Abstract
Peri-implant diseases and conditions are prevalent, accounting for approximately 40% of implants. According to the most updated disease classification by the joint effort between the American Academy of Periodontology (AAP) and the European Federation of Periodontology (EFP) in 2017, four categories have been proposed, namely implant health, peri-implant mucositis, peri-implantitis, and soft- and hard-tissue deficiencies. Clinical parameters to differentiate these four diseases and conditions are bleeding upon probing (BOP), radiographic bone loss, probing depth, hard- and soft-tissue dimension, and presence of pus/suppuration. Current methods to measure these clinical parameters have their own limitations and disadvantages, which will be highlighted in this chapter. High-frequency ultrasonography has advantages of being non-ionizing, point-of-care, and cross-sectional; therefore, it can be a first-line tool to evaluate peri-implant tissue health during implant maintenance phase. Recent research efforts by our group suggests ultrasound B-mode is accurate in delineating soft tissue thickness around implants, marginal bone level, and thickness. Additionally, it can also be used to measure peri-implant papilla height, mid-facial soft tissue height, bony fenestration, etc. Besides B-mode imaging, functional ultrasound modes, e.g. color flow and power flow, can estimate blood velocity and volume, currently in 2-dimension. These quantitative ultrasound markers may be related to the degree of inflammation around implants and are under active investigation. Once validated, these functional modes can become objective markers to estimate peri-implant inflammation. This chapter will demonstrate the potential of ultrasound to diagnose peri-implant diseases and conditions by presenting ultrasound images of clinical cases.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
8.1 Introduction
Implant therapy has become the standard of care for replacing missing teeth. It is estimated two to four million implants will be placed in the USA by 2020. While implant therapy has enjoyed a high survival rate, incidences of biological and esthetic complications are on the rise. The biological complication is mostly referring to peri-implantitis, an infectious disease affecting peri-implant hard and soft tissues. It is estimated approximately 20% implants are affected by this disease [1]. The end outcome of this disease is peri-implant bone loss and eventually implant loss. The less severe and reversible form that does not involve progressive bone loss is peri-implant mucositis. The two diseases originate and progress in soft tissues with bacterial challenge and dysbiosis before bone hemostasis disruption. In addition, nowadays, patients have a higher esthetic expectation. To achieve and sustain an esthetic outcome, a thorough soft- and hard-tissue evaluation at the treatment phase and subsequently at the maintenance phase is important. Therefore, ultrasound, being a superior imaging modality for evaluating soft tissue features, will play an important role in diagnosing peri-implantitis and evaluating tissue phenotype. This chapter will briefly discuss the current clinical methods to evaluate peri-implant structures and their limitations. Images of peri-implant tissues with various disease severity, defined by the 2017 AAP-EFP (American Academy of Periodontology/European Federation of Periodontology) World Workshop will be presented to illustrate the potential usefulness of ultrasound in diagnosing peri-implant diseases. Proper diagnosis and evaluation of peri-implant tissues will lay a foundation for decision making in treatment options and outcome assessment.
8.2 2017 AAP/EFP Classification on Peri-Implant Diseases and Conditions
In 2017 an international task force proposed a classification on peri-implant diseases and condition and formed the current foundation for studying and treating these related diseases and conditions. For details the authors can refer to the manuscripts published in 2018 [2,3,4,5,6,7]. In brief, four categories have been listed: (1) peri-implant health, (2) peri-implant mucositis, (3) peri-implantitis, and (4) soft- and hard-tissue deficiencies. The case definitions are summarized in Table 8.1. A healthy implant is surrounded by bone, with the its coronal part sealed by mucosa. This mucosa contains a core of connective tissue, comprised of mainly type 1 collagen fibers and matrix elements (85%), fibroblasts (3%), and vascular units (5%). The outer (oral) surface of the connective tissue is normally covered by keratinized epithelium. The mucosa that is in direct contact with the implant, abutment, and crown contains two components, the epithelium and the connective tissue. In health, the peri-implant mucosa height is about 3–4 mm with an epithelium that is about 2 mm long. A healthy implant should not have signs indicative of inflammation, including bleeding on gentle bleeding (BOP), erythema, swelling, suppuration. It should not have increased probing depth, mucosal recession, and pathologic bone loss. Peri-implant mucositis has signs of inflammation but a lack of bone loss beyond remodeling. On the other hand, peri-implantitis, in addition to signs of inflammation, has pathologic bone loss. Soft- and hard-tissue deficiencies, as the name indicated, have mucosal recession and/or thin mucosa and/or loss of bone in the absence of overt tissue inflammation. Therefore, diagnosis and differentiation of these diseases and conditions center on evaluation of the soft tissue inflammatory status, tissue phenotype, and dimensions of the peri-implant hard and soft tissues.
8.3 Current Methods to Measure Peri-Implant Bone Loss and Limitations
Peri-implant bone loss is the hallmark of peri-implantitis, a prevalent disease that occurs in approximately 20% of dental implants [1]. Costly and traumatic surgical interventions impact patients’ quality of life tremendously. Demands for improving quality of implant therapy have driven expansion of clinical research and patient care in this field. To provide definitive evidence, development of well-founded outcome measures and standardized diagnostic criteria are critical. Currently, peri-implant bone level measured from two-dimensional intraoral radiographs is the primary measure [9]. However, 2D radiography is incapable of providing a comprehensive evaluation of peri-implant bone level (Fig. 8.1). It only shows superimposed interproximal bone level, much less the radicular (facial and palatal/lingual) bone level and thickness. Bone thickness is another important outcome measure, especially related to esthetics and long-term peri-implant bone stability [10, 11]. This inherent limitation reduces its ability to assess disease severity and treatment outcome [9, 12, 13]. Other limitations include ionizing radiation and image distortions, etc.
An implant with peri-implant bone loss shown on the periapical radiograph (Right) and intraoperatively (Left). Interproximal bone level at mesial (BL-m) and distal sites (BL-d) is measured vertically from implant platform as a reference (R) to the first implant-bone contact. However, the radiographic interproximal bone level is only at its best the superimposition of the facial and palatal bone levels. Individual bone levels on the facial and palatal sides as well as bone thickness (BT), measured horizontally from the implant surface to the bone surface, are important parameters for disease characters and treatment selection and could be measured with the proposed sonography-based method
8.4 Current Methods to Estimate Tissue Phenotype and Limitations
Methods to evaluate tissue phenotype are summarized in Table 8.2. Visual evaluation is not an objective method to identify the tissue phenotype, since it was accurately identified in only about half of the cases, irrespective of the clinician’s experience [14]. Probe transparency is another way to determine tissue phenotype. However, it is only accurate when the mucosal thickness is either too thin, i.e. less than 0.6 mm or more than 1.2 mm. Moderate tissue thickness cannot be differentiated with this method [15]. Bone sounding is not commonly applied because it is invasive, usually performed under local anesthesia. Injection of local anesthetic solution causes patient discomfort and is also associated with a transient local tissue volume increase. When bone is thin, penetration of the sharp instrument into bone, overestimates soft tissue thickness. Use of cone-beam computed tomography (CBCT) has also shown a high diagnostic accuracy in assessing mucosal thickness, demonstrating minimal discrepancy with clinical and radiographic measurements [16]. However, routine uses of CBCT for this purpose may not recommended [17]. Optical scanners can record tissue surfaces changes overtime, e.g. tissue thickness changes before and after a given procedure. However, it cannot measure the “absolute” soft tissue thickness. Ultrasound is an established tool to measure soft tissue thickness and therefore optimal for estimate tissue phenotype [18,19,20,21,22].
8.5 Rationale of Ultrasound as an Adjunctive Diagnostic Method
Unlike teeth, facial bone around implants is more susceptible for resorption [23, 24], resulting in non-uniform bone loss in 34–45% of infected implants [25, 26]. Two-dimensional X-rays are not adequate to evaluate facial and lingual/palatal bone loss. CBCT might provide accurate 3D bone level values but issues like imaging artifacts arising from metal implants and radiation concerns are unsolved (Fig. 8.2) [12, 27, 28].
Comparisons of 2D radiograph, CT and ultrasound (US) images for assessing “facial” peri-implant bone of a human subject. The 2D radiograph can show superimposed interproximal bone but not facial bone. A CT cross-sectional scan example is shown; however, because of artifacts, thin facial bone could not be seen in this case. Here a representative US cross-sectional image clearly shows facial peri-implant bone surface and its spatial relation to the implant. ST soft tissue
There have been promising research efforts to apply ultrasonography for evaluating periodontal bone level [29,30,31], including recent works from the authors’ group [32,33,34]. Peri-implant bone level was evaluated by Bertram et al. [35] using ultrasound with a linear 12.5 MHz transducer. A total of 29 buccal bone defects in 25 patients who were diagnosed with peri-implantitis and scheduled for a revision surgery were recruited. The results showed that measurements made at moderate bone loss levels (3–6 mm) were the most reliable (ICC = 0.81 for reproducibility and 0.76 for accuracy). The mean absolute difference is 0.1 mm. However, the correlations in normal (< 3 mm) and advanced bone loss (> 6 mm) cases were moderate to poor (ICC = 0.63 to 0.73). The mean absolute difference is 0.6 mm. Recent advances in device miniaturization and image resolution improvement, coupled with other desirable properties, e.g. real-time, cost-effective, and non-ionizing make ultrasonography a reality for working in the oral cavity. 3D ultrasonography can image both interproximal and radicular (facial and palatal/lingual) bone loss. This comprehensive assessment would be especially valuable for evaluating long-term bone level stability and measuring treatment efficacy because of its non-ionizing and point-of-care nature. Figure 8.3 illustrates cross-sectional ultrasound images of the mid-facial site of an implant. The facial bone level and thickness can be determined on the image that can assist in diagnosis of peri-implant diseases and conditions. The measurements can be confirmed with a transverse image stack, as shown in Fig. 8.4.
An example of a cross-sectional ultrasound scan of an implant from a human subject. The examiners will acquire images like this example, on which the crown (C), implant (I), bone surface (B), and soft tissue (S) can be clearly identifiable. On the images, bone level (BL) and thickness (BT) will be measured and calibrated with the standard examiner
Two ultrasound images in transverse view extracted from a volume scan. The motor drove the ultrasound probe at a constant speed in the corono-apical direction so a series of spatially oriented transverse slices could be collected. The top ultrasound image was at the level of the implant platform, whereas the bottom image was at the marginal bone level. At the marginal bone level, only the outermost part of the implant surface (I) is seen; because of attenuation, roots (R) of the adjacent teeth behind bone (B) could not be seen on ultrasound. Bone thickness (BT) can be measured on this slice. The vertical distance between these two slices can be calculated to represent the marginal bone level
8.6 Ultrasound Case Demonstration Based on the New Classification
8.6.1 Case of Peri-Implant Health
Case Description
A healthy implant should not have clinical signs of inflammation, including BOP, erythema, swelling, suppuration. It should not have probing depth increase, mucosal recession, and pathologic bone loss. Figure 8.5 exemplifies such a case. The probing depth is 3 mm without BOP. On the radiograph, the marginal bone loss is within normal range. Ultrasound images provides additional useful information, including the soft tissue height, soft tissue thickness, crestal bone thickness, etc. (see Fig. 8.5). The ultrasound soft tissue height may correlate with the probing depth. The soft tissue thickness and crestal bone thickness are measures of tissue phenotype.
Ultrasound images of a healthy implant, in relation to the clinical photo and 2D radiograph. B bone, I implant, IP implant platform, CB crestal bone, P papilla, A abutment, C crown. Published with permission from [8]
8.6.2 Case of Peri-Implant Mucositis
Case Description
Peri-implant mucositis has clinical signs of inflammation but a lack of bone loss beyond normal bone remodeling, as demonstrated in Fig. 8.6. In this case, there is increased tissue inflammation and tissue swelling, as evidenced by visual examination and BOPs, as well as increased PD. Radiographic marginal bone loss is within normal range as a result of the initial healing process. Ultrasound images show normal soft tissue height, thickness, crestal bone level and thickness.
Ultrasound images of an implant with peri-implant mucositis, in relation to the clinical photo and 2D radiograph. B bone, I implant, IP implant platform, CB crestal bone, M mucosa, P papilla, A abutment, C crown. Published with permission from [8]
8.6.3 Case of Peri-Implantitis
Case Description
In additional to clinical inflammation, increased bone loss is a cardinal sign of peri-implantitis. Figure 8.7 demonstrates a case defined as early stage of peri-implantitis. There is increased PD and tissue inflammation (BOPs). Radiographic marginal bone loss is more evident and beyond normal remodeling. On ultrasound images, there is increased distance between the implant platform (IP) and the crestal bone (CB), indicative of bone loss. Increased soft tissue height is also an indication of soft tissue swelling and PD increase.
Ultrasound images of an implant with peri-implantitis, in relation to the clinical photo and 2D radiograph. B bone, I implant, IP implant platform, CB crestal bone, A abutment, C crown. Published with permission from [8]
8.6.4 Case of Peri-Implant Soft- and Hard-Tissue Deficiency
Case Description
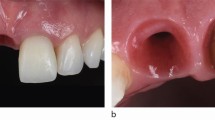
A case diagnosis of peri-implant soft- and hard-tissue deficiency presents as mucosal recession and/or thin mucosa and/or loss of bone in the absence of overt tissue inflammation. In a case shown in Fig. 8.8, the PD is within normal range (3 mm) without clinical inflammation. There is some radiographic marginal bone loss, as evidenced on radiographs. Ultrasound shows evidences of soft tissue deficiency, with 0.74 mm in soft tissue thickness, as well as hard-tissue deficiency, with close to 0 mm in crestal bone thickness on the mid-facial site. The amount of bony fenestration, i.e. implant exposure, is evident on the mid-facial site. On the ultrasound image at the mid-facial site, the implant surface with a threaded pattern is clearly seen.
Ultrasound images of an implant with tissue deficiency, in relation to the clinical photo and 2D radiograph. B bone, I implant, IP implant platform, CB crestal bone, A abutment, C crown. Published with permission from [8]
In addition to anatomical images, ultrasound is able to provide various modes, including the back scatter, elasticity, color flow, power Doppler, and photoacoustic, that could be used to quantify tissue inflammation and tissue loss, which are key to evaluate aggressiveness and status of peri-implant diseases and conditions (Table 8.3). These areas are highly interesting and in active investigations. In the near future, clinicians may start to adapt to this novel technology as a tool to diagnose peri-implant diseases and conditions.
8.7 Conclusions
Peri-implant diseases and conditions are emerging epidemic complications that do not have adequate and standard diagnostic methods currently. Conventional clinical evaluation and 2D radiographs may not grasp the whole picture of the diseases. Therefore, there is a delay in developing optimal solutions to these complications. Ultrasound can provide cross-sectional peri-implant anatomical information. It may add to the diagnostic value by offering tissue-destruction and tissue inflammation related parameters. Research ground work is being actively conducted in this field. Once validated, ultrasound can become a standard care in diagnosing peri-implant diseases and conditions in the foreseeable future.
References
Derks J, Tomasi C. Peri-implant health and disease. A systematic review of current epidemiology. J Clin Periodontol. 2015;42 Suppl 16:S158–71. ISSN:1600-051X (Electronic) 0303-6979 (Linking). https://doi.org/10.1111/jcpe.12334. http://www.ncbi.nlm.nih.gov/pubmed/25495683.
Araujo MG, Lindhe J. Peri-implant health. J Periodontol. 2018;89 Suppl 1:S249–56. ISSN:1943-3670 (Electronic) 0022-3492 (Linking). https://doi.org/10.1002/JPER.16-0424. https://www.ncbi.nlm.nih.gov/pubmed/29926949.
Berglundh T, et al. Peri-implant diseases and conditions: consensus report of workgroup 4 of the 2017 world workshop on the classification of periodontal and peri-implant diseases and conditions. J Periodontol. 2018;89 Suppl 1:S313–18. ISSN:1943-3670 (Electronic) 0022-3492 (Linking). https://doi.org/10.1002/JPER.17-0739. https://www.ncbi.nlm.nih.gov/pubmed/29926955.
Hammerle CHF, Tarnow D. The etiology of hard- and soft-tissue deficiencies at dental implants: a narrative review. J Periodontol. 2018;89 Suppl 1:S291–303. ISSN:1943-3670 (Electronic) 0022-3492 (Linking). https://doi.org/10.1002/JPER-16-0810. https://www.ncbi.nlm.nih.gov/pubmed/29926950.
Heitz-Mayfield LJA, Salvi GE. Peri-implant mucositis. J Periodontol. 2018;89 Suppl 1:S257–66. ISSN:1943-3670 (Electronic) 0022-3492 (Linking). https://doi.org/10.1002/JPER-16-0488. https://www.ncbi.nlm.nih.gov/pubmed/29926954.
Renvert S, et al. Peri-implant health, peri-implant mucositis, and peri-implantitis: case definitions and diagnostic considerations. J Periodontol. 2018;89 Suppl 1:S304–12. ISSN:1943-3670 (Electronic) 0022-3492 (Linking). https://doi.org/10.1002/JPER.17-0588. https://www.ncbi.nlm.nih.gov/pubmed/29926953.
Schwarz F, et al. Peri-implantitis. J Periodontol. 2018;89 Suppl 1:S267–90. ISSN:1943-3670 (Electronic) 0022-3492 (Linking). https://doi.org/10.1002/JPER.16-0350. https://www.ncbi.nlm.nih.gov/pubmed/29926957.
Bhaskar V, Chan H-L, MacEachern M, Kripfgans OD. Updates on ultrasound research in implant dentistry: a systematic review of potential clinical indications. Dentomaxillofac Radiol. 2018;47(6):20180076. https://doi.org/10.1259/dmfr.20180076. Epub 2018 Jun 6.
Sanz M, Chapple IL, Working Group 4 of the VIII European Workshop on Periodontology. Clinical research on peri-implant diseases: consensus report of Working Group 4. J Clin Periodontol. 2012;39 Suppl 12:202–6. ISSN:1600-051X (Electronic) 0303-6979 (Linking). https://doi.org/10.1111/j.1600-051X.2011.01837.x. http://www.ncbi.nlm.nih.gov/pubmed/22533957.
Spray JR, et al. The influence of bone thickness on facial marginal bone response: stage 1 placement through stage 2 uncovering. Ann Periodontol. 2000;5.1:119–28. ISSN:1553-0841 (Print) 1553-0841 (Linking). https://doi.org/10.1902/annals.2000.5.1.119. http://www.ncbi.nlm.nih.gov/pubmed/11885170.
Evans CD, Chen ST. Esthetic outcomes of immediate implant placements. Clin Oral Implants Res. 2008;19.1:73–80. ISSN:0905-7161 (Print) 0905-7161 (Linking). https://doi.org/10.1111/j.1600-0501.2007.01413x. https://www.ncbi.nlm.nih.gov/pubmed/17956569.
Ritter L, et al. Accuracy of peri-implant bone evaluation using cone beam CT digital intra-oral radiographs and histology. Dentomaxillofac Radiol. 2014;43.6:20130088. ISSN: 0250-832X (Print) 0250-832X (Linking). https://doi.org/10.1259/dmfr.20130088. http://www.ncbi.nlm.nih.gov/pubmed/24786136.
Schwarz F, et al. Comparison of naturally occurring and ligature-induced peri-implantitis bone defects in humans and dogs. Clin Oral Implants Res. 2007;18.2:161–70. ISSN:0905-7161 (Print) 0905-7161 (Linking). https://doi.org/10.1111/j.1600-0501.2006.01320.x. http://www.ncbi.nlm.nih.gov/pubmed/17348880.
Eghbali A, et al. The gingival biotype assessed by experienced and inexperienced clinicians. J Clin Periodontol. 2009;36.11:958–63. ISSN:1600-051X (Electronic) 0303-6979 (Linking). https://doi.org/10.1111/j.1600-051X.2009.01479.x. https://www.ncbi.nlm.nih.gov/pubmed/19811580.
De Rouck T, et al. The gingival biotype revisited: transparency of the periodontal probe through the gingival margin as a method to discriminate thin from thick gingiva. J Clin Periodontol. 2009;36.5:428–33. ISSN:0303-6979.
Fu JH, et al. Tissue biotype and its relation to the underlying bone morphology. J Periodontol. 2010;81.4:569–74. ISSN:1943-3670 (Electronic) 0022-3492 (Linking). https://doi.org/10.1902/jop.2009.090591. https://www.ncbi.nlm.nih.gov/pubmed/20367099.
Benavides E, et al. Use of cone beam computed tomography in implant dentistry: the international congress of oral implantologists consensus report. Implant Dent. 2012;21.2:78–86. ISSN:1056-6163.
Eger T, Muller HP, Heinecke A. Ultrasonic determination of gingival thickness. Subject variation and influence of tooth type and clinical features. J Clin Periodontol. 1996;23.9:839–45. ISSN:0303-6979 (Print) 0303-6979 (Linking). http://www.ncbi.nlm.nih.gov/pubmed/8891935.
Kloukos D, et al. Gingival thickness assessment at the mandibular incisors with four methods: a cross-sectional study. J Periodontol. 2018;89.11:1300–09. ISSN:1943-3670 (Electronic) 0022-3492 (Linking). https://doi.org/10.1002/JPER-180125. https://www.ncbi.nlm.nih.gov/pubmed/30043972.
Muller HP, Barrieshi-Nusair KM, Kononen E. Repeatability of ultrasonic determination of gingival thickness. Clin Oral Investig. 2007;11.4:439–42. ISSN:1432-6981 (Print) 1432-6981 (Linking). https://doi.org/10.1007/s00784-007-0125-0. http://www.ncbi.nlm.nih.gov/pubmed/17522899
Muller HP, Kononen E. Variance components of gingival thickness. J Periodontal Res. 2005;40.3:239–44. ISSN:0022-3484 (Print) 0022-3484 (Linking). https://doi.org/10.1111/j1600-0765.2005.00798.x. http://www.ncbi.nlm.nih.gov/pubmed/15853970.
Zweers J, et al. Characteristics of periodontal biotype, its dimensions, associations and prevalence: a systematic review. J Clin Periodontol. 2014;41.10:958–71. ISSN:1600-051X (Electronic) 0303-6979 (Linking). https://doi.org/10.1111/jcpe.12275. https://www.ncbi.nlm.nih.gov/pubmed/24836578.
Kehl M, Swierkot K, Mengel R. Three-dimensional measurement of bone loss at implants in patients with periodontal disease. J Periodontol. 2011;82.5:689–99. ISSN:1943-3670 (Electronic) 0022-3492 (Linking). https://doi.org/10.1902/jop.2010.100318. https://www.ncbi.nlm.nih.gov/pubmed/21080785.
Parlar A, et al. Effects of decontamination and implant surface characteristics on re-osseointegration following treatment of peri-implantitis. Clin Oral Implants Res. 2009;20.4:391–9. ISSN:1600-0501 (Electronic) 0905-7161 (Linking). https://doi.org/10.1111/j.1600-0501.2008.01655.x. https://www.ncbi.nlm.nih.gov/pubmed/19298293
Schwarz F, Claus C, Becker K. Correlation between horizontal mucosal thickness and probing depths at healthy and diseased implant sites. Clin Oral Implants Res. 2017;28.9:1158–63. ISSN:1600-0501 (Electronic) 0905-7161 (Linking). https://doi.org/10.1111/clr.12932. http://www.ncbi.nlm.nih.gov/pubmed/27458093.
Serino G, Turri A, Lang NP. Probing at implants with peri-implantitis and its relation to clinical peri-implant bone loss. Clin Oral Implants Res. 2013;24.1:91–5. ISSN:1600-0501 (Electronic) 0905-7161 (Linking). https://doi.org/10.1111/j.1600-0501.2012.02470.x. http://www.ncbi.nlm.nih.gov/pubmed/22462625.
Benic GI, et al. Dimensions of buccal bone and mucosa at immediately placed implants after 7 years: a clinical and cone beam computed tomography study. Clin Oral Implants Res. 2012;23.5:560–6. ISSN:0905-7161.
Fienitz T, et al. Accuracy of cone beam computed tomography in assessing peri-implant bone defect regeneration: a histologically controlled study in dogs. Clin Oral Implants Res. 2012;23.7:882–7. ISSN:1600-0501 (Electronic) 0905-7161 (Linking). https://doi.org/10.1111/j.1600-0501.2011.02232.x. http://www.ncbi.nlm.nih.gov/pubmed/21707753.
Marotti J, et al. Recent advances of ultrasound imaging in dentistry–a review of the literature. Oral Surg Oral Med Oral Pathol Oral Radiol. 2013;115.6:819–32. ISSN:2212-4411 (Electronic). https://doi.org/10.1016/j.oooo.2013.03.012. http://www.ncbi.nlm.nih.gov/pubmed/23706922.
Tsiolis FI, Needleman IG, Griffiths GS. Periodontal ultrasonography. J Clin Periodontol. 2003;30.10:849–54. ISSN:0303-6979 (Print) 0303-6979 (Linking). http://www.ncbi.nlm.nih.gov/pubmed/14710764.
Nguyen KT, et al. High-resolution ultrasonic imaging of dento-periodontal tissues using a multi-element phased array system. Ann Biomed Eng. 2016;44.10:2874–86. ISSN:1573-9686 (Electronic) 0090-6964 (Linking). https://doi.org/10.1007/s10439-016-1634-2. http://www.ncbi.nlm.nih.gov/pubmed/27160674.
Chan HL, et al. Non-invasive evaluation of facial crestal bone with ultrasonography. PLoS One. 2017;12.2:e0171237. ISSN:1932-6203 (Electronic) 1932-6203 (Linking). https://doi.org/10.1371/journal.pone.0171237. http://www.ncbi.nlm.nih.gov/pubmed/28178323.
Chan HL, et al. Ultrasonography for noninvasive and real-time evaluation of peri-implant tissue dimensions. J Clin Periodontol. 2018;45.8:986–95. ISSN:1600-051X (Electronic) 0303-6979 (Linking). https://doi.org/10.1111/j.cpe.12918. https://www.ncbi.nlm.nih.gov/pubmed/29757464.
Chan HL, et al. Non-ionizing real-time ultrasonography in implant and oral surgery: a feasibility study. Clin Oral Implants Res. 2017;28.3:341–347. ISSN:1600-0501 (Electronic) 0905-7161 (Linking). https://doi.org/10.1111/clr.12805. http://www.ncbi.nlm.nih.gov/pubmed/26992276.
Bertram S, Emshoff R. Sonography of periimplant buccal bone defects in periodontitis patients: a pilot study. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2008;105.1:99–103. ISSN:1528-395X (Electronic) 1079-2104 (Linking). https://doi.org/10.1016/j.tripleo.2007.01.014. http://www.ncbi.nlm.nih.gov/pubmed/17482844.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Chan, HL.(., Kripfgans, O.D. (2021). Ultrasonic Imaging for Evaluating Peri-Implant Diseases. In: Chan, HL.(., Kripfgans, O.D. (eds) Dental Ultrasound in Periodontology and Implantology. Springer, Cham. https://doi.org/10.1007/978-3-030-51288-0_8
Download citation
DOI: https://doi.org/10.1007/978-3-030-51288-0_8
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-51287-3
Online ISBN: 978-3-030-51288-0
eBook Packages: MedicineMedicine (R0)