Abstract
In arid and semi-arid areas, the water quantity and quality are a great problem. Salinization is the major threat in the region of Menzel Habib (north-western Gabès, southeastern Tunisia). The region is a large basin which is essentially represented by sandy-clay formations and bordered by cretaceous reliefs. Geochemical and statistical approaches are reported in the Menzel Habib aquifer system to examine groundwater salinization processes and factors controlling its mineralization. Geochemical studies were developed in 25 groundwater samples from the shallow aquifer to identify the origin of groundwater salinization. Groundwater geochemistry shows a high correlation between salinity and Na, Cl, Ca, Mg and SO4. These elements are mainly associated to the evaporitic Triassic by dissolution of halite, anhydrite and gypsum which occur on the area and are related to the tectonic context of the region. Additionally, bivariate diagram between Na and Cl, and Ca and SO4 have also provided a comprehensive understanding of other salinization processes that are involving in Menzel Habib shallow aquifer such as cation exchange and reverse cation exchange.
Access provided by Autonomous University of Puebla. Download conference paper PDF
Similar content being viewed by others
Keywords
1 Introduction
Most of the scenarios of the future water resources are predicting water scarcity, with a decrease in precipitation and limitation in groundwater recharge for the next five decades (Doll and Fiedler 2005). Thus, salinization of groundwater aquifers is one of the principal causes of degradation of water resources worldwide. This phenomenon is a major drawback for water use for irrigation and drinking water supply in arid and semi-arid regions, such as Menzel Habib. Menzel Habib aquifer is in the northwest of Gabès, southeastern Tunisia, between latitudes 34° and 34° 20′ N and longitudes 9° 15′ and 9° 58′ E. The average annual precipitation and temperature are 150 mm and 20 °C, respectively. The low recharge and the agricultural activities are an evidence to suggest that the quality of groundwater is threatened by the salinization risk and water quality problems superpose to a general quantitative limitation of freshwater availability. Previous studies have revealed that groundwater salinization could have many origins occurring alone or concomitantly, with dominant mechanisms, described as natural mechanisms, such as seawater intrusion (Kharroubi et al. 2012; Ben Hamouda et al. 2013), dissolution of evaporites (Rosenthal et al. 2007) and infiltration of saline surface water; but also anthropogenic sources (e.g., agricultural, industrial and domestic activities).
The origin groundwater salinization could be investigated using different approaches: from geochemical analysis to isotopic tracers and using statistical approaches. Indeed, the main subject of this study is to investigate the sources of groundwater salinization in Menzel Habib shallow aquifer using geochemical tools.
2 Geological and Hydrogeological Settings
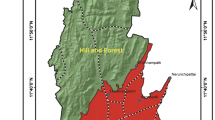
Menzel Habib area is represented by a large syncline basin with plio-quaternary filling which is essentially represented by sandy-clay and sandy-loam formations and bordered by cretaceous reliefs. Menzel Habib area is complex and was affected by different series of geological structures (Fig. 1). The Triassic lithologies have been identified as diapiric extrusions in Hadifa Mountain and are related to the tectonic context of the region (Ouled-Ghrib and Slimane 1994).
The aquifer system of Menzel Habib is composed of three layers. The reservoir formations are distributed between quaternary deposits and limestones of the upper cretaceous (Cenomanian-Turonian and Senonian). The quaternary layer is logged in a sandy-loam formation, containing a stack of aquifer levels dependent on rain inputs that are too limited in this region, the depth of water varies from 7 to 60 m. The Senonian is logged in marl levels with limestone passages, and this aquifer is exploited in the region of Menzel Habib by some public boreholes partly meeting the needs of agricultural activities. The Cenomanian-Turonian layer contained in limestone and marl-limestone levels, and it is characterized by a strong karstification and contains a water of high salinity exceeding 19 g/L preventing its exploitation for human supply and agriculture.
3 Results
3.1 Geochemical Data
In this study, 25 water samples were taken from the shallow aquifer of Menzel Habib. Electrical conductivity (EC) and pH had been measured in the field using a C933 multi-parameter analyzer. All samples were filtered with a 0.45 µm Millipore filter paper. Bicarbonate (HCO3−) was determined through the titration method with hydrochloric acid. Major cations (Na+, K+, Ca2+, Mg2+) and major anions (F-, Cl-, Br-, SO42-) were measured by an ionic liquid chromatography Metrohm 850 Professional IC in the Integrated Laboratory of Water Sciences in the Higher Institute of Water Sciences and Techniques of Gabès (Tunisia). The pH values vary from 6.95 to 7.91 with an average of 7.63 and a standard deviation of 0.168 indicating a slight alkalinity of groundwater samples. Electrical conductivity ranges from 3.06 to 14.98 ms cm−1 with an average of 7.84 and a standard deviation of 2.98. Total dissolved solids (TDS) is heterogeneous and varies strongly from 1.598 to 8.706 g/L with an average and standard deviation of 4.33 g/L and 1.77 g/L, respectively.
The major ion composition is represented in Table 1 and shows that sodium is the major cation and sulfate is the dominant anion in groundwater composition. Piper diagram (Fig. 2) shows three types of water: Na-Cl, Na-SO4 and mixed type.
3.2 Spatial Correlations
To examine spatial relations between TDS and chemical elements of groundwater, bivariate diagrams were plotted. As a result, they show a positive high correlation (r > 0.7 at 5% significance level) between TDS and Cl, SO4, Na, Mg and Ca (Fig. 3).
4 Discussion
The bivariate diagrams are an important tool to identify the origin of the water salinization of an aquifer. The high correlation between TDS and Cl, SO4, Na, Mg and Ca may be explained by the dissolution of evaporites such as halite, gypsum and anhydrite (Agoubi et al. 2013). This process can be justified by the dissolution of halite and gypsum/anhydrite (Fig. 4). The diagram of sodium versus chloride could be identified on the distinction of the different mechanisms for acquiring salinity in arid and semi-arid regions. The positive correlation (r = 0.977) suggests that sodium and chloride are derived from the same origin which is the dissolution of halite that occurs in the aquifer. These elements could have its origin on the Triassic diapir of Hadifa or the evaporation dominance that characterizes the arid and semi-arid areas. However, some of groundwater samples have a relative excess or deficit of sodium content relative to the halite dissolution line, explained by the intervention of another phenomenon controlling their salinization. The deficit of sodium content which characterizes some groundwater samples indicate the presence of reverse cation exchange mechanism, which contributes to Na adsorption on clay minerals and simultaneous release of Ca. Some other water samples have an enriched Na against Cl and a deficit on Ca against SO4. This relation could be explained by another mechanism that can also be involved in the contamination of Menzel Habib aquifer, which is the cation exchange phenomenon, with a release of sodium and binding of calcium.
5 Concluding Remarks
The results of geochemical and statistical approaches have shown that the salinity of Menzel Habib shallow aquifer is due to different mechanisms, such as dissolution of evaporites, cation exchange, reverse cation exchange and dominance of evaporation that can threat the shallow groundwater. To resolve this problem, a sustainable management of aquifer is required as a main issue in the area. Indeed, the construction of additional groundwater exploitation should not be allowed in the threatened parts of the study area, and the delimitation of groundwater protection zones should be respected. The irrigation with saltwater must be avoided with the consequence of soil salinization and therefore, an increase of water salinity by infiltration. The simultaneous pumping of adjacent wells must be avoided with a daily temporal control on wells pumping. Therefore, artificial recharge can also be applied for dilution and thus decreasing of salinity.
References
Agoubi B, Kharroubi A, Ta Abichou, Abida H (2013) Hydrochemical and geoelectrical investigation of Marine Jeffara Aquifer, southeastern Tunisia. Appl Water Sci 15:415–430
Ben Hamouda MF, Carreira P, Marques JM, Eggenkamp H (2013) Geochemical and isotopic investigations to study the origin of mineralization of the coastal aquifer of Sousse, Tunisia. Proc Earth Planet Sci 7:61–64
Doll P, Fiedler M (2005) Global-scale estimation of diffuse groundwater recharge. Hydrol Earth Syst Sci 12:863–885
Kharroubi A, Tlahigue F, Agoubi B, Azri C, Bouri S (2012) Hydrochemical and statistical studies of the groundwater salinization in Mediterranean arid zones: case of the Jerba coastal aquifer in southeast Tunisia. Environ Earth Sci 67(7):2089–2100
Ouled-Ghrib A, Slimane MF (1994) Nouvelles données géologiques sur l’Atlas méridional de la Tunisie: mise en évidence du Trias dans la chaîne de Gafsa. Not Serv Géol Tunisie 60:5–10
Rosenthal E, Zilberbrand M, Livshitz Y (2007) The hydrochemical evolution of brackish groundwater in central and northern Sinai (Egypt) and in the western Negev (Israel). J Hydrol 337(3):294–314
Acknowledgements
The authors gratefully acknowledge the contributions of the technical staff at the Laboratory Applied Hydrosciences Research Unit of Higher Institute of Water Sciences and Techniques of Gabès (Tunisia) for their help during laboratory analysis. This work is co-funded by the European Union through the European Regional Development Fund, based on COMPETE2020, project ICT (UID/GEO/04683/2013) with reference POCI- 01-0145-FEDER-007690 by FCT.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 Springer Nature Switzerland AG
About this paper
Cite this paper
Dhaoui, O., Antunes, I.M.H.R., Agoubi, B. (2021). Sustainability and Management of the Menzel Habib Aquifer System, Southeastern Tunisia. In: Abrunhosa, M., Chambel, A., Peppoloni, S., Chaminé, H.I. (eds) Advances in Geoethics and Groundwater Management : Theory and Practice for a Sustainable Development. Advances in Science, Technology & Innovation. Springer, Cham. https://doi.org/10.1007/978-3-030-59320-9_110
Download citation
DOI: https://doi.org/10.1007/978-3-030-59320-9_110
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-59319-3
Online ISBN: 978-3-030-59320-9
eBook Packages: Earth and Environmental ScienceEarth and Environmental Science (R0)