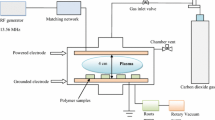
Abstract
Polypropylene membrane with the base of polyolefin exhibits promising applications such as packaging, medical tools, and especially in the filtration industry, due to its outstanding properties such as well mechanical features, recyclability, acceptable resistance to temperature and chemicals, and reasonable price. However, its applications are restricted by its hydrophobic nature. To amend the surface physical and chemical properties of polypropylene and broaden its usage, well-known methodologies such as laser modification and plasma irradiation have been investigated more than the other methods. Herein, in this chapter, the investigations on the surface refinement of polypropylene using laser and plasma are discussed. Furthermore, a comparison between the effectiveness of both methods (i.e. laser modification and plasma irradiation) is presented.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
1 Introduction
The foremost way in the filtering industry is using membrane because, in general, it controls flux rate and allows some components of a mixture to pass through it while other components are abandoned. On the other hand, with depletion of water resources and clean water shortage, wastewater treatment concerns more than previous. Hence, membranes have been widely used in various industries ranging from wastewater treatment and fuel cells to filtration industry and even medicine as artificial organs [1, 2]. The permeability feature of the membrane is widely used in the separation and filtration industry. Since the main purpose in the filtration industry is to separate a particular material from a mixture, a membrane can provide this opportunity [3]. Thereafter, the controllability property of the permeation rate of the membrane has caused a lot of attention in medicine, especially in the field of drug delivery, because the main goal in drug delivery is to regulate the permeation rate, which in general a membrane can be very efficient. In this regard, some of the most successful uses of the membrane in the medical field are artificial kidneys based on hollow-fiber membranes, artificial lungs, and drug delivery.
According to the size of particles that should be separated, in general, membranes are classified into four major categories [3]. Likewise, membranes could be divided into two categories from the point of the membrane’s structure view, isotropic and anisotropic membranes [3]. Isotropic membranes have a uniform structure throughout, and they can be subdivided into three types, microporous, nonporous, and electrically charged membrane. On the other hand, anisotropic membranes consist of layers with different chemically and or physically heterogeneous structure and permeability. Indeed, they have a skin/core structure in such a way that an extremely thin surface layer (i.e. the skin) supported on an open much thicker porous core [3]. There is quite an extensive diversity of techniques to fabricate membranes such as phase inversion, track etching, and stretching which in the following a brief description of these techniques will be outlined.
To begin with, phase inversion is one of the applicable techniques for membrane fabrication. It is a mixed way; a solution of liquid polymer from which a membrane is to be made is prepared in a selected solvent. The precipitation of the solution results in the formation of two phases of solid and liquid [3]. The solid which is a polymer-rich part forms the polymer matrix and the membrane pores are made of liquid. One of the major drawbacks of this method probably is solvent prediction since there is no straight strategy to select the appropriate solvent [3].
The track etching method is performed in such a way that the polymer sheet or foil is first exposed to the radiation of the metal ions. In the following, the exposed polymer foil is etched in an acid or alkaline medium. While the porosity of the membrane affects by the duration of radiation, the etching time, as well as temperature, determine its pore size [4]. As porosity increases, the membrane becomes fragile, so pore size is limited.
Stretching technique has been used in which the sketching of a polymer film or sheet is fundamental aspect [3]. This technique relies on having one of the following: filler particles, a mixed solvent, beta or hexagonal unit cell crystals, or a stacked lamellar structure [3]. For example, uniaxial stretching is used for a lamellar structure as follows: (1) creating a film by lamellar morphology, (2) annealing the precursor film, and (3) stretching at the desired temperature.
Having stunning properties, polymers excite large attention in membrane fabrication and are used in the wastewater industry with ease [5]. The performance of a polymeric membrane occasionally depends on several factors, including surface roughness, its material, the chemical properties of the polymer surface, and its structure [1, 6]. The membrane properties relate to its fabrication process and materials themselves. To date, to aim of reducing cost and time, synthetic-polymeric membranes have been used in various applications like reverse osmosis, wastewater treatment, microfiltration, and ultrafiltration. The polymeric membrane technology excited a plethora of attention because of its low cost, low energy consumption, and environmental adaptation. However, hydrophobicity of a membrane is an impractical characteristic, which causes inconvenience in different industrial sectors. The fundamental rationale for the hydrophobicity of a material is the lack of hydrogen bonding with water molecules. So, if we could increase the percentage of oxygen content, as a result, the hydrophobic membrane turns to a hydrophilic one [1, 7]. Wetting changes of a membrane could be scrutinized using water contact angle measurement.
The hydrophobic nature of some polymeric membranes (e.g. polypropylene) does not allow them to adhere to hydrophilic substrates and it shows a major barrier in wastewater, sea desalination, and separators in battery industries [1, 8,9,10]. Besides, a hydrophobic membrane could not contribute to the reversible process and it is a major drawback especially in dealing with aqueous solutions. Most synthetic-polymeric membranes, used commercially, are hydrophobic in nature [6]. The hydrophobic membrane has a high level of mechanical and chemical stability than the hydrophilic one which makes them appropriate candidates. Additionally, the previous studies suggested that in the case of hydrophilic membranes, a very thin layer of water could exist on the skin layer of it which contributes to reducing fouling components adsorption on it, and as a consequence increasing the antifouling characteristic of the membrane [1, 11]. Considering the aforementioned statements, a desirable membrane, which is mechanically tough and durable, antifoul, and chemically stable, could be achieved by modification of a hydrophobic polymeric membrane without changing its bulk properties [1, 5, 12, 13]. Hence, to date, several different methods for the functionalization of polymeric membranes have been introduced and applied. Techniques which are successfully used to improve a polymeric membrane are corona, chemical, UV irradiation, flame, plasma, and excimer laser [9, 10, 14]. The surface properties of polymeric membranes such as wettability, roughness, and chemical structure are determining factors in choosing a membrane for a particular application [15]. Accordingly, a few polymers having been used as polymeric membrane materials including cellulose, polyvinylidene fluoride, polysulfone, polypropylene, polyethylene, polyacrylonitrile, polyamide, and polyimide. Among the aforementioned polymers, We focus on polypropylene (PP) membrane.
2 Polypropylene Membrane
Polypropylene membrane with the base of polyolefin exhibits promising applications such as packaging, medical tools, separator in lithium-ion batteries, and especially in the filtration industry [16,17,18,19]. These vast applications are attributed to its outstanding properties such as well mechanical features, recyclability, acceptable resistance to temperature and chemicals, and reasonable price [1, 17, 20,21,22]. Yet, due to low surface energy, its hydrophobicity, and the lack of some functional groups, polypropylene exhibits several weak properties, which in turn limits its applications [1, 17, 19, 23, 24]. Of course, it should be added that there are solutions to this problem that significantly improve its poor features and broadens its usage more. Since it is the most widely used material in the filtration industry, its hydrophobicity causes proteins and other hydrophobic compounds to absorb into the membrane’s pores which count as an undeniable obstacle in the filtering process [25, 26]. Admittedly, it usually leads to a reduction in liquid permeability and membrane useful life, increasing energy usage to overcome the persistence of fouling and have a stable flux [26]. In addition, we need to replace the used membrane after a while which makes the filtration process costly. The fouling phenomenon could be categorized into two types of irreversible and reversible fouling which the intrinsic properties of the membrane (i.e. membrane wettability) determine the type of fouling. To put it another way, the reversible fouling in which membrane can be revived happens when reversible organic matters are adsorbed on hydrophilic surfaces [26]. While in contrast, adsorption of the irreversible organic matter on a hydrophobic membrane results in irreversible fouling which only chemical cleaning can partly remove precipitate [1, 26]. The fouling phenomenon could be explained based on the bonding between membrane and hydrogen of water molecules.
PP serves as the most important polymeric membrane material due to its high stability and low price. PP never shows a disposal problem than other materials (like PVDF) due to not having halogen content. Besides, the PP membrane has a vital role in microfiltration and ultrafiltration processes. The hydrophobic nature of PP could be considered as a disadvantageous factor in industrial and wastewater processes. In wastewater treatment and bio-separation, hydrophobicity is a major handicap when it comes to the treatment of solutions consist of the organic matter content which causes irreversible fouling of the membrane, and membrane fouling leads to membrane deterioration. Under this condition, some strategies such as cross flushing and backwashing can partly recover the membrane [26]. In biomedical applications, the usage of polypropylene is restricted by its hydrophobicity because of biomolecules such as enzymes prone to adsorb on hydrophobic surfaces [6, 9, 15, 17, 27, 28]. This could lead to infection and membrane fouling and change in biomolecules performance [16]. Accordingly, to avoid irreversible fouling even in hydrophobic membranes, it seems that for membranes to increase the hydrophilicity offers a practicable solution. Membrane surface treatment can indeed be applied to modify surface wettability, improve biocompatibility, and chemical properties [9, 15].
3 Surface Modification Strategies
The main purpose of surface tailoring is modification outer layer of polymers aim to add some useful functional groups which improve adhesion, hydrophilicity/hydrophobicity, anti-fouling, and dye uptake [1, 6, 29]. Eliminating the drawbacks of polymers led scientists to utilize different methods, which have been categorized in physical, chemical, and biological methods [30,31,32]. These categories are described as follows.
3.1 Chemical
An accepted route to introduce desired effects is the wet chemical method, in which the surface of polymers was exposed to some chemical reagents such as chromic acid, nitric acid, and potassium hydroxide. Utilizing this technique for polypropylene and polyethylene resulted in generating some carbonyl groups, hydroxyl groups, and carboxylic acid groups on the surface of polymers. Introducing such functional groups contribute to improve the wettability and adhesion of the surface.
3.2 Biological
Thanks to lower cost and high purity, biological modification methods have been celebrated as a convenient route for use in medical applications. In this method biomolecules such as enzymes, antibodies, and proteins were loaded on the surface of polymers through physical or chemical attachment. Physical adsorption is the easiest way compared to chemical interaction; it must be considered that chemical attachment was occurred by covalent bonding which offers a more stable biomolecule material.
3.3 Physical
To eliminate chemical agents utilized in traditional chemical routes, which impose irreparable damages to the environment, methods based on physical principles have been developed. The physical methods have been justified based on their principles in two categories plasma and radiation-assisted such as corona discharge and laser, respectively. Introducing oxygen containing functional groups, which has been provided by physical treatment, endows the surface of polymers with suitable properties like adhesion, wettability, and printability.
Among different methods, the further sections are dedicated to justify physical methods in detail.
4 Radiation
The energy coming from a source and passing through a medium is named radiation. When an electron drops down from higher energy levels to lower ones, radiation could be created [30]. Diverse types of radiation could be classified into two major categories including ionizing and non-ionizing radiation, based on their source and power [30]. Ionizing radiations have high energy which able to create an ion by removing an electron from the matter [30]. On the contrary, non-ionizing radiations do not have enough energy to create ions during exposure [30]. To be more accurate, not having acceptable parameters, some materials cannot excite interest unless to be modified through an appropriate method. These methods are classified by their technology such as chemical, plasma, and laser methods. In polymer surface modification, plasma and laser modification techniques are commonly used to improve membrane properties. In exceeding sections, a brief description of plasma and laser is exhibited.
4.1 Plasma and Plasma Modification Technique
In a broad statement, a gas that consists of ions, electrons, molecular, and atomic components in the electromagnetic field is plasma [1, 15, 33, 34]. Plasma is formed by applying an electric field between powered and grounded electrodes through a gas medium [9, 35, 36]. The most important factor to produce plasma is breakdown voltage to split gas into its species. Non-thermal (usually named as cold plasma) and thermal plasmas are commonly used for polymers to functionalize [34, 37]. Plasma is classified according to electron density and type of power supply. Among all power supplies, radio frequency (RF) or microwave sources are most common [34]. When plasma treatment is implemented to a polymer, then drastic changes are simultaneously done on the top surface of the polymer such as cross-linking (including enhancement of its hydrophilicity and/or improving its adhesion), removing contaminants, etching [10, 17, 24, 34, 38]. Accordingly, the interactions of plasma and polymer can be divided into three categories, surface reaction, polymerization of plasma, and cleaning [13]. Surface reactions occur when surface and gas species react with each other; thus, functional groups are introduced on the polymer surface. Under this condition, reactions between surface species result in cross-linking [30]. Previous studies revealed that surface reactions are done when plasma modification is performed using carbon oxides, hydrogen, oxygen, water, neon, nitrogen and its oxides, and ammonia. Polymerization occurs when a monomer in plasma (such as C2H6, C2F4, CH4, C3F6) is polymerized on the polymer surface [13]. In this condition, reactions including gas species and surface species, as well as between surface species might occur. A typical process occurring during the plasma modification is surface cleaning using etching the surface.
The performance of the plasma is that the gas-forming molecules prone to activation by the possible collisions with other species in the gas and then they hit the surface of the polymer [1]. In this method, the excited molecules of a gas are thrown to the surface of the polymer; so, physical phenomenon occurs in such a way that plasma irradiation causes heating the polymer surface resulting in the chemical bonds breaking. The necessary conditions for chemical reactions on the polymer surface are prepared. Consequently, plasma prepares the necessary environment for chemical groups to combine [9, 39].
Plasma could promote surface properties using the interaction between gas’s species and material’s surface and similarly via restructuring the surface of materials. The first one is achieved by oxidation and the second is done by degradation and formation of oxygen groups. It could be argued that nitrides and oxides are created from plasma irradiation [1]. Accordingly, the key factor in plasma functionalization to attain favorable properties is selecting the gas because the properties of the top layer of polymer which have been exposed to plasma strongly affected by the type of gas [1, 38, 40, 41].
Surface properties affect directly by plasma gas type and its nature (reactant or non-reactant). However, the most practical feature of plasma modification is altering the surface of matter while preserving the bulk properties [9, 13]. To date, many studies have been employed for the investigation of the effect of plasma on PP membrane [42, 43]. According to kinds of literature, plasma hydrophilization has been successfully performed on polymers and it has been confirmed that in some cases the water contact angle drops to less than 20° which is considerably low [1, 44, 45].
Besides, surface characterizations have confirmed the formation of polar groups during plasma exposure which is a sensible reason for hydrophilization and increasing water uptake [1, 24, 46]. Plasma modification is conducted on polymers with different active gases including oxygen, nitrogen, carbon dioxide, air, fluorine, argon, and ammonia, or a mixture of these gases [34, 44, 47,48,49]. To reduce the modification cost, oxygen and argon-containing gas are commonly utilized. On the other hand, plasma could apply at a broad range of pressure from atmosphere pressure to higher ones which the applied pressure strongly depends on the gas type and the main goal of modification [9, 34]. Low-pressure plasma results in more induced free radicals on the polymer surface according to the literature [9, 24]. Generally, it could be asserted that plasma modification is one of the few environmentally-friendly, dry, time-efficient methods which only affect the top layer of the polymer [9, 17, 24, 34].
Oxygen plasma treatment is a common method with the aim of fabricating suitable materials in many research fields. Oxygen is a reactant gas; it contributes to fabricate a desirable material by reacting with its surface. During oxygen plasma treatment, two processes may occur including itching and formation of oxygen functional groups. These mechanisms down to the highly aggressive features should be controlled by operation parameters. As a result, oxygen-containing functional groups such as C–O, C=O, O–C–O, C–O–O, and CO3 are made on the surface due to oxygen plasma radiation [10, 34]. Changing the wettability of polymers relied on creating polar functional groups which possesses many benefits [46]. Wettability has a vital role in using polymeric materials in the industry and medical science. In general, oxygen-assisted plasma is commonly performed to increase surface energy to dramatically increase the hydrophilicity [10, 13]. Additionally, oxygen plasma leads to clean the surface from organic contaminants. While in contrast, fluorine-assisted plasma contributes to surface etching and/or surface energy reduction and, as a consequence, increasing hydrophobicity [10, 13]. In general, polymer’s chemical structure does not affect the etching rate of a particular type of polymer. However, it can be stated that the etching rate in polymers with a lower melting point is higher than that of other ones [34]. The etching rate relates to the plasma gas which is used. For example, etching rate varies based on the assisted gas according to such sequence: O2 > air > CO2 > CF4 > Ar [34]. Adhesive improvement of polypropylene has been reported due to the oxygen plasma modification. It was outlined that the formation of oxygen-containing functional groups (carboxyl, hydroxyl, and carbonyl) are responsible for adhesive improvement [50].
Previous studies suggest that the surface morphology of polymer, as well as its roughness, is altered by means of low-pressure argon-assisted plasma [23]. Indeed, some oxygen functional groups appear after argon plasma modification [23]. It has been reported that after the end of the plasma irradiation process, the plasma surface can include free radicals and active species, which, if the polymer surface is exposed to the atmosphere, will cause oxygen-containing functional groups [24, 51, 52]. Novák and co-workers have reported that oxygen functional groups have been generated after atmospheric plasma modification of the polypropylene fabrics which formation of these functional groups was confirmed employing XPS and FTIR characterization techniques [24]. Among the many types of polymers, polypropylene has been shown to be most effective in argon plasma radiation, so that its wettability is increased significantly [9]. In a recent study, Mansuroglu et al. examined the effect of argon and nitrogen plasma and also RF plasma power on polypropylene crystallinity [17]. It was found that new structures such as clusters and valleys, having different order, size, and shape, are created during the plasma irradiation which was attributed to its crystallinity [17]. Rezinckova et al. investigated argon-plasma surface modification of polypropylene [53]. In this study, it was revealed that argon-plasma treatment induces dramatic changes in terms of surface roughness and morphology on polypropylene [53]. In another research, the surface of the PP membrane was treated by argon plasma [49]. The authors inferred that outcome polypropylene samples have high oxygen concentration and, as a result, less water contact angle. However, the oxygen concentration of the polypropylene surface decreased with the aging process and its water contact angle increased again over time [49]. Nitrogen-containing plasma is often used to introduce amino groups and improve printability, biocompatibility, and wettability [10, 13]. Micro-porous polypropylene membrane was exposed to Ar and He plasma aiming to modify surface properties [54], a schematic illustration of experimental was represented in Fig. 1a. To study the influence of plasma treatment, the modified PP membrane was investigated by water contact angle, and the results confirm increase of hydrophilicity of the membrane which was further used as the battery separator. The uptake ratio of electrolyte for samples was assessed and the results show it was raisin from 300 to 600% after treatment; the increase was attributed to high polarity and pore structure. The electrochemical performances resulted, in respect of unmodified PP membrane, after treatment by Ar and He plasma capacity was increased about 48% and 50%, respectively. Figure 1 depicted the percent of electrolyte uptake as a function of modification time and the performance of the battery.

(Reproduced from Liang et al. [54])
a A schematic illustration of experimental setup, b the percent of electrolyte uptake, and c charge/discharge profile of battery.
To investigate the influence of working gas on surface properties, polypropylene samples were tailored by low pressure plasma in the presence of oxygen and air as working gas [55]. The investigation of contact angle, surface chemistry and morphology, and roughness was used for this purpose. The results depicted creating polar species and introducing oxygen functional groups contribute to decrease water contact angle; and as a result, hydrophilicity was increased. The XPS analysis suggested that the increasing ratio of O/C is more significant than N/C. The AFM analysis shows that for 50 W treatment, roughness changing was considerable. For example, for 180 s of applying air and oxygen treatment for 50 W, the roughness was about 900 nm and 400 nm, respectively. The contact angle, surface roughness, and AFM analysis were presented in Fig. 2.

(Reproduced from Hegemann et al. [5])
(I) contact angel, (II) surface roughness, and (III) AFM analysis for a untreated and plasma treated PP substrates, in particular: b Air treatment 50 W-180 s; c air treatment 125 W-180 s; d air treatment 200 W-180 s; e oxygen treatment 50 W-180 s; f oxygen treatment 125 W-180 s; g oxygen treatment 20.
The effect of low-pressure radio-frequency (RF) methane (CH4)/oxygen (O2) mixture plasma was assessed by changing properties which occurred on microporous polypropylene (PP) membranes [56]. The deionized water contact angle was measured to study surface wettability and the results suggest that the angle was declined from 150° to lower than 30° and 70° in the glow and remote region, respectively, at the power of 15 W. The comparison of the surface energy of PP membrane shows increasing polar components, such as CO, contribute to increasing surface energy, which means wettability was increased. The FTIR spectra revealed that increasing hydroxyl (OH) and carbonyl (C=O) took place after plasma modification. Morphology investigation shows that the surface was altered after plasma irradiation; indeed, the smooth surface of the PP membrane became rough and porous after plasma modification. The XPS analysis, a supplement for FTIR, shows oxygen containing functional groups appeared for both glow and remote regions. Eventually, the comparison of glow and remote regions for CH4/O2 suggests that radical reaction could be promoted as well as etching effect could be restrained. Water contact angle, FTIR analysis, SEM, and the XPS spectrum of C1s were presented in Fig. 3.

(Reproduced from Juang et al. 2016)
a Water contact angle, b FTIR, and c SEM and d C1s spectrum of 5 W, 20 s glow/direct.
The aging phenomenon is an important feature in plasma treatment, using additives or further steps were recommended as convenient ways to stable properties and prevent downfall [57]. In this respect, a single-step gliding arc plasma-based method was deployed to modify biaxially-oriented polypropylene (BOPP). The samples were exposed in different exposure times and extent of hydrophobic nature was surveyed for 5 weeks. The roughness was increased after modification and the results show a maximum of 40 s of exposure. The SEM analysis of untreated sample shows some spots which are disappeared after 10 s of treatment, these spots attributed to available additives in the commercial BOPP. Increasing treatment time led to appearing plasma etching, consequently, the porous structure was observed in 30 s tailoring with the size of less than 50 nm. The results did not show a regular attitude between pore size and exposure time. The water contact angle was investigated immediately after plasma treatment and the results suggest that it was declined from 90° to 55° for 120 s of treatment. The aging phenomenon was assessed by the water contact angle of samples after 3 h of sample storage. The results affirmed a fast decline of WCA from 55° to 37°; ironically, the hydrophilicity was increased, in other words in other cases after aging hydrophobicity was recovered. The WCA was observed for prolonged time, and the results were surprisingly illustrated steady hydrophilicity. To elucidate chemical composition, the ratio of O/C was measured and it was grown after sample storage from 1 to 5 weeks. The O/C growing probably due to the active surface area of BOPP which could react by atmospheric air. The results of studies on plasma modification of polypropylene are tabulated in Table 1.
4.2 Laser-Assisted Polypropylene Modification
Nowadays, laser systems which are common types of radiation have various functions in the field of medicine. On the other hand, lasers are considered as one of the effective tools to modify polymer surfaces [30]. Since conventional surface modification techniques usually could not introduce all the required properties on the polymer surface and may cause unwanted contamination on the surface, some alternative methods are proposed to improve surface properties [19]. In this respect, laser-based modifications that induce functional groups on polymeric surfaces were substituted for conventional methods [19]. Laser-based techniques are classified into three groups, laser patterning, laser structuring, and laser texturing [67]. As mentioned previously, laser surface modification is another method to tailor the surface properties of polypropylene. Nowadays, thanks to laser power and high precision, modification of polymeric materials using lasers attract a great deal of attention [27, 68, 69]. UV lasers are one of the most favorable laser systems which are utilized in many studies and most of the fields [10]. Laser modification is considered the simplest, one-step, and medium-cost technique to amend surface-chemistry and/or –morphology [70].
Micron patterns could be induced on the polymer surface using UV lasers with no damage to its bulk properties. Laser-based techniques of polymers modification, generally, are performed in two ways which are based on the alteration of the surface chemistry and/or surface deformation (increasing roughness). Consequently, surface morphology is altered which in turn leads to adhesion improvement. As clarified previously, the laser modification technique is based on the interaction of the laser beam and polymer [67]. This process begins with focusing the laser beam on the frontal surface of the target (e.g. PP membrane) [67]. Absorption of the laser beam on the polymer surface leads to polymer heating and/or vaporizing which in turn induces chemical and/or morphological changes [27, 71]. The laser beam and polymer surface could have photochemically or thermally interaction in the exposed area [71]. Because of the low absorption coefficient of polypropylene, it was asserted that the majority of the laser’s energy penetrates the polypropylene [18].
In general, laser-based modifications could introduce oxygen-containing groups on a polymeric surface [19]. Riveiro and coworkers investigated the effect of the irradiation of a diode end-pumped Nd:YVO4 laser source operating in its fundamental wavelength, second, and third harmonics on PP samples (1064, 532, and 355 nm, respectively) for biomedical application [27]. At first, the optical response of the PP samples was surveyed. They coated PP samples with a carbon thin layer to address the high transparency (40%–62%) of the PP samples to all of the emitting wavelengths and so to increase the PP sample’s energy absorption. This group concludes that the studied laser wavelengths increase the average roughness of the samples to higher than 1 μm which is a minimum value to enhance the PP membrane’s properties for biomedical purposes. Although the studied laser wavelengths led to an increase in the average roughness of PP samples and generation of hydroxyl and carbonyl functional groups, the wettability study suggested that PP samples which are exposed to the laser wavelengths of 1064 and 335 nm demonstrate better hydrophilicity. Indeed, the mechanical performance of PP samples was not significantly improved through laser irradiation. As the response of the human tissue depends on the surface properties of the material which is in contact with it, the laser-based surface modification, which enhances the surface energy and wettability and also changes the chemistry of the surface, could be an alternative approach. In an early work, Mandolfino et al. studied the adhesion enhancement of polypropylene surface deploying two laser systems operating at 1064 and 355 nm wavelength [19]. This study suggests that polypropylene surface treatment by the laser source of 1064 nm (infrared region) leads to surface damage [19]. While in contrast, surface treatment by laser source of 355 nm presents better results with no surface damaging. This research group scrutinized laser parameters such as frequency and overlapping value. The authors inferred that using low values of frequency and overlapping results in better adhesive performance and superior mechanical behavior [19]. Park et al. searched the variation in pore size concerning laser parameters such as processing time and laser fluence to reach favorable results [18]. It was revealed that as the laser fluence increases the pores increase, provided there is sufficient processing time [18]. A femtosecond Titanium: Sapphire laser source was used to tailor surface morphology of PP to enhance automotive applications and surface changes were surveyed by different characterizations [72]. Laser modification contributes to introduce micrometric sized dimple- and groove-like structures, which changed wettability without altering surface chemical features. The measurement of water contact angle shows an obvious raising of wettability; in the other words the angle of water was reduced from 80° to 35°. μ-Raman spectroscopy data confirmed oxygen-containing functional groups was increased on the surface of PP. In contrast, the intensity of C=O was declined but the structure of PP was almost maintained. The surface adhesion was investigated by standard ink and methods, and the results suggest that femto-second laser treatment improve painting features and illuminate others surface activation step. The experimental setup and water contact angle were represented in Fig. 4.

(Reproduced from Guarnaccio et al. [72])
a A illustration of experimental rout and water contact angel for b untreated PP and c treated PP. the insets show optical microscopic images.
An ArF excimer laser was used to enhance the wettability of PP in water by replacing OH functional group by H atoms [73]. Indeed, C–H and C–H3 bonds are the prominent important for PP chemical stability. The XPS results show that the increase number of laser shots led to increasing O1s concentration in the presence of water on the surface sample. Additionally, the IR-ATR analysis affirmed that after laser treatment the OH stretching bond appeared at the center of 3300 cm−1, which turn the surface from hydrophobic to hydrophilic. The water contact angle was measured to elucidate the wettability of PP against the number of laser shot and the results exhibited increasing hydrophilicity because of decreasing water contact angle from 93° to 65° at 10,000 shots. The treated PP successfully bonded with an epoxy adhesive and the tensile shear strength of treated PP was increased about seven times higher than the untreated sample. To overcome lack of wettability of PP surface, the ArF excimer laser was utilized in air medium with different fluences (50, 100, 150, 200 mJ/cm2) [74]. The surface analysis was deployed to survey the effect of laser and water flux was measured to study storing capability of membrane. The AFM analysis affirmed decreasing of roughness from 48 to 17 nm. Furthermore, laser treatment changed the chemical composition confirmed by a peak located at 1720 cm−1 which is a signal of C=O functional group. According to XPS analysis, increasing of carbon bond and O/C was detected and formation of C–O and O–C=O, as well as increasing C=O, contribute to enhancement of wettability. WCA measurement was conducted and the results exhibited laser-modified by 200 mJ/cm2 possesses the lowest WCA about 75°. Water flux was tested for different applied pressure and the samples treated by 50 and 100 mJ/cm2 show an obvious increase compared to control sample. The experimental illustration of laser-assisted and water flux test, SEM image and percent of water flux as a function of pressure were illustrated in Fig. 5.

(Reproduced from Mohammadtaheri et al. [74])
a A schematic illustration of experimental details, b illustration for water flux experiment, c the SEM of PP morphology for 100 mJ/cm2, and d water flux as a function of pressure.
It should be underlined that laser-based techniques have not been extensively studied on polymers. Therefore, the optimum irradiation parameters (such as optimum fluence, wavelength, etc.) by which the better results (better surface morphology and chemistry) can be achieved have not been investigated in detail. Hence, several impacting factors remain unclear and investigation of these factors can stimulate researchers to more study about this technique [67].
5 Comparison of the Laser Treatment and Plasma Modification
Some polymers suffered from their low surface wettability or adhesion, which lead to hinder their application. Using laser and plasma are pioneering methods to modify the surface properties of polymers to achieve desirable products. A comparison discussion of their principles is necessary to get a clear view of the differences between plasma and laser. The interaction of plasma and polymer relied on the interaction of ions or charge carriers [10]. To be more direct, the species of the used gas in plasma could provide different reactions by polymer’s surface and the radicals, which are produced during plasma treatment, could contribute to happen other reactions. If the duration of plasma treatment excided, etching may be taken place during it and the losing of material is relied on treatment duration and plasma power [75].
The interaction of laser and polymers is attributed to coherence light, and the influence of laser beam on polymer surface relied on laser properties, such as fluence, the applying wavelength, and power [76]. Laser has been produced in different wavelengths and the interaction of laser beam and polymer surface can be divided by the wavelength range, i.e. the wavelength of laser is of importance to predict the type of interaction in advance. The UV irradiation can break molecular bonding down to photolytic interaction, which is unattainable in IR range. Indeed, in the IR region, the molecular of polymer are excited and the energy state of polymer reached a higher level. Heat generation is the consequence of molecular excitation and leads to breaking bonds and providing an opportunity for thermal reaction. In the visible region, the majority of interaction is associated to absorbing agents, which are decomposed during irradiation [76]. If laser fluence is lower than ablation threshold, surface modification is accomplished without losing weight [77].
From the review of literatures, it is obvious that the argon and nitrogen plasma modification offer multitudinous opportunity to create desired roughness and wetting in the polypropylene [30]. For example, better conductivity for polypropylene is obtained using nitrogen-plasma modification which is well above the conductivity of the untreated one [30]. To now, introducing hydroxyl groups which was attained by plasma modification carried out extensively on polymers have provided superior adhesion and hydrophilicity [27]. Considering the significant potential of laser to alter the surface properties of polymeric materials is crucial [68, 69]. The laser modification technique enjoys advantages which are highlighted as follows. An environmentally friendly polymer modification method with medium cost could be achieved by laser ablation, which is provided high speed and simple process; it is worth mentioning it could apply to large scale with limited damages [27, 67, 78]. Eliminating contamination significantly could decrease by using laser, because laser provides non-contact process. Performing in one-step, laser facilitates surface modification in the absence of chemical components [67]. Accordingly, surface modification of roughness and chemical properties for functionalization of polymer surface is attainable with ease.
Admittedly, it should be considered the low absorption coefficient of polypropylene in the wavelength range of 400 nm to 1600 nm restricts operating lasers which is an obvious handicap [27, 79]. The transparency of polypropylene in the aforementioned wavelength rang has been reported and to tackle this repercussion different methods such as using fillers, carbon pigment, and dyes are utilized [80, 81]. The optical absorption coefficient has been significantly enhanced in the near infrared region [81].
Generally, any method used to modify the surface properties of polymers has advantages and disadvantages. In other words, it is not possible to create all the desired features using one method. We can mention some of these benefits and drawbacks of the plasma modification approach according to the provided descriptions. Restricting the bulk properties changes of the polypropylene, the selection of gas type to make accurate changes, eliminating chemical reagents used in conventional approaches, and the reaching uniformity of surface are classified as obvious merits [34]. In contrast, the shortcoming could be clarified including: providing high vacuum situation, the dependence of modification degree to plasma parameters, costly process, and the absence of ability to control the formation of functional groups during process. One drawback in the use of lasers for treating polymers is their high transparency to the laser wavelength resulting in the minimum absorption of the laser beam [27].
6 Summary
Polypropylene as a promising polymer has a vital role in the industry. Its implementations, however, are constrained by its impractical properties, such as hydrophobicity. Accordingly, plasma and laser modification techniques as two popular methods have been used to develop polypropylene’s applications. Any method used to modify the surface properties of polymers has advantages and disadvantages. However, implementing environmentally-friendly and low-cost techniques is of particular interest. The present chapter provided a study on laser and plasma modification techniques of polypropylene membrane. These techniques were explained in terms of applying method and interaction and also compared on the base of effectiveness. In a conclusion, the laser modification technique is as effective as plasma modification with different active gases. The plasma modification technique is applied extensively on polypropylene and the formation of the polar functional groups is observed after the process. The reported results revealed that argon-plasma treatment induces dramatic changes in terms of surface roughness and morphology on polypropylene. Laser-based modifications could, in general, introduce groups containing oxygen on the polymeric surface. Surface treatment by laser source presents better results with no surface damaging and since this process is a non-contacting method, the outcome polypropylene presents better surface properties without additional contamination. Admittedly, the low absorption coefficient of polypropylene is the major handicap putting a limit on using laser sources. However, adding some fillers, carbon pigments, and dyes are recommended as good approaches to enhance absorption coefficient in the near infrared region. It seems that with more interest to use one-step modification techniques such as the laser method, the effect of processing parameters will be cleared which will be a significant achievement in the surface modification topic.
References
Ariono, D., Wardani, A.K.: Modification and applications of hydrophilic polypropylene membrane. In: IOP Conference Series: Materials Science and Engineering, vol. 1, p. 012014. IOP Publishing (2017)
Arora, P., Zhang, Z.: Battery separators. Chem. Rev. 104(10), 4419–4462 (2004)
Baker, R.W.: Membrane Technology and Applications, 2 edn. Wiley, Hoboken (2004)
Liu, F., Hashim, N.A., Liu, Y., Abed, M.M., Li, K.: Progress in the production and modification of PVDF membranes. J. Membr. Sci. 375(1–2), 1–27 (2011)
Hegemann, D., Brunner, H., Oehr, C.: Plasma treatment of polymers for surface and adhesion improvement. Nucl. Instrum. Methods Phys. Res., Sect. B 208, 281–286 (2003)
Wan, L.-S., Liu, Z.-M., Xu, Z.-K.: Surface engineering of macroporous polypropylene membranes. Soft Matter 5(9), 1775–1785 (2009)
Zou, L., Vidalis, I., Steele, D., Michelmore, A., Low, S., Verberk, J.: Surface hydrophilic modification of RO membranes by plasma polymerization for low organic fouling. J. Membr. Sci. 369(1–2), 420–428 (2011)
Goel, N., Bhardwaj, Y., Manoharan, R., Kumar, V., Dubey, K., Chaudhari, C., Sabharwal, S.: Physicochemical and electrochemical characterization of battery separator prepared by radiation induced grafting of acrylic acid onto microporous polypropylene membranes. eXPRESS Polym. Lett. 3(5), 268–278 (2009)
Akbar, D.: Surface modification of polypropylene (PP) using single and dual high radio frequency capacitive coupled argon plasma discharge. Appl. Surf. Sci. 362, 63–69 (2016)
Chan, C.-M., Ko, T.-M., Hiraoka, H.: Polymer surface modification by plasmas and photons. Surf. Sci. Rep. 24(1–2), 1–54 (1996)
Kochkodan, V.M., Sharma, V.K.: Graft polymerization and plasma treatment of polymer membranes for fouling reduction: a review. J. Environ. Sci. Health Part A 47(12), 1713–1727 (2012)
Bae, B., Chun, B.-H., Kim, D.: Surface characterization of microporous polypropylene membranes modified by plasma treatment. Polymer 42(18), 7879–7885 (2001)
Caiazzo, F., Canonico, P., Nigro, R., Tagliaferri, V.: Electrode discharge for plasma surface treatment of polymeric materials. J. Mater. Process. Technol. 58(1), 96–99 (1996)
Strobel, M., Jones, V., Lyons, C.S., Ulsh, M., Kushner, M.J., Dorai, R., Branch, M.C.: A comparison of corona-treated and flame-treated polypropylene films. Plasmas Polym. 8(1), 61–95 (2003)
Gomathi, N., Sureshkumar, A., Neogi, S.: RF plasma-treated polymers for biomedical applications. Curr. Sci. 1478–1486 (2008)
Galante, A., Leu, P.: Investigating antibacterial properties of plasma cleaned polypropylene. In: Highlighting Undergraduate Research at the University of Pittsburgh Swanson School of Engineering, p. 31 (2017)
Mansuroglu, D., Mecit, G., Uzun-Kaymak, I.U.: A study of crystallization in plasma modified polypropylene. Mater. Today Proc. 18, 1964–1971 (2019)
Park, C., Shin, B.-S., Kang, M.-S., Ma, Y.-W., Oh, J.-Y., Hong, S.-M.: Experimental study on micro-porous patterning using UV pulse laser hybrid process with chemical foaming agent. Int. J. Precis. Eng. Manuf. 16(7), 1385–1390 (2015)
Mandolfino, C., Pizzorni, M., Lertora, E., Gambaro, C.: Laser surface pre–treatment of polyolefin substrates for adhesive bonding. In: AIP Conference Proceedings 2019, vol. 1, p. 070002. AIP Publishing LLC
Fonouni, M., Yegani, R., Tavakkoli, A., Mollazadeh, S.: Investigating the effect of various oxidizing agents on the surface functionalization of microporous polypropylene membranes. J. Text. Polym. 4, 92–100 (2016)
Yin, M., Huang, J., Yu, J., Chen, G., Qu, S., Wang, X., Li, C.: The polypropylene membrane modified by an atmospheric pressure plasma jet as a separator for lithium-ion button battery. Electrochim. Acta 260, 489–497 (2018)
Himma, N.F., Wardani, A.K., Wenten, I.G.: Preparation of superhydrophobic polypropylene membrane using dip-coating method: the effects of solution and process parameters. Polym.-Plast. Technol. Eng. 56(2), 184–194 (2017)
Kwon, O.-J., Myung, S.-W., Lee, C.-S., Choi, H.-S.: Comparison of the surface characteristics of polypropylene films treated by Ar and mixed gas (Ar/O2) atmospheric pressure plasma. J. Colloid Interface Sci. 295(2), 409–416 (2006)
Novák, I., Špitalský, Z., Rástočná-Illová, D., Žigo, O., Mičušík, M., Nógellová, Z., Kleinová, A.: Polypropylene fabrics pre-treated by atmospheric plasma. Proc. Aprochem 2017, 224–229 (2017)
Saffar, A., Carreau, P.J., Kamal, M.R., Ajji, A.: Hydrophilic modification of polypropylene microporous membranes by grafting TiO2 nanoparticles with acrylic acid groups on the surface. Polymer 55(23), 6069–6075 (2014)
Saffar, A., Carreau, P.J., Ajji, A., Kamal, M.R.: Development of polypropylene microporous hydrophilic membranes by blending with PP-g-MA and PP-g-AA. J. Membr. Sci. 462, 50–61 (2014)
Riveiro, A., Soto, R., Del Val, J., Comesaña, R., Boutinguiza, M., Quintero, F., Lusquiños, F., Pou, J.: Texturing of polypropylene (PP) with nanosecond lasers. Appl. Surf. Sci. 374, 379–386 (2016)
Slepička, P., Kasálková, N.S., Stránská, E., Bačáková, L., Švorčík, V.: Surface characterization of plasma treated polymers for applications as biocompatible carriers. Express Polym. Lett. 7(6) (2013)
Guo, H., Ulbricht, M.: Surface modification of polypropylene microfiltration membrane via entrapment of an amphiphilic alkyl oligoethyleneglycolether. J. Membr. Sci. 349(1–2), 312–320 (2010)
Jaganathan, S.K., Balaji, A., Vellayappan, M.V., Subramanian, A.P., John, A.A., Asokan, M.K., Supriyanto, E.: Radiation-induced surface modification of polymers for biomaterial application. J. Mater. Sci. 50(5), 2007–2018 (2015)
Fabbri, P., Messori, M.: Surface modification of polymers: chemical, physical, and biological routes. In: Modification of Polymer Properties, pp. 109–130. Elsevier, Amsterdam (2017)
Siracusa, V.: Surface modification of polymers for food science. In: Surface Modification of Polymers: Methods and Applications, pp. 347–361 (2019)
Ciszewski, A., Gancarz, I., Kunicki, J., Bryjak, M.: Plasma-modified polypropylene membranes as separators in high-power alkaline batteries. Surf. Coat. Technol. 201(6), 3676–3684 (2006)
Abourayana, H.M., Dowling, D.P.: Plasma processing for tailoring the surface properties of polymers. In: Surface Energy, pp. 123–152. InTech, Rijeka (2015)
Aouinti, M., Chateigner, D., Gibaud, A., Poncin-Epaillard, F.: Planar texture developed in plasma treated polypropylene films. In: Materials Science Forum 2002, vol. 2, pp. 1579–1584. Transtec Publications (1999)
Jung, J.-S., Myung, S.-W., Choi, H.S.: Surface modification of polypropylene by nitrogen-containing plasma. Korean J. Chem. Eng. 25(5), 1190–1194 (2008)
Nehra, V., Kumar, A., Dwivedi, H.: Atmospheric non-thermal plasma sources. Int. J. Eng. 2(1), 53–68 (2008)
Bhat, N., Upadhyay, D.: Plasma-induced surface modification and adhesion enhancement of polypropylene surface. J. Appl. Polym. Sci. 86(4), 925–936 (2002)
Klemberg-Sapieha, J., Küttel, O., Martinu, L., Wertheimer, M.: Dual-frequency N2 and NH3 plasma modification of polyethylene and polyimide. J. Vac. Sci. Technol. A Vac. Surf. Films 9(6), 2975–2981 (1991)
Shahidi, H.: Journalism in Iran: From Mission to Profession. Routledge, London (2007)
Chaivan, P., Pasaja, N., Boonyawan, D., Suanpoot, P., Vilaithong, T.: Low-temperature plasma treatment for hydrophobicity improvement of silk. Surf. Coat. Technol. 193(1–3), 356–360 (2005)
Desmet, T., Morent, R., De Geyter, N., Leys, C., Schacht, E., Dubruel, P.: Nonthermal plasma technology as a versatile strategy for polymeric biomaterials surface modification: a review. Biomacromol 10(9), 2351–2378 (2009)
Li, X.-M., Reinhoudt, D., Crego-Calama, M.: What do we need for a superhydrophobic surface? A review on the recent progress in the preparation of superhydrophobic surfaces. Chem. Soc. Rev. 36(8), 1350–1368 (2007)
Dorai, R., Kushner, M.J.: A model for plasma modification of polypropylene using atmospheric pressure discharges. J. Phys. D: Appl. Phys. 36(6), 666 (2003)
Gomathi, N., Neogi, S.: Surface modification of polypropylene using argon plasma: Statistical optimization of the process variables. Appl. Surf. Sci. 255(17), 7590–7600 (2009)
Himma, N.F., Anisah, S., Prasetya, N., Wenten, I.G.: Advances in preparation, modification, and application of polypropylene membrane. J. Polym. Eng. 36(4), 329–362 (2016)
Yu, H.-Y., He, X.-C., Liu, L.-Q., Gu, J.-S., Wei, X.-W.: Surface modification of polypropylene microporous membrane to improve its antifouling characteristics in an SMBR: N2 plasma treatment. Water Res. 41(20), 4703–4709 (2007)
Yu, H.-Y., Liu, L.-Q., Tang, Z.-Q., Yan, M.-G., Gu, J.-S., Wei, X.-W.: Surface modification of polypropylene microporous membrane to improve its antifouling characteristics in an SMBR: air plasma treatment. J. Membr. Sci. 311(1–2), 216–224 (2008)
Slepička, P., Vasina, A., Kolská, Z., Luxbacher, T., Malinský, P., Macková, A., Švorčík, V.: Argon plasma irradiation of polypropylene. Nucl. Instrum. Methods Phys. Res. Sect. B 268(11–12), 2111–2114 (2010)
Ebnesajjad, S., Ebnesajjad, C.: Plasma treatment of polymeric materials. In: Andrew, W. (eds.) Surface Treatment of Materials for Adhesive Bonding, pp. 227–269 (2014)
Chen, J.-P., Chiang, Y.-P.: Surface modification of non-woven fabric by DC pulsed plasma treatment and graft polymerization with acrylic acid. J. Membr. Sci. 270(1–2), 212–220 (2006)
Poncin-Epaillard, F., Legeay, G.: Surface engineering of biomaterials with plasma techniques. J. Biomater. Sci. Polym. Ed. 14(10), 1005–1028 (2003)
Řezníčková, A., Kolská, Z., Hnatowicz, V., Stopka, P., Švorčík, V.: Comparison of glow argon plasma-induced surface changes of thermoplastic polymers. Nucl. Instrum. Methods Phys. Res. Sect. B 269(2), 83–88 (2011)
Liang, C.-H., Li, C., Chen, T.-H., Cheng, C.-Y., Huang, C.: Surface evaluation of reactive plasma-modified microporous polypropylene membrane by static contact angle analysis. Polym. Degrad. Stab. 160, 89–95 (2019)
Mandolfino, C., Lertora, E., Gambaro, C., Pizzorni, M.: Functionalization of neutral polypropylene by using low pressure plasma treatment: effects on surface characteristics and adhesion properties. Polymers 11(2), 202 (2019)
Juang, R.-S., Hou, W.-T., Huang, Y.-C., Tseng, Y.-C., Huang, C.: Surface hydrophilic modifications on polypropylene membranes by remote methane/oxygen mixture plasma discharges. J. Taiwan Inst. Chem. Eng. 65, 420–426 (2016)
Darvish, F., Sarkari, N.M., Khani, M., Eslami, E., Shokri, B., Mohseni, M., Ebrahimi, M., Alizadeh, M., Dee, C.F.: Direct plasma treatment approach based on non-thermal gliding arc for surface modification of biaxially-oriented polypropylene with post-exposure hydrophilicity improvement and minus aging effects. Appl. Surface Sci. 509, 144815 (2020)
Yun, Y.I., Kim, K.S., Uhm, S.-J., Khatua, B.B., Cho, K., Kim, J.K., Park, C.E.: Aging behavior of oxygen plasma-treated polypropylene with different crystallinities. J. Adhes. Sci. Technol. 18(11), 1279–1291 (2004)
Yu, H.-Y., Xie, Y.-J., Hu, M.-X., Wang, J.-L., Wang, S.-Y., Xu, Z.-K.: Surface modification of polypropylene microporous membrane to improve its antifouling property in MBR: CO2 plasma treatment. J. Membr. Sci. 254(1–2), 219–227 (2005)
Yan, M.-G., Liu, L.-Q., Tang, Z.-Q., Huang, L., Li, W., Zhou, J., Gu, J.-S., Wei, X.-W., Yu, H.-Y.: Plasma surface modification of polypropylene microfiltration membranes and fouling by BSA dispersion. Chem. Eng. J. 145(2), 218–224 (2008)
Tsai, C.-Y., Juang, R.-S., Huang, C.: Surface modification of polypropylene membrane by RF methane/oxygen mixture plasma treatment. Jpn. J. Appl. Phys. 50(8S2), 08KA02 (2011)
Arefi, F., Andre, V., Montazer-Rahmati, P., Amouroux, J.: Plasma polymerization and surface treatment of polymers. Pure Appl. Chem. 64(5), 715–723 (1992)
Shaw, D., West, A., Bredin, J., Wagenaars, E.: Mechanisms behind surface modification of polypropylene film using an atmospheric-pressure plasma jet. Plasma Sources Sci. Technol. 25(6), 065018 (2016)
Honarvar, Z., Farhoodi, M., Khani, M.R., Mohammadi, A., Shokri, B., Ferdowsi, R., Shojaee-Aliabadi, S.: Application of cold plasma to develop carboxymethyl cellulose-coated polypropylene films containing essential oil. Carbohyd. Polym. 176, 1–10 (2017)
Sato, T., Akiyama, H., Horiuchi, S., Miyamae, T.: Characterization of the polypropylene surface after atmospheric pressure N2 plasma irradiation. Surf. Sci. 677, 93–98 (2018)
Sanbhal, N., Mao, Y., Sun, G., Xu, R.F., Zhang, Q., Wang, L.: Surface modification of polypropylene mesh devices with cyclodextrin via cold plasma for hernia repair: Characterization and antibacterial properties. Appl. Surf. Sci. 439, 749–759 (2018)
Riveiro, A., Maçon, A.L., del Val, J., Comesaña, R., Pou, J.: Laser surface texturing of polymers for biomedical applications. Front. Phys. 6, 16 (2018)
Riveiro, A., Soto, R., Comesana, R., Boutinguiza, M.d., Del Val, J., Quintero, F., Lusquiños, F., Pou, J.: Laser surface modification of PEEK. Appl. Surf. Sci. 258(23), 9437–9442 (2012)
Riveiro, A., Soto, R., Del Val, J., Comesaña, R., Boutinguiza, M., Quintero, F., Lusquiños, F., Pou, J.: Laser surface modification of ultra-high-molecular-weight polyethylene (UHMWPE) for biomedical applications. Appl. Surf. Sci. 302, 236–242 (2014)
Etsion, I.: State of the art in laser surface texturing. J. Tribology 127(1), 248–253 (2005)
Kurella, A., Dahotre, N.B.: Surface modification for bioimplants: the role of laser surface engineering. J. Biomater. Appl. 20(1), 5–50 (2005)
Guarnaccio, A., Belviso, C., Montano, P., Toschi, F., Orlando, S., Ciaccio, G., Ferreri, S., Trevisan, D., Mollica, D., Parisi, G.P.: Femtosecond laser surface texturing of polypropylene copolymer for automotive paint applications. Surf. Coat. Technol. 406, 126727 (2021)
Murahara, M., Okoshi, M.: Photochemical surface modification of polypropylene for adhesion enhancement by using an excimer laser. J. Adhes. Sci. Technol. 9(12), 1593–1599 (1995)
Mohammadtaheri, S., Jaleh, B., Mohazzab, B.F., Eslamipanah, M., Nasrollahzadeh, M., Varma, R.S.: Greener hydrophilicity improvement of polypropylene membrane by ArF excimer laser treatment. Surf. Coatings Technol. 399, 126198 (2020)
Friedrich, J.: The Plasma Chemistry of Polymer Surfaces. Advanced Techniques for Surface Design. Wiley, Hoboken (2012)
Mittal, K.L., Bahners, T.: Laser Surface Modification and Adhesion. Wiley, Hoboken (2014)
Slepička, P., Slepičková Kasálková, N., Kolská, Z., Švorčík, V.: Surface modification of polymer substrates for biomedical applications. In: Surface Modification of Polymers: Methods and Applications, pp. 399–426 (2019)
Mandolfino, C., Pizzorni, M., Lertora, E., Gambaro, C.: Laser surface texturing of polypropylene to increase adhesive bonding. In: AIP Conference Proceedings 2018, vol. 1, p. 060004. AIP Publishing LLC
Klein, R.: Laser Welding of Plastics: Materials, Processes and Industrial Applications. Wiley, Hoboken (2012)
Glaser, S.: Colorants and special additives for laser welding. Join. Plast. 25–26 (2006)
Kagan, V., Bray, R., Kuhn, W.: Laser transmission welding of semi-crystalline thermoplastics—Part I: optical characterization of nylon based plastics. J. Reinf. Plast. Compos. 21(12), 1101–1122 (2002)
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Jaleh, B., Mohazzab, B.F., Mohazzab, B.F., Moradi, A. (2022). Comparison of the Effect of Excimer Laser Irradiation and Plasma Treatment on Polypropylene Membrane Surface. In: Baneesh, N.S., Sari, P.S., Vackova, T., Thomas, S. (eds) Plasma Modification of Polyolefins. Engineering Materials. Springer, Cham. https://doi.org/10.1007/978-3-030-52264-3_7
Download citation
DOI: https://doi.org/10.1007/978-3-030-52264-3_7
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-52263-6
Online ISBN: 978-3-030-52264-3
eBook Packages: Chemistry and Materials ScienceChemistry and Material Science (R0)