Abstract
Presbycusis is the most prevalent age-related, non-reversible, sensorineural hearing loss. Evidence supporting the relationship between auditory dysfunction and cognitive degeneration has grown over the years. Because of the aging of the world population, an early identification of the disease and an audiological recovery could mitigate the rate of cognitive decline with positive consequences for quality of elderly’ social life. A group of 50 patient (70–92 years) underwent audiometric tonal examination to evaluate hearing ability. Only 50% (active group) were equipped with a bilateral hearing aid. After three years, all patients were retested. Among the active group, the Mini-Mental State Examination was administered to 7 pathological patients to assess cognitive status at the begin and at the end of the research. The results show that the active group has achieved a significantly higher minimum audibility threshold than the control group (p < 0.01) and a cognitive benefit.
Access provided by Autonomous University of Puebla. Download conference paper PDF
Similar content being viewed by others
Keywords
1 Introduction
World Health Organization (WHO) in 2012 quantified disabling hearing loss for people around the world, based on current data of 42 population-based studies [1]. In particular, disabling hearing loss, defined as hearing loss greater than 40 dB in the better hearing ear in adults, is the fourth highest cause of disability globally. Disabling hearing disorders affect over 5% of the world’s population, or 466 million people (432 million adults and 34 million children) [1] and are very common in older age: at least one-third of persons above 65 years presents Presbycusis, the most prevalent age-related, non-reversible, sensorineural hearing loss [2]. During the next few decades, the share of global population aged 60 or more is likely to grow significantly. According to World Population Prospects 2019 [3], by 2050, 1 in 6 people in the world will be aged 65 years or over. Consequently, the number of persons with hearing loss will grow proportionately, due to population growth and ageing.
Presbycusis is more common in men than women [4] and represents the third most prevalent chronic condition, after hypertension and arthritis among elderly [5]. Usually bilateral, it affects the high frequencies of hearing [2] and is characterized by reduced hearing thresholds and speech understanding and discriminating, which cause a tremendous impact on life quality of older individuals. Unfortunately, Presbycusis remains an often undetected, underestimated and neglected condition in the geriatric population. If untreated, its impact on patients and the society as a whole would be significant [5]. Frequently, the first symptoms occur in conversation with background noise, in echoing rooms, when there is a large distance from the speaker, in directional hearing. When advanced, the hearing impairment affects the person’s ability to follow and understand one-to-one conversation also in a quiet environment and in daily living (watching television, listening to the radio, speaking on the telephone) [2]. The inability to hear prevents the elderly from communicating efficiently with family members and maintaining links to the world, reduces cognitive abilities, contributing to their isolation, causing social withdrawal, depression and possibly dementia [6].
Evidence supporting the relationship between auditory dysfunctions and subsequent cognitive degeneration has grown over the years [7]. Patients with mild, moderate, and severe hearing loss very often show high risk of developing dementia and increased hazard ratios of accelerated volumetric declines in the whole brain and Alzheimer’s disease [8].
An early diagnosis and identification of the disease and an audiological recovery with hearing aid (HA) is then important to mitigate the rate of cognitive decline with positive consequences for quality of elderly social life.
According to Eurostat data, the period from the diagnosis of hearing loss to the first application of a HA is 7 years [9]. During this time, an incorrect perception of sound stimuli could lead even to a regression of the acoustic nerve, accelerating the aging process and experiencing an altered cognitive sensitivity. Even mild hearing loss can cause significant changes in the brain and a reorganization of the auditory cortex. If the brain does not receive sounds well, mechanisms are set up to compensate for hearing loss, such as greater involvement of the sight (reading of the lips) in order to obtain the same information that was previously conveyed only by hearing. If areas of the auditory cortex are recruited from vision, this slowly results in functional changes in the cerebral cortex. The more the loss increases, the greater the effort the brain makes to feel. The use of HA, even for mild hearing loss, slows down these cortical changes, with a better perception of speech and less effort in listening.
The purpose of this study is to evaluate hearing ability and the cognitive benefit in a group of elderly patients using HA respect to a control group. Through a retrospective observational study, the research wants to demonstrate that the application of HA could slow down the aging of the hearing organ, maintaining the minimum audibility threshold.
2 Materials and Methods
A group of 50 patient aged 70–92 years, where the median age is 77, 6 y, affected by Presbycusis, was recruited through the otorhinolaryngology ambulatory of the Sapienza University Rome. In the group, 7 patients were affected by Alzheimer’s disease. All patients underwent audiometric tonal test.
Only 50% of patients was equipped with a bilateral behind-the-ear technology HA (active group: 44% female and 56% male), while the remaining 50% (control group: female: 48%, male 52%) did not accept HA. After three years, all patients were retested by diagnostic audiometer (Medsen Itera II, Otometrics) [10].
Audiometric tonal examinations of the two groups (the active and the control group) were studied. Two tests have been performed for each patient of both groups, pre (T0) and post (T1) application of the HA for the active group, three years apart. Center frequencies from 500 Hz to 4000 Hz were considered.
Among the active group, the Mini-Mental State Examination (MMSE test, Table 1) was administered to the seven pathological patients to assess cognitive status at the begin and at the end of the research.
The MMSE is an 11-item test which provide a brief quantitative measure to screen for cognitive impairment, to estimate the severity of cognitive impairment at a given point in time, to follow the course of cognitive changes in an individual over time, and to document an individual’s response to treatment [11]. MMSE consists of tests of Orientation (spatial and temporal), Registration, Attention and calculation, Recall, Language, Language-repetition, Language-understanding, Reading and written comprehension, Generation of written sentence and visual-spatial skills. The MMSE is scored on a scale of 0–30. Severe cognitive impairment: 0–17, mild cognitive impairment: 18–23, no cognitive impairment: 24–30. The score of this test is recalculated by means of corrective coefficients that take into account the age and years of schooling of each subject examined. The results collected have been statistically analysed (t test, p < 0,01).
3 Results
The results show that in the active group, the variability for T0 ranges from a minimum equal to 25, to a maximum equal to 65. After three years, among the same group, the minimum value for T0 was 33,75 while the maximum 66,25, with a statistically significant difference between the T0 and T1 data collected in the two surveys three years apart (p < 0.01). Globally, in the active group, the mean value for T0 was 44,9 while 49,9 for T1. The results show that in the active group, the difference between T0 and T1 highlights a hearing loss of 5 dB.
In the control group, the variability for T0 ranges from a minimum equal to 11,25, to a maximum equal to 55. After three years, among the same group, the minimum value for T0 was 22,5 while the maximum 75, with a statistically significant difference between the T0 and T1 data collected in the two surveys three years apart (p < 0.01). Globally, in the control group, the mean value for T0 was 34 while 49,2 for T1 (Fig. 1). The results show that in the control group, the difference between T0 and T1 highlights a hearing loss of 15,19 dB.
The collection of audiological data shows that the active group has a significantly higher minimum audibility threshold in the three years than the control group. The average variation in hearing loss in the group of prosthesis is 5 dB, in the control group the value is 15,19 dB, a hearing loss coefficient about three times greater (p < 0,01).
The box plots of mean hearing losses on the two groups show that although the control group at T0 had lower mean hearing losses, at T1 relative auditory deterioration is greater than the active group (Fig. 2). In addition, the control group highlights a greater dispersion of data.
Figure 3 shows the average of hearing loss for frequency by age group: auditory deterioration is greater for control group (without HA), where the greatest losses are in the frequencies range up 1000 Hz and emphasized for the age group >80 years. For the active group, a much more contained auditory deterioration can be detected; wearing HA preserve the auditory function, especially in the frequencies from 500 to 2000 Hz.
In conclusion, the use of HA seems to protect and preserve the decay of the auditory function, also due to aging.
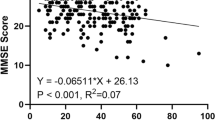
Among the seven pathological patients in the active group, the results of the MMSE test administration show that only one patient presents a mild cognitive impairment (22,3) with a severe sensorineural hearing loss, while the others showed a severe cognitive impairment (minimum value for MMSE 12,2, maximum 17,8) with a medium-grade sensorineural hearing loss. Among the patients, 43% were binaurally prosthesized with retro-auricular base-band technology, 43% with a mid-range retroauricular technology, 14% with a medium-high end retroauricular technology. At the end of the research, the results of the MMSE test administration show for 71,4% of the pathological patients examined, an improvement in cognitive performance: 43% of patients passed from severe cognitive impairment (MMSE test score 0–17), to mild cognitive impairment (MMSE test score 18–23). This improvement was particularly relevant for a patient with mild cognitive impairment (22,3) to no cognitive impairment (26,3) at the end of the research. Significant improvements in mnemonic abilities were also found in 57,1% of patients.
The results show that these pathological subjects found a more or less marked improvement in cognitive performance probably thanks to the continuous use of HA. The best results have been obtained for item 2 (Orientation) and item 5 (Recall). This improvement, especially in terms of social interactions, could be associated with a reduction in cognitive effort. The proper functioning of the auditory system is in fact essential for the purpose of an active participation in conversations and interpersonal relationships in general.
4 Conclusions
The results show that an approach to acoustic rehabilitation is fundamental for the improvement of sound perception, for the maintenance of hearing loss and for the slowdown of the auditory decay. In fact, in patients with sensorineural hearing loss belonging to the control group, the decay of the minimum hearing threshold is much more pronounced than in the active group. The auditory deprivation over time of certain sound stimuli could make discrimination more difficult; a lack of signal afferent to the cerebral cortex could have contributed to a lower efficiency of the acoustic nerve and of the acoustic pathways.
Then, an adequate and prompt hearing aid application can lead to a maintenance of minimum auditory threshold over time and to an improvement in cognitive performance, certainly associated with a reduction in the cognitive effort of the patient. The benefits for the patient mainly affect social interactions. The proper functioning of the auditory system is essential for the elderly to increase their relational skills. Long-term benefits are obtained for society in the whole. In the face of a constant increase in average life expectancy and a reduction in the birth rate, especially in western countries, reduction of the cognitive decline of the older population becomes a topic with social and economic implications no longer negligible.
References
World Health Organization: WHO global estimates on prevalence of hearing loss. Mortality and Burden of Diseases and Prevention of Blindness and Deafness (2012)
Löhler, J., Cebulla, M., Shehata-Dieler, W., Volkenstein, S., Völter, C., Walther, L.E.: Hearing impairment in old age. Deutsches Ärzteblatt Int, Dtsch Arztebl Int 116, 301–310 (2019)
United Nations, Department of Economic and Social Affairs, World Population Ageing 2019 Highlights (2019)
Blevins, N.: Presbycusis. UpToDate, Wolters Kluwer (2018)
Li-Korotky, H.S.: Age-related hearing loss: quality of care for quality of life. Gerontologist 52(2), 265–271 (2012)
Zhang, M., Gomaa, N., Ho, A.: Presbycusis: a critical issue in our community. Int. J. Otolaryngol. Head Neck Surg. 2, 111–120 (2013)
Jayakody, D., Friedland, P.L., Martins, R.N., Sohrabi, H.R.: Impact of aging on the auditory system and related cognitive functions: a narrative review. Front. Neurosci. 12, 125 (2018)
Swords, G., Nguyen, L.: Incorporating audiological measurements into Alzheimer’s diagnosis. Hear. J. 71(6), 6 (2018)
Hearing care and hearing aid use in Europe, Joint AEA EFHOH, EHIMA report, Hearing Care and Hearing Aid Use in Europe, A Europe Wide Strategy (2016)
Orlando, M.P., Giliberti, C., Lo Castro, F., Mariconte, R., Longo, L.: Effectiveness in prosthetic adaptation and users’ satisfaction: comparison between different technologies. Adv. Intell. Syst. Comput. 970, 217–227 (2020)
Folstein, M.F., Folstein, S.E., McHugh, P.R.: Mini-mental state. A practical method for grading the cognitive state of patients for the clinician. J. Psychiatr. Res. 12(3), 189–198 (1975)
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 The Editor(s) (if applicable) and The Author(s), under exclusive license to Springer Nature Switzerland AG
About this paper
Cite this paper
Longo, L., Lucchetti, A., Pasqualotto, M., Mariconte, R., Giliberti, C. (2020). Correlation Between Hearing Aid Use and Cognitive Impairment in the Elderly. In: Kalra, J., Lightner, N. (eds) Advances in Human Factors and Ergonomics in Healthcare and Medical Devices. AHFE 2020. Advances in Intelligent Systems and Computing, vol 1205. Springer, Cham. https://doi.org/10.1007/978-3-030-50838-8_50
Download citation
DOI: https://doi.org/10.1007/978-3-030-50838-8_50
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-50837-1
Online ISBN: 978-3-030-50838-8
eBook Packages: EngineeringEngineering (R0)