Abstract
It is well known that dynamic structural reorganization occurs in the adult mammalian neurohypophysis (NH) in response to chronic physiological stimulation such as osmotic stimulation and lactation. Neurohypophysial glial cells, pituicytes engulf axon terminals and interpose between the axon terminals and fenestrated capillaries under healthy normal conditions, whereas chronic physiological stimulation increases the neuro-vascular contact area via the retraction of pituicyte cellular processes. Recent evidence shows that an activity-dependent shape conversion of perivascular pericytes also participates in increasing the neuro-vascular contact area by extension of the pericyte cellular processes. In addition to the rapid activity-dependent responses of pituicytes and pericytes, angiogenesis and gliogenesis also occur to maintain a proper population density of pituicytes and endothelial cells. I will describe in this chapter how glial–neuronal or axonal–glial interactions modulate neuropeptide diffusion from the NH into the blood circulation. In conclusion, the NH has more dynamic and complicated mechanisms of structural reorganization than we have previously thought.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
The pituitary gland is an endocrine gland weighing five grams in humans that protrudes from the bottom of the hypothalamus at the base of the brain. In 150 AD, the Greek physician Galen first described the pituitary gland, but he misinterpreted its role to drain the phlegma (pituita), a cesspool of waste products derived from the brain. From 1928 to 1937, Drs. Ernst and Berta Scharrer opened up an entirely new field in the neurosciences, the field of neuroendocrinology. These two eminent and pioneering scientists hypothesized that nerve cells in the fish brain secreted hormones, which was a revolutionary idea at the time because the scientific dogma was that nerve cells conduct electrical impulses and endocrine cells secrete hormones, and that no cells exist that possess both capabilities. A specialized staining method enabled one to visualize Gomori-positive substances (e.g., neuropeptide–neurophysin complex by Gomori’s chrome alum hematoxylin stain) in the supraoptic nucleus (SON), the paraventricular nucleus (PVN), and the neurohypophysis (NH), and the ligation of the pituitary stalk resulted in an accumulation of Gomori-positive substances on the proximal side of the ligation. These Gomori-positive substances were synthesized in the hypothalamic magnocellular neurons of the SON and PVN and were transported in the magnocellular axons to the NH. A short time later, the Gomori-positive substances were purified and identified as oxytocin (OXT) and arginine vasopressin (AVP). These neurosecretory cells resemble non-neural endocrine cells since they release peptides into the circulation and control specific physiological responses. But, like classic neurons, they also propagate electrical impulses and release their neuropeptides in response to electrical activity. Thus, the hypothalamo-neurohypophysial system was the first identified peptidergic nervous system in the brain.
2 Cellular Components of the Neurohypophysis
2.1 Axonal Terminals
The adult NH is largely comprised of glial cells and the axonal terminals of AVP and OXT magnocellular neurons (Fig. 3.1). The terminals of the NH contain both small synaptic vesicles (SSVs) that carry classical neurotransmitters such as acetylcholine, glutamate, GABA, and glycine, and large dense-core vesicles (LDCVs) that store amines and neuropeptides. Exocytosis of SSVs is triggered rapidly in response to a single action potential, whereas that of LDCVs shows slow release upon repetitive firing of action potentials. Axonal terminals of magnocellular neurons have numerous AVP- and OXT-containing LDCVs and directly contact the outer basement membrane of fenestrated capillaries in the NH. Two types of binding proteins, neurophysin I and neurophysin II, are present in LDCVs by binding to OXT and AVP, respectively. In addition, axonal terminals of magnocellular neurons have many SSVs containing glutamate and ATP.
An electron micrograph showing axonal terminals of AVP and OXT magnocellular neurons contacting with the basement membrane or perivascular space of a rat fenestrated capillary. Some axonal terminals (green) make direct contact with the outer basement membrane (open arrowheads), whereas cellular processes of pituicytes (red) engulf axonal terminals and often separate axonal terminals from basement membrane by intervening between them. Pituicytes have lipid droplets (open arrows) that are the intracellular sites for neutral lipid storage. Inset: Higher magnification view shows LDCV (solid arrow) and SSV (solid arrowhead) at top right. Red, pituicytes; green, axonal terminals; blue, perivascular space; purple, endothelial cell. Scale bars = 100 nm (inset at top right), 1 μm (lower right). Micrographs were modified from Miyata et al. (2001) with permission
2.2 Pituicytes
Pituicytes are specialized glial cells in the NH that have a star-shaped morphology and form a sponge-like network. They comprise 43% of the total NH cellular population (Virard et al. 2008). Since pituicyte morphology resembles that of another type of glial cell, the astrocyte, they are sometimes called neurohypophysial astrocytes. But, pituicytes differ from astrocytes. First, pituicytes engulf unmyelinated axons and axonal terminals (also called Herring bodies), whereas astrocytes surround mainly somata and dendrites of neurons and sometimes locate closely to presynaptic terminals in the brain. Second, although pituicytes express high levels of an intermediate filament protein, glial fibrillary acidic protein (GFAP), like astrocytes, they also express the intermediate protein, vimentin, and microtubule-associated protein 2D, which are not present in astrocytes (Virard et al. 2008; Matsunaga et al. 1999). Furthermore, S100β, another marker of glial cells, is expressed in most pituicytes, but GFAP is expressed in a subpopulation of these cells, indicating that the pituicyte population is heterogeneous.
2.3 Oligodendrocyte Progenitor Cells
Another type of glial cell, oligodendrocyte progenitor cells (OPCs), are also present in the adult NH, although fully differentiated oligodendrocytes are absent. OPCs derived from the explants of the adult NH differentiate into myelinated mature oligodendrocytes in the presence of appropriate growth factors. Neurohypophysial OPCs express typical OPC markers, platelet-derived growth factor receptor β and NG2. They can proliferate in vivo and form primary spheres and differentiate into pituicytes and oligodendrocytes in vitro (Virard et al. 2008). These data indicate that OPCs are glial progenitor cells that generate new pituicytes, renew pituicytes, and thus maintain the pituicyte population.
Box 3.1. Oligodendrocyte Progenitor Cells
OPCs express the proteoglycan NG2 and the transcription factor Olig2, and can generate oligodendrocytes in the developing and adult central nervous system. OPCs can generate a subset of astrocytes during perinatal development in vivo and give rise to only one type of astrocyte when cultured in the presence of serum or bone morphogenetic proteins. This type of astrocyte differentiated from OPCs is named the type-2 astrocyte to distinguish it from type-1 astrocytes that generally exist in the central nervous system in vivo and in vitro. Therefore, OPCs are sometimes called oligodendrocyte-type-2 astrocyte (O2-A) progenitor cells. Type-1 and type-2 astrocytes express an intermediate filament GFAP, while the A2B5 antigen is expressed only in type 2, but not type-1, astrocytes.
2.4 Microglia
Microglia are resident immune cells in the central nervous system and have functions such as clearing cellular debris and dead cells from tissue through the process of phagocytosis (e.g., cell eating) (see Chap. 1). Microglia are developmentally derived from the embryonic mesoderm, whereas other neuroglia in the central nervous system are derived from neuroectoderm. Microglia sometimes engulf axonal terminals of OXT or AVP-containing neurons, and some phagosomes and secondary lysosomes possess morphologically intact neurosecretory granules and others contain partially destroyed LDCVs in the NH. Microglia are responsible for remodeling of axonal terminal arborization of neurosecretory neurons. In addition, other non-neuronal components such as endothelial cells, pericytes, and fibroblasts are present.
3 Fundamental Characteristics of Neurohypophysial Capillaries
3.1 Wide Perivascular Space and Thick Basement Membrane
Fenestrated capillaries of the NH largely differ from continuous capillaries present in the general adult brain. Furthermore, the NH has a wide perivascular space between the inner and outer basement membranes, and the surface of fenestrated capillaries is complicated or markedly rough (Miyata et al. 2001; Miyata 2017; Nishikawa et al. 2017) (Fig. 3.2). Continuous capillaries in the general brain vasculature have outer and inner basement membranes, which are frequently fused together and are seen as a single layer of basement membrane. The rough profile of perivascular space in the NH results from the irregular alignment of pericyte cellular processes (Nishikawa et al. 2017). Pericytes align parallel to endothelial cells and tightly contact with the endothelial cell layer in continuous capillaries of the general brain vasculature. Neurohypophysial pericytes, however, often elongate their cellular processes into the interstitial space between axonal terminals. Extending cellular processes of pericytes are always covered with the outer basement membrane and this specialized extension is named the “perivascular protrusion.” Quantitative ultrastructural analysis estimates that the occurrence of wide perivascular space and perivascular protrusions increases the surface area of neuro-vascular contacts of fenestrated capillaries by approximately 1.5 times. This system resembles microvilli that increase surface area of nutrient absorption without cell-surface enlargement in the gastrointestinal tract.
Light and electron micrographs revealing the wide perivascular space and unique alignment of pericyte cellular processes of the adult mouse NH. Laminin is main component of the basement membrane and its immunohistochemistry is useful to visualize the profile of fenestrated capillaries. The profile of the fenestrated capillary in the NH is larger in size and more complicated (upper left) compared with that of the continuous capillary in the cerebral cortex (upper right). Pericytes, vascular mural cells, often localize parallel to the endothelial cell layer and extend short cellular processes into the interstitial space between axonal terminals and sometimes elongate a long cellular process, or perivascular protrusion (arrows in bottom panel), whose inside is occupied with long cellular processes of pericytes. The electron microscope image in the bottom panel shows LDCVs labeled with 5 nm gold particles of neurophysin antibody (see inset at top right of bottom panel). Scale bars = 50 μm (top panels), 1 μm (bottom panel), 100 nm (inset). Micrographs were modified from Miyata (2017) and Nishikawa et al. (2017) with permission
In addition to increasing the surface area of neuro-vascular contact, perivascular protrusions act as the main diffusion route for low-molecular weight (MW) tracer substances (Nishikawa et al. 2017). In the adenohypophysis, dextran 20,000, corresponding to the size of growth hormone, moves rapidly from the interstitial space between growth hormone-secreting cells to the perivascular space and then diffuses slowly from the perivascular space to the circulation (Lafont et al. 2010). Thus, the perivascular space and protrusions are the main diffusion route of AVP and OXT.
In the NH, moreover, both the inner and outer basement membranes are thicker than those in the continuous capillaries of the general brain vasculature. The expression of collagen IV is higher at the inner basement membrane than the outer one, whereas the expression of laminin is more prominent at the outer basement membrane compared with that of the inner one (Nishikawa et al. 2017). Specialized pericyte alignment and a thick basement membrane result in a complicated profile of perivascular space, which acts to increase the vascular surface area contacting axonal terminals for efficient diffusion of AVP and OXT to the blood circulation.
3.2 Lack of Endothelial Blood–Brain Barrier
The vascular system of the general adult brain possesses a blood–brain barrier (BBB) that precludes free entry of various unnecessary or harmful exogenous substances (e.g., glutamate, GABA, neuropeptides, potassium ions) into the brain parenchyma by an endothelial cellular sheet endowed with tight junctions (Zlokovic 2011; Daneman 2012). However, the lack of typical tight junction proteins (e.g., claudin-1, claudin-5, occluding, ZO-1, ZO-2) means the endothelial BBB is absent in the vasculature of the secretory circumventricular organs (CVOs), such as the NH and median eminence (ME) (Nishikawa et al. 2017; Langlet et al. 2013). Exogenous substances in the blood do not cause any damage to the NH even in the absence of the BBB, because the NH lacks neural elements (e.g., somata and dendrites) and also contains cellular elements (pituicytes, microglia) which can phagocytize unwanted material. Thus, fenestrated capillaries in the NH enable AVP and OXT released from axonal terminals to diffuse into the blood circulation.
Box 3.2. Blood–Brain Barrier
In most parts of the body, a narrow space is present between endothelial cells so that blood-derived substances can move readily between the inside and outside of the capillary. In the brain, however, endothelial cells are tightly connected to each other by tight and adherence junctions and most substances, including ions and even water, cannot pass out of the bloodstream. Some substances required by the brain, such as various nutrients, ions, organic anions, and high-MW substances, are transported into the brain from the blood by specific transporters or specialized channels (e.g., aquaporin). To constitute a complete BBB, pericytes envelop and make intimate connections with adjacent endothelial cells and glial cells, astrocytes, to form a layer around brain blood vessels. The BBB protects the brain from unnecessary or harmful exogenous substances in the blood circulation that may cause damage to the brain neurons, and the BBB thereby maintains a constant brain environment.
Box 3.3. Circumventricular Organs
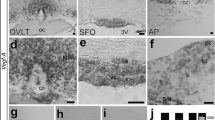
The term “circumventricular organs” was originally coined by anatomist Helmut Hofer in 1958. CVOs are specialized brain regions characterized by dense fenestrated capillaries and act as blood–brain interfaces. The CVOs are classified into the sensory and secretory CVOs on the basis of their main functions. The sensory CVOs, comprised of the organum vasculosum of the lamina terminalis (OVLT), the subfornical organ (SFO), and the area postrema (AP), express a variety of receptors and channels that allow direct sensing of blood-derived signals and send them to other brain regions. The secretory CVOs, including the median eminence (ME) and neurohypophysis (NH), mainly consist of axonal terminals and glia. The secretory CVOs can release neuropeptides from axonal terminals of hypothalamic neurons into the blood circulation.
3.3 Size-Limited Vascular Permeability
The fenestrated capillaries of the NH and ME exhibit size-limited permeability. Intravenous administration of fluorescent tracers with low MW, less than 3000 kDa, can diffuse and reach the interstitial space in the NH and ME (Morita and Miyata 2012; Miyata 2015) (Fig. 3.3). Further detailed analysis reveals that low-MW substances are likely to diffuse through perivascular protrusions. High-MW substances, more than 10,000 kDa, can scarcely pass the outer basement membrane to reach the interstitial space in the NH and ME. This size-limited permeability of fenestrated capillaries in the NH and ME is quite reasonable when considering the MW of OXT (MW = 1007), AVP (MW = 1084), and adenohypophysial hormone-releasing hormones, which range from thyrotropin-releasing hormone (MW = 362.4) to growth-hormone releasing hormone (MW = 5040.4). Low-MW soluble substances with a molecular radius less than 3 nm are presumed to move passively through endothelial intercellular clefts in capillaries lacking the endothelial BBB (Komarova and Malik 2010). The theoretical peptide molecular radius is estimated at 1.1 nm (5 kDa), 1.42 nm (10 kDa), 1.78 nm (20 kDa), and 2.4 nm (50 kDa), if the protein has the simplest spherical shape (Erickson 2009). High-MW tracers such as horse radish peroxidase and lectins are incorporated by endocytosis and transcytosis , which are often mistaken for passage through the perivascular space and endothelial intercellular clefts, although high-MW substances with more than a molecular radius of 3 nm cannot pass through endothelial intercellular clefts. Thus, the endothelial intercellular cleft in the NH acts as a physical barrier, or “ultrafiltration membrane,” that only allows the passage of substances less than approximately 5 kDa.
High vascular permeability of fenestrated capillaries in the CVOs of the adult mouse. Fluorescein isothiocyanate (FITC) is able to bind covalently to the primary amine groups of cellular components to form a stable thiourea link and is therefore useful to visualize exact vascular permeability by combing immunohistochemistry. The BBB in continuous capillaries of adult brains generally restricts free movement of substances, but fenestrated capillaries in the CVOs allow vascular permeability of FITC. Low-MW tracer FITC is permeable through fenestrated capillaries in the CVOs, but not through continuous capillaries of other adjacent brain regions. The secretory CVOs including the ME and NH show higher vascular permeability and higher penetration of FITC green fluorescence than the sensory CVOs comprising the organum vasculosum of the lamina terminalis (OVLT), subfornical organ (SFO), and area postrema (AP). Scale bar = 50 μm. Photographs were modified from Morita and Miyata (2012) with permission
3.4 Dynamics of Capillary Density by Angiogenesis
Another important difference between the vascular system in the NH and other general brain regions is the occurrence of continuous angiogenesis in the NH. Angiogenesis is robust during the development and growth of the brain, but it becomes completely quiescent in the mature adult brain. In the NH, however, proliferating endothelial cells have been observed even in healthy normal adult mouse (Furube et al. 2014) (Fig. 3.4). The vascular endothelial growth factor-A (VEGF-A) and VEGF receptor-2 are expressed in pituicytes and endothelial cells in the NH, respectively. The inhibition of VEGF signaling decreases the proliferation of endothelial cells in the NH, and a robust increase in proliferation of endothelial cells occurs in the NH after cessation of VEGF inhibition. The inhibition of VEGF signaling also causes a synchronous decrease in the density of AVP- and OXT-containing axonal terminals and endothelial cells (Fig. 3.5). Continuous angiogenesis is also present in the other CVOs under healthy normal conditions. The reader is directed to a review for an in-depth description of continuous angiogenesis in the CVOs (Miyata 2015). Thus, the population of endothelial cells is regulated coordinately with that of axonal terminals in the adult NH in a VEGF-dependent manner.
Continuous proliferation of endothelial cells under normal conditions and augmented proliferation after the withdrawal of VEGF signaling inhibition in the mouse NH. The proliferation marker BrdU, a thymidine analog, is detected in CD31-immunopositive endothelial cells under normal unstimulated conditions (left panel). Many more BrdU-labeled proliferating endothelial cells (arrowheads) are detected after the cessation of VEGF signaling inhibition (right panel). Scale bar = 50 μm. Photographs were modified from Furube et al. (2014) with permission
Inhibition of VEGF signaling simultaneously decreases the density of endothelial cells and axonal terminals in the adult mouse NH. The inhibition of VEGF signaling largely decreases the number of CD31-immunolabeled endothelial cells compared with the control NH (upper panels). The inhibition of VEGF signaling also causes a robust reduction in the density of AVP axonal terminals (lower panels). Scale bar = 50 μm. Photographs are modified from Furube et al. (2014) with permission
Box 3.4. Angiogenesis
Angiogenesis is the process by which new blood vessels are formed from elaboration of the existing vasculature. The turnover of endothelial cells is normally very low in adulthood, whereas angiogenesis occurs in the female reproductive tract and wound or injury regions. Endothelial “tip cells” lead sprouting vessels by extending filopodia and migrate in response to gradients of vascular endothelial growth factor-A (VEGF-A), while adjacent endothelial “stalk cells” trail the endothelial tip cells to make the trunk of new vessels. VEGF-A and its receptor VEGF receptor 2 (VEGFR2) are predominant angiogenic signaling factors that control the proliferation and sprouting of endothelial cells (Gerhardt et al. 2003).
4 Activity-Dependent Structural Reorganization
4.1 Activity-Dependent Increase in Neuro-vascular Contacts
Chronic physiological stimulation such as lactation and dehydration not only causes a continuous increase of neural activity in AVP and OXT magnocellular neurons, but also causes structural reorganization of somata and dendrites in the SON and PVN and axonal terminals in the NH (Miyata and Hatton 2002). In the SON and PVN, the structural reorganization is characterized by the formation of multiple synapses of afferent inputs, increased direct neuronal membrane apposition of somata, and dendritic bundling by the retraction of astrocytic cellular processes. The structural reorganization of somata and dendrites in the hypothalamic nuclei is associated with coordinated population activity to respond appropriately to altered physiological circumstances (Tasker et al. 2012) (see Chap. 2).
In addition to the hypothalamic nuclei, chronic physiological stimulation also induces structural reorganization in the NH. Wittkowski and Brinkmann (1974) observed that the relative extent of neuro-vascular contacts was significantly increased by chronic osmotic stimulation. Electron microscopic observation shows that pituicytes generally engulf the axonal terminals of magnocellular neurons and intervene between axonal terminals and the vascular basement membrane under normal unstimulated conditions (Tweedle and Hatton 1982; Miyata and Hatton 2002; Miyata 2017). Upon chronic osmotic stimulation, however, a reduction in the number of neurosecretory axons enveloped by cellular processes of pituicytes results in an increase in both the length of individual nerve terminals and the number of terminals (Tweedle and Hatton 1982; Miyata et al. 2001). Therefore, the structural reorganization of the NH is caused by the coordinate rearrangement of axonal terminals, the outer basement membrane, and glial cells, rather than enlargement or sprouting of the magnocellular terminals themselves. Furthermore, chronic osmotic stimulation leads to increased vascular permeability of low-MW substances (Nishikawa et al. 2017; Miyata 2017) (Fig. 3.6). Thus, the activity-dependent increase of neuro-vascular contacts contributes to efficiency of AVP and OXT diffusion into the blood circulation.
Activity-dependent increase in vascular permeability to low-MW fluorescent tracer FITC in the adult mouse NH. The FITC fluorescence intensity is higher in the NH of an animal that received chronic osmotic stimulation, drinking of 2% NaCl for 5 days (left micrograph), compared to that of the control animal (right micrograph). Quantitative analysis shows that the relative intensity of FITC fluorescence was significantly increased in osmotically stimulated mice compared with the control mice (right graph). Scale bar = 50 μm. * p < 0.05; ** p < 0.01 by ANOVA with Tukey’s post hoc test. Micrographs were modified from Nishikawa et al. (2017) with permission
4.2 Dynamic Alteration of Neuro-vascular Contacts by Shape Conversion of Pituicytes and Pericytes
Activity-dependent neuro-vascular structural reorganization is caused by retraction of cellular process of pituicytes (see Sect. 3.4.1). Shape conversion or retraction of cellular process of pituicytes is mediated by β-adrenergic receptors in cultured pituicytes (Rosso and Mienville 2009; Miyata 2017) (Fig. 3.7) and in the isolated NH in vitro (Smithson et al. 1990). Moreover, shape conversion of pituicytes is observed in vivo during chronic osmotic stimulation (Matsunaga et al. 1999) (Fig. 3.7). The reader is directed to reviews for an in-depth description of shape conversion of pituicytes (Rosso and Mienville 2009; Miyata 2017). Taken together, these results indicate that the retraction of pituicyte cellular processes engulfing axonal terminals results in an increase in neuro-vascular contacts in the NH.
Neurotransmitter-induced shape conversion of cultured pituicytes and activity-dependent morphological change of pituicytes in the adult rat NH. An agonist of adrenergic β-receptors, isoproterenol, changes the shape of cultured pituicytes from flat to stellate (upper panels). Chronic osmotic stimulation via water deprivation causes the retraction of the cellular processes of pituicytes (open arrows), which have well-branched cellular processes (closed arrows) in control osmotic conditions (lower panels). Scale bars = 50 μm. Photographs were modified from Miyata et al. (1999) and Matsunaga et al. (1999) with permission
Until recently, activity-dependent neuro-vascular structural reorganization was considered to be caused simply by the shape conversion of pituicytes. There was no information on changes in perivascular structure, because the fenestrated capillaries were believed to be resistant to change. Pericytes are vascular contractile mural cells that modify vascular ultrastructure and alter gene expression in endothelial cells in response to brain microenvironment alterations. Recently, my research group demonstrated dynamic changes of the perivascular space through shape conversion of pericytes in the adult mouse NH (Nishikawa et al. 2017) (Fig. 3.8). Pericytes extend their cellular processes into the extracellular space between axonal terminals so that the surface area of the neuro-vascular contacts is increased (see Sect. 3.3.1). Besides, chronic osmotic stimulation further increased the number of perivascular protrusions by 2.72-fold without changing the density of pericytes, the area of perivascular space and endothelial cells, or the diameter of vessels (Nishikawa et al. 2017).
Activity-dependent morphological changes in vascular mural cells, pericytes, in the adult mouse NH. Upper panel: An electron micrograph revealing that chronic osmotic stimulation causes dramatic morphological changes in pericytes (red) that are accompanied by extension of perivascular protrusions (arrows) or expansion of perivascular space (purple). Green, axonal terminals. Left lower panels: Chronic osmotic stimulation increases the fine cellular processes of pericytes. Right lower graph: Quantitative analysis reveals that chronic osmotic stimulation significantly increases the number of perivascular protrusions. * p < 0.01, Student’s t-test. Micrographs were modified from Nishikawa et al. (2017) with permission
Platelet-derived growth factor-B (PDGF-B) is highly expressed in endothelial cells in the developing brain vasculature. The gradient of PDGF-B is necessary for attachment to endothelial cells and migration of pericytes. In the adult mouse NH, the PDGF receptor β (PDGFRβ ) is strongly expressed in pericytes, whereas PDGF-B is present in LDCVs in axon terminals of OXT magnocellular neurons (Nishikawa et al. 2017). This observation indicates that dynamic shape conversion of pericytes is probably caused by activity-dependent release of PDGF-B from axonal terminals of OXT magnocellular neurons. Thus, activity-dependent neuro-vascular reorganization requires shape conversion of both glial cell pituicytes and vascular mural cell pericytes.
4.3 Activity-Dependent Change in Glial Proliferation
Chronic osmotic stimulation promotes the proliferation of OPCs and pituicytes, but does not change total cell number due to ongoing apoptosis (Virard et al. 2008). OPCs, but not pituicytes, express PDGFRα in the adult mouse NH and the proliferation of OPCs is mediated by PDGFRα signaling, like in other brain regions (Furube et al. 2014). The pituicyte population in the NH is regulated by a proliferation of pituicytes and OPCs and possibly contributes to make a space or “cushion” to maintain neurohypophysial volume regardless of changes in pituicyte and pericyte shape conversion, proliferation of endothelial cells, or changes in capillary density during activity-dependent structural reorganization.
5 Perspectives
Table 3.1 summarizes characteristics of cellular components in the adult NH. Pituicytes engulf axonal terminals and interpose between axonal terminals and fenestrated capillaries under healthy normal conditions; neuro-vascular contact is increased during chronic physiological stimulation by the retraction of cellular processes of pituicytes depending on increased demand of neuropeptide secretion. The activity-dependent shape conversion of perivascular pericytes also participates in increasing neuro-vascular contact by extending their cellular processes and increasing the number of perivascular protrusions. In addition to shape conversion of pituicytes and pericytes, angiogenesis and gliogenesis maintain the proper population density of pituicytes and endothelial cells. The expression of cell adhesion molecules, cytoskeletal proteins, receptors, and extracellular matrix, some of which are detected only during early periods of development and are unusual in fully mature cells, enable neurohypophysial reorganization. Recent findings reveal that the NH has more dynamic and complicated mechanisms of structural reorganization than we have thought. Understanding the molecular basis and mechanisms for crosstalk among axonal terminals, pituicytes, OPCs, pericytes, and endothelial cells will be necessary to achieve an eventual comprehensive description of the structural reorganization of the adult NH.
6 Key Literature
-
Miyata (2015) Review of characteristics of fenestrated capillaries in the CVOs.
-
Miyata (2017) Review of recent new evidence for structural reorganization of the NH.
-
Rosso and Mienville (2009) Review mechanisms for shape conversion of pituicytes.
-
Virard et al. (2008) Provides precise information for glial composition and characteristics in the NH.
References
Daneman R (2012) The blood–brain barrier in health and disease. Ann Neurol 2:648–672
Erickson HP (2009) Size and shape of protein molecules at the nanometer level determined by sedimentation, gel filtration, and electron microscopy. Biol Proc 11:32–51
Furube E, Mannari T, Morita S, Nishikawa K, Yoshida A, Itoh M, Miyata S (2014) VEGF-dependent and PDGF-dependent dynamic neurovascular reconstruction in the neurohypophysis of adult mice. J Endocrinol 221:161–179
Gerhardt H, Golding M, Fruttinger M, Ruhrberg C, Lundkvist A, Abramsson A, Jeltsch M, Mitchell C, Alitalo K, Shima D, Betsholtz C (2003) VEGF-A guides angiogenic sprouting utilizing endothelial tip cell filopodia. J Cell Biol 161:1163–1177
Komarova Y, Malik AB (2010) Regulation of endothelial permeability via paracellular and transcellular transport pathways. Annu Rev Physiol 72:463–493
Lafont C, Desarménien MG, Cassou M, Molino F, Lecoq J, Hodson D, Lacampagne A, Mennessier G, El Yandouzi T, Carmignac D, Fontanaud P, Christian H, Coutry N, Fernandez-Fuente M, Charpak S, Le Tissier P, Robinson IC, Mollard P (2010) Cellular in vivo imaging reveals coordinated regulation of pituitary microcirculation and GH cell network function. Proc Natl Acad Sci USA 107:4465–4470
Langlet F, Mullier A, Bouret SG, Prevot V, Dehouck B (2013) Tanycyte- like cells form a blood-cerebrospinal fluid barrier in the circumventricular organs of the mouse brain. J Comp Neurol 521:3389–3405
Matsunaga W, Miyata S, Kiyohara T (1999) Redistribution of MAP2 immunoreactivity in the neurohypophysial astrocytes of adult rats during dehydration. Brain Res 829:7–17
Miyata S (2015) New aspects in fenestrated capillary and tissue dynamics in the sensory circumventricular organs of adult brains. Front Neurosci 9:390
Miyata S (2017) Advances in understanding of structural reorganization in the hypothalamic neurosecretory system. Front Endocrinol 8:275
Miyata S, Hatton GI (2002) Activity-related, dynamic neuron-glial interactions in the hypothalamo- neurohypophysial system. Microsc Res Tech 56:143–157
Miyata S, Furuya K, Nakai S, Bun H, Kiyohara T (1999) Morphological plasticity and rearrangement of cytoskeletons in pituicytes cultured from adult rat neurohypophysis. Neurosci Res 33:299–306
Miyata S, Takamatsu H, Maekawa S, Matsumoto N, Watanabe K, Kiyohara T, Hatton GI (2001) Plasticity of neurohypophysial terminals with increased hormonal release during dehydration: ultrastructural and biochemical analyses. J Comp Neurol 434:413–427
Morita S, Miyata S (2012) Different vascular permeability between the sensory and secretory circumventricular organs of adult mouse brain. Cell Tissue Res 349:589–603
Nishikawa K, Furube E, Morita S, Horii-Hayashi N, Nishi M, Miyata S (2017) Structural reconstruction of the perivascular space in the adult mouse neurohypophysis during an osmotic stimulation. J Neuroendocrinol 29(2)
Rosso L, Mienville JM (2009) Pituicyte modulation of neurohormone output. Glia 57:235–243
Smithson KG, Suarez I, Hatton GI (1990) Beta-adrenergic stimulation decreases glial and increases neural contact with the basal lamina in rat neurointermediate lobes incubated in vitro. J Neuroendocrinol 2:693–699
Tasker JG, Oliet SH, Bains JS, Brown CH, Stern JE (2012) Glial regulation of neuronal function: from synapse to systems physiology. J Neuroendocrinol 24:566–576
Tweedle CD, Hatton GI (1982) Magnocellular neuropeptidergic terminals in neurohypophysis: rapid glial release of enclosed axons during parturition. Brain Res Bull 8:205–209
Virard I, Gubkina O, Alfonsi F, Durbec P (2008) Characterization of heterogeneous glial cell populations involved in dehydration-induced proliferation in the adult rat neurohypophysis. Neuroscience 151:82–91
Wittkowski W, Brinkmann H (1974) Changes of extent of neuro-vascular contacts and number of neuro-glial synaptoid contacts in the pituitary posterior lobe of dehydrated rats. Anat Embryol 146:157–165
Zlokovic BV (2011) Neurovascular pathways to neurodegeneration in Alzheimer’s disease and other disorders. Nat Rev Neurosci 12:723–738
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Miyata, S. (2021). Fenestrated Capillary and Dynamic Neuro-Glial-Vascular Reorganization of the Adult Neurohypophysis. In: Tasker, J.G., Bains, J.S., Chowen, J.A. (eds) Glial-Neuronal Signaling in Neuroendocrine Systems. Masterclass in Neuroendocrinology, vol 11. Springer, Cham. https://doi.org/10.1007/978-3-030-62383-8_3
Download citation
DOI: https://doi.org/10.1007/978-3-030-62383-8_3
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-62382-1
Online ISBN: 978-3-030-62383-8
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)