Abstract
Plant cell produces a wide range of chemical compounds needed for its survival. Mostly secondary metabolite and phytochemicals including neurotransmitters are essential for the reallocation of resources in plants in response to changing environmental factors. Among them, dopamine which is a catecholamine neurotransmitter is found in plants as well as in animals. Many plants species of different families were reported to contain significant amounts of dopamine. It mediates many physiological processes in plants. However, the role of dopamine in plants is poorly documented. They are involved in much aspect of growth, development and their synthesis is regulated by stress condition. Studies have addressed the effect of dopamine on plants as allelochemical that provides defence against herbivore, processes such as nitrogen fixation, flowering and prevention against IAA oxidation, intercellular regulation of ion permeability and photophosphorylation of chloroplast. It has been proposed to be a precursor for various alkaloids benzylisoquinolines like papaverine and morphine or of the hallucinogenic alkaloid. In this chapter current knowledge on role of dopamine in plants are documented. Dopamine, noradrenaline and adrenaline were shown to participate in intercellular regulation of ion permeability and photophosphorylation of chloroplasts. Dopamine is involved in many functions like precursor for various alkaloids, antioxidative, sugar metabolism and coordinates with phytohormones to affect plant growth. In this chapter, current knowledge on role of dopamine in plants is documented.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
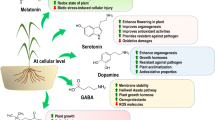
Consumption of various nutrient rich fruits and vegetables is suggested as a potential treatment for depression, due to the presence of psychoactive neurotransmitters such as serotonin, dopamine and melatonin in many plant foods. Plants produce a wide range of phytochemicals that mediate cell function, plant stress and many of these are human neuroregulatory molecules. Neurotransmitters found in plants to date include acetylcholine, epinephrine, dopamine, levodopa, γ-aminobutyrate, γ-aminobutyric acid (GABA), glutamate, indole-3-acetic acid, 5-hydroxyindoleacetic acid, melatonin and serotonin (Kulma and Szopa 2007). In animals, these compounds are stored in neurons and released into a narrow space between connecting cells, the synaptic space, in response to a stimulus. They are involved in many physiological roles in the plants. In plant cells, acetylcholine is produced in the stems, leaves, nodes and roots in response to heat shock (Momonoki and Momonoki 1991). The neurotransmitter GABA accumulates in plants following stress and is involved in pH regulation, nitrogen storage, plant development and act as antiherbivory compounds and provides defence against insects (Shelp et al. 1999, 2003; Kinnersley and Turano 2000). Indoleamines are a class of human neurotransmitters derived from tryptophan that includes serotonin and melatonin. Melatonin (N-acetyl-5-methoxytryptamine) was first isolated from the bovin epineal gland (Lerner et al. 1958). Low quantities of plant melatonin were found in foodstuffs and melatonin is absorbed and active in animal model systems (Hattori et al. 1995; Wurtman et al. 1963; Yu and Reiter 1993). Many studies are on the presence of melatonin in diverse species ranging from mammals, insects, planatians, mollusks, dinoflagellates and algae to grains, vegetables and medical plants. The first identifications of melatonin in plant tissues were made in 1995 (Dubbels et al. 1995; Hattori et al. 1995) and the discovery of melatonin in intact plants was fairly recent (Murch et al. 1997, 2000). More recently melatonin is reported in 108 plant species used in traditional Chinese medicines (Chen et al. 2003; Murch et al 1997). Melatonin is also found in sour cherries (Manchester et al. 2000), variety of foodstuffs (Badria et al. 2002) and walnuts (Reiter 2005). In plants, melatonin respond to light and dark cycle and photoperiod (Reiter et al. 2001). The highly conserved nature of indoleamines and the diversity of species containing melatonin may indicate an important role for the compound in the growth or survival of higher plants (Murch and Saxena 2002). Serotonin is a neurotransmitter that has been identified across all forms. In recent years the presence and function of serotonin in plants (phytoserotonin) are becoming an increasingly active area of research. Serotonin has been found to function as a plant growth regulator and a stress defence molecule. Through these functions serotonin has been implicated in mediation of morphogenesis, vegetative growth, reproductive development, seed germination and survival, abiotic and biotic stress survival and mediation of plant signalling and mediation of plant cycles life (Lauren et al. 2019).
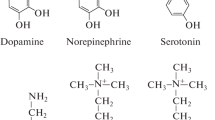
Catecholamines are also found at elevated levels in plant tissues exposed to stress (Swiedrych et al. 2004). Neurohormones, catecholamines, promote flowering in short-day plants (Khurana et al. 1987) and their synthesis is regulated by stress conditions (Swiedrych et al. 2004; Bowen et al. 2018). They are found in 44 plant families including at least 29 species grown for human consumption (Smith 1980) but, little is known about their roles, modes of action, mechanism of detection or the associated biosynthesis and regulatory pathways (Kulma and Szopa 2007). Catecholamines are an amine derived from the amino acid tyrosine that acts as neurotransmitters. They include dopamine, norepinephrine (noradrenaline) along with epinephrine (adrenaline). These are a group of biogenic amines possessing a 3,4-dihydroxy-substituted phenyl ring and widely distributed in animals and well known neurotransmitter in the mammals. The best understood example of the hormonal action epinephrine and non-epinephrine in mammals is glycogen mobilizing function. Some studies with plants have demonstrated that catecholamine has an antioxidative capacity and precursor for various alkaloids (Guinaudeau and Bruneton 1993; Smith 1980). There are reports showing that mucuna metabolizes L-DOPA to dopamine in leaves as a protective mechanism against the toxicity of L-DOPA (Matsumoto 2011). Noradrenaline and dopamine are major bioactive components of Portulaca oleracea, a traditional herbal medicine (Yue et al. 2005). One of catecholamines, i.e. dopamine is a natural product of the catecholamine pathway, widespread in animals especially in mammals (Wang et al. 2018) and has also been detected in many plant families (Kulma and Szopa 2007). In contrast to the vast amount of knowledge about its role in mammals, few reports are found on the physiological significance of dopamine in plants. In plants, studies have shown that effects of dopamine are associated with defence against herbivores, processes such as nitrogen fixation, flowering and prevention of IAA oxidation, intercellular regulation of ion permeability and photophosphorylation of chloroplasts (Weir et al. 2004; Allen 2003; Van Alstyne et al. 2006; Kuklin and Conger 1995a, b; Khurana et al. 1987) (Table 1).
2 Dopamine
This water-soluble molecule was first identified in plants as having strong antioxidative capability that was greater than glutathione, catechin, the flavonol quercetin and the flavone luteolin, and similar to that of gallocatechin gallate and ascorbic acid (Kulma and Szopa 2007). Dopamine influences sugar metabolism and coordinates with phytohormones to affect plant growth (Jung et al. 2000). It can accelerate cell expansion on a growth medium supplemented with indole acetic acid and kinetin but is useless for cells incubated on a basal medium (Protacio et al. 1992). Dopamine is found at high concentration in potato (Solanum tubersum) plants, spathes of Araceae inflorescence (Kulma and Szopa 2007; Kanazawa and Sakakibara 2000), the pulp of yellow banana (Musa acuminate), red banana (Musa sapientum var Baracoa), plantain (Plantago major) and fuerte avocado (Persea americana) (Kanazawa and Sakakibara 2000; Fieldman et al. 1987). It has been proposed to be precursor for various alkaloids benzylisoquinolines like papaverine and morphine or of the hallucinogenic alkaloid mescaline (Lundström and Agurell 1971). Some studies have addressed the effect of dopamine on plants and have revealed that it has attributes typical of an allelochemical (Inderjit and Duke 2003).
3 Dopamine Biosynthesis in Plants
The shikmic acid pathway (Fig. 1) converts simple carbohydrate precursors derived from glycolysis and pentose phosphate pathway to the aromatic amino acid tyrosine, phenylalanine, tryptophan and it participates in the biosynthesis of most plant phenolics (Taiz and Zeiger 2012). L-DOPA is a catecholamine formed by the hydroxylation of tyrosine residues by the copper containing enzyme tyrosine hydroxylase in the company of molecular oxygen and is precursor of many neurologically important molecules such as dopamine, adrenaline and nonadrenaline (Kulma and Szopa 2007).
The compound can undergo tyrosine decarboxylation in presence of tyrosine decarboxylase, resulting in tyramine synthesis. Dopamine is produced via hydroxylation of tyramine or decarboxylation of L-DOPA and dopamine hydroxylation leads to norepinephrine production which in turn methylates to give rise to epinephrine (Kulma and Szopa 2007; Kong et al. 1998; Steiner et al. 1996; Smith 1980). Although both initiating steps are fully active, different plants favour different synthetic routes. For example, in sweet banana (M.sapientum) dopamine originates from tyramine hydroxylation; but in Scotch broom (Cytisus scoparius), peyote cactus (L.williamsi) and callus of Portulacca dopamine formation occurs from DOPA (Lundstrom 1971; Smith 1980). L-DOPA precursor of dopamine is also an essential precursor in the biosynthesis of melanin which is present in much tissue of the plants (Guidotti et al. 2013). The physiological mechanism of action of dopamine is a well known neurotransmitter in mammals. Its synthesis in both plants as well mammals are analogous to each other and absence in nerve cells can cause Parkinson disease (Soares et al. 2007; Soares et al. 2014).
4 Metabolism of Catecholamines Including Dopamine
Methylation, oxidation and conjugation with other phenolic compounds are three pathways involved in metabolism of catecholamine. The methylated derivatives of catecholamine and both 3-methoxy-4-hydroxy mandelic acid and homovanillic acid are the final products of their catabolism in mammalian cell. Mandelic and homovanilc acids are absent in plants. Instead, it contains normethenephrine (Szopa et al. 2001). There is possibility that methylation can serve as a way for catecholamine deactivation. From study of animal cells it is known that methylation causes catecholamine inactivation (Li et al. 2005). Even though there were never extensive studies done, some data suggest that methylated compounds are no longer active in plants, at least in some aspects of their activity (Kamisaka 1979). Various derivatives also get synthesized by the methylation of catecholamine. Extensive studies of the catecholamine metabolism in Dona Ana cactus (Coryphantha macromerix) reveal production and accumulation of various methylated catecholamine derivative. Of these phenylamines normacromerine (N-methyl-3,4-dimethoxy-beta-hydroxyphenethylamine) is by far the most abundant (Keller and Yeary 1980). Dopamine in particular is an intermediate in alkaloid biosynthesis, most importantly of benzylisosoquinolines like paperverine and morphine of the hallucinogenic alkaloid mescaline, identified in many cactus species (Lundstrom 1971).
Studies of plants tissue cultures grown in presence of labelled tyramine and dopamine showed that catabolism of catecholamine also occurs via oxidation and oxidative polymerization (Meyer and Barz 1978). The plant amine oxidase (Medda et al. 1978) acts on monoamines oxidizing them to the corresponding aldehydes and thus participate in amine degradation. One of the more important chemical changes in dopamine oxidation is by lipoxygenase leading to melanin (Rosei et al. 1994). Catecholamine and their derivatives can also form conjugates with phenolic acids, i.e. p-coumaryladrenaline are involved in plant defence (Roepenack et al. 1974). Catecholamine also serves as substrate of other compounds active in plant cells. Catecholamines have been shown to be precursors of a series of tetrahydrobenzylisoquinoline alkaloids. The peyote cactus (Lophophora williams) contains high concentrations of the hallucinogen mescaline which is synthesized after hydroxylation of tyrosine to L-dopa or by decarboxylation to tyramine (Smith 1980).
5 Plant Stress Modulation and Dopamine
Drought stress is often one of the most limiting factors for plant growth. Plant growth is affected by drought in several ways, e.g. reduced leaf water potential, altered plant water and nutrient relationships (Boomsa and Vyn 2008; Pugnaire and Chapin 1992). Studies have shown that drought treatment led to a decline in many parameters and exogenous application of dopamine eased those inhibitory effects (Liang et al. 2018). When potato (Solanum tuberosum) plants are exposed to drought, treatment with abscisic acid or ultraviolet light can significantly increase their concentration of dopamine (Swiedrych et al. 2004). Dopamine treatment mitigates the inhibitory effects of drought on plant growth and helps to maintain strong photosynthesis, chlorophyll level and stomata functioning. The interaction between drought and dopamine indicated that responses of these growth parameters to watering regime were significantly influenced by the application of dopamine. Dopamine has also been identified as a key factor in the growth of Lactuca sativa hypocotyls and its level in potato is also significantly increased under drought conditions (Swiedrych et al. 2004). Transcripts levels of a key cholorophyll degradation gene, pheide a oxygenase and senescence associate gene 12 were elevated upon drought treatment, dopamine significantly suppressed the up regulation of these genes under stress condition (Liang et al. 2018). Exogenous treatments of dopamine inhibit nutrition stress as well as salt-induced stress (Li et al. 2015; Liang et al. 2017). In salt-stressed rice (Oryza sativa), exogenous dopamine regulates the expression of the aquaporin gene OsPIP1-3 (Abdelkader et al. 2012). Dopamine can also alleviate salt-induced stress in apple (Malus hupehensis) (Li et al. 2015). Under salinity stress, activity of tyrosine decarboxylase, a key enzyme in the dopamine synthesis pathway, is enhanced (Swiedrych et al. 2004). Dopamine is coordinated with phytochrome activity to regulate growth and enable plants to fine-tune their stress responses (Kulma and Szopa 2007). These observations indicate that dopamine has an important anti-senescence effect that might be helpful for regulating nutrient uptake, transport and resortion and ultimately influencing overall plant growth. Understanding the role of dopamine in drought tolerance introduces new possibilities to use this compound for agricultural purpose. Some other reports also give the evidence of its function during stress conditions. For example, catecholamine synthesis is much higher in darkness in Portulacca callus. In contrast in the subantartic crucifer Pringlea antiscorbutica, levels of dopamine decrease during heat stress (Hennion and Martin-Tabguy 2000).
6 Dopamine as an Allelochemical
Due to their sessile way of life, plants cannot relocate when environmental conditions become unfavourable; they adopt much survival strategy among which one is allelopathy. They rely upon the release of chemical compounds such as cyanogenic glyocosides, glucosinolates, alkaloids, terpenoids, phenolics and nitrogen containing compounds for their defence strategy (Schoonhoven et al. 2005; Mithofer and Boland 2012). Allelochemicals typically inhibit seed germination and seedling growth. Moreover, they alter several physiological and biochemical processes including water utilization, mineral uptake, foliar expansion, photosynthesis, amino acid metabolism, protein synthesis, glycolysis, mitochondrial respiration and ATP synthesis among others (Weir et al. 2004). Dopamine is one of these compounds and has also been detected in many plant families (Kulma and Szopa 2007; Golisz et al. 2011; Topal and Kocacaliskan 2006). Data accumulated suggest that dopamine can be used as an antiherbivore defence compounds by the green alga Ulvaria obscura (Kathryn et al. 2005).
Velvetbean (Mucuna pruriens) is widely used in tropical regions for intercropping with maize, sorghum and millet and for providing benefits, such as suppression of the nematode population, weed smothering, symbiotic nitrogen fixation, nutrient recycling and control of erosion (Ananya 1999; Soares et al. 2012, 2014; Fuji 2013). Many secondary compounds are produced by velvetbean. Using HPLC coupled with mass spectrometry, dopamine in 2–3-week-old leaves of Mucuna were detected (Wichers et al. 1993). The dopamine content of the leaves even exceeded the content of L-DOPA, the most abundant allelochemical in Mucuna (Jander and de Vos 2011). However, in the roots, stems and seeds, no dopamine could be detected at any stage of development. Mucuna metabolizes L-DOPA to dopamine in leaves as a protective mechanism against the toxicity of L-DOPA (Matsumoto 2011). Dopamine has also been detected in many other plant families. Southern armyworn larvae fed a diet containing seeds of velvet bean or synthetic L-DOPA precursor of dopamine showed an increased mortality (Rehr et al. 1973). Non-protein amino acid accumulates massively in many plants and seems to play an important role in resistance to herbivores (Furstenberg et al. 2013; Huang et al. 2011). There are reports on catecholamines and their derivatives as deterrents to insect predators and foraging animals (Smith 1980; Nishihara et al. 2004). The resistance of sugarbeet (Beta vulgaris) to the fungus Cercospora beticola has been suggested to be due to the presence of dopamine (Odjakova and Hadjiivanova 1997). Catecholamines, and especially dopamine, are involved in the protection against penetration of the outer plant surface. Thus, wounds in some papaver species are sealed by a brown melanin-like substance accompanied by release of dopamine and polyphenolase (Homeyer and Roberts 1984). Wound tissue formation in the saguaro cactus Carnegiea gigantea is accompanied by increase in dopamine concentration (Kuklin and Conger 1995a, b). This compound is exuded from the roots, where its concentration can reach 1 ppm in water culture solution and 50 ppm in the immediate vicinity of the roots. This concentration is high enough to reduce the growth of neighbouring plants. This growth inhibition can even be seen in agar-medium culture in a mixed culture (Fujii et al. 1991). Investigation into kinetics of DOPA in volcanic ash soil, at various pH values showed that L-DOPA is easily absorbed and transformed in the soil. Thus the concentration of allelochemicals bearing catechol moiety in soil may decrease rapidly owing to adsorption and transformation reactions and this decrease will be faster in soil with high pH or high adsorption ability which can result in a reduction in its plant growth inhibitory activity (Furubayashi et al. 2007).
7 Interaction of Dopamine with Plant Hormones
Catecholamine is associated with ethylene production, prevention of 3-indole acetic acid oxidation and Gibberline signalling (Dai et al. 1993; Kuklin and Conger 1995a, b). A threefold increase in the rate of ethylene production from tobacco thin cell layers was observed after inclusion of catecholamines in the medium (Protacio et al. 1992). Exogenous dopamine at concentrations of 5–100 μM stimulates ethylene biosynthesis in illuminated chloroplast lamellae from sugar beet leaves (Elstner et al. 1976). Dopamine, noradrenaline and adrenaline stimulated ethylene production in potato suspension cultures (Dai et al. 1993). According to the studies, dopamine affects plant development by acting with hormones leading in elevated contents of auxin (Protacio et al. 1992). It was shown that dopamine can inhibit IAA oxidation in vitro as well as in vivo via the inhibition of IAA oxidase (Kuklin and Conger 1995a, b). It is known that auxins promote the growth of stems and coleoptile and inhibit the growth of roots. It is likely that roots may require a minimum concentration of auxin to grow, but growth is strongly inhibited by concentrations of auxin required to promote elongation of stems and coleoptiles (Taiz and Zeiger 2012) Thus, if dopamine actually inhibits IAA oxidase, thereby increasing the auxin content and high levels of this hormone in the roots inhibit growth (Guidotti et al. 2013), it is no exaggeration to suggest that this could be one of the modes of action of dopamine applied to the roots of soybean seedlings. Catecholamines have been suggested to play a role in the catabolism of indoleacetic acid (Khurana et al. 1987). As described by Kamisaka (1979) catecholamine stimulate gibberylin action that induces lettuce hipocotyl elongation. The activity of enzymes involved in catecholamine, i.e. tyrosine decarboxylase, tyrosine hydroxylase and L-DOPA decarboxylase was increased in potato leaves treated with abscisic acid (Kulma and Szopa 2007).
8 Dopamine and Photosynthesis
Dopamine, noradrenaline and adrenaline were shown to participate in intercellular regulation of ion permeability and photophosphorylation of chloroplasts due to its reduction power that ends with the scavenging of free radicals (Kuklin and Conger 1995a, b; Roshchina 1990). These experiments are indirect support for the existence of specific receptors for catecholamines. Studies on the effects of applying exogenous dopamine to water-depleted soil investigated its long-term effects on nutrient status and leaf senescence under drought conditions have shown that when stressed apple plants received supplemental dopamine (100 μM), they exhibited improved growth and photosynthesis (Liang et al. 2018). Dopamine regulates chlorophyll concentrations and stomatal behaviour, while also altering the uptake, transport, partitioning, and restoration of nutrients within the whole plant (Liang et al. 2018). Addition of dopamine significantly delayed the process of drought stress-induced leaf senescence. So it is anti-senescence and has a positive influence on drought tolerance and offers new opportunities for its use in agriculture, especially in regions that are challenged by such stress conditions in the field (Liang et al. 2017).
9 Dopamine and Organogenesis
The abundance of aromatic amines and especially of dopamine and tyramine in spathes of Araceae inflorescences (Ponchet et al. 1982) prompts for a role in reproductive organogenesis (Sharma et al. 1987). The spathe is a floral leaf developing before the sex organs and is thus important for reproduction of monocotyledonous plants. Tropical species of the families Philodendroideae and Monsteroideae mostly tropical creepers contain high concentrations of catecholamines in the leaves, stems, adventious roots but highest concentrations were estimated in their inflorescences (Kuklin and Conger 1995a, b). Changes in the growth of roots by dopamine have been reported in a few plant species. Catecholamines caused a stimulation of growth in root cultures of Acmella oppositifolia and Nicotiana tabacum cultures (Protacio et al. 1992). However there are reports on dopamine-induced inhibition in soybean roots via damage caused by reactive oxygen species (Guidotti et al. 2013).
10 Dopamine and Other Cellular Activities
In contrast to the vast amount of knowledge about its role and effects in mammals, little is known about the physiological significance of dopamine in plants. Since the early days of catecholamine discovery, plant researchers have been intrigued by the question of the physiological significance of these compounds. They alter several physiological and biochemical processes including utilization, amino acid metabolism, protein synthesis, glycolysis, mitochondrial respiration and ATP synthesis among others (Weir et al. 2004).
11 Pro-oxidant and Antioxidant Properties of Dopamine Precursor
Catecholamines in animals are known to mediate toxicity by receptor-mediated and oxidative mechanism (Arno 2000). They may also be protective through their antioxidant properties. The balance of this dual nature, therefore, is dictated by ambient conditions such as concentration, pH, oxygen, content and localization.
12 Pro-oxidant Properties
Studies have shown that biosynthesis of melanin due to auto-oxidation of L-DOPA, one of the precursors of dopamine, generate ROS (Hachinohe and Matsumoto 2007, Hachinohe and Matsumoto 2005; Hachinohe et al. 2004; Pattison et al. 2002). As enzymes are not involved, the rate is enhanced by the trace concentrations of Fe3+and Cu2+ ions. The loss of an electron from L-DOPA results the formation of semiquinone radical DOPA-SQ− (Soares et al. 2014). This may be oxidized into dopaquinone (DOPA-Q), an intermediate in the L-DOPA oxidation pathway. DOPA-Q can also be generated by the direct loss of two electrons from L-DOPA by enzymatic reaction. In this way, it has been proposed that the oxidation of L-DOPA may result in damage to other molecule through either direct or indirect responses. DOPA-SQ− can transfer electrons to other molecules or remove hydrogen atoms (Takasaki and Kawakishi 1997). Indirect damage may occur by production of ROS, direct reduction of peroxides or via reduction of molecule O2 to O2− and subsequent dismutation to H2O2 species. In the presence of certain transition metal ion, H2O2 can form HO. Radicals. DOPA-Q can be oxidized and products of this process are indole compounds which can undergo further reactions to form melanin dependent (Pattison et al. 2002). (Fig. 2). Similar studies have indicated the toxic nature of dopamine. Dopamine can be enzymatically or spontaneously metabolized by molecular oxygen in physiological solutions to form ROS, leading to the formation of melanins. These ROS as well as semiquinone and quinine products of catecholamine oxidation can interact with protein, lipids. Nucleic acid and membrane components thus cause cell damage (Guidotti et al. 2013). Incubation of free DOPA, protein-bound DOPA (PB-DOPA) and related catecholamine with DNA, proteins and lipids has been shown to result in oxidative damage to the target molecule (Pattison et al. 2002). Antioxidant and pro-oxidant capacity of catecholamines and related compounds was also observed in pheochromocytoma PC12 cells (Sofic et al. 2001; Soares et al. 2012; Kruk et al. 1999; Pattison et al. 2002).
The ROS was also generated during L-DOPA oxidation and can also cause severe damage to cell proteins. For example, the iron/sulphur complexes of metalloprotein particularly Fe-S enzyme as aconitase and fumrase are rapidly destroyed by O2− with inactivation of the enzymes in the carbon fixation cycle and other metabolic pathways by oxidizing thiol functional groups and also capable of causing peroxidation of lipids and pigments (Dietz 2003). As mentioned above, L-DOPA can be oxidized towards melanin. In this context studies have shown that exposure to L-DOPA led to a greater accumulation of melanin in lettuce than in barnyard grass (Hachnohe and Matsumoto 2007). In barnyard grass, this compound is metabolized to phenylalanine, tyrosine and dopamine which were not observed in lettuce (Hachinohe et al. 2004). This reduces ROS formation and consequently membrane damage caused by lipid peroxidation. In this context reported that L-DOPA (0.1–1.0 mM) increased PPO activity and melanin synthesis (root become black) in soyabean (Soares et al. 2011). The results showed that the increase in the PPO activity was associated with browning root, suggesting that melanin synthesis came from the oxidation of L-DOPA.
13 Antioxidant Properties
Antioxidative properties of dopamine is well established in animals, whereas few reports on plants. High antioxidative potency of dopamine than glutathione, food additives such as butylated hydroxyanisole and hydroxytolune flavones, luteolin, flavonol, quercetin and catechin and similar potency to the strongest antioxidants gallocatechin gallate and ascorbic acid was found (Yasunari et al. 2000). Banana contained dopamine at high levels in both the peel and pulp. Dopamine levels ranged from 100 g in peel and 2.5–10 mg in pulp, even in ripened bananas, ready to eat. Banana is thus one of the antioxidative foods (Kanazawa and Sakakibara 2000). It enables organisms to fight with their stress responses, partly because of its antioxidative properties (Kulma and Szopa 2007). Whereas, L-DOPA has contradictory characteristics with respect to the formation of ROS. Some studies have reported an antioxidant activity of L-DOPA (Marinova and Yanishliev 2004). Importantly, L-DOPA has the structure of a phenolic acid similar to caffeic acid differing only in the presence of an amino group in the aliphatic chain of the latter. L-DOPA is the main phenolic of the seeds of Mucuna spp. When tested for their antioxidant activity it was noted that Mucuna extract showed strong antioxidant activity by decreasing the concentrations of the 1,1-diphenyl-2-picryl-hydrazyl (DPPH.) radical and ROS including nitric oxide (NO) when compared with different standards such as the antioxidant butylated hydroxytoluene (BHT), L-ascorbic acid, curcumin, quecetin and alpha-tocopherol (Rajeshwar et al. 2005).
14 Dopamine Receptors in Plants
Several experiments indirectly support the view that receptors for adrenaline or nonadrenaline are present in plants (Roshina 1990). Catecholamines were found to bind to membrane with same way as adrenoreceptors in mammals (Yasunari et al. 2000). Studies showed that propanol an antagonist of beta-adrenergic receptors in animals has been shown to suppress partially flowering of duckweed and this effect was relieved by the addition of adrenaline (Khurana et al. 1987; Högenauer 1978). Potato plants grown on alprenolol, a catecholamine agonist, were characterized by a bushy phenotype and yellowish leaves. Human dopamine receptor D1 was also expressed in potato plant that resulted in remarkable increase in catecholamine levels and changes in sugar metabolism (Skirycz et al. 2005). Newly identified DoH-CB proteins could mediate catecholamine action. This class of proteins contains both dopamine-beta-hydroxylase activity and a cyt b561 electron transport domain (CB) and thus combine in one protein with properties of two enzymes necessary for adrenaline production (Verelst and Asard 2004). In silico analysis of DoH-CB proteins from Arabidopsis thaliana shows that structural features of both CB and DoH domains are well conserved. It is interesting that some DoH-CB proteins were found to be inducible. The DoH domain was also identified in another auxin-inducible protein AIR12. These proteins are very good candidates for mediators of catecholamine function in plants and can provide a link between auxin and catecholamine action since some of the proteins containing the dopamine binding domain are induced in response to auxin. Catecholamine receptor has been identified in transformed potato plants with a cDNA encoding human dopamine receptor (HD1) (Szopa et al. 2001).
15 Conclusion
Initially after the discovery of neurotransmitter substances in plants it was proposed that they might function as a deterrent to insect predator and foraging animals. Data accumulated showed that dopamine can be used as an antiherbivore defence compounds. It was suggested that dopamine might be simply products of synthesis and degradation pathway of other metabolite (Odjakova and Hadjiivanova 1997). Recent evidences on dopamine function have shown it is more complex. They influence many aspects of plant physiology. Involvement in oxidative stress, action in concert with phytohormone in regulation of plant growth, stress responses and regulation of sugar metabolism indicate that they have important regulatory functions. They display a rapid transient increase in plant leaves submitted to wounding, water stress and ABA treatment. Dopamine is required in very small quantities and they are readily modified (methylated) during course of action. The metabolic effect produced by plant dopamine is specific in regulating starch breakdown; Also characteristic is that they are produced mainly in leaves but affect specific physiological responses in another part (tubers) of the organism. Thus taken together the entire data presented make it conceivable that dopamine might play a general role in plant physiology. The molecular mechanism is yet poorly understood and further investigation will be helpful in final elucidation of their function in plants.
The study of dopamine as plant petrochemicals will lead to new understanding of both human health and plant physiology (Fig. 3). It is interesting that some of the same compounds that affect human brain function also affect the growth and development of higher plants. However studies on dopamine are at the initial stage when compared to other branches. Recently developed transgenic technology is a new tool for studying physiological relevance of this compound for plant physiology. A metabolic engineering approach has now provided direct evidence for the role of dopamine in carbohydrate metabolism and plant response to stresses. It is however not known in detail about the compound catabolism and the perspective of the use of plant overproduced dopamine for oral treatment of patients with Parkinson disease. Since dopamine biosynthesis is affected by stresses the potential coordination of their synthesis with other compounds of pathway might occur. Nothing is as yet known in detail on dopamine signal transduction in plant. By using genetic engineering approach the respective receptor and mediating signal transduction compounds can be finally identified. Much further research is required to fully understand the role of neurologically active compounds in plants.
Abbreviations
- ABA:
-
Abscisic acid
- BHT:
-
Butylated hydroxytoluene
- Cu2+:
-
Copper
- DOPA:
-
(3,4-Dihydroxyphenylalanine)
- DOPA-Q:
-
Dopaquinone
- DOPA-SQ−:
-
Semiquinone radical of Dopamine
- DPDH:
-
1,1-Diphenyl-2-picryl-hydrazyl
- Fe3+:
-
Ferric ion
- H2O2:
-
Hydrogen peroxide
- HO:
-
Hydroxyl Radicals
- HPLC:
-
High Performance Liquid Chromatography
- IAA:
-
Auxin
- NO:
-
Nitric Oxide
- O2:
-
Oxygen
- O2−:
-
Superoxide
- OSPIP:
-
Aquaporin gene
- PPO:
-
Polyphenol oxidase
- ROS:
-
Reactive oxygen species
- SH:
-
Group thiol group
References
Abdelkader AF, El-khawas S, El-Sherif NASE, Hassanein RA, Emam MA, Hassan RE (2012) Expression of aquaporin gene (Os PIP1-3) in salt-stressed rice (Oryzasativa L.) plants pre-treated with the neurotransmitter (dopamine). Plant Omics 5:532–541
Allen JF (2003) Superoxide as an obligatory catalytic intermediate in photosynthetic reduction of oxygen by adrenaline and dopamine. Antioxid Redox Signal 5:7–14
Anaya AL (1999) Allelopathy as a tool in the management of biotic resources in agroecosystems. Crit Rev Plant Sci 18:697–739
Arno GS (2000) Antioxidant and prooxidant nature of catecholamine. A thesis submitted in conformity with the requirements for the degree of Master of Science Graduate Department of Pharmacology University of Toronto
Badria FA (2002) Melatonin, serotonin, and tryptamine in some Egyptian food and medicinal plants. J Med Food 5:153–157
Barz ME (1978) Degradation of phenylethylamine in plant suspension cultures. Planta Med 33:336–344
Boomsma CR, Vyn TJ (2008) Maize drought tolerances: potential improvements through arbuscular mycorrhizal symbiosis. Field Crops Res 108:14–31
Bowen L, Gao T, Zhao Qi, Ma C, Chen Qi, Wei Z, Li C, Li C, Ma F (2018) Effect of exogenous dopamine on the uptake transport and resorption of Apple Ionome under moderate drought. Front Plant Sci 9:755–761s
Chen GF, Huo YS, Tan DX (2003) Melatonin in Chinese medicinal herbs. Life Sci 73:19–26
Dai YR, Michaels PJ, Flores HE (1993) Stimulation of ethylene production by catecholamine and phenyl ethyl amine in potato cell suspension culture. Plant Growth Regul 12:219–222
Dietz KJ (2003) Plant peroxiredoxins. Annu Rev Plant Biol 54:93–107
Dubbels R, Reiter RJ, Klenke E, Goebel A, Schnakenberg E, Ehlers C, Schiwara HW, Schloot W (1995) Melatonin in edible plants identified by radioimmunoassay and by high performance liquid chromatography-mass spectrometry. J Pineal Res 18:28–31
Elstner EF, Konze JR, Selman BR, Stoffer C (1976) Ethylene formation in sugar beet leaves: evidence for the involvement of 3-hydroxytyramine and phenoloxidase after wounding. Plant Physiol 58:163–168
Fieldman JM, Lee EM, Castleberry CA (1987) Catecholamine and serotonin content of foods: effect on urinary excretion of homovanillic and 5-hydroxyindoleacetic acid. J Am Diet Assoc 87:1031–1035
Fuji Y (2003) Allelopathy in the natural and agricultural ecosystem and isolation of potent allelochemicals from Velvet bean (Macuna pruriens) and heiry vetch (Vicia villosa). Biol Sci Space 17:6–13
Fujii Y, Shibuya T, Yasuda T (1991) L-3,4-dihydroxyphenylalanine as an allelochemical candidate from Mucuna pruriens. Agric Biol Chem 55:617-618
Furstenberg-Hagg J, Zagrobelny M, Bak S (2013) Plant defence against insect herbivore. Int J Mol Sci 14:10242–10297
Furubayashi A, Hiradate S, Fujii Y (2007) Role of catechol structure in the adsorption and transformation reactions of L-DOPA in soils. J Chem Ecol 33:239-350
Golisz A, Sugano M, Hiradate S, Fujii Y (2011) Microarray analysis of Arabidopsis plants in response to allelochemical L-DOPA. Planta 233:231–240
Guidotti BB, Gomes BR, de Cassia R, Soares S, Soares AR, Ferrarese-Filho O (2013) The effects of dopamine on root growth and enzyme activity in soybean seedlings. Plant Signal Behav 8:9–12
Guinaudeau H, Bruneton J (1993) Isoquinolins alkaloid and sulphur compounds. In: Watermann PG, Dey PM, Harborne JB (eds) Methods in plant biochemistry, vol 8. London Academic Press, pp 373–419
Hachinohe M, Matsumoto H (2005) Involvement of reactive oxygen species generated from melanin synthesis pathway in phytotoxicity of L-DOPA. J Chem Ecol 31:237–246
Hachinohe M, Matsumoto H (2007) Mechanism of selective phytotoxicity of L-3,4dihyfroxyphenylalanine (L-DOPA) in barnyard grass and lettuce. J Chem Ecol 33:1919–1926
Hachinohe M, Sunohara Y, Matsumoto H (2004) Absorption, translocation and metabolism of DOPA in barnyardgrass and letucce: their involvement in species-selective phytotoxic action. Plant Growth Regul 43:237–243
Hattori A, Migitata H, Masayuki I, Itoh M, Yamamoto K, Ohtani-Kaneko R, Hara M, Suzuki T, Reiter RJ (1995) Identification of melatonin in plants and its effects on plasma melatonin levels and binding to melatonin receptors in vertebrates. Biochem Mol Biol Int 35:627–634
Hennion F, Martin-Tanguy J (2000) Amines of the subantartic crucifer Pringlea antiscorbutica are responsive to temperature conditions. Physiol Plant 109:232–245
Högenauer G, Kreil G, Bernheimer H (1978) Studies on the binding of DOPA (3,4-dihydroxyphenylalanine) to tRNA. FEBS Lett 88:101–104
Homeyer BC, Roberts MF (1984) Dopamine accumulation in Papaver somniferum latex. Z Naturforsch 39c:1034–1037
Huang T, Jander G, de Vos M (2011) Non-protein amino acids in plant defence against insect herbivore: representative cases and opportunities for further functional analysis. Phytochemistry 72:1531-1537
Inderjit S, Duke SO (2003) Ecophysiological aspects of allelopathy. Planta 217:529–539
Jander TG, de Vos M (2011) Non-protein amino acids in plant defence against insect herbivores: representative cases and opportunities for further functional analysis. Huang Phytochem 72:13:1531–137
Jung SY, Kim JS, Cho KY, Tae GS, Kang BG (2000) Antioxidant responses of cucumber (Cucumis sativus) to photoinhibition and oxidative stress induced by norflurazon under high and low PPFDs. Plant Sci 153:145–154
Kamisaka S (1979) Catecholamine stimulation of gibberylin action that induces lettuce hipocotyl elongation. Plant Cell Physiol 20:1199–1207
Kanazawa K, Sakakibara H (2000) High content of dopamine, a strong antioxidant in Cavendish banana. J Agric Food Chem 48:844–848
Keller W, Yeary R (1980) Catecholamine metabolism in a psychoactive cactus. Clin Toxicol 16:233–243
Khurana JP, Tamot BK, Maheshwari N, Maheshwari SC (1987) Role of catecholamine in promotion of flowering plants in a short day Duckweeed. Lemma paucicostata 6746. Plant Physiol 85(1):10–12
Kinnersley AM, Turano FJ (2000) Gamma aminobutyric (GABA) and plant responses to stress. Crit Rev Plant Sci 19:479–509
Kong KH, LeeJL PHJ, Cho SH (1998) Purification and characterization of the tyrosinase isozyme of pine needles. Biochem Mol Biol Int 45:717–724
Kruk I, Lichszteld K, Bounias M, Kadna A, KuberaNowakowska L (1999) Formation of active oxygen species during autoxidation of Dopa. Chemosphere 39:443–453
Kuklin AI, Conger BV (1995a) Catecholamine in plants. J Plant Growth Regul 14:91–97
Kuklin AI, Conger BV (1995b) Enhancement of somatic embryogenesis in orchard grass leaf culture by epinephrine. Plant Cell Rep 14:641–644
Kulma A, Szopa J (2007) Catecholamine are active compound in plants. Plant Sci 172:433–440
Lauren AE, Erland, Christina E Turi, Praveen K Saxena (2019) Chapter 2—Serotonin in plants: origin, functions, and implications. Academic Press, pp 23–46
Lerner AB, Case JD, Takahashi Y, Lee TH, Mori N (1958) Isolation of melatonin, pineal factor that lightens melanocytes. J Am Chem Soc 80:2587–2593
Li Y, Yang X, van Breeman RB, Bolton JL (2005) Characterization of two new varients of human catechol-O-methyltransferase in vitro. Cancer Lett 230:81–89
Li C, Sun XK, Chang C, Jia DF, Wei ZW, Li CY et al (2015) Dopamine alleviatessalt-induced stress in Malushupehensis. Physiol Plant 153:584–602
Liang B, Gao T, Zhao Q, Ma C, Chen Q, Wei Z, Li C, Li C, Ma F (2018) Effect of exogenous Dopamine on the uptake, transport and resortion of apple lonome under moderate drought. Plant Sci: 1–14
Liang BW, Li CY, Ma CQ, Wei ZW, Wang Q, Huang D et al (2017) Dopamine alleviates nutrient deficiency-induced stress in Malus hupehensis. Plant Physiol Biochem 119:346–359
Lundstorm J (1971) Biosynthsis of mescaline and tetrahydroisoquinoline alkaloids in Lophophora williamsii (Lemm) Coult Occurance of biosynthesis of catecholamine and other intermediates Acta Cam Scand 25:3489–3499
Lundström J, Agurell S (1971) Biosynthesis of mescaline and tetrahydroisoquinoline alkaloids in Lophophora williamsii (Lem.) Coult. Acta Pharm Suec 8:261–274
Manchester LC, Tan DX, Reither RJ, Park W, Monis K, Qi WB (2000) High levels of melatonin in the seeds of edible plants—possible function in germ tissue protection. Life Sci 67:3023–3029
Odjakova M, Hadjiivanova C (1997) Animal neurotransmitter substances in plants. Bulg J Plant Physiol 23(1–2):94–102
Marinova EM, Yanishlieva NV (2004) Inhibited oxidation of lipids II: comparison of the antioxidative properties of some hydroxy derivatives of benzoic and cinnamic acids. Eur J Lipid Sci Technol 94:428–432
Matsumoto H (2011) The mechanisms of phytotoxic action and selectivity of non-protein aromatic amino acids L-DOPA and m-tyrosine. J Pestic Sci 36:1–8
Medda R, Padiglia A, Floris G (1978) Plant Copper-amine oxidase. Phytochemistry 33:336–344
Mithofer A, Boland W (2012) Plant defence against herbivore. Int J Mol Sci 14:10242–10297
Momonoki YS, Momonoki T (1991) Changes in acetylcholine levels following leaf wilting and leaf recovery by heat stress in plant cultivars. Jpn J Crop Sci 60:283–290
Murch SJ, Saxena PK (2002) Melatonin: a potential regulator of plant growth and development? In Vitr Cell Dev Biol Plants 38:531–536
Murch SJ, Simmons CB, Saxena PK (1997) Melatonin in feverfew and other medicinal plants. Lancet 350:1598–1599
Murch SJ, KrishnaRaj S, Saxena PK (2000) Tryptophan is a precursor for melatonin and serotonin biosynthesis in in vitro regenerated St. John’s wort (Hypericum perforatum L. cv. Anthos) plants. Plant Cell Rep 19:698–704
Nishihara E, Parwez MM, Araya H, Fujji Y (2004) Germination growth response of different plant species to the allelochemical L-3,4-dihydroxyphenylalanine (L-DOPA). Plant Growth Regul 42:181–189
Pattison DI, Dean RT, Davies MJ (2002) Oxidation of DNA, proteins and lipids by DOPA, protein-bound DOPA and related catelcholamine. Toxocology 177:23–37
Ponchet M, Martin-Tanguy J, Marais A, Martin C (1982) Hydroxycinnamoyl acid amides and aromatic amines in the inflorescences of some Araceae species. Phytochemistry 21:2865–2869
Protacio CM, Dai YR, Lewis EF, Flores HE (1992) Growth stimulation by catecholamines in plant tissue/organ cultures. Plant Physiol 98:89–96
Pugnaire FI, Chapin FS (1992) Environmental and physiological factors governing nutrient resorption efficiency in barley. Oecologia 90:120–126
Rajeshwar Y, Senthil KGP, Gupta M, Mazumder UK (2005) Studies on in vitro antioxidant activities of methanol extract of Mucuna pruriens (fabaceae) seeds. Eur Bull Drug Res 13:31–39
Rehr SS, Janzen DH, Feeny PP (1973) L-DOPA in legume seeds: a chemical barrier to insects attack. Science 181:81–82
Reiter RJ, Tan DX, Burkhardt S, Manchester LC (2001) Melatonin in plants. Nutr Rev 59(9):286–290
Reiter RJ (2005) Melatonin in walnuts: Influence on levels of melatonin and total antioxidant capacity of blood. Nutrition 21(9):920–924
Roepenack-Lahaye E, Newman MA, Schornack S, Hammond Kosack, Lahaye T, Jones JDG, Daniels MJ, Dow JM (1974) p-Coumaroylnoradrenaline, a novel plant metabolite implicated in tomato defence against pathogens. J Biol Chem 278:43373–43383
Rosei MA, Blarzino C, Foppoli C, Mosca L, Coccia R (1994) Lipoxygenase catalysed oxidation of catecholamine. Biochem Biophys Res Commun 200:344–350
Roshchina V (1990) Biomediators in chloroplasts of higher plants. Effect of dopamine on photochemical activity. Photosynthetica 24:117–121
Roshina VV (1990) Biomediators in choloroplast of higher plants, 4 Reception of photosynthetic membrane. Photosynthesis 24:539–549
Schoonhoven LM, Loon JAV, Dicke M (2005) Insect-plant biology. Oxford University Press, Oxford, U.K.
Sharma JP, Turner BK, Maheshwari N, Maheshwari SC (1987) Role of catecholamine in promotion of flowering in a short-day duckweed, Lemna paucicostata. Plant Physiol 85:1012–1016
Shelp BJ, Bown AW, McLean MD (1999) Metabolism and functions of gamma-aminobutyric acid. Trends Plant Sci 4:446–452
Shelp BJ, Van Cauwenberghe OR, Bown AW (2003) Gamma aminobutyrate: From intellectual curiosity to practical pest control. Can J Bot 81:1045–1048
Skirycz A, Swiedrych A, Szopa J (2005) Expression of human dopamine receptor in potato (Solanum tubersum) results in altered tuber carbon metabolism. BMC Plant Biol 5:16–21
Smith TA (1980) Plant amines. In: Secondary plant products, Encyclopedia of plant physiology. In: Bell IA, Charlwood BV (eds). New series, vol 8. Springer, Berlin, pp 433–460
Soares AR, Cássia Siqueira-Soares R, Salvador VH, Lourdes Lucio Ferrarese M, Ferrarese-Filho O (2012) The effects of L-DOPA on root growth, lignification and enzyme activity in soybean seedlings. Acta Physiol Plant 34:1811–1817
Soares AR, de Lourdes Lucio Ferrarese M, de Cassia Siqueira-Soares R, Marchiosi R, Finger-Teixeira A, Ferrarese-Filho O, (2007) L-DOPA increases lignifications associated with Glycine max root growth-inhibition. J Chem Ecol 33:265–275
Soares AR, de Lourdes, Lucio Ferrarese M, de cassia Siqueira Soares R, Marchiosi R, Finger-Teixeira A, Ferrarese-Filho O (2011) The Allelochemical L-DOPA increases melanin production and reduces reactive oxygen species in soyabean roots. J Chem Ecol 37:891–8
Soares AR, Marchiosi R, de cassia Siqueira Soares R, de Lima RB, dos Sentos WD, Ferrarece-Filho O (2014) The role of L-DOPA in plants. Plant Signal Behav 9:e28275
Sofic E, Denisova N, Youdim K, Vatrenjak-Velagic V, De Filippo C, Mehmedagic A, Causevic A, Cao G, Joseph JA, Prior RL (2001) Antioxidant and pro-oxidant capacity of catecholamines and related compounds. J Neural Transm 108:541–557
Steiner U, Schliemann W, Stracl D (1996) Assay for tyrosine hydroxylation activity of tyrosinase from betalain-forming plants and cell culture. Anal Biochem 238:72–75
Swiedrych A, Lorenc-Kukula K, Skirycz A, Szopa J (2004) The catecholamine biosynthesis route in potato is affected by stress. Plant Physiol Biochem 42:593–600
Szopa J, Wilczynski G, Fiehn O, Wenczel A, Willmitizer L (2001) Identification and quantification of catecholamine in potato plants (Solanum tuberosum) by GC-MS. Phytochemistry: 315–320
Taiz L, Zeiger E (2012) Plant physiology. Sinauer Associates
Takasaki S, Kawakishi S (1997) Formation of protein-bound 3,4-dihydroxyphenilalanine and 5-S-Cysteinyl-3,4-dihydroxyphenylalanine as new cross-linkers in gluten. J Agric Food Chem 45:3472–3475
Topal S, Kocacaliskan I (2006) Allelopathic effects of DOPA against four weed species DPU. Fen Bilmleri Enstitusus 11:27–32
Van Alstyne KL, Nelson AV, Vyvyan JR, Cancilla DA (2006) Dopamine functions as an antiherbivore defence in the temperate green alga Ulvaria obscura. Oecologia 148: 304–311
Verelst W, Sard H (2004) Analysis of an Arabidopsis thaliana protein family, structurally related to cytochrome b561 and potentially involved in catecholamine biochemistry in plants. J Plant Physiol 24:539–549
Wang S, Che T, Levit A, Shoichet BK, Wacker D, Roth BL (2018) Structure of the D2 dopamine receptor bound to the a typical antipsychotic drug risperidone. Nature 555:269–273
Weir TL, Park S-W, Vivanco JM (2004) Biochemical and physiological mechanisms mediated by allelochemicals. Curr Opin Plant Biol 7:472–479
Wichers HJ, Visser JF, Huizing HJ, Pras N (1993) Occurrence of L-DOPA and dopamine in plants and cell cultures of Mucuna pruriens and effects of 2,4-D and NaCI on these compounds. Plant Cell Tissue Organ Cult 33:259–264
Wurtman RJ, Axelrod J, Chu EW (1963) Melatonin, a pineal substance: effect on the rat ovary. Science 141:277–278
Yasunari K, Kohno M, Kano H, Minami J, Yoshikawa J (2000) Dopamine as a novel antioxidative agent for rat vascular smooth muscle cells through dopamine D1-like receptors. Circulation 101:2302–2308
Yu HS, Reiter RJ (1993) Melatonin: biosynthesis, physiological effects and clinical applications. CRC Press, Boca Raton, FL
Yue M-E, Jiang T-F, Shi Y-P (2005) Simultaneous determination of noradrenaline and dopamine in Portulaca oleracea L. by capillary zone electrophoresis. J Sep Sci 28:360–364
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Bala, K. (2020). Beyond a Neurotransmitter: Physiological Role of Dopamine in Plants. In: Baluška, F., Mukherjee, S., Ramakrishna, A. (eds) Neurotransmitters in Plant Signaling and Communication. Signaling and Communication in Plants. Springer, Cham. https://doi.org/10.1007/978-3-030-54478-2_9
Download citation
DOI: https://doi.org/10.1007/978-3-030-54478-2_9
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-54477-5
Online ISBN: 978-3-030-54478-2
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)