Abstract
Cervical cancer screening is an example of a successful preventative strategy that markedly reduces the incidence of invasive cervical cancer (ICC) and mortality from ICC among women. Evolving screening guidelines utilizing cytology from Pap tests and high-risk human papillomavirus (hrHPV) detection have improved the identification of precursor lesions which are treated to prevent the progression to ICC. Guidelines differ by patient age and immune status, but all persons with a cervix of ages 21–65 years, regardless of sexual orientation, sexual history, or gender identity, need to be screened regularly. Understanding the natural history of HPV and cervical abnormalities is essential for the proper triage of patients with positive testing to either repeat testing or referral for colposcopy. The HPV vaccine prevents infection with hrHPV and holds promise to significantly reduce the future incidence of cervical cancer and other cancers caused by hrHPV in men and women. Significant disparities exist in the United States and worldwide in HPV vaccination rates, cervical cancer screening rates, and the evaluation, treatment, and follow-up of abnormal findings in women. These disparities continue to allow cervical cancer to be a major cause of morbidity and mortality for women worldwide.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Cervical cancer
- Pap test
- HPV vaccine
- Screening guidelines
- HPV testing
- ASCUS
- CIN
- Disparities in cervical cancer
- Colposcopy
- DES
- hrHPV
- High-risk HPV
- Cervical cytology
-
1.
Discuss the epidemiology and pathogenesis of cervical cancer.
-
2.
Review current guidelines for cervical cancer screening and HPV testing.
-
3.
Compare the recommendations for screening in special populations: HIV positive, immune-compromised, DES exposed, and post-hysterectomy patients.
-
4.
Describe current recommendations for HPV vaccination.
-
5.
Interpret and manage the results of an abnormal cytology or HPV test.
-
6.
Evaluate and discuss disparities in cervical cancer prevention, screening, and treatment.
Nina is a 53-year-old woman who presents to establish care. She emigrated from the Congo one year ago. She lives with her three children and sister and works as a medical assistant at a local clinic. She has a son of age 14 and two daughters aged 18 and 21. She delivered all of her children at home in the Congo without complications. She did not have routine healthcare in her home country. She has never had a Pap or HPV test. Nina would like to know when she should bring in her two daughters for Pap tests.
Epidemiology
Cervical cancer is a leading cause of cancer death in women worldwide, but in the United States and other high-income countries (HIC), where Pap tests are routinely performed, invasive cervical cancer (ICC) deaths are uncommon. Cervical cancer ranks as the 14th cause of cancer death in the United States but as the second or third leading cause of cancer death in many low-income countries [1]. Persistent infection with high-risk strains of human papillomavirus (hrHPV or HPV) causes >99% of cervical cancer worldwide, which “implies the highest worldwide attributable fraction so far reported for a specific cause of any major human cancer” [2]. Cervical cancer can be of squamous cell (approximately 80%), glandular cell (adenocarcinoma), or mixed adenosquamous origin, all of which are associated with HPV infection. Rarely sarcoma, lymphoma, melanoma, and clear cell adenocarcinoma occur on the cervix. Squamous cell abnormalities can progress to cervical intraepithelial neoplasia (CIN), which is most common in the fourth decade of life. ICC peaks in the fifth decade of life. Mortality rates increase with age, especially in women greater than 45 years [3]. Risk factors associated with the development of cervical cancer are related to both persistent infection with high-risk HPV viral strains and host vulnerability.
Host vulnerability may include the following:
-
HPV acquisition. Women with early age of sexual debut or history of multiple sexual partners have increased vulnerability to HPV acquisition.
-
The cervical transformation zone. Infection of the cervical transformation zone with HPV can predispose to cervical cancer for those with: a young age of first pregnancy, a history of more than three pregnancies, STI coinfection, or oral contraceptive use for more than 5–10 years.
-
Host immunity or susceptibility. Women may have increased susceptibility to HPV due to HIV infection, immunosuppression, smoking, or in utero DES exposure.
-
Screening and follow-up. Women may lack appropriate screening and follow-up which can lead to inadequate screening for cervical cancer, inadequate follow-up of an abnormal screening test, or inadequate treatment of lower genital tract neoplasias [2,3,4,5,6,7,8].
It is estimated that over 50% of all new cases of ICC occur in women who have never been screened or have been inadequately screened for cervical cancer [6]. Women lost to follow-up after treatment for CIN or ICC are also at high risk. Worldwide, more than 80% of ICC cases occur in developing or low- and medium-income countries (LMIC) where HPV vaccination, cervical cancer screening, and cervical cancer treatment are limited [7].
In the United States, significant disparities exist in the incidence of and mortality from ICC. Between the years 2010 and 2014, the age-adjusted incidence of cervical cancer in women of all ages was 7.4 cases per 100,000 women and over 9 cases per 100,000 in Hispanic and Black women, respectively [3]. In 2014, 12,578 women in the United States were diagnosed with ICC, and 4115 women died of ICC. Mortality rates are highest in Black women in the US. Published data and statistical analyses underestimate the racial disparity by up to 44% when corrections are not made for the high rate of prior hysterectomy in Black women [9]. Low-income, minority, chronically ill, uninsured or poorly insured, lesbian, transgender, immigrant women, and those with poor healthcare access are at higher risk for morbidity and mortality due to cervical cancer, an inequity which must be addressed by healthcare providers and policy-makers [4, 9,10,11]. (See section on disparities below.)
Screening and Prevention
Invasive cervical cancer (ICC) is, in theory, entirely preventable. High-risk strains of human papillomavirus (hrHPV) are the etiologic agents of 99% of all ICC and there are highly effective vaccines to prevent the acquisition and spread of hrHPV. Low-risk strains of HPV do not cause cervical cancer, and all references to HPV testing or screening refer to screening for high-risk strains. Screening women with cervical cytology (previously referred to as the Pap test) and/or HPV testing identifies individuals at risk of ICC and detects those with abnormal cervical changes. The development of ICC from initial HPV infection takes more than 15 years in many cases, and thus the detection and treatment of precursor lesions is highly effective in preventing disease progression.
HPV is easily transmitted through contact between individuals from hand, skin, oral, vaginal, anal, penile, and scrotal contact. It is not blood-borne. HPV is also spread by autoinoculation from one part of the body to another in an individual. Women who are virgins or have sex only with women (WSW) can be HPV positive. Transgender men with a cervix are also at risk. For these reasons, it is recommended that all persons who have a cervix be screened regardless of sexual history or gender identity. Screening rates are unacceptably low in underserved populations, in those with multiple chronic illnesses, and in persons who lack or have poor access to insurance. Women under 30 and over 60 are less likely to be screened [9]. Immigrants from LMIC countries are at high risk as many have never been screened in their country of origin and may not have been screened since their arrival in the United States [12].
Cervical Anatomy: The Transformation Zone
The development of ICC begins with the infection of the transformation zone (TZ) of the cervix (Fig. 14.1). The transformation zone is the area of demarcation in which the squamous epithelium from the vagina meets the columnar epithelium from the endocervix. The TZ is also referred to as the squamocolumnar junction (SCJ). The TZ undergoes metaplasia in which columnar cells transform into squamous cells. The TZ is not visible on the cervix until puberty, but is found within the endocervix. With the onset of puberty, the TZ moves from the endocervix to the ectocervix. The ectocervix is the visible surface of the cervix. Estrogen stimulation with puberty, oral contraceptive use, and pregnancy cause the transformation zone to become more prominent, erythematous, and metabolically active with cell turnover. Increased cell replication and differentiation supports viral persistence and neoplastic changes. During the teen and young adult years, the TZ is visible on the cervix and is more exposed to possible HPV infection. In adult women over 25–30 years of age, who are not pregnant or taking OCPs, the TZ recedes into the endocervix, rendering the ectocervix more resistant to HPV infection and carcinomatous changes. The vulnerability of the TZ in the early teen years is an important argument to encourage teens to delay sexual activity, have fewer sexual partners, be selective of sexual partners, use condoms, and to be vaccinated against HPV. (See Chap. 13 on “Sexually Transmitted Infections”.)
Screening for Cervical Cancer
The implementation of cervical cancer screening programs over the past 40+ years has successfully reduced cervical cancer incidence and mortality among women who have undergone Pap or cytology testing. Throughout this chapter, and in the literature, the term Pap test is used interchangeably with cytology testing. The incidence of cervical cancer in the United States has decreased from 14.8 per 100,000 women a year in 1975 to 6.8 per 100,000 women a year in 2014. The mortality rate from ICC in 1975 was 5.5 compared to 2.26 in 2014 per 100,000 women a year [2]. The continued mortality is thought to be due to ICC cases presenting in advanced stages in unscreened and inadequately screened women, in women who have been lost to follow-up after abnormal screening, or in woman who have received partial treatments.
There are two major components to cervical cancer screening: cytology and HPV testing. HPV testing identifies women at risk for cervical abnormalities, and cytology identifies actual cervical cell abnormalities. Neither test is 100% sensitive; however, the slow progression from HPV acquisition, to cervical cell abnormalities, to ICC allows for the detection of abnormalities on repeated sampling when screening guidelines are followed. Screening recommendations vary by age and risk category.
Cervical Cytology: The Papanicolaou (Pap) Test
The Pap test technique was first developed in the 1920s by George Papanicolaou who studied microscopic vaginal secretions from guinea pigs and learned to distinguish cancerous from noncancerous cells. This technique was not noticed by the medical community until the 1940s [13]. The Pap test has been the mainstay of cervical cancer screening and has evolved over the years from a yearly smear on a glass slide—the “Pap smear”—to liquid-based “thin prep” cytology specimen collection every 3–5 years. The thin prep was approved in 1996 by the FDA as the preferred option for obtaining cervical specimens, and the percentage of unsatisfactory cytology specimens has decreased since its use has become widespread. Additionally, the liquid-based test has the advantage of allowing the testing for gonorrhea, chlamydia, and trichomonas in the same vial. The Pap collection technique is as follows: the woman is asked to place her legs in foot rests in the dorsolithotomy position on an examination table, and she brings her bottom to the edge of the examination table. A speculum, moistened with water or a small amount of water-based lubricant, is gently inserted into her vagina and then opened, and the cervix is visualized using a light source. (See Chap. 3 on “The Female Sex and Gender Specific History and Examination”.)
To optimize the adequacy of the Pap test sample, mucus, discharge, or blood should not be removed from the cervix prior to the Pap collection. Women should avoid tampons, douching, or intercourse prior to collection. The plastic spatula and cytobrush combination is preferred, which is the most likely to adequately sample the transformation zone. The contoured end of the plastic spatula is gently scraped against the cervix for 360 degrees to collect cervical cells. The brush is inserted most of the way into the cervical os and rotated for a ¼ to ½ turn. Rotation that is too vigorous may cause bleeding and thus decrease endocervical cell collection. The spatula and brush are placed in the collection vial and swirled vigorously 10 times in the liquid and gently scraped with each other to remove cells, or the collection ends may be broken off the endocervical brush and spatula and placed into the liquid collection vial. Endocervical brushes should not be used during pregnancy by primary care clinicians [14, 15]. If the os is stenotic and will not allow endocervical cell collection, or if there are visible cervical abnormalities, gynecology should be consulted.
The collection vial must be properly labeled with the patient’s identifying information, or it will be rejected by the lab. The collection vial is sent to the laboratory with appropriate orders. The laboratory analyzes the cervical cell cytology and will also perform any additional testing that is ordered, including HPV and sexually transmitted infection screening. For a specimen to be satisfactory, sufficient squamous cells must be visible without obscuring inflammation or blood. The presence of endocervical cells and cells from the transformation zone should be present and will be commented upon by the pathologist [16].
High-Risk Human Papillomavirus (hrHPV)
HrHPV is the causative agent of cervical cancer and is the most common STI in the United States. During 2013–2014, the prevalence of genital HPV was 42.5% in the US adults aged 18–59 years. The highest prevalence was among the Black population at 64.1%, followed by the Hispanic population at 41.4% and was lowest in the Asian population at 23.8%. The prevalence was 50–60% in women aged 25–34 and twice as high in women between the ages of 25 and 29 when compared to women between the ages of 30 and 39 [17, 18]. HPV is transmitted to the anogenital region through mucosa to mucosa or skin to skin contact. HPV causes vaginal, vulvar, anal/rectal, penile, and oropharyngeal neoplasia in men and women; however, the discussion of these malignancies is beyond the scope of this chapter. (See Chap. 12 on “Vaginitis and Vulvar Conditions” and Chap. 15 on “Gynecologic Malignancies” for a discussion of vaginal and vulvar cancers.)
Once transmitted, HPV can result in acute asymptomatic infection. High-risk strains are carcinogenic and increase cervical cancer risk; low-risk strains cause genital warts. HPV type 16 is the highest risk strain, followed by type 18. Together these two strains account for about 70% of cervical cancers and other HPV-related cancers of the genitourinary tract, anus, and oropharynx. In unvaccinated women, HPV types 16 and 18 account for approximately 35.46% of the HPV infections noted on Pap tests. The HPV genotypes 31, 33, 45, 52, and 58 are classified “other high risk” and are also associated with a higher risk of high-grade lesions in unvaccinated women. Low-risk strains 6 and 11 cause most genital warts. The HPV vaccine used in the United States, Gardasil-9, covers these nine strains of HPV: 16, 18, 31, 33, 45, 52, 58, 6, and 11. Less common strains of carcinogenic HPV, and of low-risk HPV, are not covered by the currently available vaccination [19,20,21]. The majority of women who contract HPV will clear the virus spontaneously within 1–2 years of infection, but approximately 10% of women remain positive for 5 or more years [22] (Table 14.1).
The regression of previously positive HPV infections is presumed to be due to an adequate cell-mediated immune response, while an increased persistence of HPV is observed in immunocompromised populations. It is not clear whether HPVs are completely cleared or are maintained in a latent state in women who convert from HPV positive to HPV negative on co-testing [21].
Women with a history of CIN, cervical cancer, recent abnormal Pap tests, HIV infection, organ transplant recipients, DES exposure, and other causes of immunosuppression are at higher risk of ICC and are screened more frequently than average-risk women (discussed in section on screening guidelines). Patients with systemic lupus erythematosus or rheumatoid arthritis on biologic disease-modifying antirheumatic drug (DMARD) therapies and other biologic therapies appear to have a higher risk of cervical cancer. It is important for clinicians to be up to date on screening guidelines and HPV vaccination recommendations in these populations. Although studies are limited, annual screening is recommended for women with immunosuppression and for those on biologic therapies [23, 24].
Smokers are at higher risk of cervical cancer and have been found to obtain less frequent cervical cancer screening [8]. Tobacco use negatively impacts HPV clearance and increases the risk of persistent HPV infections. The discovery of HPV infection and ASCUS or low-grade lesions can be a strong impetus for women to stop smoking: smoking cessation is associated with increased regression of abnormal cervical lesions [25].
Cervical Cancer Screening Guidelines
Cervical cancer screening guidelines have traditionally been based on consensus by expert groups, because evidence from randomized-controlled trials is limited. Initially, Pap smears were done yearly, and then in the mid-1990s until 2012, Pap screening frequency decreased to every 2–3 years. In 2012, the US Preventive Services Task Force (USPSTF ) updated the 2003 recommendation on cervical cancer screening [26]. This was an important update as it incorporated human papillomavirus (HPV) testing into the recommendations. Between 2009 and 2011, the American Cancer Society (ACS), American Society for Colposcopy and Cervical Pathology (ASCCP), and the American Society for Clinical Pathology (ASCP) developed a working group to jointly prepare cervical cancer screening guidelines. Available evidence was reviewed and updated guidelines incorporating co-testing with cytology and HPV screening were published [27].
In 2018, the USPSTF published new updated guidelines [28] on screening for cervical cancer. It commissioned a decision analysis model [29] to evaluate at which age to begin and end screening, the optimal interval for screening, the effectiveness of different screening strategies, and the related benefits and harms of different screening strategies. Screening recommendations apply to all women who possess a cervix regardless of sexual orientation, sexual history, or gender identity [30] (Table 14.2). The guidelines below do not apply to women with a history of precancerous cervical lesions (CIN2 or greater on biopsy), cervical cancer, HIV-positive status, immunocompromised status, or exposure to diethylstilbestrol in utero, who need more intensive screening based on expert opinion [27, 30]. Women under the age of 21 should not be routinely screened with a Pap test regardless of sexual activity.
The evidence for each of these recommendations is discussed below.
-
1.
Women under the age of 21 should not be screened with a Pap test regardless of sexual activity
There is very little evidence to support cervical cancer screening in women under age 21 because cervical cancer is rare in this age group. Although HPV is acquired during sexual intercourse, there are multiple steps in the progression to cancer; in this age group, abnormal test results are transient as HPV tends to clear on its own. In addition, screening in this age group may increase harm associated with screening due to the pain, anxiety, and cost associated with unnecessary screening and possible cervical procedures. Multiple cervical procedures, such as Loop Electrosurgical Excision Procedure (LEEP) or conization, may also have the untoward consequence of cervical incompetence, negatively impacting future childbearing [29, 31].
-
2.
Women aged 21–29 years should have a Pap test every 3 years. An HPV test should only be performed for abnormal cytology results
The evidence for screening women under the age of 30 is largely based on modeling studies. There is very little data looking at the optimal screening interval in this population. By extending screening to every 3 years, the number of colposcopies needed to evaluate abnormal Pap tests is reduced compared to annual screening [32]. When two- versus three-year screening intervals were compared, there was no difference in cervical cancer burden after a 10-year follow-up interval. Other studies have supported the conclusion that there is very little added benefit in having a two-year screening interval compared to a three-year screening interval in women aged 21–29 years [32,33,34]. Randomized controlled trials (RCTs) have not supported reducing the screening interval, even when a woman has a history of previous abnormal cytology results [34, 35]. (See section on management of abnormal Pap tests below.)
The prevalence of HPV is high in young women and is usually transient. Dunne et al. found that in women aged 20–24 years, the prevalence of high- and low-risk HPV was 15.2% and 17.8%, respectively. After the age of 21–29 years, the prevalence of the high-risk HPV decreases in women [35]. HPV testing either alone or as a co-test is not recommended in the 21–29 age group because there is not an added benefit over cytology alone. Women would be exposed to increased harms from overdiagnosis and overtreatment of transient infections, with increased pain, bleeding, anxiety, cervical procedures, and risks to future childbearing, similar to women under age 21 [29, 31].
Age of Initial Screening
A consensus conference in Italy in 2015 addressing cervical cancer screening in women already vaccinated against HPV recommended increasing the age of first initial screening for cervical cancer to 30 years old for girls vaccinated at age 12. This is based on the assumption that at the age of 12, most girls have not had sexual intercourse and hence have not been exposed to hrHPV. It is important to note that the national rate of HPV vaccination is 71% in cohorts of 12-year-old girls in Italy [36]. As the prevalence of HPV vaccination increases in the US, an increased age for initial screening might be considered.
-
3.
Women aged 30–65 years should be screened with cytology alone every 3 years, with HPV testing alone every 5 years, or with co-testing every 5 years
HPV testing alone every 5 years in women 30 and older: The USPSTF reviewed several randomized control trials comparing modalities of screening and interval between screenings to develop their 2018 recommendations. In four trials that included >250,000 women, HPV testing alone with referral to colposcopy increased the rate of detection of CIN3+ lesions and cancerous lesions compared to cytology alone. In one trial, the rate of detection of invasive cervical cancer at 5 years was higher with HPV testing alone (0.03%) compared to cytology alone (0.01%). The colposcopy rates were higher in HPV testing alone compared to cytology alone in one of the trials but comparable in the other 2 trials [28].
Cytology alone every 3 years: Modeling studies suggest that co-testing, or HPV testing every 5 years, offered comparable benefits with cytology alone every 3years with regard to the detection and prevention of ICC. Modeling studies done in 2012 and cited in the 2018 USPSTF recommendations also examined screening intervals from 1 to 5 years and have not found evidence to support the use of screening intervals longer than 3 years with cytology alone even in women with a history of negative cytology tests [29, 31]. In other words, if HPV testing is not done, Pap tests are required every 3 years.
HPV testing alone in women over 25 years as an emerging primary screening strategy: The cobas ™ hrHPV test is used for co-testing with Pap and is FDA approved [37] as a primary cervical cancer screening test for women >25 years old. The strategy of using HPV screening starting at age 25 is yet to be embraced in the US, but in Australia, HPV screening alone is recommended every 5 years to women of ages 25–74 via a national screening program [38].
Arguments in favor of HPV testing alone: HPV testing alone has a higher sensitivity for detecting CIN3 or higher lesions at 76.1% in comparison to a sensitivity of 61.7% for the hybrid strategy similar to current US screening guidelines involving reflex HPV screening for ASCUS and 47.8% for cytology alone [17]. Over a 5-year period, the probability of developing lesions of CIN3 and above is similar between primary HPV testing and co-testing. Co-testing therefore does not provide increased protection against CIN3 when compared to HPV testing alone. The concern with primary HPV testing as a screening tool for women starting at 25 years of age is that women less than 30 years have a high frequency of HPV infections that later regresses. There is the potential for overdiagnosis and overtreatment in women less than 30 years, which increases harm from cervical cancer screening.
-
4.
Women over the age of 65 years who have had adequate screening can exit screening
Women who have a history of CIN2 or greater, are immunosuppressed, or have not been adequately screened in the prior 10 years , with normal results, are exceptions. Physicians may discontinue routine screening in women over the age of 65 who have been screened according to recommended guidelines for the past 10 years and have met the following criteria within the 10 years before ceasing screening:
-
Three negative consecutive Pap tests (3 years apart) or
-
Two negative HPV tests (5 years apart), with the last testing having occurred within the last 5 years
The acquisition of new sexual partners after age 65 does not change this approach to screening.
History of Neoplasia, Inadequate Screening, or Smoking
In women over the age of 65 who have had cervical lesions CIN2 or greater, Pap screening is continued for 20 years after diagnosis. Some experts recommend continued Pap screening for women over 65 who smoke, are DES exposed, or are immunosuppressed. In women over the age of 65 who have not been adequately screened, screening for hrHPV and abnormal cytology should be undertaken and repeated at least once (editor’s view). Underserved, minority, and immigrant women are at risk for inadequate, or no, screening and should be screened appropriately [14].
-
5.
Women who have had a total hysterectomy with removal of the cervix for benign indications do not need Pap tests
Women who undergo a total hysterectomy with complete removal of the cervix for benign indications such as fibroids or menorrhagia no longer need screening for cervical cancer. Women who have a history of CIN2 or greater, are immunosuppressed, have a history of DES exposure, or have not been previously screened are exceptions. A Pap test in a woman without a cervix screens for vaginal cancer, which is extremely rare except as a recurrence of cervical cancer [39, 40] or as a primary cancer in a woman exposed to DES. If the cervix is left in place, as in the case of a supracervical or “partial” hysterectomy, then routine screening guidelines should be followed. In many patients, it is not clear whether the cervix was removed during hysterectomy. The patient is often unaware of the distinctions in the types of hysterectomy. Add this information to your problem list. Clues include the following:
-
Why was the hysterectomy done? If possible, check or send for gynecology notes. If for cancer, the cervix was removed but continued screening is recommended.
-
Was it a vaginal hysterectomy? A vaginal hysterectomy removes the cervix, but be sure it was for a benign condition before you discontinue screening.
-
Was it a laparoscopic hysterectomy? It may be a partial hysterectomy which leaves the cervix intact.
-
Check for abdominal wall scars which would indicate a possible abdominal “partial” hysterectomy, leaving the cervix intact.
-
If not sure: Examine the woman and make a note if the cervix is present. Check hrHPV and Pap test once. If HPV is positive, and/or if unable to visualize the cervix for Pap, refer to GYN. If negative, use clinical judgment about further evaluation [40].
-
6.
Pap testing should still be performed in HPV-vaccinated women
Women who have received the HPV vaccine continue to undergo cervical cancer screening according to current guidelines. Some modeling studies have looked at screening women in this population at a later age and with less frequency but this is not a currently accepted recommendation [41].
Recommendations for Specific Populations and Exceptions to Routine Guidelines
Cervical cancer screening guidelines in special populations are primarily determined by expert opinion.
Immunocompromised Women
HIV-Infected Women
There is limited evidence supporting the current screening guidelines in HIV-positive women. For women under the age of 30, if a baseline Pap test is normal, annual cytology should be performed. After three consecutive normal annual screening tests, the interval is spaced to 3 years. Co-testing is not recommended in HIV-positive women under the age of 30. Women with HIV who are 30 years of age or older can be screened with cytology alone or with co-testing. If three consecutive annual tests are normal, then screening can be extended to 3 years. If co-testing is done with a normal result, the screening can extend to 3 years [42]. The incidence of invasive cervical cancer (ICC) has not been found to decrease in HIV-positive women treated with antiretroviral medications or in women who have rising CD4 counts due to treatment; therefore, increased screening intervals are recommended in these women despite adequate HIV therapy [43].
Immunosuppression from Drug Therapy
Given that HPV is less likely to be transient in an immunosuppressed population, screening is recommended at closer intervals. For women with organ transplants, cervical cancer screening is recommended annually with both cytology and HPV co-testing [44]. Women exposed to chronic immunosuppression such as those on biologic therapies may have a higher rate of cervical dysplasia and/or carcinoma but guidelines based upon evidence on how to screen this population is limited. Current recommendations are for annual Pap tests [23].
Diethylstilbestrol (DES) Exposure in Utero
Between 1938 and 1971, many women used DES as it was thought to improve pregnancy outcomes. At the time, it was not known that DES would be associated with an increased risk for squamous cell carcinoma and adenocarcinoma of the cervix, clear cell adenocarcinoma of the cervix and vagina, and an increased risk of breast cancer in females who were exposed in utero. In the cohort of in utero DES-exposed women , who are now close to 50 years of age or older, screening of both the cervix and vagina is recommended with separate specimens obtained from each site [45]. The specimens can be placed in the same vial as long as there is clear labeling that samples have been obtained from both sites. A four-quadrant Pap test should be obtained, which involves sampling of all walls of the vagina. Given the increased risk of cervical neoplasia DES-exposed women, annual cytology testing is recommended [46].
After educating Nina about cervical cancer screening, she undergoes a Pap test and the result shows abnormal squamous cells of uncertain significance (ASCUS) cytology and positive HPV “other high-risk” subtypes. The results are explained to her, and she is advised that repeat cytology could be repeated in 1 year or she could be referred for colposcopy. She indicates that she would prefer to wait, but is having some family issues and may be moving soon. She is advised that she should be evaluated as soon as possible and is warned of the dangers of not following up on abnormal tests. A referral to gynecology is placed with a note indicating that there are concerns about the patient getting lost to follow-up.
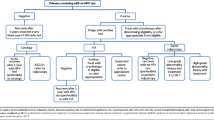
Management of Abnormal Screening Results
Pap Collection – Results
To be “satisfactory for evaluation,” squamous cells, endocervical cells, and transformation zone (TZ) cells must be present in the cytology sample, and excess blood or inflammatory cells cannot obscure results. Generalists should be familiar with the management of abnormal Pap test results in order to determine when repeat testing or referrals are appropriate. Algorithms are available for managing abnormal Pap tests through the American Society for Colposcopy and Cervical Pathology (ASCCP) at http://www.asccp.org/asccp-guidelines. The ASCCP also has an app containing algorithms for screening, management, and follow-up that can be downloaded for a nominal fee to a smartphone from www.asccp.org algorithms [40]. Pap cytology terms are listed in Table 14.3. Low-grade and high-grade intraepithelial lesions (LGSIL and HGSIL) used in this context are distinct from the histopathology terms of low-grade and high-grade lesions which are used to describe biopsy specimens outlined in the section on colposcopy and listed in Table 14.5.
Algorithms are complicated. In general, women who are
-
1.
Positive for HPV 16 or 18 – refer for colposcopy regardless of cytology results.
-
2.
Other high-risk HPV – co-test in 1 year, and refer if infection persists after 1 year.
-
3.
Pap with a persistent unsatisfactory or ASCUS finding – refer for colposcopy.
-
4.
Pap results of LGSIL or worse – refer for colposcopy.
-
5.
Atypical glandular cells or worse – refer for colposcopy.
When in doubt, referral to a gynecologist is always recommended (Table 14.4).
Given the anxiety associated with abnormal test results, providers should discuss with patients the natural history of cervical cancer. HPV is usually cleared from the body in 1–2 years. Persistent HPV infection results in a small number of cases progressing to cancer. ASCUS and LGSIL on Pap are likely to regress: only 15% persist or progress. After biopsy, CIN lesions and some higher-grade lesions regress on their own. The lag time between the development of cancerous lesions from precancerous lesions is measured in years. Women at highest risk are those who were never screened, were inadequately screened, or are lost to follow-up (Table 14.5).
If the patient tests positive for HPV 16 or 18, refer for colposcopy. The rate of CIN3+ in persistent HPV 16 infection is 8.9% at 3 years, 23.8% at 5 years, and 47.4% at 12 years. Failure to treat HPV 16 has high rate of progression to malignancy. If HPV testing alone is used for screening, then only “other high-risk” HPV results need cytology to guide the referral decision. If cytology is negative, repeat co-test in 1 year. With “other hrHPV” persistent infections on two tests, the risk of CIN3+ at 12 years is 19.3% [47, 48]. The rates of progression are the reason that evaluation, treatment, and close follow-up are essential to prevent ICC.
Colposcopy and Biopsy
A full discussion of colposcopy and biopsy is beyond the scope of this chapter. Briefly, the woman is asked to place her legs in foot rests in the dorsolithotomy position and the cervix is visualized with a speculum. The cervix is painted with acetic acid to reveal suspicious “acetowhite” lesions which reveal HPV infection or other changes. The cervix is examined with the colposcope which is basically a mounted microscope to magnify the cervix. Biopsy forceps and a tenaculum are used to take samples from the acetowhite lesions for pathology. A sampling of endocervical cells, called endocervical curettage (ECC), is obtained and sent to pathology. The designation of cervical intraepithelial neoplasia (CIN) refers to squamous cell abnormalities.
Abnormalities of glandular cells (the mucus-producing cells in the cervix) are referred to as AGC, AIS, or adenocarcinoma. The majority of cervical cancers arise from squamous cells, but adenocarcinoma has been increasing over the past few decades and comprises 20% of cervical cancers. Both types of cancers are caused by HPV and are treated in a similar manner.
The recommendations for the management of colposcopy and biopsy results are published by the ASCCP and are subject to change (see above). Principles of treatment are given in Table 14.6, for the purpose of counseling patients. Actual treatment and follow-up decisions are made in conjunction with gynecologic or gynecologic oncology specialist recommendations.
Nina is seen by gynecology, and a colposcopy and biopsy are performed. Her results return CIN1. She is educated about the results, and shared decision making is used to discuss her options. Although this lesion is likely to regress, and co-testing in 1 year is an option, there are concerns that she will return to the Congo and that she may get lost to follow-up. Gynecology is asked to discuss definitive treatment options with her including ablative and excisional therapies.
Treatment Options for Cervical Neoplasia
The full discussion of algorithms and treatments for CIN2/3 are beyond the scope of this chapter. (See Chap. 15 on “Gynecologic Malignancies, Cervical Cancer” section.)
In general, there are five primary treatments which are used in the treatment of precancerous lesions and carcinoma in situ (CIN3):
-
1.
Cryotherapy – liquid nitrogen or freezing probe used to freeze acetowhite lesions from cervix. Advantage: low cost, used in “see and treat strategy” in some low-resource settings. Disadvantage: ablative, no biopsy specimen.
-
2.
Loop electrosurgical excision procedure (LEEP) – heated semicircular wire slices off abnormal tissue or removes TZ. Advantage: biopsy specimen for pathology, can check margins. Avoid in young women.
-
3.
Laser ablation – ablative therapy with laser.
-
4.
Conization – LEEP or cold knife removes transformation zone. Avoid in young women.
-
5.
Hysterectomy – avoid in young women.
Ablative therapies are rarely used in the United States. Close monitoring is preferred over ablative or excisional treatments in younger women, due to the high incidence of regression and because harm results from overscreening and overtreatment. Younger women include those who have not completed childbearing. In older women, if treatment is needed, LEEP is the most common modality. LEEP conization is preferred to cold-knife conization, as LEEP can be done in the office setting [49].
Nina asks about preventive care for her children. She is advised that her daughters should start Pap screening at the age of 21 and should be vaccinated for HPV with three doses starting as soon as possible. Her 14-year-old son should receive two doses of the HPV vaccine: one now and one in 6–12 months.
HPV Vaccination
HPV vaccination was introduced in the United States in June 2006 to prevent hrHPV infection with the intention of decreasing the incidence, morbidity, and mortality related to cervical cancer. HPV vaccination is currently recommended for all children and is best administered before sexual debut and exposure to the virus. The HPV vaccine is recommended at ages 11–12 for both girls and boys but can be initiated at age 9 (see Table 14.7 below). Gardasil™, a quadrivalent vaccine against 16, 18, 6, and 11, was approved in 2006 and was initially offered to girls. The bivalent vaccine Cervarix ™ was licensed in 2009 and covers high-risk types 16 and 18. HPV vaccination for boys was officially recommended by the Advisory Committee on Immunization Practices (ACIP) in 2011. The latest vaccine, Gardasil 9™, was approved in 2014 and is currently the only HPV vaccine available in the US. Gardasil 9™ covers hrHPV 16 and 18; it also covers “other high-risk” strains 31, 33, 45, 52, and 58 which account for 15% of cervical cancers. The addition of these other strains has the potential to increase the prevention of cervical cancer from 70 to 90% with vaccination. Gardasil-9™ also covers strains 6 and 11 which cause genital warts [50].
Based on clinical trials with the 9 valent HPV vaccine (Gardasil-9™), the Advisory Committee on Immunization Practices (ACIP) issued a recommendation to administer a two-dose series to both females and males less than 15 years, a recommendation approved by the FDA in October 2016 [50]. The second dose is administered between 6 and 12 months after the initial dose. When given at these early ages, immunogenicity is excellent with only two doses, with significantly higher titers obtained in those who received the two-dose series before age 15 as compared to those who were vaccinated with two doses at a later age. Seroconversion rates of those who receive the two-dose vaccine before age 15 and those who receive a three-dose vaccine are comparable.
The new two-dose schedule for teens less than 15 has several advantages:
-
Increased completion rates: the dose schedule facilitates the completion of the vaccination series at two visits. The visits can be 12 months apart which correlates with the spacing of many annual visits.
-
Patient convenience: It is convenient for adolescents receiving the vaccine, the parents, and the providers through the elimination of extra clinical visits.
-
Cost reduction: Overall costs are reduced with fewer vaccine dosages, lower administration costs, savings of parent time, and fewer costs associated with bringing children to the clinic for extra visits.
-
If the HPV vaccination series is initiated after the age of 15 years, however, the three-dose series at 0, 2, and 6 months is still recommended.
Approach to Previously Vaccinated Populations
For primary care providers who treat primarily adult patients, it is important to develop an approach to counseling patients who received partial HPV vaccination or who received HPV vaccination with non-Gardasil-9 immunizations. The basic principles are as follows:
-
1.
If a person initiated vaccination for HPV prior to age 15 and received two doses of any of the three approved vaccinations at the recommended schedule, a third vaccine is not needed.
-
2.
The 9 valent HPV vaccine can be used to complete a vaccination series that was started with either a bivalent or tetravalent vaccine.
-
3.
For persons who have previously completed an earlier HPV vaccine series, there is no recommendation for additional vaccination with the new 9 valent HPV vaccine.
-
4.
For interrupted vaccination series, continuation is recommended with no need to restart the series unless one or more of the intervals between doses was shorter than recommended.
Since the introduction of the HPV vaccine, there has been a decline in hrHPV infections and a decrease in high-risk lesions. In the US, data from the HPV-IMPACT Project demonstrated that cervical intraepithelial neoplasia (CIN2) lesions and higher attributed to hrHPV 16/18 decreased from 53.6% in 2008 to 28.45 in 2012 among women 18 years or older who had received at least one dose of the vaccine. Rates of initiation and completion of HPV vaccination had been suboptimal in the United States at that time. As of the year 2017, 66% of girls between 13 and 17 had received at least one dose of the vaccine, and only 49% were up to date on the HPV vaccine. Fortunately, vaccination rates have been increasing by 5% per year in the United States and there is a movement to provide HPV vaccination worldwide, including low- and middle-income countries (LMICs) [51, 52]. As vaccination rates improve, the effectiveness of the HPV vaccine for preventing HPV infections and subsequent cellular changes in the cervix will be more fully realized.
Vaccination is recommended for all persons age 9–26, ideally at age 9–12. Catch-up vaccination for those not previously vaccinated for HPV is recommended for all persons through age 26, regardless of sexual activity or gender. In 2018, the FDA approved Gardasil-9™ use until age 45. Clinical judgment and shared decision making should be used to make vaccination decisions in adults aged 27–45 until guidelines are updated. Vaccination in adults over age 45 is not currently recommended. Further study is needed to fully understand the risks and benefits of vaccination in the older cohort [53, 54].
HPV Vaccination in Specific Populations
The following recommendations are given for specific populations:
-
Children with a history of sexual abuse or assault: initiate vaccine at age 9, sooner than the normal 11–12 years of age.
-
Medical conditions: for primary and secondary immunocompromising conditions, the ACIP recommends the three-dose HPV vaccination in those aged 9–26 due to the potential for reduction in cell-mediated or humoral immunity. Examples include patients with HIV or autoimmune disease, patients taking immunosuppressive therapy, patients who have had a transplant, or those receiving treatment for malignant neoplasms.
Disparities in Cervical Cancer Prevention and Mortality
There are significant disparities in the prevention, incidence, treatment, and mortality from cervical cancer in the United States and around the world. Patient, physician, social, economic, and system issues contribute to the discrepancy. Race, structural racism, socioeconomic status, access to healthcare, geographic isolation, educational level, insurance, poverty, and other chronic medical illness adversely affect outcomes.
Disparities in Incidence
Between 2011 and 2015, the Hispanic population had the highest incidence of cervical cancer at a rate of 9.4 cases per 100,000, 95% CI (9.1–9.9), compared to the Black population at 9.0 cases per 100,000, 95% CI (8.8–9.2); the White population at 7.4 cases per 100,000, 95% CI (7.4–7.7); and the Alaskan and Indian Native population at 6.5 cases per 100,000, 95% CI (5.3–7.6) [17].
Disparities in Mortality
Race
For the reasons noted above, the Black (African-American non-Hispanic) population in the United States has the highest mortality rate from cervical cancer (per 100,000 women) at 3.7 deaths, compared to Hispanic women at 2.6 deaths, White women at 2.2 deaths, and Alaskan Indian and Pacific Islander both at 1.8 deaths [3, 10, 11]. The mortality among Black women is significantly underestimated for the following reason: women who have had a total hysterectomy for benign reasons are no longer at risk for cervical cancer. Data that does not correct for hysterectomy status underestimates the mortality rate for cervical cancer in all races. Between 2000 and 2012, the unadjusted overall mortality rate for all women was 3.4 deaths per 100,000 women, compared to a higher rate of 5.0 deaths per 100,000 women when adjusted for hysterectomy status. This underestimation is greatest among Black women. When stratified by race, Black women had a higher correction factor due to the higher rates of hysterectomy in this population, with rates increasing from 5.7 to 10.1 per 100,000 women, compared to a change from 3.2 to 4.7 per 100,000 White women [55].
Socioeconomic status and differential access to care appear to be major factors contributing to disparities in cancer mortality. Studies show higher mortality rates from cervical cancer in women of lower socioeconomic status. Women in isolated geographic areas and those in medically underserved areas, e.g., Appalachian women, have higher mortality rates compared to other White women and to the US average [55].
Disparities in Screening
Age
Women of all races and socioeconomic status at the extreme ends of the screening age recommendations between the ages of 23 and 29 and between 60 and 65 were less likely to be screened than women ages 30–59 [6].
Race
American Indian and Alaska natives are least likely to receive a Pap within the previous 3 years according to the CDC [56] at a rate of 60.9%. Native Hawaiians and Pacific Islanders were screened at 64.9%, Hispanic at 68.6%, White women at 68.4%, and Black women at 74.6%.
Insurance
Uninsured and underinsured women were most likely not to be screened. In the 2015 survey data referenced above, there was an 80.5% Pap screening rate within 3 years for those with insurance compared to 59.3% among those without insurance.
Chronic Disease
Women with one or more chronic diseases (e.g., kidney disease, arthritis, depression) are less likely to be screened [9, 56].
Disparities: Follow-Up of Abnormal Pap Test
Inadequate follow-up of abnormal results in women who have been screened is a significant risk to women and is caused by poor or inadequate access to care, nonexistent or inadequate insurance coverage, inadequate surveillance systems to track abnormal results and follow-up, and clinician failure to adhere to recommended guidelines for the follow-up of abnormal Pap test results causing delays in care.
It is estimated that about 50 million women undergo Pap tests per year, and 3.5% of these have cytological abnormalities requiring further follow-up. An analysis of data from a program whose goal is to increase access to screening, diagnostic, and follow-up services among low-income and uninsured women between 1991 and 2000 showed poor adherence to guidelines for follow-up of abnormal tests in medically underserved areas. Only 44% of women with two abnormal tests were followed in accordance with the guidelines at the time. Black or African-American women had the lowest percentage of follow-up compared to other ethnic groups, and Alaskan Natives and Native Americans had the highest number of third Pap tests performed instead of a colposcopy [57].
In a New Zealand study, among women with CIN3 who have had punch or wedge biopsies with no subsequent treatment, the rate of cancer was 31.3% within 30 years. Of these women in whom CIN3 persisted for 2 years, and punch or wedge biopsy had been the only treatment modality, 50.3% developed cancer within 30 years. The failure to receive adequate treatment results in an extreme risk of progression to cervical cancer. However, when treated and followed appropriately, the development of cervical cancer after 30 years was 0.7% [48].
HPV Prevalence
Between 2013 and 2014, the prevalence of any genital HPV in the United States in individuals between the ages of 18 and 59 according to the National Health and Nutrition Examination Survey (NHANES) was highest among the Black population (64.1%). The Hispanic population had a prevalence of 41.4%, the White non-Hispanic population had a prevalence of 40.0%, and the Asian population a prevalence of 23.8% [18].
HPV Vaccination
In the HPV Vaccine Impact Monitoring (HPV-IMPACT) Project [58], a significant difference in vaccination rates was observed based on race/ethnicity and insurance coverage. Of the vaccinated women in the study, non-Hispanic White women had a vaccination rate of 67.45% compared to 18% vaccination rate in non-Hispanic Black women and 10.3% vaccination rate in Hispanic women. Women with private insurance were more likely to be vaccinated (65.1%) compared to those with public insurance (27.9%) and those without insurance (2.3%).
In the National Immunization Survey Teen (NIS-Teen) for adolescents aged 13–17 years, Black or Hispanic adolescents and adolescents living below the federal poverty level were significantly less likely to complete vaccination series [59].In addition to access to care and cost, research has demonstrated mistrust in vaccination, which stems from a legacy of unethical medical research, patients lived experiences of racism in medical settings, and lack of accessible healthcare. Specifically regarding HPV vaccination, vaccine acceptance is lower amongst parents who expressed mistrust in government health agencies, though trust in health information from a physician or healthcare professional was not predictive of vaccine acceptance [60]. Ongoing efforts by healthcare systems and government agencies to repair this trust are needed.
Private insurances generally cover the cost of HPV vaccination. The National Vaccine Program, Vaccines for Children (VFC), provides free vaccination for children and adolescents through 18 years of age for people who would otherwise not be able to afford the vaccine [61]. Barriers to completion among low-income groups – lack of transportation, limited healthcare access, and work schedules – can result in incomplete vaccination series even when vaccine programs pay for the initial dose.
A higher level of maternal education, having continuous insurance coverage from age 11, and living in the Northeast were all associated with higher rates of vaccine completion [59].
Disparities Among Foreign-Born Women
Data from the 2013 National Health Interview Survey (NHIS) conducted by the National Center for Health Statistics reveal that HPV vaccination initiation was higher among American-born women aged less than 26 years compared to foreign-born women (27.1% verses 17.2%) [12]. Even when controlling for confounders in a multivariate logistic analysis (demographic, economic, and healthcare variables), the difference remained unchanged. Regardless of the place of birth, females were more likely to initiate vaccination compared to males. Overall, younger foreign-born males had the lowest access to healthcare compared to all other groups.
Insurance status and access to healthcare account for most of the differential rates in HPV vaccination. This is most evident in the undocumented foreign-born persons who are not eligible for public health insurance and may not have valid social security numbers or funds to pay for private insurance. This is also supported in part by the finding that foreign-born women in the higher-income group had similar HPV vaccination rates compared to their American-born high-income group counterparts [12].
A Potential Solution: Vaginal Sample Collection
This strategy is aimed at improving screening rates in unscreened, high-risk women who have barriers to regular screening including discomfort, costs, and clinical accessibility. Patients may collect vaginal samples at home and send them in, with positive results necessitating a clinical visit and follow-up. It is not clear how this test compares to the accuracy of office-based screening, or whether follow-up of positive results would be adequate. Vaginal sample collection is not currently approved by the FDA. It is however endorsed by the World Health Organization (WHO) and is currently being studied in the United States [62, 63].
Conclusion
Cervical cancer continues to be a major public health burden throughout the world and among underserved populations in the United States. HPV vaccination, cervical cancer screening, precursor lesion treatment, and adequate follow-up of all women will advance the goal of saving the lives of women who die needlessly from invasive cervical cancer each year, a largely preventable disease.
Summary Points
-
1.
Ninety-nine percent of cervical cancer is caused by persistent high-risk HPV strains that infect the transformation zone of the cervix and lead to precancerous and cancerous changes.
-
2.
Routine cervical cancer screening guidelines call for Pap testing alone every 3 years from age 21 to 29. Women ages 30–65 should be screened with either co-testing every 5 years, hrHPV testing every 5 years, or Pap testing alone every 3 years. With some exceptions, women who have undergone a complete hysterectomy for benign conditions and women over 65 may exit screening assuming they have received adequate recommended testing in the prior 10 years.
-
3.
HIV-positive, immune-compromised, DES-exposed, and women with a history of CIN2 or greater are at higher risk of cervical cancer and are screened more intensely than those outlined in the routine guidelines.
-
4.
HPV vaccination with Gardasil-9™ is approved for use in persons aged 9–45. Current recommendations are for most children to be vaccinated with two doses at ages 11 and 12. Persons who start immunization older than 15 years of age should receive three doses at 0, 2, and 6 months. Immunocompromised persons should receive three doses, even if started at the younger age.
-
5.
Algorithms for the management of abnormal Pap smears and biopsy results are available online via a downloadable app from the ASCCP. Persons positive for HPV 16 or 18, with persistent “other high-risk strains,” with cytology of ASC-H or greater, or with ASCUS/LGSIL with HPV+ should be referred for colposcopy. CIN1 is low grade and often regresses, whereas CIN2/3 are high-grade changes which need increased monitoring and/or treatment by gynecologists.
-
6.
Significant disparities exist among certain populations in the United States and also in low and middle income countries including differences in HPV vaccination, cervical cancer screening, follow-up and treatment of abnormal results, and mortality. These disparities are particularly notable for low-income, minority, chronically ill, immigrant, poorly insured women and those with poor access to healthcare. Continued effort to reach a goal of universal HPV vaccination and universal cervical cancer screening will help close these gaps in care.
References
Group USCSW. United States cancer statistics: 1999–2014 incidence and mortality web-based report 2017 cited 2017. Available from: http://www.cdc.gov/uscs
Walboomers JM, Jacobs MV, Manos MM. Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. J Pathol. 1999;189(1):12–9.
Cancer Stat Facts: Cervical Cancer: NIH: National Cancer Institute Surveillance, Epidemiology, and End Results Program. Available from: https://seer.cancer.gov/statfacts/html/cervix.html.
Hillemanns P, Soergel P, Hertel H, Jentschke M. Epidemiology and early detection of cervical cancer. Oncol Res Treat. 2016;39:501–6.
Chelimo C, Wouldes TA, Cameron LD, Elwood JM. Risk factors for and prevention of human papillomaviruses (HPV), genital warts and cervical cancer. J Infect. 2013;66:207–17.
Benard VB, Thomas CC, King J, Massetti GM, Doria-Rose VP, Saraiya M, et al. Vital signs: cervical cancer incidence, mortality, and screening - United States, 2007-2012. MMWR Morb Mortal Wkly Rep. 2014;63(44):1004–9.
Castellsague X. Natural history and epidemiology of HPV infection and cervical cancer. Gynecol Oncol. 2008;110(3 Suppl 2):S4–7.
Fonseca-Moutinho JA. Smoking and cervical cancer. Obstet Gynecol. 2011;2011:847684. https://doi.org/10.5402/2011/847684.
Crawford A, Benard V, King J, Thomas CC. Understanding barriers to cervical cancer screening in women with access to care, behavioral risk factor surveillance system, 2014. Prev Chronic Dis. 2016;13:E154.
Cervical Cancer Rates by Race and Ethnicity Centers for Disease Control and Prevention: Division of Cancer Prevention and Control. Centers for Disease Control and Prevention; 2017 updated June 19. Available from: https://www.cdc.gov/cancer/cervical/statistics/race.htm
Viens LJHS, Watson M, Markowitz LE, Thomas CC, Thompson TD, Razzaghi H, Saraiya M, Centers for Disease Control and Prevention (CDC). Human papillomavirus–associated cancers—United States, 2008–2012. MMWR Morb Mortal Wkly Rep. 2016;65:661–6. https://doi.org/10.15585/mmwr.mm6526a1
De P, Budhwani H. Human papillomavirus (HPV) vaccine initiation in minority Americans. Public Health. 2017;144:86–91.
Tan SY, Tatsumura Y. George Papanicolaou (1883–1962): discoverer of the Pap smear. Singap Med J. 2015;56(10):586–7. https://doi.org/10.11622/smedj.2015155.
https://www.cytopathology.org/specimen-collection-adequacy-requisition/. Accessed 23 May 2018.
Tibbs RF, Wong JY, Logrono R. Enhancing recovery of endocervical component on gynecologic cytology specimens processed by thin-layer technology. Acta Cytol. 2003;47:172–6.
Elumir-Tanner L, Doraty M, for the Southern Alberta Primary Care Research Network (SAPCReN). Management of Papanicolaou test results that lack endocervical cells. CMAJ. 2011;183(5):563–8.
Wright TC, Stoler MH, Behrens CM, Sharma A, Zhang G, Wright TL. Primary cervical cancer screening with human papillomavirus: end of study results from the ATHENA study using HPV as the first-line screening test. Gynecol Oncol. 2015;136(2):189–97.
McQuillan G, Kruszon-Moran D, Markowitz LE, Unger ER, Paulose-Ram R. Prevalence of HPV in Adults Aged 18–69: United States, 2011–2014. NCHS data brief. 2017(280):1–8.
Paz-Zulueta M, Alvarez-Paredes L, Rodriguez Diaz JC, Paras-Bravo P, Andrada Becerra ME, Rodriguez Ingelmo JM, et al. Prevalence of high-risk HPV genotypes, categorized by their quadrivalent and nine-valent HPV vaccination coverage, and the genotype association with high-grade lesions. BMC Cancer. 2018;18(1):112.
Stanley M. Immunobiology of HPV and HPV vaccines. Gynecol Oncol. 2008;109:S15–21.
Schiffman M, Castle PE, Jeronimo J, Rodriguez AC, Wacholder S. Human papillomavirus and cervical cancer. Lancet. 2007;370(9590):890–907.
Rodriguez A, Schiffman M, Wacholder HR, et al. Rapid clearance of human papillomavirus and implications for clinical focus on persistent infections. J Natl Cancer Inst. 2008;100(7):513–7. https://doi.org/10.1093/jnci/djn044.
Allegretti JR, Barnes EL, Cameron A. Are patients with inflammatory bowel disease on chronic immunosuppressive therapy at increased risk of cervical high-grade dysplasia/cancer? A meta-analysis. Inflamm Bowel Dis. 2015;21(5):1089–97. https://doi.org/10.1097/MIB.0000000000000338.
Kim SC, Schneeweiss S, Liu J, et al. Biologic disease-modifying antirheumatic drugs and risk of high-grade cervical dysplasia and cervical cancer in rheumatoid arthritis: a cohort study. Arthritis Rheumatol. 2016;68(9):2106–13. https://doi.org/10.1002/art.39689.
Matsumoto K, Oki A, Furuta R, Maeda H, Yasugi T, et al. Tobacco smoking and regression of low-grade cervical abnormalities. Cancer Sci. 2010;101:2065–73.
Moyer VA. Screening for cervical cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2012;156(12):880–91, w312.
Saslow D, Solomon D, Lawson HW, Killackey M, Kulasingam SL, Cain J, et al. American Cancer Society, American Society for Colposcopy and Cervical Pathology, and American Society for Clinical Pathology screening guidelines for the prevention and early detection of cervical cancer. Am J Clin Pathol. 2012;137(4):516–42.
US Preventive Services Task force. Screening for cervical cancer. US Preventive Services Task Force recommendation statement. JAMA. 2018;320(7):674–86.
Kulasingam SL, Havrilesky LJ, Ghebre R, Myers ER. Screening for cervical cancer: a modeling study for the US Preventive Services Task Force. J Low Genit Tract Dis. 2013;17(2):193–202.
Agenor M, Muzny CA, Schick V, Austin EL, Potter J. Sexual orientation and sexual health services utilization among women in the United States. Prev Med. 2017;95:74–81.
Stout NK, Goldhaber-Fiebert JD, Ortendahl JD, Goldie SJ. Trade-offs in cervical cancer prevention: balancing benefits and risks. Arch Intern Med. 2008;168(17):1881–9.
Sasieni P, Adams J, Cuzick J. Benefit of cervical screening at different ages: evidence from the UK audit of screening histories. Br J Cancer. 2003;89(1):88–93.
Miller MG, Sung HY, Sawaya GF, Kearney KA, Kinney W, Hiatt RA. Screening interval and risk of invasive squamous cell cervical cancer. Obstet Gynecol. 2003;101(1):29–37.
Sawaya GF, McConnell KJ, Kulasingam SL, Lawson HW, Kerlikowske K, Melnikow J, et al. Risk of cervical cancer associated with extending the interval between cervical-cancer screenings. N Engl J Med. 2003;349(16):1501–9.
Dunne EF, Unger ER, Sternberg M, McQuillan G, Swan DC, Patel SS, et al. Prevalence of HPV infection among females in the United States. JAMA. 2007;297(8):813–9.
Giorgi Rossi P, Carozzi F, Federici A, Ronco G, Zappa M, Franceschi S, et al. Cervical cancer screening in women vaccinated against human papillomavirus infection: recommendations from a consensus conference. Prev Med. 2017;98:21–30.
http://www.ascopost.com/issues/august-10-2018/fda-approves-hpv-test/. Accessed 16 Oct 2018.
http://www.cancerscreening.gov.au/internet/screening/publishing.nsf/Content/cervical-screening-1. Accessed 15 Oct 2018.
Pearce KF, Haefner HK, Sarwar SF, Nolan TE. Cytopathological findings on vaginal Papanicolaou smears after hysterectomy for benign gynecologic disease. N Engl J Med. 1996;335(21):1559–62.
American Society for Colposcopy and Cervical Pathology (ASCCP) guidelines for managing abnormal PAP tests. http://www.asccp.org/asccp-guidelines
Kim JJ, Burger EA, Sy S, Campos NG. Optimal cervical cancer screening in women vaccinated against human papillomavirus. J Natl Cancer Inst. 2017;109(2):djw216.
(ACOG) TACoOaG. Gynecologic care of women and adolescents with human immunodeficiency virus. Washington, DC: Lippincott Williams & Wilkins; 2016. Available from: https://www.acog.org/Clinical-Guidance-and-Publications/Practice-Bulletins/Committee-on-Practice-Bulletins-Gynecology/Gynecologic-Care-for-Women-and-Adolescents-With-Human-Immunodeficiency-Virus.
Heard I. Prevention of cervical cancer in women with HIV. Curr Opin HIV AIDS. 2009;4(1):68–73.
Inamoto Y, Shah NN, Savani BN, Shaw BE, Abraham AA, Ahmed IA, et al. Secondary solid cancer screening following hematopoietic cell transplantation. Bone Marrow Transplant. 2015;50(8):1013–23.
Herbst AL. The current status of the DES-exposed population. Obstet Gynecol Annu. 1981;10:267–78.
Troisi R, Hatch EE, Palmer JR, et al. Prenatal diethylstilbestrol exposure and high-grade squamous cell neoplasia of the lower genital tract. Am J Obstetr Gynecol. 2016;215(3):322.e1–8. https://doi.org/10.1016/j.ajog.2016.03.007.
Gynecologists ACoOa. Abnormal cervical cancer screening test results. Washington, DC: Lippincott Williams & Wilkins; 2016. Available from: https://www.acog.org/Patients/FAQs/Abnormal-Cervical-Cancer-Screening-Test-Results
McCredie MR, Sharples KJ, Paul C, Baranyai J, Medley G, Jones RW, et al. Natural history of cervical neoplasia and risk of invasive cancer in women with cervical intraepithelial neoplasia 3: a retrospective cohort study. Lancet Oncol. 2008;9(5):425–34.
Martin-Hirsch PL, Paraskevaidis E, Kitchener H. Surgery for cervical intraepithelial neoplasia. Cochrane Database Syst Rev. 2013;12:CD001318.
Meites E, Kempe A, Markowitz LE. Use of a 2-dose schedule for human papillomavirus vaccination - updated recommendations of the advisory committee on immunization practices. MMWR Morb Mortal Wkly Rep. 2016;65(49):1405–8.
HPV Vaccination coverage data. https://www.cdc.gov/hpv/hcp/vacc-coverage/index.html. Accessed 14 Oct 2018.
WHO recommendations for routine immunizations. Updated August 2018. https://www.who.int/immunization/policy/Immunization_routine_table1.pdf?ua=1. Accessed 16 Oct 2018.
https://www.acog.org/Clinical-Guidance-and-Publications/Committee-Opinions/Committee-on-Adolescent-Health-Care/Human-Papillomavirus-Vaccination. Accessed 12 Sept 2018.
Meites E, Szilagyi PG, Chesson HW, Unger ER, Romero JR, Markowitz, LE. Human Papillomavirus Vaccination for Adults: Updated Recommendations of the Advisory Committee on Immunization Practices. MMWR Morb Mortal Wkly Rep. 2019;68:698–702.
Beavis AL, Gravitt PE, Rositch AF. Hysterectomy-corrected cervical cancer mortality rates reveal a larger racial disparity in the United States. Cancer. 2017;123(6):1044–50.
National Center for Health Statistics. Use of Pap smears among women aged 18 and over, by selected characteristics: United States, selected years 1987–2015. Available from: https://www.cdc.gov/nchs/fastats/pap-tests.htm
Benard VB, Lawson HW, Eheman CR, Anderson C, Helsel W. Adherence to guidelines for follow-up of low-grade cytologic abnormalities among medically underserved women. Obstet Gynecol. 2005;105(6):1323–8.
Hariri S, Bennett NM, Niccolai LM, Schafer S, Park IU, Bloch KC, et al. Reduction in HPV 16/18-associated high grade cervical lesions following HPV vaccine introduction in the United States - 2008-2012. Vaccine. 2015;33(13):1608–13.
Niccolai LM, Mehta NR, Hadler JL. Racial/Ethnic and poverty disparities in human papillomavirus vaccination completion. Am J Prev Med. 2011;41(4):428–33.
Nan X, Daily K, Richards A, Holt C, Wang MQ, Tracy K, Qin Y. The role of trust in health information from medical authorities in accepting the HPV vaccine among African American parents. Hum Vaccin Immunother. 2019;15(7–8):1723–31. https://doi.org/10.1080/21645515.2018.1540825. Epub 2018 Nov 5. PMID: 30396312; PMCID: PMC6746524.
Vaccines for Children (VCF) Program. Available from: https://www.cdc.gov/features/vfcprogram/#vfclist
WHO. Comprehensive cervical cancer control: a guide to essential practice Second edition. Department of Reproductive Health and Research. Switzerland; 2014.
Des Marais A, ZhaoHome Y, Hobbs M, et al. Self-collection by mail to test for human papillomavirus and sexually transmitted infections. Obstet Gynecol. 2018;132(6):1412–20. https://doi.org/10.1097/AOG.0000000000002964.
https://aidsinfo.nih.gov/guidelines. Accessed 15 Oct 2018.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Review Questions
Review Questions
-
1.
A 19-year-old woman presents to the clinic to establish care with a doctor for adults. She had routine care with her pediatrician and completed the HPV vaccine series. She has been sexually active for 2 years and is on oral contraceptives. She was recently diagnosed with chlamydia and treated. Her mother told her that she needs a Pap smear. Which of the following is recommended?
-
A.
She should be tested for high-risk HPV now.
-
B.
She should have a Pap test with HPV co-testing now.
-
C.
She should have a Pap test at age 21.
-
D.
HPV testing should be performed at age 21.
The correct answer is C. Pap testing should not start prior to age 21, even in sexually active women. HPV testing is not recommended for screening in women under age 30 (25 in some countries) except as a reflex test for abnormal Pap results. Her recent chlamydia diagnosis does not change these recommendations [17, 30].
-
A.
-
2.
A 66-year-old woman, who moved to the United States from India 1 year ago, comes in to establish care. She does not believe she has ever had a Pap test. She has stopped menstruating and denies any postmenopausal vaginal bleeding or discharge. Which of the following is correct?
-
A.
She is asymptomatic and over age 65. Pap screening is not needed.
-
B.
She should be tested with yearly Pap smears for the next 20 years because of her unknown history.
-
C.
She should receive the three-dose HPV vaccination series.
-
D.
She should undergo Pap and HPV co-testing today.
The correct answer is D. Women over 65 may exit screening if they have been screened adequately in the past 10 years, with the most recent test in the last 5 years. Clinical judgment should be used in recommendations for her screening, but she should be screened now for HPV and cervical cancer and again in 3–5 years since she has not been adequately screened in the past 10 years. Yearly Pap smears are recommended for some high-risk women, but would not apply unless she was DES exposed, infected with HIV, immunosuppressed, or had a history of cervical or vaginal malignancy within the last 20 years. HPV vaccination is not approved for persons over 45 years of age [17, 30].
-
A.
-
3.
An undocumented 40-year-old Hispanic woman, G1P1, presents to the free mobile clinic for a Pap test. She and her family move frequently to find work. She does not remember how long ago she had her last Pap, but thinks it may have been abnormal. She is unsure if she has ever had a colposcopy. She undergoes Pap and HPV co-testing today and the clinic social worker meets with her to discuss ways to get insurance and housing for her family, because they are homeless.
Which of the following is the most important next step before the patient leaves the clinic today?
-
A.
Tell her that she is high risk for cervical cancer and refer her to gynecology.
-
B.
Have her sign a release of information to get old records from all the prior healthcare facilities where she received care.
-
C.
Determine how she should be contacted to receive her Pap test results, and fully explain why follow-up is very important.
-
D.
Discuss that colon cancer screening starts at age 45 if she does not have a prior history of colorectal cancer.
The correct answer is C. Healthcare disparities should be considered when seeing patients, and lack of follow-up for abnormal results is a serious issue in certain populations. Hispanic patients are an ethnic group that often has poor follow-up for abnormal test results, and undocumented persons are at particularly high risk. There must be a secure plan to reach the patient and arrange for follow-up if the Pap test is abnormal. Telling her that she is high risk for cervical cancer is premature and may cause unnecessary anxiety. Obtaining prior medical records should be attempted, but the ability to follow-up on the current testing is more important at this visit. Colon cancer screening is important, but will not be needed for 5 years [57].
-
A.
-
4.
A 30-year-old woman presents with 9-year-old twins, a girl and a boy. She asks if her daughter should get the HPV vaccine. What are the current recommendations for HPV vaccination?
-
A.
Both children should be vaccinated at age 11 with two doses.
-
B.
The daughter should be vaccinated at age 11, the son at age 9.
-
C.
The daughter should be vaccinated with three doses, the son with two doses.
-
D.
Both children should be vaccinated now with two doses.
The correct answer is A. For children aged 9–14, the ACIP recommends a two-dose schedule for male and female patients, usually at ages 11–12. Children are vaccinated at age 9 in cases of immunosuppression or sexual abuse. The vaccination is approved for persons age 9–45, so clinical judgment can be used when making vaccination recommendations for adults [50].
-
A.
-
5.
A 31-year-old woman with HIV comes in to establish primary care. She was diagnosed with HIV at age 27 and has had three normal annual Pap smears. Her most recent testing at age 30 was normal cytology and HPV negative. How often should she receive a Pap test in the future?
-
A.
Every 3 years for life.
-
B.
Every 5 years with HPV co-testing until age 65.
-
C.
Annually, may discontinue at age 65.
-
D.
Biannually for life.
The correct answer is A. The current guidelines for HIV-positive women, which is based on limited data, recommend annual Pap starting at the time of diagnosis. At age 30, co-testing should be done. If co-testing is normal, the next testing can be delayed for 3 years. Screening does not end at age 65. These recommendations do not change based on the use of antiretroviral therapy, CD4 counts, or viral load [64].
-
A.
-
6.
A 32-year-old woman presents for care. She states that she is a virgin and is refusing a pelvic examination or Pap test. She said that she was told by her last physician that she was at very low risk for cancer, and therefore, Paps were not needed. Which of the following statements is correct according to current guidelines?
-
A.
She is at risk of cervical cancer despite her sexual history, and she should be made to sign an “against medical advice” form if she refuses testing.
-
B.
All women should be screened for cervical cancer despite sexual history starting at age 21.
-
C.
She should self-test for HPV and only get a Pap if the results are positive.
-
D.
Women who have sex with women, nuns, and virgins do not need Pap tests.
The correct answer is B. All persons with a cervix should be screened for cervical cancer between the ages of 21 and 65, regardless of sexual history, sexual orientation, or gender identity. Although persons who have never had penetrating sexual intercourse with a man may be at lower risk, HPV is spread through other forms of contact. A prior history of sexual contact or sexual assault may be denied or not remembered by the patient. The idea of self-collection of a vaginal collection for HPV screening has merit and is being suggested for screening in some lower-resource settings, but there are no current guidelines to guide its use. If she refuses pelvic examination, she should not be pressured or traumatized, but relationship building and patient education may, in time, change her mind about the screening. Refusals of recommended care, and discussions of the risks to the patient of not screening, should be clearly documented in the medical record [17].
-
A.
Rights and permissions
Copyright information
© 2020 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Oleng’, N.A., Sobel, H.G., Kwolek, D. (2020). Cervical Cancer and Human Papillomavirus: Prevention and Screening. In: Tilstra, S.A., Kwolek, D., Mitchell, J.L., Dolan, B.M., Carson, M.P. (eds) Sex- and Gender-Based Women's Health. Springer, Cham. https://doi.org/10.1007/978-3-030-50695-7_14
Download citation
DOI: https://doi.org/10.1007/978-3-030-50695-7_14
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-50694-0
Online ISBN: 978-3-030-50695-7
eBook Packages: MedicineMedicine (R0)