Abstract
Marijuana has been utilized as a medicinal plant to treat a variety of conditions for nearly five millennia. Over the past few years, there has been an unprecedented interest in using cannabis extracts to treat epilepsy, spurred on by a few refractory pediatric cases featured in the media that had an almost miraculous response to cannabidiol-enriched marijuana extracts. This review attempts to answer the most important questions a clinician may have regarding the use of marijuana in epilepsy. First, we review the preclinical and human evidences for the anticonvulsant properties of the different cannabinoids, mainly tetrahydrocannabinol (THC) and cannabidiol (CBD). Then, we explore the safety data from animal and human studies. Lastly, we attempt to reconcile the controversy regarding physicians’ and patients’ opinions about whether the available evidence is sufficient to recommend the use of marijuana to treat epilepsy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
For nearly 5000 years, cannabis has been used widely to treat a variety of medical conditions. These include nausea, anorexia, glaucoma, pain, muscle spasticity, asthma, depression, anxiety, among many others [1]. Marijuana has been a particularly interesting candidate for epilepsy treatment. Some of the first attempts in using marijuana to treat epilepsy date back to the nineteenth century when prominent English neurologists, Reynolds and Gowers, described anecdotal successes. [2•, 3]. From the late nineteenth century till the mid twentieth century, there is a paucity of data regarding the use of cannabis to treat seizures in the English language medical literature. By World War II, the US 1937 Marijuana Tax act virtually terminated all research on cannabis. As the political climate has changed and many states have recently allowed the medical use of marijuana, this has led to increased interest in research. A few pioneer studies in the late 1970s and 80s showed some or no efficacy of cannabidiol (CBD)-enriched cannabis extracts for epilepsy; however, these studies suffered from insufficient patient numbers and conflicting results. More robust preclinical data exist showing that the two main biologically active cannabinoids—tetrahydrocannabinol (THC) and cannabidiol (CBD)—clearly have anticonvulsant properties in animal models of acute seizures and epilepsy [4–9]. Experts from the American Academy of Neurology recently reviewed the available scientific studies on the safety and effectiveness of cannabis in certain neurological conditions. In their published guideline, they concluded that the strongest scientific evidence exists for oral cannabis extracts as a treatment of spasticity and central pain in multiple sclerosis. For epilepsy, they concluded that the efficacy of oral cannabinoids was unknown based on the available evidence [10]. For a list of selected cannabinoid-based pharmaceutical drugs, see Table 1.
Despite the availability of over 20 antiepileptic medications, close to 30 % of patients with epilepsy still remain refractory. The morbidity and potential mortality of epilepsy as well as the burden of medication side effects prompt patients and their loved ones to seek alternative therapies and new treatments. Medical marijuana for epilepsy received significant attention in the past few years after the social media highlighted cases of pediatric refractory epilepsies that appeared almost miraculously cured by cannabis extracts. Since then, many families whose members are affected by epilepsy have moved to states like Colorado where medical marijuana is legal and easily accessible in order to treat their loved ones. In a recent survey, 84 % of parents reported seizure reduction using CBD-enriched cannabis [11]. The survey was conducted online with parents from a Facebook group supporting the use of CBD-enriched cannabis. The children included in the survey, ranging from age 2 to 16 years, had treatment-resistant epilepsy, mostly Dravet syndrome. Cannabis extracts high in CBD, which is a non-psychoactive constituent of the cannabis plant, were well tolerated according to this survey. In fact, some parents reported other positive effects including improved alertness and better sleep and mood. Driven in part by patients and the social media, several prospective studies using purified CBD are currently on-going and are showing somewhat promising preliminary results. However, as noted above, there is a scarcity of blinded placebo-controlled studies assessing the safety and efficacy of marijuana in the treatment of epilepsy. The American Epilepsy Society (AES) issued a statement on their position on medical marijuana. In their publication, they raised the concern of a lack of robust scientific evidence for the safety and effectiveness of marijuana in epilepsy and called for further research. The AES concluded that the risk/benefit ratio does not support use of marijuana for treatment of seizures at this time. In this era of particular interest in medicinal marijuana, this article is an attempt to answer some of the most important questions a clinician may come across regarding the use of marijuana in epilepsy.
Do Cannabinoids Possess Anticonvulsant Properties?
Although cannabis has been used for centuries as a medicinal plant to treat several conditions including epilepsy, to date, there is a lack of high-quality clinical evidence to prove or disprove its efficacy.
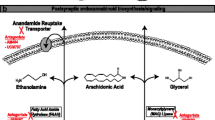
The plant cannabis sativa produces over 80 terpeno-phenol compounds called “cannabinoids.” Two major biologically active cannabinoids, THC and CBD, have been studied the most. Tetrahydrocannabinol (Δ9-THC), which is produced from the corresponding acid derivative following heating, conveys this plant its popularity given its psychotropic effects. Cannabidiol on the other hand is believed to counteract some of the THC psychoactivity and enhance its tolerability.
Although there have been anecdotal data regarding marijuana use in epilepsy for over 150 years, interest increased in 2013 following sporadic reports of pediatric cases, which appeared to be miraculously cured with CBD-rich extracts of cannabis [11, 12]. The news quickly spread to the social media, including the famous case of Charlotte Figi that was highlighted by CNN [13]. Charlotte, a little girl with SCN1A confirmed Dravet syndrome, was suffering from 300+ convulsions a week. After treating her with a cannabis strain with a high CBD to THC ratio (now known as Charlotte’s web), her seizure frequency improved more than 90 % with only two to three convulsions a month.
Her success story motivated a flurry of families to relocate to states like Colorado where medical marijuana is legal and available.
This media hype, although understandable from the point of view of desperate parents, may be deleterious for the epilepsy community as patients’ high expectations influence clinical trials and may detract from obtaining unbiased and reliable high quality data.
Since then, several uncontrolled observational studies have been published exploring patients’ experience using CBD-enriched cannabis in states that liberalized marijuana laws [11, 14]. These studies involved a refractory pediatric population and report a significant seizure reduction with only mild side effects. These results, however, need to be viewed with caution, especially given the experience in Colorado where families who moved to that state to treat their loved ones were two times more likely to have a greater than 50 % seizure reduction compared to patients who already lived in the state [14]. Family member’s feelings and expectations about a treatment can influence their judgments about its effectiveness [15]. In fact in experimental models, analgesic and motor placebo responses appear to be mediated by verbally induced expectations [16]. In the case of Colorado, where families left their jobs and friends behind to seek a new treatment to their suffering child, the expectations could have easily played a significant role.
Over the last three decades, significant advances have been made in understanding the pharmacology and mechanism of action of cannabinoids. The anticonvulsant properties of these molecules have been studied in various acute seizure models. Most of the data convincingly show that the two major biologically active cannabinoids (THC and CBD) have anticonvulsant properties. There has been some evidence, however, of pro-convulsive properties of THC in healthy animals, mostly at higher doses [17, 18] including in a recent comprehensive review of the preclinical data [19••]. The mechanism by which THC exerts its anticonvulsive properties is believed to be through the CB1 receptors, which are G protein-coupled cell membrane receptors expressed ubiquitously in neurons of the central nervous system [6, 20]. They are also expressed to a lesser degree in the lungs, liver, and kidneys. The CB2 receptor is expressed mainly in the immune system and hematopoietic cells. [21] In the case of CBD, the mechanism of action is not well understood but it has become clear that its anticonvulsant properties do not involve a cannabinoid receptor (CBR)-dependent mechanism [22].
The anticonvulsant properties of CBD have been shown to be dose dependent in several animal experiments and follow a bell-shaped curve [23].
A recent Cochrane study reviewed the available literature regarding the anticonvulsive properties of cannabis in patients with epilepsy. They searched the literature for randomized controlled studies, whether blinded or not. Four studies were reviewed which included a total of 48 patients. All included CBD as a treatment. They excluded 16 studies which were mainly case reports or retrospective studies. Due to the small sample size and low quality of these studies, the authors could not draw any conclusions on the efficacy of cannabinoids in epilepsy [24••].
A multicenter, uncontrolled, unblinded prospective study is underway using a purified 98 % oil-based CBD extract (Epidiolex) as an add-on treatment for medically resistant epilepsy in children and young adults. At 3 months of treatment, there was a reported 32 % median reduction of seizures. These preliminary results were particularly promising for patients with Dravet syndrome as 3/9 were seizure-free at 3 months [25]; however, further randomized controlled studies are clearly needed to confirm these findings.
Are Cannabinoids Safe?
Side effects from medical treatments have a major impact on quality of life in epilepsy patients [26]. Given the enormous burden of side effects associated with traditional antiepileptic medicines, patients and caregivers often look for “natural remedies.” This naturalistic fallacy or false belief that natural medicines are safer than prescription drugs, may potentially bias patient/caregiver reporting of side effects in most of the currently available uncontrolled studies; thus, controlled and blinded studies are crucial with these compounds. Keeping that in mind, most of the studies using purified CBD or high CBD content cannabis report only minor side effects, such as somnolence or fatigue, and only a few patients withdrew treatment due to side effects [11, 14, 24••]. On the other hand, THC has significant addictive potential given its psychoactive properties. There have also been reports of worsening seizures in animal and human studies, particularly those involving high THC content cannabis [17, 27] .
Despite popular belief that marijuana is safe, there is well-documented evidence of adverse health effects of cannabis use, particularly as a recreational drug. The acute effects of cannabis use, which are mostly related to its psychoactive properties, are well known an include impaired short-term memory, decreased motor coordination which may lead to motor vehicle accidents or injuries, and altered judgment which may lead to high risk behavior. Also, cannabis intoxication may lead to paranoia and psychosis, particularly at high doses. With chronic marijuana use, there are also concerns related to its effect on brain development leading to neuropsychological decline, particularly concerning given the current interest in the pediatric population [28]. Diffusion-weighted MRI and connectivity mapping have shown decreased connectivity in brain regions, particularly the fornix and corpus callosum, in long-term cannabis users [29]. These abnormalities were greatest in subjects who began regular marijuana use during early adolescence. These results using advanced MRI techniques may explain several reports of lower IQ in young adults and adolescents with chronic heavy cannabis use [30, 31]. It has also been reported that early marijuana use is associated with poor school performance and increased risk of dropping out of school [28].
In addition to the addictive potential and effects on cognitive performance, there are concerns for adverse health effects, in particular with smoking cannabis, which may lead to chronic bronchitis, airway infections, and potentially, cancer.
There is also recent evidence that links marijuana to an increase vulnerability to stroke [32].
Are there Potential Drug Interactions?
The two main cannabinoids, THC and CBD, are metabolized by the cytochrome P450 enzyme system. In vitro studies have shown that constituents of cannabis are potent and broad-spectrum inhibitors of key drug-metabolizing enzymes and transporters, including CYP2C9, CYP2C19, CYP2D6, CYP2E1, and CYP3A4 [33–35]. However, inhibition is observed at THC and CBD doses significantly higher than the ones used in clinical trials.
Limited human data exist for potential drug interactions with other antiepileptic drugs (AEDs). Preliminary data from a prospective observational study using a purified CBD extract (Epidiolex) showed that a subset of patients had experienced an increase in clobazam and its active metabolite (norclobazam) serum concentration and required subsequent dose reduction [36, 37]. The median change in clobazam levels was of 8.3 % with a very wide range (−64 to 478 %, N:17). Further studies are needed to assess the pharmacokinetic interactions of cannabis products; however, potential interactions with AEDs are likely and serum levels of AEDs should be monitored in all trials.
What do Patients and Doctors Think?
Neurologists are often asked by their patients about the use of cannabis for epilepsy and other neurological conditions. According to some studies, about 20 % of epilepsy patients are actively using cannabis [38, 39].
Many patients believe marijuana is an effective treatment for epilepsy. In a telephone survey in a tertiary epilepsy center in Canada, 24 % of patients claimed marijuana was effective at controlling their seizures [39]. Another survey of parents of children with treatment-resistant epilepsy showed that 84 % found a positive effect of cannabidiol-enriched cannabis in reducing seizure frequency. In addition to seizure reduction, parents also reported other beneficial effects including increased alertness, better mood, and improved sleep [11]. These reports, however, need to be analyzed with caution as they lack placebo controls and blinded outcome and solely represent self-assessment of efficacy and tolerability, which may be subject to bias.
Physicians, on the other hand, appear to have a different opinion than their patients. In a recent online survey conducted by Epilepsia, only a minority of neurologists and epileptologists reported that there was sufficient data to support the efficacy and safety of cannabinoids in epilepsy and only about half of them would recommend its use even in severe cases of medically refractory seizures. Interestingly, the same survey included patient and general public opinion showing that more than 95 % subjects believe there is enough safety and efficacy data and would recommend the use of marijuana in epilepsy. Interestingly, general physicians’ responses were closer to those of the patients and the general public, as greater than 80 % would advise using marijuana in severe cases of epilepsy [40].
Although these surveys are subject to many limitations, they show that there is a great discrepancy in self-assessment of the available data. It appears that the specialists do not generally endorse marijuana in treating epilepsy, while patients and the general public, perhaps tired by the slow progress in science, are willing to recommend cannabinoids for epilepsy without waiting for solid scientific data.
Conclusions
Extensive preclinical data support the anticonvulsant properties of the main biologically active cannabinoids, THC and CBD, with better data for CBD. Solid clinical data in patients with epilepsy, however, are lacking. Despite the plethora of circumstantial data collected over a period of 150 years and a handful of small randomized trials, good quality placebo-controlled blinded studies are sorely needed.
Emerging preliminary data from uncontrolled studies using purified CBD extracts show encouraging results in terms of efficacy, particularly in certain refractory epilepsy syndromes. The numbers of studies are small, however, and possibly subject to bias. Surveys of parents living in states where medical marijuana is legal paint a picture where CBD is highly effective for refractory pediatric epilepsy cases with minimal adverse effects. A recent study conducted by the editors of Epilepsia showed that almost all the patients and public surveyed believe there is sufficient efficacy and safety data for the use of marijuana in epilepsy. This opinion was only shared by a minority of general neurologists and epileptologists. This is mostly due to the lack of objective data making physicians less enthusiastic than their patients.
Given the proven anticonvulsant effects from preclinical studies and the lack of psychoactive properties, CBD could be a good candidate for a new antiepileptic medication. Although it appears to be safe with only minor side effects reported so far in the clinical trials, the long-term effects are unknown, and how it compares to other AEDs is unknown. Given the efficacy results of CBD in pediatric epilepsies, it is particularly important to assess the long-term neuropsychological effects in the developing brain.
Many questions raised in this article remain unanswered: How effective is cannabis in pediatric vs. adult patients? Is there a particular type of epilepsy or syndrome such as Dravet for which cannabinoids are particularly effective? Are purified forms of non-psychoactive cannabinoids (cannabidiol and cannabidivarin) safer for the developing brain? One thing is clear: further studies are needed to assess the role cannabinoids will play in the armamentarium against epilepsy. We feel it is premature to recommend cannabinoids to patients with epilepsy at this time; however, we encourage participation in a controlled and double-blinded study.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance•• Of major importance
Petersen, R.C., Marihuana research findings. 1976. Summary. NIDA Res Monogr. 1977:14:1–37.
Koppel BS et al. Systematic review: efficacy and safety of medical marijuana in selected neurologic disorders: report of the Guideline Development Subcommittee of the American Academy of Neurology. Neurology. 2014;82(17):1556–63. Koppel et al. reviewed the available literature on medical marijuana to treat symptoms of multiple sclerosis, epilepsy and movement disorders. It found sufficient articles with class I evidence to support a role for cannabis in the symptomatic treatment of multiple sclerosis (MS), but its efficacy in Huntington disease (HD), Parkinson disease (PD) and epilepsy is unknown.
WG, Epilepsy and other chronic convulsive disorders. Churchill, 1881.
Reynolds, J., Epilepsy: its symptoms, treatment, and relation to other chronic convulsive diseases. John Churchill, 1861
Consroe P, Wolkin A. Cannabidiol—antiepileptic drug comparisons and interactions in experimentally induced seizures in rats. J Pharmacol Exp Ther. 1977;201(1):26–32.
Consroe P et al. Effects of cannabidiol on behavioral seizures caused by convulsant drugs or current in mice. Eur J Pharmacol. 1982;83(3–4):293–8.
Wallace MJ et al. Assessment of the role of CB1 receptors in cannabinoid anticonvulsant effects. Eur J Pharmacol. 2001;428(1):51–7.
Wallace MJ, Martin BR, DeLorenzo RJ. Evidence for a physiological role of endocannabinoids in the modulation of seizure threshold and severity. Eur J Pharmacol. 2002;452(3):295–301.
Jones NA et al. Cannabidiol displays antiepileptiform and antiseizure properties in vitro and in vivo. J Pharmacol Exp Ther. 2010;332(2):569–77.
Karler R, Cely W, Turkanis SA. Anticonvulsant properties of delta 9-tetrahydrocannabinol and other cannabinoids. Life Sci. 1974;15(5):931–47.
Porter BE, Jacobson C. Report of a parent survey of cannabidiol-enriched cannabis use in pediatric treatment-resistant epilepsy. Epilepsy Behav. 2013;29(3):574–7.
Saade D, Joshi C. Pure cannabidiol in the treatment of malignant migrating partial seizures in infancy: a case report. Pediatr Neurol. 2015;52(5):544–7.
Maa E, Figi P. The case for medical marijuana in epilepsy. Epilepsia. 2014;55(6):783–6.
Press CA, Knupp KG, Chapman KE. Parental reporting of response to oral cannabis extracts for treatment of refractory epilepsy. Epilepsy Behav. 2015;45:49–52.
Grelotti DJ, Kaptchuk TJ. Placebo by proxy. BMJ. 2011;343:d4345.
Benedetti F et al. Conscious expectation and unconscious conditioning in analgesic, motor, and hormonal placebo/nocebo responses. J Neurosci. 2003;23(10):4315–23.
Martin P, Consroe P. Cannabinoid induced behavioral convulsions in rabbits. Science. 1976;194(4268):965–7.
Stadnicki SW et al. Delta9-tetrahydrocannabinol: subcortical spike bursts and motor manifestations in a Fischer rat treated orally for 109 days. Life Sci. 1974;14(3):463–72.
Devinsky O et al. Cannabidiol: pharmacology and potential therapeutic role in epilepsy and other neuropsychiatric disorders. Epilepsia. 2014;55(6):791–802. This manuscript is an evidence-based review of the preclinical and clinical evidence for the use Cannabidiol (CBD) in epilepsy and other neuropsychiatric disorders. The authors highlighted the limited evidence supporting medical marijuana and CBD to treat epilepsy and acknowledged the encouraging preclinical evidence and called for rigorous research.
Matsuda LA et al. Structure of a cannabinoid receptor and functional expression of the cloned cDNA. Nature. 1990;346(6284):561–4.
Pacher P, Mechoulam R. Is lipid signaling through cannabinoid 2 receptors part of a protective system? Prog Lipid Res. 2011;50(2):193–211.
Pertwee RG. The diverse CB1 and CB2 receptor pharmacology of three plant cannabinoids: delta9-tetrahydrocannabinol, cannabidiol and delta9-tetrahydrocannabivarin. Br J Pharmacol. 2008;153(2):199–215.
Cilio MR, Thiele EA, Devinsky O. The case for assessing cannabidiol in epilepsy. Epilepsia. 2014;55(6):787–90.
Gloss D, Vickrey B. Cannabinoids for epilepsy. Cochrane Database Syst Rev. 2014;3:CD009270. This article is an evidence-based systematic review that screened randomized controlled studies using cannabinoids for the treatment of epilepsy. Four studies met their inclusion criteria. Although two of the studies found limited improvements, all four suffered from methodological issues, including small sample size and, in some cases, inadequate blinding. The authors could not draw any reliable conclusions regarding the efficacy of cannabinoids in epilepsy based on the available evidence. They also acknowledged the lack of significant side effects in any of the patients studied except in one study (Ames 1985) which reported mild drowsiness.
Devinsky O. et al. Efficacy and safety of Epidiolex (cannabidiol) in children and young adults with treatment-resistant epilepsy: Initial data from an expanded access program. 2014.(Abst. 3.303).
Wheless JW. Intractable epilepsy: a survey of patients and caregivers. Epilepsy Behav. 2006;8(4):756–64.
Keeler MH, Reifler CB. Grand mal convulsions subsequent to marijuana use. Case report. Dis Nerv Syst. 1967;28(7 Pt 1):474–5.
Volkow ND et al. Adverse health effects of marijuana use. N Engl J Med. 2014;370(23):2219–27.
Zalesky A et al. Effect of long-term cannabis use on axonal fibre connectivity. Brain. 2012;135(Pt 7):2245–55.
Bolla KI et al. Dose-related neurocognitive effects of marijuana use. Neurology. 2002;59(9):1337–43.
Fried P et al. Current and former marijuana use: preliminary findings of a longitudinal study of effects on IQ in young adults. CMAJ. 2002;166(7):887–91.
Wolff V et al. Cannabis-related stroke: myth or reality? Stroke. 2013;44(2):558–63.
Yamaori S et al. Potent inhibition of human cytochrome P450 3A isoforms by cannabidiol: role of phenolic hydroxyl groups in the resorcinol moiety. Life Sci. 2011;88(15–16):730–6.
Yamaori S et al. Comparison in the in vitro inhibitory effects of major phytocannabinoids and polycyclic aromatic hydrocarbons contained in marijuana smoke on cytochrome P450 2C9 activity. Drug Metab Pharmacokinet. 2012;27(3):294–300.
Yamaori S et al. Cannabidiol, a major phytocannabinoid, as a potent atypical inhibitor for CYP2D6. Drug Metab Dispos. 2011;39(11):2049–56.
Friedman D.e.a. The effect of Epidiolex (cannabidiol) on serum levels of concomitant anti-epileptic drugs in children and young adults with treatment-resistant epilepsy in an expanded access program. 2014(Abst. 2.309).
Bruno P.e.a. Effect of cannabidiol on the antiepileptic drug clobazam. (Abst. 2.428).
Hamerle M et al. Cannabis and other illicit drug use in epilepsy patients. Eur J Neurol. 2014;21(1):167–70.
Gross DW et al. Marijuana use and epilepsy: prevalence in patients of a tertiary care epilepsy center. Neurology. 2004;62(11):2095–7.
Mathern GW, Beninsig L, Nehlig A. Fewer specialists support using medical marijuana and CBD in treating epilepsy patients compared with other medical professionals and patients: result of Epilepsia’s survey. Epilepsia. 2015;56(1):1–6.
Compliance with Ethics Guidelines
Conflict of Interest
Kamil Detyniecki has received research grants from Eisai, Lundbeck, and Sunovion and an honoraria payment from Eisai.
Lawrence Hirsch has received consultancy fees from Lundbeck, Upsher-Smith, and GlaxoSmithKline, grants from UCB-Pharma, Upsher-Smith, Lundbeck, Eisai, and Sunovion, and royalty payments from UpToDate, Inc. and Medlink Corporation.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Author information
Authors and Affiliations
Corresponding author
Additional information
This article is part of the Topical Collection on Epilepsy
Rights and permissions
About this article
Cite this article
Detyniecki, K., Hirsch, L. Marijuana Use in Epilepsy: The Myth and the Reality. Curr Neurol Neurosci Rep 15, 65 (2015). https://doi.org/10.1007/s11910-015-0586-5
Published:
DOI: https://doi.org/10.1007/s11910-015-0586-5