rinn JL, Luft FC, Bähring S (2018) Reorganization of inter-chromosomal interactions in the 2q37-deletion syndrome. EMBO J 37:e96257
Manvelyan M, Hunstig F, Mrasek K, Bhatt S, Pellestor F, Weise A, Liehr T (2008a) Position of chromosomes 18, 19, 21 and 22 in 3D-preserved interphase nuclei of human and gorilla and white hand gibbon. Mol Cytogenet 1:9
Manvelyan M, Hunstig F, Bhatt S, Mrasek K, Pellestor F, Weise A, Simonyan I, Aroutiounian R, Liehr T (2008b) Chromosome distribution in human sperm – a 3D multicolor banding-study. Mol Cytogenet 1:25
Manvelyan M, Kempf P, Weise A, Mrasek K, Heller A, Lier A, Höffken K, Fricke HJ, Sayer HG, Liehr T, Mkrtchyan H (2009) Preferred co-localization of chromosome 8 and 21 in myeloid bone marrow cells detected by three dimensional molecular cytogenetics. Int J Mol Med 24:335–341
Meaburn KJ, Misteli T, Soutoglou E (2007) Spatial genome organization in the formation of chromosomal translocations. Semin Cancer Biol 17:80–90
Othman MAK, Lier A, Junker S, Kempf P, Dorka F, Gebhart E, Sheth FJ, Grygalewicz B, Bhatt S, Weise A, Mrasek K, Liehr T, Manvelyan M (2012) Does positioning of chromosomes 8 and 21 in interphase drive t(8;21) in acute myelogenous leukemia? BioDiscovery 4:4
Roediger J, Hessenkemper W, Bartsch S, Manvelyan M, Huettner SS, Liehr T, Esmaeili M, Foller S, Petersen I, Grimm MO, Baniahmad A (2014) Supraphysiological androgen levels induce cellular senescence in human prostate cancer cells through the Src-Akt pathway. Mol Cancer 13:214
Roix JJ, McQueen PG, Munson PJ, Parada LA, Misteli T (2003) Spatial proximity of translocation-prone gene loci in human lymphomas. Nat Genet 34:287–291
Steinhaeuser U, Starke H, Nietzel A, Lindenau J, Ullmann P, Claussen U, Liehr T (2002) Suspension (S)-FISH, a new technique for interphase nuclei. J Histochem Cytochem 50:1697–1698
Sun HB, Shen J, Yokota H (2000) Size-dependent positioning of human chromosomes in interphase nuclei. Biophys J 79:184–190
Walter J, Joffe B, Bolzer A, Albiez H, Benedetti PA, Müller S, Speicher MR, Cremer T, Cremer M, Solovei I (2006) Towards many colors in FISH on 3D-preserved interphase nuclei. Cytogenet Genome Res 114:367–378
Weise A, Starke H, Heller A, Claussen U, Liehr T (2002) Evidence for interphase DNA decondensation transverse to the chromosome axis: a multicolor banding analysis. Int J Mol Med 9:359–361
Weise A, Mrasek K, Fickelscher I, Claussen U, Cheung SW, Cai WW, Liehr T, Kosyakova N (2008) Molecular definition of high-resolution multicolor banding probes: first within the human DNA sequence anchored FISH banding probe set. J Histochem Cytochem 56:487–493
Weise A, Bhatt S, Piaszinski K, Kosyakova N, Fan X, Altendorf-Hofmann A, Tanomtong A, Chaveerach A, de Cioffi MB, de Oliveira E, Walther JU, Liehr T, Chaudhuri JP (2016) Chromosomes in a genome-wise order: evidence for metaphase architecture. Mol Cytogenet 9:36
Williams RE, Fisher AG (2003) Chromosomes, positions please! Nat Cell Biol 5:388–390
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Liehr, T. (2020). Chromosome Architecture Studied by High-Resolution FISH Banding in Three-Dimensionally Preserved Human Interphase Nuclei. In: Iourov, I., Vorsanova, S., Yurov, Y. (eds) Human Interphase Chromosomes. Springer, Cham. https://doi.org/10.1007/978-3-030-62532-0_8
Download citation
DOI: https://doi.org/10.1007/978-3-030-62532-0_8
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-62531-3
Online ISBN: 978-3-030-62532-0
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)