Abstract
The human endometrium is a unique, highly dynamic tissue that undergoes cyclic changes of cell proliferation, differentiation, and death. Endometrial cancer is the most common malignancy among women in developed countries. Importantly, the incidence of endometrial cancer is rising in high-income countries. Currently histological classification is used for subtyping of endometrial cancer, while ongoing research is evaluating markers for more accurate molecular classification. Evolutionary conserved Notch signaling pathway regulates diverse cellular processes such as proliferation, differentiation, and cell invasion. Accumulating evidence links aberrant Notch signaling with diseases such as hyperplasia and endometrial cancer. This chapter summarizes the current state of Notch signaling investigations in the endometrium, endometriosis, and endometrial cancer.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
Notch Signaling in the Normal Endometrium
The human endometrium is a unique, dynamic system that undergoes cyclic changes regulating cell proliferation, differentiation, and death during every menstrual cycle and pregnancy. Physiological changes that occur in fertile women are tightly regulated by hormones, specifically estrogen, progesterone, and chorionic gonadotropin (Banerjee and Fazleabas 2011; Maruyama and Yoshimura 2008). Therefore, it is not surprising that evolutionary conserved Notch pathway, due to its role in proliferation, differentiation, and angiogenesis, is actively involved in endometrium remodeling as well as in diseases such as hyperplasia and cancer.
Morphologically the endometrium is divided into functional and basal layers. The functional layer occupies two-thirds of the endometrial thickness and it is responsible for proliferation and secretion. During menstruations, the functional layer separates from the basal layer, while the basal layer serves as a base for endometrial regeneration and remains intact during menstruation. The endometrium is composed of different compartments: the luminal and the glandular epithelium, the stroma with stromal fibroblastic cells, and the vascular compartment (Diedrich et al. 2007; Ruiz-Alonso et al. 2012).
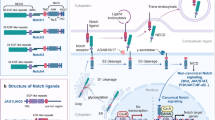
Notch is crucial for uterine development. In fact, it was shown that overexpression of NICD1 in mouse uterus leads to complete infertility, absence of uterine glands, and dysregulation of progesterone and estrogen signaling (Su et al. 2016). Mikhailik et al. have examined transcripts and demonstrated that Notch signaling is active in both the epithelial and stromal cells of the human endometrium. They have shown that all four NOTCH receptors are ubiquitously expressed in endometrial cells, whereas ligands JAG1 and DLL4 and targets HES1 and HEY are predominantly expressed in endometrial epithelial cells and are scarce in stromal cells. In addition, JAG1 induces Notch signaling activity in the stromal cells resulting in regulation of 452 genes (Mikhailik et al. 2009). It is important to note that this analysis was performed following cell isolation and culturing. Other investigation has demonstrated that NOTCH1 and NOTCH3 primarily localize to the glandular epithelium, whereas NOTCH4 localize to the stroma (Cobellis et al. 2008; Mitsuhashi et al. 2012).
During menstrual cycle the human endometrium undergoes various phases including proliferative phase of endometrium regeneration, followed by a mid-secretory phase in which endometrial stromal fibroblasts undergo differentiation known as decidualization to secretory “epithelioid” cells and last late-secretory phase where the endometrium is shed in a nonpregnancy cycle. When menopause begins, the endometrium loses the ability to proliferate and differentiate and loses its functional layers (Salamonsen 2019; Mori et al. 2012; Evans et al. 2016). Notch pathway is active in the human endometrium and highly dynamic; expression patterns of Notch receptors and ligands are different according to cell type and menstrual cycle phase (Mikhailik et al. 2009; Mitsuhashi et al. 2012). Notch signaling is more active in cycling endometrium than in menopause, as the expression levels of three Notch signaling molecules, NOTCH1, NOTCH4, and JAG1, are decreased in postmenopause endometrium (Cobellis et al. 2008).
There are only a few reports regarding the role of Notch signaling in remodeling of the endometrium, and the available data is sometimes conflicting (Table 4.1). Mitsuhashi et al. have found no cyclic changes in expression of NOTCH1, JAG1, and DLL4 proteins during menstrual cycle. Only receptor NOTCH3 expression level was increased during the secretory phase in the stroma cells. The expression of these signaling molecules was investigated predominantly in glandular cells of the normal endometrium (Mitsuhashi et al. 2012). Schuring et al. have not detected cycle-dependent changes in expression of NOTCH1 and NUMB (a negative regulator of Notch) when compared to proliferative and secretory endometrium (Schuring et al. 2018). Meanwhile Cobelis et al. have performed immunohistochemical analysis of 60 samples of physiological endometrium (20 of each in proliferative and secretory phase and in menopause) and observed an increased expression level of NOTCH1 and JAG1 in the secretory phase and the opposite trend for NOTCH4. Authors proposed that these results indicate the role of NOTCH4 in cell proliferation as an attribute of proliferative phase of the cycle, whereas NOTCH1 possibly associates with cell differentiation as a characteristic of secretory phase in the human endometrium (Cobellis et al. 2008). These opposite results were obtained despite the fact that Mitsuhashi and Cobelis groups had used the same antibodies against NOTCH1 for immunodetection, however at different concentrations. Suzuki et al. have also shown the decreased level of NOTCH4 expression during secretory phase if compared to the proliferative phase (Suzuki et al. 2000). The immunostaining of the endometrium has demonstrated that the expression of NOTCH1 receptor increased significantly in both glandular and luminal epithelium in the mid-secretory phase in comparison to the early- and the late-secretory phases. While NOTCH3 increased in the luminal epithelium during proliferative phase compared to secretory phase, no changes in expression were detected in glandular epithelium. Ligand JAG1 was detected upregulated in the luminal epithelium, and ligand DLL1 and NUMB were found in the glandular epithelium in the mid-secretory compared to the proliferative phase (Van Sinderen et al. 2014). It has been determined that DLL4 expression increases in the secretory phase of the menstrual cycle in both glandular and luminal epithelium (Cuman et al. 2014). Previously, it has been shown that NOTCH mediates uterine stromal decidualization by preventing stromal fibroblast apoptosis and regulates gene expression and cytoskeleton reorganization in mouse (Afshar et al. 2012). It has been revealed, by microarray studies, that NOTCH2, NOTCH3, and JAG2 are expressed by the trophectoderm (polarized transporting single cell layer) of human blastocysts (Aghajanova et al. 2012).
The endometrium is involved in implantation and placental formation during establishment of pregnancy. Disorders in this process are a major reason for infertility. Critical role in implantation plays interaction of blastocyst and the endometrium. It was also demonstrated that Notch signaling is involved in implantation and placentation (Cuman et al. 2013; Cuman et al. 2014). NOTCH1, JAG1, and DLL1 are downregulated in the endometrium of women with unexplained infertility (Van Sinderen et al. 2014).
Notch Signaling in the Endometriosis
Endometriosis is a gynecological condition characterized by the growth of ectopic endometrial cells outside the uterus. This tissue similarly to eutopic endometrium undergoes regeneration and shedding during the menstrual cycle. Endometriosis affects one in ten women of childbearing age and infertility is often associated with this disease (Hickey et al. 2014). Endometriosis shares mechanisms of estrogen stimulation and chronic inflammation with endometrial cancer; therefore it may be associated with this type of cancer (Yu et al. 2015). The deregulated expression components of the Notch signaling in endometriosis suggest an involvement of this pathway in the pathogenic process. Su et al. reported that Notch receptors NOTCH1 and NOTCH4, ligands JAG2 and DLL4, as well as target genes HEY1and HES5 were downregulated in eutopic endometrium of endometriosis patients suggesting that suppressed Notch signaling contributes to decidualization defects and is responsible for decreased fertility in woman with endometriosis (Su et al. 2015). NOTCH2 has also been shown as a regulator of decidualization (Otti et al. 2014). It was demonstrated that NOTCH1, JAG1, JAG2, and survivin significantly decrease in women with endometriosis, polycystic ovary syndrome, or repeated implantation failure, concluding that Notch signaling molecule might be associated with implantations problems and poor outcomes observed in these diseases (Amjadi et al. 2019). In addition, the expression of gene FOXO1 (NOTCH1 coactivator) was activated in decidualization. Interestingly, NOTCH1 also regulate FOXO1 expression. In the case of endometriosis, the suppression of Notch signaling results in decreased FOXO1 expression and decidualization failure (Brar et al. 2001). In contrast to the aforementioned studies, several groups have obtained opposite results. Expression of NOTCH1 in patients with deep infiltrating endometriosis and NUMB in luminal epithelium was significantly higher as compared with controls (Schuring et al. 2018). In the findings of another group, the expression levels of NOTCH1 and JAG1were upregulated in ectopic endometria than in their eutopic and normal counterparts. Moreover, estrogen regulates cell invasion in the endometriosis via activation of estrogen receptor alpha and the enhancement of Notch signaling (Li et al. 2018). After ultra-deep targeted sequencing, mutations of NOTCH1 and NOTCH2 genes were observed in the ectopic endometrium and atypical endometriosis, but not in normal endometrium tissue (Er et al. 2016).
Endometrial Cancer
Endometrial cancer (EC) is the sixth most common malignancy among women and 15th cancer in general. The rates of new diagnoses have been rising by about 1% each year. The frequency of this cancer varies among different countries – the incidence of this disease is highest in Central and Eastern Europe and North America, and it is lowest in Middle and Western Africa. Importantly, the incidence of EC is rising in high-income countries; this might be attributable to high rates of obesity, physical inactivity, late menopause, and extended life expectancy. Endometrial cancer accounts for 2% of cancer deaths in women. Fortunately, EC often causes specific symptoms at early stages, and when diagnosed at an early stage, 5-year survival rate is relatively high, reaching 69%. On the other hand, a delayed diagnosis leads to advanced stage and lower chance of survival. The majority of EC are sporadic, and 5–10% of women inherit cancer susceptibility (Sundar et al. 2017; Amant et al. 2018; Ferlay et al. 2018; World Cancer Research Fund 2019).
Endometrial carcinoma has been traditionally classified into two histological types described by Bokhman (Bokhman 1983). Type I tumors make up 80–90% of endometrial cancers and are typically characterized by a low-grade endometrioid histology (endometrioid endometrial cancer, EEC) , arising on a background of atypical hyperplasia. EEC is characterized by estrogen and progesterone receptor positivity and has a favorable prognosis in most cases. Factors leading to an excess of estrogen relative to progesterone are associated with this type of cancer (Sanderson et al. 2017; Amant et al. 2018). Type II cancer is determined in 10–20% of endometrial cancers. This type of cancer is associated with typically high-grade non-endometrioid histology (serous endometrial cancer, SEC; clear cell endometrial cancer, CCEC; uterine carcinosarcoma, UCS), arising in atrophic endometria, and is usually estrogen independent. The precursor of lesion in this type endometrial carcinoma is not yet fully established. This cancer type has higher risk for metastases and less favorable prognosis (Pathiraja et al. 2013; Akhtar et al. 2019).
Aside from morphologic differences between type I and type II, the endometrial cancers are distinguished by genetic alterations. The mutations of the tumor suppressor gene PTEN (phosphatase and tensin homolog) are the most frequent genetic alteration (up to 83%) in endometrioid endometrial cancer. This mutation coexists with other mutations in PIK3CA (phosphatidylinositol 3-OH kinase), CTNNB1 (which encodes β-catenin), KRAS (proto-oncogene, GTPase), and ARID1A (AT-rich interaction domain 1A) and defects in DNA mismatch repair. Mutations in tumor protein TP53, component of ubiquitin ligase Skp1-Cullin1-F-box complex FBXW7 (F-box with 7 tandem WD40), and PPP2R1A (protein phosphatase 2 scaffold subunit alpha) are found in non-endometrioid endometrial cancer. Other frequent alterations in this type of cancer is the overexpression of oncogene HER2/neu (erb-b2 receptor tyrosine kinase 2) and p16 (cyclin-dependent kinase inhibitor 2A, CDKN2A) loss of function leading to uncontrolled cell growth and aneuploidy (McConechy et al. 2012; Matias-Guiu and Prat 2013; O’Hara and Bell 2012).
However, the accuracy of histologic classification is not sufficient in order to distribute patients into optimal treatment subgroups, since various endometrial cancer types may exhibit shared characteristics. Recently, significant progress has been made in understanding molecular events in EC, and a division of tumors into distinct prognostic groups was suggested. The Cancer Genome Atlas (TCGA) provides the most comprehensive molecular study, involving whole-genome sequencing, exome sequencing, MSI assay, and a copy number analysis. Endometrial cancers could be classified into four distinct groups: (1) POLE (polymerase ξ exonuclease) ultramutated, which have POLE exonuclease domain mutations; (2) MSI hypermutated tumors, which have MSI-H (microsatellite instability-high) status and have hypermethylated MLH1 promoter; (3) copy-number low, MSS (microsatellite stabile) tumors, characterized by a low copy number aberrations and frequent CTNNB1 mutation; and (4) and copy-number high which are characterized by high-level copy number alterations and frequent TP53 mutation. Women in POLE-mutated subgroup exhibit the best prognosis, whereas women from copy-number high subgroup have the worst prognosis in progression-free survival. This classification is currently not applied in routine clinical practice, but due to the evolution of the methodologies involved, it will, hopefully, be ready in the near future (Cancer Genome Atlas Research et al. 2013; Urick and Bell 2019; Carlson and McCluggage 2019).
Notch Signaling in the Endometrial Cancer
Expression of Notch Signaling Components
Deregulated expression of Notch receptors and Notch ligands has been found in an increasing number of human solid tumors. However, there are only a limited number of reports of Notch signaling in the endometrial cancer. Moreover, as in the case of normal endometrium/endometriosis, the role of Notch signaling in endometrial cancer is ambiguous and seems to depend on analyzed Notch receptor/ligand as well as on the analysis methods. For Notch signaling component expression analysis, in most cases immunohistochemical staining was used, and the expression in the endometrium was compared to normal tissue from separate patients. Suzuki et al. have published the first report about Notch signaling in endometrial cancer in 2000 (Suzuki et al. 2000). They reported that endometrial cancer cells express a significantly lower level of NOTCH4 compared to normal endometria proposing the role of NOTCH4 in endometrial cancer development. Cobellis et al. studied normal (n = 60) and pathological (n = 60) endometrium samples by immunohistochemical analysis. They detected Notch signaling changes in different normal endometrium phases. The elevated expression of NOTCH1 in hyperplasia and carcinoma compared to polyps was found, whereas NOTCH4 and JAG1 expression decrease correlated to histological grade. In support, the expression of NOTCH1 and NOTCH4 correlated to p21 and cyclin D expression level (Cobellis et al. 2008). Mitsuhashi et al. demonstrated, using immunohistochemistry, the expression of NOTCH1, NOTCH3, JAG1, and DLL4 proteins was higher in endometrial cancer (n = 76) versus normal endometrium (n = 37) from non-cancer patients. Additionally, the elevated level of NOTCH1 correlated with cancer aggressiveness such as invasion into the myometrial layer and metastasis. High expression of NOTCH1 and JAG1 was associated with poor patient’s prognosis (Mitsuhashi et al. 2012). Mori et al. using immunohistochemistry staining also obtained similar results that NOTCH1 expression in endometrial adenocarcinoma (n = 21) was significantly higher than in normal endometrium (Mori et al. 2012). When the amount of proteins NOTCH1, NOTCH3, NOTCH4, and JAG2 was determined by Western blot analysis of endometrial cancer and adjacent nontumor tissue from the same patient, the level of proteins NOTCH1 and NOTCH3 was unchanged. Meanwhile the relative amount of proteins NOTCH4 and JAG2 was decreased in the majority of stage I endometrial cancer samples, compared to nontumor endometrium of the same patient (Sasnauskiene et al. 2014).
Gene expression analysis between normal and malignant patient samples showed significant elevation of JAG2 level in endometrial cancer tissues, but it has no impact on cancer patient survival (Townsend et al. 2019). Jonusiene et al. found the decreased expression of Notch receptors (NOTCH1, NOTCH2, NOTCH3, and NOTCH4), ligands (JAG1, JAG2, and DLL1), and Notch target gene HES1 in endometrial cancer tissue compared to normal endometrium of the same patient at mRNA level. For this analysis the samples of endometrial cancer and adjacent nontumor endometrial tissue from the same woman were used (n = 50) (Jonusiene et al. 2013). This study supports the notion that Notch signaling is less active in endometrial cancer and supposes that it can function as tumor suppressor. In Williams et al. investigation, it was discovered that early endometrial cancer cells lose the apicobasal polarity in the low-grade endometrial cancer. In addition, it was observed the mislocalization of Notch receptor. The decreased expression of NOTCH4 receptor, ligand JAG1, and downstream targets HES1 and HEY1 in low-grade endometrial cancer was detected by RT-PCR analysis, indicating that overall Notch signaling is suppressed in low-grade endometrial cancer, while the expression of NOTCH1 and NOTCH2 was unchanged (Williams et al. 2017).
Polychronidou et al. analyzed the expression of NOTCH2, NOTCH3, and JAG1 in endometrial carcinoma tissue (n = 204) by immunostaining. It was found that more than 70% of tumor were negative for all three proteins. The study has been performed only using cancer tissues. The analysis showed that expressions of NOTCH2 and JAG1 have opposite prognostic impacts. The expression of NOTCH2 has the unfavorable prognostic impact in endometrial cancer, while JAG1 expression reflects a favorable prognosis. Moreover, expression of JAG1 was favorable in the absence NOTCH2/3 and was very similar to patients with low or undetectable expression of all three markers. The expression of NOTCH3 did not yield significant results possibly, due to small number of NOTCH3-positive patients (Polychronidou et al. 2018).
The data of a retrospective study obtained from The Cancer Genome Atlas (545 tumor and 35 adjacent tissues) concluded that expression of Notch ligand DLL3 was upregulated in endometrial cancer tissues. Evaluation depends on patient age, FIGO stage, and grade. It was also discovered that upregulation of DLL3 expression was associated with the shortest overall survival in patients with endometrial cancer (Wang et al. 2018).
All available data concerning Notch signaling member’s expression in endometrial cancer are summarized in Table 4.1. All investigations coincide on the only one point: the expression levels of NOTCH4 protein and mRNA are decreased in endometrial cancer. Meanwhile, the expression status of other Notch signaling members is scarce and sometimes contradictory, and more data are needed to draw the conclusions (Table 4.2).
Stem Cells in Endometrial Cancer
A small population of adult stem cells, including epithelial progenitors, mesenchymal stem cells, and side-population cells, have been identified in the human endometrium. These cells contribute to regenerative capacity of the endometrium (Evans et al. 2016). Cancer stem cells (CSCs) are cells with stem-like properties crucial for generation of neoplastic cell population; they are responsible for invasiveness and formation of drug resistance (Hanahan and Weinberg 2011; Carvalho et al. 2015). Different molecules were studied as markers of CSC in endometrial cancer, including CD133, CD44, CD117(c-kit), and aldehyde dehydrogenase 1 (ALDH1) (Tempest et al. 2018; Giannone et al. 2019). Different signaling pathways regulate stemness in EC, including Notch signaling.
The cell surface marker CD133 is known as prominin; it identifies stem-like cell population. CD133+ cells have exhibited a more aggressive proliferation in vitro and higher resistance to chemotherapeutic drugs cisplatin and paclitaxel (Elbasateeny et al. 2016). Analysis of endometrial cancer Ishikawa cells, separated into two CD133+and CD133− subpopulations, demonstrated the increased level of NOTCH1 protein in cancer stem-like cells CD133+. The blockade of the Notch signaling with γ-secretase inhibitor DAPT suppressed CSC proliferation. Moreover, a treatment of Ishikawa cells with DAPT and other therapeutic target, EGFR inhibitor, was more efficient than treating with any compound alone. Authors concluded that Notch signaling seems to be a promising therapeutic target for CSCs (Shang et al. 2018).
Another stem cells marker in EC is RNA-binding protein Musashi-1. Gotte et al. found an increased protein level of Musashi-1 in endometriosis and endometrial cancer. siRNA silencing of Musashi-1 resulted in decreased expression of NOTCH1 protein and its downstream target HES1 in Ishikawa cells. At the functional level, these changes promote reduced cell proliferation and apoptosis induction (Gotte et al. 2011). It was also shown that patients with upregulated Musashi-1 expression have poor survival rate, which may be an independent prognostic factor for endometrial cancer (Ma et al. 2015).
Crosstalk of Notch and Obesity Signals
Increasing body mass index is associated with a significant increase in the risk of endometrial cancer (Reeves et al. 2007; Renehan et al. 2008). It has been demonstrated that in comparison with all obesity-related cancers, increasing body mass index is most strongly associated with endometrial cancer incidence and mortality (Schmandt et al. 2011). Although the correlation between obesity and cancer incidence is identified, the molecular mechanisms linking these processes remain the area of intensive studies. Obesity is characterized by excess of adipose tissue, which drives the dysregulation of complex metabolic and endocrine activities (Crean-Tate and Reizes 2018). Leptin is an adipose tissue-secreted hormone, which correlates with the level of adiposity and body mass index in women. Leptin signaling has been shown to induce breast cancer growth and progression (Ando et al. 2014). It was demonstrated that Notch, IL-1, and leptin crosstalk outcome (NILCO) is involved in the induction of breast cancer cell proliferation and migration, where leptin upregulates Notch ligands, receptors, and target genes (Guo and Gonzalez-Perez 2011). In analogy to breast cancer, the group of Gonzalez-Perez hypothesized that NILCO could be a link between obesity and endometrial cancer progression (Daley-Brown et al. 2015). This group has demonstrated that leptin is an inducer of Notch receptors (NOTCH1–4), ligands (JAG1 and DLL4), and downstream effectors (survivin, HEY2) and leptin (OB-R) and IL-1 (IL-1R tI) receptors in endometrial cancer cells (Daley-Brown et al. 2019). The impact of leptin was higher for the poorly differentiated and more aggressive cell lines An3Ca and KLE, resembling type II endometrial cancer. Leptin also upregulated the expression of NOTCH1, NOTCH3, and NOTCH4 receptors in the more differentiated HEC-1A and Ishikawa cells, resembling more differentiated type I endometrial cancer. Moreover, it was demonstrated that leptin induces cell cycle progression and proliferation of endometrial cancer cells. The importance of leptin signaling for endometrial cancer has to be proved using animal models.
Mutations in Notch-Related Genes
DNA repair system plays a crucial role in recognition and repairing of insertions or deletions in microsatellites – the repeated sequences of DNA. Abnormal function of a repair system causes the creation of novel microsatellite fragments resulting in microsatellite instability (MSI). Some cancer types, including endometrial cancer, exhibit higher rates of MSI (16.5%). Higher rates of MSI tumor selectively share alterations in genes of common pathways including Notch and Wnt proposing possibilities of pathway-targeted therapies (Trabucco et al. 2019). Mutations of NOTCH1 and NOTCH2 genes were identified in endometriosis-associated ovarian cancer, and it may predispose endometriotic lesion to malignant transformation (Er et al. 2016).
miRNAs
A class of a small noncoding RNAs, miRNAs, are important for gene regulation, and they are differentially expressed in various malignant tissues. It was identified that 138 miRNAs are differently expressed in endometrial cancer in comparison to the normal endometrium. Among deregulated miRNAs was miR-34a, regulating members of Notch family NOTCH1, NOTCH2, JAG1, and DLL. In addition, NOTCH1 was regulated by miR-34* and miR-27b* (Jurcevic et al. 2014). Upregulation of miR-34 led to a significant decrease of NOTCH1 and DLL1 at mRNA level, while downregulation led to a significant increase in this mRNA (Jurcevic et al. 2016). Devor et al. reported a significant downregulation of miRNA-181c in endometrial cancer. The decrease of miRNA-181c was in part attributed to upregulation of NOTCH2 (Devor et al. 2017).
Conclusion
The aberrant expression of Notch signaling receptors and ligands suggests that this pathway is important for changes in cycling endometrium and in disorders such as endometriosis or endometrial cancer. There are controversial suggestions concerning the role of Notch signaling in the endometrium that are supported by sometimes contradictory results about expression changes of Notch molecules. Therefore, additional functional studies are required to reveal the importance of Notch signaling for endometrial cancer progression. Future challenges in the field include choosing of the right methods and approaches to analyze the importance of Notch signaling for endometrial cancer. It is necessary to understand how this pathway interacts with other signaling pathways, including Wnt and Hedgehog. These new studies may offer new potential markers for endometrial cancers molecular classification and prognostic or therapeutic targets.
References
Afshar Y, Jeong JW, Roqueiro D, DeMayo F, Lydon J, Radtke F, Radnor R, Miele L, Fazleabas A (2012) Notch1 mediates uterine stromal differentiation and is critical for complete decidualization in the mouse. FASEB J 26(1):282–294. https://doi.org/10.1096/fj.11-184663
Aghajanova L, Shen S, Rojas AM, Fisher SJ, Irwin JC, Giudice LC (2012) Comparative transcriptome analysis of human trophectoderm and embryonic stem cell-derived trophoblasts reveal key participants in early implantation. Biol Reprod 86(1):1–21. https://doi.org/10.1095/biolreprod.111.092775
Akhtar M, Al Hyassat S, Elaiwy O, Rashid S, Al-Nabet A (2019) Classification of endometrial carcinoma: new perspectives beyond morphology. Adv Anat Pathol 26(6):421–427. https://doi.org/10.1097/PAP.0000000000000251
Amant F, Mirza MR, Koskas M, Creutzberg CL (2018) Cancer of the corpus uteri. Int J Gynaecol Obstet 143(Suppl 2):37–50. https://doi.org/10.1002/ijgo.12612
Amjadi F, Salehi E, Zandieh Z, Rashidi M, Taleahmad S, Javedani Masrour M, Aflatoonian R, Mehdizadeh M (2019) Comparative evaluation of NOTCH signaling molecules in the endometrium of women with various gynecological diseases during the window of implantation. Iran J Basic Med Sci 22(4):426–431. https://doi.org/10.22038/ijbms.2019.32961.7874
Ando S, Barone I, Giordano C, Bonofiglio D, Catalano S (2014) The multifaceted mechanism of leptin signaling within tumor microenvironment in driving breast cancer growth and progression. Front Oncol 4:340. https://doi.org/10.3389/fonc.2014.00340
Banerjee P, Fazleabas AT (2011) Extragonadal actions of chorionic gonadotropin. Rev Endocr Metab Disord 12(4):323–332. https://doi.org/10.1007/s11154-011-9193-1
Bokhman JV (1983) Two pathogenetic types of endometrial carcinoma. Gynecol Oncol 15(1):10–17. https://doi.org/10.1016/0090-8258(83)90111-7
Brar AK, Handwerger S, Kessler CA, Aronow BJ (2001) Gene induction and categorical reprogramming during in vitro human endometrial fibroblast decidualization. Physiol Genomics 7(2):135–148. https://doi.org/10.1152/physiolgenomics.00061.2001
Cancer Genome Atlas Research N, Kandoth C, Schultz N, Cherniack AD, Akbani R, Liu Y, Shen H, Robertson AG, Pashtan I, Shen R, Benz CC, Yau C, Laird PW, Ding L, Zhang W, Mills GB, Kucherlapati R, Mardis ER, Levine DA (2013) Integrated genomic characterization of endometrial carcinoma. Nature 497(7447):67–73. https://doi.org/10.1038/nature12113
Carlson J, McCluggage WG (2019) Reclassifying endometrial carcinomas with a combined morphological and molecular approach. Curr Opin Oncol 31(5):411–419. https://doi.org/10.1097/CCO.0000000000000560
Carvalho MJ, Laranjo M, Abrantes AM, Torgal I, Botelho MF, Oliveira CF (2015) Clinical translation for endometrial cancer stem cells hypothesis. Cancer Metastasis Rev 34(3):401–416. https://doi.org/10.1007/s10555-015-9574-0
Cobellis L, Caprio F, Trabucco E, Mastrogiacomo A, Coppola G, Manente L, Colacurci N, De Falco M, De Luca A (2008) The pattern of expression of Notch protein members in normal and pathological endometrium. J Anat 213(4):464–472. https://doi.org/10.1111/j.1469-7580.2008.00963.x
Crean-Tate KK, Reizes O (2018) Leptin regulation of cancer stem cells in breast and gynecologic Cancer. Endocrinology 159(8):3069–3080. https://doi.org/10.1210/en.2018-00379
Cuman C, Menkhorst EM, Rombauts LJ, Holden S, Webster D, Bilandzic M, Osianlis T, Dimitriadis E (2013) Preimplantation human blastocysts release factors that differentially alter human endometrial epithelial cell adhesion and gene expression relative to IVF success. Hum Reprod 28(5):1161–1171. https://doi.org/10.1093/humrep/det058
Cuman C, Menkhorst E, Winship A, Van Sinderen M, Osianlis T, Rombauts LJ, Dimitriadis E (2014) Fetal-maternal communication: the role of Notch signalling in embryo implantation. Reproduction 147(3):R75–R86. https://doi.org/10.1530/REP-13-0474
Daley-Brown D, Oprea-Ilies GM, Lee R, Pattillo R, Gonzalez-Perez RR (2015) Molecular cues on obesity signals, tumor markers and endometrial cancer. Horm Mol Biol Clin Invest 21(1):89–106. https://doi.org/10.1515/hmbci-2014-0049
Daley-Brown D, Harbuzariu A, Kurian AA, Oprea-Ilies G, Gonzalez-Perez RR (2019) Leptin-induced Notch and IL-1 signaling crosstalk in endometrial adenocarcinoma is associated with invasiveness and chemoresistance. World J Clin Oncol 10(6):222–233. https://doi.org/10.5306/wjco.v10.i6.222
Devor EJ, Miecznikowski J, Schickling BM, Gonzalez-Bosquet J, Lankes HA, Thaker P, Argenta PA, Pearl ML, Zweizig SL, Mannel RS, Brown A, Ramirez NC, Ioffe OB, Park KJ, Creasman WT, Birrer MJ, Mutch D, Leslie KK (2017) Dysregulation of miR-181c expression influences recurrence of endometrial endometrioid adenocarcinoma by modulating NOTCH2 expression: an NRG Oncology/Gynecologic Oncology Group study. Gynecol Oncol 147(3):648–653. https://doi.org/10.1016/j.ygyno.2017.09.025
Diedrich K, Fauser BC, Devroey P, Griesinger G, Evian Annual Reproduction Workshop G (2007) The role of the endometrium and embryo in human implantation. Hum Reprod Update 13(4):365–377. https://doi.org/10.1093/humupd/dmm011
Elbasateeny SS, Salem AA, Abdelsalam WA, Salem RA (2016) Immunohistochemical expression of cancer stem cell related markers CD44 and CD133 in endometrial cancer. Pathol Res Pract 212(1):10–16. https://doi.org/10.1016/j.prp.2015.10.008
Er TK, Su YF, Wu CC, Chen CC, Wang J, Hsieh TH, Herreros-Villanueva M, Chen WT, Chen YT, Liu TC, Chen HS, Tsai EM (2016) Targeted next-generation sequencing for molecular diagnosis of endometriosis-associated ovarian cancer. J Mol Med 94(7):835–847. https://doi.org/10.1007/s00109-016-1395-2
Evans J, Salamonsen LA, Winship A, Menkhorst E, Nie G, Gargett CE, Dimitriadis E (2016) Fertile ground: human endometrial programming and lessons in health and disease. Nat Rev Endocrinol 12(11):654–667. https://doi.org/10.1038/nrendo.2016.116
Ferlay J, Colombet M, Soerjomataram I, Dyba T, Randi G, Bettio M, Gavin A, Visser O, Bray F (2018) Cancer incidence and mortality patterns in Europe: estimates for 40 countries and 25 major cancers in 2018. Eur J Cancer 103:356–387. https://doi.org/10.1016/j.ejca.2018.07.005
Giannone G, Attademo L, Scotto G, Genta S, Ghisoni E, Tuninetti V, Aglietta M, Pignata S, Valabrega G (2019) Endometrial cancer stem cells: role, characterization and therapeutic implications. Cancers 11(11). https://doi.org/10.3390/cancers11111820
Gotte M, Greve B, Kelsch R, Muller-Uthoff H, Weiss K, Kharabi Masouleh B, Sibrowski W, Kiesel L, Buchweitz O (2011) The adult stem cell marker Musashi-1 modulates endometrial carcinoma cell cycle progression and apoptosis via Notch-1 and p21WAF1/CIP1. In J Cancer 129(8):2042–2049. https://doi.org/10.1002/ijc.25856
Guo S, Gonzalez-Perez RR (2011) Notch, IL-1 and leptin crosstalk outcome (NILCO) is critical for leptin-induced proliferation, migration and VEGF/VEGFR-2 expression in breast cancer. PLoS One 6(6):e21467. https://doi.org/10.1371/journal.pone.0021467
Hanahan D, Weinberg RA (2011) Hallmarks of cancer: the next generation. Cell 144(5):646–674. https://doi.org/10.1016/j.cell.2011.02.013
Hickey M, Ballard K, Farquhar C (2014) Endometriosis. BMJ 348:g1752. https://doi.org/10.1136/bmj.g1752
Jonusiene V, Sasnauskiene A, Lachej N, Kanopiene D, Dabkeviciene D, Sasnauskiene S, Kazbariene B, Didziapetriene J (2013) Down-regulated expression of Notch signaling molecules in human endometrial cancer. Med Oncol 30(1):438. https://doi.org/10.1007/s12032-012-0438-y
Jurcevic S, Olsson B, Klinga-Levan K (2014) MicroRNA expression in human endometrial adenocarcinoma. Cancer Cell Int 14(1):88. https://doi.org/10.1186/s12935-014-0088-6
Jurcevic S, Klinga-Levan K, Olsson B, Ejeskar K (2016) Verification of microRNA expression in human endometrial adenocarcinoma. BMC Cancer 16:261. https://doi.org/10.1186/s12885-016-2296-z
Li N, Zhang L, Li Q, Du Y, Liu H, Liu Y, Xiong W (2018) Notch activity mediates oestrogen-induced stromal cell invasion in endometriosis. Reproduction 157(4):371–381. https://doi.org/10.1530/REP-18-0326
Ma L, Xu YL, Ding WJ, Shao HF, Teng YC (2015) Prognostic value of Musashi-1 in endometrioid adenocarcinoma. Int J Clin Exp Pathol 8(5):4564–4572
Maruyama T, Yoshimura Y (2008) Molecular and cellular mechanisms for differentiation and regeneration of the uterine endometrium. Endocr J 55(5):795–810. https://doi.org/10.1507/endocrj.k08e-067
Matias-Guiu X, Prat J (2013) Molecular pathology of endometrial carcinoma. Histopathology 62(1):111–123. https://doi.org/10.1111/his.12053
McConechy MK, Ding J, Cheang MC, Wiegand K, Senz J, Tone A, Yang W, Prentice L, Tse K, Zeng T, McDonald H, Schmidt AP, Mutch DG, McAlpine JN, Hirst M, Shah SP, Lee CH, Goodfellow PJ, Gilks CB, Huntsman DG (2012) Use of mutation profiles to refine the classification of endometrial carcinomas. J Pathol 228(1):20–30. https://doi.org/10.1002/path.4056
Mikhailik A, Mazella J, Liang S, Tseng L (2009) Notch ligand-dependent gene expression in human endometrial stromal cells. Biochem Biophys Res Commun 388(3):479–482. https://doi.org/10.1016/j.bbrc.2009.07.037
Mitsuhashi Y, Horiuchi A, Miyamoto T, Kashima H, Suzuki A, Shiozawa T (2012) Prognostic significance of Notch signalling molecules and their involvement in the invasiveness of endometrial carcinoma cells. Histopathology 60(5):826–837. https://doi.org/10.1111/j.1365-2559.2011.04158.x
Mori M, Miyamoto T, Ohno S, Miyake Y, Sakaguchi T, Ohno E (2012) Diagnostic utility of notch-1 immunocytochemistry in endometrial cytology. Acta Cytol 56(2):166–170. https://doi.org/10.1159/000335485
O’Hara AJ, Bell DW (2012) The genomics and genetics of endometrial cancer. Adv Genom Genet 2012(2):33–47. https://doi.org/10.2147/AGG.S28953
Otti GR, Saleh L, Velicky P, Fiala C, Pollheimer J, Knofler M (2014) Notch2 controls prolactin and insulin-like growth factor binding protein-1 expression in decidualizing human stromal cells of early pregnancy. PLoS One 9(11):e112723. https://doi.org/10.1371/journal.pone.0112723
Pathiraja P, Dhar S, Haldar K (2013) Serous endometrial intraepithelial carcinoma: a case series and literature review. Cancer Manag Res 5:117–122. https://doi.org/10.2147/CMAR.S45141
Polychronidou G, Kotoula V, Manousou K, Kostopoulos I, Karayannopoulou G, Vrettou E, Bobos M, Raptou G, Efstratiou I, Dionysopoulos D, Chatzopoulos K, Lakis S, Chrisafi S, Tsolakidis D, Papanikolaou A, Dombros N, Fountzilas G (2018) Mismatch repair deficiency and aberrations in the Notch and Hedgehog pathways are of prognostic value in patients with endometrial cancer. PLoS One 13(12):e0208221. https://doi.org/10.1371/journal.pone.0208221
Reeves GK, Pirie K, Beral V, Green J, Spencer E, Bull D, Million Women Study C (2007) Cancer incidence and mortality in relation to body mass index in the Million Women Study: cohort study. BMJ 335(7630):1134. https://doi.org/10.1136/bmj.39367.495995.AE
Renehan AG, Tyson M, Egger M, Heller RF, Zwahlen M (2008) Body-mass index and incidence of cancer: a systematic review and meta-analysis of prospective observational studies. Lancet 371(9612):569–578. https://doi.org/10.1016/S0140-6736(08)60269-X
Ruiz-Alonso M, Blesa D, Simon C (2012) The genomics of the human endometrium. Biochim Biophys Acta 1822(12):1931–1942. https://doi.org/10.1016/j.bbadis.2012.05.004
Salamonsen LA (2019) WOMEN IN REPRODUCTIVE SCIENCE: my WOMBan’s life: understanding human endometrial function. Reproduction 158(6):F55–F67. https://doi.org/10.1530/REP-18-0518
Sanderson PA, Critchley HO, Williams AR, Arends MJ, Saunders PT (2017) New concepts for an old problem: the diagnosis of endometrial hyperplasia. Hum Reprod Update 23(2):232–254. https://doi.org/10.1093/humupd/dmw042
Sasnauskiene A, Jonusiene V, Krikstaponiene A, Butkyte S, Dabkeviciene D, Kanopiene D, Kazbariene B, Didziapetriene J (2014) NOTCH1, NOTCH3, NOTCH4, and JAG2 protein levels in human endometrial cancer. Medicina 50(1):14–18. https://doi.org/10.1016/j.medici.2014.05.002
Schmandt RE, Iglesias DA, Co NN, Lu KH (2011) Understanding obesity and endometrial cancer risk: opportunities for prevention. Am J Obstet Gynecol 205(6):518–525. https://doi.org/10.1016/j.ajog.2011.05.042
Schuring AN, Dahlhues B, Korte A, Kiesel L, Titze U, Heitkotter B, Ruckert C, Gotte M (2018) The endometrial stem cell markers notch-1 and numb are associated with endometriosis. Reprod Biomed Online 36(3):294–301. https://doi.org/10.1016/j.rbmo.2017.11.010
Shang C, Lang B, Meng LR (2018) Blocking NOTCH pathway can enhance the effect of EGFR inhibitor through targeting CD133+ endometrial cancer cells. Cancer Biol Ther 19(2):113–119. https://doi.org/10.1080/15384047.2016.1250985
Su RW, Strug MR, Joshi NR, Jeong JW, Miele L, Lessey BA, Young SL, Fazleabas AT (2015) Decreased Notch pathway signaling in the endometrium of women with endometriosis impairs decidualization. J Clin Endocrinol Metab 100(3):E433–E442. https://doi.org/10.1210/jc.2014-3720
Su RW, Strug MR, Jeong JW, Miele L, Fazleabas AT (2016) Aberrant activation of canonical Notch1 signaling in the mouse uterus decreases progesterone receptor by hypermethylation and leads to infertility. Proc Natl Acad Sci U S A 113(8):2300–2305. https://doi.org/10.1073/pnas.1520441113
Sundar S, Balega J, Crosbie E, Drake A, Edmondson R, Fotopoulou C, Gallos I, Ganesan R, Gupta J, Johnson N, Kitson S, Mackintosh M, Martin-Hirsch P, Miles T, Rafii S, Reed N, Rolland P, Singh K, Sivalingam V, Walther A (2017) BGCS uterine cancer guidelines: recommendations for practice. Eur J Obstet Gynecol Reprod Biol 213:71–97. https://doi.org/10.1016/j.ejogrb.2017.04.015
Suzuki T, Aoki D, Susumu N, Udagawa Y, Nozawa S (2000) Imbalanced expression of TAN-1 and human Notch4 in endometrial cancers. Int J Oncol 17(6):1131–1139. https://doi.org/10.3892/ijo.17.6.1131
Tempest N, Maclean A, Hapangama DK (2018) Endometrial stem cell markers: current concepts and unresolved questions. Int J Mol Sci 19(10). https://doi.org/10.3390/ijms19103240
Townsend MH, Ence ZE, Felsted AM, Parker AC, Piccolo SR, Robison RA, O’Neill KL (2019) Potential new biomarkers for endometrial cancer. Cancer Cell Int 19:19. https://doi.org/10.1186/s12935-019-0731-3
Trabucco SE, Gowen K, Maund SL, Sanford E, Fabrizio DA, Hall MJ, Yakirevich E, Gregg JP, Stephens PJ, Frampton GM, Hegde PS, Miller VA, Ross JS, Hartmaier RJ, Huang SA, Sun JX (2019) A novel next-generation sequencing approach to detecting microsatellite instability and pan-tumor characterization of 1000 microsatellite instability-high cases in 67,000 patient samples. J Mol Diagnost 21(6):1053–1066. https://doi.org/10.1016/j.jmoldx.2019.06.011
Urick ME, Bell DW (2019) Clinical actionability of molecular targets in endometrial cancer. Nat Rev Cancer 19(9):510–521. https://doi.org/10.1038/s41568-019-0177-x
Van Sinderen M, Cuman C, Gamage T, Rainczuk K, Osianlis T, Rombauts L, Dimitriadis E (2014) Localisation of the Notch family in the human endometrium of fertile and infertile women. J Mol Histol 45(6):697–706. https://doi.org/10.1007/s10735-014-9587-y
Wang J, Zhang K, Liu Z, Wang T, Shi F, Zhang Y, Su J, Jia Y (2018) Upregulated delta-like protein 3 expression is a diagnostic and prognostic marker in endometrial cancer: a retrospective study. Medicine 97(51):e13442. https://doi.org/10.1097/MD.0000000000013442
Williams E, Villar-Prados A, Bowser J, Broaddus R, Gladden AB (2017) Loss of polarity alters proliferation and differentiation in low-grade endometrial cancers by disrupting Notch signaling. PLoS One 12(12):e0189081. https://doi.org/10.1371/journal.pone.0189081
World Cancer Research Fund. https://www.wcrf.org/dietandcancer/cancertrends/endometrial-cancer-statistics. Accessed October 23, 2019
Yu HC, Lin CY, Chang WC, Shen BJ, Chang WP, Chuang CM, Task Force on Carcinogenesis of Endometrial C (2015) Increased association between endometriosis and endometrial cancer: a nationwide population-based retrospective cohort study. Int J Gynecol Cancer 25(3):447–452. https://doi.org/10.1097/IGC.0000000000000384
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Jonusiene, V., Sasnauskiene, A. (2021). Notch and Endometrial Cancer. In: Reichrath, J., Reichrath, S. (eds) Notch Signaling in Embryology and Cancer. Advances in Experimental Medicine and Biology, vol 1287. Springer, Cham. https://doi.org/10.1007/978-3-030-55031-8_4
Download citation
DOI: https://doi.org/10.1007/978-3-030-55031-8_4
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-55030-1
Online ISBN: 978-3-030-55031-8
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)