Abstract
Prolonged lifetime estrogen exposure due to early puberty, delayed menopause, environmental estrogen/phytoestrogen exposure, and/or exogenous hormone therapy has been correlated with an increased risk of estrogen-responsive cancers including breast, endometrial, and ovarian carcinomas. Accumulating evidence links aberrant Notch signaling with these estrogen-responsive cancers, and mechanisms of cross talk between the estrogen signaling pathway and Notch are beginning to emerge. Notch signaling is a tightly regulated process that is controlled temporally and spatially by the cellular environment, and Notch can behave as an oncogene or as a tumor suppressor in a cell-, tissue-, and timing-specific manner. The role played by Notch in cancer stem cells as a mediator of hormone therapy resistance is becoming increasingly clear, most notably in breast cancer, wherein combinatorial therapeutic strategies are being designed to target not only the bulk of tumor cells but also endocrine-resistant cancer stem cells. This chapter seeks to outline the recent history and current state of the estrogen-Notch interaction in estrogen-dependent cancers.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
- Notch
- Estrogen
- Breast
- Endometriosis
- Endometrial cancer
- Ovarian
- Estrogen receptor
- Estrogen-related receptor
- Cancer
- Angiogenesis
- Stem Cell
14.1 Introduction to Estrogen Signaling
Mechanisms of hormone action were first proposed by Jensen over 50 years ago and included a description of direct hormone binding to nuclear receptors [1]. The role of estrogen and its receptors has since expanded beyond the direct ligand-receptor interaction to include mechanisms of DNA binding, non-genomic effects, and receptor-mediated non-ligand hormone activities. In addition to their role in gonadal function, estrogens are now known to impact many cellular processes in systems as varied as immune, neuroendocrine, vascular, and skeletal, as well as play a key role in disease such as cancers, endometriosis, uterine fibroids, autoimmune disease, and obesity [2]. Understanding the function of estrogens (and all steroid hormones) in both the normal and diseased state is critical for developing relevant therapeutic strategies for hormone-dependent pathologies.
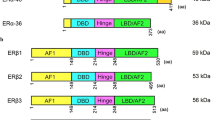
Estrogens freely cross the cellular membrane to interact with estrogen receptors. Upon ligand binding, these receptors dimerize and activate gene transcription in the nucleus by binding to estrogen response elements (EREs) in the DNA, displacing corepressors and/or recruiting coactivators [3]. In mammals, there are two classical estrogen receptors, ERα and ERβ [4, 5], as well as three orphan nuclear receptors called estrogen-related receptors (ERRα/NR3B1, ERRβ/NR3B2, and ERRγ/NR3B3). ERRs have significant amino acid homology with ERα/β, yet do not bind to naturally occurring estrogens. Elevated expression of ERRα correlates with poor prognosis in breast and ovarian cancers [6, 7] and tumor aggressiveness in ovarian and endometrial cancers [8, 9]. Interestingly, ERRγ expression is linked with favorable outcomes and improved progression-free survival in ovarian and breast cancers [7, 10]. The role of ERRs in cancer has been reviewed recently [11], and this chapter will focus on the role of the classical estrogen receptors, ERα and ERβ.
Estrogens are present in both males and females, and ERα/β are differently distributed across tissues. Estrogen receptors are also present on the cellular membrane where they can initiate rapid, non-genomic signaling [12,13,14]. Mitochondria-localized estrogen receptor transcription factors have also been described [15], and GPR30/GPER, a G protein-coupled receptor, was identified in the endoplasmic reticulum where it binds estrogen leading to mobilization of intracellular calcium and production of phosphatidylinositol 3,4,5-trisphosphate, an upstream regulator of AKT in the nucleus [16, 17]. A recent review of estrogen biology is available [3].
Prolonged exposure to estrogens due to precocious puberty, delayed menopause, or the presence of environmental estrogens/phytoestrogens is associated with an increased risk of estrogen-responsive cancers [18]. Small molecule agonists and/or antagonists of estrogen receptors have been used as therapeutic strategies for estrogen-responsive cancers, but tumor recurrence and resistance limit the success of these approaches. Additionally, molecules with selective action in particular tissues have also been designed (termed selective estrogen receptor modulators, SERMs). These compounds function as agonists in some tissues and antagonists in others toward the goal of minimizing side effects while maximizing efficacy. For example, tamoxifen is a SERM that is used as a first-line therapy for estrogen receptor-positive breast cancer in premenopausal patients [19]. Tamoxifen is an ER antagonist in the breast but an agonist in other tissues such as the bone, endometrium, and vascular endothelium. Raloxifene, a second-generation SERM with a slightly different estrogenic profile, has decreased side effects compared to tamoxifen and is beneficial in the bone with decreased risk of endometrial cancer and cardiovascular events. Conversely, the pure ER antagonist ICI 182,780 (fulvestrant) is antiestrogenic in all tissues and causes degradation of ER proteins [20]. A complete understanding of the molecular pathways modulated by steroid hormones could lead to improved and novel therapeutics for hormone-related pathologies and prevention of therapy-resistant cancers. The signaling mechanisms by which steroid receptors regulate their many processes have been the subject of a number of reviews [16, 21, 22]. This chapter seeks to highlight data accumulated over the last 20 years on the intersection of estrogen and the Notch signaling pathways.
14.2 Introduction to Notch Signaling
Notch signaling is an evolutionarily conserved pathway that is involved in a number of cellular processes including cell self-renewal, proliferation, differentiation, and death. Notch activation occurs via juxtacrine activation of Notch receptors by ligands present on neighboring cells. In mammals, there are four notch receptors (Notch1–Notch4) and five known Notch ligands (Jagged-1 and Jagged-2 and Delta-like 1, 3, and 4). Of these, Delta-like 1 and 4 are activating ligands, while Delta-like 3 functions as a negative regulator [23]. Notch receptors are synthesized as single-chain proteins and are cleaved into extracellular and transmembrane subunits in the Golgi apparatus. Once present at the cellular membrane, binding to ligand induces a second cleavage event by ADAM10, which removes the extracellular subunit. ADAM17/TACE can also cleave Notch during ligand-independent activation [24, 25]. A third cleavage event by the γ secretase complex releases the Notch intracellular domain (NICD) which translocates into the nucleus and regulates transcription of Notch target genes by interacting with the CSL transcription factor complex. This interaction displaces corepressors and recruits coactivators to regulate the expression of Notch targets such as the HES and HEY families of genes [26]. Notch is known to regulate transcription of many genes involved in the cell cycle [26], apoptosis [27], and stem cell maintenance [28], and recent genome-wide studies suggest the number of Notch transcriptional target genes is even higher than initially thought [29].
Notch can also signal in a noncanonical fashion, wherein Notch affects cell survival and metabolism by interacting with the mitochondria in the cytoplasm, instead of in the nucleus [30]. Posttranslational modifications regulate Notch activity, with phosphorylation, glycosylation, and ubiquitination playing key roles in Notch availability and degradation [31]. Notch is known to cross talk with other important cellular signaling pathways such as the TNFα [32], interleukin 1β [33], VEGF [34], and TGFβ [35] signaling pathways and modulate pathways involved in cell survival and proliferation like NF-κB [36] and ErbB2 [37]. As a result of these layers of regulation, the effects of Notch signaling are tightly controlled in a dose-, time-, and cell context-dependent manner. As a result, Notch signaling in hormone-dependent cancers can have oncogenic or tumor-suppressive activity, depending on the cellular environment, tissue type, and strength of signal. Deregulation of the Notch pathway has been described in a variety of tumors including estrogen-responsive solid tumors of the breast [38, 39], endometrium [40], and ovary [41].
14.3 Notch-Estrogen Cross Talk in Cancers
14.3.1 Breast Cancer
Estrogens play a major role in the proliferation of normal mammary epithelia, and lifetime exposure to unopposed estrogens via early puberty, late menopause, and/or exogenous exposure through oral contraceptives or hormone replacement therapy has been linked to increased breast cancer risk [42]. Breast cancer itself is a heterogeneous disease that is split into clinico-pathological subcategories based on immunohistochemical staining, ERα positive, Her2/neu positive, and triple negative (cancers that lack expression of ERα, progesterone receptor, and Her2/neu), with ERα-positive cancers making up more than 80% of breast malignancies in developed countries. More recently, breast cancers have been further subcategorized at the molecular level by gene expression profiles into luminal A, luminal B, basal-like, Her2-enriched, and claudin-low subtypes [43, 44]. Of these, luminal A and luminal B tumors are ERα positive but have different molecular profiles. Luminal B tumors tend to have a worse prognosis, a higher proliferation rate as measured by Ki67, and a higher likelihood of developing endocrine resistance [45]. An even more granular molecular classification identifies ten subgroups on the basis of mutational and gene expression profiles [46]. Attempts have been made to correlate molecular signatures of breast cancers with patient outcomes for personalized breast cancer therapy. Two gene expression-based tests, Oncotype DX and MammaPrint/BluePrint, do predict clinical outcomes in early-stage breast cancer and provide information on the likelihood of benefit from chemotherapy [47, 48]. However, more complete analyses are required before genetic signatures can guide clinical decision-making processes, especially in late-stage cancers [46, 49].
14.3.1.1 Notch Receptors and Ligands
Aberrant activation of the Notch signaling pathway has been implicated in breast cancer pathogenesis [37, 50] due to elevated levels of Notch signaling pathway components, including Notch receptors, ligands, and target genes [51, 52]. For example, high levels of Jagged-1 and Notch1 expression correlate with poor overall survival [53,54,55], and loss of the Numb-mediated inhibitory control of Notch signaling is found in 50% of human breast cancers [56]. On the other hand, Notch2 appears to reverse the oncogenic impact of Notch1 and Notch4 in some breast cancer cells [57], and its expression tracks with more differentiated tumors [58].
The first evidence of cross talk between the Notch signaling pathway and estrogens was generated by Rizzo et al. studying breast cancer cell lines [59]. Despite the high expression of Jagged-1 and Notch1 mRNA in breast cancer specimens, Notch transcriptional activity did not correlate with receptor overexpression in breast cancer cell lines. In ERα-positive cells, estrogen inhibited Notch transcriptional activity through decreased Notch1 ICD levels that led to an accumulation of Notch at the cellular membrane. This effect was reversed by treatment with tamoxifen or raloxifene, demonstrating the involvement of ERα [59]. Further, this effect was independent of ligand, since estrogen had no effect on Jagged-1 protein levels, and this effect was also observed upon coculture of MCF7 cells with Jagged-1 overexpressing feeder cells. These data suggested that Notch signaling may be reactivated by the use of common first-line endocrine therapies for breast cancer [59]. Other investigators have generated conflicting results using cDNA arrays followed by semiquantitative RT-PCR, demonstrating an increase in Jagged-1 and Notch1 expression in MCF7 cells [60]. Differences in therapeutic approaches may account for this discrepancy in results. The data by Rizzo et al. were further confirmed through knockdown studies wherein Notch1 and Notch4 were ablated and with studies using γ secretase inhibitors. All of these approaches resulted in significant decreases in endpoints of tumorigenesis and increases in cellular apoptosis [37, 59]. GPER has also been shown to facilitate estrogen-Notch cross talk in breast cancer, independent of ERα. In ERα-negative cells, Pupo et al. report an increase in γ secretase-dependent activation of Notch1 and increased levels of the Notch target gene Hes1 upon stimulation with the GPER ligand G1 or estrogen [61].
Reactivation of Notch in the context of resistance to antiestrogen therapy or estrogen withdrawal results in the activation of ERα target genes, and overexpression of Notch1 has been measured in tamoxifen-resistant breast cancer samples [62]. Notch1 can activate transcription of ERα target genes via recruitment of Notch-CSL-MAML1 transcriptional complexes to promoter regions of ERα target genes [63]. CSL binding elements are frequently in close proximity to EREs, and the presence of ERα recruits p300. Data generated by Hao et al. suggests cross talk between p300 and the Notch transcriptional complex to activate ERα-responsive genes in the absence of estrogen [63]. PKCα overexpression in clinical specimens predicts endocrine therapy resistance [64]. Yun et al. demonstrate that overexpression of PKCα correlates with Notch4 expression. PKCα was shown to selectively increase Notch4, but not Notch1, in endocrine-resistant breast cancer cell lines through an AP-1-dependent mechanism [65]. DMXL2, a modulator of Notch signaling, is overexpressed in ERα-positive metastatic breast cancers that progress after endocrine therapy [66]. Another study reports that elevated levels of nicastrin, a subunit of the γ secretase complex, correlate with elevated Notch4 in estrogen therapy-resistant cells [67]. Treatment of cells with anti-nicastrin monoclonal antibody or a γ secretase inhibitor (GSI) attenuates the invasiveness of endocrine therapy-resistant cells by blocking endothelial to mesenchymal transition. On the other hand, overexpression of nicastrin induces Notch4, resulting in increased tamoxifen resistance and invasiveness [67].
The therapeutic implications of these studies are paramount and suggest that in response to antiestrogen therapy , ERα-positive breast cancers develop additional mechanisms through the Notch pathway to activate estrogen signaling. Therefore, the efficacy of endocrine therapy can be improved by the addition of Notch inhibition, and several studies have been reported which support this hypothesis. Preclinically, MCF7 xenografts treated intratumorally with tamoxifen combined with γ secretase inhibitor decrease tumor growth better than either agent individually [59]. Haughian et al. demonstrate that in luminal breast cancers, there is often an expansion of “luminobasal” cells upon antiestrogen therapy. Notch inhibitors block the expansion of luminobasal cells and increase the efficacy of antiestrogen therapy [68]. Yun et al. demonstrate increased tamoxifen sensitivity in ERα-positive, PKCα overexpressing cells in culture and in vivo that have been treated with Notch inhibitors [65]. Genome-wide chromatin remodeling studies demonstrate that there is a global change in the chromatin landscape in resistant breast cancers. Classical ERα signaling is “epigenetically disengaged,” while Notch signaling is hyperactive. Blocking Notch signaling with γ secretase inhibitors attenuated growth of endocrine-resistant breast cancer cells [69]. Activation of the Notch pathway in serial xenografts in mice results in acquired resistance to tamoxifen, which can then be reversed by treatment with γ secretase inhibitors [62].
14.3.1.2 Stem Cells in Breast Cancer
Cancer stem cells (CSC) have been identified in breast cancer and are generally accepted to be responsible for tumor recurrence [70]. Despite being ERα negative, the growth of breast CSCs is affected by estrogen, and both tamoxifen and siRNA silencing of ERα inhibit the proliferation of breast cancer cell lines enriched with cancer stem cells. This signaling is thought to occur between non-CSC and CSC, similar to the paracrine communication between stromal and stem cells in the tumor microenvironment. Notch signaling was investigated by Harrison et al., and treatment with a γ secretase inhibitor blocked the response of CSCs to estrogen both in vitro and in vivo [71]. In contrast, Simoes et al. report a decrease in the number of CSCs in response to estrogen. In this study, the embryonic stem cell genes NANOG, OCT4, and SOX2 decreased upon estrogen treatment, implying differentiation and hence a decrease of the available CSC pool [72]. Other studies report that antagonism of ERα increases the number and self-renewing capability of CSCs and suggest that this activity may be responsible for endocrine therapy resistance. For example, tamoxifen treatment increased the number of MCF7 mammospheres [72], and in a different study, mammospheres were resistant to high doses of tamoxifen [73]. In preclinical in vitro and clinical studies after endocrine or chemotherapy, resistant cells and tumor biopsies are enriched for tumor-initiating cells as measured by markers of breast CSCs [74, 75].
Notch signaling is also required for proliferation of breast CSCs and is strongly linked to endocrine therapy resistance [76, 77]. Short-term treatment with endocrine therapies enriches for Jagged-1/Notch4 activated CSCs in patient tumor samples as well as PDX models. Two independent ERα-positive patient cohorts demonstrate that a Notch4/Hes/Hey gene signature predicts poor response to hormone therapy [77]. Further, hormone therapy has been reported to promote resistant, self-renewing CSCs through a mechanism involving Notch and ERα switching. In this study, initial responses to hormone therapy abrogated oxidative phosphorylation, increased paracrine levels of IL6, and resulted in a population of cells that were deficient in self-renewal, CD133hi/ERlo/OXPHOSlo. These cells become metabolically active and utilize oxidative phosphorylation in the absence of ERα. Inhibition of IL6-Notch switches the CD133hi CSC dependence on IL6/Notch to dependence on ER by activating expression of ERα. Thus, through an oxidative phosphorylation mechanism presumably regulated by Notch, hormone therapy drives self-renewal of dormant CSCs and mediates metastatic progression [78].
Clinically, there are a number of trials investigating the combination of Notch inhibitors with tamoxifen and other standard of care chemotherapeutics to increase sensitivity of bulk tumor cells while simultaneously targeting CSCs. Studies have been performed to investigate the safety and target engagement profiles of γ secretase inhibitors MK-0752 (Merck), RO4929097 (Roche), and PF03084014 (Pfizer) in combination therapy with tamoxifen or letrozole for breast cancer. Additional phase II/III studies are in the planning stages. Second generation GSIs such as LY3039478 are currently being investigated in breast cancer in combination with endocrine therapy. For a comprehensive list of current breast cancer clinical trials, see clinicaltrials.gov.
14.3.2 Endometriosis/Endometrial Cancer
Endometrial cancer is the most common gynecological malignancy in the United States with an estimated 60,000 new cases diagnosed and more than 10,000 deaths in 2016 alone [79]. Endometrial cancers are classified by histological staging and appearance. Approximately 70–80% of endometrial cancers are estrogen-dependent and are classified as endometrioid adenocarcinoma, type I [80]. The remaining 20–30% are type II non-endometrioid cancers (typically serous papillary and clear cell carcinoma along with mixed Müllerian tumors) and are estrogen independent. The 5-year survival rate in patients with low-grade, localized disease is approximately 80%, with 15–20% of patients developing metastasis and tumor recurrence. Treatments have limited efficacy for advanced-stage disease due to chemoresistance [81]. Approximately 90% of endometrial cancers are sporadic, and 10% are inherited. Genetic mutations in PTEN, PI3CA and K-ras have been identified in endometrioid endometrial cancer along with alterations in DNA repair pathways involving MLH1, MSH6, and microsatellite instability [82, 83]. Mutation in p53 is associated with type II endometrial cancer, along with inactivating mutations in p16 and overexpression of Her2/neu [82].
During the reproductive years, normal uterine endometrium undergoes regular cycles of differentiation and remodeling throughout the menstrual cycle. This process is mediated by a variety of factors including hormones (specifically estrogen, progesterone, and chorionic gonadotropin), changes in cell cycle activities, differentiation of endometrial cells, and vascular remodeling to produce a receptive environment for implantation. Notch pathway components are present in the endometrium throughout the menstrual cycle [84,85,86], and the dysregulation of Notch signaling has been implicated in this tumor type.
14.3.2.1 Notch Receptors and Ligands in the Endometrium
In endometrial carcinoma , the presence/absence of individual Notch receptors and ligands remains an area of active debate. Reported results appear to be extremely dependent on the Notch receptor analyzed, menopausal state of the patient, phase of menstrual cycle at time of analysis, and tumor stage. Using immunohistochemistry, Mitsuhashi et al. reported elevated levels of Notch1, Notch3, Jagged-1, and Delta-like-4 in endometrial cancer (n = 76) versus normal endometrium from unmatched, non-cancer patients (n = 37) [85]. Further, the elevation of Notch1 increased in later-stage cancers and correlated with cancer aggressiveness measures such as ovarian metastasis and invasion into the myometrial layer of the uterus. Elevated Notch3 remained constant across all cancer stages and did not correlate with metastasis or invasion; however, elevation of Notch1 and Notch3 correlated with poorer patient outcomes [85]. The Mitsuhashi study did not analyze Notch4.
Another study by Cobellis et al. examined the levels of Notch1, Notch4, and Jagged-1 by immunohistochemistry in normal endometrial samples (n = 60) of pre- and postmenopausal women, along with unmatched pathologic endometrial samples (n = 60) from patients with polyps, endometrial hyperplasia, and carcinoma. In this study, Notch1 and Notch4 had equivalent expression in the normal proliferative phase, while Notch1 increased and Notch4 decreased in the normal secretory phase. The authors propose that this result indicates a key role for Notch4 in cellular proliferation as characterized by the proliferative phase of the menstrual cycle, while Notch1 plays a more significant role in cellular differentiation as is characteristic of the secretory phase. These results are consistent with the notion of unopposed estrogen inhibiting Notch1 activation, as Rizzo et al. observed in breast cancer cells [84]. Further, Notch1, Notch4, and Jagged-1 all decreased significantly in normal menopausal endometrium indicating a decreased role for Notch signaling in the normal postmenopausal endometrium. In pathologies, Notch1 demonstrated elevated expression in hyperplasia and carcinoma compared to polyps, whereas Notch4 and Jagged-1 displayed striking decreases with increasing histological grade. Notch often functions as an oncogene in tissues where its normal role is a regulator of progenitor or stem cell fate and as a tumor suppressor in cases when normal function is the induction of terminal differentiation. In the Cobellis study, the decrease in Notch4 and Jagged-1 protein from polyps to carcinoma suggests a role for Notch4 signaling as a tumor suppressor and perhaps Notch1 as an oncogene in endometrial carcinoma [84]. This study did not analyze Notch3, and there is no data on the menopausal status of the patients from whom pathological endometrial samples were obtained.
The Didžiapetrienė laboratory reports that Notch receptors (Notch1-4), ligands (Jagged-1, Jagged-2, and Delta-like 1), and target gene Hes1 are all significantly decreased at the RNA level (via q-PCR) in endometrial carcinoma (n = 20) when compared to matched, adjacent non-tumor endometrium (n = 20). This suggests that Notch signaling plays a tumor-suppressive role in endometrial cancers [87]. Further, at the RNA level, Notch1, Notch4, and Delta-like 1 were decreased significantly more in stage IB than stage 1A cancers. At the protein level as measured by Western blot, only Notch4 and Jagged-1 were decreased in endometrial cancer, leading the authors to propose that a change in the stability of Notch receptors and ligands may happen in the cancerous state [88]. A comprehensive, complete evaluation of known Notch receptors, ligands, and target genes is still necessary in a larger, well-controlled study of matched tumor/normal sample pairs of known estrogen status to understand the role of the different Notch receptors in endometrial pathology.
Endometrioid endometrial cancers are typically estrogen receptor positive and proliferate in response to estrogen. As mentioned above, aberrant Notch signaling has been proposed as a key mechanism in endometrial cancer. Wei et al. performed studies in Ishikawa (ER-positive) endometrial carcinoma cells and demonstrated that estrogen stimulated cell proliferation due to induction of Notch1 and that this effect could be abolished by using the γ secretase inhibitor, N-[N-(3,5-difluorophenacetyl)-L-alanyl]-S-phenylglycine t-butyl ester (DAPT). Blocking with the ER antagonist ICI 182,780 also blocked Notch signaling and induced growth arrest.
MicroRNAs are a class of small, non-coding RNAs that inhibit gene expression. Jurcevic et al. identified 138 miRNAs that were differentially expressed in endometrial carcinoma in comparison to normal endometrium [89]. One of these miRNAs, miR-34a, regulates the Notch signaling pathway by targeting both Notch1 and Delta-like 1. Using miR-34a mimetics and inhibitors, the effect of miR-34a on Notch1 and Delta-like 1 was confirmed in Ishikawa cells in vitro, suggesting that miR-34a mimetics may be another future avenue of therapeutic potential.
14.3.2.2 Stem Cells in Endometrial Carcinoma
Endometrial carcinoma stem-like cells are identified by the cell surface marker CD133. CD133+ cells have active Notch signaling resulting in increased proliferation and low rates of apoptosis and play a critical role in retaining the self-renewing properties of cancer stem cells. Epidermal growth factor receptor (EGFR) is overexpressed, mutated, or otherwise functionally altered in many epithelial malignancies, including endometrial carcinoma. EGFR is an important histological marker for invasive potential and is predictive of recurrence and overall outcomes of endometrial cancers [90]. EGFR is also a therapeutic target for endometrial carcinoma through antibody or small molecule-based therapies. Treatment of Ishikawa cells with DAPT or AG1478 was more efficacious than treating with either compound alone suggesting that combination therapy targeting Notch and EGFR may have improved outcomes in endometrial cancer [91].
Another stem cell marker in endometrial carcinoma is Musashi-1 [92]. Endometrial carcinomas have significantly more Musashi-1 positive cells than normal endometrium. SiRNA knockdown of Musashi-1 resulted in increased expression of Notch1 mRNA. However, since Musashi-1 is a transcriptional repressor of Numb, which induces Notch internalization and degradation by ubiquitination, the loss of Musashi-1 resulted in significantly decreased levels of Notch1 and Hes1 protein. Further, the loss of Musashi-1 resulted in an accumulation of cells in the G1 phase indicating a block in cell cycle progression [92]. Musashi-1 may emerge as a putative therapeutic target for endometrial carcinoma stem cell therapy.
The expression of microRNAs may also play a role in regulating endometrial cancer stem cells . miRNA-134 is significantly downregulated in endometrial cancer stem cells. miRNA-134 is a member of the genetically imprinted DLK1-DIO3 region present on 14q23 which contains genes for large and small RNAs, for paternally expressed genes such as Delta-like homolog 1 (DLK1) and iodothyronine deiodinase 3 (DIO3) and also the maternally expressed genes MEG3, MEG8, and the antisense retrotransposon-like 1 (RTL1) [93]. Overexpression of miR-134 decreased proliferation, decreased the cell’s ability to develop chemoresistance, and suppressed the migratory ability of human endometrial cancer stem cells. Further, overexpression of miR-134 decreased Notch pathway signaling in human endometrial cancer stem cells [94]. Whereas this miRNA has only been tested in stem cells from type II endometrial carcinoma, this pathway may also have utility in type I endometrial cancers. Additional studies are required to further elucidate the direct target(s) of miR-134 and their role in endometrial carcinoma.
14.3.2.3 Endometriosis and Infertility
Endometriosis is the aberrant overgrowth of hormonally responsive endometrial cells outside the uterine cavity that results in severe pelvic pain, dysmenorrhea, and infertility. Endometriosis affects one in ten women of childbearing age [95]. During the mid- to late secretory phase of the menstrual cycle, estrogen and progesterone induce the stromal cells of the endometrium to differentiate such that pregnancy will ensue if implantation occurs [96]. Notch is regulated by chorionic gonadotropin and progesterone to mediate uterine stromal differentiation and decidualization via several mechanisms [97, 98]. In mice, the lack of Notch1 decreases cellular proliferation by altering the activity of cell cycle proteins and by increasing apoptosis, suggesting that Notch signaling is crucial to promoting successful implantation. Given the role of Notch1 in decidualization, Su et al. studied the role of Notch1 in women with endometriosis, as well as in a baboon model of spontaneous endometriosis. They demonstrated that receptors Notch1 and Notch4, ligands Jagged-2 and Delta-like 4, and Notch target genes HES5 and HEY1 were decreased in endometriosis compared to normal endometrial tissue, suggesting that suppressed Notch signaling is responsible for decreased fertility in patients with endometriosis [99]. Additionally, one of the early genes activated in decidualization is FOXO1 [100], which acts as a Notch1 coactivator by interacting with CSL. In the endometrium, FOXO1 expression is also regulated by Notch1 such that in the case of endometriosis, suppression of the Notch signaling pathway also suppresses FOXO1 and inhibits decidualization [99]. Interestingly in normal endometrium, the mechanism through which Notch1 activates FOXO1 expression is by cross talk with the liganded progesterone receptor at the promoter of FOXO1 [97]. However, in endometriosis, progesterone resistance inhibits Notch1 activity, results in decreased FOXO1 expression and decidualization failure [99].
14.3.3 Ovarian Cancer
Ovarian cancer is by far the most lethal gynecologic cancer in the United States. It is estimated that over 22,000 new cases will be diagnosed in 2017, and ovarian cancer will be responsible for over 14,000 deaths [101]. This mortality rate is due in part to the lack of molecular markers to identify early ovarian cancers and the observation that more than 75% of patients present at diagnosis with stage III or IV disease. First-line therapy often involves debulking surgery followed by aggressive chemotherapy, but recurrence rates are high, and tumors are often resistant to further chemotherapy, resulting in a 5-year survival rate of 46.5% [101]. Treatment options for recurrent, resistant ovarian cancer are few, highlighting the necessity for new, targeted molecular therapies for this devastating disease.
The majority of ovarian cancers are classified as ovarian adenocarcinomas that derive from the ovarian surface epithelium. There are numerous histological subtypes, of which the most common is serous adenocarcinoma, followed by endometrioid and mucinous carcinomas as well as other, less common subtypes [102]. Although the etiology of ovarian cancer is unknown, there are several loss-of-function mutations in well-described tumor suppressor genes that have been correlated with ovarian cancer, for example, TP53 [103], PTEN [104], and BRCA1/2 in familial cancer [105, 106]. Similarly, overexpression or gene duplication of oncogenes has also been described for PI3K [107], AKT2 [108], EFGR [109], c-Myc [110], K-ras [111], and Her2/neu [112]. Disruptions in the Notch signaling pathway have also been correlated with ovarian cancer formation.
14.3.3.1 Notch1
Initial studies of Notch1 signaling in ovarian cancer were performed by Hopfer et al. [113] on a collection of 32 ovarian cancers (17 ovarian adenocarcinoma, 12 ovarian adenoma, 3 borderline tumors), 3 ovarian cancer-derived cell lines (A2780, OVCAR-3, 2008), and 1 ovarian surface epithelial cell line (IOSE-144). At both the mRNA and protein levels, the group demonstrated a consistent increase in Jagged-2, DLL-1, and Manic Fringe in adenocarcinoma compared to adenoma. Overexpression of the Notch1 ICD in ovarian cancer cells led to an increase in proliferation and anchorage-independent growth, suggesting a role for Notch1 in ovarian tumorigenesis [113]. Analysis of Notch1 by Rose et al. demonstrates that NICD is overexpressed in 76% of human ovarian adenocarcinomas when measured by Western blotting and is consistent with the expression of NICD measured in ovarian cancer cell lines [114]. Knockdown of NICD using siRNAs to Notch1 ICD resulted in decreased proliferation in three ovarian cancer cell lines [114]. Further, Notch1 expression was shown to correlate with the stage and differentiation status of ovarian cancers. Using immunohistochemistry, Wang et al. demonstrated elevated expression of Notch1 in 95% of ovarian cancers compared to patient-matched opposite side normal ovarian tissue. These results were confirmed using RT-PCR and Western blotting, and additional stratification of these data indicates Notch1 expression increased in samples with poor differentiation and elevated FIGO staging scores [115]. Additionally, Notch1, Notch3, and Notch ligand DLL4 were elevated in 18 ovarian cancers compared to healthy ovarian tissues [116], and in a smaller study (n = 10), Notch1, Jagged-1, and DLL1 were elevated and correlated with metastatic ovarian cancers [117]. However, conflicting data has also been reported. Using a novel immunohistochemical method to detect Notch1 ICD in 147 ovarian cancer samples, none demonstrated increased Notch1, even though NICD was detected in other cancers with known Notch activation [118]. More recently, the prognostic utility of Notch receptors and ligands was assessed and correlated with patient outcomes using the Kaplan-Meier plotter [119] (http://kmplot.com) to analyze publically available ovarian cancer gene expression datasets from the Cancer Biomedical Informatics Grid (caBIG, https://biospecimens.cancer.gov/relatedinitiatives/overview/caBIG.asp), the Gene Expression Omnibus (GEO; http://www.ncbi.nlm.nih.gov/geo), and The Cancer Genome Atlas (TCGA; https://cancergenome.nih.gov) [120]. Elevated Notch2 and Notch3 expression was correlated with poor progression-free survival, whereas high Notch4 expression was associated with overall survival. These results suggest that the different Notch receptors may have different prognostic value in ovarian cancers.
Next-generation sequencing has also been performed to identify a molecular signature specific for a subtype of ovarian cancer that is associated with endometriosis termed endometriosis-associated ovarian cancer or EAOC. Recent studies have suggested that endometriosis may be a precursor lesion to this form of ovarian cancer and a molecular profile would aid diagnosis in preneoplastic lesions. Notch1, Notch2, and Notch4 showed recurrent missense mutations in EAOC specimens [121].
14.3.3.2 Notch3
Notch3 was also initially identified through studies designed to identify early biomarkers of ovarian cancer. In one study, Affymetrix microarrays were used to analyze transcriptional profiles of 42 ovarian cancers versus normal ovarian epithelium; Notch3 was upregulated more than threefold in this sample set [122]. Another study in ovarian cancer cell lines identified Jagged-2 when compared to immortalized ovarian surface epithelial cell lines [123]. Gene amplification was studied by Park et al. to identify chromosomal regions with copy number variations in 31 late-stage ovarian cancers [124]. Single nucleotide polymorphism (SNP) array and digital karyotyping both identified an amplified region on chromosome 19 – the region containing Notch3. Amplification of Notch3 was identified in 20% of the samples and was confirmed by increased protein expression as measured by fluorescent in situ hybridization and immunohistochemistry [124]. Notch3 knockdown by siRNA or inhibition of γ secretase decreased DNA synthesis as a measure of proliferation and increased apoptosis in ovarian cancer cell lines [124] suggesting that Notch3 activation may play an important role in ovarian cancer development. A similar study confirmed these results in a different sample set [125], while a genome-wide study of ovarian carcinoma conducted by The Cancer Genome Atlas (TCGA) revealed similar results with Notch3 genetic changes identified in 50% of ovarian cancer cases [41]. Further studies implicated Notch3 as a prognostic indicator, demonstrating that elevated mRNA for Notch3, Jagged-1, and Jagged-2 as well as elevated Notch3 protein correlated with chemoresistance and poor overall survival [126, 127].
The identification of Notch3 as a prognostic marker and driver of ovarian carcinoma led to mechanistic studies aimed at identifying the primary Notch ligand responsible for Notch3 activation. Choi et al. analyzed the expression levels of all known Notch ligands in ovarian cancer and found Jagged-1 to have the highest expression. Knockout of Jagged-1 in feeder cell cocultures negatively impacted the proliferative and adhesive properties of ovarian cancer cells, whereas constitutive expression of Notch3 ICD had the reverse effect [128]. Further studies confirm the presence of Notch3 and Jagged-1 expression in ovarian cancer samples and propose that dynamin-dependent endocytosis is a key step in the Jagged-1 activation of Notch3 [129].
Notch3 was found to exert its effect through the actions of target genes such as Pbx1. Pbx1 is a known proto-oncogene that has been studied in leukemias and was recently identified as a Notch3 target gene in ovarian cancer [130]. Chen et al. used a systems biology approach to identify Notch3 target genes by combining transcriptome analysis with ChIP-on-chip analysis. From this, they were able to demonstrate that the target genes identified by ChIP were often the same transcriptional regions regulated by Notch3 in ovarian cancer cells and were able to identify DLGAP5 as a new Notch3 target gene in ovarian cancer [131].
Studies of the epigenetic regulation and gene methylation modifications present in ovarian cancer were performed by Ivan et al. [132]. These studies used TCGA data to highlight the clinical relevance of epigenetic modification of genes in the Notch signaling pathway by examining the overlap between epigenetic regulation by methylation and miRNAs and overall patient survival. Using this approach, the authors found an inverse relationship between DNA methylation and the gene expression of CCND1, PPARG, and RUNX1, all genes involved in the Notch pathway. Further, the expression level of these genes along with the DNA methylation status was predictive of patient outcomes with low DNA methylation/high expression being indicative of poorer overall survival. miRNA correlations demonstrated a similar trend with an inverse relationship between miRNA levels and gene expression of CCND1, PPARG, and RUNX1. As with DNA methylation, patients with low miRNA expression/high gene expression demonstrated poorer overall survival [132].
In an effort to identify proteins involved in modulating the Notch3 signaling pathway in ovarian cancer, Jung et al. used a human proteome microarray to screen for Notch3-ICD interacting proteins [133]. The E3 ubiquitin-protein ligase WWP2 was identified as an interacting partner of Notch3 that specifically binds Notch3 over the other Notch receptors. Further, WWP2 attenuates Notch3 pathway activity and leads to cell cycle arrest. Analysis of TCGA data revealed that the majority of ovarian carcinomas carry inactivating mutations in WWP2, suggesting that in the normal ovarian epithelium, WWP2 acts as a tumor suppressor by inhibiting Notch3 activity [133].
14.3.3.3 Angiogenesis in Ovarian Cancer
In general, Notch is actively involved in angiogenesis and vessel patterning [134, 135], with Notch1, Notch4, DLL1, DLL4, and Jagged-1 being the most highly expressed Notch pathway components involved in the differentiation between the tip and tube cellular phenotype in a developing vessel [136,137,138,139,140]. In ovarian cancer, Lu et al. specifically examined the gene expression profiles of endothelial cells from normal ovarian tissue or aggressive ovarian cancer and detected 2.5X elevated expression of Jagged-1 among other genes [141]. Jagged-1 has been shown to be a critical regulator of tip formation and sprouting through competitive, antagonistic regulation of DLL4-activated Notch signaling [142], confirming hypotheses that the equilibrium between available Notch ligands can have significant effects on the outcome of pathway activation. DLL4 has been extensively studied as a regulator of angiogenic activities in ovarian tumor endothelium [135, 143, 144] and other tumors [145]. In one study, DLL4 was overexpressed in 72% of tumors analyzed and correlated with poor clinical outcomes. The investigators noted that DLL4 was lowest in tumors responding to anti-VEGF therapies and that the combination of anti-VEGF therapies plus knockdown of DLL4 in mouse models decreased tumor proliferation better than either therapy alone [146]. Subsequently, Kuhnert et al. reported efficacy using a humanized DLL4 monoclonal antibody (REGN421) in mouse xenograft models of ovarian cancer. Antagonism of DLL4 in this system led not only to a reduction in tumor volume but also the formation of nonfunctional blood vessels. As with the Hu study, combination of DLL4 monoclonal antibody with anti-VEGF therapy showed decreased tumor proliferation and decreased angiogenesis than either therapy alone [143]. Additionally, use of a γ secretase inhibitor in a mouse model of ovarian cancer resulted in decreased microvessel density, suggesting that Notch pathway inhibition by these compounds may also be a mechanism to block angiogenesis in tumors resistant to anti-VEGF therapies [147]. Estrogen also enhances angiogenic branching via signaling of the VEGF-DLL4/Notch pathway in human umbilical vein endothelial cells [148]. This effect is attenuated by inhibition of Notch signaling further supporting the combination of anti-DLL4/Notch and anti-VEGF as a putative therapy for estrogen-dependent cancers.
14.3.3.4 Stem Cells in Ovarian Carcinoma
Aberrant activation of the Notch signaling pathway plays a key role in chemoresistance and recurrence in ovarian cancer. This is generally attributed to the presence of a population of cancer stem cells (CSCs) that have the capacity to initiate tumor formation and self-renew through asymmetric division [149]. Cancer stem-like cells in ovarian cancer are identified by a variety of cell surface markers including CD44, CD24, CD117, CD133, and ALDH1. Depending on the published series, combinations of these various markers exhibit different CSC characteristics. For example, in a recent publication, CD133+ and ALDH1+ cells were implicated as CSCs, with the presence of CD133+ cells in a primary ovarian cancer strongly correlating with poor survival and the coexpression of both CD133+ and ALDH1+ indicative of a decreased progression-free interval and poor overall survival [150]. Interestingly, of patients with CD133/ALDH1 positivity in primary tumors, 85% lost these surface markers in recurrent tumors where cancer stem-like cells would be expected to be more prominent. This may represent cellular differentiation or other changes that would lead to the loss of CSC surface markers [150]. Further studies are warranted to clarify this issue . In earlier studies, Bapat et al. reported a subpopulation of CD44+ stem-like cells with tumor-initiating activity [151], and Zhang et al. isolated CD44+ and CD117+ cells from ovarian tumors with self-renewing and tumor-initiating properties [152]. This diversity in surface and functional markers may reflect the heterogeneity of ovarian cancer and ovarian CSC, and further studies are necessary to clarify this issue. Comprehensive reviews of the many stem cell marker studies have been recently published [153, 154].
Notch signaling is one of the signal transduction pathways that has been implicated in CSC stemness, along with Wnt/β-catenin, IL6/JAK/STAT, Hedgehog, NF-kB, and PI3K/AKT [28, 155, 156]. In a study of 45 matched primary and recurrent tumor samples, genes involved in these pathways, including Notch, were significantly increased in recurrent disease [157]. More recently, Kang et al. demonstrated that galectin-3 supports CSC stemness by activating Notch signaling via Notch1 ICD. Galectin-3 was overexpressed in advanced-stage ovarian cancers, and in vitro modulation of galectin-3 reduced the levels of cleaved Notch1 ICD and expression of Notch target genes Hes1 and Hey1 [158].
14.3.3.5 Epithelial-to-Mesenchymal Transition
Another characteristic of CSCs is their ability to acquire mesenchymal traits and the ability of cells to develop increased migratory and invasiveness characteristics. Notch signaling is an inducer of the epithelial-to-mesenchymal transition (EMT) along with TGFβ, Hedgehog, and Wnt signaling pathways and promotes tumor invasion, metastasis, and chemoresistance through the activation of EMT-associated transcription factors such as Snail, Slug, Twist, and ZEB [159, 160]. Notch3 was demonstrated to induce EMT, block carboplatin-induced apoptosis, and attenuate ERK phosphorylation in ovarian cancer cell lines [161]. Subsequent studies implicated Ras-associated protein Rap1A as the upstream activator of ERK and Notch in EMT [162]. Further accumulated evidence suggests that EMT can be blocked by Notch inhibition as a therapeutic strategy [163, 164]. Indeed, the γ secretase inhibitor DAPT blocked TGFβ-induced EMT in ovarian cancer cell lines [165, 166].
14.3.3.6 Targeting Notch in Ovarian Cancer
Preclinical studies demonstrate that inhibition of Notch pathway components is a viable strategy in ovarian cancer. Inhibition of Jagged-1 by siRNA delivered intravenously by chitosan nanoparticle delivery in an orthotopic mouse model of ovarian cancer demonstrated significant reductions in tumor volume and microvessel density. Further, knockdown of Jagged-1 sensitized cells to subsequent docetaxel treatment [167]. Several studies have demonstrated that Notch inhibition sensitizes ovarian cancer cells (particularly CSCs) to chemotherapy [168,169,170,171,172,173]. McAuliffe et al. show that overexpression of Notch3 results in the expansion of CSCs and increased resistance to platinum-based chemotherapy [170]. Treatment with a γ secretase inhibitor had the reverse effect, leading to depleted CSCs and increased sensitivity to platinum therapy. Importantly, the combination of γ secretase inhibitor and cisplatin was a synergistic effect that eliminated CSC and bulk tumor cells through enhanced DNA damage response, cell cycle arrest, and apoptosis [170]. Inhibition with the MRK-003 γ secretase inhibitor in combination with standard chemotherapy agent paclitaxel demonstrated decreases in Notch signaling and paclitaxel resistance in ovarian cancer model systems [169]. Pretreatment of chemotherapy-resistant ovarian cancer cell lines with the γ secretase inhibitors DAPT or MK-0752 also downregulates Notch and decreases proliferation [168, 171]. Yen et al. presented a novel strategy by inhibiting Notch2/3 with an antagonist antibody alone or in combination with paclitaxel [174]. Again, inhibition of Notch signaling in addition to standard chemotherapy demonstrated a decrease in CSCs and a delay in tumor recurrence in preclinical models. Similarly, inhibition of DLL4 with anti-DLL4 antibodies in combination with anti-VEGF therapy aflibercept was efficacious in reducing tumor volume in preclinical models of ovarian cancer [143, 175]. Finally, combination of Notch inhibition by DAPT in combination with Bay11-7085 decreased proliferation of ovarian cancer cell lines suggesting that this combination therapy may have efficacy in ovarian cancer [176].
The preclinical successes with Notch inhibition have led to clinical trials of Notch γ secretase inhibitors alone and in combination with other therapies. Several phase I trials have been reported, including the use of enoticumab, a humanized DLL4 monoclonal antibody [177], and three γ secretase inhibitors (MK-0752, RO4929097, and LY900009) [178,179,180]. The RO4929097 γ secretase inhibitor has completed a phase II clinical trial in patients with platinum therapy-resistant ovarian cancer but demonstrated insufficient activity to warrant further study as a monotherapy [181]. For a complete, up-to-date listing of clinical trials in progress, visit www.clinicaltrials.gov.
14.4 Conclusions
The regulation of estrogen signaling in cancers encompasses a much broader range of pathways than initially appreciated, and there is still much to be learned. The newly discovered mechanisms of cross talk between Notch and estrogen signaling pathways that have been identified in breast cancers may also be applicable and relevant to other estrogen-dependent cancers such as endometrial and ovarian cancers. The intersection of estrogen and Notch signaling pathways has opened up a new direction for future investigation into the etiology of hormone-dependent cancers. More importantly, these new studies are offering translational approaches that may have clinical utility in the form of combination therapies utilizing Notch inhibitors along with traditional chemotherapy regimens.
References
Jensen, E. V. (1962). On the mechanism of estrogen action. Perspectives in Biology and Medicine, 6, 47–59.
Burns, K. A., & Korach, K. S. (2012). Estrogen receptors and human disease: An update. Archives of Toxicology, 86, 1491–1504.
Hamilton, K. J., Hewitt, S. C., Arao, Y., & Korach, K. S. (2017). Estrogen hormone biology. Current Topics in Developmental Biology, 125, 109–146.
Kuiper, G. G., Enmark, E., Pelto-Huikko, M., Nilsson, S., & Gustafsson, J. A. (1996). Cloning of a novel receptor expressed in rat prostate and ovary. Proceedings of the National Academy of Sciences of the United States of America, 93, 5925–5930.
Mangelsdorf, D. J., Thummel, C., Beato, M., Herrlich, P., Schutz, G., Umesono, K., Blumberg, B., Kastner, P., Mark, M., Chambon, P., & Evans, R. M. (1995). The nuclear receptor superfamily: The second decade. Cell, 83, 835–839.
Ariazi, E. A., & Jordan, V. C. (2006). Estrogen-related receptors as emerging targets in cancer and metabolic disorders. Current Topics in Medicinal Chemistry, 6, 203–215.
Sun, P., Sehouli, J., Denkert, C., Mustea, A., Konsgen, D., Koch, I., Wei, L., & Lichtenegger, W. (2005). Expression of estrogen receptor-related receptors, a subfamily of orphan nuclear receptors, as new tumor biomarkers in ovarian cancer cells. Journal of Molecular Medicine (Berlin), 83, 457–467.
Fujimoto, J., Alam, S. M., Jahan, I., Sato, E., Sakaguchi, H., & Tamaya, T. (2007). Clinical implication of estrogen-related receptor (ERR) expression in ovarian cancers. The Journal of Steroid Biochemistry and Molecular Biology, 104, 301–304.
Fujimoto, J., & Sato, E. (2009). Clinical implication of estrogen-related receptor (ERR) expression in uterine endometrial cancers. The Journal of Steroid Biochemistry and Molecular Biology, 116, 71–75.
Ariazi, E. A., Clark, G. M., & Mertz, J. E. (2002). Estrogen-related receptor alpha and estrogen-related receptor gamma associate with unfavorable and favorable biomarkers, respectively, in human breast cancer. Cancer Research, 62, 6510–6518.
Tam, I. S., & Giguere, V. (2016). There and back again: The journey of the estrogen-related receptors in the cancer realm. The Journal of Steroid Biochemistry and Molecular Biology, 157, 13–19.
Gu, S., Papadopoulou, N., Gehring, E. M., Nasir, O., Dimas, K., Bhavsar, S. K., Foller, M., Alevizopoulos, K., Lang, F., & Stournaras, C. (2009). Functional membrane androgen receptors in colon tumors trigger pro-apoptotic responses in vitro and reduce drastically tumor incidence in vivo. Molecular Cancer, 8, 114.
Gustafsson, K. L., Farman, H., Henning, P., Lionikaite, V., Moverare-Skrtic, S., Wu, J., Ryberg, H., Koskela, A., Gustafsson, J. A., Tuukkanen, J., Levin, E. R., Ohlsson, C., & Lagerquist, M. K. (2016). The role of membrane ERalpha signaling in bone and other major estrogen responsive tissues. Scientific Reports, 6, 29473.
Valadez-Cosmes, P., Vazquez-Martinez, E. R., Cerbon, M., & Camacho-Arroyo, I. (2016). Membrane progesterone receptors in reproduction and cancer. Molecular and Cellular Endocrinology, 434, 166–175.
Sepuri, N. B., Tammineni, P., Mohammed, F., & Paripati, A. (2017). Nuclear transcription factors in the Mitochondria: A new paradigm in fine-tuning Mitochondrial metabolism. Handbook of Experimental Pharmacology, 240, 3–20.
Shi, H., Kumar, S. P., & Liu, X. (2013). G protein-coupled estrogen receptor in energy homeostasis and obesity pathogenesis. Progress in Molecular Biology and Translational Science, 114, 193–250.
Revankar, C. M., Cimino, D. F., Sklar, L. A., Arterburn, J. B., & Prossnitz, E. R. (2005). A transmembrane intracellular estrogen receptor mediates rapid cell signaling. Science, 307, 1625–1630.
Key, T. J., Appleby, P. N., Reeves, G. K., Travis, R. C., Alberg, A. J., Barricarte, A., Berrino, F., Krogh, V., Sieri, S., Brinton, L. A., Dorgan, J. F., Dossus, L., Dowsett, M., Eliassen, A. H., Fortner, R. T., Hankinson, S. E., Helzlsouer, K. J., Hoff man-Bolton, J., Comstock, G. W., Kaaks, R., Kahle, L. L., Muti, P., Overvad, K., Peeters, P. H., Riboli, E., Rinaldi, S., Rollison, D. E., Stanczyk, F. Z., Trichopoulos, D., Tworoger, S. S., & Vineis, P. (2013). Sex hormones and risk of breast cancer in premenopausal women: A collaborative reanalysis of individual participant data from seven prospective studies. The Lancet Oncology, 14, 1009–1019.
Maximov, P. Y., Lee, T. M., & Jordan, V. C. (2013). The discovery and development of selective estrogen receptor modulators (SERMs) for clinical practice. Current Clinical Pharmacology, 8, 135–155.
Jia, M., Dahlman-Wright, K., & Gustafsson, J. A. (2015). Estrogen receptor alpha and beta in health and disease. Best Practice & Research. Clinical Endocrinology & Metabolism, 29, 557–568.
Gustafsson, J. A. (2016). Historical overview of nuclear receptors. The Journal of Steroid Biochemistry and Molecular Biology, 157, 3–6.
Schwartz, N., Verma, A., Bivens, C. B., Schwartz, Z., & Boyan, B. D. (2016). Rapid steroid hormone actions via membrane receptors. Biochimica et Biophysica Acta, 1863, 2289–2298.
Zhang, J., Gao, H., & Zhang, Y. (2017). Differential expression of the Notch1 receptor, and its ligands Dll1, Dll3 and Dll4 in distinct human pituitary adenoma subtypes. Oncology Letters, 13, 4533–4539.
Hirata, N., Yamada, S., Shoda, T., Kurihara, M., Sekino, Y., & Kanda, Y. (2014). Sphingosine-1-phosphate promotes expansion of cancer stem cells via S1PR3 by a ligand-independent Notch activation. Nature Communications, 5, 4806.
Palmer, W. H., & Deng, W. M. (2015). Ligand-independent mechanisms of Notch activity. Trends in Cell Biology, 25, 697–707.
Crabtree, J. S., Singleton, C. S., & Miele, L. (2016). Notch signaling in neuroendocrine tumors. Frontiers in Oncology, 6, 94.
Afshar, Y., Stanculescu, A., Miele, L., & Fazleabas, A. T. (2007). The role of chorionic gonadotropin and Notch1 in implantation. Journal of Assisted Reproduction and Genetics, 24, 296–302.
Takebe, N., Miele, L., Harris, P. J., Jeong, W., Bando, H., Kahn, M., Yang, S. X., & Ivy, S. P. (2015). Targeting Notch, Hedgehog, and Wnt pathways in cancer stem cells: Clinical update. Nature Reviews. Clinical Oncology, 12, 445–464.
Guruharsha, K. G., Kankel, M. W., & Artavanis-Tsakonas, S. (2012). The Notch signalling system: Recent insights into the complexity of a conserved pathway. Nature Reviews. Genetics, 13, 654–666.
Ayaz, F., & Osborne, B. A. (2014). Non-canonical notch signaling in cancer and immunity. Frontiers in Oncology, 4, 345.
Borggrefe, T., & Liefke, R. (2012). Fine-tuning of the intracellular canonical Notch signaling pathway. Cell Cycle, 11, 264–276.
Quillard, T., Devalliere, J., Chatelais, M., Coulon, F., Seveno, C., Romagnoli, M., Barille Nion, S., & Charreau, B. (2009). Notch2 signaling sensitizes endothelial cells to apoptosis by negatively regulating the key protective molecule survivin. PLoS One, 4, e8244.
Verginelli, F., Adesso, L., Limon, I., Alisi, A., Gueguen, M., Panera, N., Giorda, E., Raimondi, L., Ciarapica, R., Campese, A. F., Screpanti, I., Stifani, S., Kitajewski, J., Miele, L., Rota, R., & Locatelli, F. (2015). Activation of an endothelial Notch1-Jagged1 circuit induces VCAM1 expression, an effect amplified by interleukin-1beta. Oncotarget, 6, 43216–43229.
Gu, J. W., Rizzo, P., Pannuti, A., Golde, T., Osborne, B., & Miele, L. (2012). Notch signals in the endothelium and cancer “stem-like” cells: Opportunities for cancer therapy. Vascular Cell, 4, 7.
Kurpinski, K., Lam, H., Chu, J., Wang, A., Kim, A., Tsay, E., Agrawal, S., Schaffer, D. V., & Li, S. (2010). Transforming growth factor-beta and notch signaling mediate stem cell differentiation into smooth muscle cells. Stem Cells, 28, 734–742.
Osipo, C., Golde, T. E., Osborne, B. A., & Miele, L. A. (2008). Off the beaten pathway: The complex cross talk between Notch and NF-kappaB. Laboratory Investigation; A Journal of Technical Methods and Pathology, 88, 11–17.
Rizzo, P., Osipo, C., Pannuti, A., Golde, T., Osborne, B., & Miele, L. (2009). Targeting Notch signaling cross-talk with estrogen receptor and ErbB-2 in breast cancer. Advances in Enzyme Regulation, 49, 134–141.
Miele, L., Golde, T., & Osborne, B. (2006). Notch signaling in cancer. Current Molecular Medicine, 6, 905–918.
Stylianou, S., Clarke, R. B., & Brennan, K. (2006). Aberrant activation of notch signaling in human breast cancer. Cancer Research, 66, 1517–1525.
Daley-Brown, D., Oprea-Ilies, G. M., Lee, R., Pattillo, R., & Gonzalez-Perez, R. R. (2015). Molecular cues on obesity signals, tumor markers and endometrial cancer. Hormone Molecular Biology and Clinical Investigation, 21, 89–106.
N. Cancer Genome Atlas Research. (2011). Integrated genomic analyses of ovarian carcinoma. Nature, 474, 609–615.
Rice, M. S., Eliassen, A. H., Hankinson, S. E., Lenart, E. B., Willett, W. C., & Tamimi, R. M. (2016). Breast cancer research in the nurses’ health studies: Exposures across the life course. American Journal of Public Health, 106, 1592–1598.
Herschkowitz, J. I., Simin, K., Weigman, V. J., Mikaelian, I., Usary, J., Hu, Z., Rasmussen, K. E., Jones, L. P., Assefnia, S., Chandrasekharan, S., Backlund, M. G., Yin, Y., Khramtsov, A. I., Bastein, R., Quackenbush, J., Glazer, R. I., Brown, P. H., Green, J. E., Kopelovich, L., Furth, P. A., Palazzo, J. P., Olopade, O. I., Bernard, P. S., Churchill, G. A., Van Dyke, T., & Perou, C. M. (2007). Identification of conserved gene expression features between murine mammary carcinoma models and human breast tumors. Genome Biology, 8, R76.
Perou, C. M., Sorlie, T., Eisen, M. B., van de Rijn, M., Jeffrey, S. S., Rees, C. A., Pollack, J. R., Ross, D. T., Johnsen, H., Akslen, L. A., Fluge, O., Pergamenschikov, A., Williams, C., Zhu, S. X., Lonning, P. E., Borresen-Dale, A. L., Brown, P. O., & Botstein, D. (2000). Molecular portraits of human breast tumours. Nature, 406, 747–752.
Sorlie, T., Perou, C. M., Tibshirani, R., Aas, T., Geisler, S., Johnsen, H., Hastie, T., Eisen, M. B., van de Rijn, M., Jeffrey, S. S., Thorsen, T., Quist, H., Matese, J. C., Brown, P. O., Botstein, D., Lonning, P. E., & Borresen-Dale, A. L. (2001). Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proceedings of the National Academy of Sciences of the United States of America, 98, 10869–10874.
Curtis, C., Shah, S. P., Chin, S. F., Turashvili, G., Rueda, O. M., Dunning, M. J., Speed, D., Lynch, A. G., Samarajiwa, S., Yuan, Y., Graf, S., Ha, G., Haffari, G., Bashashati, A., Russell, R., McKinney, S., M. Group, Langerod, A., Green, A., Provenzano, E., Wishart, G., Pinder, S., Watson, P., Markowetz, F., Murphy, L., Ellis, I., Purushotham, A., Borresen-Dale, A. L., Brenton, J. D., Tavare, S., Caldas, C., & Aparicio, S. (2012). The genomic and transcriptomic architecture of 2,000 breast tumours reveals novel subgroups. Nature, 486, 346–352.
Paik, S., Shak, S., Tang, G., Kim, C., Baker, J., Cronin, M., Baehner, F. L., Walker, M. G., Watson, D., Park, T., Hiller, W., Fisher, E. R., Wickerham, D. L., Bryant, J., & Wolmark, N. (2004). A multigene assay to predict recurrence of tamoxifen-treated, node-negative breast cancer. The New England Journal of Medicine, 351, 2817–2826.
Cardoso, F., van’t Veer, L. J., Bogaerts, J., Slaets, L., Viale, G., Delaloge, S., Pierga, J. Y., Brain, E., Causeret, S., DeLorenzi, M., Glas, A. M., Golfinopoulos, V., Goulioti, T., Knox, S., Matos, E., Meulemans, B., Neijenhuis, P. A., Nitz, U., Passalacqua, R., Ravdin, P., Rubio, I. T., Saghatchian, M., Smilde, T. J., Sotiriou, C., Stork, L., Straehle, C., Thomas, G., Thompson, A. M., van der Hoeven, J. M., Vuylsteke, P., Bernards, R., Tryfonidis, K., Rutgers, E., Piccart, M., & Investigators, M. (2016). 70-gene signature as an aid to treatment decisions in early-stage breast cancer. The New England Journal of Medicine, 375, 717–729.
Rivenbark, A. G., O’Connor, S. M., & Coleman, W. B. (2013). Molecular and cellular heterogeneity in breast cancer: Challenges for personalized medicine. The American Journal of Pathology, 183, 1113–1124.
Miele, L. (2006). Notch signaling. Clinical Cancer Research, 12, 1074–1079.
Yao, K., Rizzo, P., Rajan, P., Albain, K., Rychlik, K., Shah, S., & Miele, L. (2011). Notch-1 and notch-4 receptors as prognostic markers in breast cancer. International Journal of Surgical Pathology, 19, 607–613.
Bednarz-Knoll, N., Efstathiou, A., Gotzhein, F., Wikman, H., Mueller, V., Kang, Y., & Pantel, K. (2016). Potential involvement of Jagged1 in metastatic progression of human breast carcinomas. Clinical Chemistry, 62, 378–386.
Reedijk, M., Odorcic, S., Chang, L., Zhang, H., Miller, N., McCready, D. R., Lockwood, G., & Egan, S. E. (2005). High-level coexpression of JAG1 and NOTCH1 is observed in human breast cancer and is associated with poor overall survival. Cancer Research, 65, 8530–8537.
Dickson, B. C., Mulligan, A. M., Zhang, H., Lockwood, G., O’Malley, F. P., Egan, S. E., & Reedijk, M. (2007). High-level JAG1 mRNA and protein predict poor outcome in breast cancer. Modern Pathology, 20, 685–693.
Reedijk, M., Pinnaduwage, D., Dickson, B. C., Mulligan, A. M., Zhang, H., Bull, S. B., O’Malley, F. P., Egan, S. E., & Andrulis, I. L. (2008). JAG1 expression is associated with a basal phenotype and recurrence in lymph node-negative breast cancer. Breast Cancer Research and Treatment, 111, 439–448.
Pece, S., Serresi, M., Santolini, E., Capra, M., Hulleman, E., Galimberti, V., Zurrida, S., Maisonneuve, P., Viale, G., & Di Fiore, P. P. (2004). Loss of negative regulation by Numb over Notch is relevant to human breast carcinogenesis. The Journal of Cell Biology, 167, 215–221.
O’Neill, C. F., Urs, S., Cinelli, C., Lincoln, A., Nadeau, R. J., Leon, R., Toher, J., Mouta-Bellum, C., Friesel, R. E., & Liaw, L. (2007). Notch2 signaling induces apoptosis and inhibits human MDA-MB-231 xenograft growth. The American Journal of Pathology, 171, 1023–1036.
Parr, C., Watkins, G., & Jiang, W. G. (2004). The possible correlation of Notch-1 and Notch-2 with clinical outcome and tumour clinicopathological parameters in human breast cancer. International Journal of Molecular Medicine, 14, 779–786.
Rizzo, P., Miao, H., D’Souza, G., Osipo, C., Song, L. L., Yun, J., Zhao, H., Mascarenhas, J., Wyatt, D., Antico, G., Hao, L., Yao, K., Rajan, P., Hicks, C., Siziopikou, K., Selvaggi, S., Bashir, A., Bhandari, D., Marchese, A., Lendahl, U., Qin, J. Z., Tonetti, D. A., Albain, K., Nickoloff, B. J., & Miele, L. (2008). Cross-talk between notch and the estrogen receptor in breast cancer suggests novel therapeutic approaches. Cancer Research, 68, 5226–5235.
Soares, R., Balogh, G., Guo, S., Gartner, F., Russo, J., & Schmitt, F. (2004). Evidence for the notch signaling pathway on the role of estrogen in angiogenesis. Molecular Endocrinology, 18, 2333–2343.
Pupo, M., Pisano, A., Abonante, S., Maggiolini, M., & Musti, A. M. (2014). GPER activates Notch signaling in breast cancer cells and cancer-associated fibroblasts (CAFs). The International Journal of Biochemistry & Cell Biology, 46, 56–67.
Martz, C. A., Ottina, K. A., Singleton, K. R., Jasper, J. S., Wardell, S. E., Peraza-Penton, A., Anderson, G. R., Winter, P. S., Wang, T., Alley, H. M., Kwong, L. N., Cooper, Z. A., Tetzlaff, M., Chen, P. L., Rathmell, J. C., Flaherty, K. T., Wargo, J. A., McDonnell, D. P., Sabatini, D. M., & Wood, K. C. (2014). Systematic identification of signaling pathways with potential to confer anticancer drug resistance. Science Signaling, 7, ra121.
Hao, L., Rizzo, P., Osipo, C., Pannuti, A., Wyatt, D., Cheung, L. W., Sonenshein, G., Osborne, B. A., & Miele, L. (2010). Notch-1 activates estrogen receptor-alpha-dependent transcription via IKKalpha in breast cancer cells. Oncogene, 29, 201–213.
Assender, J. W., Gee, J. M., Lewis, I., Ellis, I. O., Robertson, J. F., & Nicholson, R. I. (2007). Protein kinase C isoform expression as a predictor of disease outcome on endocrine therapy in breast cancer. Journal of Clinical Pathology, 60, 1216–1221.
Yun, J., Pannuti, A., Espinoza, I., Zhu, H., Hicks, C., Zhu, X., Caskey, M., Rizzo, P., D’Souza, G., Backus, K., Denning, M. F., Coon, J., Sun, M., Bresnick, E. H., Osipo, C., Wu, J., Strack, P. R., Tonetti, D. A., & Miele, L. (2013). Crosstalk between PKCalpha and Notch-4 in endocrine-resistant breast cancer cells. Oncogene, 2, e60.
Faronato, M., Nguyen, V. T., Patten, D. K., Lombardo, Y., Steel, J. H., Patel, N., Woodley, L., Shousha, S., Pruneri, G., Coombes, R. C., & Magnani, L. (2015). DMXL2 drives epithelial to mesenchymal transition in hormonal therapy resistant breast cancer through Notch hyper-activation. Oncotarget, 6, 22467–22479.
Lombardo, Y., Faronato, M., Filipovic, A., Vircillo, V., Magnani, L., & Coombes, R. C. (2014). Nicastrin and Notch4 drive endocrine therapy resistance and epithelial to mesenchymal transition in MCF7 breast cancer cells. Breast Cancer Research, 16, R62.
Haughian, J. M., Pinto, M. P., Harrell, J. C., Bliesner, B. S., Joensuu, K. M., Dye, W. W., Sartorius, C. A., Tan, A. C., Heikkila, P., Perou, C. M., & Horwitz, K. B. (2012). Maintenance of hormone responsiveness in luminal breast cancers by suppression of Notch. Proceedings of the National Academy of Sciences of the United States of America, 109, 2742–2747.
Magnani, L., Stoeck, A., Zhang, X., Lanczky, A., Mirabella, A. C., Wang, T. L., Gyorffy, B., & Lupien, M. (2013). Genome-wide reprogramming of the chromatin landscape underlies endocrine therapy resistance in breast cancer. Proceedings of the National Academy of Sciences of the United States of America, 110, E1490–E1499.
Reya, T., Morrison, S. J., Clarke, M. F., & Weissman, I. L. (2001). Stem cells, cancer, and cancer stem cells. Nature, 414, 105–111.
Harrison, H., Simoes, B. M., Rogerson, L., Howell, S. J., Landberg, G., & Clarke, R. B. (2013). Oestrogen increases the activity of oestrogen receptor negative breast cancer stem cells through paracrine EGFR and Notch signalling. Breast Cancer Research, 15, R21.
Simoes, B. M., Piva, M., Iriondo, O., Comaills, V., Lopez-Ruiz, J. A., Zabalza, I., Mieza, J. A., Acinas, O., & Vivanco, M. D. (2011). Effects of estrogen on the proportion of stem cells in the breast. Breast Cancer Research and Treatment, 129, 23–35.
Cariati, M., Naderi, A., Brown, J. P., Smalley, M. J., Pinder, S. E., Caldas, C., & Purushotham, A. D. (2008). Alpha-6 integrin is necessary for the tumourigenicity of a stem cell-like subpopulation within the MCF7 breast cancer cell line. International Journal of Cancer, 122, 298–304.
Creighton, C. J., Li, X., Landis, M., Dixon, J. M., Neumeister, V. M., Sjolund, A., Rimm, D. L., Wong, H., Rodriguez, A., Herschkowitz, J. I., Fan, C., Zhang, X., He, X., Pavlick, A., Gutierrez, M. C., Renshaw, L., Larionov, A. A., Faratian, D., Hilsenbeck, S. G., Perou, C. M., Lewis, M. T., Rosen, J. M., & Chang, J. C. (2009). Residual breast cancers after conventional therapy display mesenchymal as well as tumor-initiating features. Proceedings of the National Academy of Sciences of the United States of America, 106, 13820–13825.
Kabos, P., Haughian, J. M., Wang, X., Dye, W. W., Finlayson, C., Elias, A., Horwitz, K. B., & Sartorius, C. A. (2011). Cytokeratin 5 positive cells represent a steroid receptor negative and therapy resistant subpopulation in luminal breast cancers. Breast Cancer Research and Treatment, 128, 45–55.
Harrison, H., Farnie, G., Howell, S. J., Rock, R. E., Stylianou, S., Brennan, K. R., Bundred, N. J., & Clarke, R. B. (2010). Regulation of breast cancer stem cell activity by signaling through the Notch4 receptor. Cancer Research, 70, 709–718.
Simoes, B. M., O’Brien, C. S., Eyre, R., Silva, A., Yu, L., Sarmiento-Castro, A., Alferez, D. G., Spence, K., Santiago-Gomez, A., Chemi, F., Acar, A., Gandhi, A., Howell, A., Brennan, K., Ryden, L., Catalano, S., Ando, S., Gee, J., Ucar, A., Sims, A. H., Marangoni, E., Farnie, G., Landberg, G., Howell, S. J., & Clarke, R. B. (2015). Anti-estrogen resistance in human breast tumors is driven by JAG1-NOTCH4-dependent cancer stem cell activity. Cell Reports, 12, 1968–1977.
Sansone, P., Ceccarelli, C., Berishaj, M., Chang, Q., Rajasekhar, V. K., Perna, F., Bowman, R. L., Vidone, M., Daly, L., Nnoli, J., Santini, D., Taffurelli, M., Shih, N. N., Feldman, M., Mao, J. J., Colameco, C., Chen, J., DeMichele, A., Fabbri, N., Healey, J. H., Cricca, M., Gasparre, G., Lyden, D., Bonafe, M., & Bromberg, J. (2016). Self-renewal of CD133(hi) cells by IL6/Notch3 signalling regulates endocrine resistance in metastatic breast cancer. Nature Communications, 7, 10442.
Siegel, R. L., Miller, K. D., & Jemal, A. (2016). Cancer statistics, 2016. CA: a Cancer Journal for Clinicians, 66, 7–30.
Evans, T., Sany, O., Pearmain, P., Ganesan, R., Blann, A., & Sundar, S. (2011). Differential trends in the rising incidence of endometrial cancer by type: Data from a UK population-based registry from 1994 to 2006. British Journal of Cancer, 104, 1505–1510.
Amant, F., Moerman, P., Neven, P., Timmerman, D., Van Limbergen, E., & Vergote, I. (2005). Endometrial cancer. Lancet, 366, 491–505.
Bansal, N., Yendluri, V., & Wenham, R. M. (2009). The molecular biology of endometrial cancers and the implications for pathogenesis, classification, and targeted therapies. Cancer Control, 16, 8–13.
Hecht, J. L., & Mutter, G. L. (2006). Molecular and pathologic aspects of endometrial carcinogenesis. Journal of Clinical Oncology, 24, 4783–4791.
Cobellis, L., Caprio, F., Trabucco, E., Mastrogiacomo, A., Coppola, G., Manente, L., Colacurci, N., De Falco, M., & De Luca, A. (2008). The pattern of expression of Notch protein members in normal and pathological endometrium. Journal of Anatomy, 213, 464–472.
Mitsuhashi, Y., Horiuchi, A., Miyamoto, T., Kashima, H., Suzuki, A., & Shiozawa, T. (2012). Prognostic significance of Notch signalling molecules and their involvement in the invasiveness of endometrial carcinoma cells. Histopathology, 60, 826–837.
Van Sinderen, M., Cuman, C., Gamage, T., Rainczuk, K., Osianlis, T., Rombauts, L., & Dimitriadis, E. (2014). Localisation of the Notch family in the human endometrium of fertile and infertile women. Journal of Molecular Histology, 45, 697–706.
Jonusiene, V., Sasnauskiene, A., Lachej, N., Kanopiene, D., Dabkeviciene, D., Sasnauskiene, S., Kazbariene, B., & Didziapetriene, J. (2013). Down-regulated expression of Notch signaling molecules in human endometrial cancer. Medical Oncology, 30, 438.
Sasnauskiene, A., Jonusiene, V., Krikstaponiene, A., Butkyte, S., Dabkeviciene, D., Kanopiene, D., Kazbariene, B., & Didziapetriene, J. (2014). NOTCH1, NOTCH3, NOTCH4, and JAG2 protein levels in human endometrial cancer. Medicina (Kaunas, Lithuania), 50, 14–18.
Jurcevic, S., Olsson, B., & Klinga-Levan, K. (2014). MicroRNA expression in human endometrial adenocarcinoma. Cancer Cell International, 14, 88.
Cai, S., Zhang, Y. X., Han, K., & Ding, Y. Q. (2017). Expressions and clinical significance of COX-2, VEGF-C, and EFGR in endometrial carcinoma. Archives of Gynecology and Obstetrics, 296, 93–98.
Shang, C., Lang, B., & Meng, L. R. (2016). Blocking NOTCH pathway can enhance the effect of EGFR inhibitor through targeting CD133+ endometrial cancer cells. Cancer Biology & Therapy, 19(2), 113–119.
Gotte, M., Greve, B., Kelsch, R., Muller-Uthoff, H., Weiss, K., Kharabi Masouleh, B., Sibrowski, W., Kiesel, L., & Buchweitz, O. (2011). The adult stem cell marker Musashi-1 modulates endometrial carcinoma cell cycle progression and apoptosis via Notch-1 and p21WAF1/CIP1. International Journal of Cancer, 129, 2042–2049.
Benetatos, L., Hatzimichael, E., Londin, E., Vartholomatos, G., Loher, P., Rigoutsos, I., & Briasoulis, E. (2013). The microRNAs within the DLK1-DIO3 genomic region: Involvement in disease pathogenesis. Cellular and Molecular Life Sciences, 70, 795–814.
Gao, Y., Liu, T., & Huang, Y. (2015). MicroRNA-134 suppresses endometrial cancer stem cells by targeting POGLUT1 and Notch pathway proteins. FEBS Letters, 589, 207–214.
Eskenazi, B., & Warner, M. L. (1997). Epidemiology of endometriosis. Obstetrics and Gynecology Clinics of North America, 24, 235–258.
Ramathal, C. Y., Bagchi, I. C., Taylor, R. N., & Bagchi, M. K. (2010). Endometrial decidualization: Of mice and men. Seminars in Reproductive Medicine, 28, 17–26.
Afshar, Y., Miele, L., & Fazleabas, A. T. (2012). Notch1 is regulated by chorionic gonadotropin and progesterone in endometrial stromal cells and modulates decidualization in primates. Endocrinology, 153, 2884–2896.
Afshar, Y., Jeong, J. W., Roqueiro, D., DeMayo, F., Lydon, J., Radtke, F., Radnor, R., Miele, L., & Fazleabas, A. (2012). Notch1 mediates uterine stromal differentiation and is critical for complete decidualization in the mouse. The FASEB Journal, 26, 282–294.
Su, R. W., Strug, M. R., Joshi, N. R., Jeong, J. W., Miele, L., Lessey, B. A., Young, S. L., & Fazleabas, A. T. (2015). Decreased Notch pathway signaling in the endometrium of women with endometriosis impairs decidualization. The Journal of Clinical Endocrinology and Metabolism, 100, E433–E442.
Brar, A. K., Handwerger, S., Kessler, C. A., & Aronow, B. J. (2001). Gene induction and categorical reprogramming during in vitro human endometrial fibroblast decidualization. Physiological Genomics, 7, 135–148.
National Cancer Institute SEER Program website at https://seer.cancer.gov/statfacts/html/ovary.html, accessed on 21 June 2017.
Koshiyama, M., Matsumura, N., & Konishi, I. (2017). Subtypes of ovarian cancer and ovarian cancer screening. Diagnostics (Basel), Mar 2;7(1). pii: E12. doi: https://doi.org/10.3390/diagnostics7010012. Review.PMID:28257098
Kupryjanczyk, J., Thor, A. D., Beauchamp, R., Merritt, V., Edgerton, S. M., Bell, D. A., & Yandell, D. W. (1993). p53 gene mutations and protein accumulation in human ovarian cancer. Proceedings of the National Academy of Sciences of the United States of America, 90, 4961–4965.
Obata, K., Morland, S. J., Watson, R. H., Hitchcock, A., Chenevix-Trench, G., Thomas, E. J., & Campbell, I. G. (1998). Frequent PTEN/MMAC mutations in endometrioid but not serous or mucinous epithelial ovarian tumors. Cancer Research, 58, 2095–2097.
Ford, D., Easton, D. F., Stratton, M., Narod, S., Goldgar, D., Devilee, P., Bishop, D. T., Weber, B., Lenoir, G., Chang-Claude, J., Sobol, H., Teare, M. D., Struewing, J., Arason, A., Scherneck, S., Peto, J., Rebbeck, T. R., Tonin, P., Neuhausen, S., Barkardottir, R., Eyfjord, J., Lynch, H., Ponder, B. A., Gayther, S. A., Zelada-Hedman, M., et al. (1998). Genetic heterogeneity and penetrance analysis of the BRCA1 and BRCA2 genes in breast cancer families. The Breast Cancer Linkage Consortium. American Journal of Human Genetics, 62, 676–689.
Kote-Jarai, Z., & Eeles, R. A. (1999). BRCA1, BRCA2 and their possible function in DNA damage response. British Journal of Cancer, 81, 1099–1102.
Philp, A. J., Campbell, I. G., Leet, C., Vincan, E., Rockman, S. P., Whitehead, R. H., Thomas, R. J., & Phillips, W. A. (2001). The phosphatidylinositol 3′-kinase p85alpha gene is an oncogene in human ovarian and colon tumors. Cancer Research, 61, 7426–7429.
Cheng, J. Q., Godwin, A. K., Bellacosa, A., Taguchi, T., Franke, T. F., Hamilton, T. C., Tsichlis, P. N., & Testa, J. R. (1992). AKT2, a putative oncogene encoding a member of a subfamily of protein-serine/threonine kinases, is amplified in human ovarian carcinomas. Proceedings of the National Academy of Sciences of the United States of America, 89, 9267–9271.
Kohler, M., Janz, I., Wintzer, H. O., Wagner, E., & Bauknecht, T. (1989). The expression of EGF receptors, EGF-like factors and c-myc in ovarian and cervical carcinomas and their potential clinical significance. Anticancer Research, 9, 1537–1547.
Tashiro, H., Miyazaki, K., Okamura, H., Iwai, A., & Fukumoto, M. (1992). c-myc over-expression in human primary ovarian tumours: Its relevance to tumour progression. International Journal of Cancer, 50, 828–833.
Enomoto, T., Weghorst, C. M., Inoue, M., Tanizawa, O., & Rice, J. M. (1991). K-ras activation occurs frequently in mucinous adenocarcinomas and rarely in other common epithelial tumors of the human ovary. The American Journal of Pathology, 139, 777–785.
Slamon, D. J., Godolphin, W., Jones, L. A., Holt, J. A., Wong, S. G., Keith, D. E., Levin, W. J., Stuart, S. G., Udove, J., Ullrich, A., et al. (1989). Studies of the HER-2/neu proto-oncogene in human breast and ovarian cancer. Science, 244, 707–712.
Hopfer, O., Zwahlen, D., Fey, M. F., & Aebi, S. (2005). The Notch pathway in ovarian carcinomas and adenomas. British Journal of Cancer, 93, 709–718.
Rose, S. L., Kunnimalaiyaan, M., Drenzek, J., & Seiler, N. (2010). Notch 1 signaling is active in ovarian cancer. Gynecologic Oncology, 117, 130–133.
Wang, M., Wang, J., Wang, L., Wu, L., & Xin, X. (2010). Notch1 expression correlates with tumor differentiation status in ovarian carcinoma. Medical Oncology, 27, 1329–1335.
Wang, H., Huang, X., Zhang, J., Shao, N., Chen, L. O., Ma, D., & Ji, C. (2014). The expression of VEGF and Dll4/Notch pathway molecules in ovarian cancer. Clinica Chimica Acta, 436, 243–248.
Oktem, G., Sanci, M., Bilir, A., Yildirim, Y., Kececi, S. D., Ayla, S., & Inan, S. (2012). Cancer stem cell and embryonic development-associated molecules contribute to prognostic significance in ovarian cancer. International Journal of Gynecological Cancer, 22, 23–29.
Kluk, M. J., Ashworth, T., Wang, H., Knoechel, B., Mason, E. F., Morgan, E. A., Dorfman, D., Pinkus, G., Weigert, O., Hornick, J. L., Chirieac, L. R., Hirsch, M., Oh, D. J., South, A. P., Leigh, I. M., Pourreyron, C., Cassidy, A. J., Deangelo, D. J., Weinstock, D. M., Krop, I. E., Dillon, D., Brock, J. E., Lazar, A. J., Peto, M., Cho, R. J., Stoeck, A., Haines, B. B., Sathayanrayanan, S., Rodig, S., & Aster, J. C. (2013). Gauging NOTCH1 activation in cancer using immunohistochemistry. PLoS One, 8, e67306.
Szasz, A. M., Lanczky, A., Nagy, A., Forster, S., Hark, K., Green, J. E., Boussioutas, A., Busuttil, R., Szabo, A., & Gyorffy, B. (2016). Cross-validation of survival associated biomarkers in gastric cancer using transcriptomic data of 1,065 patients. Oncotarget, 7, 49322–49333.
Chen, C., Wang, X., Huang, S., Wang, L., Han, L., & Yu, S. (2017). Prognostic roles of Notch receptor mRNA expression in human ovarian cancer. Oncotarget, 8, 32731–32740.
Er, T. K., Su, Y. F., Wu, C. C., Chen, C. C., Wang, J., Hsieh, T. H., Herreros-Villanueva, M., Chen, W. T., Chen, Y. T., Liu, T. C., Chen, H. S., & Tsai, E. M. (2016). Targeted next-generation sequencing for molecular diagnosis of endometriosis-associated ovarian cancer. Journal of Molecular Medicine (Berlin), 94, 835–847.
Lu, K. H., Patterson, A. P., Wang, L., Marquez, R. T., Atkinson, E. N., Baggerly, K. A., Ramoth, L. R., Rosen, D. G., Liu, J., Hellstrom, I., Smith, D., Hartmann, L., Fishman, D., Berchuck, A., Schmandt, R., Whitaker, R., Gershenson, D. M., Mills, G. B., & Bast, R. C., Jr. (2004). Selection of potential markers for epithelial ovarian cancer with gene expression arrays and recursive descent partition analysis. Clinical Cancer Research, 10, 3291–3300.
Euer, N. I., Kaul, S., Deissler, H., Mobus, V. J., Zeillinger, R., & Weidle, U. H. (2005). Identification of L1CAM, Jagged2 and Neuromedin U as ovarian cancer-associated antigens. Oncology Reports, 13, 375–387.
Park, J. T., Li, M., Nakayama, K., Mao, T. L., Davidson, B., Zhang, Z., Kurman, R. J., Eberhart, C. G., Shih Ie, M., & Wang, T. L. (2006). Notch3 gene amplification in ovarian cancer. Cancer Research, 66, 6312–6318.
Nakayama, K., Nakayama, N., Jinawath, N., Salani, R., Kurman, R. J., Shih Ie, M., & Wang, T. L. (2007). Amplicon profiles in ovarian serous carcinomas. International Journal of Cancer, 120, 2613–2617.
Jung, S. G., Kwon, Y. D., Song, J. A., Back, M. J., Lee, S. Y., Lee, C., Hwang, Y. Y., & An, H. J. (2010). Prognostic significance of Notch 3 gene expression in ovarian serous carcinoma. Cancer Science, 101, 1977–1983.
Rahman, M. T., Nakayama, K., Rahman, M., Katagiri, H., Katagiri, A., Ishibashi, T., Ishikawa, M., Iida, K., Nakayama, S., Otsuki, Y., & Miyazaki, K. (2012). Notch3 overexpression as potential therapeutic target in advanced stage chemoresistant ovarian cancer. American Journal of Clinical Pathology, 138, 535–544.
Choi, J. H., Park, J. T., Davidson, B., Morin, P. J., Shih Ie, M., & Wang, T. L. (2008). Jagged-1 and Notch3 juxtacrine loop regulates ovarian tumor growth and adhesion. Cancer Research, 68, 5716–5723.
Hu, W., Liu, T., Ivan, C., Sun, Y., Huang, J., Mangala, L. S., Miyake, T., Dalton, H. J., Pradeep, S., Rupaimoole, R., Previs, R. A., Han, H. D., Bottsford-Miller, J., Zand, B., Kang, Y., Pecot, C. V., Nick, A. M., Wu, S. Y., Lee, J. S., Sehgal, V., Ram, P., Liu, J., Tucker, S. L., Lopez-Berestein, G., Baggerly, K. A., Coleman, R. L., & Sood, A. K. (2014). Notch3 pathway alterations in ovarian cancer. Cancer Research, 74, 3282–3293.
Park, J. T., Shih Ie, M., & Wang, T. L. (2008). Identification of Pbx1, a potential oncogene, as a Notch3 target gene in ovarian cancer. Cancer Research, 68, 8852–8860.
Chen, X., Thiaville, M. M., Chen, L., Stoeck, A., Xuan, J., Gao, M., Shih Ie, M., & Wang, T. L. (2012). Defining NOTCH3 target genes in ovarian cancer. Cancer Research, 72, 2294–2303.
Ivan, C., Hu, W., Bottsford-Miller, J., Zand, B., Dalton, H. J., Liu, T., Huang, J., Nick, A. M., Lopez-Berestein, G., Coleman, R. L., Baggerly, K. A., & Sood, A. K. (2013). Epigenetic analysis of the Notch superfamily in high-grade serous ovarian cancer. Gynecologic Oncology, 128, 506–511.
Jung, J. G., Stoeck, A., Guan, B., Wu, R. C., Zhu, H., Blackshaw, S., Shih Ie, M., & Wang, T. L. (2014). Notch3 interactome analysis identified WWP2 as a negative regulator of Notch3 signaling in ovarian cancer. PLoS Genetics, 10, e1004751.
Phng, L. K., Potente, M., Leslie, J. D., Babbage, J., Nyqvist, D., Lobov, I., Ondr, J. K., Rao, S., Lang, R. A., Thurston, G., & Gerhardt, H. (2009). Nrarp coordinates endothelial Notch and Wnt signaling to control vessel density in angiogenesis. Developmental Cell, 16, 70–82.
Li, J. L., Sainson, R. C., Shi, W., Leek, R., Harrington, L. S., Preusser, M., Biswas, S., Turley, H., Heikamp, E., Hainfellner, J. A., & Harris, A. L. (2007). Delta-like 4 Notch ligand regulates tumor angiogenesis, improves tumor vascular function, and promotes tumor growth in vivo. Cancer Research, 67, 11244–11253.
Favre, C. J., Mancuso, M., Maas, K., McLean, J. W., Baluk, P., & McDonald, D. M. (2003). Expression of genes involved in vascular development and angiogenesis in endothelial cells of adult lung. American Journal of Physiology. Heart and Circulatory Physiology, 285, H1917–H1938.
Hofmann, J. J., & Iruela-Arispe, M. L. (2007). Notch signaling in blood vessels: Who is talking to whom about what? Circulation Research, 100, 1556–1568.
Villa, N., Walker, L., Lindsell, C. E., Gasson, J., Iruela-Arispe, M. L., & Weinmaster, G. (2001). Vascular expression of Notch pathway receptors and ligands is restricted to arterial vessels. Mechanisms of Development, 108, 161–164.
Xie, Q., Cheng, Z., Chen, X., Lobe, C. G., & Liu, J. (2017). The role of Notch signalling in ovarian angiogenesis. Journal of Ovarian Research, 10, 13.
Vorontchikhina, M. A., Zimmermann, R. C., Shawber, C. J., Tang, H., & Kitajewski, J. (2005). Unique patterns of Notch1, Notch4 and Jagged1 expression in ovarian vessels during folliculogenesis and corpus luteum formation. Gene Expression Patterns, 5, 701–709.
Lu, C., Bonome, T., Li, Y., Kamat, A. A., Han, L. Y., Schmandt, R., Coleman, R. L., Gershenson, D. M., Jaffe, R. B., Birrer, M. J., & Sood, A. K. (2007). Gene alterations identified by expression profiling in tumor-associated endothelial cells from invasive ovarian carcinoma. Cancer Research, 67, 1757–1768.
Benedito, R., Roca, C., Sorensen, I., Adams, S., Gossler, A., Fruttiger, M., & Adams, R. H. (2009). The notch ligands Dll4 and Jagged1 have opposing effects on angiogenesis. Cell, 137, 1124–1135.
Kuhnert, F., Chen, G., Coetzee, S., Thambi, N., Hickey, C., Shan, J., Kovalenko, P., Noguera-Troise, I., Smith, E., Fairhurst, J., Andreev, J., Kirshner, J. R., Papadopoulos, N., & Thurston, G. (2015). Dll4 blockade in stromal cells mediates antitumor effects in preclinical models of ovarian cancer. Cancer Research, 75, 4086–4096.
Patel, N. S., Li, J. L., Generali, D., Poulsom, R., Cranston, D. W., & Harris, A. L. (2005). Up-regulation of delta-like 4 ligand in human tumor vasculature and the role of basal expression in endothelial cell function. Cancer Research, 65, 8690–8697.
Trindade, A., Djokovic, D., Gigante, J., Mendonca, L., & Duarte, A. (2017). Endothelial Dll4 overexpression reduces vascular response and inhibits tumor growth and metastasization in vivo. BMC Cancer, 17, 189.
Hu, W., Lu, C., Dong, H. H., Huang, J., Shen, D. Y., Stone, R. L., Nick, A. M., Shahzad, M. M., Mora, E., Jennings, N. B., Lee, S. J., Roh, J. W., Matsuo, K., Nishimura, M., Goodman, B. W., Jaffe, R. B., Langley, R. R., Deavers, M. T., Lopez-Berestein, G., Coleman, R. L., & Sood, A. K. (2011). Biological roles of the Delta family Notch ligand Dll4 in tumor and endothelial cells in ovarian cancer. Cancer Research, 71, 6030–6039.
Shah, M. M., Zerlin, M., Li, B. Y., Herzog, T. J., Kitajewski, J. K., & Wright, J. D. (2013). The role of Notch and gamma-secretase inhibition in an ovarian cancer model. Anticancer Research, 33, 801–808.
Caliceti, C., Aquila, G., Pannella, M., Morelli, M. B., Fortini, C., Pinton, P., Bonora, M., Hrelia, S., Pannuti, A., Miele, L., Rizzo, P., & Ferrari, R. (2013). 17beta-estradiol enhances signalling mediated by VEGF-A-delta-like ligand 4-notch1 axis in human endothelial cells. PLoS One, 8, e71440.
Lupia, M., & Cavallaro, U. (2017). Ovarian cancer stem cells: Still an elusive entity? Molecular Cancer, 16, 64.
Ruscito, I., Cacsire Castillo-Tong, D., Vergote, I., Ignat, I., Stanske, M., Vanderstichele, A., Ganapathi, R. N., Glajzer, J., Kulbe, H., Trillsch, F., Mustea, A., Kreuzinger, C., Benedetti Panici, P., Gourley, C., Gabra, H., Kessler, M., Sehouli, J., Darb-Esfahani, S., & Braicu, E. I. (2017). Exploring the clonal evolution of CD133/aldehyde-dehydrogenase-1 (ALDH1)-positive cancer stem-like cells from primary to recurrent high-grade serous ovarian cancer (HGSOC). A study of the Ovarian Cancer Therapy-Innovative Models Prolong Survival (OCTIPS) Consortium. European Journal of Cancer, 79, 214–225.
Bapat, S. A., Mali, A. M., Koppikar, C. B., & Kurrey, N. K. (2005). Stem and progenitor-like cells contribute to the aggressive behavior of human epithelial ovarian cancer. Cancer Research, 65, 3025–3029.
Zhang, S., Balch, C., Chan, M. W., Lai, H. C., Matei, D., Schilder, J. M., Yan, P. S., Huang, T. H., & Nephew, K. P. (2008). Identification and characterization of ovarian cancer-initiating cells from primary human tumors. Cancer Research, 68, 4311–4320.
Foster, R., Buckanovich, R. J., & Rueda, B. R. (2013). Ovarian cancer stem cells: Working towards the root of stemness. Cancer Letters, 338, 147–157.
Ottevanger, P. B. (2017). Ovarian cancer stem cells more questions than answers. Seminars in Cancer Biology, 44, 67–71.
Iqbal, W., Alkarim, S., AlHejin, A., Mukhtar, H., & Saini, K. S. (2016). Targeting signal transduction pathways of cancer stem cells for therapeutic opportunities of metastasis. Oncotarget, 7, 76337–76353.
Matsui, W. H. (2016). Cancer stem cell signaling pathways. Medicine (Baltimore), 95, S8–S19.
Steg, A. D., Bevis, K. S., Katre, A. A., Ziebarth, A., Dobbin, Z. C., Alvarez, R. D., Zhang, K., Conner, M., & Landen, C. N. (2012). Stem cell pathways contribute to clinical chemoresistance in ovarian cancer. Clinical Cancer Research, 18, 869–881.
Kang, H. G., Kim, D. H., Kim, S. J., Cho, Y., Jung, J., Jang, W., & Chun, K. H. (2016). Galectin-3 supports stemness in ovarian cancer stem cells by activation of the Notch1 intracellular domain. Oncotarget, 7, 68229–68241.
Singh, A., & Settleman, J. (2010). EMT, cancer stem cells and drug resistance: An emerging axis of evil in the war on cancer. Oncogene, 29, 4741–4751.
Marchini, S., Fruscio, R., Clivio, L., Beltrame, L., Porcu, L., Fuso Nerini, I., Cavalieri, D., Chiorino, G., Cattoretti, G., Mangioni, C., Milani, R., Torri, V., Romualdi, C., Zambelli, A., Romano, M., Signorelli, M., di Giandomenico, S., & D’Incalci, M. (2013). Resistance to platinum-based chemotherapy is associated with epithelial to mesenchymal transition in epithelial ovarian cancer. European Journal of Cancer, 49, 520–530.
Gupta, N., Xu, Z., El-Sehemy, A., Steed, H., & Fu, Y. (2013). Notch3 induces epithelial-mesenchymal transition and attenuates carboplatin-induced apoptosis in ovarian cancer cells. Gynecologic Oncology, 130, 200–206.
Lu, L., Wang, J., Wu, Y., Wan, P., & Yang, G. (2016). Rap1A promotes ovarian cancer metastasis via activation of ERK/p38 and notch signaling. Cancer Medicine, 5, 3544–3554.
Espinoza, I., & Miele, L. (2013). Deadly crosstalk: Notch signaling at the intersection of EMT and cancer stem cells. Cancer Letters, 341, 41–45.
Espinoza, I., Pochampally, R., Xing, F., Watabe, K., & Miele, L. (2013). Notch signaling: Targeting cancer stem cells and epithelial-to-mesenchymal transition. OncoTargets and Therapy, 6, 1249–1259.
Pazos, M. C., Abramovich, D., Bechis, A., Accialini, P., Parborell, F., Tesone, M., & Irusta, G. (2017). Gamma secretase inhibitor impairs epithelial-to-mesenchymal transition induced by TGF-beta in ovarian tumor cell lines. Molecular and Cellular Endocrinology, 440, 125–137.
Zhou, J., Jain, S., Azad, A. K., Xu, X., Yu, H. C., Xu, Z., Godbout, R., & Fu, Y. (2016). Notch and TGFbeta form a positive regulatory loop and regulate EMT in epithelial ovarian cancer cells. Cellular Signalling, 28, 838–849.
Steg, A. D., Katre, A. A., Goodman, B., Han, H. D., Nick, A. M., Stone, R. L., Coleman, R. L., Alvarez, R. D., Lopez-Berestein, G., Sood, A. K., & Landen, C. N. (2011). Targeting the notch ligand JAGGED1 in both tumor cells and stroma in ovarian cancer. Clinical Cancer Research, 17, 5674–5685.
Chen, X., Gong, L., Ou, R., Zheng, Z., Chen, J., Xie, F., Huang, X., Qiu, J., Zhang, W., Jiang, Q., Yang, Y., Zhu, H., Shi, Z., & Yan, X. (2016). Sequential combination therapy of ovarian cancer with cisplatin and gamma-secretase inhibitor MK-0752. Gynecologic Oncology, 140, 537–544.
Groeneweg, J. W., DiGloria, C. M., Yuan, J., Richardson, W. S., Growdon, W. B., Sathyanarayanan, S., Foster, R., & Rueda, B. R. (2014). Inhibition of notch signaling in combination with Paclitaxel reduces platinum-resistant ovarian tumor growth. Frontiers in Oncology, 4, 171.
McAuliffe, S. M., Morgan, S. L., Wyant, G. A., Tran, L. T., Muto, K. W., Chen, Y. S., Chin, K. T., Partridge, J. C., Poole, B. B., Cheng, K. H., Daggett, J., Jr., Cullen, K., Kantoff, E., Hasselbatt, K., Berkowitz, J., Muto, M. G., Berkowitz, R. S., Aster, J. C., Matulonis, U. A., & Dinulescu, D. M. (2012). Targeting Notch, a key pathway for ovarian cancer stem cells, sensitizes tumors to platinum therapy. Proceedings of the National Academy of Sciences of the United States of America, 109, E2939–E2948.
Wang, M., Ma, X., Wang, J., Wang, L., & Wang, Y. (2014). Pretreatment with the gamma-secretase inhibitor DAPT sensitizes drug-resistant ovarian cancer cells to cisplatin by downregulation of Notch signaling. International Journal of Oncology, 44, 1401–1409.
Feng, Z., Xu, W., Zhang, C., Liu, M., & Wen, H. (2017). Inhibition of gamma-secretase in Notch1 signaling pathway as a novel treatment for ovarian cancer. Oncotarget, 8, 8215–8225.
Kang, H., Jeong, J. Y., Song, J. Y., Kim, T. H., Kim, G., Huh, J. H., Kwon, A. Y., Jung, S. G., & An, H. J. (2016). Notch3-specific inhibition using siRNA knockdown or GSI sensitizes paclitaxel-resistant ovarian cancer cells. Molecular Carcinogenesis, 55, 1196–1209.
Yen, W. C., Fischer, M. M., Axelrod, F., Bond, C., Cain, J., Cancilla, B., Henner, W. R., Meisner, R., Sato, A., Shah, J., Tang, T., Wallace, B., Wang, M., Zhang, C., Kapoun, A. M., Lewicki, J., Gurney, A., & Hoey, T. (2015). Targeting Notch signaling with a Notch2/Notch3 antagonist (tarextumab) inhibits tumor growth and decreases tumor-initiating cell frequency. Clinical Cancer Research: An Official Journal of the American Association for Cancer Research, 21, 2084–2095.
Huang, J., Hu, W., Hu, L., Previs, R. A., Dalton, H. J., Yang, X. Y., Sun, Y., McGuire, M., Rupaimoole, R., Nagaraja, A. S., Kang, Y., Liu, T., Nick, A. M., Jennings, N. B., Coleman, R. L., Jaffe, R. B., & Sood, A. K. (2016). Dll4 inhibition plus aflibercept markedly reduces ovarian tumor growth. Molecular Cancer Therapeutics, 15, 1344–1352.
Majidinia, M., Alizadeh, E., Yousefi, B., Akbarzadeh, M., Mihanfar, A., Rahmati-Yamchi, M., & Zarghami, N. (2017). Co-inhibition of Notch and NF-kappaB signaling pathway decreases proliferation through downregulating IkappaB-alpha and Hes-1 expression in human ovarian cancer OVCAR-3 cells. Drug Research (Stuttg), 67, 13–19.
Chiorean, E. G., LoRusso, P., Strother, R. M., Diamond, J. R., Younger, A., Messersmith, W. A., Adriaens, L., Liu, L., Kao, R. J., DiCioccio, A. T., Kostic, A., Leek, R., Harris, A., & Jimeno, A. (2015). A phase I first-in-human study of enoticumab (REGN421), a fully human Delta-like ligand 4 (Dll4) monoclonal antibody in patients with advanced solid tumors. Clinical Cancer Research, 21, 2695–2703.
Brana, I., Berger, R., Golan, T., Haluska, P., Edenfield, J., Fiorica, J., Stephenson, J., Martin, L. P., Westin, S., Hanjani, P., Jones, M. B., Almhanna, K., Wenham, R. M., Sullivan, D. M., Dalton, W. S., Gunchenko, A., Cheng, J. D., Siu, L. L., & Gray, J. E. (2014). A parallel-arm phase I trial of the humanised anti-IGF-1R antibody dalotuzumab in combination with the AKT inhibitor MK-2206, the mTOR inhibitor ridaforolimus, or the NOTCH inhibitor MK-0752, in patients with advanced solid tumours. British Journal of Cancer, 111, 1932–1944.
Pant, S., Jones, S. F., Kurkjian, C. D., Infante, J. R., Moore, K. N., Burris, H. A., McMeekin, D. S., Benhadji, K. A., Patel, B. K., Frenzel, M. J., Kursar, J. D., Zamek-Gliszczynski, M. J., Yuen, E. S., Chan, E. M., & Bendell, J. C. (2016). A first-in-human phase I study of the oral Notch inhibitor, LY900009, in patients with advanced cancer. European Journal of Cancer, 56, 1–9.
Richter, S., Bedard, P. L., Chen, E. X., Clarke, B. A., Tran, B., Hotte, S. J., Stathis, A., Hirte, H. W., Razak, A. R., Reedijk, M., Chen, Z., Cohen, B., Zhang, W. J., Wang, L., Ivy, S. P., Moore, M. J., Oza, A. M., Siu, L. L., & McWhirter, E. (2014). A phase I study of the oral gamma secretase inhibitor R04929097 in combination with gemcitabine in patients with advanced solid tumors (PHL-078/CTEP 8575). Investigational New Drugs, 32, 243–249.
Diaz-Padilla, I., Wilson, M. K., Clarke, B. A., Hirte, H. W., Welch, S. A., Mackay, H. J., Biagi, J. J., Reedijk, M., Weberpals, J. I., Fleming, G. F., Wang, L., Liu, G., Zhou, C., Blattler, C., Ivy, S. P., & Oza, A. M. (2015). A phase II study of single-agent RO4929097, a gamma-secretase inhibitor of Notch signaling, in patients with recurrent platinum-resistant epithelial ovarian cancer: A study of the Princess Margaret, Chicago and California phase II consortia. Gynecologic Oncology, 137, 216–222.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2018 Springer Science+Business Media, LLC, part of Springer Nature
About this chapter
Cite this chapter
Crabtree, J.S. (2018). Notch Signaling in Estrogen-Dependent Cancers. In: Miele, L., Artavanis-Tsakonas, S. (eds) Targeting Notch in Cancer. Springer, New York, NY. https://doi.org/10.1007/978-1-4939-8859-4_14
Download citation
DOI: https://doi.org/10.1007/978-1-4939-8859-4_14
Published:
Publisher Name: Springer, New York, NY
Print ISBN: 978-1-4939-8857-0
Online ISBN: 978-1-4939-8859-4
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)