Abstract
Some concerns regarding the depletion of non-renewable sources of energy along with the environmental damage resulting from their use have motivated the search for alternative fuels. Ethanol is a renewable and important energy alternative for Otto-cycle engines. In such context, this study proposes the evaluation of carbon monoxide (CO) and total hydrocarbons (THC) concentrations and the performance of a spark-ignited non-road engine fueled with gasoline types A and C (GC, with 27% ethanol) and kerosene as adulterant in different proportions. Quality parameters of the fuel blends were also evaluated. The results revealed some difficulties in the identification of gasoline adulteration, relating to the current legal parameters in the conditions of mixtures and tests used in the study. Regarding the gaseous emissions, the addition of the adulterant resulted in an increase of the CO and THC concentrations to 74% and 78%, respectively, in gasoline type C with 30% adulterant, when compared to gasoline C free of adulterant. The addition of ethanol to the gasoline resulted in a reduction of the CO and THC concentrations to 64% and 56%, respectively, in relation to the pure gasoline (without ethanol), evidencing greater combustion efficiency due to the presence of the oxygenated fuel (ethanol).
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
Introduction
Nowadays, energy demand is mainly supplied by fossil fuels, which greatly contribute to the deterioration of air quality. This fact, along with the successive oil crisis, has promoted a search for alternative fuels (Lin et al. 2010; Schirmer et al. 2017) such as ethanol.
Ethanol can be used both as a vehicle fuel and as a gasoline additive, aiming at octane enhancement (Schifter et al. 2013; Walter 2009). In addition, its properties (such as greater heat of vaporization and antiknock characteristics) are advantageous, since they can improve engine efficiency and increase compression ratios (Lin et al. 2010).
Currently in Brazil, the commercialized gasolines are types A and C. Gasoline type A is pure, that is, without the addition of ethanol and is sold only by producers and importers. Gasoline type C contains the addition of anhydrous ethyl alcohol (AEA) and is the gas sold to the final consumers (ANP 2016). The literature shows that both the content of the ethanol added to the gasoline and the engine operation parameters (such as the loads applied) directly influence gas emissions (Schirmer et al. 2017). In general, the presence of ethanol in the blend reduces carbon monoxide (CO) and hydrocarbon emissions (HC) (Schirmer et al. 2017; Masum et al. 2015; Gravalos et al. 2013; Lin et al. 2010; Ribeiro et al. 2018; Yao et al. 2013).
According to the Brazilian National Agency of Petroleum, Natural Gas and Biofuels (ANP, in Portuguese), there are several norms regulating gasoline, which set forth fuel specifications all over the country. Gasoline that is not in compliance with the specifications set forth by the ANP is considered a non-compliant product, known as adulterated.
The adulteration of gasoline is generally obtained through the addition of solvents such as kerosene, benzene, turpentine, diesel, etc. or even excess anhydrous ethyl alcohol (AEA), since these products represent lower prices and lower tax charges when compared to gasoline (Babu et al. 2017; Kalligeros et al. 2003; Obodeh and Akhere 2010; Oliveira et al 2004). The use of adulterated gasoline might provoke damage to the engine as well as increase the pollutant gas emissions to the atmosphere, due to the alterations of the gasoline physicochemical properties such as density, vapour pressure and distillation curve (Fonseca et al. 2007; Gawande and Kaware 2013; Takeshita 2006). Kerosene, for example, being a heavier compound than gasoline (therefore, more difficult to burn), tends to result in an increase in fuel consumption, poor atomization, engine corrosion, reduction in octane quality, and general wear. In addition to reducing the engine performance, the irregular burning of kerosene-added gasoline increases emissions of gases such as CO, NOx and HC. Also, a characteristic odor is noticed when the gasoline-kerosene blend is used (Tharby 2002; Gawande and Kaware 2013; Takeshita 2006). Tharby (2002) highlights the damage that might occur to engines using adulterated gasoline, such as problems in the fuel injection system, valves, spark plugs, oxygen sensors, etc. even in cases when adulterants are used in small proportions.
Olanyk et al. (2014) evaluated the effect of the addition of different percentages (10, 20, 30 and 40% v/v) of “rubber solvent” to type A gasoline on the performance and emissions of a single-cylinder engine with a volumetric displacement of 196 cm3 and a four-stroke cycle. Those authors verified an increase in fuel mass consumption of up to 7% with the gradual addition of adulterant, as well as a rise in CO and HC concentrations of 38% and 16%, respectively.
Obodeh and Akhere (2010) investigated the effect of gasoline adulterated with kerosene in the proportions of 10, 20, 30, 40 and 50% v/v (and pure gasoline, as a basis for comparison) on the emissions and performance of an Otto-cycle engine, four-stroke and four-cylinders. The results showed an increase in the exhaustion emissions as the kerosene content in the blend was increased (ranging from 21.7 to 53% CO, from 23.4 to 57.1% HC and from 2.4 to 8.25% particulate matter) and in specific fuel consumption (34–36%) when compared to pure gasoline.
While the use of blends of oxygenated fuels in small spark-ignition non-road engines has been widely studied, little research has been developed in relation to the use of adulterated fuels in this category of engines. Gravalos et al. (2013) pointed out that non-road gasoline engines differ from other automotive engines in relation to technical specifications, which might imply differences related to these engines’ emissions and performance with the use of different fuels and their mixtures.
In this context, this study proposes the evaluation and comparison of gas emissions and mass and brake specific consumptions of a small spark-ignited non-road engine fuelled with kerosene (as adulterant) added to type C (with 27%v/v AEA) gasoline, which operated under different conditions of load applied to the engine.
Material and Methods
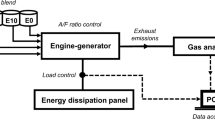
Fuel Blends, Properties and Experimental Apparatus
The tests were carried out with gasoline type A (pure), type C (Brazilian commercial gasoline, currently with 27% anhydrous ethyl alcohol—AEA) and kerosene, purchased from a chemical industry. To verify the effects of AEA and kerosene on fuel consumption and gas emissions, the following fuel blends were used: Gasoline C (GC); Gasoline C with 15% kerosene as adulterant (GC15); Gasoline C with 30% kerosene as adulterant (GC30) and Gasoline A (GA). Some properties of the fuels used are presented in Table 2.1.
Some of the main fuel quality parameters as specified by Resolution no 40/2013 of the Brazilian National Agency of Petroleum, Natural Gas and Biofuels (ANP) were also determined in the laboratory, as follows: color, visual aspect, density, distillation curve, final boiling point (FPB) and residue, according to the methods and specifications presented in Table 2.2. Such parameters are directly associated with the combustion quality and, therefore, the gas emissions resulting from the burning of the fuel blends investigated.
The engine-generator used was a single cylinder, four-stroke cycle with a volumetric displacement of 196 cc and maximum power of 6.5 hp (4.85 kW), with carburetor, coupled to a generator with maximum operating power of 2.5 kW (TG2800 model, Toyama brand) (Toyama 2018).
For the variation of the loads applied to the engine-generator, an energy dissipation panel of 10 halogen lamps (with 0.3 kW each one) was used (Olanyk 2013). During the operation, the loads were measured with a digital clamp multimeter.
The concentrations of total hydrocarbons (THC) and carbon monoxide (CO) were measured using a PC-MULTIGAS gas analyzer (by NAPRO). The analyzer specifications are shown in Table 2.3.
Combustion Assays Varying the Fuel Blends and the Loads Applied to the System
The combustion tests evaluated the THC and CO concentrations and fuel consumption as a function of the different fuel mixtures (GC, GC15, GC30, GA) and the loads applied to the system (2.0, 1.5, 1.0, 0.5 and 0 kW), pursuant to the Brazilian Norm ABNT NBR ISO 8178-4 (ABNT 2012).
The throttle of the engine was adjusted in the totally open region during the tests, following manufacturer’s instructions, characterizing, therefore, a lean mixture condition. Prior to each test, the engine was heated for approximately 20 min at 2.0 kW. All the measurements were carried out in triplicate, generating a total of six emission reports for each condition of load × mixture evaluated. The temperature of the exhaustion gases was measured every 30 s with an infrared thermometer (Minipa brand). The data regarding CO and THC gas concentration were obtained with the software PC-MULTIGAS, installed in a portable computer connected to the system.
The fuel mass consumption (Mc) (g min−1) was determined (in triplicate) by the variation of the fuel mass in each test for a period of fifteen minutes of working engine. From the mass consumption and the power (P) dissipated by the energy dissipation panel coupled to the generator (kW), it was possible to obtain data regarding the brake specific fuel consumption (bsfc) (g (kWh)−1), using Eq. 2.1 (Masum et al. 2015).
Statistical Analysis of the Experiment
The mean results obtained in triplicate for the CO and THC concentrations were statistically analyzed using the software Action Stat (Estatcamp 2014). Initially, the Bartlett test was applied to verify the variance homogeneity. After homogeneity was observed, the variance analysis (ANOVA) was carried out, followed by the Scott-Knott test, at 5% probability level, for the comparison of means between the treatments.
Results and Discussion
Fuel Quality Parameters
Table 2.4 presents the results of the parameters destined to verify the quality of the fuels under investigation, according to Resolution ANP n. 40/2013 (Table 2.2).
The density values in the commercialized gasoline “C” must be between 730 and 770 kg m−3, pursuant to the norms for fuel commercialization determined by the regulating agency (ANP 2011). Table 2.4 shows that all density values for Gasoline C and its blends with the adulterant (kerosene) are within the band determined by ANP. Regarding gasoline A, since the mandatory percentages of anhydrous ethyl alcohol (AEA) in the commercialized gasoline “C” have been varying in values by 20% v/v since the 1990s, the density of the type A gasoline was expected to be below the standards set forth for gasoline C, mainly for not containing AEA in its composition (794 kg m−3 density, according to Broustail et al. 2011).
Distillation curves represent a relevant parameter in the evaluation of fuel mixture volatility. When compared to gasoline, ethanol shows lower vapor pressure, higher latent heat of vaporization and a lower boiling point; therefore, when they are mixed, the formation of azeotropic mixtures occurs, implying variations in the final boiling point (FPB) and the temperature bands of distillation curves (Andersen et al. 2010; Bielaczyc et al. 2013; Koç et al. 2009; Silva et al. 2008). When kerosene is added to gasoline, the mixture volatility is reduced and the boiling point increases, which might hamper the burning of the fuel mixture, damaging the engine over time and increasing the emission of unburned hydrocarbons and particulate material in the exhaustion gases (Gwillian et al. 2004). Such behavior is observed in Table 2.4: when the amount of kerosene added to the GC increases, the distillation temperatures gradually increase; in the formulation containing 30% kerosene, the maximum values established are exceeded for the distillates of 10 and 50% evaporation. It seems relevant to mention that Fonseca et al. (2007), when studying the adulteration of type C gasoline (≅24% AEA) with kerosene mixed in different proportions (2, 4, 6, 10 and 20% in volume), observed that all maximum values specified were exceeded corresponding to distillates of 10, 50 and 90%, for the mixtures with 20% kerosene.
The parameters of color and visual aspect indicate the presence of contaminants and materials in suspension in the fuel. The current ANP Resolution 40/2013 determines a “colorless to yellowish” color and a “clear and impurity free” aspect. The addition of AEA to type A gasoline gives the mixture a yellowish aspect, which was observed in the blends investigated; even so, the visual analysis showed that even the samples with kerosene were within the requirements. Also, none of the mixtures showed the presence of impurities.
Regardless of the parameter evaluated (visual aspect, FPB, distillation curves, density), some difficulty was found in identifying adulteration in the gasoline, mainly regarding lower adulterant concentrations. In this study, for example, the values obtained for GC15 were within the technical specifications and only some values were in excess when the kerosene added to type C gasoline reached 30%. Also, considering that the variations of each one of these parameters depend on the adulterant characteristics (many of them presenting physicochemical characteristics very close to those of the gasoline), a revision of the parameters set forth in the ANP 40/2013 seems to be advisable, or even the adoption of other parameters/analyses that might be able to identify fuel adulteration with lower levels of adulterant concentration.
Exhaust Emissions—CO and THC
Figures 2.1 and 2.2 show, respectively, the concentrations of carbon monoxide (CO) and total hydrocarbons (THC) by varying the fuel blends and loads applied to the engine-generator.
Figures 2.1 and 2.2 show that the samples with AEA (GC, GC15 and GC30) presented lower CO and THC concentrations, when compared to the GA samples. AEA has 35% oxygen in its composition and a stoichiometric air–fuel ratio lower than the gasoline (mainly composed of hydrocarbons); in this case, a greater content of oxygen in mass in the fuel contributed to the oxidation of THC to CO2, which can be considered an effect of the pre-mixed oxygen to the fuel in liquid phase (KOÇ et al. 2009). Moreover, the addition of AEA promotes an improvement in the combustion balance and efficiency due to the reduction of hydrocarbons heavy fractions and a better evaporation of the AEA-gasoline mixtures, since the latent heat of vaporization and the octane number of the AEA-gasoline mixtures are commonly higher than that of the pure gasoline (type A) (Schifter et al. 2018; Turner et al. 2011). Other studies investigating types of engines and varied operation conditions also verified a reduction in CO emissions and hydrocarbons with ethanol addition to the gasoline (Elfasakhany 2015; Gravalos et al. 2013; Koç et al. 2009; Nwufo et al. 2017; Ribeiro et al. 2018; Schirmer et al. 2017).
As expected, the variance analysis revealed statistically significant differences in CO and THC emissions, considering the load and fuel used (p-value = 7.71E−24, for CO and p-value = 1.14E−24, for THC). The results of the mean comparison test are presented in Tables 2.5 and 2.6 for all treatments.
Regarding fuel adulteration, the statistical analysis pointed out that the addition of 30% keronese to Type C gasoline significantly increased the CO emissions when compared to pure type C gasoline (for all loads investigated). In relation to THC emissions, the 30% adulterant addition resulted in an increase in emissions, mainly in greater load conditions applied to the engine (1.0, 1.5 and 2.0 kW). The addition of kerosene to the gasoline reduced the volatility and the fuel mixture octane number, mainly due to an increase in the heavy fractions of hydrocarbons (which present a trend to remain in their liquid phase), as well as an increase in the mixtures; these aspects might cause the phenomenon called engine knocking, resulting in damage to the engine and higher levels of CO and THC emissions (Gawande and Kaware 2013; Gwillian et al. 2004; Heywood 1988; Sinha and Shivgotra 2012). Also, with the increase in the hydrocarbon heavy fractions of the fuel mixtures, there is a possibility of carbon deposits in the spark system, engine piston and valve. In general, adulterating gasoline with kerosene causes problems related to the fuel detonation, due to alterations in volatility, formation and accumulation of residues in the engine cylinders, incomplete burning of hydrocarbons, and reduction in the engine efficiency (Dahadha et al. 2013; Heywood 1988; Fonseca et al. 2007).
Regarding the behavior of CO and THC concentrations as a function of the load variation, the increase in the load applied to the engine results in the admission of a greater fuel volume (per time) in the engine cylinder (increase in the fuel mass consumption), in order to supply the demand for more rotation of the engine axis and greater intensities in the generator magnetic field (Barakat et al. 2016; Olanyk 2013). Indeed, an increasing trend in CO and THC emissions was observed with the load increase in the system operation.
Performance of the Engine-Generator Set
The mass consumption and brake specific fuel consumption values are presented in Figs. 2.3 and 2.4, respectively, for all the blends and loads evaluated.
Figures 2.3 and 2.4 show that the mass consumption and bsfc results did not vary much as a function of different formulations of gasoline type A and type C with the adulterant. When AEA is added to the gasoline, the fuel consumption is affected, due to the lower calorific value of the oxygenated compounds in relation to hydrocarbons (Gibbs et al. 2009; Wu et al. 2004). According to Channiwala and Parikh (2002), the calorific value of liquid fuels depends directly on their elemental base (chemical composition), and carbon and hydrogen are the greatest contributors to the generation of heat in the combustion. Costagliola et al (2016) and Hsieh et al (2002) highlighted that despite ethanol’s lower heating value, its addition to the gasoline provokes a leaning effect, which results in better use of the fuel to generate energy in the engine. In this study, the low variability in fuel consumption as a function of the blends might be explained by the compensation of the improvement in the combustion efficacy due to the leaning effect, in relation to a lower energy content of the blends with different proportions of AEA.
The trend of increase in mass consumption with the increase in the load applied to the engine, for all blends investigated, might be justified by the demand for higher torques from the engine axis and higher intensities of the magnetic field of the generator in higher load conditions (Barakat et al. 2016; Olanyk 2013), which demand greater fuel volume. Figure 2.4 shows, in all blends investigated, a reduction in bsfc with the increase in the load applied to the engine. When the fuel mass consumption increases as a function of the increase in the load, there is a trend towards a better thermodynamic use of the fuel and a reduction in bsfc.
Conclusions
The following conclusions can be drawn based on our tests:
The presence of anhydrous ethyl alcohol (AEA) in gasoline (commercially available in Brazil in the 27% proportion) resulted in a significant reduction in CO and THC concentrations to 64% and 56%, respectively, when compared to pure gasoline (type A, ethanol free), therefore evidencing greater combustion efficiency with the presence of oxygenated fuel (ethanol) in the blend.
Taking into consideration the test conditions adopted in this study (type of adulterant, engine, loads, etc.) as well as the current specifications set forth by the fuel regulating Brazilian agency (ANP), the legal parameters to detect fuel adulteration were seen to present limitations, mainly regarding lower concentrations of adulterant. Thus, from the legal standpoint, it seems relevant to consider the adoption of more restrictive limits or even the inclusion of other analysis parameters, so that the adulteration can be more easily detected.
The addition of kerosene to type C gasoline resulted in an increase in the CO and THC concentrations of up to 74% and 78%, respectively (this fact was observed for higher percentages of adulterant).
In the test conditions adopted, fuel consumption was seen to be more sensitive to the load variations applied than to the type of fuel used.
Further studies should be developed on emission and consumption tests with higher loads applied to the engine, as well as the adoption of other adulterants, to evaluate the influence of these factors in the system’s general performance.
References
ABNT—ASSOCIAÇÃO BRASILEIRA DE NORMAS TÉCNICAS. NBR ISO 8178-4 (2012) Motores alternativos de combustão interna—Medição da emissão de gases de exaustão. Parte 4: Ciclos de ensaio em regime constante para diferentes aplicações de motor. Rio de Janeiro: ABNT. https://www.abntcatalogo.com.br/norma.aspx?ID=090271. Accessed 25 Apr 2019
AGÊNCIA NACIONAL DO PETRÓLEO, GÁS NATURAL E BIOCOMBUSTÍVIEIS (2011) Cartilha do Posto Revendedor de Combustíveis. 5ª ed. Available in: https://www.larcopetroleo.com.br/wp-content/uploads/2013/10/cartilha_anp.pdf
AGÊNCIA NACIONAL DO PETRÓLEO, GÁS NATURAL E BIOCOMBUSTÍVIEIS (2013) Resolução ANP No 40. Available in http://www.anp.gov.br/legislacao/qualidade
AGÊNCIA NACIONAL DO PETRÓLEO, GÁS NATURAL E BIOCOMBUSTÍVIEIS (2016) Gasolina. Available in http://www.anp.gov.br/producao-de-derivados-de-petroleo-e-processamento-de-gas-natutal/producao-centrais-de-materias-primas-petroquimicas-cpq/gasolina
Andersen VF, Anderson JE, Wallington TJ, Mueller SA, Nielsen OJ (2010) Distillation curves for alcohol-gasoline blends. Energy Fuels 24(4):2683–2691. https://doi.org/10.1021/ef9014795
Babu V, Krishna R, Mani N Review on the detection of adulteration in fuels through computational techniques (2017) Mater Today Proc 4(2):1723–1729. https://doi.org/10.1016/j.matpr.2017.02.013
Barakat Y, Awad EN, Ibrahim V (2016) Fuel consumption of gasoline ethanol blends at different engine rotational speeds. Egypt J Petrol 25(3):309–315. https://doi.org/10.1016/j.ejpe.2015.07.019
Bielaczyc P, Woodburn J, Klimkiewicz D, Pajdowski P, Szczotka A (2013) An examination of the effect of ethanol–gasoline blends’ physicochemical properties on emissions from a light-duty spark ignition engine. Fuel Process Technol 107:50–63. https://doi.org/10.1016/j.fuproc.2012.07.030
Broustail G, Seers P, Halter F, Moréac G, Mounaim-Rousselle C (2011) Experimental determination of laminar burning velocity for butanol and ethanol iso-octane blends. Fuel 90(1):1–6. https://doi.org/10.1016/j.fuel.2010.09.021
Chang DY, Lopez I, Yocklovich SG (2008) Determination of kerosene and #2 diesel in soil by purge and trap versus extraction procedure. J Soil Contamination 1(3):239–251. https://doi.org/10.1080/15320389209383414
Channiwala SA, Parikh PP (2002) A unified correlation for estimating HHV of solid, liquid and gaseous fuel. Fuel 81(8):1051–1063. https://doi.org/10.1016/S0016-2361(01)00131-4
Costagliola MA, Prati MV, Florio S, Scorletti P, Terna D, Iodice P, Buono D, Senatore A (2016) Performances and emissions of a 4-stroke motorcycle fuelled with ethanol/gasoline blends. Fuel 183(1):470–477. https://doi.org/10.1016/j.fuel.2016.06.105
Dahadha AA, Talat N, Barakat S (2013) Study of the research octane number depression of domestic kerosene-doped automotive gasoline. Advances in Applied Science Research 4(4):129–134. ISSN: 0976–8610
Elfasakhany A (2015) Investigations on the effects of ethanol-methanol-gasoline blends in a spark-ignition engine: Performance and emissions analysis. Eng Sci Technol Int J 18(4):713–719. https://doi.org/10.1016/j.jestch.2015.05.003
Estatcamp. Software Action (2014) Consultoria em Estatística e Qualidade. São Carlos. Available in https://www.estatcamp.com.br/empresa/action-stat#in
Fonseca MM, Yoshida MI, Fortes ICP, Pasa VMD (2007) Thermogravimetric study of kerosene-doped gasoline. J Therm Anal Calorim 87(2):499–503. https://doi.org/10.1007/s10973-005-7457-2
García CA, Manzini F, Islas JM (2017) Sustainability assessment of ethanol production from two crops in Mexico. Renew Sustain Energy Rev 72:1199–1207. https://doi.org/10.1016/j.rser.2016.10.035
Gawande AP, Kaware JP (2013) Fuel adulteration consequences in India: a review. Sci Rev Chem Commun 3(3):161–171. Available in https://www.tsijournals.com/articles/fuel-adulteration-consequences-in-india--a-review.pdf
Gibbs L, Anderson B, Barnes K, Engeler G, Freel J, Horn J, Ingham M, Kohler D, Lesnini D, Macarthur R, Mortier M, Peyla D, Taniguchi B, Tiedemann A, Welstand S, Bernhardt D, Collini K, Farr A, Jones J, Lind J, Tom C (2009) Motor gasolines technical review. Chevron Corporation. Available in https://www.academia.edu/4857002/Motor_Gasolines_Technical_Review_Chevron_Products_Company_Motor_Gasolines_Technical_Review_Recycled_Recyclable_paper
Gravalos I, Moshou D, Gialamas Th, Xyradakis P, Kateris D, Tsiropoulos Z (2013) Emissions characteristics of spark ignition engine operating on lower-higher molecular mass alcohol blended gasoline fuels. Renew Energy 50:27–32. https://doi.org/10.1016/j.renene.2012.06.033
Gwilliam K, Kojima M, Johnson T (2004) Reducing air pollution from urban transport. Washington D.C. Available in https://documents.worldbank.org/curated/en/989711468328204490/pdf/304250PAPER0Reducing0air0pollution.pdf
Heywood JB (1988) Internal combustion engine fundamentals. McGraw Hill, 1st edn, 930p. ISBN 0-07-028637-X
Hsieh WD, Chen RH, Wu TL, Lin TH (2002) Engine performance and pollutant emission of an SI engine using ethanol–gasoline blended fuels. Atmos Environ 36(3):403–410. https://doi.org/10.1016/S1352-2310(01)00508-8
Kalligeros S, Zannikos F, Stournas S, Lois E (2003) Fuel adulteration issues in Greece. Energy 28(1):15–26. https://doi.org/10.1016/S0360-5442(02)00091-9
Koç M, Sekmen Y, Topgül T, Yücesu HS (2009) The effects of ethanol-unleaded gasoline blends on engine performance and exhaust emissions in a spark-ignition engine. Renew Energy 34(10):2101–2106. https://doi.org/10.1016/j.renene.2009.01.018
Lin WY, Chang YY, Hsieh YR (2010) Effect of ethanol-gasoline blends on small engine generator energy efficiency and exhaust emission. J Air Waste Manag Assoc 60(2):142–148. https://doi.org/10.3155/1047-3289.60.2.142
Masum BM, Masjuki HH, Kalam MA, Palash SM, Habibullah M (2015) Effect of alcohol-gasoline blends optimization on fuel properties, performance and emissions of a SI engine. J Clean Product 86(1):230–237. https://doi.org/10.1016/j.jclepro.2014.08.032
Napro (2018) Manual de operação, analisador de gases modelo 10.02/10J. Available in http://www.napro.com.br/analisadores.html
Nwufo OC, Nwaiwu CF, Ononogbo C, Igbokwe JO, Nwafor OMI, Anyanwu EE (2017) Performance, emission and combustion characteristics of a single cylinder spark ignition engine using ethanol–petrol-blended fuels. Int J Ambient Energy 39(8):792–801. https://doi.org/10.1080/01430750.2017.1354318
Obodeh O, Akhere NC (2010) Experimental study on the effects of kerosene-doped gasoline on gasoline-powered engine performance characteristics. J Petrol Gas Eng 1(2):37–40. Available in https://pdfs.semanticscholar.org/e177/bd80cee1e9c0323464305d2dc9f490d13736.pdf
Olanyk LZ (2013) Avaliação das emissões gasosas de um motor monocilindro ciclo Otto utilizando diferentes misturas de gasolina com etanol e adulterante. Universidade Estadual do Centro-Oeste, Guarapuava. Available in https://www.unicentro.br/posgraduacao/mestrado/bioenergia/dissertacoes/Disserta_o_Luciano_Zart_Olanyk_52448bfa35e4a.pdf
Olanyk LZ, Schirmer WN, Gueri MVD, Guedes CLB, Borsato D, Rodrigues PRP, Quessada TP, Oliveira DS (2014) Avaliação das emissões gasosas, parâmetros de qualidade e desempenho de consumo de um motor de combustão interna operando com misturas gasolina/adulterante em diferentes proporções. CIATEC 6(2):15–30. https://doi.org/10.5335/ciatec.v6i2.3814
Oliveira FS, Teixeira LSG, Araujo MCU, Korn M (2004) Screening analysis to detect adulterations in Brazilian gasoline samples using distillation curves. Fuel 83(7–8):917–923. https://doi.org/10.1016/j.fuel.2003.09.018
Ribeiro CB, Martins KG, Gueri MVD, Pavanello GP, Schirmer WN (2018) Effect of anhydrous ethanol/gasoline blends on performance and exhaust emissions of spark-ignited non-road engines. Environ Sci Pollut Res 25(24):24192–24200. https://doi.org/10.1007/s11356-018-2476-2
Schifter I, Diaz L, Gómez JP, Gonzalez U (2013) Combustion characterization in a single cylinder engine with mid-level hydrated ethanol–gasoline blended fuels. Fuel 103:292–298. https://doi.org/10.1016/j.fuel.2012.06.002
Schifter I, González U, Díaz L, Rodríguez R, Mejía-Centeno I, González-Macías C (2018) From actual ethanol contents in gasoline to mid-blends and E-85 in conventional technology vehicles. Emission control issues consequences. Fuel 219(1):239–247. https://doi.org/10.1016/j.fuel.2018.01.118
Schirmer WN, Olanyk LZ, Guedes CLB, Quessada TP, Ribeiro CB, Capanema MA (2017) Effects of air/fuel ratio on gas emissions in a small spark-ignited non-road engine operating with different gasoline/ethanol blends. Environ Sci Pollut Res 24(25):20354–20359. https://doi.org/10.1007/s11356-017-9651-8
Shoba, TT, Crua C, Heikal MR, Gold MR (2011) Optical characterisation of diesel, RME and kerosene sprays by microscopic imaging. In: European conference on liquid atomization and spray systems (ILASS), 24th. Estoril. Available in https://research.brighton.ac.uk/en/publications/optical-characterisation-of-diesel-rme-and-kerosene-sprays-by-mic
Silva R, Menezes EW, Cataluña R (2008) Rendimento térmico e emissões de contaminantes atmosféricos de gasolinas formuladas com etanol. MTBE E TAEE Química Nova 31(5):980–984. https://doi.org/10.1590/S0100-40422008000500007
Sinha SN, Shivgotra VK (2012) Environmental monitoring of adulterated gasoline with kerosene and their assessment at exhaust level. J Environ Biol 33:729–734. Available in https://www.jeb.co.in/journal_issues/201207_jul12/paper_09.pdf
Takeshita EV (2006) Adulteração de gasolina por adição de solventes: Análise dos parâmetros físico-químicos. Universidade Federal de Santa Catarina, Florianópolis. Available in https://repositorio.ufsc.br/bitstream/handle/123456789/89520/226279.pdf?sequence=1
Tharby R (2002) Catching gasoline and diesel adulteration. The World Bank. Urban air pollution: South Asia urban air quality management briefing note 7:4. Available in http://documents1.worldbank.org/curated/en/223591468164352248/pdf/multi0page.pdf
Toyama (2018) Geradores gasolina (31-1110—TG 2800CX). Available in https://www.toyama.com.br/?menu=produtos&produto=TG2800CX&cod=239
Turner D, Xu H, Cracknell RF, Natarajan V, Chen X (2011) Combustion performance of bio-ethanol at various blend ratios in a gasoline direct injection engine. Fuel 90(5):1999–2006. https://doi.org/10.1016/j.fuel.2010.12.025
Walter A (2009) Bio-ethanol development(s) in Brazil. In: Soetaert W, Vandamme EJ (eds) Biofuels. Wiley, pp 55–75. https://doi.org/10.1002/9780470754108.ch4
Wu CW, Chen RH, Pu JY, Lin TH (2004) The influence of air–fuel ratio on engine performance and pollutant emission of an SI engine using ethanol-gasoline-blended fuels. Atmos Environ 38(40):7093–7100. https://doi.org/10.1016/j.atmosenv.2004.01.058
Yao YC, Tsai JH, Wang IT (2013) Emissions of gaseous pollutant from motorcycle powered by ethanol–gasoline blend. Appl Energy 102:93–100. https://doi.org/10.1016/j.apenergy.2012.07.041
Acknowledgements
The authors are thankful to Conselho Nacional de Desenvolvimento Científico e Tecnológico (National Council for Scientific and Technological Development) (CNPq, regarding the Universal Call MCTI/CNPq no. 14/2014) for the financial support.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 The Editor(s) (if applicable) and The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Gauer, M.A., Ribeiro, C.B., Buratto, W.G., Schirmer, W.N. (2020). Evaluating Gaseous Emissions and Performance of a Spark-Ignited Non-road Engine Fueled with Gasoline, Ethanol and Adulterant Blends. In: Leal Filho, W., de Andrade Guerra, J.B.S. (eds) Water, Energy and Food Nexus in the Context of Strategies for Climate Change Mitigation. Climate Change Management. Springer, Cham. https://doi.org/10.1007/978-3-030-57235-8_2
Download citation
DOI: https://doi.org/10.1007/978-3-030-57235-8_2
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-57234-1
Online ISBN: 978-3-030-57235-8
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)