Abstract
Schizophrenia is a neuropsychiatric disease with 1% worldwide prevalence and characterized by a deep distortion in thought and perception, cognitive dysfunction, and social behavioral deficits. After the discovery of the antipsychotic effects of chlorpromazine, a large body of evidence pointed out to the neurotransmission misbalance as the main factor in the development of this pathology. Nowadays, it is known that schizophrenia is related to a pluri-factorial etiopathogenesis where gene factors, neuroinflammation, and brain microenvironment’s alterations are taken into account as well. In this sense, glial cells (oligodendrocytes, astrocytes, and microglial cells) are essential pieces in brain microenvironment with crucial roles in synaptic establishment and function, neuroinflammation, and metabolic and ion homeostasis, among others. Currently, glial cells are the target of numerous researches on the race to puzzle out the schizophrenia etiopathology.
Among the multiplicity of regulatory substances involved in glial cell functionality, it becomes outstanding the newly described roles for brain angiotensin II (Ang II). This neuropeptide, through its AT1 receptors (AT1-R), expressed in neurons and glial cells modulates brain homeostasis and several neurotransmission systems (dopaminergic, glutamatergic, and GABAergic) and has a pro-inflammatory role in pathological conditions. In this way, Ang II has been involved in cognition processes, stress responses, and mental disorders such as schizophrenia, addiction, Parkinson’s, and Alzheimer’s diseases.
In this chapter, we aimed to summarize the role of the glial cells in the schizophrenia with a special reference to AT1-R involvement in this complex scenario.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
Introduction
Schizophrenia is a severe mental disorder with 1% worldwide prevalence, which means that it affects the life quality and longevity of more than 21 million people worldwide according to the World Health Organization [1]. This pathology is characterized by profound disruption of thoughts, language, perception, and the sense of self, which often includes psychotic experiences, such as hallucinations and delusions. Together, they are called positive symptoms and were initially considered as the main psychological signs of this pathology. However, schizophrenia presents other psychological features such as social behavioral deficits, lack of motivation, and anhedonia – grouped as the negative symptoms – and cognitive dysfunction [1]. Historically, the classical neuron-centric view that has long-dominated neuroscience and the pharmacological research, fundamentally by using antipsychotic drugs, constituted the largest part of the characterization of schizophrenia etiopathology. In this sense, the bibliography is full of reports pointing out to neurotransmission misbalance – essentially dopaminergic or glutamatergic – as the main factor in the development of this pathology. Over the last years, the dopaminergic-centrist theories have given place to a more complex interpretation as schizophrenia becomes a multifactorial puzzle where glial cells are one of the new targets of interest. Glial cells (oligodendrocytes, astrocytes, and microglial cells) are essential pieces in brain microenvironment function as they play crucial roles in metabolic and ion homeostasis control, synaptic establishment and function, modulation of several neurotransmission systems, as well as neuroprotection, tissue repair, and inflammation [2, 3]. Regarding these glial functions , it is not surprising that alterations in their functionality and integrity could be related to psychiatric disorder development. In this sense, it has been reported, in human and animal research, that glial cells are involved in several mental diseases including Parkinson’s disease, major depressive disorder, addiction, and schizophrenia [2]. The intricate patterns involved in both etiopathology and symptomatology make schizophrenia an unreadable enigma for the moment. Indeed, more than 100 years after its first description by Kraepelin and other psychiatrists, there is not a theory that explains all the features observed throughout its development and the consequent symptoms. Genetic, neurodevelopmental, and environmental theories can be mentioned among the multiplicity of the hypothesis that attempts to clarify the complex scenario of this pathology. Each one of them could represent different components, of the underlying cascade, that trigger the neurotransmitter imbalance and the subsequent schizophrenic symptom expression. These series of concatenated pathological events would occur throughout life, producing deficits in dendritic spine formation, due to an interplay between genetic factors and obstetric complication, along with an excessive apoptosis and synaptic pruning during adolescence, leading to brain disconnection and the subsequent psychotic symptoms [4]. Moreover, the different stages of schizophrenia development are transversally crossed by neuroinflammation. Some of the first insights of a possible association between the schizophrenic syndrome and neuroinflammation come from a century ago, when an increase of schizophrenia diagnosis rates was reported after an influenza epidemic that had happened in 1918 [5]. Although with some inconsistencies, more recent studies are in line with this asseveration, where schizophrenia development seems to be related with influenza as well as other maternal infection like herpes simplex virus type 2, Toxoplasma gondii, and nonspecific bacterial infections [6]. Taking into account the heterogeneity of etiological infections, the increased risk to suffer schizophrenia probably involve alterations related to immune system activation and not due to specific pathogenic pathways of each microorganism. To this respect, increased pro-inflammatory cytokine levels during pregnancy have been related to higher risk to suffer schizophrenia [6]. On the other hand, high genetic contribution to schizophrenia development was observed in studies made in twin with a heritability up to 80% [7]. Located on the short arm of chromosome 6, major histocompatibility complex (MHC) was consistently related to this high contribution to schizophrenia susceptibility [8,9,10,11]. In this region, at least 250 genes that encode human leukocyte antigens and many other immune and nonimmune genes are present. Variations on many genes encoded in this genome’s region could induce an unsuitable immune reaction leading to exacerbate response and the consequent neuroinflammation throughout life. Together with the genetic vulnerability, the early neuroinflammatory insults abovementioned could imprint long-life marks over microglia cells, displaying an increased immune reactivity throughout the life of schizophrenic patients [12]. Both processes could explain the high levels of inflammatory markers, such as pro-inflammatory cytokines and C-reactive protein, that have been described in the blood and cerebrospinal fluid of schizophrenia patients [13, 14]. This immune hyperactivity during brain development could lead to less dendritic spine formation and to an enhanced mesencephalic progenitor differentiation into dopaminergic neurons, promoting some features of schizophrenia, like disruption of cortical synaptic connectivity and hyperdopaminergia [12, 15].
The central brain angiotensin II (Ang II) effects are mediated mainly by the two G protein-coupled receptors, the Ang II type 1 receptor (AT1-R) and Ang II type 2 receptor (AT2-R) . AT1-R is present in astrocytes, microglia, and brain endothelial cells pointing out its crucial role in neuroinflammatory responses [16, 17]. To this last respect, Ang II, via AT1-R, is one of the most important inflammation and oxidative stress inducers by the nicotinamide adenine dinucleotide phosphate (NADPH) oxidase complex activation [18]. Nowadays, a large body of evidence supports the existence of a local renin-angiotensin system (RAS) with all of its components synthesized in the central nervous system (CNS). The presence and synthesis of angiotensinogen have been described in neurons and astrocytes. Whereas angiotensinogen production in neurons is restricted to some brain regions, its astrocytes’ synthesis is the most important and widespread source [9,10,11,12]. Moreover, it has been reported an intra- and extracellular location of renin, while the Ang II converter enzyme (ACE) has been found extracellularly, as soluble and membrane-bound forms. Additionally, Karamyan [19] described alternative central pathways for Ang II synthesis that involved elastase, proteinase 3, cathepsin G, and tonin activity. Further evidence supports intraneuronal generation and activity of Ang II, as well, in brain microvessels [17, 20, 21].
In the present chapter, we attempt to summarize the role of the glial cells in the schizophrenia unmasking the AT1-R involvement in the complex glial scenario.
Glial Cells in Schizophrenia
Microglia
Microglia , resident macrophages of the brain, participates in the progressive loss of synaptic connection during normal neurodevelopment after birth . This physiological process called synaptic pruning could lead, beyond a critical threshold, to cortical disconnection and psychotic symptoms. Moreover, a similar pathological state could be triggered in normal brains after an excessive microglia activation with an excessive synaptic pruning [15]. Furthermore , it has been hypothesized that an inadequate immune activation to a predominant type 2 response could induce an excess of kynurenic acid (KYNA) formation, an endogenous N-methyl-d-aspartate (NMDA) antagonist released mainly by astrocytes, known to lead to a glutamatergic hypofunction, an important hallmark of schizophrenia [12]. Moreover, it has been observed that about 10% of patients with an initial schizophrenia diagnosis have NMDA receptor antibodies [22]. In addition, a meta-analysis performed by Miller et al. shows that some cytokines, like interleukin-1β (IL-1β), interleukin-6 (IL-6), and tumor growth factor β (TGF-β), could be markers for acute exacerbations, since their levels were high during psychotic episodes and normal after antipsychotic treatment [23].
Anti-inflammatory effects of antipsychotic drugs are another important finding that relates immune imbalance with schizophrenia etiopathology. In this sense, a meta-analysis performed by Tourjman shows that antipsychotic treatment reduces the plasma levels of pro-inflammatory cytokines IL-1β and interferon-γ and increases soluble interleukin-2 receptor [24]. Further evidence, which supports an inflammatory response, came from the benefits of nonsteroidal anti-inflammatory drugs (NSAIDs) in the treatment of schizophrenia. In this case, the addition of NSAIDs to antipsychotic treatment generated an improvement, although modest, in symptom control [25, 26].
Under physiological conditions, Ang II and AT1-R are expressed mainly in neurons and astrocytes; meanwhile, in nonactivated microglia, they are present at really low levels. However, under a pathological state, such as neuroinflammation, stroke, or multiple sclerosis, Ang II and AT1-R increase their expression in activated microglia promoting an inflammatory feedback. Bhat [27] has reported that lipopolysaccharide (LPS)-induced gliosis is associated with RAS overactivation evidenced by increased Ang II level and AT1-R expression in both astroglial and microglial cells. Moreover, in the same study, they showed that AT1-R blockade blunted the neuroinflammation via suppression of glial activation and imbalance in inflammatory cytokines in both cell types. To this last respect, a large body of evidence reported that Ang II, via AT1-R, plays a key role in oxidative stress and inflammation, promoting NADPH oxidase activation. Moreover, a cross talk between AT1-R and the microglial toll-like receptor 4 contributes to Ang II pro-inflammatory signal inducing ROS production, NF- κB, and pro-inflammatory cytokine release, including IL-1β, IL-6, and TNF-α, and leads the microglial cell activation [28, 29]. In microglial cells, the NADPH oxidase activity is the main regulator of the shift between M1/pro-inflammatory and M2/immunoregulatory microglial phenotypes, where high oxidative levels promote the pro-inflammatory and inhibit the immunoregulatory phenotype [30]. In this sense, it is known that an M1/M2 phenotype balance is necessary to prevent brain damage, so it is not a surprise that the Ang II overactivation could lead to a chronic neuroinflammatory state. In this sense, the AT1-R involvement in microglial neuroinflammation has been extensively studied in models of Parkinson’s disease and hypertension [18, 29, 31,32,33]. To this last respect, both M1 and M2 markers have been found upregulated in an Ang II-induced mouse model of hypertension, whereas the microglial depletion reduced the neuroinflammation and the blood pressure, as well as the levels of peripheral hypertensive hormones in a mice model of hypertension [34, 35]. In the same way, in an animal model of parkinsonism with MPTP, it has been shown that the Ang II/AT1-R activation of the microglia is involved in the dopaminergic degeneration. This neurotoxin induces an increase in the RhoA/Rho-kinase pathway, which plays a critical role in the inflammatory and oxidative effects of Ang II that is inhibited by AT1-R blockade or depletion [36]. Moreover, Rho-kinase activation upregulated AT1-R expression in microglial cells leading to a feedforward mechanism. AT1-R regulates the microglial response through two main pathways , Rho-kinase and NADPH oxidase, thus controlling superoxide generation, microglial motility and phagocytosis, and the release of inflammatory cytokines [29].
Astrocytes
Astrocytes are the neurovascular unit’s center of integration mediating the vascular response to couple the neuronal activity, through the activation of their metabotropic glutamatergic receptors, Ca+2 oscillations, and release of gliotransmitters and vasoactive substances. In this way, astrocytes control the blood flow in response to the neuronal function (functional hyperemia), and the metabolites exchange through the blood-brain barrier. Furthermore, astrocytes exert a critical contribution to neurotransmission systems since they are responsible for the metabolism, recycling, and/or degradation of glutamate and GABA. Classical examples of these astrocytes’ functions are glutamine-glutamate or KYNA circle. On the other hand, astroglia is involved in the immune response and tissue repair after damage [3, 37,38,39]. Taking all together, since astrocytes have a critical role in maintaining neuronal function, it is not surprising that alterations in their functions have been associated with several psychiatric disorders. However, regarding schizophrenia, the astrocytic contribution is not clear [37, 40]. Initial studies have reported signs of gliosis in a regional-dependent manner and usually closely related to the illness’ history and severity [41, 42]. In this sense, Arnold [43] reported gliosis only in a schizophrenia subgroup of patients with a high prevalence of severe cognitive impairment and functional disability. However, later studies that focused on glial fibrillary acidic protein (GFAP) mRNA or protein analyses found no changes [44,45,46,47] or decreased expression [48,49,50,51,52]. In another study, augmented astrogliosis –described for schizophrenia – was reported to be concomitant with increased neuroinflammatory marker expression, and the authors suggested that astrocyte reactivity could be due to external factors (i.e., differences in psychiatric etiopathology, illnesses coexistence, or treatments received) [44]. In line with this hypothesis , it has been shown an increase in GFAP immunoreactivity after chronic antipsychotic treatment [53]. However, in animal models (rats), the treatment with haloperidol – typical antipsychotic – has no effects over GFAP expression [51]. Focusing the attention over the astroglial enzymes, products, or gliotransmitters, the reports over astrocytes’ role in schizophrenia become more inconsistent. Among the main astrocyte’s enzymes, the glutamine synthetase (GS) is a critical component of glutamine-glutamate cycling expressed mainly in astrocytes. This enzyme catalyzes the ATP-dependent condensation of ammonia and glutamate to form glutamine, playing a fundamental role in the glutamate neurotransmission and homeostasis, as well as neurotoxicity prevention by clearing of ammonia [54]. In the available bibliography, there is no agreement about GS involvement in this pathology, considering that the expression of this enzyme has been reported to be increased, decreased, or with no changes in schizophrenic patients [51, 53, 55,56,57]. Similar results were found for glutaminase, another glutamine-glutamate cycling enzyme, responsible for glutamine-glutamate transformation [55, 57]. However, it has been suggested that these changes could be due to antipsychotic treatment. In this sense, in astrocyte cultures, risperidone increased GS as well as the glutamate uptake and glutathione content; meanwhile, haloperidol increased reactive oxygen species (ROS) but did not show any effect over these astrocyte functions [58]. Moreover, it has been shown an increase in glutamine synthetase-like protein in patients with schizophrenia, which becomes even higher after the treatment with olanzapine [59]. Other critical players in the glutamate-glutamine circle are the excitatory amino acid transporters (EAATs), expressed primarily on astrocytes. The evidence suggests that abnormalities in these glutamate transporter localization and function could underlie alterations in the kinetics of perisynaptic glutamate buffering, clearance, and cycling, contributing to the glutamatergic dysfunction described in schizophrenia [60, 61]. Regarding this last respect, it has been reported a decreased expression of these transporters in critical areas implicated in schizophrenia physiopathology [61]. Interestingly, McCullumsmith [60] showed an increased expression of these glutamate transporters in neurons and suggested that this could be a way to balance out the loss of astroglial reuptake capacity. Moreover, KYNA was found to be increased in critical regions of the CNS in schizophrenic patients, an effect that contributes to the reduction of the glutamatergic neurotransmission [62]. Studies performed in rats showed that the treatment with antipsychotics, like haloperidol, clozapine, and raclopride, caused a reduction in KYNA levels in the caudate putamen, hippocampus, and frontal cortex [63]. Another astroglial marker is the S100B, a neurotrophic factor released from several cell types, but within the CNS, it is released by astrocytes, and it is used as an astrocyte integrity indicator. Through its paracrine and autocrine role, in low concentration, S100B regulates proliferation and differentiation of neurons and glia and modulates dopamine and glutamatergic synaptic function. On the contrary, the over-release of this factor has been related to neuronal dysfunction and apoptosis due to increased expression of inducible nitric oxide synthase (iNOS) or pro-inflammatory cytokines [64, 65]. Regarding schizophrenia patients, several studies have revealed increased S100B levels in the peripheral blood and cerebrospinal fluid (CSF) of patients [65,66,67,68,69,70]. This increase in S100B has been associated to a more severe negative psychopathology and cognitive deficit, supporting the key role of astroglia in the schizophrenia etiopathology [65, 68, 69, 71]. Moreover, it has been reported higher levels of this factor at the cellular level in the early stages or acute paranoid schizophrenia that could be associated with astro- and oligodendroglial activation. These evidences suggest that glial activation and structural damage lead to a neurodegenerative-like process in schizophrenia [65, 72, 73]. Interestingly, an increased inflammatory markers’ expression has been linked to higher levels of S100B in CSF of schizophrenic patients, suggesting that inflammatory processes could lead or exacerbate the glial dysfunction in these patients [66]. Furthermore, different reports showed a decrease in the S100B levels in CSF of patients after antipsychotic treatment. However, this effect appears to be selective for patients with predominantly positive symptoms, since the patients with negative symptomatology showed high levels of S100B even after antipsychotic treatment [65, 69, 72, 74]. Indeed, Qi et al. [67] reported that S100B was significantly higher in patients with refractory schizophrenia which were treated with both clozapine and typical antipsychotics, without significant difference between these two treatment groups . In animal research (monkeys), it has been reported lower S100B expression after chronic haloperidol or olanzapine treatment [75].
Succeeding the description of Ang II activity within the CNS, the first evidence of brain locally produced components was observed as the co-localization of angiotensinogen and the main astrocytic marker – GFAP – whereas the local synthesis of the precursor by astrocytes was confirmed short after [76,77,78]. Furthermore, it was simultaneously evidenced that Ang II binds to receptors in glial cells with similar binding properties and different functionality when compared to angiotensin actions over neurons [79]. These receptors were later identified as the AT1-R subtype, promoting PLC activation and inositol-phosphate hydrolysis [80, 81]. Moreover, it was observed that Ang II, through AT1-R, increased intracellular Ca+2 levels as an initial peak (via IP3) followed by a sustained plateau (for Ca+2 influx from extracellular sources) [82, 83]. Afterward, several cellular effects through different signaling pathways have been described for these receptors in cultured astrocytes including CREB phosphorylation, JACK2/STAT3 and MAPK/ERK activation, and inducible early gene transcription [84,85,86,87]. Overall, nowadays it is widely accepted the constitutive presence and expression of AT1-R in astrocytes through which Ang II autoregulates its activity by controlling the synthesis of all RAS components [88, 89]. However, under certain pathological conditions that involve neuroinflammation, AT1-R overactivity in astrocytes has been found to be detrimental. In this sense, AT1-R activation stimulates ROS production and IL-6 synthesis and release in cultured glial cells via the NF-κB signaling pathway [87, 89], whereas Ang II promotes human astrocyte senescence by superoxide production, after membrane translocation of NADPH oxidase’s subunits. Interestingly, these effects were blunted by AT1-R blockade and the antioxidant tempol [90]. Moreover, in vitro studies showed that the inflammatory condition stimulated by LPS involves AT1-R activation for the upcome of astrogliosis, Ang II synthesis, and AT1-R upregulation, concomitant with NF-κB nuclear translocation, ROS production, and TNF-α release [27]. In the same direction, experimental autoimmune encephalomyelitis in rodents implies AT1-R overexpression in glial cells, triggering the upregulation and activation of transforming growth factor-β (TGF-β) and sustaining the inflammatory condition. Specifically, the AT1-R blockade decreased thrombospondin-1 (TSP-1) secretion from astrocytes, which later promotes TGF-β activation, and improved clinical scores in rodents [91]. Furthermore, AT1-R antagonists effectively blocked TNF-α release by astrocytes under hypoxic conditions [92]. Interestingly, the immunomodulatory actions of dopamine in neurodegenerative conditions involve the misbalance of RAS component production by astrocytes [93]. This interaction has also been observed after amphetamine exposure, where the psychostimulant-induced astrocyte reactivity involves AT1-R activation, concomitant with vascular-network rearrangement and apoptosis in cortical areas [94]. This way, accumulating evidence supports the synergic activity of AT1-R, pro-inflammatory mediators, and ROS in astrocytes contributing to the upcome of neuroinflammatory scenario [95, 96].
Oligodendrocytes
Oligodendrocytes are the glial cells responsible for the myelination processes. The appearance of myelinating oligodendrocytes facilitated the conduction of the nervous impulse, making the synaptic transmission faster and more efficient and leading to vertebrate CNS increase in complexity . In this sense, it is known that myelination processes have a critical role in cognitive functions, such as attention, learning, and memory. In humans, the myelin structure formation begins postnatally, and it is completed in young adulthood, around the time of the first psychotic episode expression and schizophrenia detection [97, 98]. Initial researches reported changes in gray and white matters and in the size of the ventricles. These findings gave a new meaning to oligodendrocytes and the myelination process in the schizophrenia etiopathology. Furthermore, several studies showed an atypical myelination in patients; the association observed in healthy controls between age, education, and the myelin water fraction in the frontal lobe’s white matter is not found in schizophrenic subjects [99]. Indeed, a decreased myelin fraction was observed in schizophrenic patients on their first episode, suggesting that these myelin changes precede the pathology occurrence and the possible pharmacological treatments [99]. Moreover, it has been reported that white matter density alterations are related to illness’ severity, where the low density of the corpus callosum and anterior commissure suggests an aberrant interhemispheric connectivity of anterior cortical and subcortical brain regions and reflecting decreased hemispheric specialization in schizophrenia [100]. All together , these results support the reduced lateralization observed in schizophrenic patients [101, 102]. On the other hand, these myelin structural alterations are companied by oligodendrocyte functional alterations, integrity loss, and/or decreased population [103]. To this last respect, it has been reported a reduction of oligodendrocyte density in the hippocampus , frontal cortex, and anterior cingulate cortex [99, 104,105,106,107,108]. In the same way, decreased oligodendrocyte- and myelin-related genes have been found reduced in schizophrenic patients [108,109,110,111,112,113]. One of the main genetic risk factors in schizophrenia are the disrupted-in-schizophrenia 1 (DISC1) genes, expressed in oligodendrocytes regulating negatively their differentiation and maturation. Bernstein et al. [114] have shown augmented oligodendrocyte-positive expression for DISC1 genes in patients with paranoid schizophrenia . Furthermore, the use of knockout mouse models, missing oligodendrocyte-, or the myelin-related genes linked to schizophrenia recreates the demyelination observed in the human disease in animal research [113]. Interestingly, it has been shown that sub-chronic olanzapine improved oligodendrocyte- and myelin-related gene expression in rats [115]. In the same way, in cellular culture and in vivo, it has been reported that antipsychotics, such as haloperidol, olanzapine, and quetiapine, promote the differentiation of oligodendrocytes through transcription factors 1 and 2 (Olig1 and Olig2), without having any effect over their proliferation [97, 116, 117]. Other studies described an oligodendrocyte development stage-dependent effect for haloperidol. In this sense, in a proliferation phase, haloperidol promotes the cellular spread but inhibits their differentiation in the maturation process [118]. It seems that there is a special susceptibility period during oligodendrocyte development, mainly in gestational and perinatal phases. In this sense, it has been reported that inflammatory processes during early gestational stages produce a decreased number of oligodendrocytes and myelination alterations in the offspring’s adult brain [119, 120]. These results are supported reciprocally with prenatal or gestational inflammation, since, as mentioned above, schizophrenia has been strongly related to maternal inflammatory insults.
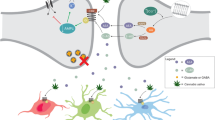
Only a few studies have been focused on Ang II involvement over oligodendroglial functions. However, the confirmation of AT1-R expression on oligodendrocytes suggests undescribed physiological roles of Ang II over these cells [88]. Several lines of evidences suggest an indirect Ang II involvement in re-myelinization through its action over astrocytes or microglial cells. In this sense , angiotensinogen, the precursor of Ang II, has been suggested as a potential biomarker of progression of multiple sclerosis, an illness that results in myelin sheath damage [121]. Moreover, clearance and recycling of lipid debris are necessary processes for an adequate myelination and depend on a suitable lipid transport. The main apolipoprotein (Apo) involved in this lipid transport in the brain is Apo E, which is produced by astrocyte. Since Ang II, through its AT1-R, modulates astroglial function, this peptide could alter Apo E synthesis and indirectly myelin transport and recycling [122, 123] (Fig. 16.1).
The available evidences underpinning the glial system as a key player in the etiopathology of schizophrenia. The AT1-R broad role over neurotransmission systems and glial function encourages their consideration in the development of this pathology and allows to postulate them as a new target for treatment. Indeed, it is important to be conscious that the actual knowledge is the tip of the iceberg in this multifactorial pathology and more studies become necessary to fill out the blank in the schizophrenia puzzle
References
WHO | Schizophrenia [Internet]. WHO [Internet]. World Health Organization; 2014.
Kim R, Healey KL, Sepulveda-Orengo MT, Reissner KJ. Astroglial correlates of neuropsychiatric disease: From astrocytopathy to astrogliosis. Prog Neuropsychopharmacol Biol Psychiatry. 2018;87(Pt A):126–46.
Marchese NA, Casarsa BS, Baiardi GC, Bregonzio C. Neurovascular cognitive alterations: implication of brain renin–angiotensin system. In: Psychiatry and Neuroscience Update update. Cham: Springer International Publishing; 2015. p. 101–17.
Anderson IM. Selective serotonin reuptake inhibitors versus tricyclic antidepressants: a meta-analysis of efficacy and tolerability. J Affect Disord. 2000;58(1):19–36.
Menninger KA. Influenza and schizophrenia. An analysis of post-influenzal “dementia precox”, as of 1918, and five years later further studies of the psychiatric aspects of influenza. 1926. Am J Psychiatry. 1994;151(6 Suppl):182–7.
Khandaker GM, Zimbron J, Lewis G, Jones PB. Prenatal maternal infection, neurodevelopment and adult schizophrenia: a systematic review of population-based studies. Psychol Med. 2013;43(02):239–57.
Sullivan PF, Kendler KS, Neale MC. Schizophrenia as a complex trait: evidence from a meta-analysis of twin studies. Arch General Psychiatry. 2003;60(12):1187.
Corvin A, Morris DW. Genome-wide association studies: findings at the major histocompatibility complex locus in psychosis. Biol Psychiatry. 2014;75(4):276–83.
International Schizophrenia Consortium IS, Purcell SM, Wray NR, Stone JL, Visscher PM, O’Donovan MC, et al. Common polygenic variation contributes to risk of schizophrenia and bipolar disorder. Nature. 2009;460(7256):748–52.
Shi J, Levinson DF, Duan J, Sanders AR, Zheng Y, Pe’er I, et al. Common variants on chromosome 6p22.1 are associated with schizophrenia. Vol. 460. Nature. 2009;460(7256):753–7.
Stefansson H, Ophoff RA, Steinberg S, Andreassen OA, Cichon S, Rujescu D, et al. Common variants conferring risk of schizophrenia. Nature. 2009;460(7256):744–7.
Müller N, Weidinger E, Leitner B, Schwarz MJ. The role of inflammation in schizophrenia. Front Neurosci. 2015;9:372.
Potvin S, Stip E, Sepehry AA, Gendron A, Bah R, Kouassi E. Inflammatory cytokine alterations in schizophrenia: a systematic quantitative review. Biol Psychiatry. 2008;63(8):801–8.
Miller BJ, Culpepper N, Rapaport MH. C-reactive protein levels in schizophrenia. Clin Schizophr Relat Psychoses. 2014;7(4):223–30.
Cannon TD. How schizophrenia develops: cognitive and brain mechanisms underlying onset of psychosis. Trends Cogn Sci. 2015;19(12):744–56.
von Bohlen und Halbach O, Albrecht D. The CNS renin-angiotensin system. Cell Tissue Res. 2006;326(2):599–616.
Zhou J, Pavel J, Macova M, Yu Z-X, Imboden H, Ge L, et al. AT1 receptor blockade regulates the local angiotensin II system in cerebral microvessels from spontaneously hypertensive rats. Stroke. 2006;37(5):1271–6.
Wu C-Y, Zha H, Xia Q-Q, Yuan Y, Liang X-Y, Li J-H, et al. Expression of angiotensin II and its receptors in activated microglia in experimentally induced cerebral ischemia in the adult rats. Mol Cell Biochem. 2013;382(1–2):47–58.
Karamyan VT, Speth RC. Enzymatic pathways of the brain renin–angiotensin system: unsolved problems and continuing challenges. Regul Pept. 2007;143(1–3):15–27.
Basmadjian OM, Occhieppo VB, Marchese NA, Baiardi G, Bregonzio C. Brain Angiotensin II Involvement in chronic mental disorders, Protein Pept Lett 2017;24(9):817–826.
Deliu E, Brailoiu GC, Eguchi S, Hoffman NE, Rabinowitz JE, Tilley DG, et al. Direct evidence of intracrine angiotensin II signaling in neurons. Am J Physiol Cell Physiol. 2014;306(8):C736–44.
Steiner J, Walter M, Glanz W, Sarnyai Z, Bernstein H-G, Vielhaber S, et al. Increased prevalence of diverse N -methyl-D-aspartate glutamate receptor antibodies in patients with an initial diagnosis of schizophrenia. JAMA Psychiatry. 2013;70(3):271.
Miller BJ, Buckley P, Seabolt W, Mellor A, Kirkpatrick B. Meta-analysis of cytokine alterations in schizophrenia: clinical status and antipsychotic effects. Biol Psychiatry. 2011;70(7):663–71.
Tourjman V, Kouassi É, Koué M-È, Rocchetti M, Fortin-Fournier S, Fusar-Poli P, et al. Antipsychotics’ effects on blood levels of cytokines in schizophrenia: a meta-analysis. Schizophr Res. 2013 Dec;151(1–3):43–7.
Sommer IE, van Westrhenen R, Begemann MJH, de Witte LD, Leucht S, Kahn RS. Efficacy of anti-inflammatory agents to improve symptoms in patients with schizophrenia: an update. Schizophr Bull. 2014;40(1):181–91.
Sommer IE, de Witte L, Begemann M, Kahn RS. Nonsteroidal anti-inflammatory drugs in schizophrenia. J Clin Psychiatry. 2012;73(04):414–9.
Bhat SA, Goel R, Shukla R, Hanif K. Angiotensin receptor blockade modulates NF$κ$B and STAT3 signaling and inhibits glial activation and neuroinflammation better than angiotensin-converting enzyme inhibition. Mol Neurobiol. 2016;53(10):6950–67.
Biancardi VC, Stranahan AM, Krause EG, de Kloet AD, Stern JE. Cross talk between AT1 receptors and toll-like receptor 4 in microglia contributes to angiotensin II-derived ROS production in the hypothalamic paraventricular nucleus. Am J Physiol Heart Circ Physiol. 2016;310(3):H404–15.
Borrajo A, Rodriguez-Perez AI, Diaz-Ruiz C, Guerra MJ, Labandeira-Garcia JL. Microglial TNF-α mediates enhancement of dopaminergic degeneration by brain angiotensin. Glia. 2014;62(1):145–57.
Labandeira-Garcia JL, Rodríguez-Perez AI, Garrido-Gil P, Rodriguez-Pallares J, Lanciego JL, Guerra MJ. Brain Renin-angiotensin system and microglial polarization: implications for aging and neurodegeneration. Front Aging Neurosci. 2017;9:129.
Sun H, Wu HQ, Yu X, Zhang GL, Zhang R, Zhan SQ, et al. Angiotensin II and its receptor in activated microglia enhanced neuronal loss and cognitive impairment following pilocarpine-induced status epilepticus. Mol Cell Neurosci. 2015;65:58–67.
Rodriguez-Perez AI, Borrajo A, Rodriguez-Pallares J, Guerra MJ, Labandeira-Garcia JL. Interaction between NADPH-oxidase and rho-kinase in angiotensin II-induced microglial activation. Glia. 2015;63(3):466–82.
Labandeira-Garcia JL, Rodriguez-Pallares J, Rodríguez-Perez AI, Garrido-Gil P, Villar-Cheda B, Valenzuela R, et al. Brain angiotensin and dopaminergic degeneration: relevance to Parkinson’s disease. Am J Neurodegener Dis. 2012;1(3):226–44.
Kapoor K, Bhandare AM, Farnham MMJ, Pilowsky PM. Alerted microglia and the sympathetic nervous system: a novel form of microglia in the development of hypertension. Respir Physiol Neurobiol. 2016;226:51–62.
Shi P, Grobe JL, Desland FA, Zhou G, Shen XZ, Shan Z, et al. Direct pro-inflammatory effects of prorenin on microglia. PLoS One. 2014;9(10):e92937.
Villar-Cheda B, Dominguez-Meijide A, Joglar B, Rodriguez-Perez AI, Guerra MJ, Labandeira-Garcia JL. Involvement of microglial RhoA/rho-kinase pathway activation in the dopaminergic neuron death. Role of angiotensin via angiotensin type 1 receptors. Neurobiol Dis. 2012;47(2):268–79.
Xia M, Abazyan S, Jouroukhin Y, Pletnikov M. Behavioral sequelae of astrocyte dysfunction: focus on animal models of schizophrenia. Schizophr Res. 2016;176(1):72–82.
Goudriaan A, de Leeuw C, Ripke S, Hultman CM, Sklar P, Sullivan PF, et al. Specific glial functions contribute to schizophrenia susceptibility. Schizophr Bull. 2014;40(4):925–35.
Hansson E, Rönnbäck L. Glial neuronal signaling in the central nervous system. FASEB J. 2003;17(3):341–8.
Bernstein H-G, Steiner J, Guest PC, Dobrowolny H, Bogerts B. Glial cells as key players in schizophrenia pathology: recent insights and concepts of therapy. Schizophr Res. 2015;161(1):4–18.
Rajkowska G, Miguel-Hidalgo JJ, Makkos Z, Meltzer H, Overholser J, Stockmeier C. Layer-specific reductions in GFAP-reactive astroglia in the dorsolateral prefrontal cortex in schizophrenia. Schizophr Res. 2002;57(2–3):127–38.
Bruton CJ, Crow TJ, Frith CD, Johnstone EC, Owens DG, Roberts GW. Schizophrenia and the brain: a prospective clinico-neuropathological study. Psychol Med. 1990;20(2):285–304.
Arnold SE, Franz BR, Trojanowski JQ, Moberg PJ, Gur RE. Glial fibrillary acidic protein-immunoreactive astrocytosis in elderly patients with schizophrenia and dementia. Acta Neuropathol. 1996;91(3):269–77.
Catts VS, Wong J, Fillman SG, Fung SJ, Shannon WC. Increased expression of astrocyte markers in schizophrenia: association with neuroinflammation. Aust N Z J Psychiatry. 2014;48(8):722–34.
Garey L. When cortical development goes wrong: schizophrenia as a neurodevelopmental disease of microcircuits. J Anat. 2010;217(4):324–33.
Fatemi SH, Laurence JA, Araghi-Niknam M, Stary JM, Schulz SC, Lee S, et al. Glial fibrillary acidic protein is reduced in cerebellum of subjects with major depression, but not schizophrenia. Schizophr Res. 2004;69(2–3):317–23.
Casanova MF. Astrocytosis and schizophrenia. Schizophr Res. 1991;5(3):186–7.
Najjar S, Pearlman DM. Neuroinflammation and white matter pathology in schizophrenia: systematic review. Schizophr Res. 2015;161(1):102–12.
Hercher C, Chopra V, Beasley CL. Evidence for morphological alterations in prefrontal white matter glia in schizophrenia and bipolar disorder. J Psychiatry Neurosci. 2014;39(6):376–85.
Williams MR, Hampton T, Pearce RKB, Hirsch SR, Ansorge O, Thom M, et al. Astrocyte decrease in the subgenual cingulate and callosal genu in schizophrenia. Eur Arch Psychiatry Clin Neurosci. 2013;263(1):41–52.
Steffek AE, McCullumsmith RE, Haroutunian V, Meador-Woodruff JH. Cortical expression of glial fibrillary acidic protein and glutamine synthetase is decreased in schizophrenia. Schizophr Res. 2008;103(1–3):71–82.
Webster MJ, O’Grady J, Kleinman JE, Weickert CS. Glial fibrillary acidic protein mRNA levels in the cingulate cortex of individuals with depression, bipolar disorder and schizophrenia. Neuroscience. 2005;133(2):453–61.
Toro CT, Hallak JEC, Dunham JS, Deakin JFW. Glial fibrillary acidic protein and glutamine synthetase in subregions of prefrontal cortex in schizophrenia and mood disorder. Neurosci Lett. 2006;404(3):276–81.
Rose CF, Verkhratsky A, Parpura V. Astrocyte glutamine synthetase: pivotal in health and disease. Biochem Soc Trans. 2013;41(6):1518–24.
Katsel P, Byne W, Roussos P, Tan W, Siever L, Haroutunian V. Astrocyte and glutamate markers in the superficial, deep, and white matter layers of the anterior cingulate gyrus in schizophrenia. Neuropsychopharmacology. 2011;36(6):1171–7.
Burbaeva GS, Boksha IS, Tereshkina EB, Savushkina OK, Starodubtseva LI, Turishcheva MS, et al. Systemic neurochemical alterations in schizophrenic brain: glutamate metabolism in focus. Neurochem Res. 2007;32(9):1434–44.
Bruneau EG, McCullumsmith RE, Haroutunian V, Davis KL, Meador-Woodruff JH. Increased expression of glutaminase and glutamine synthetase mRNA in the thalamus in schizophrenia. Schizophr Res. 2005;75(1):27–34.
Quincozes-Santos A, Bobermin LD, Tonial RPL, Bambini-Junior V, Riesgo R, Gottfried C. Effects of atypical (risperidone) and typical (haloperidol) antipsychotic agents on astroglial functions. Eur Arch Psychiatry Clin Neurosci. 2010;260(6):475–81.
Burbaeva GS, Boksha IS, Tereshkina EB, Savushkina OK, Turishcheva MS, Starodubtseva LI, et al. Effect of olanzapine treatment on platelet glutamine synthetase-like protein and glutamate dehydrogenase immunoreactivity in schizophrenia. World J Biol Psychiatry. 2006;7(2):75–81.
McCullumsmith RE, O’Donovan SM, Drummond JB, Benesh FS, Simmons M, Roberts R, et al. Cell-specific abnormalities of glutamate transporters in schizophrenia: sick astrocytes and compensating relay neurons? Mol Psychiatry. 2016;21(6):823–30.
Shan D, Lucas EK, Drummond JB, Haroutunian V, Meador-Woodruff JH, McCullumsmith RE. Abnormal expression of glutamate transporters in temporal lobe areas in elderly patients with schizophrenia. Schizophr Res. 2013;144(1–3):1–8.
Müller N, Myint A-M, Krause D, Weidinger E, Schwarz MJ. Anti-inflammatory treatment in schizophrenia. Prog Neuro-Psychopharmacol Biol Psychiatry. 2013;42:146–53.
Ceresoli-Borroni G, Rassoulpour A, Wu H-Q, Guidetti P, Schwarcz R. Chronic neuroleptic treatment reduces endogenous kynurenic acid levels in rat brain. J Neural Transm (Vienna, Austria: 1996). 2006;113(10):1355–65.
O’Connell K, Thakore J, Dev KK. Levels of S100B are raised in female patients with schizophrenia. BMC Psychiatry. 2013;13(1):146.
Rothermundt M, Falkai P, Ponath G, Abel S, Bürkle H, Diedrich M, et al. Glial cell dysfunction in schizophrenia indicated by increased S100B in the CSF. Mol Psychiatry. 2004;9(10):897–9.
Hong W, Zhao M, Li H, Peng F, Wang F, Li N, et al. Higher plasma S100B concentrations in schizophrenia patients, and dependently associated with inflammatory markers. Sci Rep. 2016;6(1):27584.
Qi LY, Xiu MH, Chen DC, Wang F, Kosten TA, Kosten TR, et al. Increased serum S100B levels in chronic schizophrenic patients on long-term clozapine or typical antipsychotics. Neurosci Lett. 2009;462(2):113–7.
Pedersen A, Diedrich M, Kaestner F, Koelkebeck K, Ohrmann P, Ponath G, et al. Memory impairment correlates with increased S100B serum concentrations in patients with chronic schizophrenia. Prog Neuro-Psychopharmacol Biol Psychiatry. 2008;32(8):1789–92.
Rothermundt M, Missler U, Arolt V, Peters M, Leadbeater J, Wiesmann M, et al. Increased S100B blood levels in unmedicated and treated schizophrenic patients are correlated with negative symptomatology. Mol Psychiatry. 2001;6(4):445–9.
Wiesmann M, Wandinger KP, Missler U, Eckhoff D, Rothermundt M, Arolt V, et al. Elevated plasma levels of S-100b protein in schizophrenic patients. Biol Psychiatry. 1999;45(11):1508–11.
Ling S, Tang Y, Jiang F, Wiste A, Guo S, Weng Y, et al. Plasma S-100B protein in Chinese patients with schizophrenia: comparison with healthy controls and effect of antipsychotics treatment. J Psychiatr Res. 2007;41(1–2):36–42.
Zhang XY, Xiu MH, Song C, Chen DC, Wu GY, Haile CN, et al. Increased serum S100B in never-medicated and medicated schizophrenic patients. J Psychiatr Res. 2010;44(16):1236–40.
Steiner J, Bernstein H-G, Bielau H, Farkas N, Winter J, Dobrowolny H, et al. S100B-immunopositive glia is elevated in paranoid as compared to residual schizophrenia: a morphometric study. J Psychiatr Res. 2008;42(10):868–76.
Schroeter ML, Abdul-Khaliq H, Frühauf S, Höhne R, Schick G, Diefenbacher A, et al. Serum S100B is increased during early treatment with antipsychotics and in deficit schizophrenia. Schizophr Res. 2003;62(3):231–6.
Konopaske GT, Dorph-Petersen K-A, Pierri JN, Wu Q, Sampson AR, Lewis DA. Effect of chronic exposure to antipsychotic medication on cell numbers in the parietal cortex of macaque monkeys. Neuropsychopharmacology. 2007;32(6):1216–23.
Deschepper CF, Bouhnik J, Ganong WF. Colocalization of angiotensinogen and glial fibrillary acidic protein in astrocytes in rat brain. Brain Res. 1986;374(1):195–8.
Imboden H, Harding JW, Hilgenfeldt U, Celio MR, Felix D. Localization of angiotensinogen in multiple cell types of rat brain. Brain Res. 1987;410(1):74–7.
Stornetta RL, Hawelu-Johnson CL, Guyenet PG, Lynch KR. Astrocytes synthesize angiotensinogen in brain. Science (New York, N.Y.). 1988;242(4884):1444–6.
Raizada MK, Phillips MI, Crews FT, Sumners C. Distinct angiotensin II receptor in primary cultures of glial cells from rat brain. Proc Natl Acad Sci U S A. 1987;84(13):4655–9.
Sumners C, Tang W, Zelezna B, Raizada MK. Angiotensin II receptor subtypes are coupled with distinct signal-transduction mechanisms in neurons and astrocytes from rat brain. Proc Natl Acad Sci U S A. 1991;88(17):7567–71.
Tallant EA, Higson JT. Angiotensin II activates distinct signal transduction pathways in astrocytes isolated from neonatal rat brain. Glia. 1997;19(4):333–42.
Wang D, Martens JR, Posner P, Sumners C, Gelband CH. Angiotensin II regulation of intracellular calcium in astroglia cultured from rat hypothalamus and brainstem. J Neurochem. 1996;67(3):996–1004.
Gebke E, Müller AR, Jurzak M, Gerstberger R. Angiotensin II-induced calcium signalling in neurons and astrocytes of rat circumventricular organs. Neuroscience. 1998;85(2):509–20.
Delaney J, Chiarello R, Villar D, Kandalam U, Castejon AM, Clark MA. Regulation of c-fos, c-jun and c-myc gene expression by angiotensin II in primary cultured rat astrocytes: role of ERK1/2 MAP kinases. Neurochem Res. 2008;33(3):545–50.
Holownia A, Braszko JJ. The effect of angiotensin II and IV on ERK1/2 and CREB signalling in cultured rat astroglial cells. Naunyn Schmiedeberg’s Arch Pharmacol. 2007;376(3):157–63.
Alanazi AZ, Patel P, Clark MA. p38 Mitogen-activated protein kinase is stimulated by both angiotensin II and angiotensin III in cultured rat astrocytes. J Recept Signal Transduct Res. 2014;34(3):205–11.
Kandalam U, Clark MA. Angiotensin II activates JAK2/STAT3 pathway and induces interleukin-6 production in cultured rat brainstem astrocytes. Regul Pept. 2010;159(1–3):110–6.
Fogarty DJ, Matute C. Angiotensin receptor-like immunoreactivity in adult brain white matter astrocytes and oligodendrocytes. Glia. 2001;35(2):131–46.
Gowrisankar YV, Clark MA. Angiotensin II induces interleukin-6 expression in astrocytes: role of reactive oxygen species and NF-κB. Mol Cell Endocrinol. 2016;437:130–41.
Liu G, Hosomi N, Hitomi H, Pelisch N, Fu H, Masugata H, et al. Angiotensin II induces human astrocyte senescence through reactive oxygen species production. Hypertens Res. 2011;34(4):479–83.
Lanz TV, Ding Z, Ho PP, Luo J, Agrawal AN, Srinagesh H, et al. Angiotensin II sustains brain inflammation in mice via TGF-beta. J Clin Invest. 2010;120(8):2782–94.
Danielyan L, Lourhmati A, Verleysdonk S, Kabisch D, Proksch B, Thiess U, et al. Angiotensin receptor type 1 blockade in astroglia decreases hypoxia-induced cell damage and TNF alpha release. Neurochem Res. 2007;32(9):1489–98.
Dominguez-Meijide A, Rodriguez-Perez AI, Diaz-Ruiz C, Guerra MJ, Labandeira-Garcia JL. Dopamine modulates astroglial and microglial activity via glial renin-angiotensin system in cultures. Brain Behav Immunity. 2017;62:277–90.
Occhieppo VB, Marchese NA, Rodriguez ID, Basmadjian OM, Baiardi G, Bregonzio C. Neurovascular unit alteration in somatosensory cortex and enhancement of thermal nociception induced by amphetamine involves central AT1 receptor activation. Eur J Neurosci. 2017;45:1586–93.
de Kloet AD, Liu M, Rodríguez V, et al. Role of neurons and glia in the CNS actions of the renin-angiotensin system in cardiovascular control. Am J Physiol Regul Integr Comp Physiol. 2015;309:R444–58.
O’Connor AT, Clark MA. Astrocytes and the renin angiotensin system: relevance in disease pathogenesis. Neurochem Res. 2018;43(7):1297–307.
Fang F, Zhang H, Zhang Y, Xu H, Huang Q, Adilijiang A, et al. Antipsychotics promote the differentiation of oligodendrocyte progenitor cells by regulating oligodendrocyte lineage transcription factors 1 and 2. Life Sci. 2013;93(12–14):429–34.
Takahashi N, Sakurai T, Davis KL, Buxbaum JD. Linking oligodendrocyte and myelin dysfunction to neurocircuitry abnormalities in schizophrenia. Prog Neurobiol. 2011;93(1):13–24.
Flynn SW, Lang DJ, Mackay AL, Goghari V, Vavasour IM, Whittall KP, et al. Abnormalities of myelination in schizophrenia detected in vivo with MRI, and post-mortem with analysis of oligodendrocyte proteins. Mol Psychiatry. 2003;8(9):811–20.
Hulshoff Pol HE, Schnack HG, Mandl RCW, Cahn W, Collins DL, Evans AC, et al. Focal white matter density changes in schizophrenia: reduced inter-hemispheric connectivity. NeuroImage. 2004;21(1):27–35.
Fei Ho N, Li Z, Ji F, Wang M, Kuswanto CN, Yi Sum M, et al. Hemispheric lateralization abnormalities of the white matter microstructure in patients with schizophrenia and bipolar disorder. J Psychiatry Neurosci. 2017;42(4):242–51.
Levitt JJ, Nestor PG, Levin L, Pelavin P, Lin P, Kubicki M, et al. Reduced structural connectivity in frontostriatal white matter tracts in the associative loop in schizophrenia. Am J Psychiatry. 2017;174(11):1102–11.
Vikhreva OV, Rakhmanova VI, Orlovskaya DD, Uranova NA. Ultrastructural alterations of oligodendrocytes in prefrontal white matter in schizophrenia: a post-mortem morphometric study. Schizophr Res. 2016;177(1–3):28–36.
Falkai P, Malchow B, Wetzestein K, Nowastowski V, Bernstein H-G, Steiner J, et al. Decreased Oligodendrocyte and Neuron Number in Anterior Hippocampal Areas and the Entire Hippocampus in Schizophrenia: A stereological Postmortem study. Schizophr Bull. 2016;42(suppl 1):S4–12.
Hof PR, Haroutunian V, Friedrich VL, Byne W, Buitron C, Perl DP, et al. Loss and altered spatial distribution of oligodendrocytes in the superior frontal gyrus in schizophrenia. Biol Psychiatry. 2003;53(12):1075–85.
Vostrikov V, Orlovskaya D, Uranova N. Deficit of pericapillary oligodendrocytes in the prefrontal cortex in schizophrenia. World J Biol Psychiatry. 2008;9(1):34–42.
Farkas N, Lendeckel U, Dobrowolny H, Funke S, Steiner J, Keilhoff G, et al. Reduced density of ADAM 12-immunoreactive oligodendrocytes in the anterior cingulate white matter of patients with schizophrenia. World J Biol Psychiatry. 2010;11(3):556–66.
Felsky D, Voineskos AN, Lerch JP, Nazeri A, Shaikh SA, Rajji TK, et al. Myelin-associated glycoprotein gene and brain morphometry in schizophrenia. Front Psychiatry. 2012;3:40.
Shen P-C, He L-Q, Yang X-J, Cao H-X. Renal protection of losartan 50 mg in normotensive Chinese patients with nondiabetic chronic kidney disease. J Investig Med. 2012;60(7):1041–7.
Chavarria-Siles I, White T, de Leeuw C, Goudriaan A, Lips E, Ehrlich S, et al. Myelination-related genes are associated with decreased white matter integrity in schizophrenia. Eur J Human Gene. 2016;24(3):381–6.
Tkachev D, Mimmack ML, Ryan MM, Wayland M, Freeman T, Jones PB, et al. Oligodendrocyte dysfunction in schizophrenia and bipolar disorder. Lancet. 2003;362(9386):798–805.
Mauney SA, Pietersen CY, Sonntag K-C, Woo T-UW. Differentiation of oligodendrocyte precursors is impaired in the prefrontal cortex in schizophrenia. Schizophr Res. 2015;169(1–3):374–80.
Segal D, Koschnick JR, Slegers LHA, Hof PR. Oligodendrocyte pathophysiology: a new view of schizophrenia. Int J Neuropsychopharmacol. 2007;10(4):503–11.
Bernstein H-G, Jauch E, Dobrowolny H, Mawrin C, Steiner J, Bogerts B. Increased density of DISC1-immunoreactive oligodendroglial cells in fronto-parietal white matter of patients with paranoid schizophrenia. Eur Arch Psychiatry Clin Neurosci. 2016;266(6):495–504.
Ersland KM, Skrede S, Stansberg C, Steen VM. Subchronic olanzapine exposure leads to increased expression of myelination-related genes in rat fronto-medial cortex. Transl Psychiatry. 2017;7(11):1262.
Xiao L, Xu H, Zhang Y, Wei Z, He J, Jiang W, et al. Quetiapine facilitates oligodendrocyte development and prevents mice from myelin breakdown and behavioral changes. Mol Psychiatry. 2008;13(7):697–708.
Yamauchi T, Tatsumi K, Makinodan M, Kimoto S, Toritsuka M, Okuda H, et al. Olanzapine increases cell mitotic activity and oligodendrocyte-lineage cells in the hypothalamus. Neurochem Int. 2010;57(5):565–71.
Niu J, Mei F, Li N, Wang H, Li X, Kong J, et al. Haloperidol promotes proliferation but inhibits differentiation in rat oligodendrocyte progenitor cell cultures. This paper is one of a selection of papers published in this special issue entitled “Second International Symposium on Recent Advances in Basic”. Biochem Cell Biol. 2010;88:611–20.
Favrais G, van de Looij Y, Fleiss B, Ramanantsoa N, Bonnin P, Stoltenburg-Didinger G, et al. Systemic inflammation disrupts the developmental program of white matter. Ann Neurol. 2011;70(4):550–65.
Graf AE, Haines KM, Pierson CR, Bolon BN, Houston RH, Velten M, et al. Perinatal inflammation results in decreased oligodendrocyte numbers in adulthood. Life Sci. 2014;94(2):164–71.
Ottervald J, Franzén B, Nilsson K, Andersson LI, Khademi M, Eriksson B, et al. Multiple sclerosis: identification and clinical evaluation of novel CSF biomarkers. J Proteome. 2010;73(6):1117–32.
Haroutunian V, Katsel P, Roussos P, Davis KL, Altshuler LL, Bartzokis G. Myelination, oligodendrocytes, and serious mental illness. Glia. 2014;62(11):1856–77.
Deza-Ponzio R, Herrera ML, Bellini MJ, Virgolini MB, Hereñú CB. Aldehyde dehydrogenase 2 in the spotlight: the link between mitochondria and neurodegeneration. Neurotoxicology. 2018;68:19–24.
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Occhieppo, V.B. et al. (2021). Glial Cells in the Schizophrenia Puzzle: Angiotensin II Role. In: Gargiulo, P.Á., Mesones Arroyo, H.L. (eds) Psychiatry and Neuroscience Update. Springer, Cham. https://doi.org/10.1007/978-3-030-61721-9_16
Download citation
DOI: https://doi.org/10.1007/978-3-030-61721-9_16
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-61720-2
Online ISBN: 978-3-030-61721-9
eBook Packages: MedicineMedicine (R0)