Abstract
Vertebrate homoeostasis is regulated by secretion of neurohormones from specialized neuroendocrine neurovascular interfaces such as the hypothalamic–neurohypophyseal system (HNS). Fish are shown to possess an additional caudal neurosecretory system (CNSS), which is termed urophysis, due to its anatomical location at the caudal spinal cord and its structural similarity to the hypophysis gland. The urophysis is a vascularized gland-like structure, which is interfaced by exceptionally large neurons termed Dahlgren cells. In contrast to the well-studied HNS of fish and mammals, the development and function of the urophysis/CNSS are not well understood, and related research has strongly declined in the last three decades. In this chapter, we summarize the main knowledge regarding the evolution, development and structure of the two neuroendocrine interfaces. Additionally, we describe the main knowledge regarding their regulatory and functional roles in fish homoeostasis. Where applicable, a general comparison to non-piscine vertebrates is described.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
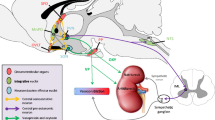
Neuroendocrine regulation of homoeostasis in most vertebrates is mainly orchestrated by the hypothalamus, a brain region whose neurons either affect the anterior pituitary gland by means of a vascular portal system, or directly form neurovascular interfaces with the capillary network of the posterior pituitary gland, the neurohypophysis, to release neurohormones into the circulation (Wircer et al. 2016; Biran et al. 2018). Interestingly, piscines uniquely possess an additional homoeostatic neurovascular interface known as the caudal neurosecretory system (CNSS, Fig. 4.1). In 1914, Dahlgren identified huge secretory cells residing in the spinal cord of elasmobranchs (Dahlgren 1914). A few years later, Speidel performed a systematic analysis of the caudal spinal cord of various fish species and identified the cells of Dahlgren in 26 out of 30 species he examined. Moreover, Speidel found that in more evolved fishes, Dahlgren cells innervate a vascularized glandular structure that shares high structural homology to the neurohypophysis (Speidel 1922). This glandular structure was later termed the urophysis. These discoveries initiated a great deal of research which resulted in the identification of novel neuropeptides affecting blood pressure—the urotensins, which were later shown to be functionally important in other vertebrates. Importantly, while the neurohypophysis releases its neuropeptides to the adenohypophysis and to the general circulation, vascular drainage of the urophysis delivers caudal neurohormones into the kidney, liver and swim bladder (Bern 1985). Despite the above findings, in the last twenty years there has hardly been any published information concerning the urophysis. This might be due to the uniqueness of the urophysis to fish species and the failure to identify a robust physiological function, which could be directly attributed to the CNSS (Bern 1985). In this chapter, we review some of the major findings regarding the piscine neurohypophysis and urophysis and their suggested neuroendocrine physiological functions in fishes.
Schematic representation of the neurohypophysis and urophysis in fish. The neurohypophysis is located in the posterior pituitary of the zebrafish brain, with axonal projections coming in from the hypothalamus. The axonal projections are interspersed within the vascular plexus of the posterior pituitary (magnified schema). The urophysis is located on the caudal region of the spinal cord with projections coming in from the Dahlgren cells located along the spine
2 The Hypothalamo–Neurohypophysis
The hypothalamo–neurohypophyseal system (HNS) is a neurosecretory interface, which is conserved across all vertebrate organisms. The fish HNS comprises two distinct populations of neurons that secrete the arginine-vasopressin-like (AVPL) and oxytocin-like (OXTL) neuropeptides, also known as arginine-vasotocin and isotocin, respectively, in all fish species other than zebrafish. In the interest of simplicity, we will henceforth refer to the piscine neurohypophyseal neurohormones as OXTL and AVPL. These cells reside in the piscine neurosecretory preoptic region (NPO) and posterior tuberculum (PT) of the fish diencephalon and project their axons onto the posterior pituitary, also known as the neurohypophysis, where they secrete their neurohormone cargo through a neuroendocrine–vascular interface (Biran et al. 2018). The termini of these neurons have distinctive swellings along their length which serve as synaptic release sites for their neurohormones (Tweedle et al. 1989). Upon their release, OXTL and AVPL are taken up by the fenestrated, i.e. permeable, capillary plexus of the neurohypophysis. The vasculature of this particular region is an extension of the cerebral vascular network; however, it possesses distinct qualities that allow for its selective permeability. Together, they also represent one of the key neurovascular interfaces, which will be discussed in length in a later section.
In addition to these components, the neurohypophysis also contains specialized astrocyte-like cells called pituicytes (Anbalagan et al. 2018; Chen et al. 2020). The pituicytes extend processes that engulf the secretory axonal termini, likely to act as a regulatory barrier to neurosecretion (Miyata 2017), like the glia of the fish urophysis, which were named urocytes (Kriebel 1980),
2.1 Evolution and Ontology of the Neurohypophysis
Box 4.1 The historical tale of the neurohypophysis
The hypothalamo–neurohypophyseal system has long posed enigmatic questions regarding its existence, and later its true function. The Dutch physiologist Van Rijnberk stated in 1901 that the posterior pituitary is a functionless rudimentary organ (Described in: Hackenberg and Etminan 2003). In 1908, Herring (Herring 1908) alluded to the presence of nerve fibres and neuroglia in the posterior pituitary, and described what would later be referred to as Herring bodies. A year later, Blair-Bell described the effects of pituitary extracts on atonic uteri during labour (Bell 1909); this work was in line with that of others, suggesting the physiological effects of pituitary extracts (Von den Velden 1913). However, the concept of neurosecretion in the neurohypophysis was first suggested in 1917, by Speidel (Scharrer 1987). This idea was carried forward by the seminal work of Ernst Scharrer in 1928 who described the histology of the European Minnow nucleus magnocellularis preopticus and revealed vacuoles/vesicles in nerve-gland cells in the diencephalon that may secrete hormones into the neurohypophysis (Scharrer 1928). In 1940, Ernst and Berta Scharrer published their neurosecretion concept of the hypothalamo–neurohypophyseal system (HNS), which was shown to be conserved across multiple vertebrate species (Scharrer and Scharrer 1940). The functionality of the HNS as a pathway for the secretion of neurohormones was finally accepted unanimously in 1949 after neurosecretory material in the hypothalamic neurons and the neurohypophysis were shown to be one and the same (Bargmann and Hild 1949).
The pars nervosa of the posterior pituitary is a conserved structure across all vertebrates, including over 34,000 piscine species. The structure and morphology of the HNS vary from the most primitive fishes, Cyclostomes, through to the higher vertebrates, including humans, however, the basic components of the system seem to remain constant.
Primitive fish, or the Elasmobranchs, lack a clear demarcation between the magnocellular (i.e. larger cells) and parvocellular cells (i.e. smaller cells) of the preoptic nuclei. Within the same class, we see larger cells, indistinguishable from each other, in the subclass of Holocephali. In more evolved fishes, the presence of two distinct populations of cells, the parvocellular and the magnocellular neurons, is noted from pre-teleosts through to the advanced teleosts (Wircer et al. 2016; Perks 1969).
As in pre-teleost bony fish, primitive teleosts such as the European eel (Anguilla anguilla) present a neurohypophyseal structure that is a thickened extension of the infundibular stalk. In advanced teleosts, this structure is better represented as a pituitary core, surrounded by adenohypophyseal tissue. Axonal innervations pass through the hypophyseal tract into the pars nervosa, characterized by bead-like droplets along their length, carrying neurosecretory material. Multiple studies have shown the presence of granules or elementary vesicles in these swellings, containing neurohormones (Holmes and Knowles 1960; Navone et al. 1989; Anbalagan et al. 2019). While some researchers reported that the sizes of these elementary vesicles were similar to those found in the preoptic nucleus, other works demonstrated that two distinct elementary vesicles containing two different peptides may be present in the pars nervosa (Knowles et al. 1966; Leatherland and Dodd 1967).
Within the pars nervosa, the axonal termini are distributed amongst a dense capillary network that conveys neurosecretory material into the systemic circulation (Anbalagan et al. 2019). Within the purview of vascular structures of the pars nervosa, pre-teleostean fish, such as the longnose gar (Lepidosteus osseus), seem to possess a vascular portal system, while the teleostean structure lacks it (Sathyanesan and Chavin 1967).
The presence of neurohypophyseal glia, the pituicytes, is consistent across teleostean species. A striking result regarding these cells was observed in a few unrelated teleosts, European eel, European conger (Conger conger) and goldfish (Carrassius auratus) (Knowles and Vollrath 1966; Leatherland 1972). These studies showed that axonal bundles carrying neurosecretory material terminated not only around the dense capillary plexus, but also on the surface of the pituicytes (Knowles and Vollrath 1965). In primitive fish like the Cyclostomes, the pituicytes were described as being derived from the ependymal cells of the ventricles which proliferated into the pars nervosa (Green and Maxwell 1959). These ependymal cells were also found to be present in the Elasmobranchs, the spiny dogfish (Squalus acanthias), and were then described as “parenchymatous pituicytes” (Van de Kamer and Verhagen 1955).
2.2 Development of the Neurohypophysis
The neurohypophysis is formed as an invagination of the diencephalon floor, deepening to form the infundibular cavity. In zebrafish (Danio rerio), precursor cell clusters on both sides of the diencephalon merge to form a pituitary cluster at about 28 h post-fertilization (Glasgow et al. 1997; Chapman et al. 2005). Within 36 h post-fertilization, cell bodies from the NPO generate axonal convergence along the midline at the developing neurohypophysis (Gutnick et al. 2011). Structural analysis of the HNS in the adult European eel shows that axonal fibres projecting into the neurohypophysis are separated into bundles by the radial pituicytes (Knowles and Vollrath 1965). This suggests the possibility that the pituicytes reside in the pars nervosa during, and perhaps prior to axonal enervation.
Over the next 36 h, i.e. 72 h post-fertilization, the embryonic neurohypophysis undergoes vascularization, probably from angiogenic cues released by the axonal termini and astroglia in the region (Gutnick et al. 2011). The hypophyseal artery and vein grow into the developing region, conceivably from existing cerebral vasculature. Thus, endothelial vessels in the ventral diencephalon sprout towards the palatocerebral artery from 48 h post-fertilization, giving rise to the hypophyseal artery. At the same time, the primary head sinus sprouts bilaterally towards the midline giving rise to the hypophyseal vein. By 72 h post-fertilization, these vascular branches fuse to create a loop-like structure dubbed the hypophyseal capillary (Gutnick et al. 2011). Thereafter, the hypophyseal capillary makes tight connections with the axonal termini, and along with the resident pituicytes, forms the basis of a functional neurovascular interface of the neurohypophysis (Fig. 4.2). As the animal develops further, the density of the axonal projections increases along with the complexity of the vasculature, forming an anterior and posterior capillary plexus with dense innervation of nerve fibres, and numerous pituicytes (Anbalagan et al. 2018; Gordon et al. 2019).
Neurohypophysis in juvenile zebrafish. A confocal microscope image of a transgenic juvenile zebrafish (30-day old) in which both the hypothalamo–neurohypophyseal oxytocin neurons and blood vessels are genetically tagged with fluorescent proteins. The image shows the hypophyseal capillary plexus (in red) which is innervated by hypothalamic axonal projections (in grey) forming multiple neuro-vascular interfaces through which the oxytocin neurohormone is released into the peripheral blood circulation. Oxtl oxytocin-like, kdrl vascular endothelial growth factor receptor kdr-like
The adult neurohypophyseal structure bears clear differences in the structure of the pars nervosa (as outlined in the previous section) between the different classes of fish as well as in the interaction with intermediate lobes and the adenohypophysis parts of the pituitary. Non-teleostean fish, from the Elasmobranchs up to the Holosteans, show the presence of neuronal projections from the pars nervosa projecting into the intermediate lobe of the pituitary. Remarkably, in teleosts the neural tissue invaginates into parts of the intermedia, extending all the way into the adenohypophysis (Perks 1969).
2.3 Neurohypophyseal Function
As in other vertebrates, the Piscine neurohypophyseal system is the primary region of secretion of two major homeostatic hormones, OXTL and AVPL. Together, these two neuropeptides regulate the homeostatic responses to various internal and external physiological challenges, ranging from water balance to social behaviour.
2.3.1 Osmoregulation
In mammals, AVP was first identified as an antidiuretic hormone, maintaining water balance in the organism by affecting water reabsorption rates from the kidney (Baratz and Ingraham 1959). AVP in the mammalian kidneys acts on AVP-V2 receptor to increase the expression of aquaporins in the membrane of nephron tubule cells, thereby increasing water reuptake rates. In teleost species, the V2 receptor was shown to be expressed in the nephros and the gills. For example, freshwater eels exposed to salt water had a marked increase in plasma AVPL levels. This result was also replicated when the freshwater eels were injected with saline solution intraperitoneally (Warne and Balment 1995).
Interestingly, the mRNA of avplv1 receptor was found to be expressed in the gills of the freshwater eel and the density of its expression was found to change depending on the osmolarity of the environment, salt water inducing increased expression of the receptor compared to freshwater (Balment et al. 2006). This suggests that osmoregulation in teleosts is mediated by coordination between neurohypophyseal AVPL and expression of its receptors in the gills.
These effects, while predominantly studied in the context of AVPL, were also observed in the case of OXTL. Studies in banded houndshark (Triakis scyllium) identified an increase in plasma OXTL after exposure of freshwater fish to salt water (Hyodo et al. 2004). OXTL has also been implicated in regulating ionocyte differentiation in zebrafish, thus affecting ion exchange to maintain optimal internal salt balance (Chou et al. 2011).
In the elasmobranch dogfish (Scyliorhinus canicular), perfusion of AVPL into in situ preparations of the kidney showed a marked antidiuretic effect. This study also implicated the addition of AVPL in decreased glomerular filtration rates, a possible mechanism by which the dogfish acclimatizes to reduced salinity (Wells et al. 2002).
2.3.2 Reproduction
OXT has been widely studied in mammalian models in the context of pregnancy, childbirth and lactation (Russell et al. 2003). In mammals, oxytocin receptors are widely found in both female and male reproductive tissues, affecting uterine contractions during labour, menstrual cycles and ovulation, milk ejection reflex during lactation, sperm shedding from the testis and ejaculation (Burbach et al. 2006). In piscine species, however, OXTL has been implicated to play a major role in courting behaviour, egg-laying and sexual health of teleosts (Altmieme et al. 2019; Piccinno et al. 2014; Viveiros et al. 2003).
Male zebrafish subjected to female pheromones demonstrated increased courtship behaviour, which was inhibited following administration of OXTL and AVPL antagonists; it was, therefore, suggested that triggering the release of these neurohormones stimulates the central behavioural pathways, thereby increasing the possibility of reproductive success (Altmieme et al. 2019).
The two neurohypophyseal peptides also play a key role in the release of oocytes from fish females through their action on the smooth muscle cells of the ovarian wall. Ovarian wall contractility of gilthead seabream (Sparus aurata L.) was shown to be induced by in vitro administration of OXTL in vitellogenic non-spawning females (Piccinno et al. 2014). Similarly, when exposed to OXTL in vitro, testes slices of the African catfish (Clarias gariepinus) increased semen release to the media (Viveiros et al. 2003). These data demonstrate the involvement of OXTL and AVPL peptides in the regulation of reproductive functions at both central and gonadal levels in both mammals and fishes.
2.3.3 Behaviour
AVPL and OXTL exert major effects on mammalian behaviour, specifically in the context of social behaviour and dysfunction. In non-human mammalian models, these peptides allowed for better social recognition (Ross et al. 2009; Veenema et al. 2012), maternal behaviour (Bosch and Neumann 2012), and conversely played a role in cognitive impairments (Abramova et al. 2020).
Some of the mammalian phenotypes are recapitulated in fish shoaling and mating behaviours. In the case of the goldfish, these two peptides were shown to act in a conflicting fashion. While OXTL increased the tendency to social approaching, AVPL inhibited it (Thompson and Walton 2004). Additional complexity is added by the demonstration that HNS hormones also alter sex-specific social tendencies, as seen in the case of Porichthys notatus where the acoustic behavioural responses decreased in males and females only on exposure to AVPL and OXTL, respectively (Goodson and Bass 2000a, 2000b). Within the purview of hierarchical behaviour, exposing shoaling Neolamprologus pulcher cichlids to OXTL increased their awareness towards the dominant individuals (Reddon et al. 2012; Balshine et al. 2014).
Zebrafish are a social species in that they display collective behaviour in the formation of small, loose groups, known as shoals (Robinson et al. 2019; Miller and Gerlai 2012; Suriyampola et al. 2016). The absence of OXTL-mediated signalling was shown to reduce their shoaling tendencies, the converse of which was true when they were exposed to the peptide (Landin et al. 2020). Zebrafish OXTL receptor regulates memory recognition of familiar vs novel conspecifics (Ribeiro et al. 2020a, 2020b). The zebrafish receptor is also involved in the perception of biological motion, but not conspecific shape—two specific visual features that zebrafish use to appraise and react to social cues (Nunes et al. 2020).
It can be proposed that the effect of OXTL on fish behaviour is context dependent, as put forward in Ramsey’s analysis of the social salience hypothesis that oxytocin expression allows improved cognitive processing in social contexts (Ramsey et al. 2019). The same can also be said in the case of AVPL expression in teleosts. Much like OXTL, the effects of AVPL on their behaviour seem to be context dependent. For example, intraperitoneal injection of AVPL into bluehead wrasse (Thalassoma bifasciatum) was shown to decrease aggression in territorial males while increasing it in non-territorial males (Semsar et al. 2001). Cohesively, the administration of Manning compound (AVPL receptor antagonist) was seen to inhibit these behavioural effects.
A key feature that should be noted is the differential effect of centrally and peripherally released hormones. In white perch (Morone Americana), intracerebroventricular administration of AVPL peptide showed strong activation of circuits involved in mating behaviour while circulating intraperitoneal AVPL injection had negligible effects on this behaviour (Salek et al. 2002).
Finally, although the classical effects of hormones, including OXT, is to activate or facilitate specific behavioural responses in an acute manner, OXT can have organizational effects on the developing social brain as well. Thus, pharmacological treatment of neonatal rats with OXT had long-term effects on behaviour in the adult (Noonan et al. 1989). Recently, it has been shown that OXTL can shape the structure of the developing forebrain as well as the functional connectivity of the so called social decision making network (SDMN) in zebrafish (Nunes et al. 2021). Thus, perturbation of zebrafish OXTL neurons during early but not late development disrupts the behavioural display of social drive in the adult, affecting the neurodevelopment of specific dopaminergic clusters associated with visual processing and reward (Nunes et al. 2021). Taken together, these data suggest that OXTL in fish regulates complex social behaviours including the ability to assimilate and process more social cues and information.
2.4 Neurovascular Interface
The teleost HNS neuronal populations are mostly investigated for their roles in the central regulation of homeostatic processes. However, the role of HNS vasculature and non-neuronal cells in this regulation is less clear. Importantly, understanding the mechanism through which the HNS exerts its systemic influence requires us to elaborate on a crucial topic—the neurovascular interface.
As described earlier, the axonal fibres in the neurohypophyseal tissue form direct contact with the capillary plexus. This capillary plexus in adult zebrafish arises from a simple loop-like structure of the embryonic HNS (Gutnick et al. 2011). While this capillary is an extension of the cerebral vasculature, it lacks a blood–brain barrier and instead, the vasculature of this region is highly fenestrated, i.e. permeable, allowing the exchange of blood-borne proteins and hormones between the brain and the peripheral circulation (Gordon et al. 2019; Anbalagan et al. 2018).
Functionally, the presence of fenestrated capillaries in the neurohypophysis is of great significance as it allows for the HNS to respond to peripheral stimuli and allows the direct release of neurohormones into the blood circulation. This versatile structure is maintained by factors released by the resident pituicytes. We have recently shown that several angioactive ligands released by the pituicytes inhibit the formation of tight junctions between the vascular endothelia while maintaining the endothelial cell fenestrations (Anbalagan et al. 2018). Electron microscopy images of this region in adult zebrafish demonstrated the presence of neurosecretory termini seated near the basement membrane of the vasculature with pituicyte processes ensheathing them (Anbalagan et al. 2018, 2019).
3 Urophysis
Box 4.2 The urophysis—an underexplored neuroendocrine interface
The first indication of a caudal secretory system came from Weber in 1827 (Weber 1826) when dissection of the carp spinal cord indicated a caudal structure at the termini. This was further investigated almost a century later when Dahlgren described large cells along the spine of skates, which secreted granules into the blood (Dahlgren 1914). In 1925, Favaro (1925) showed the morphological similarities between a caudal bulge of teleosts and that of the neurohypophysis. Enami and Imai (1955) showed the conserved anatomical organization of this structure across fish species. By this point, it had become evident that this caudal neurosecretory system existed only in fishes, and it was suggested that it could serve as a neuroendocrine interface. A seminal study concerning the caudal neurosecretory system (CNSS) was the 1959 description of Dahlgren cells and their axonal projections into the vasculature of the urophysis (Enami 1959). The presence of neurosecretory products released in the urophysis was elucidated in 1969 where urophyseal extracts were shown to be functionally significant in blood pressure (Bern and Lederis 1969) and later in maintaining osmolality (Loretz and Bern 1981). By the 1990s (Conlon et al. 1996), urotensin II had been identified in species that lacked a caudal neurosecretory system, indicating a conserved role for the ancient hormone.
Fishes are unique amongst vertebrates, due to the presence of an additional neurosecretory organ associated with the spinal cord at its caudal end (Fig. 4.1). Importantly, the posterior pituitary interface bears a strong resemblance to the previously described caudal neurosecretory system in fish (Kriebel 1980). As demonstrated in the Pomolobus aestivalis, the urophysis consists of an axonal-vascular entanglement termed the neurohaemal zone/urophysis, which is roughly comparable to the hypophyseal neurovascular interface. Axonal fibres terminating in the urophysis are surrounded by fenestrated capillaries, with a predominant perivascular space. The ultrastructure of this region also shows the presence of neurosecretory granules contained in axonal swellings, dubbed Herring bodies, similar to what is observed in the pars nervosa (Kriebel et al. 1979). In 1914, Dahlgren identified giant neurosecretory cells located at the distal end of the spinal cord of skates (Rajidae) (Dahlgren 1914) and in 1927 Weber found these localized swellings in the posterior end of the spinal cord (Weber 1927). This was later shown to generate a neurovascular structure, which was designated urophysis/urohaemal-organ, and the giant neural secretory cells were later termed Dahlgren cells (Enami 1959). Dahlgren cells are large magnocellular neurons projecting into the urophysis through thick non-myelinated axon endings. The axon endings are rich in secretory granules and have an intimate relationship with endothelial cells for the transfer of neurosecretory products (Holmgren 1964). The structural anatomical assembly of Dahlgren cells with the urophysis is referred to as the CNSS. The simplest organized form of the urophysis was commonly found in elasmobranchs (Chondrichthyes) whereas the highest organized form was found to be present in all bony fishes (Osteichthyes). Teleosts develop a discrete CNSS which shows a structural analogy to the cranial HNS (Bern 1985; Winter et al. 2000). The piscine CNSS is located at the distal end of the spinal cord, and in teleosts it spans the last three vertebrae (Holmgren 1964). The urophysis resides at the end of the spinal cord, posterior to the last spinal nerve (Fig. 4.1). In some species, the urophysis is innervated by means of a stalk through which the nerves and ependymal fibres enter, while in other species innervation is more diffused (Bern and Takasugi 1962). The urophysial outpouching structure is covered by meninges that arise from the end of the spinal cord and was shown to be populated by glial cells. Furthermore, ependymal and glial fibres from the spinal cord and vasculature generate an anatomical network (Amin et al. 1992; Fridberg 1962).
3.1 Evolutionary Aspects of Urophysis
Understanding the evolutionary aspects of Dahlgren cells and urophysis can give additional insights regarding piscine evolution (Fig. 4.3). More evolved fish display elongation or extension of Dahlgren cell terminals innervating a distinct neurohaemal organ which reflects a well-developed urophysis. Elasmobranchs are known as primitive ancestral fish, and exhibit more dispersed Dahlgren cells with shorter axons and a less anatomically discrete urophysis (Fridberg and Bern 1968; Qureshi et al. 1978). Accordingly, the neurosecretory Dahlgren-like cells of less evolved fish are widely distributed and form a diffuse neurohaemal zone. For example, in the neurohaemal zone of elasmobranchs small terminals of the Dahlgren cells are connected to the ventral part of the spinal cord and directly contact the capillary bed (Bern and Hagadorn 1959). Traces of such arrangement of Dahlgren cells were also noticed in some cyprinids, where the processes of Dahlgren cells terminate at the ventral part of the spinal cord and come close to the meningeal sheath to contact the blood vessels (Fridberg 1962). The anatomical isolation of Dahlgren cell terminals from the spinal cord occurs in the course of evolution. It was proposed that the isolation of Dahlgren cell terminals occurs in three stages: (i) In elasmobranchs and the early developmental stage of CNSS in some teleosts the terminals of Dahlgren cells are present within the spinal cord. (ii) In elasmobranchs as well as in the intermediate developmental stage of CNSS in teleosts the nerve terminals penetrate the meningeal sheath. (iii) In more evolved teleost species, Dahlgren cell terminals move out from the spinal cord to penetrate the urophysis and terminate at the capillary bed, resulting in a lobular structure of the urophysis (Fig. 4.3) (Saenko 1978).
Evolution of the piscine urophysis. Schematic representation of the urophyseal structure in fish showing the evolution of the caudal neurosecretory system from primitive fish (Elasmobranch) to the teleosts. Dahlgren cells evolve through the piscine phylogenetic tree to send projections from the caudal spinal cord into the neurohaemal interface of the urophysis
An evolutionarily related change in Dahlgren cell morphology was suggested for the evolution of teleosts from primitive fish (Speidel 1922). In early evolved piscines, the cells are small and similar to other nerve cells, without any morphological resemblance to Dahlgren cells. The second group of fish species, which are more evolved, possess small to moderate-sized cells, with limited resemblance to Dahlgren cells. The third and most evolved species show large-sized Dahlgren cells with modified morphology and are commonly seen in most teleost species (Speidel 1922). Notably, in the early developmental stages of teleosts, moderately sized cells are present which later develop into large-sized Dahlgren cells in the CNSS of mature fish (Cioni et al. 2000). This developmental differentiation of Dahlgren cells from neuronal populations of teleost embryos further supports an evolutionary speciation process. In this view, small cells of the spinal cord initially served as specialized nerve cells in primitive piscines and later evolved into large glandular cells in teleosts.
3.2 Ontogenesis of CNSS
Embryonic development of Dahlgren cells and the urophysis was studied through various immunoreactive, histological and microscopy studies. Histological studies demonstrated that morphogenesis of the urophysis is initiated in the early larval stages, however, its mature organ form is finalized only after several months from hatching (Cioni et al. 2000; Fridberg 1962; Imai 1965; Sano and Kawamoto 1959). In chum salmon (Oncorrhynchus keta), immunohistochemistry of Urotensin1 (UI) and Urotensin2 (UII) localized in immature Dahlgren cells (i.e. appearing as agranular ovoidal cells) and fibres near the caudal region of the neural tube of forty-day-old embryos before hatching. Two weeks from hatching, the UI- and UII-positive cells and fibres increase in number, however, pronounced capillary formation is only detected in 3-month-old larvae and the maturation of the CNSS is finalized 5 months after hatching (Oka et al. 1993). It is interesting to note that although the HNS develops earlier than the CNSS, synthesis of UI and UII is identified in the embryonal CNSS before its appearance in the HNS (Oka et al. 1993). It was suggested that the urophysis differentiates from the meningeal tissue of the spinal cord and that Dahlgren cells originate from embryonic neuroectodermal cells, which differentiate first at the anterior region, gain secretory properties and migrate to the caudal region (Fridberg 1962; Fridberg and Bern 1968). A later study in chum salmon demonstrated that Dahlgren cells originate from neuroblasts and differentiate in the lateral plate of the caudal neural tube (Oka et al. 1993).
In Nile tilapia (Oreochromis niloticus), UI and UII immunoreactive perikarya and fibres were identified for the first time only in four days post-hatching larvae. At this stage, two bundles of neurosecretory fibres were observed at the future site of the urophysis. The initial differentiation of the tilapia urophysis is observed near the caudal region at 24 days post-hatching. The budding urophysis comprises a ventral swelling of the spinal cord in association with protruding dilated vessels. Further development occurs through increasing the number of neurosecretory terminals and branching of blood vessels. Meanwhile, neurosecretory cells rise in number and start to differentiate morphologically. The mature or fully formed urophysis is observed in four-month-old juveniles (Cioni et al. 2000). Obviously, additional work is needed with transgenic marker lines that will help to clearly uncover the embryonal origins of the CNSS. Nonetheless, it seems that functional speciation of Dahlgren cells begins at the initiation of hatching and free swimming and requires several months to reach the mature CNSS organ formation.
3.3 Physiology
The CNSS serves as the main neuroendocrine site for the synthesis of several neuropeptides with key importance in the homeostatic regulation of physiological functions. Nonetheless, although it has been recognized for more than a century, an exclusive critical role of the CNSS in physiological homoeostasis has yet to be elucidated. The CNSS is known as the major site for synthesis and release of urotensins (Ichikawa et al. 1982; Pearson et al. 1980). These neuropeptides show close similarities with other cortistatin and somatostatin peptides expressed by the central nervous system and other tissues of higher vertebrates, from reptiles and birds to mammals and humans (Lu et al. 2008; Vaudry et al. 2010). From an evolutionary perspective, this signifies the functional importance of these urotensins. The CNSS also produces and secretes additional neuropeptides such as corticotrophin-releasing factor (CRF), parathyroid hormone-related protein (PTHrP), OXTL and AVPL (Gozdowska et al. 2013; Ingleton et al. 2002; Lederis et al. 1982). Little is known regarding the functional and physiological importance of their secretion from the CNSS, however, they were found to be involved mainly in osmoregulation, reproduction and blood circulation.
3.3.1 Osmoregulation
UI and UII exert a direct effect on ion transport through epithelial cells in the kidney, which support their involvement in osmoregulation (Loretz et al. 1983; Marshall and Bern 1979; Ashton 2006). The importance of CNSS as an osmoregulatory structure is supported by: (i) the urophysis displays structural modifications with respect to the osmotic stress, (ii) urophysectomy affects the osmotic balance and (iii) urotensins secretion from the urophysis result in altered renal function of fish (Berlind 1973; Chan 1975). Several studies demonstrated that the urophysis undergoes structural and secretory modifications in response to altered salinity. Bonefish (Albula vulpus) raised in ponds with fluctuating salinity display increased intracellular cytoplasmic invagination and a higher level of secretory product was measured in their urophysis than in bonefish collected from open sea (Fridberg et al. 1966a). Cytological variations and altered urophyseal secretion were also detected in euryhaline brook trout (Salvelinus fontinalis) exposed to variable ion concentrations. Brook trout raised in a freshwater environment have an irregular shape of nucleus, elongated endoplasmic reticulum and Golgi bodies with reduced secretory granules. When maintained for a few days in deionized water, the cell organelles were shown to be highly developed, with increased numbers of secretory granules. Nonetheless, prolonged exposure to deionized water does not lead to increased neurosecretory activity, including changes in secretory granules. When exposed to 25% sea water for 24 h, brook trout exhibited increased secretory activity in the cells while prolonged exposure to increased salinity reduced the secretory activity in the urophysis (Chevalier 1976). These findings support the involvement of the CNSS in the homeostatic regulation of osmotic stress, mainly in response to acute environmental fluctuations. The Mozambique tilapia (Oreochromis mossambicus) is a hardy euryhaline fish that can grow in variable salinities from freshwater to sea water (Chourasia et al. 2018). Freshwater-adapted tilapia that were urophysectomized and exposed to brackish water displayed significantly increased Na+, K+ and Ca++ in their blood than sham-operated controls. Contrastingly, sea water-adapted urophysectomized fish exhibited decreased Na+ and K+ in the bloodstream compared to sham-operated control fish. These results indicate that the urophysis has a role in maintaining osmotic balance in the fish (Baldisserotto et al. 1994). Similar results were obtained in urophysectomized Mozambique tilapia exposed to water containing 1.7% NaCl (Takasugi and Bern 1962). However, as treated fish exhibited increased mortality and weight loss with high level of serum chloride that was not identified in the later experiment, it was suggested that the lack of calcium in NaCl salinated water increased the osmotic stress (Baldisserotto et al. 1994; Takasugi and Bern 1962). Molecular analysis of urotensin expression in the euryhaline flounder (Platichthys flesus) suggested that UII is highly important for water and electrolyte homoeostasis and has an active role in preventing dehydration and salt deposition in high salinity conditions such as haemodilution in freshwater conditions (Lu et al. 2006). Urotensins were found to affect ion absorption in the urinary bladder of fish. Urinary bladders of longjaw mudsuckers (Gillichthys mirabilis) were exposed in vitro to physiological doses of UII, which directly altered ion transport in surface epithelia, a known component of osmoregulation. Moreover, India ink injection into the caudal vein demonstrated a direct but separate connectivity of the urophysis to the kidney and urinary bladder, further supporting a direct effect UII on the urinary bladder (Loretz and Bern 1981). It was also demonstrated by similar means that UII stimulates the absorption of Na+ and Cl− ions in the posterior intestine in 5% sea water-adapted longjaw mudsuckers (Loretz et al. 1983). These studies suggest that the CNSS directly modulates the main tissues known to be involved in water and electrolyte homoeostasis in fish both under baseline and osmotic stress conditions.
3.3.2 Reproduction
Analysis of urophysis protein extracts and molecular gene expression analysis of piscine CNSS during reproductive cycle and spawning period has demonstrated a role for the CNSS in fish reproduction. UII was found to be increased in the blood of white suckers (Catostomus commersoni) three months prior to the spawning period and it declined by half during and after spawning (Lederis 1973). Analysis of CNSS structure during the goldfish reproductive cycle demonstrated that the size of Dahlgren cells is altered with respect to ovarian development. Dahlgren cell size increases towards spawning initiation and decreases at the end of spawning season (Chen and Mu 2008). Importantly, while several studies demonstrated that urophysial extracts can modulate the contraction of ovary, oviduct and sperm ducts in some bony fishes (Berlind 1972; Lederis 1970), only one report demonstrated the direct effect of UII on ovarian smooth muscle contraction (Leonard et al. 1993). Urophysial extracts were found to be inefficient for spawning induction in several teleost species, further supporting their role in gonadal contraction and not as gonadal maturation factors (Behr et al. 2000). Gonad-localized and follicular-stage dependent UI levels were identified in the ovary of olive flounders (Paralichthys olivaceus), supporting the involvement of urotensins in piscine ovarian development (Zhou et al. 2019), however, the possible connection and interaction between gonadal and CNSS urotensins remains to be determined.
3.3.3 Other Physiological Roles
Urotensins were reported to play a role in the stress regulation and muscle contraction of fishes. Dahlgren cell structure and its peptide secretion varied with temperatures. The firing frequency of Dahlgren cells was shown to increase with temperature, suggesting the role of the urophysis in thermoregulation. The response to thermal stress was suggested to be mediated through the transient receptor potential cation channel family (TRPs) (Yuan et al. 2020b). CNSS expression of UI, UII and corticotropin-releasing hormone (CRH) as well as plasma cortisol, CRH but not UII were shown to increase in olive flounders on exposure to acute hypothermal stress, returning to baseline levels following 8 days of adaptation (Yuan et al. 2020a). Chronic but not acute hyperthermal challenge led to increased expression of CNSS CRH and UI but not UII (Yuan et al. 2020a). In addition, the urophysis was suggested to be involved in the regulation of blood circulation, vascular smooth muscle contraction and the digestive system of fish (Fridberg 1962; Lederis 1977). Overall, current literature suggest pleiotropic functions of CNSS, which is not surprising considering the expression of multiple neurohormones in this structure. Further research regarding urophysial functions in homeostatic fish physiology is needed.
4 Conclusions and Outlook
The importance of the HNS and its related neurohormones in the regulation of homoeostatic and physiological functions is obvious given its structural and functional evolutionary conservation. Nonetheless, the existence of the CNSS in fishes as well as its evolvement in piscine species support an unidentified but highly important urophysiological role(s).
Some of the failures in underpinning major CNSS functions may be explained by the 2–3 weeks required for full regeneration of this system following complete removal of all CNSS neurohemal components (Fridberg et al. 1966b). Paradoxically, this very rapid regeneration further supports the high importance of the CNSS in fish physiology. Importantly, new and relevant pharmacological and genetic tools have been developed for the urotensin system (Lescot et al. 2008; Zhang et al. 2018) and some were also developed for non-neuronal components of the HNS (Anbalagan et al. 2018; Gordon et al. 2019). These tools may prove valid for studying both neural and non-neural components of the CNSS aiming to identify specific physiological functions of this system.
While the HNS is fully functional during early embryonal stages, CNSS components begin to differentiate at later developmental stages and its structural establishment occurs only several months later. This suggests that the CNSS functions are of importance to adult fish physiology and possibly connected to sexual maturation. As described above, euryhaline fish exhibit more developed CNSS anatomy, which further supports this concept. Nonetheless, the ability of urophysial extracts and hormones to modulate water and electrolyte homoeostasis, as well as the CNSS anatomy, has led to an inherent bias as most fish species used to study this system were euryhaline, making at least some of the findings questionable with regard to stenohaline piscines.
Finally, much effort has been invested in recent years in understanding the regulatory mechanisms of HNS neuropeptide secretion. However, the anatomical location and complex connectivity of the HNS with additional brain centres hinder these efforts. The close morphological, cellular and structural similarities between HNS and CNSS and the ability to analyze CNSS ex vivo make the CNSS a potentially unique model for the study of neurohormone secretion.
References
Abramova O, Zorkina Y, Ushakova V, Zubkov E, Morozova A, Chekhonin V (2020) The role of oxytocin and vasopressin dysfunction in cognitive impairment and mental disorders. Neuropeptides 83:102079
Altmieme Z, Jubouri M, Touma K, Coté G, Fonseca M, Julian T et al (2019) A reproductive role for the nonapeptides vasotocin and isotocin in male zebrafish (Danio rerio). Comp Biochem Physiol B Biochem Mol Biol 238:110333. https://doi.org/10.1016/j.cbpb.2019.110333
Amin AB, Mortensen L, Poppe TT (1992) Histology atlas: normal structure of salmonids. Bodo: Akvapatologisk Laboratorium, Postboks 773
Anbalagan S, Gordon L, Blechman J, Matsuoka RL, Rajamannar P, Wircer E et al (2018) Pituicyte cues regulate the development of permeable neuro-vascular interfaces. Dev Cell 47(6):711–726.e5. https://doi.org/10.1016/j.devcel.2018.10.017
Anbalagan S, Blechman J, Gliksberg M, Gordon L, Rotkopf R, Dadosh T, Shimoni E, Levkowitz G (2019) Robo2 regulates synaptic oxytocin content by affecting actin dynamics. elife 8:e45650. https://doi.org/10.7554/eLife.45650
Ashton N (2006) Renal and vascular actions of urotensin II. Kidney Int 70(4):624–629
Baldisserotto B, Mimura OM, Salomão LC (1994) Urophyseal control of plasma ionic concentration in Oreochromis mossambicus (Pisces) exposed to osmotic stress. Ciência e Natura, 12. https://doi.org/10.5902/2179460x26385
Balment RJ, Lu W, Weybourne E, Warne JM (2006) Arginine vasotocin a key hormone in fish physiology and behaviour: a review with insights from mammalian models. Gen Comp Endocrinol 147(1):9–16. https://doi.org/10.1016/j.ygcen.2005.12.022
Balshine S, O’Connor CM, Reddon AR, Voisin MR (2014) Isotocin and sociality in the cooperatively breeding cichlid fish, Neolamprologus pulcher. Behaviour 151(10):1389–1411. https://doi.org/10.1163/1568539X-00003190
Baratz RA, Ingraham RC (1959) Sensitive bioassay method for measuring antidiuretic hormone in mammalian plasma. Proc Soc Exp Biol Med 100(2):296–299. https://doi.org/10.3181/00379727-100-24605
Bargmann W, Hild W (1949) Über die morphologie der neurosekretorischen verknüpfung von hypothalamus und neurohypophyse. Cells Tissues Organs 8(3):264–280
Behr ER, Baldisserotto B, Parra WG, Brandão DA, Herke Z (2000) Urophysial and pituitary extracts for spawning induction in teleosts. Ciência Rural 30:897–898
Bell WB (1909) The pituitary body and the therapeutic value of the infundibular extract in shock, uterine atony, and intestinal paresis. Br Med J 2(2553):1609–1613. https://doi.org/10.1136/bmj.2.2553.1609
Berlind A (1972) Teleost caudal neurosecretory system: sperm duct contraction induced by urophysial material. J Endocrinol 52(3):567–574. https://doi.org/10.1677/joe.0.0520567
Berlind A (1973) Caudal neurosecretory system: a physiologist’s view. Am Zool 13(3):759–770. https://doi.org/10.1093/icb/13.3.759
Bern HA (1985) The elusive urophysis—Twenty-five years in pursuit of caudal neurohormones. Am Zool 25(3):763–770. https://doi.org/10.1093/icb/25.3.763
Bern H, Hagadorn I (1959) A comment on the elasmobranch caudal neurosecretory system. In: Comparative endocrinology. Wiley, New York, pp 725–727
Bern H, Lederis K (1969) A reference preparation for the study of active substances in the caudal neurosecretory system of teleosts. J Endocrinol 45(1):Suppl: xi–xii
Bern HA, Takasugi N (1962) The caudal neurosecretory system of fishes. Gen Comp Endocrinol 2:96–110. https://doi.org/10.1016/0016-6480(62)90032-1
Biran J, Blechman J, Wircer E, Levkowitz G (2018) Development and function of the zebrafish neuroendocrine system. In: Ludwig M, Levkowitz G (eds) Model animals in neuroendocrinology: from worm to mouse to man. Wiley-Blackwell, New York, pp 101–131. https://doi.org/10.1002/9781119391128.ch5
Bosch OJ, Neumann ID (2012) Both oxytocin and vasopressin are mediators of maternal care and aggression in rodents: from central release to sites of action. Horm Behav 61(3):293–303
Burbach J, Young LJ, Russell J (2006) Oxytocin: synthesis, secretion, and reproductive functions. Knobil Neill’s Physiol Reprod 2:3055–3128
Chan DK (1975) Cardiovascular and renal effects of urotensins, I and II in the eel, Anguilla rostrata. Gen Comp Endocrinol 27(1):52–61. https://doi.org/10.1016/0016-6480(75)90052-0
Chapman SC, Sawitzke AL, Campbell DS, Schoenwolf GC (2005) A three-dimensional atlas of pituitary gland development in the zebrafish. J Comp Neurol 487(4):428–440. https://doi.org/10.1002/cne.20568
Chen H, Mu R (2008) Seasonal morphological and biochemical changes of Dahlgren cells implies a potential role of the caudal neurosecretory system (CNSS) in the reproduction cycle of teleostean fish. Fish Physiol Biochem 34(1):37–42. https://doi.org/10.1007/s10695-007-9143-8
Chen Q, Leshkowitz D, Blechman J, Levkowitz G (2020) Single-cell molecular and cellular architecture of the mouse neurohypophysis. eneuro 7(1):ENEURO.0345-19.2019. https://doi.org/10.1523/ENEURO.0345-19.2019
Chevalier G (1976) Ultrastructural changes in the caudal neurosecretory cells of the trout Salvelinus fontinalis in relation to external salinity. Gen Comp Endocrinol 29(4):441–454. https://doi.org/10.1016/0016-6480(76)90027-7
Chou M-Y, Hung J-C, Wu L-C, Hwang S-PL, Hwang P-P (2011) Isotocin controls ion regulation through regulating ionocyte progenitor differentiation and proliferation. Cell Mol Life Sci 68(16):2797–2809. https://doi.org/10.1007/s00018-010-0593-2
Chourasia TK, D’Cotta H, Baroiller JF, Slosman T, Cnaani A (2018) Effects of the acclimation to high salinity on intestinal ion and peptide transporters in two tilapia species that differ in their salinity tolerance. Comp Biochem Physiol A Mol Integr Physiol 218:16–23. https://doi.org/10.1016/j.cbpa.2018.01.004
Cioni C, Francia N, Greco A, De Vito L, Bordieri L, Crosetti D (2000) Development of the caudal neurosecretory system of the nile tilapia Oreochromis niloticus: an immunohistochemical and electron microscopic study. J Morphol 243(2):209–218. https://doi.org/10.1002/(sici)1097-4687(200002)243:2<209::Aid-jmor9>3.0.Co;2-j
Conlon JM, Yano K, Waugh D, Hazon N (1996) Distribution and molecular forms of urotensin II and its role in cardiovascular regulation in vertebrates. J Exp Zool A Ecol Genet Physiol 275(2–3):226–238
Dahlgren U (1914) The electric motor nerve centers in the skates (Rajidae). Science 40(1041):862–863. https://doi.org/10.1126/science.40.1041.862
Enami M (1959) The morphology and functional significance of the caudal neurosecretory system of fishes. Comp Endocr:697–724
Enami M, Imai K (1955) Studies in neurosecretion V. Caudal neurosecretory system in several freshwater teleosts. Endocrinol Jpn 2(2):107–116
Favaro G (1925) Contribution à l’étude morphologique de l’hypophyse caudale (renflement caudal de la moelle épinière) des téléostéens. Avec 3 Planches. Résumé de l’A. Archives Italiennes de Biologie 75(15):164–170
Fridberg G (1962) Studies on the caudal neurosecretory system in teleosts. Acta Zool 43(1):1–77. https://doi.org/10.1111/j.1463-6395.1962.tb00068.x
Fridberg G, Bern HA (1968) The urophysis and the caudal neurosecretory system of fishes. Biol Rev 43(2):175–199. https://doi.org/10.1111/j.1469-185X.1968.tb00958.x
Fridberg G, Bern HA, Nishioka RS (1966a) The caudal neurosecretory system of the isospondylous teleost, Albula vulpes, from different habitats. Gen Comp Endocrinol 6(2):195–212
Fridberg G, Nishioka RS, Bern HA, Fleming WR (1966b) Regeneration of the caudal neurosecretory system in the cichlid teleost Tilapia mossambica. J Exp Zool 162(3):311–335. https://doi.org/10.1002/jez.1401620308
Glasgow E, Karavanov AA, Dawid IB (1997) Neuronal and neuroendocrine expression of lim3, a LIM class homeobox gene, is altered in mutant zebrafish with axial signaling defects. Dev Biol 192(2):405–419. https://doi.org/10.1006/dbio.1997.8761
Goodson JL, Bass AH (2000a) Forebrain peptides modulate sexually polymorphic vocal circuitry. Nature 403(6771):769–772. https://doi.org/10.1038/35001581
Goodson JL, Bass AH (2000b) Vasotocin innervation and modulation of vocal-acoustic circuitry in the teleost Porichthys notatus. J Comp Neurol 422(3):363–379. https://doi.org/10.1002/1096-9861(20000703)422:3<363::aid-cne4>3.0.co;2-8
Gordon L, Blechman J, Shimoni E, Gur D, Anand-Apte B, Levkowitz G (2019) The fenestrae-associated protein Plvap regulates the rate of blood-borne protein passage into the hypophysis. Development 146(23). https://doi.org/10.1242/dev.177790
Gozdowska M, Ślebioda M, Kulczykowska E (2013) Neuropeptides isotocin and arginine vasotocin in urophysis of three fish species. Fish Physiol Biochem 39(4):863–869. https://doi.org/10.1007/s10695-012-9746-6
Green J, Maxwell D (1959) Comparative anatomy of the hypophysis and observations on the mechanism of neurosecretion. In: Comparative endocrinology. Wiley, New York, pp 368–392
Gutnick A, Blechman J, Kaslin J, Herwig L, Belting HG, Affolter M et al (2011) The hypothalamic neuropeptide oxytocin is required for formation of the neurovascular interface of the pituitary. Dev Cell 21(4):642–654. https://doi.org/10.1016/j.devcel.2011.09.004
Hackenberg KAM, Etminan N (2003) Chapter 8 Supraoptic and paraventricular nucleus (SON, PVN). In: Swaab DF (ed) Handbook of clinical neurology. Elsevier, London, pp 163–237. https://doi.org/10.1016/s0072-9752(03)80015-5
Herring PT (1908) The histological appearances of the mammalian pituitary body. Quart J Exp Physiol 1(2):121–159. https://doi.org/10.1113/expphysiol.1908.sp000007
Holmes RL, Knowles FGW (1960) ‘Synaptic vesicles’ in the neurohypophysis. Nature 185(4714):710–711
Holmgren U (1964) Neurosecretion in teleost fishes: the caudal neurosecretory system. Am Zool 4:37–45. https://doi.org/10.1093/icb/4.1.37
Hyodo S, Tsukada T, Takei Y (2004) Neurohypophysial hormones of dogfish, Triakis scyllium: structures and salinity-dependent secretion. Gen Comp Endocrinol 138(2):97–104. https://doi.org/10.1016/j.ygcen.2004.05.009
Ichikawa T, McMaster D, Lederis K, Kobayashi H (1982) Isolation and amino acid sequence of urotensin I, a vasoactive and ACTH-releasing neuropeptide, from the carp (Cyprinus carpio) urophysis. Peptides 3(5):859–867. https://doi.org/10.1016/0196-9781(82)90028-6
Imai K (1965) Development of the caudal and hypothalamic neurosecretory systems of the eel, Anguilla japonica. Embryologia (Nagoya) 9(1):66–77. https://doi.org/10.1111/j.1440-169x.1965.tb00216.x
Ingleton PM, Bendell LA, Flanagan JA, Teitsma C, Balment RJ (2002) Calcium-sensing receptors and parathyroid hormone-related protein in the caudal neurosecretory system of the flounder (Platichthys flesus). J Anat 200(5):487–497. https://doi.org/10.1046/j.1469-7580.2002.00036.x
Knowles F, Vollrath L (1965) Synaptic contacts between neurosecretory fibres and pituicytes in the pituitary of the eel. Nature 206(989):1168–1169. https://doi.org/10.1038/2061168a0
Knowles F, Vollrath L (1966) The structure and innervation of the pars distalis at different stages of the life-cycle. Phil Trans R Soc Lond Ser B Biol Sci 250(768):329–342
Knowles F, Vollrath L, Zuckerman S (1966) Neurosecretory innervation of the pituitary of the eels Anguilla and Conger I. The structure and ultrastructure of the neuro-intermediate lobe under normal and experimental conditions. Phil Trans R Soc Lond Ser B Biol Sci 250(768):311–327. https://doi.org/10.1098/rstb.1966.0005
Kriebel RM (1980) The caudal neurosecretory system of Poecilia sphenops (Poeciliidae). J Morphol 165(2):157–165. https://doi.org/10.1002/jmor.1051650204
Kriebel RM, Burke JD, Meetz GD (1979) Morphologic features of the caudal neurosecretory system in the blueback herring, Pomolobus aestivalis. Anatom Record 195(3):553–571. https://doi.org/10.1002/ar.1091950314
Landin J, Hovey D, Xu B, Lagman D, Zettergren A, Larhammar D et al (2020) Oxytocin receptors regulate social preference in zebrafish. Sci Rep 10(1):5435. https://doi.org/10.1038/s41598-020-61073-4
Leatherland JF (1972) Histophysiology and innervation of the pituitary gland of the goldfish, Carassius auratus L.: a light and electron microscope investigation. Can J Zool 50(6):835–844. https://doi.org/10.1139/z72-113
Leatherland JF, Dodd JM (1967) Types of secretory neurones in the pre-optic nucleus of the European eel, Anguilla anguilla L. Nature 216(5115):586–587. https://doi.org/10.1038/216586a0
Lederis K (1970) Active substances in the caudal neurosecretory system of bony fishes. Memb Soc Endocrinol 18:465–484
Lederis K (1973) Current studies on urotensins. Am Zool 13(3):771–773. https://doi.org/10.1093/icb/13.3.771
Lederis K (1977) Chemical properties and the physiological and pharmacological actions of urophysial peptides. Am Zool 17(4):823–832. https://doi.org/10.1093/icb/17.4.823
Lederis K, Letter A, McMaster D, Moore G, Schlesinger D (1982) Complete amino acid sequence of urotensin I, a hypotensive and corticotropin-releasing neuropeptide from Catostomus. Science 218(4568):162–165. https://doi.org/10.1126/science.6981844
Leonard JBK, Bartley SM, Taylor MH (1993) Effects of ions and bioactive substances on ovarian contraction in Fundulus heteroclitus. J Exp Zool 267(4):468–473. https://doi.org/10.1002/jez.1402670413
Lescot E, Bureau R, Rault S (2008) Nonpeptide urotensin-II receptor agonists and antagonists: review and structure–activity relationships. Peptides 29(5):680–690. https://doi.org/10.1016/j.peptides.2007.09.019
Loretz CA, Bern HA (1981) Stimulation of sodium transport across the teleost urinary bladder by urotensin II. Gen Comp Endocrinol 43(3):325–330. https://doi.org/10.1016/0016-6480(81)90291-4
Loretz CA, Freel RW, Bern HA (1983) Specificity of response of intestinal ion transport systems to a pair of natural peptide hormone analogs: Somatostatin and urotensin II. General Comp Endocr 52(2):198–206. https://doi.org/10.1016/0016-6480(83)90113-2
Lu W, Greenwood M, Dow L, Yuill J, Worthington J, Brierley MJ et al (2006) Molecular characterization and expression of urotensin II and its receptor in the flounder (Platichthys flesus): a hormone system supporting body fluid homeostasis in euryhaline fish. Endocrinology 147(8):3692–3708. https://doi.org/10.1210/en.2005-1457
Lu W, Abdel-Razik AE, Ashton N, Balment RJ (2008) Urotensin II: lessons from comparative studies for general endocrinology. Gen Comp Endocrinol 157(1):14–20. https://doi.org/10.1016/j.ygcen.2008.03.010
Marshall WS, Bern HA (1979) Teleostean urophysis: urotensin II and ion transport across the isolated skin of a marine teleost. Science 204(4392):519–521
Miller N, Gerlai R (2012) From schooling to shoaling: patterns of collective motion in zebrafish (Danio rerio). PLoS One 7(11):e48865. https://doi.org/10.1371/journal.pone.0048865
Miyata S (2017) Advances in understanding of structural reorganization in the hypothalamic neurosecretory system (Review). Front Endocrinol 8(275). https://doi.org/10.3389/fendo.2017.00275
Navone F, Di Gioia G, Jahn R, Browning M, Greengard P, De Camilli P (1989) Microvesicles of the neurohypophysis are biochemically related to small synaptic vesicles of presynaptic nerve terminals. J Cell Biol 109(6 Pt 2):3425–3433. https://doi.org/10.1083/jcb.109.6.3425
Noonan LR, Continella G, Pedersen CA (1989) Neonatal administration of oxytocin increases novelty-induced grooming in the adult rat. Pharmacol Biochem Behav 33(3):555–558. https://doi.org/10.1016/0091-3057(89)90386-9
Nunes AR, Carreira L, Anbalagan S, Blechman J, Levkowitz G, Oliveira RF (2020) Perceptual mechanisms of social affiliation in zebrafish. Sci Rep 10(1):3642. https://doi.org/10.1038/s41598-020-60154-8
Nunes AR, Gliksberg M, Varela SAM, Teles M, Wircer E, Blechman J, Petri G, Levkowitz G, Oliveira RF (2021) Developmental effects of oxytocin neurons on social affiliation and processing of social information. J Neurosci:JN-RM-2939-20. https://doi.org/10.1523/JNEUROSCI.2939-20.2021
Oka S, Chiba A, Honma Y, Iwanaga T, Fujita T (1993) Development of the caudal neurosecretory system of the chum salmon, Oncorhynchus keta, as revealed by immunohistochemistry for urotensins I and II. Cell Tissue Res 272(2):221–226. https://doi.org/10.1007/BF00302727
Pearson D, Shively JE, Clark BR, Geschwind II, Barkley M, Nishioka RS et al (1980) Urotensin II: a somatostatin-like peptide in the caudal neurosecretory system of fishes. Proc Natl Acad Sci USA 77(8):5021–5024. https://doi.org/10.1073/pnas.77.8.5021
Perks A (1969) The neurohypophysis. Fish Physiol 2:111–205
Piccinno M, Zupa R, Corriero A, Centoducati G, Passantino L, Rizzo A et al (2014) In vitro effect of isotocin on ovarian tunica albuginea contractility of gilthead seabream (Sparus aurata L.) in different reproductive conditions. Fish Physiol Biochem 40(4):1191–1199. https://doi.org/10.1007/s10695-014-9915-x
Qureshi MA, Swarup H, Qureshi TA (1978) Caudal neurosecretory system and the neurohemal organ of Tor tor (Ham.). Anat Anz 143(2):183–191
Ramsey ME, Fry D, Cummings ME (2019) Isotocin increases female avoidance of males in a coercive mating system: assessing the social salience hypothesis of oxytocin in a fish species. Horm Behav 112:1–9. https://doi.org/10.1016/j.yhbeh.2019.03.001
Reddon AR, O’Connor CM, Marsh-Rollo SE, Balshine S (2012) Effects of isotocin on social responses in a cooperatively breeding fish. Anim Behav 84(4):753–760. https://doi.org/10.1016/j.anbehav.2012.07.021
Ribeiro D, Nunes AR, Gliksberg M, Anbalagan S, Levkowitz G, Oliveira RF (2020a) Oxytocin receptor signalling modulates novelty recognition but not social preference in zebrafish. J Neuroendocrinol 32(4):e12834. https://doi.org/10.1111/jne.12834
Ribeiro D, Nunes AR, Teles MC, Anbalagan S, Blechman J, Levkowitz G et al (2020b) Genetic variation in the social environment affects behavioral phenotypes of oxytocin receptor mutants in zebrafish. eLife 9:e56973. https://doi.org/10.7554/eLife.56973
Robinson KJ, Bosch OJ, Levkowitz G, Busch KE, Jarman AP, Ludwig M (2019) Social creatures: model animal systems for studying the neuroendocrine mechanisms of social behaviour. J Neuroendocrinol 31(12):e12807. https://doi.org/10.1111/jne.12807
Ross HE, Cole CD, Smith Y, Neumann ID, Landgraf R, Murphy AZ et al (2009) Characterization of the oxytocin system regulating affiliative behavior in female prairie voles. Neuroscience 162(4):892–903. https://doi.org/10.1016/j.neuroscience.2009.05.055
Russell JA, Leng G, Douglas AJ (2003) The magnocellular oxytocin system, the fount of maternity: adaptations in pregnancy. Front Neuroendocrinol 24(1):27–61. https://doi.org/10.1016/s0091-3022(02)00104-8
Saenko II (1978) Caudal neurosecretory system in acipenseridae and some aspects of its evolution. In: Bargmann W, Oksche A, Polenov AL, Scharrer B (eds) Neurosecretion and neuroendocrine activity. Springer, Berlin, pp 353–356
Salek SJ, Sullivan CV, Godwin J (2002) Arginine vasotocin effects on courtship behavior in male white perch (Morone americana). Behav Brain Res 133(2):177–183. https://doi.org/10.1016/s0166-4328(02)00003-7
Sano Y, Kawamoto M (1959) Entwicklungsgeschichtliche beobachtungen an der neurophysis spinalis caudalis von Lebistes reticulatus Peters. Z Zellforsch Mikrosk Anat 51(1):56–64
Sathyanesan AG, Chavin W (1967) Hypothalamo-hypophyseal neurosecretory system in the primitive actinopterygian fishes (Holostei and Chondrostei). Acta Anat (Basel) 68(2):284–299. https://doi.org/10.1159/000143034
Scharrer E (1928) Die lichtempfindlichkeit blinder elritzen. (Untersuchungen über das zwischenhirn der fische I.). Z Vgl Physiol 7(1):1–38
Scharrer B (1987) Neurosecretion: beginnings and new directions in neuropeptide research. Annu Rev Neurosci 10(1):1–18
Scharrer E, Scharrer B (1940) Secretory cells within the hypothalamus. Res Publ Assoc Res Nerv Ment Dis 20:170–194
Semsar K, Kandel FL, Godwin J (2001) Manipulations of the AVT system shift social status and related courtship and aggressive behavior in the bluehead wrasse. Horm Behav 40(1):21–31. https://doi.org/10.1006/hbeh.2001.1663
Speidel CC (1922) Further comparative studies in other fishes of cells that are homologous to the large irregular glandular cells in the spinal cord of the skates. J Comp Neurol 34(3):303–317. https://doi.org/10.1002/cne.900340303
Suriyampola PS, Shelton DS, Shukla R, Roy T, Bhat A, Martins EP (2016) Zebrafish social behavior in the wild. Zebrafish 13(1):1–8. https://doi.org/10.1089/zeb.2015.1159
Takasugi N, Bern HA (1962) Experimental studies on the caudal neurosecretory system of Tilapia mossambica. Comp Biochem Physiol 6:289–303. https://doi.org/10.1016/0010-406x(62)90133-0
Thompson RR, Walton JC (2004) Peptide effects on social behavior: effects of vasotocin and isotocin on social approach behavior in male goldfish (Carassius auratus). Behav Neurosci 118(3):620–626. https://doi.org/10.1037/0735-7044.118.3.620
Tweedle CD, Smithson KG, Hatton GI (1989) Neurosecretory endings in the rat neurohypophysis are en passant. Exp Neurol 106(1):20–26. https://doi.org/10.1016/0014-4886(89)90140-4
Van de Kamer JC, Verhagen TG (1955) A cytological study of the neurohypophysis of Scylliorhinus caniculus. Z Zellforsch Mikrosk Anat 42(3):229–246. https://doi.org/10.1007/BF00319284
Vaudry H, Do Rego JC, Le Mevel JC, Chatenet D, Tostivint H, Fournier A et al (2010) Urotensin II, from fish to human. Ann N Y Acad Sci 1200:53–66. https://doi.org/10.1111/j.1749-6632.2010.05514.x
Veenema A, Bredewold R, De Vries G (2012) Vasopressin regulates social recognition in juvenile and adult rats of both sexes, but in sex-and age-specific ways. Horm Behav 61(1):50–56
Viveiros ATM, Jatzkowski A, Komen J (2003) Effects of oxytocin on semen release response in African catfish (Clarias gariepinus). Theriogenology 59(9):1905–1917
Von den Velden R (1913) The renal effects of hypophyseal extract in humans [in German]. Berliner Klinische Wochenschrift 50:2083–2086
Warne JM, Balment RJ (1995) Effect of acute manipulation of blood volume and osmolality on plasma [AVT] in seawater flounder. Am J Phys 269(5 Pt 2):R1107–R1112. https://doi.org/10.1152/ajpregu.1995.269.5.R1107
Weber EH (1826) Dissection of the caudal spinal coral of carp. Archiv für Anatomie, Physiologie und Wissenschaftliche Medicin
Weber EH (1927) Kroten unpeurer faden, Mit demsichdes Ruckenmerit beiieinigen fischen endigt, namentlich beim Cyprinus carpio. Archiv für Anatomie, Physiologie und Wissenschaftliche Medicin, 316
Wells A, Anderson G, Hazon N (2002) Development of an in situ perfused kidney preparation for elasmobranch fish: action of arginine vasotocin. Am J Physiol Regul Integr Comp Physiol 282:R1636–R1642. https://doi.org/10.1152/ajpregu.00810.2000
Winter MJ, Ashworth A, Bond H, Brierley MJ, McCrohan CR, Balment RJ (2000) The caudal neurosecretory system: control and function of a novel neuroendocrine system in fish. Biochem Cell Biol 78(3):193–203
Wircer E, Ben-Dor S, Levkowitz G (2016) Non-mammalian models for neurohypophysial peptides. In: Murphy D, Gainer H (eds) Molecular Neuroendocrinology: from genome to physiology. Wiley-Blackwell, New York, pp 301–328. https://doi.org/10.1002/9781118760369.ch14
Yuan M, Li X, Long T, Chen Y, Lu W (2020a) Dynamic responses of the caudal neurosecretory system (CNSS) under thermal stress in olive flounder (Paralichthys olivaceus) (Original Research). Front Physiol 10(1560). https://doi.org/10.3389/fphys.2019.01560
Yuan M, Li X, Lu W (2020b) The caudal neurosecretory system: a novel thermosensitive tissue and its signal pathway in olive flounder (Paralichthys olivaceus). J Neuroendocrinol 32(6):e12876. https://doi.org/10.1111/jne.12876
Zhang X, Jia S, Chen Z, Chong YL, Xie H, Feng D et al (2018) Cilia-driven cerebrospinal fluid flow directs expression of urotensin neuropeptides to straighten the vertebrate body axis. Nat Genet 50(12):1666–1673. https://doi.org/10.1038/s41588-018-0260-3
Zhou H, Ge C, Chen A, Lu W (2019) Dynamic expression and regulation of urotensin I and corticotropin-releasing hormone receptors in ovary of olive flounder Paralichthys olivaceus. Front Physiol 10:1045. https://doi.org/10.3389/fphys.2019.01045
Acknowledgments and Funding
We thank Ludmila Gordon for providing the image of the zebrafish HNS. JB lab is supported by grant 20-04-0055 from the Chief Scientist of the Ministry of Agriculture and Rural Development. Figures 4.1 and 4.3 were created with BioRender.com. PR is supported by a research grant for student’s fellowship from the Benoziyo Endowment Fund for the Advancement of Science and by the Weizmann–CNRS Collaboration Program. G.L. lab is supported by the Israel Science Foundation (#1511/16 and #349/21); US-Israel Bi-National Science Foundation (#2017325); Yeda-Sela Center for Basic Research (in the frame of the Weizmann Institute) and a research grant from Sagol Institute for Longevity Research. G.L. is an incumbent of the Elias Sourasky Professorial Chair.
Recommended Readings
Biran, J., Blechman, J., Wircer, E., & Levkowitz, G. (2018). Development and function of the zebrafish neuroendocrine system. In M. Ludwig, & G. Levkowitz (Eds.), Model animals in neuroendocrinology: From worm to mouse to man (pp. 101–131). Wiley-Blackwell.
Bern, H.A., The elusive urophysis—Twenty-five years in pursuit of caudal neurohormones. American Zoologist, 1985. 25(3): p. 763–770.
Miyata, S., Advances in understanding of structural reorganization in the hypothalamic neurosecretory system. Frontiers in Endocrinology, 2017. 8(275).
Gutnick, A., et al., The hypothalamic neuropeptide oxytocin is required for formation of the neurovascular interface of the pituitary. Dev Cell, 2011. 21(4): p. 642–54.
Anbalagan, S., et al., Pituicyte cues regulate the development of permeable neuro-vascular interfaces. Developmental Cell, 2018. 47(6): p. 711–726.e5.
Balment, R.J., et al., Arginine vasotocin a key hormone in fish physiology and behaviour: a review with insights from mammalian models. General and Comparative Endocrinology, 2006. 147(1): p. 9–16.
Winter, M.J., et al., The caudal neurosecretory system: control and function of a novel neuroendocrine system in fish. Biochem Cell Biol, 2000. 78(3): p. 193–203.
Wircer, E., Ben-Dor, S., & Levkowitz, G. (2016). Non-mammalian models for neurohypophysial peptides. In D. Murphy, & H. Gainer (Eds.), Molecular neuroendocrinology: from genome to physiology (pp. 301–328). Wiley-Blackwell.
Gozdowska, M., M. Ślebioda, and E. Kulczykowska, Neuropeptides isotocin and arginine vasotocin in urophysis of three fish species. Fish Physiol Biochem, 2013. 39(4): p. 863–9.
Author information
Authors and Affiliations
Corresponding authors
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Rajamannar, P., Arokiadhas, I., Levkowitz, G., Biran, J. (2021). The Neurohypophysis and Urophysis: Ancient Piscine Neurovascular Interfaces. In: Grinevich, V., Dobolyi, Á. (eds) Neuroanatomy of Neuroendocrine Systems. Masterclass in Neuroendocrinology, vol 12. Springer, Cham. https://doi.org/10.1007/978-3-030-86630-3_4
Download citation
DOI: https://doi.org/10.1007/978-3-030-86630-3_4
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-86629-7
Online ISBN: 978-3-030-86630-3
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)