Abstract
Solid-liquid mixtures are very important for the study of molecular interactions that describe the thermodynamic properties of the components. The objective of this work was to determine the behavior of the nitrobenzene and para-xylene mixtures by means of two excess energy models, Margules and Non-Randon Two-Liquid (NRTL), comparing the calculated results with experimental data from the literature. The Margules model, whose parameters varied with temperature, was more accurate than the model that did not consider the temperature variation. The NRTL model was also accurate, presenting only a small deviation in the lower para-xylene compositions, while the ideal case and Margues model with temperature independence presented a large imprecision. Therefore, the two-parameter Margules model with temperature dependence presented the best approximation of the equilibrium system.
Access provided by Autonomous University of Puebla. Download conference paper PDF
Similar content being viewed by others
Keywords
1 Introduction
In solid-liquid mixtures, crystallization is a process where thermodynamic conditions lead the molecules to approach and group in highly organized structures, reaching a state of solid-liquid equilibrium. Among many ways to describe the solid-liquid equilibrium of a binary system, establishing the parameters of an excess energy model may be satisfactory to represent the behavior of the substances in the mixture.
In the phase equilibrium calculations, one of the most important steps is fitting adjustable parameters of activity coefficient models to experimental data [1]. Each model has a certain number of parameters, and their estimation is a common challenging problem. Considering the importance of the study of solid-liquid equilibria, the mixture of xylene and nitrobenzene emerges as an interesting case.
Xylenes are light aromatic hydrocarbons derived from petroleum and processed in petrochemical industries. Para-xylene is part of BTXs (benzene, toluene, and xylene), which may be used to react to producing compounds as nitrobenzene [2]. The study of the equilibrium of nitrobenzene and para-xylene may contribute to describing its synthesis [3]. To the best of our knowledge, the solid-liquid equilibrium modeling of nitrobenzene and para-xylene mixtures using the two-suffix Margules and the Non-Random Two-Liquid (NRTL) models was not investigated in literature before.
Thus, the aim of this study was to determine the behavior of nitrobenzene and para-xylene mixtures by means of two excess energy models, the two-suffix Margules with dependence and independence of temperature and the NRTL, identifying the eutectic point of the mixture and comparing the calculated results with those obtained experimentally by Proust and Fernandez [4].
2 Methods
From the Gibbs excess energy definition of the two-suffix Margules model (Ge) (1), using the definition of Eq. (2), we obtained the equations that determine the activity coefficients (γ) (3 and 4).
The activity coefficients were fitted using compositions obtained experimentally by Proust and Fernandez [4] for different temperatures, considering molar fractions in the liquid phase for para-xylene (x1) and for nitrobenzene (x2), and using an initial estimation for parameters A and B. The calculation of the activity coefficients was repeated considering parameters A and B temperature-dependent.
Theoretical compositions were calculated by means of the equilibrium condition with the variation of activity coefficients for each temperature. Solid and liquid heat capacity were considered the equal and the theoretical composition of the liquid phase in equilibrium was calculated for each experimental temperature (5).
The deviation between theoretical and experimental compositions (6) of the calculated liquid phase (ARD %) was minimized by varying the parameters (A and B) of activity coefficients (3 and 4). The deviations were evaluated neglecting experimental or calculated values for molar fractions equal to zero or one (pure substances).
The second model used was NRTL (7).
Taking the definition presented by Eq. (2), we obtain (8 and 9).
With the parameters defined as (10, 11, 12 and 13).
The same procedure used to obtain theoretical compositions in Margule’s excess energy model was used to obtain the theoretical compositions of the mixture for the NRTL model. Subsequently, the theoretical composition of the liquid phase of the mixture was calculated for the ideal case. Table 1 shows the properties of the pure components used in this study.
3 Results and Discussion
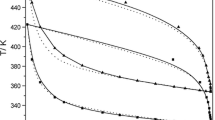
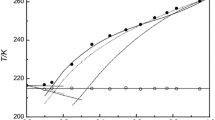
Both Figs. 1 and 2 show that different regions can be identified in the phase diagram of the models. These regions separate the zones of the existence of different phases. Eutectic line defines two regions: under it, there is a solid phase composed of para-xylene and nitrobenzene, and over it, there are three other regions. The left region is composed of a nitrobenzene solid phase and a liquid mixture of both compounds. The right region is composed of para-xylene in the solid phase and a liquid mixture. And the central upside region is composed of a liquid mixture of both compounds.

*Experimental data from Proust and Fernandez [4]
Phase diagram of the NRTL model to para-xylene (x1) and nitrobenzene mixture

*Experimental data from Proust and Fernandez [4]
Phase diagram of the Margules model of two parameters dependent on temperature to para-xylene (x1) and nitrobenzene mixture
NRTL usually leads to reasonable phase equilibria predictions [5, 6]. NRTL model (Fig. 1) provided eutectic point compatible with experimental data, both in temperature (259.22 K) and composition ([x1 = 0.38] and [x2 = 0.62]). However, it was evidenced that along the curve for low para-xylene molar fraction, there was a deviation when compared to the experimental one (ARD = 3.56%). The parameters of this model were: α equal to 0.015, b12 equal to 9.896 and b21 equal to 10.437. Also, in order to define the behavior of all parameters, some correlations were obtained as follows:
In the two-suffix Margules model of Gibbs excess energy with temperature dependence (Fig. 2), there was a better estimation of the composition as a function of temperature. Thus, the calculated eutectic point was compatible with the experimental one, both in temperature (259.22 K) and in molar composition ([x1 = 0.38] and [x2 = 0.62]). The correlations of the molar fraction of nitrobenzene with both parameters A and B are presented below. These correlations do not consider the points x1 = 0 and x1 = 1, because these points did not adapt to any model proposed.
Despite its simplicity, the Margules equations showed a reasonable quality (ARD = 0.01%) for the description of eutectic solid-liquid equilibrium profiles [7].
In the two-suffix Margules model of Gibbs excess energy with temperature independence (Fig. 3), (ARD = 27.15%) the eutectic point was also at the same temperature (259.22 K) of that obtained with experimental data. However, the composition of molar fraction in the liquid phase was different (experimental [x1 = 0.38] and [x2 = 0.62], model [x1 = 0.27], and [x2 = 0.73]). The parameter A of this model was equal to 10,833.546 and B were equal to 185.106.

*Experimental data from Proust and Fernandez [4]
Phase diagram of the Margules model of two parameters independent on temperature to para-xylene (x1) and nitrobenzene mixture
In the ideal case (Fig. 4), both the range of the para-xylene composition from 0.2–0.8 as the eutectic point was different from experimental data (ARD = 7.93%). This can be justified by the fact that in the ideal case, the interactions between distinct molecules are disregarded.

*Experimental data from Proust and Fernandez [4]
Phase diagram of the ideal case to para-xylene (x1) and nitrobenzene mixture
Margules model, whose parameters varied with temperature, was more accurate than the one without considering temperature changes. In fact, this result is in good agreement with the observations presented by Rocha and Guirardello [8] that also used the two-parameter Margules equation, with temperature dependence, to model the liquid phase in solid-liquid equilibria for many other binary systems.
Furthermore, NRTL was also accurate, showing a small deviation in the lower molar composition of para-xylene. NRTL model is also efficient to provide a great approximation of solid-liquid equilibrium to other systems [9]. Literature also brings the use of Margules and NRTL models to represent other solid-liquid equilibrium systems. Pelaquim et al. [10] used NRTL and three-suffix Margules models to describe the properties of the liquid of many solid-liquid mixtures of fatty acids. Likewise, Matos et al. [11] evaluated binary solid-liquid equilibrium of fatty acids, fatty alcohols, and triolein, in which the liquid lines of these systems were adequately described. Three-suffix Margules and NRTL models were also accurate in representing the solid-liquid equilibrium of octacosanol in three pure solvents 1-pentanol, 1-hexanol, and toluene [12].
Margules model with temperature independence, however, showed great deviation when compared to experimental data, even bigger than the ideal case deviation. This is justified by the lack of information that the absence of temperature dependence causes. This mixture shows an increase in the intensity of attractive molecular interactions with increasing system temperature [3]. The literature also highlights the need to develop unified interaction parameters to represent all possible phase equilibria [5].
4 Conclusion
This study presented an analysis of Gibbs’s excess energy models to describe the equilibrium system of the solid-liquid mixture of para-xylene and nitrobenzene. It was found that the Margules model of two parameters with temperature dependence was the best to represent the equilibrium of the system. NRTL model also provided a good prediction of the experimental data. However, Margule’s model of two parameters independent of temperature did not represent the mixture effectively. The representation of the ideal case was also not accurate.
Although some models are known to represent well mixtures, there are some cases that they can diverge, so it is very important to compare experimental data with the modeling results. The agreement between the predicted results by means of Margules and NRTL models with the experimental phase equilibrium data contributes to the modeling of solid-liquid mixtures with nitrobenzene and para-xylene.
References
Vatani, M., Asghari, M., Vakili-Nezhaad, G.: Application of Genetic Algorithm to the calculation of parameters for NRTL and Two-Suffix Margules models in ternary extraction ionic liquid systems. J. Indus. Eng. Chem. 18, 1715–1720 (2012). https://doi.org/10.1016/j.jiec.2012.03.008
Franck, H.G., Stadelhofer, J.W.: Production of benzene, toluene and xylenes. In: Industrial Aromatic Chemistry. Springer, Berlin, Heidelberg. pp. 99–131 (1988). doi: 10.1007/978–3-642-73432-8_4
Rafiee, H.R., Frouzesh, F.: The study of partial and excess molar volumes for binary mixtures of nitrobenzene and bensenaldehyde with xylene isomers from T = (298.15 to 318.15) K and P = 0.087 MPa. J. Adv. Res. 7(5), 769–780 (2016). https://doi.org/10.1016/j.jare.2015.11.003
Proust, P.C., Fernandez, J.C.: Experimental solid-liquid equilibria of binary mixtures of organic compounds. Fluid Phase Equilib. 29, 265–272 (1986)
Moudjari, Y., Louaer, W., Meniai, A.: Modeling of solid-liquid equilibrium using a modified group contribution based NRTL model. Fluid Phase Equilib. 492, 118–136 (2019). https://doi.org/10.1016/j.fluid.2019.03.021
Zhang, X., Zhao, Y., Liu, Y., Tang, W.: The effects of solvent properties on solid-liquid phase equilibrium of ethylene thiourea. J. Mol. Liq. 285, 459–467 (2019). https://doi.org/10.1016/j.molliq.2019.03.178
Maximo, G.J., Carareto, N.D.D., Costa, M.: Chapter 8—Solid-liquid equilibrium in food processes. In: Thermodynamics of Phase Equilibria in Food Engineering, pp. 335–384 (2019). https://doi.org/10.1016/b978-0-12-811556-5.00008-9
Rocha, S.A., Guirardello, R.: An approach to calculate solid-liquid phase equilibrium for binary mixtures. Fluid Phase Equilib. 281, 12–21 (2009). https://doi.org/10.1016/j.fluid.2009.03.020
Tan, Q., Leng, Y., Wang, J., Huang, C., Yuan, Y.: Correlation and prediction of the solubility of the racemic tartaric acid–ethanol-water system with the NRTL model. J. Mol. Liq. 216, 476–483 (2016). https://doi.org/10.1016/j.molliq.2016.01.080
Pelaquim, F.P., Matos, F.C., Cardoso, L.P., Batista, E.A.C., Meirelles, A.J.A., Costa, M.C.: Solid-liquid phase equilibrium diagrams of binary mixtures containing fatty acids, fatty alcohol compounds and tripalmitin using differential scanning calorimetry. Fluid Phase Equilib. 497, 19–32 (2019). https://doi.org/10.1016/j.fluid.2019.05.020
Matos, F.C., Costa, M.C., Meirelles, A.J.A., Batista, E.A.C.: 12. Binary solid-liquid equilibrium systems containing fatty acids, fatty alcohols and triolein by differential scanning calorimetry. Fluid Phase Equilib. 404, 1–8 (2015). https://doi.org/10.1016/j.fluid.2015.06.015
Cuevas, M.S., Crevelin, E.J., Moraes, L.A.B., Oliveira, A.L., Rodrigues, C.E.C., Meirelles, A.J.A.: Solubility of commercial octacosanol in organic solvents and their correlation by thermodynamic models at different temperatures. J. Chem. Thermodynamics 110, 186–192 (2017). https://doi.org/10.1016/j.jct.2017.02.025
Acknowledgements
To the Federal University of Technology—Paraná (UTFPR).
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 The Editor(s) (if applicable) and The Author(s), under exclusive license to Springer Nature Switzerland AG
About this paper
Cite this paper
Fontana, M., dos Santos Ribas, C., Schuck, P.H., Turino, R.L., Zuber, A. (2021). Modeling of Solid-Liquid Equilibrium in Nitrobenzene and Para-Xylene Mixtures. In: Iano, Y., Arthur, R., Saotome, O., Kemper, G., Borges Monteiro, A.C. (eds) Proceedings of the 5th Brazilian Technology Symposium. Smart Innovation, Systems and Technologies, vol 202. Springer, Cham. https://doi.org/10.1007/978-3-030-57566-3_36
Download citation
DOI: https://doi.org/10.1007/978-3-030-57566-3_36
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-57565-6
Online ISBN: 978-3-030-57566-3
eBook Packages: EngineeringEngineering (R0)