Abstract
Global obesity is rising at an alarming rate, with estimates predicting that by 2030 nearly 1 in 2 adults will have obesity (BMI ≥ 30 kg/m2), and 1 in 4 adults will have severe obesity (BMI ≥ 35 kg/m2). With this increasing number of patients with severe obesity and related comorbidities, there has been an increasing role for bariatric surgery in managing these conditions. The most striking upsurge was seen in the number of sleeve gastrectomies (SG) performed, rising from 17.8% of total procedures performed in 2011 to 61.4% of total procedures performed in 2018. This increasing popularity of SG over the past decade has been due to its safety profile, technical ease, and excellent long-term efficacy. However, a blanket prescription of this procedure should be avoided, and an effort towards more personalized and evidence-based procedure selection should be adopted. In this chapter, we will first explore the current indications for metabolic and bariatric surgery, followed by a criterion that makes SG a better surgical option.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
Global obesity is rising at an alarming rate, with estimates predicting that by 2030 nearly 1 in 2 adults will have obesity (BMI ≥ 30 kg/m2), and 1 in 4 adults will have severe obesity (BMI ≥ 35 kg/m2) [1]. It is well studied that obesity increases the risk of other chronic medical conditions, including type 2 diabetes mellitus, cardiovascular disease, cerebrovascular, chronic kidney disease, nonalcoholic fatty liver disease, metabolic syndrome, and many cancers. With an increasing number of patients with severe obesity and related comorbidities, there is an increasing role of bariatric surgery in managing these conditions, especially diabetes. Each year there is an increase in the number of bariatric procedures performed in the US as per the American Society for Metabolic and Bariatric Surgery (ASMBS) estimate of bariatric surgery numbers. The most significant upsurge seen in the number of sleeve gastrectomy (SG) performed rose from 17.8% of total procedures performed in 2011 to 61.4% of total procedures performed in 2018 [2, 3]. This increasing popularity of SG over the past decade has been due to its safety profile, technical ease, and excellent long-term efficacy. However, a blanket prescription of this procedure should be avoided, and an effort towards more personalized and evidence-based procedure selection should be adopted. In this chapter, we will first explore the current indications for metabolic and bariatric surgery, followed by a criterion that makes SG a better surgical option.
2 Current Eligibility Criteria for Bariatric Surgery
In 1991, National Institutes of Health (NIH) Consensus Development Panel first outlined the universally accepted guidelines for surgery for obesity and weight-related disease [4]. Since then the guidelines have been repeatedly revised and expanded over the years, most American and international societies have agreed on general guidelines for the indication of bariatric and metabolic surgery as listed below.
-
Adults with a BMI ≥ 40 kg/m2 regardless of comorbid illness.
-
Adults with a BMI 35.0–39.9 kg/m2 with comorbidities, including:
-
o
Type 2 diabetes (T2D)
-
o
Hypertension
-
o
Hyperlipidemia
-
o
Obstructive sleep apnea (OSA)
-
o
Nonalcoholic steatohepatitis (NASH)
-
o
Pseudotumor cerebri
-
o
Gastroesophageal reflux disease
-
o
Asthma
-
o
Venous stasis disease
-
o
Severe urinary incontinence
-
o
Debilitating arthritis
-
o
Impaired quality of life
-
o
-
Adults with a BMI 30.0–34.9 kg/m2 with severe comorbidities listed above, especially T2D.
-
Furthermore, potential surgical candidates must have the following conditions:
-
Inability to achieve a healthy weight loss for a while with prior weight loss efforts.
-
Absence of drug and alcohol problems
-
No uncontrolled psychological conditions
-
A capacity to understand the risks and commitment associated with the surgery.
3 Age
In their Consensus Statement of the NIH Consensus Development Conference (1991) and the International Federation for the Surgery of Obesity and Metabolic Disorders (IFSO) has age criterion—between the ages of 18 to 65 years [4, 5]. However, there is considerable flexibility in these recommendations [6]. In recent years, there is a growing interest in the use of bariatric surgery at the extremes of age. Bariatric surgery in the adolescent will be discussed in-depth in another section later in the chapter. Briefly, the ASMBS pediatric committee recommends an adult BMI threshold selection criterion as it appears to be appropriate for adolescents, with some modification about associated comorbid disease thresholds [7]. A significant concern when dealing with adolescents is their degree of maturity and onset of puberty. These need to be taken into account when surgical treatment is recommended along with other psychological factors, metabolic issues, functional comorbidities, quality of life, and attention to long-term health risks in the absence of treatment in a patient who otherwise has a long life expectancy [6].
In the advanced age (>60 years), the higher perioperative risk due to anesthesia, complex medical comorbidities, and increased risk of mortality from complications have been cited as risk factors precluding bariatric surgery some series [8]. Besides, the elderly are less compliant with new dietary and lifestyle changes. However, more recent studies have shown not only low morbidity and mortality but a clinically significant weight loss and improvement in comorbidities in older patients [9,10,11,12,13,14]. In their comparison of patients > 60 years who underwent bariatric surgery from 2009 to 2013, with those who underwent bariatric surgery from 1999 to 2005, Gebhart et al. found a significant decrease in mortality (0.3%) [15]. A systematic review by Giordano et al. of 26 articles with 8,149 patients showed a pooled mortality of 0.01%, and the overall complication rate was 15%. More recently, Nor Hanipah et al. suggested that bariatric surgery is safe and effective in patients aged ≥ 75 years older when carefully selected [14]. Similarly, Susmallian et al. analyzed the results of bariatric surgery in elderly patients (>65 years) for three years [12]. They noted a perioperative complication rate of 9%, with 1.3% needing re-operative intervention. These rates are slightly higher than those for the younger patient. However, most complications result from an increasing number of comorbidities seen in these patient populations. Often these complications do not need a major intervention. The authors believe that patients should not be denied bariatric surgery solely based on their age. It is vital to carefully counsel the elder patient about their slightly increased risks of morbidity and the possibility of less satisfactory outcomes.
4 BMI
From its inception, patient selection based on NIH Consensus statements for bariatric procedures has been based on BMI [4]. This selection criterion is inadequate as BMI is a poor indicator of adiposity, metabolic disease, and cardiovascular risk. It is a well known fact that a higher composition of visceral fat is associated with increased liver, muscle and pancreatic fat that results in a higher metabolic condition in individuals with same BMI [16,17,18,19]. BMI does not account for different body composition related to gender or age; that is, females and older patients have high body fat compared to males and younger patients, respectively. Also, there is an interracial difference in fat distribution; for example, an Asian has more body fat than a Caucasian with the same BMI [20]. The BMI criterion for bariatric procedures should be adjusted for ethnicity (e.g., 18.5–22.9 kg/m2 is healthy range, 23–24.9 kg/m2 overweight, and ≥25 kg/m2 obesity for Asians). The recent ASMBS updated position statement on bariatric surgery in class I obesity recommends patients with a BMI of 30 to 35 kg/m2 with obesity-related comorbidities be considered for surgical intervention after the failure of nonsurgical treatments [21]. This is particularly relevant for uncontrolled type 2 diabetes patients, as there are high-quality data that show significant benefit. Presently, patients in the 18 to 65 age group who have class I obesity along with severe comorbidities have the best evidence for bariatric and metabolic surgery.
5 Procedure Selection
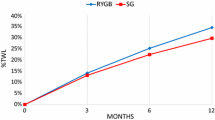
A bariatric procedure should be selected based on individualized therapy goals, personalized risk stratification based on the patient’s medical history, and available surgical expertise. Aminian et al. developed and validated an individualized metabolic surgery (IMS) score to aid the evidence-based procedure selection for T2D [22]. The IMS score uses four independent predictors of diabetes remission—preoperative duration of T2D, HbA1C level, number of diabetes medications, and insulin use before surgery. This was the largest reported cohort of patients with T2D (n = 900), which had a minimum 5-year glycemic data after RYGB and SG (median follow-up time of 7 years, range: 5–12 years). The IMS score categorizes T2D into three validated stages of severity to guide procedure selection—mild, moderate, and severe. In mild disease (IMS score 25 points or less), SG and RYGB are recommended as both are highly effective in the treatment of T2D. However, RYGB results in slightly higher long-term diabetes remission rates and a reduction in the number of diabetes medications. In severe T2D (IMS Score > 95) where there is a limited functional beta-cell reserve in the pancreas, both procedures have similarly low efficacy in achieving diabetes remission, reduction in HbA1C level, and use of diabetes medications. In the large intermediate disease group, RYGB is recommended over SG as it achieves better long-term diabetes remission, this is likely related to its more pronounced effect on the gut hormones and neuroendocrine milieu. In the original cohort with the intermediate diabetes severity, long term diabetes remission was observed in 60% of patients who underwent RYGB versus 25% of those who had SG. The IMS score calculator (smartphone application) computes a score when the patient’s data are entered, and recommendation for an average surgical risk patient is provided. Given that the IMS cohort had patients with higher BMI and involved mostly patients from the US and Spain, it may not accurately apply to patients with class I obesity or Asian ethnicity. Another limitation of this model was that it did not include bariatric surgical procedures with more potent metabolic effects such as malabsorptive procedures (Duodenal Switch, SADI). Generally, RYGB and SG appear to be similarly effective in improving hypertension, dyslipidemia, and quality-of-life indices [23,24,25,26].
6 Other Considerations in Decision-Making
A procedure may be preferred over the other depending on the individual patient’s condition [22, 25]. In patients with extremely high BMI and higher surgical risk, SG may be chosen because the limited working space makes a bypass procedure challenging and unsafe. After losing weight, these patients may be a candidate for diversionary procedures.
SG is favored in patients with multiple small bowel diseases like Crohn’s disease [27], multiple small bowel resection, dense adhesions, large complex ventral hernias due to technical reasons [25].
Compared with GI bypass procedures, SG has minimal effect on the pharmacokinetics of medications as it does not alter gut absorption. This makes SG most suitable in patients with complex psychiatric and addiction history requiring psychotropic polypharmacy [22, 24, 28].
SG is a better choice for patients who smoke [29, 30] or are dependent on chronic nonsteroidal anti-inflammatory drugs as it circumvents the risk of marginal ulceration seen in RYGB patients [25].
Similarly, in patients with history of duodenal ulcer, increased risk of gastric cancer, or patient in whom access to distal stomach, duodenum, and biliary tree would be necessary in future, SG is a prudent choice, as RYGB precludes easy access to the remnant stomach and duodenum.
RYGB would be preferred over SG in patients with severe gastroesophageal reflux disorder (GERD) or Barrett’s esophagus [28]. There are concerning reports of worsening or development of de novo GERD after SG, which may progress to Barrett’s esophagus. Although its significance has yet to be determined, GERD after SG can be effectively treated with medical therapy in most cases. In 5% to 10% of patients with GERD after SG, medical management is ineffective controlling the symptoms. In these cases, a conversion to RYGB is warranted [25].
In patients with osteoporosis or decreased bone density, SG may be a better option compared with RYGB. Calcium and vitamin D deficiencies are commonly seen after bariatric surgery that is responsible for the accelerated bone loss. Multiple studies have shown more significant bone loss after RYGB than SG at the femoral neck [31, 32]. Comparative studies have noted a significantly increase in circulating bone turnover markers such as CTX, PINP, TRAcP5b in the RYGB compared with SG [33]. This could also be related to the different hormonal responses induced by these procedures [33,34,35].
A meta-analysis of 12 studies concluded that RYGB surgery is associated with a higher risk of renal stone and increased urine oxalate and calcium oxalate supersaturation [36]. In an another meta-analysis, SG was associated with reduced kidney stones formation (pooled risk ratio of 0.37, 95% CI 0.16–0.85) compared with RYGB that was associated with increased risk (pooled risk ratio of 1.73, 95% CI 1.30–2.30) [37]. The overall pooled risk ratio of kidney stones in patients undergoing bariatric surgery was 1.22 (95% CI 0.63–2.35). It has been postulated that the fat malabsorption induced enteric hyperoxaluria results in an increased risk of kidney stones in RYGB and other malabsorptive procedures.
Portomesenteric and splenic vein thrombosis (PMSVT) is a rare but potentially severe complication after bariatric surgery. A meta-analysis and systematic review of 41 studies reported that SG is associated with remarkably increased risk of PMSVT when compared with RYGB [38]. The estimated incidence was 0.4%, and 43% of patients had a history of the hypercoagulable disorder. Hence, in patients who have hypercoagulable disorders, an RYGB would be recommended.
Patients with increased risk of colon cancer need special consideration. In general, following bariatric surgery, the risk of hormone-related cancer like breast, endometrial, and prostate cancer reduces. However, an English study has shown that gastric bypass was also associated with a greater than two-fold increase risk of colorectal cancer (odd ratio 2⋅63, 95% CI 1⋅17 to 5⋅95) [39]. Similar results were seen in an earlier Swedish study [40] and a Nordic study, they also noted the increased risk is exaggerated with longer follow-ups [41]. There are some postulations to explain this anomaly to the protective effect of bariatric surgery on overall cancer incidence. The inflammatory environment stimulates hyperproliferation of the bowel mucosa after RYGB in a rat model [42]. Furthermore, similar results of increased proliferation of rectal mucosa along with elevated bio-marker levels were seen in patients who underwent RYGB [43]. The changes in the gut microbiome that partially mediates the metabolic changes could be responsible [44, 45]. In summary, the increased risk of colorectal cancer is that following RYGB, the local mucosal changes occur secondary to the malabsorptive effects and changes in the gut microbiome. The authors recommend that patients with an inherent increase risk of colorectal cancer undergo SG.
Management of blood sugar in some patients with type 1 diabetes after bariatric surgery can be extremely challenging. Particularly, some patients with type 1 diabetes may develop severe hypoglycemia after diversionary bariatric procedures. In patients with type 1 diabetes, SG, which is associated with more predictable absorption of carbohydrates and fat-soluble nutrients, would be a preferred procedure, unless there is a reason not to perform SG [46].
Outlined in Table 1 is the bariatric procedure selection (RYGB or SG) based on the patient’s condition.
7 Summary
-
In conclusion, for better outcomes, it is crucial to guide patients towards the most suitable bariatric procedure depending on their obesity-related comorbidities and overall medical conditions.
-
BMI ≥ 40 kg/m2 or BMI ≥ 35 kg/m2 with obesity-related comorbidities are used as eligibility criteria for bariatric surgery selection. However, there are reliable data that support the positive impact of these operations in patients with lower BMI (30–35 kg/m2) with uncontrolled metabolic conditions.
-
The current review of the literature indicates that RYGB and SG have positive effects on improvement of T2D and that RYGB has a more substantial effect on remission. RYGB and SG appear to be similarly effective in improving hypertension, dyslipidemia, and quality-of-life indices.
-
The IMS score is an evidence-based and validated prediction tool that can help in a personalized selection process of metabolic surgery in patients with T2D and obesity.
-
The surgical risk, differential impact of each procedure on weight and other obesity-related diseases (e.g., GERD), presence of other medical and mental problems, patient’s behavioral factors (e.g., postoperative compliance, active smoking), medications (e.g., chronic nonsteroidal anti-inflammatory drugs, immunosuppressive medications, or psychotropic polypharmacy), and patient’s values and goals should be considered when the patient and medical team make a shared decision about the most appropriate surgical procedure [25].
References
Ward ZJ, Bleich SN, Cradock AL, et al. Projected US state-level prevalence of adult obesity and severe obesity. N Engl J Med. 2019;381(25):2440–50. https://doi.org/10.1056/NEJMsa1909301.
Surgery AS for M and B. Estimate of Bariatric Surgery Numbers, 2011–2017. WwwAsmbsOrg. 2018.
English WJ, DeMaria EJ, Hutter MM, et al. American Society for Metabolic and Bariatric Surgery 2018 estimate of metabolic and bariatric procedures performed in the United States. Surg Obes Relat Dis. 2020;16(4):457–63. https://doi.org/10.1016/j.soard.2019.12.022.
Chair MG, Barondess JM, Bellegie NJ, et al. Gastrointestinal surgery for severe obesity: Consensus statement. Nutr Today. 1991;26(5):32–5. https://doi.org/10.1097/00017285-199109000-00007.
International Federation for the surgery of obesity. IFSO—Are you a candidate.
Luca M De, Angrisani L, Himpens J, et al. for the Surgery of Obesity and Metabolic Disorders ( IFSO ). 2018;26(8):1659–1696. doi:https://doi.org/10.1007/s11695-016-2271-4.Indications
Michalsky M, Reichard K, Inge T, Pratt J, Lenders C. ASMBS pediatric committee best practice guidelines. Surg Obes Relat Dis. 2012;8(1):1–7. https://doi.org/10.1016/j.soard.2011.09.009.
Livingston EH, Langert J. The impact of age and medicare status on bariatric surgical outcomes. Arch Surg. 2006;141(11):1115–20. https://doi.org/10.1001/archsurg.141.11.1115.
Aminian A, Brethauer SA, Sharafkhah M, Schauer PR. Development of a sleeve gastrectomy risk calculator. Surg Obes Relat Dis. 2015;11(4):758–64.
Sugerman HJ, DeMaria EJ, Kellum JM, Sugerman EL, Meador JG. Wolfe LG Effects of bariatric surgery in older patients. Ann Surg. 2004;240(2):243–7. https://doi.org/10.1097/01.sla.0000133361.68436.da.
Dunkle-Blatter SE, St. Jean MR, Whitehead C, et al. Outcomes among elderly bariatric patients at a high-volume center. Surg Obes Relat Dis. 2007;3(2):163–169. doi:https://doi.org/10.1016/j.soard.2006.12.004
Susmallian S, Raziel A, Barnea R, Paran H. Bariatric surgery in older adults. Medicine (Baltimore). 2019;98(3):e13824. https://doi.org/10.1097/md.0000000000013824.
Koh CY, Inaba CS, Sujatha-Bhaskar S, Nguyen NT. Outcomes of laparoscopic bariatric surgery in the elderly population. Am Surg. 2018.
Nor Hanipah Z, Punchai S, Karas LA, et al. The Outcome of Bariatric Surgery in Patients Aged 75 years and Older. Obes Surg. 2018. https://doi.org/10.1007/s11695-017-3020-z.
Gebhart A, Young MT, Nguyen NT. Bariatric surgery in the elderly: 2009–2013. Surg Obes Relat Dis. 2015;11(2):393–8. https://doi.org/10.1016/j.soard.2014.04.014.
Müller MJ, Lagerpusch M, Enderle J, Schautz B, Heller M, Bosy-Westphal A. Beyond the body mass index: Tracking body composition in the pathogenesis of obesity and the metabolic syndrome. Obes Rev. 2012;13(SUPPL.2):6–13. doi:https://doi.org/10.1111/j.1467-789X.2012.01033.x
Okorodudu DO, Jumean MF, Montori VM, et al. Diagnostic performance of body mass index to identify obesity as defined by body adiposity: A systematic review and meta-analysis. Int J Obes. 2010;34(5):791–9. https://doi.org/10.1038/ijo.2010.5.
Gómez-Ambrosi J, Silva C, Galofré JC, et al. Body mass index classification misses subjects with increased cardiometabolic risk factors related to elevated adiposity. Int J Obes. 2012;36(2):286–94. https://doi.org/10.1038/ijo.2011.100.
Gómez-Ambrosi J, Silva C, Galofré JC, et al. Body adiposity and type 2 diabetes: Increased risk with a high body fat percentage even having a normal BMI. Obesity. 2011;19(7):1439–44. https://doi.org/10.1038/oby.2011.36.
Corbel MJ, Tolari F, Yadava VK, WHO Expert Consultation. Appropriate body-mass index for Asian populations and its implications. Lancet. 2004;363(9403):157–163. doi:https://doi.org/10.1016/S0140-6736(03)15268-3
Aminian A, Chang J, Brethauer SA, Kim JJ. ASMBS updated position statement on bariatric surgery in class I obesity (BMI 30–35 kg/m 2). Surg Obes Relat Dis. 2018;14(8):1071–87. https://doi.org/10.1016/j.soard.2018.05.025.
Aminian A, Brethauer SA, Andalib A, et al. Individualized Metabolic Surgery Score: Procedure Selection Based on Diabetes Severity. In: Annals of Surgery. ; 2017. doi:https://doi.org/10.1097/SLA.0000000000002407
Schauer PR, Bhatt DL, Kirwan JP, et al. Bariatric surgery versus intensive medical therapy for diabetes—5-year outcomes. N Engl J Med. 2017. https://doi.org/10.1056/NEJMoa1600869.
Peterli R, Wolnerhanssen BK, Peters T, et al. Effect of laparoscopic sleeve gastrectomy vs laparoscopic roux-en-y gastric bypass onweight loss in patients with morbid obesity the sm-boss randomized clinical trial. JAMA—J Am Med Assoc. 2018. https://doi.org/10.1001/jama.2017.20897.
Aminian A. Bariatric procedure selection in patients with type 2 diabetes: choice between Roux-en-Y gastric bypass or sleeve gastrectomy. Surg Obes Relat Dis. 2020;16(2):332–9.
Salminen P, Helmio M, Ovaska J, et al. Effect of laparoscopic sleeve gastrectomy vs laparoscopic roux-en-y gastric bypass onweight loss at 5 years among patients with morbid obesity the SLEEVEPASS randomized clinical trial. JAMA—J Am Med Assoc. 2018. https://doi.org/10.1001/jama.2017.20313.
Aminian A, Andalib A, Ver MR, Corcelles R, Schauer PR, Brethauer SA. Outcomes of Bariatric Surgery in Patients with Inflammatory Bowel Disease. Obes Surg. 2016. https://doi.org/10.1007/s11695-015-1909-y.
Ames GE, Clark MM, Gothe KB, Collazo-Clavell ML, Elli EF. Which Bariatric Surgery is Best for My Patient: Guiding Patients Toward the Optimal Surgical Treatment for Obesity While Supporting Autonomy Bariatric Times. Bariatric Times. 2018;15(11):18–27.
Haskins IN, Nowacki AS, Khorgami Z, et al. Should recent smoking be a contraindication for sleeve gastrectomy? Surg Obes Relat Dis. 2017. https://doi.org/10.1016/j.soard.2017.02.028.
Inadomi M, Iyengar R, Fischer I, Chen X, Flagler E, Ghaferi AA. Effect of patient-reported smoking status on short-term bariatric surgery outcomes. Surg Endosc. 2018. https://doi.org/10.1007/s00464-017-5728-1.
Lupoli R, Lembo E, Saldalamacchia G, Avola CK, Angrisani L, Capaldo B. Bariatric surgery and long-term nutritional issues. World J Diabetes. 2017;8(11):464–74. https://doi.org/10.4239/wjd.v8.i11.464.
Bredella MA, Greenblatt LB, Eajazi A, Torriani M. Yu EW Effects of Roux-en-Y gastric bypass and sleeve gastrectomy on bone mineral density and marrow adipose tissue. Bone. 2017;95:85–90. https://doi.org/10.1016/j.bone.2016.11.014.
Ivaska KK, Huovinen V, Soinio M, et al. Changes in bone metabolism after bariatric surgery by gastric bypass or sleeve gastrectomy. Bone. 2017. https://doi.org/10.1016/j.bone.2016.11.001.
Hage MP, El-Hajj FG. Bone and mineral metabolism in patients undergoing Roux-en-Y gastric bypass. Osteoporos Int. 2014. https://doi.org/10.1007/s00198-013-2480-9.
Folli F, Sabowitz BN, Schwesinger W, Fanti P, Guardado-Mendoza R, Muscogiuri G. Bariatric surgery and bone disease: From clinical perspective to molecular insights. Int J Obes. 2012. https://doi.org/10.1038/ijo.2012.115.
Upala S, Jaruvongvanich V, Sanguankeo A. Risk of nephrolithiasis, hyperoxaluria, and calcium oxalate supersaturation increased after Roux-en-Y gastric bypass surgery: a systematic review and meta-analysis. Surg Obes Relat Dis. 2016. https://doi.org/10.1016/j.soard.2016.04.004.
Thongprayoon C, Cheungpasitporn W, Vijayvargiya P, Anthanont P, Erickson SB. The risk of kidney stones following bariatric surgery: A systematic review and meta-analysis. Ren Fail. 2016. https://doi.org/10.3109/0886022X.2015.1137186.
Shoar S, Saber AA, Rubenstein R, et al. Portomesentric and splenic vein thrombosis (PMSVT) after bariatric surgery: a systematic review of 110 patients. Surg Obes Relat Dis. 2018. https://doi.org/10.1016/j.soard.2017.09.512.
Mackenzie H, Markar SR, Askari A, et al. Obesity surgery and risk of cancer. Br J Surg. 2018;105(12):1650–7. https://doi.org/10.1002/bjs.10914.
Derogar M, Hull MA, Kant P, Östlund M, Lu Y, Lagergren J. Increased risk of colorectal cancer after obesity surgery. Ann Surg. 2013;258(6):983–8. https://doi.org/10.1097/SLA.0b013e318288463a.
Tao W, Artama M, von Euler-Chelpin M, et al. Colon and rectal cancer risk after bariatric surgery in a multicountry Nordic cohort study. Int J Cancer. 2019:1–8. doi:https://doi.org/10.1002/ijc.32770
Li JV, Reshat R, Wu Q, et al. Experimental bariatric surgery in rats generates a cytotoxic chemical environment in the gut contents. Front Microbiol. 2011. https://doi.org/10.3389/fmicb.2011.00183.
Kant P, Sainsbury A, Reed KR, et al. Rectal epithelial cell mitosis and expression of macrophage migration inhibitory factor are increased 3 years after Roux-en-Y gastric bypass (RYGB) for morbid obesity: Implications for long-term neoplastic risk following RYGB. Gut. 2011. https://doi.org/10.1136/gut.2010.230755.
Palleja A, Kashani A, Allin KH, et al. Roux-en-Y gastric bypass surgery of morbidly obese patients induces swift and persistent changes of the individual gut microbiota. Genome Med. 2016. https://doi.org/10.1186/s13073-016-0312-1.
Farin W, Oñate FP, Plassais J, et al. impact of laparoscopic Roux-en-Y gastric bypass and sleeve gastrectomy on gut microbiota: a metagenomic comparative analysis. Surg Obes Relat Dis. 2020. https://doi.org/10.1016/j.soard.2020.03.014.
Kirwan JP, Aminian A, Kashyap SR, Burguera B, Brethauer SA, Schauer PR. Bariatric Surgery in Obese Patients With Type 1 Diabetes. Diabetes Care. 2016;39(6):941–8. https://doi.org/10.2337/dc15-2732.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 The Editor(s) (if applicable) and The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Shariff, F., Aminian, A. (2021). Eligibility Criteria for Sleeve Gastrectomy. In: Al-Sabah, S., Aminian, A., Angrisani, L., Al Haddad, E., Kow, L. (eds) Laparoscopic Sleeve Gastrectomy. Springer, Cham. https://doi.org/10.1007/978-3-030-57373-7_9
Download citation
DOI: https://doi.org/10.1007/978-3-030-57373-7_9
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-57372-0
Online ISBN: 978-3-030-57373-7
eBook Packages: MedicineMedicine (R0)