Abstract
Polyolefins are well-known and the most commonly used polymers worldwide. Advantages like outstanding mechanical properties, chemical resistance, low cost, and processability are neighboring with some drawbacks like relatively high gas and vapor permeability, low surface energy. This chapter introduces surface plasma modification as an environmentally friendly, fast, and versatile technique. Details regarding different plasma reactor designs, generation methods, working parameters suitable for treating polyolefins are presented. Furthermore, plasma activation, grafting, and etching are described as the most commonly used techniques for surface energy modification to enhance polyolefins' biocompatibility, printability, adhesion to materials, and other parameters. For instance, plasma activation cross-linking of the polymer chains can be achieved, which leads to gas and vapor permeability improvement. Choice of working conditions allows controlling the degree of cross-linking, the type, and the concentration of the incorporated functional groups on the surface. Plasma polymerization is introduced as a technique for coating deposition with different properties and functionality depending on the operating parameters and monomer selection. Improvement of barrier layer performance and modification of the surface energy are the main applications of plasma polymerization of polyolefins.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
1 Introduction
Polyolefins are a family of synthetic polymers prepared by the polymerization of olefin monomers. Olefins are acyclic and cyclic hydrocarbons that contain one or more carbon–carbon double bonds without an aromatic character [105]. Figure 1 shows some examples of polyolefins.
Polyolefins are the most commonly used polymers worldwide due to their low cost, processability, chemical resistance and mechanical properties (ranging from elastomeric to very rigid materials) [16]. By varying the processing parameters and the possible implementation of copolymerization and cross-linking, the desired properties can be obtained. Among the different polyolefins, polyethylene (PE) and polypropylene (PP) are most widely employed. For commercial purposes, isotactic PP (methyl side-groups located on the same side of the backbone) is most often considered due to its superior mechanical properties, originating from a higher degree of crystallinity. In contrast to PP, commercial PE can be classified based on its cross-linking degree, density and molecular weight. The most extensively used types are: (1) low-density PE (LDPE), which is the original commercial PE containing significant branching, resulting in a lower polymer chain packing density and therefore a low material density; (2) high-density PE (HDPE), being a highly linear polymer in which the polymer chains are tightly packed together, leading to a higher density and a more crystalline character and (3) linear low-density PE (LLDPE), being a similar linear polymer with short chain branches thus triggering similar densities as LDPE [120].
The application range of polyolefin-based materials is very broad, ranging from the biomedical field to the automotive industry and from packaging equipment to toys. In the biomedical field, the use of PP as hernia repair meshes, sutures and disposable items like syringes are good examples [123]. Ultra-high-molecular-weight PE (UHMWPE) has particularly gained considerable attention in this field given its improved mechanical properties engendered by the intermolecular interactions between the long chains. These properties make UHMWPE a suitable bearing material in joint replacements, for instance. LDPE, LLDPE, HDPE and PP are most commonly used for packaging of food, drinks and other consumer goods [120]. PP has also become the most employed thermoplastic material in the automobile industry [24]. Furthermore, cyclic olefin polymers (COPs) and cyclic olefin copolymers (COCs) have attracted much attention for microfluidic and biosensor applications [127]. This widespread use evidence that the aforementioned properties of polyolefins are highly advantageous for various applications. Nonetheless, some polymer characteristics are a disadvantage for certain purposes. Generally, these disadvantages can be divided in 2 main categories. The first category is the hydrocarbon nature of these polymers conferring them with a low surface energy. This makes printing on and adhesive bonding to the polymer difficult. Depositing a coating on the substrate is also challenging. Moreover, the polymer composition hampers cell adhesion and therefore subsequent cell performances such as proliferation, differentiation and migration. On the other hand, polyolefins are not completely inert, which is a problem for biomedical applications that demand either a good cell interaction or complete inertness [101, 146]. The second category is the relatively high gas and vapor permeability of the polymers, which is a major limitation for packaging applications given the resulting restricted shelf-life of the packed food or other goods. Typically, polyolefins have a moderate to good resistance against water vapor but are highly permeable to oxygen. The origin of this permeability is not completely clarified, but it is hypothesized that density and crystallinity play a role [152].
Several modification strategies have been developed to address these 2 problems. These strategies can be mainly divided based on the different underlying problems. The introduction of polar functional groups on the polymer surface is the main method to address the chemical inertness of polyolefins (problem 1). This can lead to a better adhesion in the aforementioned applications. A distinction can be made between bulk and surface functionalization, as illustrated in Fig. 2. Bulk functionalization can introduce functional groups on the polymer chains with a homogeneous distribution on the surface and in the bulk of the resulting material. This can be either done by modifying the polymerization process (a) or by functionalizing the polymer after polymerization (b). In the co-polymerization functionalization (a), monomers with the desired functional group are introduced in the polymerization process so that the resulting polymer has these groups build-in. In the post-polymerization functionalization (b), the C-H bonds in the polymer chain are preferably broken, which leads to the generation of reactive radicals on the polymer chain. Reactions with these sites lead to a functionalization of the polymer. In the surface functionalization (c), only the outer layer of the polyolefin will be modified, leaving the bulk of the material untouched. This method is advantageous for most applications, as the bulk properties that are most often desirable are not affected. Moreover, for most purposes, the interactions with the material usually take place at the surface. Additionally, the bulk modification needs an optimization of the well-established industrial material processing steps, while the surface modification is performed afterwards and is independent of these processing steps. Therefore, surface modifications provide an interesting and often sufficient strategy for material improvements.
Difference between bulk functionalization (a and b) and surface functionalization (c). a: functionalization by co-polymerization with a functional monomer. b: post-polymerization functionalization of the polymer chains before processing the material. Both techniques lead to functional groups in the bulk and on the surface of the material [16]. c: surface functionalization. * can be the desired functional group or a functional group that can be converted to the desired functional group
In order to address the gas and vapor permeability (problem 2), a number of different strategies can be applied. The most important ones are the blending with low permeable materials to form nanocomposite materials and the modification of the surface by adding a barrier layer on top of the surface or changing the permeability of the surface itself by polymer cross-linking [152]. It is clear that the first technique has the same disadvantages as the bulk functionalization technique in comparison to surface treatments, as changing the bulk can, on the one hand, alter the advantageous polyolefin properties and, on the other hand, requires an optimization of the material processing. However, applying a barrier layer on the surface requires a good adhesion, thus making the chemical inertness a problem that also needs to be solved for addressing the gas and vapor permeability problem of polyolefins.
In view of the above, it can be concluded that the surface modification of polyolefins aiming at improving their properties is an interesting approach. In particular, increasing the surface energy is of uttermost importance. This can be done by a variety of different techniques. Similar to the polymer functionalization (Fig. 2), all surface modification techniques aim at breaking the C–H or C–C bond and introducing other functionalities on the polymer chain. One of the techniques to break C–H/C–C bonds is the use of γ- or UV-irradiation. The created radicals can react with oxygen, which leads to the generation of oxygen-containing functional groups like alcohols, hydroperoxides and ketones [85]. Another technique for the oxidation of polyolefin surfaces is flame treatment, in which radicals are created in the combustion process. This leads to the generation of carbonyl, carboxyl and hydroxyl groups on the surface. Both techniques lead to an increased surface energy, which is beneficial for applications in which a good adhesion is required [43]. A third technique is the chemical treatment of the polyolefin surface. This can be done by using oxidizing agents like chromic acid or aqueous solution of ammoniacal ammonium persulfate in the presence of Ni+2 ions, for example, which leads to the introduction of polar groups such as >C = O and –COOH [36]. A fourth technique is the use of plasma surface modification, which will be the main topic of this book chapter. In comparison to the abovementioned techniques, plasma surface modification has a number of advantages. Compared to the irradiation techniques and the chemical treatments with typical treatment times in the order of hours, plasma modification is a fast technique with treatment times ranging in the order of seconds to minutes [163]. Furthermore, plasma modification is a solvent-free technique, which makes it more environmentally friendly than chemical treatments. Flame treatment is also a fast and solvent-free technique, but care should be provided to prevent polymer melting, which is not a problem with optimized plasma surface modifications. The latter technique can also treat surfaces that cannot be reached with flame treatment, enabling the modification of 3D porous objects. Plasma surface modifications of polyolefins thus provide a platform with a number of advantages over other modification strategies and are therefore found in a variety of applications and research fields. Besides changing the surface energy, plasma surface modifications are also used for improving the gas and vapor permeability of polyolefins, making it a more widely applicable technique. On the other hand, there still remain some challenges as plasma treated surfaces are prone to ageing effects and the selectivity of the treatment is limited. This book chapter will give a general overview of the different plasma surface modifications of polyolefins. The focus will be on the general concepts of these techniques with references to the application potential. Therefore, the following sections will first introduce the concept of plasma and different plasma classifications (Sect. 2) and the methods to generate plasma for polyolefin surface modification (Sect. 3). This will provide the basis for a general description of plasma-surface interactions and different plasma surface modification techniques applied to polyolefins (Sect. 4).
2 Plasma Definition and Classification
2.1 What is Plasma?
The word “plasma”, which was first introduced by Langmuir, can be defined as an environment where energetic, charged and neutral carrier assemblies move in random directions and exhibit a collective behavior [89]. The net electrical charge in this mixture of oppositely charged particles is approximately zero. Plasma is commonly referred to as the fourth state of matter and constitutes the most common state in the universe as stars, nebulae and Aurora consist of it. On earth, natural plasmas like lightning and Aurora Borealis are rare, and most common plasmas are man-made. In contrast to celestial plasmas that are generated by the addition of thermal energy, the most conventional method of plasma generation on earth is a gas breakdown, using an electric field. Even a neutral gas has some charged particles, that are generated by phenomena such as space radiations, which can accelerate in the presence of an electric field, thus hitting other species. At the right conditions, this can create new charges with the ability to collide with other molecules and atoms. This produces new electrons, negative and positive ions and radiation, leading to the generation of an avalanche of charged species until a steady state condition is reached. Besides the aforementioned species, usually a plasma also contains excited species and radicals. The produced radiation is mainly situated in the visible and ultraviolet (UV) range and is generated as a result of inelastic collisions between atoms/molecules and energetic electrons. This process can lead to excitation of the atom/molecule (A + e → A* + eʹ), among others, which can subsequently induce emission of the energy stored in this excited state via radiation in a range of different wavelengths. This results in the luminosity of plasma (A* → A + hν). Furthermore, charges can be lost by homogenous recombination (A+ + e− → A*) and/or diffusion to the wall of the discharge (A+ → A).
In plasma surface modification, the plasma particles are brought in contact with a substrate (e.g. polyolefin) by placing it in the (vicinity of a) discharge. These energetic species can interact with this substrate as schematically presented in Fig. 3, resulting in the alteration of its surface properties. The interactions between the plasma species and the surface mainly depend on the respective amount and energy of these different particles. This is influenced by a number of plasma characteristics of which the ionization degree, plasma equilibrium, working pressure and discharge driving frequency are the most relevant for polyolefin surface modifications. These characteristics will be discussed in more detail in Sect. 2.2, which will form the fundamental basis for explaining the different plasma source configurations (Sect. 2.3) and plasma modification techniques (Sect. 2.4).
2.2 Plasma Classification
2.2.1 Plasma Ionization
The degree of ionization α is defined as α = N+/(N + N+), in which N+ is the number of ions and N the number of neutral particles. It is a dimensionless number and can be denoted as a percentage. The ionization degree determines the amount of charged particles in the plasma and is, therefore, a measure of the interaction between a plasma and the electric and magnetic fields. An ionized gas can be identified as plasma when the degree of ionization is at least 10–6 [26]. Plasma is called weakly ionized if 10–6 < α < 10–1. For this type of plasma, the collisions happen more often between neutrals and electrons than between ions and electrons. A weakly ionized plasma can be called a “low-temperature plasma” or more often “cold plasma”, due to considerably low electron temperature which controls the degree of ionization in the plasma. Plasma can be referred to as “hot plasma” when it is almost or fully ionized (α ≈ 1).
2.2.2 Plasma Thermodynamic Equilibrium
Plasmas can be also classified in terms of their thermodynamic equilibrium, which is based on the relative temperature of ions, electrons and neutrals. Three different classes can be defined: thermal equilibrium, local thermal equilibrium and non-thermal equilibrium.
In thermal equilibrium plasmas, all components are in thermodynamic equilibrium with each other (Telectron = Tion = Tgas). Such an environment can be created in high temperature and high-density plasma due to frequent collisions between fast electrons and heavy species (ions and neutrals). Typically, these plasmas also have a high degree of ionization. Some examples are lightening, the solar core and thermonuclear fusion plasmas. The temperature of these plasmas is too high for the treatment of polyolefins. Therefore, they are not further discussed.
In non-thermal or non-equilibrium plasmas, the temperature of electrons, which typically is in the range of 2 to 10 eV, and the temperature of ions are significantly different (Telectron > > Tion = Tgas). The reason for this difference is that electrons gain much more energy from the applied power due to their considerably lower mass in comparison to ions. Moreover, the energy transfer from electrons to ions and neutrals is not efficient in this type of plasma because of conservation of momentum. Typically, these plasmas are weakly ionized and are therefore most often cold plasmas, however more highly ionized non-thermal plasmas also exist. Examples of non-thermal plasmas are the Earth’s ionosphere and most of the “technological plasmas”. The application range of non-thermal plasmas is broad and covers analytical chemistry, environmental engineering and biomedicine, among others. These plasmas are most suitable for the treatment of heat sensitive materials such as polyolefins.
Besides the two mentioned classes of plasma, there are also local thermal equilibrium plasmas which are in quasi-equilibrium. This means that the plasma is not far from full equilibrium, yet it is not fulfilled in all volume. Nevertheless, within the area, local thermodynamic equilibrium exists, and the electron, ion and neutral temperatures are in the same range (0.4 – 1 eV). The ion temperature is considerably higher than the one of non-thermal equilibrium plasma while the electron temperature is much lower. This type of plasma can be used for surface modification techniques like chemical and physical vapor deposition as well as plasma spraying. However, these techniques are generally not used for direct continuous polymer treatment as the overall plasma temperature will damage the surface.
2.2.3 Plasma Pressure
Plasma density is directly proportional to plasma pressure and both can be described together as one parameter. Working pressure determines plasma appearance, energy, temperature and other characteristics which are essential for surface modification applications. A low pressure plasma works at sufficiently low pressures (typically lower than 1 Torr), at which the collision between heavy particles is correspondingly low, which favors its non-thermal equilibrium character. Equally important, when the pressure is very low, high voltage and current are required to ignite and sustain the discharge due to the same reasons mentioned above. However, a low pressure plasma provides a controlled environment in which parameters like power, temperature and gas flow can be tuned for obtaining the desired surface reactions. As a result, a homogeneous and selective interaction between plasma and the substrate is feasible. At really low pressures, the lack of charged particles decreases the coupling efficiency of electromagnetic energy into the plasma, which makes it impossible to use high-frequency power sources like radiofrequency (RF) and microwave (MW) discharges (see Sect. 2.2.4).
For atmospheric pressure plasmas (APPs), the electron mean free path is significantly decreased in comparison to low pressure plasmas because of the increase in pressure. Correspondingly, the number of collisions drastically increases as well, which rises the temperature of the plasma. Therefore, an APP has a lower degree of ionization, a lower electron temperature and a higher particle and electron density as compared to low pressure plasmas. The particle density is in the order of 1012–1014 particles cm−3 for low pressure plasmas and 1015–1018 particles cm−3 for APPs. The first APP was developed as a high temperature arc-based source [51]. However, a stable homogeneous low-temperature plasma is required for the treatment of polyolefins, as the instability of the APP and the possible transition to a thermal arc are major issues because of the material’s thermal sensitivity. By the beginning of the 1990s, non-equilibrium APPs began to overcome this problem by limiting the time of each glowing duration. This can either be done by utilizing a pulsed power supply as an energy source, or by alternating the polarity of electrodes with a high frequency. Another approach to control the transition to arc and thermal equilibrium is using a dielectric barrier which is discussed in detail below [87]. Furthermore, discharge gas selection and flow rate can have a significant influence on the stability of APPs. For instance, helium (He) is a well-known gas for producing a stable plasma due to the small atom size and hence a longer mean free path. Moreover, increasing the intensity of the electric field by implementing sharp points at the edge of the powered electrode (e.g. corona discharge) is another adjustment performed for obtaining low-temperature plasmas at atmospheric pressure. APPs are more widely accepted for industrial purposes because of the convenient implementation in industrial processes, the lacking need for expensive and huge vacuum equipment and the lower complexity of the reactors.
In between the pressure range of low pressure plasmas and APPs, medium pressure plasmas can be defined. This is a less frequently explored pressure range. However, this range is also interesting because obtaining a large plasma volume is more easy at medium pressure than atmospheric pressure. Furthermore, no expensive vacuum equipment is needed [32, 108, 110].
2.2.4 Plasma Excitation Frequency
As mentioned before, one of the most common methods to transform a neutral gas into a plasma discharge is by utilizing an electric field. According to the temporal behavior of the electric field, discharges can be classified as direct current (DC), pulsed DC, alternating current (AC), RF and MW discharges. Depending on this temporal behavior, different plasma chemistries can be obtained and utilized in a variety of processes. In order to predict possible surface plasma treatment of polyolefins, it is crucial to describe the main characteristics of each class of plasma excitation frequencies.
When a potential difference of zero frequency is applied between a cathode and an anode, the gas breakdown happens by a DC discharge. In this type of plasma, secondary electrons emitted from the cathode play a crucial role as they collide with the background gas and finally reach drift velocity along the tube axis. DC discharges have attracted enormous interest because of their time-independence in the macroscopic scale, which makes them more straightforward compared to RF discharges. At higher pressure, the transition to the arc is more probable because of an increased current density, which contributes to a higher temperature and instability of the plasma. This drawback limits the application of DC discharges in case of plasma treatment of polyolefins. Furthermore, plasma can be contaminated by sputtering the cathode surface, and the electrodes can only be made of conductive materials. Usually, the voltage can vary between a few hundred Volts to a few kV based on the application and the current is in the order of mA. There are possibilities to overcome the transition to arc, such as minimizing the radial size or using a dielectric barrier or a corona discharge source configuration.
Another option to avoid the transition of a DC discharge to an arc is to apply the electric field in a discrete form of pulses from microsecond to millisecond while keeping the amplitude of voltage and current high. The relatively short pulse duration contributes to the formation of non-equilibrium plasmas. The charged particles notice the relatively short pulses before the spark creation. The main advantages of using pulsed DC discharges are minimized etching and damage of the treated substrate surface and the possibility of working at a higher power by controlling the duty cycle.
AC discharges have electromagnetic field oscillations in the range of kHz. This discharge type is appropriate for overcoming the charge-accumulation problem that occurs when non-conductive materials are placed between the electrodes (as in dielectric barrier discharges (DBDs), see Sect. 3.4). In fact, the alternating voltage between electrodes in each half cycle will produce opposite charge accumulation. The advantage of oscillating power supplies over DC discharges is that they interact with plasma by displacement currents, rather than true currents. Therefore, no contact between the electrodes and plasma is needed. The absence of the connection between the discharge and the electrodes improves the reliability, reproducibility, and lifetime of plasma reactors and the produced species. The influence of frequency on the treatment of polyolefin substrates has been previously studied and the investigations have suggested that an increase in frequency can improve the surface modification [1, 82].
Low-frequency plasmas (up to 450 kHz) are widely used due to the simplicity of the power source. However, this discharge type has a slower reaction rate and a higher transition to arc probability as compared to higher frequency plasma sources. RF discharges can be utilized to overcome these limitations. RF discharges are obtained when the gas is subjected to an oscillating electromagnetic field with frequencies in the range of MHz. The main distinction that identifies RF plasmas from lower frequency plasmas is the dynamic behavior of electrons and ions: only electrons can follow the changes in the radiofrequency electromagnetic field, while heavy ions remain almost stationary. This is opposite to a low frequency plasma where both charged species move according to the electromagnetic field. Moreover, since the quenching time of reactive plasma species is longer than half a period cycle of an RF source, the stability considerably increases. Hence, RF discharges have some advantages, such as the less pronounced electron and ion bombardment of the electrodes, negligible thermal output, better discharge stability, higher electrical efficiency due to the lower electrode loss and higher electron kinetic temperature which results in the increased number of radicals, chemical reactions and ionization processes inside the plasma. This results in a large density of reactive species at a reduced temperature in comparison to lower frequency discharges [129, 164]. All these advantages make RF plasmas suitable for surface modification of polyolefins.
MW plasmas can be generated and sustained by electromagnetic radiation in the frequency range of 300 MHz–300 GHz. The typical frequency is 2.45 GHz, which corresponds to a wavelength that is comparable to plasma reactor dimensions (12.24 cm). In comparison with other power generators, MW generators require lower gas flow rates and powers in order to ignite a plasma. More dissociation of species also occurs in this system, which may lead to deposition and etching.
3 Plasma Source Configurations
Feasibility, in combination with some attractive properties for technological and industrial demands, has led scientists to broaden the applicability of plasma sources by using different configurations. Since the main focus here is directed towards a partially ionized low-temperature plasma for polyolefin surface modifications, the description is limited to the main plasma source configurations used for this purpose.
3.1 Corona Discharge
A corona discharge can be initiated by a local breakdown of gas in atmospheric pressure when the current density is comparatively low. A sufficiently high electric field in close proximity to the edges or small radii of curvature in a plasma reactor usually gives rise to a corona discharge development. Due to the electrode geometry and configuration, ionization occurs locally around tips or sharp points in the absence of insulating surfaces. It is also possible to ignite such a discharge without the presence of a grounded electrode. In this case, the surrounding environment acts like one. Typically, corona discharges contain a drift region positioned between the ionization region near the tip of the powered electrode and the grounded (low-field) electrode. This large drift zone can enhance the excitation or recombination of species such as ions, electrons and neutrals. However, due to inelastic collisions, this region is depleted from reactive components, and consequently, only a free radical chemistry happens there. Increasing the current leads first to the production of a burst corona, which is characterized by simple avalanches. The discharge can change to a regime where the charge density is high enough to trigger the streamer mechanism by further increasing the current. This highly branched non-uniform streamer, which occurs due to inhomogeneity in a corona discharge, can go beyond the active zone but cannot propagate infinitely. In fact, if there is enough applied potential through the conductive channel, it can lead to a longer streamer that might have better applicability. The presence of free transient electrons in such streamers makes them capable of dissociating and exciting neutrals. By increasing the current even further, it is possible to have extreme secondary emission that produces a self-sustained discharge which is quite similar to a glow discharge. If the secondary emission is really efficient, the discharge can make the transition to spark and arc [21]. For the treatment of heat sensitive materials, the latter is, however, undesirable. An alternative approach is the use of a corona discharge with a shortened lifetime. It has been shown that a pulsed power supply with a pulse duration between 100 and 300 ns provides an operating time small enough to prevent the transition from streamer to spark and leads to an increase in the ionization degree [52]. Consequently, a more ionized environment can improve the efficiency of the surface treatment. Figure 4 depicts different electrode configurations that produce structurally different corona discharges [35]. Nevertheless, in corona configuration, introducing plain polyolefin film gives a superficial resemblance to DBDs as the polymer can act as a dielectric barrier. This case is not valid for polyolefin textiles and fibers.
The generation of a corona discharge with low power and low temperature at atmospheric pressure opens a wide range of possible applications including treatment of polyolefin materials. The utilization of corona in the surface treatment of polymer is commercialized. Polyolefins have been ensured to be easily and successfully treated with corona discharge resulting in a significant surface oxidation. The main advantages of this plasma are the simplicity of the design, the generation of significantly small discharge currents and the possibility to work in atmospheric pressure and using ambient air as the reagent gas. However, the last feature is beneficial only for applications that are dealing with surface oxidation while for other types of treatment, this aspect can be a necessary restriction. This limitation can be solved by working with specific gases and in defined ambient conditions. However, this is not economically efficient and hazardous substances can only be used with designated suction systems [95].
3.2 RF Discharge
As mentioned before, high-frequency discharges have become more attractive due to the high density of excited species in low-temperature plasmas which makes such sources suitable for the surface treatment of polyolefins [93, 165]. The principal division in RF plasma sources depends on how the energy is coupled to the system. Figure 5 shows the two coupling mechanisms, namely capacitive or inductive coupling.
An Inductively coupled plasma (ICP) is characterized by a magnetic field of an induction coil or inductor in which the discharge is located. The coil can have a spiral or helix shape and can be positioned inside or outside the plasma volume. In most of the ICP arrangements, a quartz tube is used to separate the coil and the discharge in order to prevent plasma flow through the coil (see Fig. 5a). ICPs can reach a high electron density while the ion energy remains low. The main advantage of ICPs is the possibility to control the ion energy fluence, which is responsible for the nanoscale surface topography, the hydrophilic behavior and chemical properties of polyolefin as illustrated for argon (Ar) treatment of LDPE [143]. Additionally, the high electron density in these systems can be used for plasma polymerization purposes.
A capacitively coupled plasma (CCP) accomplishes the power coupling by oscillating electric fields. In this configuration, the plasma is formed between two separated parallel metal electrodes, which resembles a capacitor configuration. Another essential component in CCP systems is the network to match the impedance of the generator and the reactor. This matching network reduces the power reflection and loss from the generator to the reactor. The ion energy that falls onto the polymer sample in CCP is small; thus, the possibility of polymer damage is low. Nevertheless, in order to control the ion bombardment of the substrate, dual frequency CCPs have been developed, resulting in faster treatment in case of PP [2, 77]. Furthermore, the structural modification of PP using CCP plasma has been investigated. The crystallite size of PP is prone to be modified depending on the CCP plasma operational condition [2].
3.3 MW Discharge
MW-induced plasma is mostly generated in a magnetron. In these systems, the electromagnetic wave is carried by a hollow shaped conductor waveguide to the reactor. A coaxial cable can also be used as a carrier, but this is performed less frequently, and therefore, this description focusses on waveguides. The waveguide carries high-frequency energy of a magnetron with lowered energy loss, only allowing the propagation of TE and TM wave modes into the waveguide. A few different examples of experimental set-ups will be mentioned below.
For instance, it is possible to couple the waveguide to the plasma by using a quartz tube positioned perpendicularly with respect to the waveguide where the axial high electric field exists. The discharge gas is passing through this tube which propagates the plasma. Figure 6a indicates the typical MW plasma coupled with the waveguide.
Electron-cyclotron resonance (ECR) MW reactor (Fig. 6b) is another configuration using MW power. In this configuration, plasma is formed through the interaction between the MW electric field and magnetic field created by a solenoidal electromagnet. A superposition of these two fields under resonant condition brings a higher power to the plasma. Therefore, the reactor consists of a resonance region and a process part where the polyolefin sample is being treated [27]. This type of MW configuration is suitable for situations in which a higher degree of ionization and ion energy is of great importance.
MW induced plasma sources have been widely used for the surface modification of polyolefins [70, 96, 137]. One of the advantages of MW plasma sources is the possibility to work without any electrodes, making it easier to handle and preventing electrode contamination. Besides, the ignition under different discharge gases without transition to an arc mode is almost always possible. Both MW and RF discharges are known for their high radical density because a high power input can be used. This allows the reduction in treatment time to a few milliseconds, which is crucial and eligible for industrial applications [6]. However, the spatial limitation for some MW source designs is a disadvantage which makes the use of arrays necessary for applications where the treatment of large areas is required.
3.4 DBD
Besides using a pulsed mode corona discharge to prevent spark formation at higher voltages, a dielectric barrier between the electrodes can be employed. A DBD is a gas breakdown between two separated electrodes with at least one electrode insulated with a dielectric. This non-conductive material can be glass, ceramic or a specific polymer which prevents the generation of sparks and current propagation by the formation of streamers. Furthermore, a dielectric barrier can control the amount of energy and charge of filamentary microdischarges and distribute them over an extended region, thus producing a homogeneous large-scale plasma. Generally, this plasma can be sustained by an AC, pulsed DC or high-frequency (from lower RF to MW) source in order to avoid charge accumulation and microdischarges due to direct currents. DBDs are perfect examples of how dielectric barriers can considerably suppress the large current density and limit the discharge current to avoid the transition to an arc.
Figure 7 illustrates three different electrodes configurations [17]. Other arrangements of electrodes and dielectric barriers can be subcategorized into these three configurations. In a volume DBD (Fig. 7b), the plasma filaments need to cross the gas gap without any contact with the dielectric. In a surface DBD (Fig. 7a), filaments always develop in direct contact with the dielectric. A cylindrical DBD (Fig. 7c) is another example of a potential volume DBD configuration. However, to the best of our knowledge, it is not used in polyolefin surface modification. The sample position varies for the different plasma source geometries. In volume DBDs, a sample can be positioned in the plasma active zone between the two electrodes. In surface DBDs, a sample can be placed next to the powered electrode. DBDs are suitable sources for polymer surface modification because of their simplicity of operation, the possibility to work in a wide pressure range at low temperature and the ability to have homogeneous surface modifications. Furthermore, this plasma type is easily up-scaled and has an extensive processing range [17, 19, 79]. All these listed benefits make DBDs a suitable and preferred option for industrial applications.
3.5 Trends in Plasma Source Technology
Unambiguously categorizing individual sources becomes less straightforward nowadays. In recent years, driven by the research intention to develop stable homogeneous plasma sources at ambient pressure, the majority of plasma sources described in literature today are a combination of features belonging to the above-described categories. One of the examples of non-equilibrium plasma sources working at atmospheric pressure is a microplasma. Based on Paschen’s law, the electrode separation should be small enough to obtain a lower breakdown voltage at high pressure. Therefore, a microplasma provides an improved stability by minimizing the dimensions to the sub-millimeter range. This results in a higher electron temperature accompanied by a lower gas temperature. Moreover, the accumulation of heat is hardly happening as a result of a comparatively high surface to volume ratio. This makes microplasmas suitable for the treatment of heat sensitive polyolefins [37]. Another example of a plasma source working at high pressure is the plasma jet. It is a system with a planar or coaxial geometry and a small discharge gap operating at atmospheric or elevated pressure. Usually, it blows a stream of noble gases outside of the source which allows the plasma to propagate outside the electrode arrangement. The geometry of the configuration and carrier gas flow can highly control the jet plume shape and the way it spreads. A plasma jet seems to be homogeneous at first glance while it actually consists of intermittent high-speed bullets [99]. The applied electric field has a particular influence on the way these bullets propagate in the environment. A plasma jet has the exclusive ability to work in almost the total frequency range (from MW to DC). Another feature of this plasma source is the ability to provide a vast range of temperatures, which makes it applicable for polymer treatments. A thermal plasma jet may turn to an arc and is called a “plasma torch”. A non-thermal plasma jet is found to work under different configurations, as Laroussi and Lu described (Lu et al., 2012). Figure 8 schematizes the four categories of jets: the DBD plasma jet, DBD-like jets, single electrode jet, and dielectric-free electrode jet.
DBD jets have become popular in many science areas, ranging from material processing to medical applications, due to the variety of features they offer and the possibility to pick a desirable one for each purpose. For instance, a plasma jet can be generated with up to a 100 mm length and a diameter in the range of few millimeters while keeping the gas temperature close to the room temperature due to the low power density delivered to the system.
As mentioned before, the cost reduction due to the exclusion of vacuum devices and the design simplicity is advantageous and makes the source popular for the surface treatment of polyolefins [98, 132, 153]. Another advantage is the possibility for a remote operation, in which the sample is placed in the proximity (up to few tens of millimeter) of the plasma jet. Therefore, the treatment is not performed in a confined space and contact between the substrate and the plasma jet is not necessary, leading to polymer modification in non-contact mode [11]. As a consequence, the substrate can be processed by long-lived plasma species carried by the gas effluent. The treatment with this type of active species may prevent damage to the polymer surface since the modification condition is mild. Moreover, the miniaturized dimension makes the processing of complex structures like porous 3D scaffolds, tubular structures and delicate materials more feasible [122]. Lastly, it has been shown that samples treated with a plasma jet are more effectively modified compared to volume DBD-treated samples because of an increased surface degradation during the latter treatment. Samples modified with a DBD are more exposed to charged particles and UV radiation as compared to plasma jet-modified samples. Under this condition, polymer chains are more prone to breaking thus causing degradation of the polymer [83]. However, a plasma jet can only modify the region where reactive species formed by the discharge are present. This gives rise to a restriction residing in non-homogenous surface modifications by this discharge type. Nonetheless, this limitation can be solved by implementing multiple arrays of jets and/or a displacement of the plasma jet(s), similar to MW-induced plasma [61].
4 Plasma Surface Modification Techniques
As previously mentioned, plasma is a mixture of atoms/molecules, electrons, ions, excited species, radicals and radiation in the UV–visible range. The presence of reactive species like electrons, ions, excited species, radicals and UV radiation makes plasma a suitable environment for interaction with a surface. Even the surface of polyolefins, which are considered to be quite inert, can be modified by plasma species. The reason behind this phenomenon is that the range of dissociation and fragmentation energy of the bonds in polyolefins (and polymers in general) is low in comparison to the energy of the plasma species. Furthermore, as plasma only interacts with the uppermost layer of the surface, the bulk properties can be preserved while altering the surface properties.
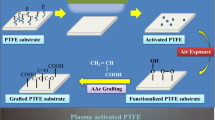
Hence, plasma treatment can be an excellent alternative for surface modification of polyolefins, as it is capable of breaking chemical bonds, which can lead to different surface modifications. The different potential surface interactions are etching (ablation), cross-linking, functionalization, grafting of specific functional groups and plasma polymerization, as displayed in Fig. 9. During etching, plasma interacts with the surface to form volatile products that are removed from the surface. Cross-linking occurs when radicals on the polymer chains recombine. Functionalization refers to the formation of functional groups on the surface, while grafting uses functionalization for the addition of other functional groups to the surface. Plasma polymerization is used to deposit a coating on the surface. Cross-linking, functionalization and grafting are processes that are all typically described by the term “plasma treatment”. Depending on the selection of the plasma parameters like the gas composition, power and pressure, the desired surface modification can be obtained [95]. The following sections will give a more detailed overview of plasma etching, plasma treatment and plasma polymerization.
It should be noted that most of the presented studies have been carried out under low-pressure and high-frequency plasma environment, despite the need for expensive and complex vacuum equipment. This choice is made because these plasmas are highly controllable systems with well selected active species, as discussed in Sect. 2.2.3. Atmospheric pressure plasmas are a suitable alternative for polyolefin modification, because of the aforementioned absence of vacuum chambers and pumping time. However, the temperature of the reactive environment should be maintained sufficiently low to prevent thermal damage of polyolefins. Nonetheless, a considerable number studies have employed non-thermal plasmas successfully [20, 103, 107, 119].
4.1 Plasma Etching
Etching refers to the removal of a boundary layer of material from the surface by either dynamic physical or chemical processes. Plasma etching is a non-equilibrium process performed in non-thermal plasma and has some unique features as it is chemically selective and usually anisotropic. This process involves the removal of material and the formation of gas phase products by bombarding the surface with high energy ions, neutrals or their combination produced by a high-density plasma (e.g. capacitively coupled plasma). Plasma etching of polymer surfaces can be performed over a broad range of pressures and frequencies. However, in order to avoid damage to the material, the power should be optimized to the used substrate and plasma. This process is associated with a functionalization of the surface. This functionalization is preliminary and is followed by etching and formation of gaseous products.
For a successful etching of polyolefin surfaces, plasma discharge gases like O2, F2, Cl2 and Ar can be used. During such plasma impact, volatile products like COx, CClx and CFx will be generated in the plasma reactor [35]. However, it should be noted that polyolefins are less susceptible to plasma etching than polymers comprising oxygen-containing groups. Plasma etching can improve the adhesion properties of polyolefins by increasing their surface roughness and imparting anchor effects. However, the functionalization can also influence the adhesion properties during etching processes [142]. Plasma etching will not improve the permeability properties, although cross-linking effects can positively influence this parameter as will be discussed in Sect. 4.2. It is worth mentioning that the combination of an RF source and a plasma jet has also triggered the etching of polyethylene, as shown in a study conducted by Fricke et al. This research has provided information on the removal of microorganisms and therefore the decontamination/sterilization of the surface [50].
Application-oriented studies that are focused on the plasma etching of polyolefins are somewhat limited. However, some more fundamental studies focusing on the etching rate exist. This parameter is controlled by several process characteristics, such as the discharge gas, gas flow rate and reactor composition. The former characteristic is studied by Kwon et al., who have shown that an Ar RF plasma has a lower etching rate than a mixture of Ar with O2, because O2 can rapidly remove the oxidized or broken bonds on the top layer of polyolefin films [84]. The comparison between N2 and O2 ICP discharges for plasma etching has demonstrated a higher etch rate and an increased roughness in case of oxygen treated samples, but similar trends in etching rate for both discharge gases [64]. On the other hand, modifying the gas flow rate enables the control of the etching rate. It is assumed that this rate increases rapidly by increasing the gas flow rate until it reaches a maximum value that depends on the discharge gas. A further increase in the gas flow rate leads to a decrease in the etching rate [22]. An example of the etching rate control via the reactor composition is the use of a grounded metal screen in the reactive region (Faraday cage). This has enabled the elimination of ions from the reaction region in the RF plasma etching of PE by fluorine gas, which resulted in a larger fluorination depth [4]. The etching rate can influence the structure of polyolefins after plasma treatment. In the case of PE, after plasma exposure, a lamellar structure of crystal or amorphous phases can be associated with a different etching rate. This structure does not even change after ageing [41, 168].
4.2 Plasma Treatment
4.2.1 Plasma Activation
This approach implies the introduction of different functionalities and/or the modification of already present functionalities on the chemically inert surface by a gas discharge, without introducing monomers. For this purpose, different gases can be used. A first distinction can be made between noble gas plasmas and other discharge gas plasmas. Theoretically, the former type of plasmas can only generate free radicals on the surface by dissociation and fragmentation of the surface bonds, as the generated plasma species will not be incorporated on the surface. Other discharges cannot only generate free radicals as the formed plasma species can also couple to the surface and induce functional group formation. In both plasma types, the remaining free radicals will react with ambient air, leading to the incorporation of oxygen-containing functionalities onto the treated surface. Furthermore, the coupling of free radicals leads to cross-linking in the polymer matrix, which competes with the degradation and functionalization of the polymer. In polyolefins, free radicals can be either created by C–C or C–H bond dissociation, as depicted in Fig. 10, which employs PE as an example. The former process leads to a functionalization with a degradation while the latter process leads to only a functionalization. Because of the excess of energy in plasma and the similarity between C–C and C–H bond dissociation, plasma parameters should be carefully tuned to obtain the desired surface modification. The functional group density after plasma activation increases with respect to the treatment time and reaches an equilibrium after a certain time [7, 151].
As mentioned before, plasma surface modification has certain advantages over other techniques, as it can cause less thermal and chemical damage to the surface under appropriate conditions, uses a significantly smaller time interval and is solvent-free. However, there are also some disadvantages of using plasma for surface activation. Firstly, completely selective surface reactions are almost impossible to achieve, which leads to surfaces that have no unique functionality. Secondly, the level of surface functionalization by plasma is smaller than by wet chemical methods that have been well studied for a long time for such purposes [18, 28]. Thirdly, plasma activated surfaces are prone to ageing. This is an effect of surface adaptation in which the surface tends to minimize the surface and interface free energy and returns to a lower energy state. Reorientation of the plasma-induced polar groups toward the interface can explain this phenomenon [140]. However, these problems pose no limitation for most polyolefin applications, as the surface energy modification is most often sufficient and the ageing can be circumvented by performing post-plasma modifications directly after the treatment.
A wide variety of plasma process parameters can influence the activation process, like the plasma power and treatment time, which can be described together as energy density, the pressure and the discharge gas. Švorčík et al. have even indicated that the chemical structure of the substrate could influence the treatment, as LDPE and HPDE have shown different properties in terms of wettability, creation of conjugated double bonds, ageing rate, degree of cross-linking, incorporated oxygen percentage and surface roughness when both were exposed to the same Ar plasma [168]. With respect to the influence of plasma pressure, Shenton et al. have compared atmospheric pressure and low-pressure RF plasma surface modification of polyolefins [139]. These two types of plasma can induce a similar treatment effect on the surface, but with a different degree. Also the involved processes differ, as electron and ion bombardment and VUV and UV photons may all play a significant role in a low-pressure plasma, while it is unlikely that these species contribute to the surface modification at atmospheric pressure.
A wide variety of discharge gases is used for the surface modification of polyolefins. In the following sections, these gases are discussed in more detail. These different gases are researched both for fundamental and application-based reasons. For the latter reason, some gases are studied for the improvement of the surface free energy and adhesive bonding, while research also focuses on addressing the problem of gas and vapor permeability as discussed in the introduction. Typically, the introduction of polar functional groups via a wide variety of gases solves the former problem, since it increases the surface free energy and improves adhesion and wettability of the polymers [39, 98]. During the activation process, the previously described process of plasma etching may also take place, which leads to a roughness increase of the surface. On the other hand, the gas and vapor permeability are examined after a plasma-induced cross-linking effect.
4.2.1.1 Ar and He Plasma
As mentioned before, Ar and He plasmas are theoretically unable to incorporate new functionalities onto the surface and thus a surface functionalization proceeds through reactions between the generated surface radicals and the ambient air. This results in the incorporation of multiple oxygen-containing functionalities such as peroxide, carboxyl, hydroxyl and carbonyl groups on the polyolefin surface. It should, however, be noted that impurities in the discharge chamber like water vapor or air could lead to similar surface oxidation during the plasma process. This leads to a lower amount of free radicals on the surface before exposure to ambient air. The introduction of oxygen-containing groups alters the surface free energy, which determines the degree of wettability and adhesive capability [53]. For example, UHMWPE performance in shoulder prostheses can be enhanced by surface activation through Ar and He DBD plasma at atmospheric pressure. It was demonstrated that oxygen incorporation on the surface increases the adhesive interactions between UHMWPE and bone cement. Moreover, an increase in the cell adhesion has been reported through this plasma activation process [155].
Besides surface functionalization, Ar and He plasma are also of interest for the cross-linking of the surface because the radical formation is not competing with the functionalization, as compared to other gases that are mentioned in Sects. 4.2.1.2–4.2.1.4 [33, 62, 86, 169]. The cross-linking effect of He plasma can be applied as a pre-treatment of the substrate to prevent ageing of a subsequent treatment. For instance, plasma pretreatment has been performed prior to NH3 treatment that is used to improve adhesion with aluminum deposited by metallization. The result showed that the adhesion degradation is minimized when applying this pretreatment [147]. Lin et al. have illustrated that Ar plasma activation with an RF source could reduce n-hexane permeation through HDPE bottles [94].
4.2.1.2 Nitrogen-Containing Plasma
Nitrogen-containing plasmas like nitrogen, ammonia and N2/H2 plasma can incorporate nitrogen functionalities like amines, imines and amides [37, 39, 45, 104, 159]. Also surface radicals can be introduced, leading to a similar oxidation as mentioned in the previous section. The introduction of these oxygen-containing functional groups can lead to an increase in the wettability [48]. In general, nitrogen-containing plasmas enhance the surface free energy which is desired for applications like dyeability, printability or biocompatibility [95]. For example, Hollahan et al. have used an ammonia-based RF plasma to introduce amine groups on different polymers like PP to improve blood compatibility. The ammonia is dissociated in the plasma, forming NH2 radicals, which can combine with carbon radicals on the polymer surface to form amine groups [67]. Furthermore, N2 pulsed arc plasma has shown potential in ion implantation of Cu and Ag antimicrobial reagents on PE surfaces, providing a considerable antibacterial performance against E.coli and enhancing cell growth [166, 167].
Nitrogen-containing plasmas do not always incorporate nitrogen functionalities. Sanchis et al. have used a nitrogen RF plasma to increase the wettability of LDPE for improved adhesion to polyolefin foams for automotive industry applications. This treatment only resulted in oxygen functionalities on the surface. The amount of these functionalities was similar to the amount introduced by the oxygen plasma. However, the mechanism of oxygen incorporation was different, as the oxygen plasma exposure resulted in direct functionalization, while the nitrogen plasma exposure introduced oxygen-containing functional groups via post-plasma reactions with ambient air [130].
4.2.1.3 Oxygen-Containing Plasma
Oxygen-containing plasma comprises a variety of possible discharge gases like CO2, CO, O2 and air. Although air mainly contains nitrogen, it can be classified in this category because it primarily produces oxygen-containing functional groups since oxygen is by far more reactive than nitrogen. Moreover, nitrogen-containing intermediates increase the atomic oxygen formation. Air and oxygen plasmas in vacuum have revealed a variety of oxygen containing functionalities [121]. A low-pressure RF plasma ignited in oxygen or air and applied for HDPE treatment, for example, was found to successfully increase its surface micro-hardness and incorporate carbonyl, carboxyl, ether and peroxide groups on the modified surface, which improved its wettability [92]. Multiple studies have focused on increasing adhesive bonding to polyolefins by air and/or oxygen plasma. A recent investigation has indicated that the shear strength of adhesive-bonded joints of two PP films made after low-pressure air or oxygen RF plasma treatment is significantly higher than the shear strength obtained from joints with only-degreased surfaces [102]. Furthermore, Murthy et al. have observed that the bond strength between glass reinforced PP and HDPE panels can be significantly increased after treatment with an air plasma jet, which could be directly associated with the increase in surface energy of both surfaces [131]. Also air DC discharge plasma has shown the ability to increase the adhesive bonding of HDPE and PP to steel as a secondary structure in assemblies for the automotive industry. In this case, the lap shear, tensile strength and surface free energy reach maximal values after which they decrease again, indicating a specific optimal plasma treatment for this particular experimental condition [14]. Other studies on an air DBD and air plasma jet, however, indicated that the surface oxidation and associated wettability reached an equilibrium after a certain treatment time [31, 75].
CO2 and CO plasma can add oxygen-containing groups on the treated surface of polyolefins due to the presence of oxygen-containing reactive species in the reacting chamber. Apart from the alcohol, ketone and carboxylic acid functional group incorporation, substrate degradation and cross-linking can occur. This stabilizes the modified surface, as was illustrated with PP [5]. Utilizing a MW discharge at low pressure can produce carboxylic acid groups on the surface. Moreover, it was demonstrated that the degradation via chain scissions has a heterogeneous effect since it mainly affected amorphous zones [106]. An addition of H2O to a CO2 MW plasma did not improve the surface functionality, as HDPE treated in the CO2/H2O plasma demonstrated a lower percentage of carboxylic acid on the modified surface [115].
4.2.1.4 Halogen-Containing Plasma
Haloform plasmas are often reported to introduce a mono-sort of halogen groups. This designation is based on the ability of these plasmas to introduce mostly one type of chemical bonds, which is distinct from other plasmas. Namely, C-X bonds are mainly incorporated on the polyolefin surface, in which X is a halogen atom. CF4 plasma is one type of a haloform plasma that typically enhances the surface hydrophobicity [30, 76]. The fluorination mainly happens due to the substitution of H by F atoms in the polyolefin chain, leading to the formation of CF, CF2 and CF3 groups on the surface. It has been demonstrated that VUV radiation enhanced the surface treatment efficiency in RF glow discharges. Fresnais et al. have used a MW discharge to ignite a mixture of CF4 with oxygen, which was proven to preserve the hydrophobic characteristics together with a lower surface roughness of the treated LDPE substrate [49]. One of the application examples of plasma fluorination is the enhancement of the PP surface properties to prevent fuel permeation through the walls of such polymeric containers. Although the wet chemical treatment can be used to achieve the same aim, it shows deeper altering effects in terms of the thickness of fluorinated surface (5000 nm) compared to a plasma functionalization (10–100 nm). Nevertheless, the barrier efficiency remains the same in both approaches [57]. Also SF6 plasmas have the capability to fluorinate polyolefins, as demonstrated by Amorim et al. The treatment introduced, besides fluorine-containing functionalities, a significant amount of oxygen and a small amount of sulfur to the PP surface. However, it still resulted in a decreased surface wettability [3]. Other examples of mono-functionalization processes are bromoform and chloroform plasmas, which can produce a single surface functionality with high yields [56, 78, 162]. One of the necessary conditions to achieve a mono-sort functionalization is performing the treatment in low-pressure plasma reactors since they provide an excellent control over the discharge parameters and thus the final surface modification. Results indicated that the bromination by using a RF plasma is very selective and has less undesired side products. Moreover, the post-plasma oxidation in this functionalization was small compared to the fluorination process [55].
4.2.1.5 Hydrogen Plasma
Hydrogen plasma differs from others because of its particularly intense broadband emissions in the VUV region (below 170 nm). These photons are also the main reason why the primary outcome of such plasma treatment is cross-linking and the formation of double bonds. These changes in the polyolefin structure increase the surface density, which results in the reduction of the permeability of the surface layer [13, 68, 124].
4.2.2 Plasma Grafting
Plasma grafting is the second type of plasma treatment in which the activation step is used to covalently bind molecules on a surface. Plasma grafting can be performed via post-plasma grafting or syn-irradiation grafting (direct grafting). In the former approach, the sample is first functionalized by a plasma activation step. Within this approach, a distinction can be made based on the surface chemistry used for grafting. In the first post-plasma grafting technique, Ar or He plasmas are most commonly used to introduce radicals onto the surface. Typically, these radicals will react with the ambient air after the treatment, leading to the formation of peroxide and hydroperoxide groups, as mentioned before, although the radicals could also be used to start a radical polymerization reaction directly by introducing the monomer in the gas phase after the plasma is turned off. The latter strategy is, however, not frequently used. The aforementioned peroxide and hydroperoxide groups are then used as initiating sites for grafting polymerization. The monomer can be added in the gas phase or the substrate can be immersed into a monomer solution, after which polymerization is initiated by an increase in temperature or by UV radiation, for example [37]. This process leads to covalent bonding of a particular functional group to the surface with a composition similar to the typical polymerization, as the monomer is not introduced during the plasma exposure. Polyolefins are good candidates for this process, as the response to the plasma surface oxidation is fast and leads to the introduction of the desired chemical functionalities. Figure 11a illustrates the first type of post-irradiation plasma grafting, which is sometimes referred to as plasma-induced graft (co)polymerization [141]. The grafting quality depends on the initial radical density, the monomer quantity and reactivity and the reaction time [73, 125]. This technique can be performed with a wide variety of monomers like acrylic acid and different acrylates, such as glycidyl methacrylate, methyl acrylate, and 2-hydroxy ethylacrylate [73]. Polyolefins functionalized with the help of post-irradiation plasma grafting are mainly researched for biomedical applications. For instance, polyethylene has been grafted with styrene via CCP Ar plasma for the enhancement of the surface biocompatibility [58]. This study has concluded that the chemical nature of the monomer grafted onto the polymer is more critical than the oxidation induced by the plasma. Biomolecules can also be immobilized on plasma grafted polyolefins. An example is the immobilization of chitosan on plasma grafted acrylic acid making use of oxygen RF plasma on PP-based sutures via a carbodiimide-mediated reaction. Additionally, different antimicrobial agents have been coupled to the suture which leads to drug release properties and an enhanced antimicrobial activity [134].
As mentioned before, a distinction in post-plasma grafting can be made based on the surface chemistry used for grafting. Besides using (hydro) peroxides or radicals for initiating polymerization, other plasma-induced functional groups can be used to immobilize molecules, as illustrated in Fig. 11b. This resembles the immobilization after plasma grafting as described above. A frequently used functional group for molecule immobilization is a primary amine. As mentioned before, nitrogen-containing plasma can activate the polyolefin surface and introduce NH2-groups [46, 159]. For example, Ghasemi et al. have immobilized the enzyme trypsin on ammonia plasma treated polyethylene films via a glutaraldehyde linker. The aldehyde functionality of the linker can react with a primary amine on the surface and the enzyme, forming an imine which is subsequently reduced by sodium cyanoborohydride leading to a chemically stable secondary amine [59]. Another employed strategy is the use of CO2 plasma for the introduction of carboxylic acid groups. Vartiainen et al. have immobilized glucose oxidase and chitosan on N2/CO2 plasma-treated PP for antimicrobial packaging applications and have compared it with N2/NH3 treatment. The N2/CO2 DBD plasma treatment was reported to generate carboxylic acid functionalities on which the bioactive molecule can be immobilized via a carbodiimide-mediated reaction. Glutaraldehyde was again used as a coupling agent for the N2/NH3 plasma treated polymer, but without the reduction step. The density of primary amines was higher than the density of carboxylic acid groups, and the enzymatic activity and amount of immobilized chitosan were both higher for the N2/NH3 plasma treated PP [156, 157]. Plasma treated polyolefins can also be modified via less conventional chemical reaction pathways. Habib et al. grafted the antioxidant ascorbic acid (ASA), which also has antibacterial characteristics, on LDPE after air plasma treatment [63]. They suggested that the post-plasma grafting reaction proceeded via deprotonation of ASA by alkoxyl radicals, which originated from the decomposition of (hydro) peroxides on the plasma treated surface, after which the ASA-radical can interact with double bonds that were also formed during the plasma treatment. As such, ASA can be covalently grafted onto the LDPE surface, imparting the material with antibacterial properties towards Escherichia coli and Staphylococcus aureus.
Syn-irradiation grafting is the other main plasma grafting approach. During this process, a monomer or molecule is adsorbed onto the substrate and subsequently exposed to plasma. During the plasma exposure, radicals are generated in the adsorbed molecule layer, which leads to a coupling of the molecules to the surface and between each other. Most often, a monomer is used, which leads to the formation of a cross-linked polymer layer. Figure 12 shows the steps of syn-irradiation plasma grafting. An example of a simple monomer syn-irradiation plasma grafting step is the study of Prachar et al., in which tetraethoxysilane (TEOS) has been adsorbed onto the surface of PP via CCP air plasma exposure. The induced silicon functionalities have led to a permanent hydrophilicity of the substrate [126]. Bigger molecules can also be grafted via this method [117]. An example is the immobilization of dyes on PP, LDPE and HDPE for the fabrication of colored materials [34]. In this type of process, the preservation of the monomer or molecule is of importance to obtain the desired surface functionality. This needs an optimization of the plasma parameters, as side reactions can occur during the plasma exposure. These side reactions increase the heterogeneity, which is usually not desired in the process of grafting. Therefore, post-plasma grafting is more favorable than syn-irradiation plasma grafting to have higher homogeneity and specificity, as the grafted molecules will only resemble the conventional polymer completely in the former technique. Syn-irradiation plasma grafting, however, forms a more straightforward method, as the wet chemical treatment step is limited to a dipping or spraying procedure.
4.3 Plasma Polymerization
The term “plasma polymerization” is often employed to describe a variety of processes in which the introduction of precursors into a plasma results in the deposition of a coating [100]. Plasma-enhanced chemical vapor deposition (PE-CVD) is the most frequently used type of plasma polymerization for polyolefin surface modification. Therefore, this specific technique will be discussed in more detail in the following paragraphs. Also, all the examples of plasma polymerization that will be explained in detail in this section refer to PE-CVD processes. PE-CVD is a coating technique in which an organic monomer in the vapor phase is introduced in a plasma, leading to the conversion of the monomer into reactive fragments and subsequent polymerization. This can occur in the gas phase or on a substrate placed in the plasma, which usually results in the deposition of highly cross-linked films [71]. Typically, ions created in the plasma phase can bombard the surface and the coating, leading to an etching of the growing film [37]. The monomer can be, but does not need to be, a molecule that can polymerize via conventional polymerization techniques. In the former case, the resulting plasma polymer will not resemble a polymer obtained via these conventional techniques because of fragmentation of the monomer structure and the aforementioned cross-linking process [100]. There is also a difference between plasma polymerization and plasma grafting. The former technique applies a coating that is based on the monomer structure onto a substrate, although the monomer functionality is not completely preserved. The latter technique, however, leads to covalent grafting of molecules to the substrate that preserves the monomer/molecule structure [37].
During the plasma polymerization process, the monomer (alone or with a carrier gas) is fed into the plasma discharge or afterglow. Similar to plasma activation, plasma polymerization can be performed at low pressure, intermediate pressure and atmospheric pressure [100, 154]. The plasma process and the resulting coating is influenced by a number of other parameters besides the pressure, like the chemical structure of the monomer, the monomer flow rate, the carrier gas flow rate and the plasma power. The most important factor that affects the plasma polymer properties is the energy applied per monomer molecule. Yasuda proposed a parameter, W/FM (with W: power (W), F: monomer flow rate (mol/s), M: molecular mass of the monomer (kg/mol)), to represent the energy input per unit mass of monomer [148]. Increasing the energy applied per monomer molecule (or increasing W/FM) leads to a higher precursor fragmentation. This is considered to be beneficial for the stability of the coatings, which is in some applications of significant importance. However, more fragmentation will usually lead to a loss of the initial monomer composition in the resulting coating as well. This can be an unwanted effect, as the preservation of the monomer functionality is crucial for some purposes [148]. To improve the monomer functionality retention, a pulsed treatment cycle can be used. During the on-periods of the pulses, which are usually in the order of microseconds, monomer activation and generation of reactive site on the surface occur, while polymerization can take place during the off-periods, usually in the order of milliseconds, in which the influence of UV-, ion-, or electron-induced damage to the growing film is limited. This leads to higher retention of the monomer functionality, while stable coatings can be obtained [12]. Plasma polymerization can be performed in a wide number of source configurations of which DBD, RF and MW are the most frequently used.
The following sections will give an overview on different monomers used for PE-CVD on polyolefin substrates. This technique is applied for solving both general problems related to polyolefins. However, some coatings are mainly studied because they can increase the barrier performance of polyolefins, like silicon-oxide coatings (Sect. 4.3.1) and carbon-hydrogen coatings (Sect. 4.3.2), while other coatings are mainly applied to modify the surface free energy, like COOH- and amine-rich coatings (Sects. 4.3.3–4.3.4). Section 4.3.5 will shortly discuss less studied coatings and the last section will discuss grafting on plasma polymerized coatings.
4.3.1 Silicon-Oxide Coatings
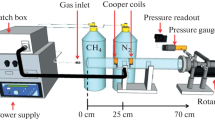
Plasma polymerization of organosilicons has been widely applied on polyolefins for the creation of silicon-oxide (SiOx) coatings [72, 102, 113, 114, 133]. These coatings have attracted interest because of their wide application range in several fields as they impart good scratch resistance, thermal stability, reduced friction, adjusted wettability, flame retardance and gas–vapor barriers. For polyolefin applications, the latter characteristic is the most important. Organosilicon precursors are characterized by their sufficient volatility near room temperature, affordability, relative non-toxicity and non-flammability [109, 113, 136, 161]. Moreover, a large variety of potential reactants are commercially available such as hexamethyldisiloxane (HMDSO), tetramethyldisiloxane (TMDSO), tetraethoxysilane (TEOS) and tetramethoxysilane (TMOS). Particularly, HMDSO is preferred over other monomers in the broad majority of studies involving organosilicon plasma polymerization due to its highly organic character, high vapor pressure and ability to deposit siloxane coatings at low temperature [88, 109]). Since polyolefins are widely used in the pharmaceutical and food packaging sectors, the enhancement of their gas-barrier properties is critically required for a better product conservation. In this sense, polyolefins started, a few decades ago, to constitute one of the main substrates in the studies of HMDSO and other organosilicon plasma polymer coatings. It is worth mentioning that among all polyolefins, PP is by far the most considered in such studies because of its more prominent position as packaging polymer given its transparency, low specific weight brilliance and low density at considerably low costs [80, 136]. HMDSO plasma polymerization will deposit coatings resembling polydimethylsiloxane (PDMS) at low powers, which compromises the barrier performance. This behavior was compensated by increasing the oxygen to HMDSO monomer ratio, which reduces the organic amount within the plasma-polymerized coatings. This leads to more inorganic quartz-like deposits generally exhibiting higher barrier performance [81]. The challenge with the inorganic coatings is to have a crack-free coating as this will lower the barrier performance. Moreover, because of the use in food and pharmaceutical packaging applications, the necessary autoclaving also tends to induce cracks on the SiOx coatings. This can be enhanced by first depositing a PDMS-like coating or by using a vertical gradient in the coating from PDMS-like to SiO2-like by progressively increasing the oxygen to HMDSO ratio. In this way a combination of favorable thermal, adhesive and mechanical properties on the one hand, and adequate barrier performance on the other hand is reached [80]. More recently, an atmospheric pressure plasma jet deposition of silica barrier coatings on PP was proposed by Scopece et al. as an alternative to the traditional high cost vacuum systems. One of the major downsides of the atmospheric plasma processes is the formation of powder during deposition. This can compromise the durability and quality of the generated coatings. Moreover, the formation of cracks was also observed on the coatings as a result of a thermal fatigue phenomenon after the repetitive heating and cooling steps induced by the repetitive plasma jet passages. Despite these drawbacks, the gas barrier performance of PP was improved by a factor of 2. Increasing the deposited coating thickness or applying multiple coating layers were shown to exhibit an additive gas barrier character [136].
4.3.2 Carbon-Hydrogen Coatings
Next to the silicon-oxide coatings, carbon-hydrogen coatings have also been deposited on polyolefins via plasma polymerization to enhance their gas-barrier properties [9, 69, 94, 145]. This polymerization can be performed using any gaseous hydrocarbon monomer. However, for an easy processability, methane (CH4), acetylene (C2H2) and ethylene (C2H4) are generally selected. The properties of the obtained amorphous coatings are mainly influenced by the cross-linking degree and to a lower extent by the use of saturated or unsaturated monomers. Methane is characterized by considerably reduced deposition rates compared to ethylene and acetylene [66]. Lin et al. have conducted a comparative study between methane and acetylene plasma polymerizations done by an RF discharge to coat the inner surface of HDPE bottles. When using methane as precursor, free radicals were generated by the hydrogen detachment solely, which yields plasma polymers with low number of trapped free radicals. However, acetylene polymerization led to the formation of di-radicals and as such, the production of plasma polymers with considerably higher number of free radicals. As a consequence of this high free radical concentration, large amounts of oxygen-containing functionalities were incorporated onto the surface, even though the base monomer had no oxygen. Therefore, the O/C ratio of the acetylene-derived plasma coatings was 6 times higher than that of methane plasma-polymerized surface. Under optimal parameters, a significant reduction in gas permeation is detected when using acetylene in comparison to methane but also to trimethylsilane, acrylic acid plasma polymerizations and Ar plasma activation. This is mainly due to a combination of surface tightness and an increase in polar functional groups on the surface [94]. When increasing the input energy, the internal stress within the hydrocarbon network intensifies leading to the generation of diamond-like carbon (DLC) coatings [66]. DLC films consist of amorphous carbon with graphite structure comprising sp2 bonds and diamond structures comprising sp3 bonds. Moreover, DLC coatings are characterized by their lubricating properties, toughness, abrasion resistance, biocompatibility and chemical stability. In addition to their prominent role as gas-barrier, DLC films are also used to enhance the adhesive properties of polyolefins. In fact, the low adhesion of polyolefins is a limiting factor occasionally preventing them from being widely used particularly in the biomedical applications demanding an enhanced adhesion [69, 145]. Takahashi et al. have indeed shown that the low-adhesive surface of LLDPE and HDPE could be substantially improved after applying a DLC coating resulting from an acetylene plasma polymerization done via a RF plasma device. However, other polyolefins such as LDPE, isotactic PP and syndiotactic PP did not show higher adhesive properties because of the low adhesion between DLC and the 3 polyolefins. This adhesion can be improved by a number of different strategies like plasma etching or activation prior to plasma polymerization. For example, Baba et al. used H2O and O2 plasma prior to acetylene plasma polymerization [9].
4.3.3 COOH-Rich Coatings
Besides the use of plasma-coated polyolefins in many technological fields necessitating barrier and protective coatings, other particular plasma coatings are also continuously being developed for other purposes. The major field for COOH-rich coatings is the enhancement of the cytocompatibility of polyolefins for their use in the biomedical field. These functionality can also be introduced by plasma activation of polyolefins, because of the carbon-hydrogen nature of these polymers. As already mentioned in the introduction, the main downside restricting the use of unmodified polyolefins in the biomedical domain is their inadequate biocompatibility stemming from their hydrophobicity and low surface energy [111, 128, 135]. Therefore, among other plasma polymers, COOH-rich coatings are the most frequently deposited on polyolefin substrates in order to improve cell-surface interactions [29, 128]. In fact, COOH groups substantially enhance polyolefin surface wettability and favor the interactions with some cellular receptors thus stimulating several signaling pathways and metabolic processes. Consequently, COOH-dense coatings support the adhesion, proliferation and differentiation of a broad variety of cell types [15, 23, 29, 65, 112, 128, 138]. Moreover, COOH functionalities are sensitive to changes in the pH of the milieu in which they are submerged making them promising candidates in drug-delivery applications involving the release of a drug in a pH-specific environment [111]. Plasma deposition of carboxylic films on polyolefins was mainly performed using the unsaturated acrylic acid (AA) or maleic anhydride (MAA) monomers at low, medium and atmospheric pressures with a high retention of COOH groups [74, 112, 149]. However, the main issue of plasma-polymerized COOH films is their low stability in aqueous environments. If deposited with unsuitable plasma working parameters, the coating can rapidly dissolve, not only abolishing the coating effect but also drastically acidifying the surroundings. This acidification constitutes a major drawback in the biomedical applications as it yields harmful effects on tissues and cells, similar to what is perceived during infection [29, 38, 47, 111]. Therefore, several studies have tackled the stability of plasma-polymerized COOH–rich coatings on polyolefins while maximally preserving the high density of COOH by two approaches: (1) enhancement of the adhesion between the coating and the substrate via a surface pre-polymerization treatment and (2) improvement of the coating cohesion strength via increasing the cross-linking by carefully selecting the plasma parameters [111, 112, 128, 135]. Typically, these type of coatings contain next to carboxyl functionalities a wide variety of other oxygen-containing functionalities like alcohols, ethers and ketons [135]. Most studies investigate this plasma polymerization on flat polyolefin surfaces, but Nistico et al. subjected hernia-repair PP meshes to an RF pulse discharge plasma polymerization of AA to confer adhesive properties and therefore, reduce undesired displacements of the meshes [118].
4.3.4 Amine-Rich Coatings
Besides COOH-rich coatings, amine-rich coatings are also regularly studied for polyolefin surface modifications for biomedical applications [10]. These coatings have a more favorable wettability in comparison to untreated polyolefins and protonated amines can introduce localized positive charges on the surface if placed in aqueous solution at physiological pH. This can lead to electrostatic interactions with negatively charged cells and proteins [8]. Different precursor gases like organic amines (e.g. allylamine), (3-Aminopropyl) triethoxysilane (APTES) and mixtures of acetylene/ethylene and nitrogen or ammonia are used to deposit amine-rich coatings [8, [40, 54, 60, 150, 158]. Typically, these coatings possess a complex nitrogen-rich chemistry that includes, among others, nitriles, imines and primary and secondary amines. Furthermore, oxygen can interact with the surface after exposure to ambient air, leading to the formation of amides and conventional oxidation products like peroxides. Traces of oxygen-containing species in the discharge can also lead to this type of functional groups in the plasma polymer [8, 150]. Different studies have shown that amine-rich coatings can improve cell adhesion. The plasma polymerization of allylamine on UHMPE in a medium pressure DBD indicated a good cell viability in comparison to the bare polymer, for example [8]. The cell-interactivity of a plasma polymerized ethylene/N2 mixture was used to synthesize micropatterns for controlled cell culturing on PP. The coatings were deposited with an atmospheric pressure DBD and the patterns were fabricated using a mask [60]. Another application that is researched is the use of amine-rich coatings on COPs. As mentioned in the introduction, COPs and COCs are emerging as biosensor devices and plasma polymerization of functional coatings can be interesting. APTES is plasma polymerized in a RF plasma source to add primary amines on the substrate, which can be used for covalent attachment of target molecules like active antibodies or DNA molecules [158].
4.3.5 Other Plasma-Polymerized Coatings
This section gives a short overview of plasma polymers with other functional groups than the aforementioned functionalities. Alcohol functional groups can be introduced via plasma polymerization of allyl alcohol. Besides hydroxyl groups, ether, epoxy, carboxyl and carbonyl groups can be found in these coatings [42, 116]. Nihlstrand et al. have performed adhesion tests between the coating, deposited by a RF plasma source on different thermoplastic polyolefins or polyolefin rubber, and lacquer. At appropriate plasma parameters, excellent lacquer adhesion could be observed. Increasing the power led to a lower amount of hydroxyl groups and a decreased adhesion performance. The variation in the adhesion performance is attributed to VUV emission during the plasma deposition, which leads to radical creation in the near-surface region of the substrate. This can cause chain scission reactions which can lower the cohesive strength of the substrate [116]. Friedrich et al. have tested the peel strength of an aluminum layer deposited on plasma polymerized allyl alcohol on PP by an RF plasma source and have compared it with plasma polymerized allylamine and acrylic acid. It was observed that the adhesion between the coating and the aluminum increased in the following order: allylamine << allyl alcohol < acrylic acid [54].
Plasma-polymerized poly (ethylene glycol) films are of interest for biomedical applications in which complete surface inertness is required, as these coatings can resemble the native polymer which is known for its antifouling behavior. As mentioned in the introduction, polyolefins are not completely inert in biological environments, which can for example lead to inflammatory responses when implanted. Choi et al. have deposited these coatings on PE in a RF plasma reactor and have performed in vivo evaluations. After 4 weeks of implantation, it could be observed that the coated substrates caused a lower degree of inflammation, fibrous tissue proliferation and toxicity as compared to the bare PE, illustrating the superior biocompatibility and minimum toxicity of these type of coatings [25].
Fluorocarbon plasma polymers are another type of coatings applied on polyolefins. Different precursors can be used like trifluoromethane/ethylene mixtures and octafluorocyclobutane [144, 160]. A hydrophobic fluor-rich coating with CF-, CF2- and CF3-bonds is usually obtained [144]. Walker et al. have observed that plasma polymerization of a trifluoromethane/ethylene mixture in a MW source results in PE with increased barrier performance to toluene [160].
4.3.6 Grafting on Plasma Polymers
Similar to plasma grafting on polyolefins, plasma polymerized coatings can also be used for immobilization purposes. A variety of studies has used plasma polymers for grafting, but only a limited amount has focused on polyolefin surfaces. An explanation for this limited interest can be due to the fact that the introduction of functional groups by plasma activation for subsequent immobilization is sufficient by itself for the intended applications, leading to a lacking need for grafting on plasma polymers. Another explanation could be that this grafting technique is mainly of importance in the biomedical field, where materials like titanium, polyethylene terephthalate and biodegradable polymers are preferred substrates. An example of grafting on a plasma polymer is the study of Lim and coworkers who have used acrylic acid plasma polymerization for protein immobilization on a HDPE facial implant [90]. The carboxyl groups on the surface reacted with the primary amine groups of a bone promoting signaling protein via a carbodiimide-mediated reaction. Another example has involved a RF plasma source to deposit plasma polymerized acrylic acid coatings on LDPE. A similar chemistry was used for subsequent attachment of heparin and highly-sulphated hyaluronic acid via a spacer molecule to avoid thrombus formation on the surface [44]. Amine-containing precursors were employed in other studies, like the work of Lee and co-workers. They have used allylamine and cyclopropylamine as precursors in a DBD plasma polymerization process for the immobilization of laccase enzymes on amine-functionalized non-woven PE/PP fibers, which can be used for dye removal from wastewater [91]. Glutaraldehyde has acted as a linking molecule, reacting with the primary amines on the surface and of the enzyme. The same strategy was also employed by Liu and co-workers to immobilize horseradish peroxidase on PP, using a plasma polymerized allylamine layer deposited with a RF plasma source. These surfaces could be useful for multiple biotechnological applications such as biosensors [97].
5 Conclusion
Polyolefins are of the most frequently used polymers because of their favorable properties. However, given their low surface energy and relatively high gas and vapor permeability, material adjustments are required for some applications. Surface modifications provide an interesting type of these adjustments, as, in addition to several advantages, they are performed independent of the well-established material processing steps. Among different surface modification approaches, plasma-based techniques are very attractive due to their fast, environmentally friendly and widely applicable aspects. A variety of different plasma reactor designs exists, of which corona discharges, DBDs, RF sources, MW sources and plasma jets are the most used for the surface treatment of polyolefins. Depending on the used plasma parameters, different plasma-surface interactions can be obtained. Plasma activation and grafting are two techniques that can introduce a variety of functional groups on the surface, with the latter providing more selectivity over these groups. Together with plasma etching, these techniques can all be used for surface energy modification. This enhances the printability, dyeability and biocompatibility of the polyolefin surface and increases the adhesive bonding to materials. Plasma activation can also induce a cross-linking of the polymer chains, which can lead to an enhancement of the gas and vapor permeability. Tuning the working conditions for plasma activation can modify the type and density of the incorporated functional groups on the surface and the degree of cross-linking. On the other hand, plasma polymerization leads to coating deposition. Depending on the monomer and other working parameters, coatings with different functionalities and properties can be obtained. Some coatings are very useful for improving the barrier layer performance. These coatings are significantly better and more useful than plasma activation for this purpose. Other coatings are applicable for modification of the surface energy, which has the same intent as plasma activated and grafted polyolefins. In general, it can be concluded that plasma modification of polyolefins is an interesting approach for the improvement of these materials, which widens their applications range.
References
Abourayana, H.M., Milosavljević, V., Dobbyn, P., et al.: Evaluation of the effect of plasma treatment frequency on the activation of polymer particles. Plasma Chem. Plasma Process. 37(4), 1223–1235 (2017)
Akbar, D.: Surface modification of polypropylene (PP) using single and dual high radio frequency capacitive coupled argon plasma discharge. Appl. Surf. Sci. 362, 63–69 (2016)
Amorim, M.K.M, Rangel, E.C., Landers, R., et al.: Effects of cold SF6 plasma treatment on a-C:H, polypropylene and polystyrene. Surf. Coat. Technol. 385, 125398 (2020)
Anand, M., Cohen, R., Baddour, R.: Surface modification of low density polyethylene in a fluorine gas plasma. Polymer 22(3), 361–371 (1981)
Aouinti, M., Bertrand, P., Poncin-Epaillard, F.: Characterization of polypropylene surface treated in a CO2 plasma. Plasmas Polym. 8(4), 225–236 (2003)
Aumann, T., Theirich, D., Engemann, J.: Rapid surface modification of polyethylene in microwave and rf-plasmas: comparative study. Surf. Coat. Technol. 142, 169–174 (2001)
Aziz, G., Cools, P., Geyter, N.D., et al.: Dielectric barrier discharge plasma treatment of ultrahigh molecular weight polyethylene in different discharge atmospheres at medium pressure: a cell-biomaterial interface study. Biointerphases 10(2), 029502 (2015)
Aziz, G., De Geyter, N., Declercq, H., et al.: Incorporation of amine moieties onto ultra-high molecular weight polyethylene (UHMWPE) surface via plasma and UV polymerization of allylamine. Surf. Coat. Technol. 271, 39–47 (2015)
Baba, K., Hatada, R.: Deposition of diamond-like carbon films on polymers by plasma source ion implantation. Thin Solid Films 506, 55–58 (2006)
Babaei, S., Sabbatier, G., Hoesli, C.A., et al.: Functional plasma polymer films for the purification of pancreatic β cells. Plasma Process. Polym. 16(10), 1900041 (2019)
Babaeva, N.Y., Kushner, M.J.: Interaction of multiple atmospheric-pressure micro-plasma jets in small arrays: He/O2 into humid air. Plasma Sources Sci. Technol. 23(1), 015007 (2014)
Bazaka, K., Jacob, M.V., Chrzanowski, W., et al.: Anti-bacterial surfaces: natural agents, mechanisms of action, and plasma surface modification. RSC Adv. 5(60), 48739–48759 (2015)
Behnisch, J., Holländer, A., Zimmermann, H.: Surface modification of polyethylene by remote dc discharge plasma treatment. J. Appl. Polym. Sci. 49(1), 117–124 (1993)
Bhowmik, S., Ghosh, P., Ray, S., et al.: Surface modification of high density polyethylene and polypropylene by DC glow discharge and adhesive bonding to steel. J. Adhes. Sci. Technol. 12(11), 1181–1204 (1998)
Bitar, R., Cools, P., De Geyter, N., et al.: Acrylic acid plasma polymerization for biomedical use. Appl. Surf. Sci. 448, 168–185 (2018)
Boaen, N.K., Hillmyer, M.A.: Post-polymerization functionalization of polyolefins. Chem. Soc. Rev. 34(3), 267–275 (2005)
Brandenburg, R.: Dielectric barrier discharges: progress on plasma sources and on the understanding of regimes and single filaments. Plasma Sources Sci. Technol. 26(5), 053001 (2017)
Briggs, D., Brewis, D.M., Dahm, R.H., et al.: Analysis of the surface chemistry of oxidized polyethylene: comparison of XPS and ToF-SIMS. Surf. Interface Anal. 35(2), 156–167 (2003)
Bruggeman, P.J., Iza, F., Brandenburg, R.: Foundations of atmospheric pressure non-equilibrium plasmas. Plasma Sources Sci. Technol. 26(12), 123002 (2017)
Carrino, L., Polini, W., Sorrentino, L.: Ageing time of wettability on polypropylene surfaces processed by cold plasma. J. Mater. Process. Technol. 153, 519–525 (2004)
Chang, J.-S., Lawless, P.A., Yamamoto, T.: Corona discharge processes. IEEE Trans. Plasma Sci. 19(6), 1152–1166 (1991)
Chapman, B.: Sputtering [Glow Discharge Process], vol. 177. Wiley, New York (1980)
Cheng, Z., Teoh, S.-H.: Surface modification of ultra thin poly (ε-caprolactone) films using acrylic acid and collagen. Biomaterials 25(11), 1991–2001 (2004)
Chirayil, C.J., Joy, J., Maria, H.J., et al.: Polyolefins in automotive industry, pp. 265–283. Fundamentals and Industrial Applications. Springer International Publishing, Polyolefin Compounds and Materials (2016)
Choi, C., Hwang, I., Cho, Y.-L., et al.: Fabrication and characterization of plasma-polymerized poly(ethylene glycol) film with superior biocompatibility. ACS Appl. Mater. Interfaces. 5(3), 697–702 (2013)
Chu, P.K., Lu, X.: Low Temperature Plasma Technology: Methods and Applications. CRC Press (2013)
Coen, M.C., Dietler, G., Kasas, S., et al.: AFM measurements of the topography and the roughness of ECR plasma treated polypropylene. Appl. Surf. Sci. 103(1), 27–34 (1996)
Cognard, J.: Some recent progress in adhesion technology and science. C. R. Chim. 9(1), 13–24 (2006)
Cools, P., Declercq, H., De Geyter, N., et al.: A stability study of plasma polymerized acrylic acid films. Appl. Surf. Sci. 432, 214–223 (2018)
Corbin, G.A., Cohen, R.E., Baddour, R.: Surface fluorination of polymers in a glow discharge plasma: photochemistry. Macromolecules 18(1), 98–103 (1985)
Cui, N.-Y., Brown, N.M.D.: Modification of the surface properties of a polypropylene (PP) film using an air dielectric barrier discharge plasma. Appl. Surf. Sci. 189(1), 31–38 (2002)
De Geyter, N., Morent, R., Leys, C.: Surface characterization of plasma-modified polyethylene by contact angle experiments and ATR-FTIR spectroscopy. Surf. Interface Anal.: Int. J. Devoted Dev. Appl. Tech. Anal. Surf. Interfaces Thin Films 40(3–4), 608–611 (2008)
De Geyter, N., Morent, R., Leys, C., et al.: Treatment of polymer films with a dielectric barrier discharge in air, helium and argon at medium pressure. Surf. Coat. Technol. 201(16), 7066–7075 (2007)
De Smet, L., Vancoillie, G., Minshall, P., et al.: Plasma dye coating as straightforward and widely applicable procedure for dye immobilization on polymeric materials. Nat. Commun. 9(1), 1123 (2018)
Denes, F.S., Manolache, S.: Macromolecular plasma-chemistry: an emerging field of polymer science. Prog. Polym. Sci. 29(8), 815–885 (2004)
Desai SM and Singh RP (2004) Surface Modification of Polyethylene. In: Albertsson A-C (ed) Long Term Properties of Polyolefins. Berlin, Heidelberg: Springer Berlin Heidelberg, pp.231–294.
Desmet, T., Morent, R., De Geyter, N., et al.: Nonthermal plasma technology as a versatile strategy for polymeric biomaterials surface modification: a review. Biomacromol 10(9), 2351–2378 (2009)
Detomaso, L., Gristina, R., Senesi, G.S., et al.: Stable plasma-deposited acrylic acid surfaces for cell culture applications. Biomaterials 26(18), 3831–3841 (2005)
Drnovska, H., Lapčík, L., Buršíková, V., et al.: Surface properties of polyethylene after low-temperature plasma treatment. Colloid Polym. Sci. 281(11), 1025–1033 (2003)
Dvořáková, H., Čech, J., Stupavská, M., et al.: Fast surface hydrophilization via atmospheric pressure plasma polymerization for biological and technical applications. Polymers 11(10), 1613 (2019)
Encinas, N., Díaz-Benito, B., Abenojar, J., et al.: Extreme durability of wettability changes on polyolefin surfaces by atmospheric pressure plasma torch. Surf. Coat. Technol. 205(2), 396–402 (2010)
Fahmy, A., Schönhals, A., Friedrich, J.: Reaction of water with (radicals in) plasma polymerized allyl alcohol (and formation of OH-rich polymer layers). J. Phys. Chem. B 117(36), 10603–10611 (2013)
Farris, S., Pozzoli, S., Biagioni, P., et al.: The fundamentals of flame treatment for the surface activation of polyolefin polymers – a review. Polymer 51(16), 3591–3605 (2010)
Favia, P., Palumbo, F., d’Agostino, R., et al.: Immobilization of heparin and highly-sulphated hyaluronic acid onto plasma-treated polyethylene. Plasmas Polym. 3(2), 77–96 (1998)
Favia, P., Stendardo, M.V., d’Agostino, R.: Selective grafting of amine groups on polyethylene by means of NH3−H2 RF glow discharges. Plasmas Polym. 1(2), 91–112 (1996)
Favia, P., Stendardo, M.V., d’Agostino, R.: Selective grafting of amine groups on polyethylene by means of NH 3− H 2 RF glow discharges. Plasmas Polym. 1(2), 91–112 (1996)
Finke, B., Schröder, K., Ohl, A.: Structure retention and water stability of microwave plasma polymerized films from allylamine and acrylic acid. Plasma Process. Polym. 6(S1), S70–S74 (2009)
Foerch, R., McIntyre, N., Sodhi, R., et al.: Nitrogen plasma treatment of polyethylene and polystyrene in a remote plasma reactor. J. Appl. Polym. Sci. 40(11–12), 1903–1915 (1990)
Fresnais, J., Chapel, J., Poncin-Epaillard, F.: Synthesis of transparent superhydrophobic polyethylene surfaces. Surf. Coat. Technol. 200(18–19), 5296–5305 (2006)
Fricke, K., Steffen, H., Von Woedtke, T., et al.: High rate etching of polymers by means of an atmospheric pressure plasma jet. Plasma Process. Polym. 8(1), 51–58 (2011)
Fridman, A.: Plasma Chemistry. Cambridge University Press, Cambridge (2008)
Fridman, A., Chirokov, A., Gutsol, A.: Non-thermal atmospheric pressure discharges. J. Phys. D Appl. Phys. 38(2), R1 (2005)
Friedrich, J.: The plasma chemistry of polymer surfaces: advanced techniques for surface design. Wiley (2012)
Friedrich, J., Kühn, G., Mix, R., et al.: Formation of plasma polymer layers with functional groups of different type and density at polymer surfaces and their interaction with Al atoms. Plasma Process. Polym. 1(1), 28–50 (2004)
Friedrich, J., Wettmarshausen, S., Hennecke, M.: Haloform plasma modification of polyolefin surfaces. Surf. Coat. Technol. 203(23), 3647–3655 (2009)
Friedrich, J.F., Mix, R., Schulze, R.-D., et al.: New plasma techniques for polymer surface modification with monotype functional groups. Plasma Process. Polym. 5(5), 407–423 (2008)
Friedrich, J.F., Wigan, L., Unger, W., et al.: Barrier properties of plasma and chemically fluorinated polypropylene and polyethyleneterephthalate. Surf. Coat. Technol. 74, 910–918 (1995)
Geckeler, K., Gebhardt, R., Grünwald, H.: Surface modification of polyethylene by plasma grafting with styrene for enhanced biocompatibility. Naturwissenschaften 84(4), 150–151 (1997)
Ghasemi, M., Minier, M.J.G., Tatoulian, M., et al.: Ammonia plasma treated polyethylene films for adsorption or covalent immobilization of trypsin: quantitative correlation between X-ray photoelectron spectroscopy data and enzyme activity. J. Phys. Chem. B 115(34), 10228–10238 (2011)
Girard-Lauriault, P.-L., Mwale, F., Iordanova, M., et al.: Atmospheric pressure deposition of micropatterned nitrogen-rich plasma-polymer films for tissue engineering. Plasma Process. Polym. 2(3), 263–270 (2005)
Guang-Liang, C., Xu, Z., Guo-Hua, L., et al.: Fabricating a reactive surface on the fibroin film by a room-temperature plasma jet array for biomolecule immobilization. Chinese Phys. B 21(10), 105201 (2012)
Guruvenket, S., Rao, G.M., Komath, M., et al.: Plasma surface modification of polystyrene and polyethylene. Appl. Surf. Sci. 236(1), 278–284 (2004)
Habib, S., Lehocky, M., Vesela, D., et al.: Preparation of progressive antibacterial LDPE surface via active biomolecule deposition approach. Polymers 11(10), 1704 (2019)
Hassan, A., Aal, S.A.A.E., Shehata, M.M., et al.: Plasma-etching and modification of polyethylene for improved surface structure, wettability and optical behavior. Surf. Rev. Lett. 26(07) (2019)
He, T., Yang, Z., Chen, R., et al.: Enhanced endothelialization guided by fibronectin functionalized plasma polymerized acrylic acid film. Mater. Sci. Eng. C 32(5), 1025–1031 (2012)
Hegemann, D.: Plasma polymerization and its applications in textiles. Indian J. Fibre Text. Res. 31, 99–115 (2006)
Hollahan, J., Stafford, B., Falb, R., et al.: Attachment of amino groups to polymer surfaces by radiofrequency plasmas. J. Appl. Polym. Sci. 13(4), 807–816 (1969)
Hong, J., Truica-Marasescu, F., Martinu, L., et al.: An investigation of plasma-polymer interactions by mass spectrometry. Plasmas Polym. 7(3), 245–260 (2002)
Hoshida, T., Tsubone, D., Takada, K., et al.: Controlling the adhesion between diamond-like carbon (DLC) film and high-density polyethylene (HDPE) substrate. Surf. Coat. Technol. 202(4), 1089–1093 (2007)
Hrycak, B., Sikora, A., Moczała, M., et al.: Atmospheric pressure microwave argon plasma sheet for wettability modification of polyethylene surfaces. IEEE Trans. Plasma Sci. 47(2), 1309–1315 (2019)
Ibrahim, J., Al-Bataineh, S.A., Michelmore, A., et al.: Atmospheric pressure dielectric barrier discharges for the deposition of organic plasma polymer coatings for biomedical application. Plasma Chem. Plasma Process. (2020)
Inagaki, N., Tasaka, S., Nakajima, T.: Preparation of oxygen gas barrier polypropylene films by deposition of SiOx films plasma-polymerized from mixture of tetramethoxysilane and oxygen. J. Appl. Polym. Sci. 78(13), 2389–2397 (2000)
Johnsen, K., Kirkhorn, S., Olafsen, K., et al.: Modification of polyolefin surfaces by plasma-induced grafting. J. Appl. Polym. Sci. 59(10), 1651–1657 (1996)
Kang, M.S., Chun, B., Kim, S.S.: Surface modification of polypropylene membrane by low-temperature plasma treatment. J. Appl. Polym. Sci. 81(6), 1555–1566 (2001)
Kawakami, R., Yoshitani, Y., Mitani, K., et al.: Effects of air-based nonequilibrium atmospheric pressure plasma jet treatment on characteristics of polypropylene film surfaces. Appl. Surf. Sci. 509, 144910 (2020)
Khairallah, Y., Arefi, F., Amouroux, J.: Surface fluorination of polyethylene films by different CF4 glow discharges: effects of frequency and electrode configuration. Thin Solid Films 241(1–2), 295–300 (1994)
Kim, H., Lee, J.: Dual radio-frequency discharges: Effective frequency concept and effective frequency transition. J. Vac. Sci. Technol. A: Vac. Surf. Films 23(4), 651–657 (2005)
Kiss, É., Samu, J., Tóth, A., et al.: Novel ways of covalent attachment of poly(ethylene oxide) onto polyethylene: surface modification and characterization by XPS and contact angle measurements. Langmuir 12(6), 1651–1657 (1996)
Kogelschatz, U.: Dielectric-barrier discharges: their history, discharge physics, and industrial applications. Plasma Chem. Plasma Process. 23(1), 1–46 (2003)
Korner L, Sonnenfeld A, Heuberger R, et al. (2010a) Oxygen permeation, mechanical and structural properties of multilayer diffusion barrier coatings on polypropylene. Journal of Physics D-Applied Physics 43(11).
Korner, L., Sonnenfeld, A., von Rohr, P.R.: Silicon oxide diffusion barrier coatings on polypropylene. Thin Solid Films 518(17), 4840–4846 (2010)
Kostov, K., Nishime, T., Hein, L., et al.: Study of polypropylene surface modification by air dielectric barrier discharge operated at two different frequencies. Surf. Coat. Technol. 234, 60–66 (2013)
Kostov, K.G., Nishime, T.M.C., Castro, A.H.R., et al.: Surface modification of polymeric materials by cold atmospheric plasma jet. Appl. Surf. Sci. 314, 367–375 (2014)
Kwon, O.-J., Myung, S.-W., Lee, C.-S., et al.: Comparison of the surface characteristics of polypropylene films treated by Ar and mixed gas (Ar/O2) atmospheric pressure plasma. J. Colloid Interface Sci. 295(2), 409–416 (2006)
Lacoste, J., Carlsson, D.J.: Gamma-, photo-, and thermally-initiated oxidation of linear low density polyethylene: a quantitative comparison of oxidation products. J. Polym. Sci., Part A: Polym. Chem. 30(3), 493–500 (1992)
Lai, J., Sunderland, B., Xue, J., et al.: Study on hydrophilicity of polymer surfaces improved by plasma treatment. Appl. Surf. Sci. 252(10), 3375–3379 (2006)
Laimer, J., Störi, H.: Recent Advances in the Research on Non-Equilibrium Atmospheric Pressure Plasma Jets. Plasma Process. Polym. 4(3), 266–274 (2007)
Lamendola, R., Favia, P., Palumbo, F., et al.: Plasma-modification of polymers: process control in PE-CVD of gas-barrier films and plasma-processes for immobilizing anti-thrombotic molecules. Eur. Phys. J.-Appl. Phys. 4(1), 65–71 (1998)
Langmuir, I.: Oscillations in ionized gases. Proc. Natl. Acad. Sci. USA 14(8), 627 (1928)
Lee, J.W., Kim, M.I., Kim, B.G., et al.: Plasma polymerization and rhBMP-2 immobilization on porous high density polyethylene for osteoinduction. Int. J. Oral Maxillofac. Surg. 44, e252 (2015)
Lee, S.H., Yeo, S.Y., Cools, P., et al.: Plasma polymerization onto nonwoven polyethylene/polypropylene fibers for laccase immobilization as dye decolorization filter media. Text. Res. J. 3578–3590 (2018)
Lehocký, M., Drnovská, H., Lapčı́ková, B., et al.: Plasma surface modification of polyethylene. Colloids Surf. A 222(1), 125–131 (2003)
Li, Y., Zhang, Z., Shi, W., et al.: Adhesion enhancement of polyethylene modified by capacitively coupled radio frequency plasma polymerization of ethanol. Surf. Coat. Technol. 259, 77–82 (2014)
Lin, Y., Yasuda, H.: Hydrocarbon barrier performance of plasma-surface-modified polyethylene. J. Appl. Polym. Sci. 60(12), 2227–2238 (1996)
Liston, E., Martinu, L., Wertheimer, M.: Plasma surface modification of polymers for improved adhesion: a critical review. J. Adhes. Sci. Technol. 7(10), 1091–1127 (1993)
Liu, H., Xie, D., Qian, L., et al.: The mechanical properties of the ultrahigh molecular weight polyethylene (UHMWPE) modified by oxygen plasma. Surf. Coat. Technol. 205(8–9), 2697–2701 (2011)
Liu, Z.-M., Tingry, S., Innocent, C., et al.: Modification of microfiltration polypropylene membranes by allylamine plasma treatment: Influence of the attachment route on peroxidase immobilization and enzyme efficiency. Enzyme Microb. Technol. 39(4), 868–876 (2006)
Lommatzsch, U., Pasedag, D., Baalmann, A., et al.: Atmospheric pressure plasma jet treatment of polyethylene surfaces for adhesion improvement. Plasma Process. Polym. 4(S1), S1041–S1045 (2007)
Lu, X., Laroussi, M., Puech, V.: On atmospheric-pressure non-equilibrium plasma jets and plasma bullets. Plasma Sources Sci. Technol. 21(3) 034005 (2012)
Macgregor, M., Vasilev, K.: Perspective on plasma polymers for applied biomaterials nanoengineering and the recent rise of oxazolines. Materials 12(1), 191 (2019)
Maitz, M.F.: Applications of synthetic polymers in clinical medicine. Biosurface Biotribology 1(3), 161–176 (2015)
Mandolfino, C.: Polypropylene surface modification by low pressure plasma to increase adhesive bonding: effect of process parameters. Surf. Coat. Technol. 366, 331–337 (2019)
Mandolfino, C., Lertora, E., Gambaro, C., et al.: Improving adhesion performance of polyethylene surfaces by cold plasma treatment. Meccanica 49(10), 2299–2306 (2014)
Mansuroglu, D., Uzun-Kaymak, I.U.: Argon and nitrogen plasma modified polypropylene: surface characterization along with the optical emission results. Surf. Coat. Technol. 358, 551–559 (2019)
McNaught, A.D., Wilkinson, A.: IUPAC. Compendium of Chemical Terminology, 2nd ed. (the “Gold Book”). WileyBlackwell; 2nd Revised edition edition)
Medard, N., Soutif, J.-C., Poncin-Epaillard, F.: Characterization of CO2 plasma-treated polyethylene surface bearing carboxylic groups. Surf. Coat. Technol. 160(2–3), 197–205 (2002)
Merche, D., Vandencasteele, N., Reniers, F.: Atmospheric plasmas for thin film deposition: a critical review. Thin Solid Films 520(13), 4219–4236 (2012)
Morent, R., De Geyter, N., Gengembre, L., et al.: Surface treatment of a polypropylene film with a nitrogen DBD at medium pressure. Eur. Phys. J. Appl. Phys. 43(3), 289–294 (2008)
Morent, R., De Geyter, N., Jacobs, T., et al.: Plasma-polymerization of HMDSO using an atmospheric pressure dielectric barrier discharge. Plasma Process. Polym. 6, S537–S542 (2009)
Morent, R., De Geyter, N., Leys, C., et al.: Surface modification of non-woven textiles using a dielectric barrier discharge operating in air, helium and argon at medium pressure. Text. Res. J. 77(7), 471–488 (2007)
Morent, R., De Geyter, N., Trentesaux, M., et al.: Stability study of polyacrylic acid films plasma-polymerized on polypropylene substrates at medium pressure. Appl. Surf. Sci. 257(2), 372–380 (2010)
Morent, R., De Geyter, N., Van Vlierberghe, S., et al.: Influence of operating parameters on plasma polymerization of acrylic acid in a mesh-to-plate dielectric barrier discharge. Prog. Org. Coat. 70(4), 336–341 (2011)
Morent, R., De Geyter, N., Van Vlierberghe, S., et al.: Organic-inorganic behaviour of HMDSO films plasma-polymerized at atmospheric pressure. Surf. Coat. Technol. 203(10–11), 1366–1372 (2009)
Mortazavi, S.H., Jahazi, A., Ghoranneviss, M.: The effect of plasma polymerization of silicon compounds on the properties of biaxially oriented polypropylene (BOPP). Polym. Bull. 77(4), 1813–1828 (2020)
Médard, N., Soutif, J.-C., Poncin-Epaillard, F.: CO2, H2O, and CO2/H2O plasma chemistry for polyethylene surface modification. Langmuir 18(6), 2246–2253 (2002)
Nihlstrand, A., Hjertberg, T., Johansson, K.: Plasma polymerization of allyl alcohol on polyolefin-based materials: characterization and adhesion properties. J. Adhes. Sci. Technol. 10(2), 123–150 (1996)
Nikitin, D., Lipatova, I., Naumova, I., et al.: Immobilization of chitosan onto polypropylene foil via air/solution atmospheric pressure plasma afterglow treatment. Plasma Chem. Plasma Process. 40(1), 207–220 (2020)
Nisticò, R., Rosellini, A., Rivolo, P., et al.: Surface functionalisation of polypropylene hernia-repair meshes by RF-activated plasma polymerisation of acrylic acid and silver nanoparticles. Appl. Surf. Sci. 328, 287–295 (2015)
Novák, I., Pollak, V., Chodak, I.: Study of surface properties of polyolefins modified by corona discharge plasma. Plasma Process. Polym. 3(4–5), 355–364 (2006)
Novák, I., Popelka, A., Špitalský, Z., et al.: Polyolefin in packaging and food industry, pp. 181–199. Fundamentals and Industrial Applications. Springer International Publishing, Polyolefin Compounds and Materials (2016)
Nuzzo, R., Smolinsky, G.: Preparation and characterization of functionalized polyethylene surfaces. Macromolecules 17(5), 1013–1019 (1984)
Onyshchenko, I., De Geyter, N., Nikiforov, A.Y., et al.: Atmospheric pressure plasma penetration inside flexible polymeric tubes. Plasma Process. Polym. 12(3), 271–284 (2015)
Owonubi, S.J., Agwuncha, S.C., Fasiku, V.O., et al.: 17 - Biomedical applications of polyolefins. In: Polyolefin Fibres, 2nd edn. Woodhead Publishing, pp.517–538 (2017)
Petasch, W., Räuchle, E., Walker, M., et al.: Improvement of the adhesion of low-energy polymers by a short-time plasma treatment. Surf. Coat. Technol. 74–75, 682–688 (1995)
Poncin-Epaillard, F., Chevet, B., Brosse, J.C.: Modification of isotactic polypropylene by a cold plasma or an electron beam and grafting of the acrylic acid onto these activated polymers. J. Appl. Polym. Sci. 53(10), 1291–1306 (1994)
Prachar, J., Novak, I., Kleinova, A., et al.: Plasma grafting of polypropylene with organosilanes and its alkylamine treatment. Vacuum 127, 38–44 (2016)
Raj, J., Herzog, G., Manning, M., et al.: Surface immobilisation of antibody on cyclic olefin copolymer for sandwich immunoassay. Biosens. Bioelectron. 24(8), 2654–2658 (2009)
Ramkumar, M., Pandiyaraj, K.N., Kumar, A.A., et al.: Evaluation of mechanism of cold atmospheric pressure plasma assisted polymerization of acrylic acid on low density polyethylene (LDPE) film surfaces: Influence of various gaseous plasma pretreatment. Appl. Surf. Sci. 439, 991–998 (2018)
Sajeev, U., Mathai, C.J., Saravanan, S., et al.: On the optical and electrical properties of RF and AC plasma polymerized aniline thin films. Bull. Mater. Sci. 29(2), 159–163 (2006)
Sanchis, R., Fenollar, O., García, D., et al.: Improved adhesion of LDPE films to polyolefin foams for automotive industry using low-pressure plasma. Int. J. Adhes. Adhes. 28(8), 445–451 (2008)
Murthy, V.S.M.D., Vaidya, U.: Improving the adhesion of glass/polypropylene (glass-PP) and high-density polyethylene (HDPE) surfaces by open air plasma treatment. Int. J. Adhes. Adhes. 95, 102435 (2019)
Sarani, A., Nikiforov, A.Y., De Geyter, N., et al.: Surface modification of polypropylene with an atmospheric pressure plasma jet sustained in argon and an argon/water vapour mixture. Appl. Surf. Sci. 257(20), 8737–8741 (2011)
Sarmadi, A.M., Ying, T.H., Denes, F.: HMDSO-plasma modification of polypropylene fabrics. Eur. Polymer J. 31(9), 847–857 (1995)
Saxena, S., Ray, A.R., Kapil, A., et al.: Development of a new polypropylene-based suture: plasma grafting, surface treatment, characterization, and biocompatibility studies. Macromol. Biosci. 11(3), 373–382 (2011)
Sciarratta, V., Vohrer, U., Hegemann, D., et al.: Plasma functionalization of polypropylene with acrylic acid. Surf. Coat. Technol. 174, 805–810 (2003)
Scopece, P., Viaro, A., Sulcis, R., et al.: SiOx-based gas barrier coatings for polymer substrates by atmospheric pressure plasma jet deposition. Plasma Process. Polym. 6, S705–S710 (2009)
Sekiguchi, H., Orimo, T.: Gasification of polyethylene using steam plasma generated by microwave discharge. Thin Solid Films 457(1), 44–47 (2004)
Seo, H.S., Ko, Y.M., Shim, J.W., et al.: Characterization of bioactive RGD peptide immobilized onto poly(acrylic acid) thin films by plasma polymerization. Appl. Surf. Sci. 257(2), 596–602 (2010)
Shenton, M., Stevens, G.: Surface modification of polymer surfaces: atmospheric plasma versus vacuum plasma treatments. J. Phys. D Appl. Phys. 34(18), 2761 (2001)
Siow, K.S., Britcher, L., Kumar, S., et al.: Plasma methods for the generation of chemically reactive surfaces for biomolecule immobilization and cell colonization-a review. Plasma Process. Polym. 3(6–7), 392–418 (2006)
Suzuki, M., Kishida, A., Iwata, H., et al.: Graft copolymerization of acrylamide onto a polyethylene surface pretreated with glow discharge. Macromolecules 19(7), 1804–1808 (1986)
Švorčík, V., Kolářová, K., Slepička, P., et al.: Modification of surface properties of high and low density polyethylene by Ar plasma discharge. Polym. Degrad. Stab. 91(6), 1219–1225 (2006)
Švorčík, V., Kotál, V., Slepička, P., et al.: Modification of surface properties of polyethylene by Ar plasma discharge. Nucl. Instrum. Methods Phys. Res. Sect. B 244(2), 365–372 (2006)
Tahara, M., Cuong, N., Nakashima, Y.: Improvement in adhesion of polyethylene by glow-discharge plasma. Surf. Coat. Technol. 174, 826–830 (2003)
Tajima, S., Komvopoulos, K.: Surface modification of low-density polyethylene by inductively coupled argon plasma. J. Phys. Chem. B 109(37), 17623–17629 (2005)
Tajima, S., Komvopoulos, K.: Physicochemical properties and morphology of fluorocarbon films synthesized on crosslinked polyethylene by capacitively coupled octafluorocyclobutane plasma. J. Phys. Chem. C 111(11), 4358–4367 (2007)
Takahashi, J., Hotta, A.: Adhesion enhancement of polyolefins by diamond like carbon coating and photografting polymerization. Diam. Relat. Mater. 26, 55–59 (2012)
Tamada, Y., Ikada, Y.: Cell adhesion to plasma-treated polymer surfaces. Polymer 34(10), 2208–2212 (1993)
Tatoulian, M., Arefi-Khonsari, F., Mabille-Rouger, I., et al.: Role of helium plasma pretreatment in the stability of the wettability, adhesion, and mechanical properties of ammonia plasma-treated polymers. Application to the Al-polypropylene system. J. Adhes. Sci. Technol. 9(7), 923–934 (1995).
Thiry, D., Konstantinidis, S., Cornil, J., et al.: Plasma diagnostics for the low-pressure plasma polymerization process: a critical review. Thin Solid Films 606, 19–44 (2016)
Thomas, M., von Hausen, M., Klages, C.P., et al.: Generation of stable coatings with carboxylic groups by copolymerization of MAA and VTMS using DBD at atmospheric pressure. Plasma Process. Polym. 4(S1), S475–S481 (2007)
Truica-Marasescu, F., Wertheimer, M.R.: Nitrogen-rich plasma-polymer films for biomedical applications. Plasma Process. Polym. 5(1), 44–57 (2008)
Unger, W., Lippitz, A., Gross, T., et al.: The use of octadecyltrichlorosilane self-assembled layers as a model for the assessment of plasma treatment and metallization effects on polyolefins. Langmuir 15(4), 1161–1166 (1999)
Valentina, S.: Food packaging permeability behaviour: a report. Int. J. Polymer Sci. 2012, 11 (2012)
Van Deynse, A., Cools, P., Leys, C., et al.: Surface modification of polyethylene in an argon atmospheric pressure plasma jet. Surf. Coat. Technol. 276, 384–390 (2015)
Van Guyse, J.F.R., Cools, P., Egghe, T., et al.: Influence of the aliphatic side chain on the near atmospheric pressure plasma polymerization of 2-alkyl-2-oxazolines for biomedical applications. ACS Appl. Mater. Interfaces 11(34), 31356–31366 (2019)
Van Vrekhem, S., Cools, P., Declercq, H., et al.: Application of atmospheric pressure plasma on polyethylene for increased prosthesis adhesion. Thin Solid Films 596, 256–263 (2015)
Vartiainen, J., Rättö, M., Paulussen, S.: Antimicrobial activity of glucose oxidase-immobilized plasma-activated polypropylene films. Packag. Technol. Sci. 18(5), 243–251 (2005)
Vartiainen, J., Rättö, M., Tapper, U., et al.: Surface modification of atmospheric plasma activated BOPP by immobilizing chitosan. Polym. Bull. 54(4), 343–352 (2005)
Volcke, C., Gandhiraman, R.P., Gubala, V., et al.: Reactive amine surfaces for biosensor applications, prepared by plasma-enhanced chemical vapour modification of polyolefin materials. Biosens. Bioelectron. 25(8), 1875–1880 (2010)
Wagner, A.J., Fairbrother, D.H., Reniers, F.: A comparison of PE surfaces modified by plasma generated neutral nitrogen species and nitrogen Ions. Plasmas Polym. 8(2), 119–134 (2003)
Walker, M., Baumgärtner, K.M., Räuchle, E.: Multilayer barrier films on polyethylene polymerized from CF3H/C2H4 plasmas. Acta Polym. 47(10), 466–469 (1996)
Wang, Y., Zhang, J., Shen, X.Y.: Surface structures tailoring of hexamethyldisiloxane films by pulse rf plasma polymerization. Mater. Chem. Phys. 96(2–3), 498–505 (2006)
Wettmarshausen, S., Kühn, G., Hidde, G., et al.: Plasmabromination – the Selective Way to Monotype Functionalized Polymer Surfaces. Plasma Process. Polym. 4(9), 832–839 (2007)
Wu, S., Zhang, J., Xu, X.: Studies on high density polyethylene (HDPE) functionalized by ultraviolet irradiation and its application. Polym. Int. 52(9), 1527–1530 (2003)
Yasuda, H., Hsu, T.: Some aspects of plasma polymerization investigated by pulsed RF discharge. J. Polym. Sci.: Polym. Chem. Ed. 15(1), 81–97 (1977)
Yenchun, L., Yenpei, F.: Inductively coupling plasma (ICP) treatment of propylene (PP) surface and adhesion improvement. Plasma Sci. Technol 11(6), 704 (2009)
Zhang, W., Ji, J., Zhang, Y., et al.: Effects of NH3, O2, and N2 co-implantation on Cu out-diffusion and antimicrobial properties of copper plasma-implanted polyethylene. Appl. Surf. Sci. 253(22), 8981–8985 (2007)
Zhang, W., Luo, Y., Wang, H., et al.: Ag and Ag/N2 plasma modification of polyethylene for the enhancement of antibacterial properties and cell growth/proliferation. Acta Biomater. 4(6), 2028–2036 (2008)
Author information
Authors and Affiliations
Corresponding authors
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Narimisa, M., Ghobeira, R., Onyshchenko, Y., De Geyter, N., Egghe, T., Morent, R. (2022). Different Techniques Used for Plasma Modification of Polyolefin Surfaces. In: Baneesh, N.S., Sari, P.S., Vackova, T., Thomas, S. (eds) Plasma Modification of Polyolefins. Engineering Materials. Springer, Cham. https://doi.org/10.1007/978-3-030-52264-3_2
Download citation
DOI: https://doi.org/10.1007/978-3-030-52264-3_2
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-52263-6
Online ISBN: 978-3-030-52264-3
eBook Packages: Chemistry and Materials ScienceChemistry and Material Science (R0)