Abstract
Cannabis-derived cannabinoids have neuroactive properties. Recently, there has been emerging interest in the use of cannabidiol (CBD)-enriched products for treatment of drug-resistant epilepsy. In 2018, the FDA approved the use of CBD-rich Epidiolex for two severe forms of epilepsy in children (Lennox-Gastaut and Dravet syndromes). Experimental research supports the use of CBD in many CNS disorders, though the mechanisms underlying its anticonvulsant and neuroprotective effects remain unclear. CBD has been shown to reduce inflammation, protect against neuronal loss, normalize neurogenesis, and act as an antioxidant. These actions appear to be due to the multimodal mechanism of action of CBD in the brain. This chapter briefly describes the current information on cannabis pharmacology with an emphasis on the clinical utility of CBD in the treatment of refractory epilepsies and other related seizure conditions. Clinical trials are ongoing for other forms of epilepsy and refractory seizures associated with infantile spasms, tuberous sclerosis, and Rett syndrome. Overall, adjunct CBD has been found to be generally safe and effective for treatment-resistant seizures in children with severe early-onset epilepsy. Whether an add-on CBD is efficacious for the long-term treatment of various epilepsy and seizure types in adults being tested in various clinical trials.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
7.1 Introduction
Epilepsy is the most complex and devastating chronic brain disorder in children and adults. Despite the influx of new anticonvulsant drugs (AEDs) over the past 50 years, 30–40% of epileptic patients still experience refractory seizures that are untreatable by current medications. These patients with treatment-resistant seizures are at a much higher risk for persistent brain damage and other secondary consequences of epileptic seizures that adversely influence quality of life. Polypharmacy to manage such seizures is associated with serious side effects such as sedation, somnolence, and cognitive impairment. This is commonly observed in children with certain types of devastating pediatric epilepsies, such as Lennox-Gastaut, Doose, and Dravet syndromes in which afflicted children have an increased risk of death compared to their peers of the same age (Autry et al. 2010). One explanation for this high percentage of refractory seizures is that preclinical research and screening for AEDs has been biased toward agents that modulate only a single pathology in epileptogenesis; i.e., focused on either reducing hyperexcitation or increasing inhibition via the modulation of ion channels or neurotransmission. This empirical approach often ignores the multimodal intracellular components that might serve as novel targets for the relief of symptomatic seizures. Therefore, the need for different therapeutic options to manage refractory forms of epilepsy is still a current issue.
Cannabis is a generic term for products of the plant Cannabis sativa L. This plant has been used therapeutically for thousands of years (Grotenhermen 2006). Marijuana is a dried mixture of cannabis leaves and flowers. It was well known that the cannabis plant had psychotropic effects, inducing a "high" or euphoric effect. Research over the past few decades led to the identification of cannabinoids, which are categorized into three primary classes: phytocannabinoids, endocannabinoids, and synthetic cannabinoids. Hemp, a strain of Cannabis sativa L. (historically grown for fibrous materials found in stalks and seeds), contains minimal amounts of THC and low levels of CBD. An oil extracted from cannabis seeds by cold pressing is called Hemp oil or hempseed oil. It contains only trace amounts of cannabinoids. CBD oil or hemp CBD oil is an extract obtained from the flowering portions of the hemp plant, then dissolved in coconut or sesame oil. It contains no THC and has no psychoactive properties. Cannabis oil is a concentrated cannabis extract, often containing very high THC levels.
Recently, the cannabis-derived phytocannabinoids have shown compelling evidence as a potential therapy for medication-resistant seizures (Friedman and Devinsky 2015; Reddy and Golub 2016; O’Connell et al. 2017). Specifically, the nonpsychoactive phytocannabinoid, cannabidiol (CBD), has been well tolerated and retains both anticonvulsant and anti-inflammatory properties, though a mechanism of action that has yet to be fully clarified (Reddy and Golub 2016; Billakota et al. 2019). Some CBD-based pharmaceuticals (Epidiolex, Realm Oil, and others) have been suggested as potential therapies for refractory epilepsy. In June 2018, the FDA approved Epidiolex for the treatment of seizures associated with two rare and very severe forms of pediatric epilepsy, Lennox-Gastaut syndrome and Dravet syndrome. Epidiolex has been approved in patients aged two and older in these syndromes; ongoing clinical trials are rapidly underway to determine the efficacy of CBD-based treatments in young, adult, and aged epileptic patients with other types of refractory seizures, as well as for many different neurological conditions.
The topic of cannabis in medical regimens has been widely debated in both the academic and political communities, with a special focus of the rationale of cannabis products for children. There are numerous stories and anecdotal findings of desperate parents seeking cannabis products to relieve their children’s seizures; however, what scientific evidence exists for cannabis’ effectiveness? This book chapter briefly discusses the pharmacology of cannabis and reviews the clinical utility of CBD in the treatment of refractory epilepsies.
7.2 Pharmacology of Cannabinoids
The cannabis plant contains over 100 compounds, collectively referred to as phytocannabinoids (ElSohly and Gul 2014). Among these products, the two best characterized cannabinoids are tetrahydrocannabinol (THC) and cannabidiol (CBD) (Fig. 7.1). Cannabidivarin (CBDV) is a variant of CBD with some neuroactive properties. Tetrahydrocannabidivarin, a structurally similar compound to THC, is actually an antagonist of THC. Δ-Tetrahydrocannabinolic acid (THCA), the most abundant cannabinoid in cannabis bred for recreational use, is a nonpsychoactive or non-euphoric precursor of THC. THCA converts to THC when heated or smoked. Most of the data on CBD and THC compounds demonstrate anticonvulsant properties in major experimental seizure models (Rosenberg et al. 2017; Patra et al. 2019; Huizenga et al. 2019). However, there has also been some evidence and discrepancy of proconvulsive properties of THC in healthy animals, generally at high doses (Stadnicki et al. 1974; Martin and Consroe 1976). THC is psychoactive and prone to tolerance, and therefore, is a less likely therapeutic option for epilepsy. Conversely, CBD does not exert psychoactive effects. Therefore, CBD has been selected for advanced studies in experimental and clinical trials for epilepsy.
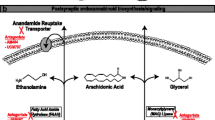
While studying how THC exerting its psychotropic effects, the endocannabinoid system was discovered within the body. The endocannabinoid system can influence many neurophysiological processes, including neuronal excitability, pain and inflammation, feeding and energy regulation, and learning and memory, as well as emotion regulation. Endocannabinoids identified so far include anandamide, 2-arachidonoylglycerol, virodhamine, N-arachidonoyl dopamine, and noladin ether. These physiological functions of endocannabinoids are mediated by cannabinoid receptors (CBRs) that have been found within the central nervous system, reproductive organs, skin, and digestive tract (Grotenhermen 2006).
The primary pharmacological effects of THC and CBD are due to their interaction with the CBRs. There are two subtypes of CBRs, CB1 and CB2, which are G-protein-coupled receptors that work primarily to inhibit adenylate cyclase activity through the Gi/o pathway (Howlett et al. 1986). Through this mechanism, CB1 activation can modulate neurotransmitter release in the brain areas the receptors are most highly expressed, i.e., the limbic structures, cerebral cortex, basal ganglia, and select areas of the midbrain and medulla (Tsou et al. 1998). CB2 receptors are located primarily on immune tissues and specific cell types such as the spleen, lymph nodes, B-cells, macrophages, and microglia (Galiegue et al. 1995). Activation of CB2 receptors on immune cells or organs may lead to immunosuppressive responses. Furthermore, there is limited expression of CB2 receptors within the CNS, located only in areas such as the hippocampus and brainstem (Stempel et al. 2016). Unlike activation of CB1 receptors in these regions, CB2 activation has been demonstrated to trigger neuronal excitation, which may explain the debate among scientists over THC’s antiseizure abilities. However, not all cannabinoids act on the endocannabinoid system, as in the case of CBD. A series of consecutive studies using the maximal electroshock and pilocarpine models of epilepsy observed that the anticonvulsant properties of THC were due to its activity at CB1 receptors, whereas the antiseizure properties of CBD were CB1 independent and may occur via a different, unknown mechanism (Wallace et al. 2001, 2002, 2003). Pharmacological mechanisms underlying the therapeutic claims for reducing the prevalence of epileptic seizures with CBD-enriched products are currently poorly understood. The following sections discuss the known pharmacokinetics and mechanisms of two major (THC and CBD) cannabinoids.
7.3 Mechanism, Bioavailability, and Metabolism of THC
THC is the most abundant compound found in cannabis and is responsible for the psychoactive effects commonly associated with the recreational smoking of marijuana. It has also been associated with changes in human cognition and perception (Hofmann and Frazier 2013). THC acts as a partial agonist of CB1 receptors in the endocannabinoid system, which are predominantly found within the CNS, with especially high concentrations in the hippocampus. It is through this binding action that THC exerts it anticonvulsive properties (Consroe et al. 1982). The hippocampus is often a focal point for many forms of epilepsies. Within the hippocampus, CB1 receptors have been observed in high levels within the CA1–3, and with highest expression in the dentate gyrus (Glass et al. 1997). These receptors have been further characterized onto presynaptic GABAergic neurons of basket cells as well as excitatory neurons (Irving et al. 2000; Kawamura et al. 2006). THC administration can reduce glutamate release in excitatory neurons, as well as inhibit release from cholinergic neurons and GABAergic interneurons (Shen et al. 1996; Gifford et al. 2000; Katona et al. 2000). Furthermore, CB1 activation has been shown to inhibit voltage-gated Ca2+ channels and increase K+ channel activity to inhibit presynaptic transmission (Shen et al. 1996).
THC also binds to a number of other targets including CB2 receptors, located primarily on the cells of the immune system; transient receptor potential cation channels TRPA1, TRPM8, and TRPV2; serotonin receptors; G-protein-coupled GPR55 receptor; and the μ- and δ- opioid receptors; as well as some subtypes of Na, K, and Ca channels (Pertwee and Cascio 2014). The mechanism by which THC produces anticonvulsant effects is believed to be through CB1 receptors (Detyniecki and Hirsch, 2015), but, as previously mentioned, there is some controversy over THC’s actual anticonvulsant effects. A recent review of THC’s anticonvulsant properties found 34 articles covering animal models of epilepsy. Antiseizure effects were found in 21/34 studies (61.8%), no significant effects were found in 11/34 studies (32.4%), and proconvulsant effects were seen in 1/34 studies (2.9%) (Rosenberg et al. 2015). The mixed results for the anticonvulsant effects of THC are likely due to the complicated pharmacology of the agent as well as the diverse effects of the endocannabinoid system, including the modulation of both excitatory glutamatergic and inhibitory GABAergic neurotransmission.
Both the absorption and bioavailability of THC are highly dependent on the route of administration. Inhaling or smoking THC has the fastest absorption, with peak plasma levels reached after 10 min and a bioavailability of about 25%. Oral consumption of THC provides approximately 6% bioavailability and slower absorption, taking anywhere from 2–6 h to reach peak plasma levels (Gaston and Friedman 2009). Following absorption, THC binds to proteins within the blood to circulate a volume of distribution of 3.4 L/Kg before hepatic metabolism by cytochrome P450 enzymes CYP2C9, CYP2C19, and CYP3A4. Excretion of THC metabolites occurs through both urine and feces (Huestis 2007).
7.4 Mechanism, Bioavailability, and Metabolism OF CBD
Contrary to THC, CBD has a very low affinity for the orthosteric sites of the endocannabinoid receptors. Numerous mechanisms have been proposed to explain the anticonvulsant action of CBD, though the exact mechanisms still remain unknown. CBD shares some targets with currently available antiepileptic drugs (AED) ethosuximide and zonisamide by blocking calcium influxes through T-type voltage-gated calcium channels (Ross et al. 2008). This activity is similar to the actions of levetiracetam, lamotrigine, and eslicarbazepine, which target L-, P/Q, and N- type calcium channels. Recent research has demonstrated that CBD can also target aberrant mutant Nav1.6 sodium channel activity, which is often associated with severe syndromic epilepsies like Dravet syndrome (Patel et al. 2016). Furthermore, Kaplan and colleagues report a substantial reduction in the frequency and duration of seizures in Scn1a mutant Dravet mice by enhancing GABAergic neurotransmission and decreasing action potential firing of excitatory neurons (Kaplan et al., 2017). These changes in the excitation/inhibition ratio were both mimicked and occluded by the inhibition of the lipid-activated G-protein coupled receptor GPR55. Their results suggest GPR55 as a target of the CBD’s anticonvulsant mechanism. Other potential targets for CBD’s anticonvulsant activity include agonism at 5-HT1A serotonin receptors, TRPA1, and TRPV1/2 (Devinsky et al. 2014b, b). Though serotonin receptors seem like an unconventional target for epilepsy, activation of 5-HT1A receptors via flenfluramine administration has demonstrated to be an effective add-on treatment in Dravet syndrome (Ceulemans et al. 2012).
It has been established that CBD binds poorly to the orthosteric sites of the CB1 and CB2 endocannabinoid receptors, through which THC exerts its major effects; however, some evidence suggests that CBD can act at CB1 receptors as a negative allosteric modulator. Recently, Straiker et al. (2018) demonstrated that CBD has no direct effect on excitatory transmission within autaptic hippocampal neurons but does inhibit two forms of endogenous cannabinoid-mediated retrograde synaptic plasticity. CBD reduced depolarization-induced suppression of excitation (DSE), an endogenous 2-arachidonoyl glycerol cannabinoid-mediated effect, as well as metabotropic suppression of excitation without affecting GABA-B receptor function (Straiker et al. 2018). These results expanded on the finding that CBD has a pharmacological profile consistent with potent negative allosteric modulation of CB1 signaling (Laprairie et al. 2015), though implications for CBD antiseizure properties are still somewhat limited.
The clinical pharmacokinetics and pharmacology profile of CBD is outlined in Table 7.1. Bioavailability and absorption of CBD is also highly dependent of the route of administration, with peak concentrations being reached after 10 min when given intranasally, 2 h when orally consumed, and over 15 h when administered through a transdermal gel (Ohlsson et al. 1986; Deianna et al. 2012). CBD has a low bioavailability of 10% due to high first-pass metabolism in the gut and liver (Devinsky et al. 2014b, b). Both the Cmax and area under the curve increase when CBD is administered with a high-fat meal or fatty vehicle (i.e., sesame oil- or coconut oil-based suspension). Similar to THC, CBD is highly lipophilic and reaches a volume of distribution of 32 L/kg by traveling through the plasma protein binding (Ohlsson et al. 1986). CBD is metabolized in the liver predominately by the cytochrome P450 enzymes CYP2C19 and CYP3A4 before excretion in feces (Gaston and Friedman 2009). The anticonvulsant effects of the circulating metabolites of CBD, 7-COOH-CBD and 6-OH-CBD, remain undefined.
7.5 Translational Studies of CBD on the Comorbities of Epilepsy
Epilepsy is known first and foremost as a seizure disorder, with patients experiencing a variety of seizure types, i.e., convulsive, absence, atonic, clonic, tonic, and myoclonic. These symptomatic seizures can be induced by genetic, mechanical injury, psychological, or viral causes. In many cases, there is a period between the initial insult and the onset of seizures. During this latency period, several restructuring processes are underway, transforming a normal brain into a hyperexcitable, epileptic one (Clossen and Reddy 2017). These processes have been mirrored in many rodent models, and include neuronal and blood-brain barrier damage, inflammation, alterations in neurogenesis, axonal/dendritic plasticity, and epigenetic changes (Reddy and Kuruba 2013; Younus and Reddy 2017). Furthermore, patients with epilepsy have a higher prevalence for psychiatric disorders such as anxiety and depression. Since epilepsy is associated with such a wide array of neurological disruptions, it is critical that new antiseizure drugs show neuroprotection against some or all these factors. This section describes the current preclinical understanding of the neuroprotective actions of CBD that are related to these epileptogenic processes and comorbidities.
7.5.1 Neuroprotective Actions of CBD
CBD has been shown to have a wide spectrum of actions, suggesting many therapeutic possibilities for the drug. CBD prevented oxidative damage in an NMDA-mediated neurotoxicity rat model (Hampson et al. 1998) and also prevents the exacerbation of reactive oxygen/reactive nitrogen species production (Campos et al. 2017). Reactive oxygen species and reactive nitrogen species (ROS/RNS) accumulation often leads to cell injury and cell death, especially in high glucose-induced mitochondrial production. CBD seems to protect against cell death by enhancing intracellular recycling of cellular components. This autophagic pathway can malfunction under stress, thereby promoting more damaging mechanisms. Hosseinzadeh et al. (2016) suggest the neuroprotective effects of CBD in pilocarpine-induced seizures may be due in part to its activation of hippocampal autophagic machinery to promote cell survivability over cell death. In one study of cerebral ischemia, CBD supplementation improved basal respiration and enhanced mitochondrial function via the pentose-phosphate pathway (Sun et al. 2017). CBD also normalizes caspase-3 expression in rats with brain iron overload and decreased β-amyloid protein deposit-induced neurodegeneration (Da Silva et al. 2014; Esposito et al. 2011).
7.5.2 Anti-inflammatory Actions of CBD
Inflammation within epileptic foci can exacerbate seizures and epileptogenesis. CBD ameliorates expression of pro-inflammatory cytokines, which can aggravate injury (Rajesh et al. 2007). During hypoxic-ischemic brain damage, CBD administration provided neuroprotection by reducing the deleterious effects of glutamate toxicity and inflammatory molecule production such as IL-6, TNF alpha, COX-2, and iNOS (Castillo et al. 2010). These effects were mediated by CBD’s activity on CB2 and adenosine receptors. Together, these studies suggest CBD acts as an antioxidant to reduce ROS/RNS, attenuates inflammatory cascades, and significantly reduces neurodegeneration in a variety of injury models.
7.5.3 CBD Facilitation of Neurogenesis
Hippocampal neurogenesis is a major source of plasticity in the brain with a variable rate throughout the lifetime. It has been demonstrated in many rodent models that seizures produce both short- and long-term changes in cell proliferation (Parent et al. 1997; Hattiangady et al. 2004). Prolonged seizure activity leads to a significant increase in neurogenesis in the dentate gyrus cells, which can contribute to acute aberrant network reorganization during epileptogenesis. Cell proliferation returns to baseline approximately 1 month following status epilepticus in rats; however, in cases with extreme neuroinflammation within the temporal regions, reduced neurogenesis after status epilepticus has also been observed (Hattiangady et al. 2004). It is suggested that seizure-generated granule cells have poorer survivability than new-born neurons in a naïve animal, and that a lowered rate of neurogenesis may be correlated with higher initial injury severity (Mohpael et al. 2004).
Neuropsychiatric disorder models provide an opportunity to study the mechanisms of neurogenesis. In rodents, exposure to chronic stressors induces dendritic remodeling and reduced adult hippocampal neurogenesis (Bessa et al. 2009). Reduced neurogenesis is recognized as one of the major mechanisms related to smaller hippocampal volume in patients suffering from mood disorders and schizophrenia (Lucassen et al. 2010). A study in 2010 demonstrated a CBD-rich diet can increase BrdU-labeled newborn neurons in the hippocampus (Wolf et al. 2010); however, the contributory role of neurogenesis in epileptogenesis is still uncertain (Danzer et al. 2019). CBD has also been shown to prevent stress-induced reduction in neurogenesis as well as prevent neurogenic disruption in a genetic mouse model of Alzheimer’s disease (Crippa et al. 2018; Esposito et al. 2011). Furthermore, CBD can modulate intracellular pathways related to synaptic remodeling including the Erk1/2 and Akt pathways (Solinas et al. 2013). In an iron overload induced-brain damage model, CBD administration normalized the expression of synaptophysin, a critical protein involved in proper synaptic and vesicular function (Da Silva et al. 2014). These actions of CBD are independent of cannabinoid receptor modulation.
7.5.4 Anxiolytic Actions of CBD
Initial studies investigating the effects of CBD on anxiety-like behaviors yielded somewhat contradictory results. In the early 1980s, Zuardi and Karniol (1983) found 10 mg/kg of CBD attenuated conditioned emotional responses in rats, whereas Silveira Filho and Tufik (1981) found no such effects with 100 mg/kg of CBD. It was later found during a dose-response study that CBD induces anxiolytic effects at low doses, but those effects diminish with higher quantities (Guimaraes et al. 1990). Since then, many studies have demonstrated the anxiolytic effects of CBD in preventing fear expression and reconsolidation in models of generalized anxiety, PTSD, panic disorder, and high-stress (Campos et al. 2012a, 2012b; Resstel et al. 2006; Stern et al. 2015). Additionally, it was also demonstrated that acute or repeated administration of CBD significantly decreased the behavioral and autonomic responses of acute restraint stress (Granjeiro et al. 2011). CBD reduced anxiogenic effects seen in acute stress via predator exposure in rats as well as in chronic unpredictable stress (Campos et al. 2012a, 2012b). The mechanism of the anxiolytic effects of CBD are poorly understood, but is most likely due to its interaction with serotonin 5HT1A receptors. This anxiolytic nature of CBD could also be associated with antidepressant like properties, which have been observed in forced-swim and bulbectomy models in rats (Zanelati et al. 2010; Linge et al. 2016). This is corroborated by a human study that shows that CBD reduces anxiety in social anxiety disorder and that this is related to its effects on activity in limbic and paralimbic brain areas (Crippa et al. 2011).
7.6 Pivotal Clinical Trials That Led to the Approval of Epidiolex
Early case reports and surveys on the use of cannabis for epilepsy have historically been limited and underpowered with inconclusive results. In the late nineteenth century, two prominent British neurologists observed reductions in seizure frequency when they treated their epileptic patients with cannabis (Reynolds 1861; Gowers 1881). Despite these successes, cannabis remained understudied as a possible therapeutic for convulsive disorders until the 1970/80s. A Cochrane review published in 2012 found four controlled studies, which examined the therapeutic potential of CBD for epilepsy (Gloss and Vickrey 2014). This review searched for randomized, controlled clinical trials that showed direct evidence of anticonvulsant effects of CBD in human seizures. They identified only four studies that fit their efficacy criteria, with a total sample of 48 participants. Of these four trials, two found limited improvements on seizure frequency, but all had some methodological flaw including small sample size and inadequate blinding (Mechoulam and Carlini 1978; Cunha et al. 1980; Ames and Cridland 1986; Trembly and Sherman 1990). Dosing ranged from 200 mg - 300 mg per day, and the only side effect reported in any of these studies was somnolence. In general, only short-term tolerability of CBD-enriched therapeutics was demonstrated in these studies.
Two case studies received a lot of media attention when patients became seizure-free after treatment with medical marijuana or extracts of its components. In one case, a 24-year-old man self-treated with 2–5 rolled cigarettes per night containing whole plant marijuana in addition to his prescribed AEDs and experienced a full reduction in refractory seizures (Consroe et al. 1975). The second case report, which convinced many parents with epileptic children to move to Colorado, featured a young girl named Charlotte, who suffered from severe Dravet syndrome. She was started on a sublingual preparation of cannabis extract with a ratio of 16:1 CBD:THC in tandem with her prescribed clobazam. After 3 months of treatment, Charlotte experienced >90% reduction in seizures (Maa and Figi 2014). This strain is now named “Charlotte’s Web” as a tribute to the success Charlotte found using this extract. In 2013, an oil-based extract of Charlotte’s Web, called Realm Oil, was tested in 13 patients whose diagnoses included Doose, Dravet, and Lennox-Gastaut syndromes. A parent-survey reported that 11/13 patients reported a weekly reduction in seizures, and of these 11, 8 experienced a 98–100% reduction in seizures (Gedde and Maa 2013). An additional caregiver survey yielded positive outcomes of other CBD-enriched products in epilepsy. In one survey, 16/19 responders (84%) reported a reduction in seizure frequency with cannabis therapy (Porter and Jacobson 2013). Response rates appear similar with all products but vary by syndrome (LGS > Dravet). Together with the steady progress in experimental research, these case reports and underwhelming trials paved the way for the neoteric clinical trials and FDA approval of Epidiolex for Lennox-Gastaut and Dravet syndromes in 2018.
Lennox-Gastaut syndrome (LGS) is a rare, severe, and drug-resistant form of epilepsy that begins in early childhood and changes throughout life. LGS is prevalent in ~4% of children with epilepsy. The classic description of LGS focuses on three typical clinical (classic triad) features: (i) multiple seizure types; (ii) cognitive impairment; and (iii) slow spike-wave EEG (Bourgeois et al. 2014). LGS involves a variety of seizure types including tonic and atypical absence; drop seizures occur in at least 50% patients. Many patients with LGS have significant cognitive impairment. A distinct EEG pattern is the third characteristic feature of LGS for most patients. LGS presentation, however, is variable and not all patients exhibit all components of the classic triad at onset. LGS with cognitive and physical impairments elicits a significant impact on patients and caregivers. LGS signs and symptoms may also change over time. Such high-risk seizures may predispose patients to status epilepticus or sudden unexpected death in epilepsy (SUDEP) and head injury (Schmidt and Bourgeois 2000). There is a high risk (14-fold) of mortality in children with LGS. Research is currently being carried out to identify genetic factors in LGS.
The effectiveness of Epidiolex for the symptomatic treatment of seizures associated with LGS was founded on two randomized, double-blind, placebo-controlled trials conducted in 2014–2015 (Thiele et al. 2018; Devinsky et al. 2018a, 2018b). Epidiolex contains >98% CBD and less than <0.15% THC and is prepared in a strawberry-flavored sesame oil-based suspension. These trials included patients aged 2–55 years old (Study 1 n = 171; Study 2 n = 225) and compared doses of 10 mg/kg/day and 20 mg/kg/day of Epidiolex versus placebo. A period of 4- weeks was used to assess baseline seizures in LGS patients. A minimum of 8 drop seizures, which were inadequately controlled by their current medications during this period, was required to participate in the clinical trial. The most common concomitant AEDs in these studies were clobazam, valproate, lamotrigine, levetiracetam, and rufinamide. A 2-week titration period followed the 4-week baseline.
The primary efficacy measure was percent change in seizure frequency during the 12-week maintenance period. In both studies, Epidiolex-treated patients observed a significantly greater reduction in seizure frequency. In Study 1 (Thiele et al. 2018), a 41% reduction in seizure was observed with 20 mg/kg/day (p = 0.01). In Study 2 (Devinsky et al. 2018a, b), a 36% reduction was seen at 10 mg/kg/day (p < 0.01) and 38% reduction at 20 mg/kg/day (p < 0.01) compared to placebo. Secondary measures included changes in Subject/Caregiver Global Impression of Change (S/CGIC) during the last visit. This scale was conducted using a 7-point comparison impression on the status of the patient’s overall condition at the beginning and ending of the clinical trial using phrases such as “much improved”, “slightly improved”, “no change”, “much worse”, etc. The S/CGIC scores for Studies 1 and 2 most closely corresponded to “slightly improved” in treated patients vs “no change” in placebo groups. An additional observational study reported 9/23 patients experience >50% decrease in seizures, with a median reduction in seizures of 32% for all patients when treated with 25 mg/kg per day of Epidiolex (cannabidiol) in tandem with prescribed AEDs (Devinsky et al. 2014b, b). A similar report was made in 2015 with 25 patients (Oldham et al., 2015).
Dravet syndrome (DS) is a developmental disorder typified by severe seizures and delayed onset of psychomotor deficits (Dravet et al. 2005). DS has distinct characteristics to confirm diagnosis. Seizures typically develop in the first year of life in infants in children with no apparent developmental disabilities. The initial seizure is often triggered by an illness and may present as a prolonged, febrile and afebrile-generalized seizures and progress to severe and often refractory epileptic encephalopathy; seizures decrease in frequency and severity with sexual maturity (Steel et al. 2017; Gataullina and Dulac 2017). DS has a broad differential diagnosis with a typical and unique quartet characteristics: (i) temperature sensitivity; (ii) prolonged seizures in otherwise normally developing infant; (iii) developmental delays following early normal development; and (iv) myoclonic seizures. DS affects motor and cognitive development. In early childhood, seizures are triggered by hyperthermia, bathing, flashing lights, emotional stress, visual patterns, and overexertion. In adolescence, seizures persist, occurring more often during sleep. In DS, seizures may be worsened by AEDs that target sodium channels. In addition to increasing the risk for SUDEP, many children are frequently taken to the ER with status epilepticus, which can lead to brain damage. The delayed social and cognitive development and movement disorders are additional clinical presentation in adolescent DS patients. In a majority of cases, mutations in the sodium channel gene SCN1A form the genetic basis for DS (Fujiwara 2006; −Steel et al. 2017). SCN1A+/− mice exhibits symptoms reminiscent of human DS; they display both thermally induced and spontaneous seizures, and develop autism-like social deficits (Rubinstein et al. 2015). The loss of function in Nav1.1 channels in SCN1A+/− mice selectively reduces sodium current and excitatory drive in GABAergic interneurons contributing to heightened epileptogenesis.
The effectiveness for Epidiolex for the treatment of seizures associated with DS was demonstrated in one randomized, double-blind, placebo-controlled trial (Devinksy et al. 2017; NCT02091375). Like the LGS trials, this study had a 4-week baseline, 2-week titration, and 12-week maintenance period. The minimum requirements for trial participation were a documented history of DS and at least 4 uncontrolled convulsive seizures while on stable AED medication during the baseline period. Primary efficacy measurement was based on percent change from baseline in seizure frequency, including atonic, tonic, clonic, and tonic-clonic seizures during the treatment period. Patients were aged between 2–18 years and at least 93% (112/120) of participants were taking two or more concomitant AEDs. Most commonly prescribed AEDs during the DS study were clobazam, valproate, stiripentol, levetiracetam, and topiramate. A reduction in seizure frequency was observed after 4-weeks of treatment with 20 mg/kg/day Epidiolex. The median frequency of convulsive seizures decreased from 12.4 per month to 5.9 per month in the treated patients, compared to a decrease from 14.9 to 14.1 seizures per month in the placebo group. Of the treated participants (n = 61), 43% had a > 50% reduction in seizures, including 5% who became seizure-free. Among these patients, cannabidiol treatment resulted in a significant reduction in convulsive seizures versus placebo.
Safety and antiseizure effectiveness of an open-label extension trial on the long-term CBD treatment in patients with Dravet syndrome were reported recently (Devinsky et al. 2019). Patients who completed GWPCARE1 Part A (NCT02091206) or Part B, or a second placebo-controlled trial, GWPCARE2 (NCT02224703), were invited to enroll in a long-term open-label extension trial, GWPCARE5 (NCT02224573). A purified CBD oral solution (100 mg/mL) was titrated from 2.5 to 20 mg/kg/day over a 2-week period, along with their existing medications. A total of 264 enrolled in this open-label extension. Common adverse events reported include diarrhea (34.5%), pyrexia (27.3%), decreased appetite (25.4%), and somnolence (24.6%). In patients from GWPCARE1 Part B, the median reduction from baseline in monthly seizure frequency assessed in 12-week periods up to week 48 ranged from 39% to 51% for total seizures. After 48 weeks of treatment, 85% of patients/caregivers reported improvement in the patient’s overall condition. Overall, this study confirms that long-term CBD treatment had a reasonable safety profile and led to sustained reductions in seizure frequency in patients with treatment-resistant DS.
Long-term safety and efficacy of CBD in children and adults with treatment resistant LGS or DS from expanded access program were reported recently (Laux et al. 2019). Since 2014, patients with severe treatment-resistant epilepsies (TREs) have been receiving add-on CBD in an ongoing, expanded access program (EAP), which closely reflects clinical practice. Twenty-eight percent of LGS/DS patients withdrew, primarily owing to lack of efficacy (20%). At 12 weeks, add-on CBD reduced monthly seizures by 50% and total seizures by 44%. At 12 weeks, the proportions of patients with ≥50%, ≥75%, and 100% reductions in major motor seizures were 53%, 23%, and 6%; the proportions with corresponding reductions in total seizures were 46%, 26%, and 5%. These results confirm that CBD had an acceptable safety and efficacy during long-term treatment in LGS or DS.
There is emerging evidence on long-term safety and efficacy data for intractable epilepsies beyond LGS and Dravet syndrome, as evident from the open-label expanded access program (Szaflarski et al. 2018). In January 2014, an expanded access program (EAP) was initiated to provide CBD to patients with treatment-resistant epilepsy (TRE). Preliminary efficacy data have been reported previously (Devinsky et al. 2016). They have published an updated paper, reporting pooled results for safety outcomes up to 144 weeks and efficacy endpoints up to 96 weeks in more than 600 patients (Szaflarski et al. 2018). Of the 607 patients treated (median treatment duration, 48 weeks; range 2–146 weeks), 76% of patients remained on treatment. CCBD was associated with 51% and 48% reductions in median monthly convulsive and total seizures, respectively, after 12 weeks of treatment. Reductions in median monthly convulsive and total seizures were similar among visit windows through 96 weeks of treatment. At visits between weeks 12 and 96, inclusive, the ≥50%, ≥75%, and 100% response rates were notable and similar among time points. Overall, these results from this ongoing EAP support previous observational and clinical trial data showing that add-on CBD may be an efficacious long-term treatment option for TRE (Szaflarski et al. 2018b). CBD was generally well tolerated; treatment-emergent adverse events were consistent with those reported previously.
To date, most clinical trials have focused on the assessment of safety and/or efficacy of CBD in combination with prescribed antiepileptic drugs. Studies focusing on genetically based epilepsies such as Dravet, Lennox-Gastaut, and West syndromes have been the most common. However, there are currently over 20 clinical trials ongoing to study the efficacy of CBD for a number of neurological conditions including the seizure-associated disorders of infantile spasms (NCT02953548; NCT02954887), tuberous sclerosis complex (NCT02544750; NCT02544763), Rett syndrome (NCT03848832), and refractory epilepsy in adults (NCT02607904; NCT02564952; NCT02565108; and NCT02286986). These investigations of CBD are providing rigorous information on the pharmacological basis of their clinical use. A higher dose of CBD is associated with a greater chance for seizure improvement, though children may respond better to lower doses of CBD than adults (Hernando et al. 2018). There is promising early indications of CBD’s effectiveness as an adjunct AED for children with intractable generalized epilepsy (Cilio et al. 2018; Carney et al. 2017). A transdermal CBD gel is proposed as an adjunctive therapy for the treatment of focal seizures in adults (Sebree et al. 2016; O’Brien et al. 2018), but further evaluation in double blind studies is warranted to confirm such predictions.
7.7 Adverse Effects and Drug Interactions
In controlled and uncontrolled trials in patients with LGS and DS, 689 patients were treated with CBD, including 533 patients treated for more than 6 months, and 391 patients treated for more than 1 year. In an expanded access program and other compassionate use programs, 161 patients with DS and LGS were treated with CBD, including 109 patients treated for more than 6 months, 91 patients treated for more than 1 year, and 50 patients treated for more than 2 years (Devinsky et al. 2014b, b; Devinksy et al. 2017; Szaflarski et al. 2018). In these clinical trials of CBD, the most common adverse reactions that occurred in CBD-treated patients (incidence at least 10% and greater than placebo) were somnolence; decreased appetite; diarrhea; transaminase elevations; fatigue, malaise, and asthenia; rash; insomnia, sleep disorder, and poor quality sleep; and infections. CBD can cause weight loss (Szaflarski et al. 2018b). However, CBD does not produce THC-like behavioral (rewarding) responses and it does not produce physical dependence. There is increasing attention on the importance of the relationship between sleep and epilepsy. CBD therapy has been associated with alterations in sleep architecture (Drake et al. 2018). Further research on the effects of CBD on sleep parameters and related measures is needed.
CBD is metabolized by CYP3A4 and CYP2C19. Therefore, co-administration with a moderate or strong inhibitor of CYP3A4 or CYP2C19 can increase CBD plasma concentrations, which may result in a greater risk of adverse reactions. Conversely, co-administration with a strong CYP3A4 or CYP2C19 inducer can decrease CBD plasma concentrations, which may lower the efficacy of the drug. Co-administration of CBD increases plasma concentrations of drugs that are metabolized by CYP2C19 (e.g., diazepam) and may increase the risk of adverse reactions with these substrates. In addition, co-administration of CBD produces a three-fold increase in plasma concentrations of N-desmethylclobazam, the active metabolite of clobazam (a substrate of CYP2C19. This drug interaction may increase the risk of clobazam-related adverse reactions. In addition, diet had a significant interaction on the pharmacokinetics of CBD. In clinical pharmacokinetic studies, high fat diet was found to increase Cmax and AUC of CBD (Epidiolex) ~4 fold (Taylor et al. 2018). In another pharmacokinetic study, purified CBD capsules were administered orally with and without food to adults with refractory epilepsy. An average of 14-fold and 4-fold increases in Cmax and AUC respectively were found in patients in the fed condition (Birnbaum et al. 2019).
Some studies were performed to investigate drug-drug interactions between CBD and frequently prescribed AEDs. These trials found significant changes in AED serum levels when CBD and clobazam were taken in tandem (Friedman et al. 2014; Geffrey et al. 2015). For those patients, the dose of clobazam was reduced. These studies also boasted a 50–55% reduction in seizures in 11/13 patients, though the remaining 2/13 patients experienced increased seizure activity (Geffrey et al. 2015). The discrepancy in efficacy could be due to varying etiologies of epilepsy, since the mechanism of CBD is still unknown.
As all completed clinical trials have tested CBD with concurrent AED medications, the clinical relevance of drug-drug interactions is extremely important. Both CBD and THC inhibit hepatic enzymes CYP2C19 at low levels, and CYP3A4 at high levels. These enzymes are induced by carbamazepine, topiramate, and phenytoin, and are inhibited by sodium valproate, and are responsible for the metabolism of many AEDs (Gaston and Friedman 2009). An open label CBD study found drug-drug interactions with several AEDs taken by both children and adults, including increases in levels of rufinamide, topiramate, zonisamide, eslicarbazepine, clobazam, and N-desmethylclobazam (Devinsky et al. 2018a, 2018b; Jiang et al. 2013). The only significant interactions observed were with clobazam and N-desmethylclobazam, which increased to levels above the therapeutic range. Clinically significant drug interactions were observed with CBD and clobazam and N-desmethylclobazam, which increased to levels above therapeutic range. Such actions of CBD have raised questions on whether the observed antiseizure effects and side effects of CBD therapy were related to a direct action of CBD, or were due to CBD’s indirect effects on elevation of N-desmethylclobazam levels when coadministered with clobazam, a antiseizure medication for DS. In a recent case report from five patients receiving adjunctive treatment with CBD exhibited increases in brivaracetam levels by 95% to 280% (Klotz et al. 2019). Another clinically significant drug interaction between BCD and tacrolimus was reported (Leino et al. 2019). Therefore, it is advised that when taking these medications concurrently with CBD, dosing may be altered to reduce risk of interactions and serious adverse effects.
7.8 Conclusions and Future Perspectives
Among epileptic patients, 30% remain untreated with currently available medications. Recent clinical trials and experimental research have brought new insights to the potential use of cannabinoids for the treatment of epileptic seizures. CBD, the active ingredient in Epidiolex, is a cannabinoid that naturally occurs in the Cannabis sativa plant. Preclinical models have demonstrated that CBD not only possesses anticonvulsant properties but may also be able to disrupt maladaptive processes associated with epileptogenesis. CBD has been shown to act as an antioxidant, reduce proinflammatory cytokines, rescue neurogenesis, and ameliorate neurodegeneration. Furthermore, CBD can modulate neuropsychiatric disorders such as anxiety and depression, which are often seen as comorbidities in epileptic patients. Clinical efforts from several well-designed trials of a plant-based CBD (Epidiolex, Greenwich Biosciences, Carlsbad, CA, USA) have demonstrated the antiseizure efficacy in patients >2 years old, especially when taken in tandem with currently prescribed AEDs (Elliott et al. 2019). Patients exhibited a favorable adverse effect profile. However, CBD therapy has associated adverse effects, with somnolence, decreased appetite, and diarrhea being the most common. Moreover, there is some concern for drug-drug interactions, specifically with clobazam, topiramate, rufinamide, phenytoin, clonazepam, and carbamazepine. Similar beneficial outcomes have been reported by an Israeli clinical study with CBD-enriched medical cannabis in a population of children and adolescents (Tzadok et al. 2016). Despite such overwhelming therapeutic claims, the molecular basis of CBD therapy for epilepsy remains unclear. It does not appear to exert its anticonvulsant effects through interaction with cannabinoid receptors. It is critical to know how CBD controls seizures so chemists can design novel synthetic compounds for epilepsy to surpass the hurdles of mixed CBD extracts such as extraction, purification, and standardization. In addition, it remains to be determined if plant-based vs synthetic CBDs are identical in terms of pharmacological outcomes, both in effectiveness and side effects. Recently, a synthetic, non-intoxicating 8,9-dihydrocannabidiol (H2CBD) has been prepared and demonstrated to have effectiveness comparable to CBD both for decreasing the frequency and severity of experimental seizures in rats (Mascal et al. 2019). It is claimed that H2CBD cannot be converted by any reasonable synthetic route into THC, and thus could be a safe, noncontroversial drug for seizure therapy.
Patient responsiveness to cannabis-enriched therapeutics varies substantially, and in some cases has been suggested to even exacerbate seizures. This inconsistency in therapeutic responses could be contributed to qualitative and quantitative chemical variability in medical products, individual differences in the etiology of seizures between patients, or even in the pathological reorganization of epileptic circuits between forms of epilepsy. Therefore, the consensus among neurologists is to first clinically test FDA-approved formulations of cannabis products in specific epilepsy syndromes. However, marketing unapproved hemp or CBD-containing products with uncertain formulations is often seen in some dispensaries and wellness aisles of grocery stores. These should not be considered substitutes or generics for FDA-approved medicines. These false advertisements can keep patients from accessing appropriate and recognized therapies (such as Epidiolex) to treat serious, and in certain cases, fatal diseases. The rigorous FDA-approval process is bypassed for such dispensary products. FDA-approved CBD products are available by prescription in both specialty and retail pharmacies, but not dispensaries. With adequate and well-controlled clinical studies, physicians will have more confidence in the drug’s potency and efficacy to support appropriate dosing in a variety of patients. To this end, the FDA approved the first CBD-based medication, Epidiolex, for two specific forms of severe pediatric epilepsy: Lennox-Gastaut and Dravet syndromes. There are ongoing clinical trials to expand upon the efficacy, safety, and dosing for other epilepsies, as well as different age groups. CBD may impact a variety of brain conditions (Pisanti et al. 2017); further research is warranted to profile the full spectrum of CBD pharmacology.
Cannabis and all its cannabinoids are controlled substances and regulated by the Drug Enforcement Administration. While most cannabinoids are classified as Schedule I (banned substances), the regulatory statutes are rapidly changing with recent legislation. As of October 30, 2018, Hemp, defined as a cannabis plant containing <0.3% THC has been descheduled. Thus, on a federal account, the Agicultural Improvement Act (also called the Farm bill) extended the protections of Hemp research to include plant products including Hemp-derived CBD. This provision recognizes the importance of experimental research to discover medicinal uses and mechanistic pathways of cannabinoids for epileptic disorders and other conditions. Presently, four products that are approved by the FDA are de-classified from this schedule. The clinically available THC and THC analogs are listed in Schedule II/III and the plant-derived CBD (Epidiolex) is listed in Schedule V. Recently, a synthetic CBD (H2CBD) has been prepared and tested in experimental seizure models (Mascal et al. 2019). It is claimed that the synthetic CBD alternative is easier to purify than a plant extract, eliminates the need to use agricultural land for hemp cultivation, and could avoid legal complications with cannabis-related products. The synthetic H2CBD and CBD were found to be equally effective for the reduction of both the frequency and severity of seizures. Unlike the plant-derived CBD, H2CBD cannot be converted by synthetic route into THC, and thus has fewer regulatory hurdles.
Conflict of Interest
None.
References
Ames FR, Cridland S (1986) Anticonvulsant effect of cannabidiol. S Afr Med J 69:14–18
Autry AR, Trevathan E, Van Naarden Braun K, Yeargin-Allsopp M (2010) Increased risk of death among children with Lennox-Gastaut syndrome and infantile spasms. J Child Neurol 25(4):441–447
Bessa JM, Ferreira D, Melo I, Marques F, Cerqueira JJ, Palha JA, Almeida OF, Sousa N (2009) The mood improving actions of antidepressants do not depend on neurogenesis but are associated with neuronal remodeling. Mol Psychiatry 14(8):764–773
Billakota S, Devinsky O, Marsh E (2019) Cannabinoid therapy in epilepsy. Curr Opin Neurol 32(2):220–226
Birnbaum AK, Karanam A, Marino SE, Barkley CM, Remmel RP, Roslawski M, Gramling-Aden M, Leppik IE (2019) Food effect on pharmacokinetics of cannabidiol oral capsules in adult patients with refractory epilepsy. Epilepsia 60(8):1586–1592
Bourgeois BF, Douglass LM, Sankar R (2014) Lennox-Gastaut syndrome: a consensus approach to differential diagnosis. Epilepsia 55(Suppl 4):4–9
Campos AC, Ferreira FR, Guimaraes FS (2012a) Cannabidiol block long-lasting behavioral consequences of predator threat stress: possible involvement of 5HT1A receptors. J Psychiatr Res 46:1501–1510
Campos AC, Fogaca MV, Scarante FF, Joca SRL, Sales AJ, Gomes FV, Sonego AV, Rodrigues NS, Galve-Roperh I, Guimaraes FS (2017) Plastic and neuroprotective mechanisms involved in the therapeutic effects of cannabidiol in psychiatric disorders. Front Pharmacol 8:269
Campos AC, Moreira FA, Gomes FV, Del Bel EA, Guimaraes FS (2012b) Multiple mechanisms involved in the large-spectrum therapeutic potential of cannabidiol in psychiatric disorders. Philos Trans R Soc Lond Ser B Biol Sci 367:3364–3378
Carney PR, Evans V, Febo M, DeMarse T, Johnson CR, Anderson C (2017) An evaluation of effectivenss of cannabidiol as an antiepileptic drug for children with intractable generalized epilepsy. In: Abstract 1.048. American Epilepsy Society, Washington, D.C
Castillo A, Tolon MR, Fernandez-Ruiz J, Romero J, Martinez-Orgado J (2010) The neuroprotective effect of cannabidiol in an in vitro model of newborn hyper-ischemic brain damage in mice is mediated by CB2 and adenosine receptors. Neurobiol Dis 37(2):434–440
Ceulemans B, Boel M, Leyssens K, Van Rossem C, Neels P, Jorens PG et al (2012) Successful use of fenfluramine as an add-on treatment for Dravet syndrome. Epilepsia 53:1131–1139
Cilio MR, Wheless JW, Dlugos D, Parikh N, Miller I (2018) Long-term safety of pharmaceutical cannabidiol oral solution as adjunctive treatment for pediatric patitents with treatment-resistant epilepsy. In: Abstract 3.294. American Epilepsy Society, New Orleans, LA
Clossen BL, Reddy DS (2017) Novel therapeutic approaches for disease-modification of epileptogenesis for curing epilepsy. Biochim Biophys Acta Mol basis Dis 1863(6):1519–1538
Consroe P, Benedito MA, Leite JR, Carlini EA, Mechoulam R (1982) Effects of cannabidiol on behavioral seizures caused by convulsants drugs or current in mice. Eur J Pharmacol 83:293–298
Consroe PF, Wood GC, Buchsbaum H (1975) Anticonvuldant nature of marihuana smoking. JAMA 234:306–307
Crippa JA, Derenusson GN, Ferrari TB, Wichert-Ana L, Duran FL, Martin-Santos R, Simões MV, Bhattacharyya S, Fusar-Poli P, Atakan Z, Santos Filho A, Freitas-Ferrari MC, McGuire PK, Zuardi AW, Busatto GF, Hallak JE (2011) Neural basis of anxiolytic effects of cannabidiol (CBD) in generalized social anxiety disorder: a preliminary report. J Psychopharmacol 25(1):121–130
Crippa JA, Guimaraes FS, Campos AC, Zuardi AW (2018) Translational investigation of the therapeutic potential of cannabidiol: Toward a new age. Front Immunol 9:2009. https://doi.org/10.3389/fimmu.2018.02009
Cunha JM, Carlini EA, Pereira AE, Ramos OL, Pimentel C, Gagliardi R, Sanvito WL, Lander N, Mechoulam R (1980) Chronic administration of cannabidiol to healthy volunteers and epileptic patients. Pharmacol 21:175–185
Da Silva VK, de Freitas BS, da Silva Dornelles A, Nery LR, Falavigna L, Ferreira RD, Bogo MR, Hallak JE, Zuardi AW, Crippa JA, Schroder N (2014) Cannabidiol normalizes caspase 3, synaptophysin, and mitochondrial fission protein DNM1L expression levels in rats with brain iron overload: implications for neuroprotection. Mol Neurobiol 49(1):222–233
Danzer SC (2019) Adult neurogenesis in the development of epilepsy. Epilepsy Curr 19(5):316–320
Deianna S, Watanabe A, Yamasaki Y, Amada N, Arthur M, Fleming S et al (2012) Plasma and brain pharmacokinetic profile of cannabidiol (CBD), cannabidivarine (CBDV), delta-9-tetrahydrocannabivarin (THCV) and cannabigerol (CBG) in rats and mice following oral and intraperitoneal administration and CBD action on obsessive-compulsive behavior. Psychopharmacology 219(3):859–873
Devinksy O, Cross JH, Laux L, Marsh E, Miller I, Nabbout R, Scheffer IE, Thiele EA, Wright S, Cannabidiol in Dravet Syndrome Study Group (2017) Trial of Cannabidiol for drug-resistant seizures in the Dravet Syndrome. N Engl J Med 376(21):2011–2020
Devinsky O, Cilio MR, Cross H, Fernandez-Ruiz J, French J, Hill C, Di Marzo V, Jutras-Aswad D, Notcutt WG, Martinez-Orgado J, Robson PJ, Rohrback BG, Thiele E, Whalley B, Friedman D (2014b) Cannabidiol: Pharmacology and potential therapeutic role in epilepsy and other neuropsychiatric disorders. Epilepsia 55:791–802
Detyniecki K, Hirsch L (2015) Marijuana use in epilepsy: The myth and the reality. Curr Neurol Rep 15, 65
Devinsky O, Nabbout R, Miller I, Laux L, Zolnowska M, Wright S, Roberts C (2019) Long-term cannabidiol treatment in patients with Dravet syndrome: An open-label extension trial. Epilepsia 60(2):294–302
Devinsky O, Marsh E, Friedman D et al (2016) Cannabidiol in patients with treatment-resistant epilepsy: an open-label interventional trial. Lancet Neurol 15:270–278
Devinsky O, Patel AD, Cross JH, Villanueva V, Wirrell EC, Privitera M, Greenwood SM, Roberts C, Checketts D, Van Landingham KE, Zuberi SM, GWCARE3 Study Group (2018b) Effect of cannabidiol on drop seizures in the Lennox-Gastaut syndrome. N Engl J Med 378(20):1888–1897
Devinsky O, Patel AD, Thiele EA et al (2018a) Randomized, dose-ranging safety trial of cannabidiol in Dravet syndrome. Neurology 90:e1204–e1211
Devinsky O, Sullivan J, Friedman D, Thiele E, Marsh E, Laux L, Hedlund J, Tilton N, Bluvstein J, Cilio M (2014a. Seattle, WA) Efficacy and safety of Epidiolex (cannabidiol) in children and young adults with treatment-resistent epilepsy: Initial data from an expanded access program. In: Proceedings of the American Epilepsy Society Annual Meeting. American Epilepsy Society, Chicago, IL
Drake A, Chapman KE, Bear JJ (2018) Alterations in sleep architecture with CBD use. In: Abstract 2.269. American Epilepsy Society, New Orleans LA
Dravet C, Bureau M, Oguni H, Fukuyama Y, Cokar O (2005) Severe myoclonic epilepsy in infancy: Dravet syndrome. Adv Neurol 95:71–102
Elliott J, DeJean D, Clifford T, Coyle D, Potter BK, Skidmore B, Alexander C, Repetski AE, Shukla V, McCoy B, Wells GA (2019) Cannabis-based products for pediatric epilepsy: A systematic review. Epilepsia 60(1):6–19
ElSohly M, Gul W (2014) Constituents of Cannabis sativa. In: Pertwee RG (ed) Handbook of Cannabis. Oxford University Press, Oxford, UK, pp 3–22
Esposito G, Scuderi C, Valenza M, Togna GI, Latina V, De Filippis D, Cipriano M, Carratu MR, Luvone T, Sterado L (2011) Cannabidiol reduces Aβ-induced neuroinflammation and promotes hippocampal neurogenesis through PPARγ involvement. PLoS One 6(12):e28668
Friedman D, Cilio MR, Tilton N, Sullivan J, Hedlund J, Rosenburg E, Bluvstein J, Devinksy O (2014. 5-9; Seattle, WA. Abst. 2.309) The effect of Epidiolex (cannabidiol) on serum levels of concomitant anti-epileptic drugs in children and young adults with treatment-resistant epilepsy in an expanded access program. In: Proceedings of the American Epilepsy Society Annual Meeting. American Epilepsy Society, Chicago, IL
Friedman D, Devinsky O (2015) Cannabinoids in the treatment of epilepsy. N Engl J Med 373:1048–1458
Fujiwara T (2006) Clinical spectrum of mutations in SCN1A gene: severe myoclonic epilepsy in infancy and related epilepsies. Epilepsy Res 70(Suppl 1):S223–S230
Galiegue S, Mary S, Marchand J, Dussossoy D, Carriere D, Carayon P et al (1995) Expression of central and peripheral cannabinoid recepors in human immune tissues and leukocyte subpopulations. Eur J Biochem 232:54–61
Gaston TE, Friedman D (2009) Pharmacology of cannabinoids in the treatment of epilepsy. Epilepsy Behav 15(2):S3–S4. https://doi.org/10.1016/j.yebeh.2009.04.027
Gataullina S, Dulac O (2017) From genotype to phenotype in Dravet disease. Seizure 44:58–64
Gedde MM, and Maa E (2013) Whole cannabis extract of high concentration cannabidiol may calm seizures in highly refractory pediatric epilepsies. In: American Epilepsy Society annual meeting, Seattle, WA. Abst. 3.330
Geffrey AL, Pollack SF, Bruno PL, Thiele EA (2015) Drug-drug interaction between clobazam and cannabidiol in children with refractory epilepsy. Epilepsia 56:1246–1251
Gifford AN, Bruneus M, Gatley SJ, Volkow ND (2000) Cannabinoid receptor-mediated inhibition of acetylcholine release from hippocampal and cortical synaptosomes. British J Pharm 131(1):645–650
Glass M, Dragunow M, Faull RL (1997) Cannabinoid receptors in the human brain: A detailed anatomical and quantitative autoradiographic study in the fetal, neonatal, and adult human brain. Neurosci 77(2):299–318
Gloss D, Vickrey B (2014) Cannabinoids for epilepsy. Cochrane Database Syst Rev 6(3):CD009270
Gowers W (1881) Epilepsy and other chronic convulsive disorders. Churchill, London
Granjeiro EM, Gomes FV, Guimaraes FS, Correa FM, Resstel LB (2011) Effects of intracisternal administration of cannabidiol on the cardiovascular and behavioral responses to acute restraint stress. Pharmacol Bicochem Behav 99(4):743–748
Grotenhermen F (2006) Cannabinoids and the endocannabinoid system. Can Underwrit 1:10–14
Guimaraes FS, Chiaretti TM, Graeff FG, Zuardi AW (1990) Antianxiety effect of cannabidiol in the elevated plus maze. Psychopharm 100(4):558–559
Hampson AJ, Grimaldi M, Axelrod J, Wink D (1998) Cannabidiol and (−)Delta9-tetrahydrocannabidiol are neuroprotective antioxidants. Proc Natl Acad Sci U S A 95(14):826–8273
Hattiangady B, Rao MS, Shetty AK (2004) Chronic temporal lobe epilepsy is associated with severely declined dentate neurogenesis in the adult hippocampus. Neurobiol Dis 17:473–490
Hernando K, Bebin EM, Gaston TE, Grayson L, Moreadith R, Szaflarski JP. (2018) Higher cannabidiol levels are associated with better seizure response following treatment with a pharmaceutical grade cannabidiol. Abstract 1.468, American Epilepsy Society, New Orleans, LA
Hofmann ME, Frazier CJ (2013) Marijuana, endocannabinoids, and epilepsy: Potential and challenges for improved therapeutic intervention. Exper Neurol 244:43–50
Hosseinzadeh M, Nikseresht S, Kohdagholi F, Naderi N, Maghsoudi N (2016) Cannabidiol post-treatment alleviates rat epileptic-related behaviors and activates hippocampal cell autophagy pathway along with antioxidant defense in chronic phase of pilocarpine-induced seizure. J Mol Neurosci 58(4):432–440
Howlett AC, Qualy JM, Khachatrian LL (1986) Involvement of gi in the inhibition of denylate cyclase by cannbimimetic drugs. Mol Pharmacol 29(3):307–313
Huestis MA (2007) Human cannabinoid pharmacokinetics. Chem Biodivers 4(8):1770–1804
Huizenga MN, Sepulveda-Rodriguez A, Forcelli PA (2019) Preclinical safety and efficacy of cannabidivarin for early life seizures. Neuropharmacology 148:189–198
Irving AJ, Coutts AA, Harvey J, Rae MG, Mackie K, Bewick GS, Pertwee RG (2000) Functional expression of cell surface cannabinoid CB1 receoptros on presynaptic inhibitiory terminals in cultured rat hippocampal neurons. Neurosci 98(2):253–262
Jiang R, Yamaori S, Okamoto Y et al (2013) Cannabidiol is a potent inhibition of the catalytic activity of cytochrome P450 2C19. Drug Metab Pharmacokinetic 28:332–338
Kaplan JS, Stella N, Catterall WA, Westenbroek RE (2017) Cannabidiol attenuates seizures and social deficits in mouse model of Dravet syndrome. Proc Natl Acad Sci USA 114(42):11229–11234
Katona I, Sperlagh B, Magloczky A, Santha E, Kofalvi A et al (2000) GABAergic interneurons are the targets of cannabinoid actions in the human hippocampus. Neurosci. 100(4):797–804
Kawamura Y, Fukaya M, Maejima T, Yoshida T, Miura E, Watanabe M et al (2006) The CB1 cannabinoid receptor is the major cannabinoid receptor at excitatory presynaptic sites in the hippocampus and cerebellum. Neurosci 26(11):2991–3001
Klotz KA, Hirsch M, Heers M, Schulze-Bonhage A, Jacobs J (2019) Effects of cannabidiol on brivaracetam plasma levels. Epilepsia 60(7):e74–e77
Laux LC, Bebin EM, Checketts D, Chez M, Flamini R, Marsh ED, Miller I, Nichol K, Park Y, Segal E, Seltzer L, Szaflarski JP, Thiele EA, Weinstock A; CBD EAP study group (2019) Long-term safety and efficacy of cannabidiol in children and adults with treatment resistant Lennox-Gastaut syndrome or Dravet syndrome: Expanded access program results. Epilepsy Res 154:13–20
Laprairie RB, Bagher AM, Kelly ME, Denovan-Wright EM (2015) Cannabidiol is a negative allosteric modulator of the cannabinoid CB1 receptor. Br J Pharmacol 172:4790–4805
Leino AD, Emoto C, Fukuda T, Privitera M, Vinks AA, Alloway RR (2019) Evidence of a clinically significant drug-drug interaction between cannabidiol and tacrolimus. Am J Transplant 19(10):2944–2948
Linge R, Jimenez-Sanchez L, Campa L, Pilar-Cuellar F, Vidal R, Pazos A, Adell A, Diaz A (2016) Cannabidiol induces rapid-acting antidepressant-like effects and enhances cortical 5-HT/glutamate neurotransmission: role of 5-HT1A receptors. Neuropharmacology 103:16–26
Lucassen PJ, Meerlo P, Naylor AS, van Dam AM, Dayer AG, Fuchs E, Oomen CA, Czeh B (2010) Regulation of adult neurogenesis by stress, sleep disruption, exercise and inflammation: Implications for depression and antidepressant action. Eur Neuropsychopharmacol 20(1):1–17
Maa E, Figi P (2014) The case for medical marijuana in epilepsy. Epilepsia 55:783–786
Martin P, Consroe P (1976) Cannabinoid induced behavioral convulsions in rabbits. Science 194(4268):965–967
Mascal M, Hafezi N, Wang D, Hu Y, Serra G, Dallas ML, Spencer JPE (2019) Synthetic, non-intoxicating 8,9-dihydrocannabidiol for the mitigation of seizures. Sci Rep 9(1):7778
Mechoulam R, Carlini EA (1978) Toward drugs derived from cannabis. Naturwissenschaften 65:174–179
Mohpael P, Ekdahl CT, Lindvall O (2004) Status epilepticus severity influences the long-term outcome of neurogenesis in the adult dentate gyrus. Neurobiol Dis 15:196–205
O’Brien TJ, Berkovic SF, French J, Messenheimer J, Gutterman D. (2018) Transdermal cannabidiol (CBD) gel for the treatment of focal epilepsy in adults. Abstract 2.253, American Epilepsy Society, New Orleans, LA
O’Connell BK, Gloss D, Devinsky O (2017) Cannabinoids in treatment-resistant epilepsy: A review. Epilepsy Behav 70(Pt B):341–348
Ohlsson A, Lindgren JE, Andersson S, Agurell S, Gillespie H, Hollister LE (1986) Single-dose kinetics of deuterium-labelled cannabidiol in man after smoking ad intravenous administration. Biomed Environ Mass Spectrom 13(2):77–83
Oldham M, Sullivan J, Singhal N, Tilton N, and Cilio M (2015) Long-term efficacy and tolerability of add-on cannabidiol for drug-resistant pediatric epilepsies, in: Proceedings of the American Epilepsy Society Annual Meeting; 2015 Dec. 4–8; Philadelphia, PA. Abst. 2.296, American Epilepsy Society, Chicago, IL
Parent JM, Yu TW, Leibowitz RT, Geschwind DH, Sloviter RS, Lowenstein DH (1997) Dentate granule cell neurogenesis is increased by seizures and contributes to aberrant network reorganization in the adult rat hippocampus. J Neurosci 17:3727–3738
Patel RR, Barbosa C, Brustovetsky T, Brustovetsky N (2016) & Cummins, T.R. Aberrant epilepsy-associated mutant Nav1.6 sodium channel activity can be targeted with cannabidiol. Brain 139:2164–2181
Patra PH, Barker-Haliski M, White HS, Whalley BJ, Glyn S, Sandhu H, Jones N, Bazelot M, Williams CM, McNeish AJ (2019) Cannabidiol reduces seizures and associated behavioral comorbidities in a range of animal seizure and epilepsy models. Epilepsia 60(2):303–314
Pertwee RG, Cascio MG (2014) Known pharmacological actions of delta-9-tetrahydrocannabinol and of four other chemical constituents of cannabis that activate cannabinoid receptors. In: Pertwee RG (ed) Handbook of cannabis. Oxford University Press, Oxford, UK
Pisanti S, Malfitano AM, Ciaglia E, Lamberti A, Ranieri R, Cuomo G, Abate M, Faggiana G, Proto MC, Fiore D, Laezza C, Bifulco M (2017) Cannabidiol: State of the art and new challenges for therapeutic applications. Pharmacol Ther 175:133–150
Porter BE, Jacobson C (2013) Report of a parent survey of cannabidiol-enriched cannabis use in pediatric treatment-resistant epilepsy. Epilepsy Behav 29:574–577
Rajesh M, Mukhopadhyay P, Batkai S, Hasko G, Liaudet L, Drel VR, Obrosova IG, Pacher P (2007) Cannabidiol attenuates high glucose-induced endothelial cell inflammatory response and barrier disruption. Am J Physiol Heart Circ Physiol 293:H610–H619
Reddy DS, Golub V (2016) The pharmacological basis of cannabis therapy for epilepsy. J Pharmacol Exp Therap 357:45–55
Reddy DS, Kuruba R (2013) Experimental models of status epilepticus and neuronal injury for evaluation of therapeutic interventions. Int J Mol Sci 14(9):18284–18318
Resstel LB, Joca SR, Moreira FA, Correa FM, Guimaraes FS (2006) Effects of cannabidiol and diazepam on behavioral and cardiovascular responses induced by contextual conditioned fear in rats. Behav Brain Res 172:294–298
Reynolds JR (1861) Epilepsy: its symptoms, treatment, and relation to other chronic convulsive diseases. UK. John Churchill, London
Rosenberg EC, Patra PH, Whalley BJ (2017) Therapeutic effects of cannabinoids in animal models of seizures, epilepsy, epileptogenesis, and epilepsy-related neuroprotection. Epilepsy Behav 70(Pt B):319–327
Rosenberg E, Tsien C, Richard W, Whalley BJ, Devinksy O (2015) Cannabinoids and epilepsy. Neurotherapeutics 12(4):747–768
Ross HR, Napier I, Conner M (2008) Inhibition of recombinant human T-type calcium channels by delta9-tetrahydrocannabinol and cannabidiol. J Bio Chem 283:16124–16134
Rubinstein M, Han S, Tai C, Westenbroek RE, Hunker A, Scheuer T, Catterall WA (2015) Dissecting the phenotypes of Dravet syndrome by gene deletion. Brain 138(8):2219–2233
Schmidt D, Bourgeois B (2000) A risk-benefit assessment of therapies for Lennox-Gastaut syndrome. Drug Saf 22(6):467–477
Sebree T, O’Neill C, Messenheimer J, Gutterman D. (2016) Safety and tolerability of ZYN002 (synthetic cannabidiol) transdermal gel in healthy subjects: two phase 1, randomized, double-blind, placebo-controlled studies. Abstract 2.214, American Epilepsy Society, Houston, TX
Shen M, Piser TM, Seybold VS, Thayer SA (1996) Cannabinoid receptor agonists inhibit glutamatergic synaptic transmission in rat hippocampal cultures. Neurosci. 16(14):4322–4334
Silveira Filho NG, Tufik S (1981) Comparative effects between cannabidiol and diazepam on neophobia, food intake, and conflict behavior. Res Commun Psychol Psychiatry Behav 6:25–26
Solinas M, Massi P, Cinquina V, Valentia M, Bolognini D, Garboldi M, Monti E, Rubino T, Parolaro D (2013) Cannabidiol, a non-psychoactive cannabinoid compound, inhibits proliferation and invasion in U87-MG and T98G glioma cells through a multitarget effect. PLoS One 8(10):e76918
Stadnicki SW, Schaeppi U, Rosenkrantz H, Braude MC (1974) Detla9-tetrahydrocannabidiol: subcortical spike bursts and motor manifestations in a Fischer rat treated orally for 109 days
Steel D, Symonds JD, Zuberi SM, Brunklaus A (2017) Dravet syndrome and its mimics: beyond SCN1A. Epilepsia 58:1807–1816
Stempel AV, Stumpf A, Zhang HY, Ozdogan T, Pannasch U, Theis AK et al (2016) Cannabinoid type 2 receptors mediate a cell type-specific plasticity in the hippocampus. Neuron 90:795–809
Stern CA, Gazarini L, Vanvossen AC, Zuardi AW, Galve-Roperh I, Guimaraes FS et al (2015) Delta9-tetrahydrocannabinol alone and combined with cannabidiol mitigate fear memory through reconsolidation disruption. Eur Neuropsychopharmacol 25:958–965
Straiker A, Dvorakova M, Zimmowitch A, Mackie K (2018) Cannabidiol inhibits endocannabinoid signaling in autaptic hippocampal neurons. Mol Pharmacol 94(1):743–748
Sun S, Hu F, Wu J, Zhang S (2017) Cannabidiol attenuates OGD/R-induced damage by enhancing mitochondrial bioenergetics and modulating glucose metabolism via pentose-phosphate pathway in hippocampal neurons. Redox Biol 11:577–585
Szaflarski JP, Bebin EM, Comi AM et al (2018a) CBD EAP Study Group. Long-term safety and treatment effects of cannabidiol in children and adults with treatment-resistant epilepsies: expanded access program results. Epilepsia 59(8):1540–1548
Szaflarski JP, Bebin EM, Cutter G, DeWolfe J, Dure LS, Gaston TE, Kankirawatana P, Liu Y, Singh R, Standaert DG, Thomas AE, Ver Hoef LW, Program UC (2018b) Cannabidiol improves frequency and severity of seizures and reduces adverse events in an open-label add-on prospective study. Epilepsy Behav 87:131–136
Thiele EA, Marsh ED, French JA, Mazurkiewicz-Beldzinska M, Benbadis SR, Joshi C, Lyons PD, Taylor A, Roberts C, Sommerville K (2018) GWPCARE4 Study Group. Cannabidiol in patients with seizures associated with Lennox-Gastaut syndrome (GWCARE4): a randomized, double-blind, placebo-controlled phase 3 trial. Lancet 391(10125):1085–1096
Taylor L, Gidal B, Blakey G, Tayo B, Morrison G (2018) A Phase I, Randomized, Double-Blind, Placebo-Controlled, Single Ascending Dose, Multiple Dose, and Food Effect Trial of the Safety, Tolerability and Pharmacokinetics of Highly Purified Cannabidiol in Healthy Subjects. CNS Drugs 32(11):1053–1067
Trembly B, Sherman M (1990. July 8-11) Double-blind clinical study of cannabidiol as a secondary anticonvulsant, presented at: marijuana ‘90 International Conference on Cannabis and Cannabinoids. International Association for Cannabinoid Medicines, Kolymbari, Crete
Tsou K, Brown S, Sanduo-pena M, Mackie K, Walker J (1998) Immunohistochemical distribution of cannabinoid CB1 receptors in the rat central nervous system. Neuroscience 83:393–411
Tzadok M, Uliel-Siboni S, Linder I, Kramer U, Epstein O, Menascu S, Nissenkorn A, Yosef OB, Hyman E, Granot D, Dor M, Lerman-Sagie T, Ben-Zeev B (2016) CBD-enriched medical cannabis for intractable pediatric epilepsy: the current Israeli experience. Seizure 35:41–44
Wallace MJ, Blair RE, Falenski KW, Martin BR, DeLorenzo RJ (2003) The endogenous cannabinoid system regulates seizure frequency and duration in a model of temporal lobe epilepsy. J Pharmacol Exp Ther 307:129–137
Wallace MJ, Martin BR, DeLorenzo RJ (2002) Evidence for a physiological rle of endocannabinoids in the modulation of seizure threshold and severity. Eur J Pharmacol 452:295–301
Wallace MJ, Wiley JL, Martin BR, DeLorenzo RJ (2001) Assessment of the role of CB1 receptors in cannabinoid anticonvulsant effects. Eur J Pharmacol 428:51–57
Wolf SA, Bick-Sander A, Fabel K, Leal-Galicia P, Tauber S, Ramirez-Rodriquez G, Muller A, Melnik A, Waltinger TP, Ullrich O, Kempermann G (2010) Cannabinoid receptor CB1 mediates baseline and activity-induced survival of new neurons in adult hippocampal neurogenesis. Cell Commun Signal 8:12
Younus I, Reddy DS (2017) Epigenetic interventions for epileptogenesis: a new frontier for curing epilepsy. Pharmacol Ther 177:108–122
Zanelati TV, Biojone C, Moreira FA, Guimaraes FS, Joca SR (2010) Antidepressant-like effects of cannabidiol in mice: possible involvement of 5-HT1A receptors. Br J Pharmcol 159:122–128
Zuardi AW, Karniol IG (1983) Changes in the conditioned emotional response of rats induced by 9-THC, CBD and mixture of the two cannabinoids. Arq Biol Tecnol 26:391–397
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Golub, V., Reddy, D.S. (2021). Cannabidiol Therapy for Refractory Epilepsy and Seizure Disorders. In: Murillo-Rodriguez, E., Pandi-Perumal, S.R., Monti, J.M. (eds) Cannabinoids and Neuropsychiatric Disorders. Advances in Experimental Medicine and Biology, vol 1264. Springer, Cham. https://doi.org/10.1007/978-3-030-57369-0_7
Download citation
DOI: https://doi.org/10.1007/978-3-030-57369-0_7
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-57368-3
Online ISBN: 978-3-030-57369-0
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)