Abstract
The skin is the largest organ of the body, ranging in size from 1.5 to 2.0 m2 in adults [1]. This highly organized composite structure fulfils a wide variety of functions critical to the maintenance of homeostasis [2]. Burns, caused by thermal, chemical, or electrical injuries, can result in severe and irreparable damage to the skin, leading to wound contracture, scar tissue formation, and a loss of functionality. In the United States, between 60,000 and 80,000 patients are hospitalized annually for the treatment of serious burns [3, 4]. The average cost of patient care, reconstruction, and rehabilitation is extremely high, especially in severe or extensive cases [5]. Improvements in resuscitation techniques now facilitate the survival of patients with major burns extending over more than 90% of their bodies [6].
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
17.1 Introduction
The skin is the largest organ of the body, ranging in size from 1.5 to 2.0 m2 in adults [1]. This highly organized composite structure fulfils a wide variety of functions critical to the maintenance of homeostasis [2]. Burns, caused by thermal, chemical, or electrical injuries , can result in severe and irreparable damage to the skin, leading to wound contracture, scar tissue formation, and a loss of functionality. In the United States, between 60,000 and 80,000 patients are hospitalized annually for the treatment of serious burns [3, 4]. The average cost of patient care, reconstruction, and rehabilitation is extremely high, especially in severe or extensive cases [5]. Improvements in resuscitation techniques now facilitate the survival of patients with major burns extending over more than 90% of their bodies [6].
Conventional treatment strategies for wound closure rely on the use of autografts. Weaknesses of the autologous approach include the need for additional surgery, the creation of donor site defects, technical difficulty, expense, and higher patient risk [7, 8]. Allogenic materials have also been implemented but are only suitable for temporary wound coverage, as they are associated with immune rejection and a failure to integrate into the host tissues [9]. Consequently, there is great need for the development of an economical tissue-engineered skin substitute. To date, numerous burn dressing biomaterials and engineered constructs have been investigated but have failed to match the wound healing properties of grafted autologous tissues [7, 10,11,12]. With continued research, it may be possible to design a device that could promote the complete regeneration of the skin, with rapid wound closure, functional recovery, and excellent cosmetic results.
17.2 Physiology of the Skin
In order to develop a successful tissue engineering strategy to promote regeneration following burn injury, it is critical to have a comprehensive understanding of the structure and function of the normal skin.
17.2.1 Basic Organization and Cellular Composition
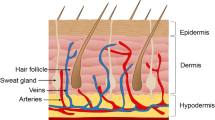
The skin, or integument, is composed of multiple tissue and cell types arranged to form a complex, multifunctional organ (Fig. 17.1). The integumentary system is multilayered and can be divided into three interconnected regions: the epidermis, the dermis, and the hypodermis [2]. Located within these layers are the accessory structures or appendages, including glands, hair follicles, and nails, that are critical to the proper functioning of the skin [13]. The basal lamina located between the epidermal and dermal layers also plays a critical role in the maintenance of skin structure and function [14]. The primary cell types found within the skin include keratinocytes, melanocytes, Merkel cells, Langerhans cells, and fibroblasts [15].
17.2.1.1 Keratinocytes
Keratinocytes are the stratified squamous epithelial cells that are the primary cellular component of the epidermis, comprising 90–95% of all cells within this region [2]. These cells function as a protective barrier and also play an important role in skin immune function, secreting numerous cytokines that mediate inflammatory and immune responses, including interleukins and interferons [16].
The keratinocyte phenotype changes as the cells progress outwards from the basal layer towards the skin surface. This alteration corresponds to a change in the state of differentiation of the keratinocytes from proliferative to cornified cells [17]. The structural changes in the cells can be correlated with variations in the expression of keratins, the main structural proteins produced by the keratinocytes [18]. These alpha-helical proteins can be classified as either acidic (type I) or basic (type II). During assembly, heterodimers, each composed of one acidic and one basic keratin, are arranged to form cytoskeletal intermediate filaments [19]. The keratinocytes in the basal layer express keratins 5 (acidic) and 14 (basic), whereas the more differentiated cells in the outer layers express keratins 1 (acidic) and 10 (basic) [20, 21].
17.2.1.2 Melanocytes
Melanocytes are pigment-producing cells that are typically found in the basal epidermal layer in an approximate ratio of 1 melanocyte to every 10 basal keratinocytes [15]. In general, these cells do not divide, and their survival is dependent on growth factors secreted by the keratinocytes, such as melanocyte-stimulating hormone (MSH), basic fibroblast growth factor (bFGF), and endothelin-1 [22, 23]. Melanocytes have many cytoplasmic processes, termed dendrites, which facilitate the delivery of the pigment melanin to the epidermal keratinocytes [24]. A single melanocyte will supply melanin to approximately 35 keratinocytes through the transfer of unique organelles termed melanosomes, which produce and store the pigment within the cells [25]. The specific mechanism of this transfer is currently unclear but may involve keratinocyte-melanocyte membrane fusion or melanosome exocytosis followed by keratinocyte coated-pit endocytosis or phagocytosis [26]. The two main types of melanin are eumelanin, which is brown to black in color, and pheomelanin, which is yellow to red in color [25, 27]. Skin color is dependent on both melanosome size and density within the keratinocytes and can be affected by factors such as ultraviolet light and MSH [28].
17.2.1.3 Merkel Cells
Merkel cells are specialized neuroendocrine cells that are found in the epidermis and dermis in varying site-specific densities. The highest concentrations of these cells are in the outer root sheaths of the hair follicles and in the epidermal ridges of the deep epidermis [29]. In some regions of the skin, these cells are arranged into units termed tactile discs or touch receptors. Upon compression, Merkel cells can function as mechanoreceptors, secreting neuropeptides that stimulate the dermal nerve endings [2]. The cells can be identified microscopically by their characteristic cell surface microvilli and dense-core cytoplasmic granules that contain various neuropeptides [30]. Epidermal Merkel cells have been shown to produce nerve growth factor (NGF), which can bind to NGF receptors expressed on dermal Merkel cells [31]. These dermal cells often form synaptic contacts with the axon terminals present in the dermis. Merkel cells can also be connected to keratinocytes via desmosomal junctions and may play a stimulatory role in the proliferation and differentiation of these cells [2].
17.2.1.4 Langerhans Cells
Langerhans cells are antigen-presenting dendritic cells that function in the immune response to pathogens and cancerous cells within the skin [32]. These cells are primarily found in the non-basal region of the epidermis but can also be found within the dermis. Overall, the Langerhans cell population is relatively small, representing 1–5% of the total number of epidermal cells. Antigens diffusing through the epidermis are likely to contact one of the many dendrites of the Langerhans cells [15, 33]. Endogenous antigens, such as those related to epidermal cancer, are also recognized [34]. The Langerhans cells engulf the antigens via phagocytosis and then migrate across the basal lamina and underlying dermis into the regional lymph node [35]. Following intracellular processing, the antigens are presented at the cell surface bound to major histocompatibility complex (MHC) class II molecules. Interaction of the T cells in the lymph node with the MHC/antigen complexes, combined with the release of stimulatory cytokines by the Langerhans cells, results in T-cell activation [32, 36, 37]. Langerhans cells are also involved in the initiation of T-cell-dependent B-cell immune responses and secondary T-cell responses [33]. For wound healing treatment strategies, it is important to note that these cells have been shown to play a role in the rejection of grafted allogenic and xenogenic skin [38].
17.2.1.5 Fibroblasts
Fibroblasts are the main cellular component of the dermis and are responsible for the production and secretion of numerous extracellular matrix (ECM) molecules found in the skin, such as collagen, elastin, fibronectin, decorin, tenascin, laminin, and various proteoglycans [39]. To allow for turnover of the ECM , these cells also secrete matrix metalloproteinases (MMPs) [40].
17.2.2 The Epidermis
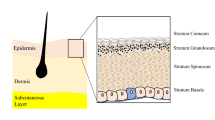
The epidermis, the outermost region of the skin, provides many of the barrier properties of the organ. This avascular, multilayered epithelium has very sparse ECM and is composed primarily of cells. The thickness of the epidermis is site-specific, with thicker layers found in the regions of the body that experience greater frictional forces, such as on the palms of the hands and the soles of the feet. The deeper layers of the epidermis are arranged into the epidermal ridges that increase the contact area between the epidermis and the dermis [2, 15, 24]. To support the continual regeneration of the epidermis, the keratinocytes differentiate in a process termed keratinization or cornification [17]. The progressive stages of differentiation can be observed in the epidermal layers: the stratum germinativum, stratum spinosum, stratum granulosum, stratum lucidum, and stratum corneum (Fig. 17.2). In humans, it takes approximately 14 days for a cell leaving the innermost epidermal layer, the stratum germinativum, to reach the outer stratum corneum. These cells then typically reside in the stratum corneum for an additional 14 days before they are shed from the body [15].
A diagrammatic and histological view of the epidermal layers. (a) The stratum corneum. (b) The stratum granulosum. (c) The stratum spinosum. (d) The stratum germinativum. (e) The underlying dermis. The integrin junctions between the basal keratinocytes and the underlying basal lamina can be seen in the diagram on the left. (Diagram on left adapted with permission from Janes et al. [43]. Copyright 2002 by John Wiley and Sons Limited. Histological image on right reprinted from www.lab.anhb.uwa.edu.au with the permission of Dr. L. Slomianka)
17.2.2.1 Stratum Germinativum
The stratum germinativum , also referred to as the stratum basale, is the innermost epidermal layer, composed of a monolayer of cells that are firmly attached via hemidesmosomal junctions to the basal lamina that separates the epidermis from the dermis [2, 14]. This cell layer primarily consists of morphologically identical, columnar basal keratinocytes, interspersed with melanocytes and Merkel cells. The basal keratinocytes can be classified as one of three types based on their proliferative capacity: stem cells, transient-amplifying cells, or post-mitotic differentiating cells [41]. Approximately 10% of the cells that reside in the basal cell layer are stem cells, which are slow-cycling cells with the potential for continual cell division [42, 43]. When a stem cell divides, one daughter cell remains a stem cell in the basal layer, while the other daughter is destined to differentiate. The cells that will differentiate, termed transient-amplifying cells, will divide a limited number of times before differentiating and moving up in the epidermal layers. The post-mitotic differentiating cells account for 5–10% of the basal keratinocyte cell population and can be identified based on the expression of early differentiation markers , such as keratin 10 and involucrin [44, 45].
17.2.2.2 Stratum Spinosum
Adjacent to the stratum germinativum , the stratum spinosum consists of 8–10 rows of polyhedral keratinocytes that have begun to differentiate. Melanocytes and Langerhans cells can also be found interspersed throughout this layer [15]. The keratinocytes in this stratum are interconnected by desmosomal junctions, which are supported intracellularly by bundles of cytoskeletal proteins termed tonofibrils [46]. This network structure gives the cells a characteristic “spiky” appearance following histological processing. The phenotype of the spinosum keratinocytes varies depending on their position within the layer. As the cells progress outwards, they lose their polygonal structure and develop a larger and flatter shape [2]. All of the keratinocytes still contain cellular organelles, including melanosomes secreted by the melanocytes [27]. In the upper layers of the stratum spinosum, lamellar granules can be observed within the keratinocytes. These granules contain proteins, such as keratohyalin, and other components that are involved in the final stages of keratinization [17]. Within this layer, the differentiating cells begin to synthesize keratin types 1 and 10. It is interesting to note that during wound healing, the expression of these two proteins is downregulated in favor of keratin types 6 and 16 [47].
17.2.2.3 Stratum Granulosum
The 2–3 rows of flattened, nucleated cells in the stratum granulosum can be identified by the uniform presence of basophilic keratohyalin (lamellar) granules [2]. As previously mentioned, the proteins included in these granules function in the formation of the outer cornified cell layer. Keratohyalin has been shown to form an intracellular matrix that envelops and strengthens the cytoskeletal keratin filaments [48]. This keratin network is further reinforced by keratin aggregation and inter-filament disulfide bond formation, promoted by the protein filaggrin, whose precursor profilaggrin is found within the granules [2, 49]. Two other granular proteins, involucrin and loricrin, are involved in the formation of the cornified cellular envelope [50, 51]. The abrupt change from granulocytic phenotype to that of a terminally differentiated corneocyte is mediated within the outer layer of the stratum granulosum. These cells produce apoptotic enzymes that facilitate the destruction of the nucleus and all cellular organelles [52].
17.2.2.4 Stratum Lucidum
The stratum lucidum , found primarily in the thick skin of the palms and soles, consists of several layers of dead, clear, flattened keratinocytes that are enriched in keratin [24].
17.2.2.5 Stratum Corneum
The outermost layer of the epidermis, the stratum corneum , is composed of multiple rows of dead, terminally differentiated keratinocytes that are continually sloughed off and replaced. These cells are responsible for the majority of the barrier properties of the epidermis and, consequently, the skin. The thickness of the corneal layer varies from 15 to hundreds of layers, depending on the site within the body [2, 15]. Variations in thickness can also be correlated to differences in sex, age, and disease state [24, 53]. In general, the corneocytes are large, flattened, polyhedral cells that are interconnected via desmosomal junctions at overlapping boundaries. Over 80% of the cell content is composed of high-molecular mass keratins [17]. As in the other epidermal layers, changes in cell phenotype and function can be observed across the layer. The outer cell layer has flatter cells, which have more rigid, cornified cellular envelopes [51]. These exterior cells also secrete proteolytic enzymes that degrade the desmosomal junctions to allow for desquamation. Due to the presence of residual interconnections, corneal cells are typically shed in large groups [54].
17.2.3 The Dermis
The dermis is rich in ECM components that impart strength, elasticity, density, and compliance to the skin. Within this region, a vast network of fibrillar and amorphous connective tissue supports the dermal cellular components, vascular and neural networks, and the appendage structures [55, 56]. Interactions between the dermis and the epidermis via the basal laminar region are critical to maintaining the proper phenotype and functioning of both regions [14, 57, 58]. In order to satisfy the metabolic needs of both the dermis and the avascular epidermis, the dermis is highly vascularized. The intricate networks of dermal blood vessels also function in the homeostatic regulation of the body temperature [59]. Neural networks of sensory and autonomic fibers present in the dermis function in the control and communication systems of the skin [60]. Lymphatic channels facilitate the controlled removal of water and other molecules from the region and play an important immunogenic role [61]. Although the dermis contains fewer cells than the epidermis, fibroblasts, smooth muscle cells, pericytes, monocytes, macrophages, dermal dendrocytes, and mast cells can be found within this region [2].
As previously mentioned, dermal connective tissue is composed of both fibrous and non-fibrous components, which are continually undergoing turnover. Collagen is the most abundant protein in the dermis, accounting for approximately 75% of the dry weight of skin [2, 62]. The most common types of collagen found in skin are types I, III, and V, which can be classified as fibrillar collagens. More specifically, approximately 80–90% of the collagen in the dermis is of type I, whereas 8–12% is of type III. Collagen type V functions to regulate fiber diameter through interactions with types I and III. Fibril-associated collagen type VI is also found throughout the dermis, associated with type I collagen [63,64,65]. This network of collagen fibers imparts tensile strength to the skin. Elastic fibers , including elastin, form a network that extends throughout the dermis, spanning from the basal laminar region into the hypodermis. These fibers impart elasticity to the skin, allowing it to return to its original structure after experiencing distending forces [62, 66].
The non-fibrous connective tissue components of the dermis include proteoglycans (PGs), glycosaminoglycans (GAGs), and glycoproteins. These matrix components form the gel-like ground substance that surrounds the fibrous networks. PGs and GAGs found in the skin include biglycan, decorin, versican, perlecan, syndecan, heparan sulfate, and chondroitin-6 sulfate [2, 67,68,69]. Hyaluronan is also expressed in the embryonic dermis and during wound healing [70]. The ability of the PGs and GAGs to bind water molecules contributes to the regulation of water loss, as well as to the maintenance of the dermal volume and compressive properties [71, 72]. These molecules also function to bind cytokines and mediate cellular attachment to other matrix components [73]. Glycoproteins , including fibronectin, laminin, vitronectin, and tenascin, function in dermal cell-matrix and matrix-matrix interactions [2]. Overall, based in part on connective tissue structure, the dermis can be subdivided into two regions, the papillary dermis and the reticular dermis.
17.2.3.1 Papillary Dermis
The papillary dermis is the more superficial dermal region that is composed of small bundles of loose connective tissue, including collagen fibrils and immature elastic fibers [65]. This region is named for the papillae or projections of the dermis into the epidermal ridges. The matrix in this region, which hosts the majority of the dermal cells, is composed primarily of type III collagen [15]. The subpapillary plexus of arterioles and post-capillary venules serves as the lower separation boundary with the reticular dermis. The capillaries that supply the epidermis and upper dermal layers extend from this plexus into the papillary dermis [59]. Sensory and autonomic axons, as well as receptors , can also be found in this region [60].
17.2.3.2 Reticular Dermis
The lower and larger portion of the dermis, termed the reticular dermis , is composed of an interconnected collagen network, intertwined with a mature system of elastic fibers [55, 65, 66]. This region, which is connected to the papillary region by extended fibrous bundles, imparts the dermis with strength and resilience. Blood vessels, appendages, and dermal cells are interspersed throughout this connective tissue meshwork. The collagen bundles and elastic fibers increase in size in the deeper reticular layers approaching the hypodermis, which is distinguished from the dermis by the appearance of adipose tissue [2, 24].
17.2.4 The Dermal-Epidermal Junction Zone
The dermal-epidermal junction (DEJ) zone is characterized by a specialized basal lamina that is critical to the maintenance of proper skin structure and function [14]. This dynamic layer of interconnected proteins is approximately 100 nm thick and has been shown to have a critical role in skin remodeling, wound healing, and embryonic development. While the DEJ structurally separates the dermis from the epidermis, the proteins found within the basal laminar layer also mediate the attachment and interactions of these two regions [74]. For skin tissue engineering purposes, it is important to note that the DEJ zone maintains the overall structural integrity of the skin. Disorders affecting this region result in weak skin that is highly prone to shearing or tearing when exposed to applied forces [58]. The basal lamina also has critical functions in regulating keratinocyte cell polarity, proliferation, differentiation, and migration. Further, this matrix also influences the barrier properties of the skin and can restrict the movement of molecules based on size and charge [14].
Three different types of anchoring complexes stabilize and strengthen the DEJ zone: hemidesmosomes, anchoring filaments, and anchoring fibers. Using microscopy techniques, these structures can be observed in four distinct regions within the DEJ [75]. The first identifiable region consists of the hemidesmosomes in the plasma membranes of the basal keratinocytes. These cell-matrix attachment structures are supported intracellularly by bundles of intermediate keratin filaments [17]. The next two regions, the lamina lucida and the lamina densa, contain the anchoring filaments primarily comprised of linkages between hemidesmosomal integrin α6β4 and DEJ laminin type 5 [76,77,78]. The anchoring filaments firmly attach the basal cells to the underlying matrix, including the rich network of collagen IV fibers found in the lamina densa. The DEJ region adjacent to the dermis, termed the sublamina densa, contains collagen type VII anchoring fibrils [79]. These specialized complexes, originating in the lamina densa, firmly attach the DEJ to the dermis by either extending into anchoring plaques in the dermis or looping back into the densa region. The fibrils that form loops are interconnected with dermal collagen type I fibers, further stabilizing the structure [57, 58]. Overall, the DEJ maintains the integrity of the skin and regulates the dermal-epidermal interactions .
17.2.5 The Hypodermis
The hypodermis , also referred to as the subcutaneous layer, is located directly below the dermis. The primary component of this region is adipose tissue [2, 24]. Although a distinct border exists between the dermis and the hypodermis, the two regions are structurally and functionally interconnected through the rich vascular, neural, and lymphatic networks that supply the regions [59,60,61]. In addition, many of the appendage structures, including hair follicles and sweat glands, extend into the subcutaneous region [2]. The primary functions of the hypodermis include energy storage and release, insulation, mechanical protection, and the maintenance of body contours [80].
17.2.6 The Appendages
The epidermal appendages, including sweat glands, sebaceous glands, hair follicles, and nails, arise from protrusions of the epidermis into the dermis [24, 81]. Consequently, these structures are lined with epithelial cells, which, through growth and differentiation, are involved in the process of re-epithelialization following epidermal injury [82]. Moreover, the appendages have critical roles in the maintenance of homeostasis.
17.2.6.1 Sweat Glands
Sweat glands can be classified as either eccrine or apocrine, based on their structure and function. Eccrine sweat glands are composed of three parts: the coiled gland, the intradermal duct, and the intraepidermal portion. The coiled gland, located deep within the dermis, is lined with secretory cells that produce eccrine sweat. This sweat is delivered to the epidermal surface via the intradermal duct that extends through the dermal region, connecting to the intraepidermal portion and ultimately terminating as an open pore [83]. These glands primarily function in the regulation of body temperature, through cooling by sweat evaporation, but are also involved in the elimination of wastes. In general, eccrine sweat glands are found over the majority of the skin surface and are most concentrated on the palms of the hands and the soles of the feet [84]. Although similar in structure to eccrine glands, apocrine glands have coiled regions that are approximately ten times larger in diameter and intradermal ducts that terminate into the uppermost region of a hair follicle. Although the exact function of apocrine sweat glands remains unclear, it is believed that they function as scent glands related to sexual attraction. More specifically, these glands, which begin functioning during puberty, secrete small amounts of viscous sweat that becomes odorous when exposed to bacteria on the outer epidermis. Apocrine glands are most concentrated in the axillae and anogenital regions [24, 85].
17.2.6.2 Sebaceous Glands
Sebaceous glands are oil-producing glands that are found throughout the skin surface in varying sizes and densities, with the exception of the palms of the hands and the soles of the feet. In general, these glands are connected to hair follicles to form pilosebaceous units. A sebaceous gland consists of one or more branched lobules (acini) connected to a hair follicle by a short duct [13, 86]. The glands produce sebum, a substance composed of fats, cholesterol, proteins, and inorganic salts. Sebum protects the skin and hair, preventing these structures from becoming dry and brittle. This oily substance also aids in the prevention of excessive water loss due to evaporation from the skin surface [87].
17.2.6.3 Hair Follicles
While primarily vestigial in humans , the principal function of hair is to provide protection from injury and environmental conditions. Hair, consisting of a free shaft and a root structure, is supported in the skin by the hair follicle. Follicles extend at oblique angles into the deep dermis or hypodermis. The outermost layer of the follicle, the external root sheath, is composed of epithelium that is continuous with the epidermis. The inner root sheath surrounds the hair root, extending from the base of the follicle, termed the bulb, to the skin surface [88]. Progenitors in a region of the bulb referred to as the matrix proliferate to form the hair shaft in alternating periods of growth (anagen) and rest (telogen). Also located at the base of the bulb is the dermal papilla, an indentation of loose connective tissue, rich in vasculature, which supports the hair growth. Above the bulb, the follicular wall thickens to form the bulge, which contains stem cells believed to function in follicular regeneration and the replenishment of the matrix progenitors [43, 89, 90]. Smooth muscle bundles originating in the dermis, termed the arrector pylori, attach to the follicle at the bulge region and function in piloerection [88]. The duct of the sebaceous gland associated with the follicle opens up into the channel above this region, closer to the epidermis [86].
17.2.6.4 Nails
Nails are modified keratin-rich epidermal cells that protect and support the tips of the fingers and toes. While primarily serving esthetic purposes in humans, deterioration in the condition of nails can often be indicative of more general health problems [24]. Nails are composed of a nail root, a nail plate, and a free edge. Growth of nails occurs from the matrix, which primarily underlies the nail root. Proliferating epidermal cells in the matrix are pressed tightly together into layers to create a plate that is slowly forced outwards, thereby forming the nail [91, 92].
17.2.7 Functions of the Skin
The skin, with its unique structure, performs a multitude of functions critical to the maintenance of total body homeostasis [2, 15]. Some of the key functions of this vital organ are detailed in Table 17.1.
17.3 Development of the Integumentary System
In order to design a successful strategy for skin regeneration following burn injury, it is important to have an understanding of the events that occur during the embryological development of the integumentary system. Epidermal development commences during the 3rd week of gestation, shortly following the completion of germ layer formation by gastrulation. The single ectodermal layer surrounding the embryo is subdivided into the epidermal ectoderm , the neural ectoderm , and the neural crest , through a process termed neurulation [93]. Underlying the epidermal ectoderm is the loose mesenchymal tissue that will ultimately develop into the mature dermis [94]. It is important to note that the basal lamina of the DEJ, which is fully developed by the 9th week of gestation, plays a critical role in skin morphogenesis [14].
17.3.1 The Epidermis
The development of the epidermal layers commences during the 4th week, with the proliferation of the epidermal ectoderm to form a secondary layer of flattened cells, termed the periderm. Signaling with the underlying dermis is critical to the formation of the epidermal layers during all morphogenic phases. By the 11th week of gestation, an intermediate epidermal layer can be observed between the germinative cells in the basal layer and the peridermal cells. During this period, the epidermal ridges also begin to form. As the basal cells continue to proliferate and differentiate, the appearance of the mature epidermal barrier commences around the 20th week of development. After this point, many of the peridermal cells undergo apoptosis and become detached from the fetal epidermis [94,95,96].
Unlike the keratinocytes, which arise directly from the superficial ectoderm, many of the other cells found in the epidermis migrate in from other regions [95]. Melanoblasts, precursors of the melanocytes, are derived from the embryonic neural crest and first migrate into the developing dermis during the 6th week. Mature melanocytes can be first detected in the epidermal region during the 10th week of gestation [25, 95]. The origin of Merkel cells is also believed to be the migrating neural crest cell population [2]. Langerhans cells, which differentiate from precursors in the bone marrow, migrate into the epidermis and first appear around the 7th week of development [60].
17.3.2 The Dermis
In general, the dermis is derived from the mesodermal cells underlying the epidermal ectoderm [95]. During the early stages of development, the neodermis is composed primarily of interconnected cells surrounded by a loose matrix of hyaluronan and glycogen [97]. Differentiation of the mesenchymal progenitors into mature fibroblasts commences during the third month of gestation. As the newly formed fibroblasts synthesize ECM components, the dermal matrix becomes more fibrillar. Vascularization of the dermis also begins during this time period [24, 94,95,96].
17.3.3 The Appendages
The appendages develop from regions of the epidermis that proliferate and extend into the dermis to form columns of epithelial cells surrounded by mesenchymal tissue. It is believed that both autocrine and paracrine signaling with the underlying mesoderm are critical in the formation of these ectodermally derived projections, which first start to appear during the fourth month of gestation [24, 81, 95]. Ultimately, the development of each of the different types of appendages may be attributed to variations in the secretion and timing of inductive factors, which affect the proliferation, differentiation, and organization of the epithelial columns. Some factors believed to be important in follicular development include transforming growth factor-β (TGF-β), sonic hedgehog (SHH) protein, noggin, and members of the bone morphogenic protein (BMP) family [95].
17.4 Burns
17.4.1 Burn Classification
Burns are traumatic events that can severely disrupt the proper structure and functioning of the skin. Burns can be classified as either first degree, partial thickness, or full thickness based on the extent of the damage to the integumentary system (Fig. 17.3). Table 17.2 lists the general characteristics of each of these types of burns [98,99,100].
The classification of partial and full thickness burns based on the extent of the damage to the integumentary system. “C” represents the necrotic tissues in the zone of coagulation, and “*” represents the regional edema in the tissues underlying the burn injury. (Figure adapted with permission from www.burnsurgery.org)
17.4.2 Principles of Burn Wound Healing
Normal wound healing is characterized by three stages of healing: the inflammatory phase, the proliferative phase, and the remodeling phase [101]. One of the primary events in the inflammatory phase is the accumulation of platelets at the site of injury, leading to blood coagulation and clot formation, which provides a preliminary matrix for cellular infiltration. Inflammatory cytokines secreted by these aggregating cells regulate the regional blood flow and have a multitude of stimulatory effects, including the promotion of leukocyte invasion and angiogenesis. The second phase of wound healing, the proliferative phase, generally commences within 24 h. Rapid re-epithelialization to achieve wound closure is facilitated through the migration of epidermal cells from the wound edges and from the residual appendage structures in the injured region (Fig. 17.4) [101,102,103]. This process is stimulated by growth factors, including TGF-β and epidermal growth factor (EGF). Angiogenesis also occurs and is promoted by the secretion of FGF by the macrophages that are localized in the wound site [101, 104, 105]. A loose ECM network, deposited by the fibroblasts that have invaded from the surrounding tissues, encompasses the newly formed blood vessels. This provisional matrix , termed granulation tissue, provides a substrate for cell migration, facilitating cellular repopulation and wound closure. The final stage of wound healing, the remodeling phase, requires months to complete. In the initial period, the fibroblasts assume a myofibroblastic phenotype and, stimulated by TGF-β and other factors, cause the contraction of the wound, expediting closure [101, 104]. Ultimately, the dermal ECM is modified with newly synthesized matrix components, resulting in scar tissue formation [101,102,103].
The proliferative phase of the wound healing process in the skin. A preliminary fibrin matrix for cell migration is formed during the blood coagulation and clotting processes following the initial injury. Invading inflammatory cells secrete growth factors that promote angiogenesis and dermal fibroblasts deposit extracellular matrix components, forming granulation tissue. Re-epithelialization occurs through the migration of epidermal cells from the wound edges and from the residual epidermal appendage structures, such as hair follicles. (Figure reprinted with permission from Martin [103]. Copyright 1997 by the American Association for the Advancement of Science)
Severe burn wounds can be classified into three regions of varying tissue injury: the zone of coagulation, the zone of stasis, and the zone of hyperemia [100, 106]. The zone of coagulation is characterized by the complete dissolution of all levels of protein structure. Further, cell death within this region, which extends downwards from the original site of injury, is uniform and irreversible. Clinically, the zone of coagulation presents as the burn eschar [107]. Adjacent to the zone of coagulation is the zone of stasis, a region in which there is a progressive deterioration in the local blood flow. Although the majority of the cells within this zone are initially alive, many die due to ischemia. Numerous factors combine to cause the impaired blood flow, including clotting events, burn edema, vasoconstriction, and heat damage to the cells and proteins [100]. Without intervention, the tissues in this region will die, thereby expanding the zone of coagulation [69]. The zone of hyperemia is located at the periphery of the injury, adjacent to the unaffected body tissues. Within this region, there is minimal damage to the protein structures and cells. In general, complete healing of these tissues is possible. Due to local inflammatory responses and the release of numerous vasoactive molecules, increased vascular flow rates and the dilation of the local vessels characterize this zone [7, 100].
In the case of burn injuries, the process of wound healing can be dramatically modified or impaired. Skin damage can be extensive, with the complete destruction of all of the skin layers over a large surface area of the body. First degree or superficial partial thickness burns will generally heal normally, with re-epithelialization occurring from the wound edges and from the undamaged appendage structures. In contrast, in deep partial thickness or full thickness burns, the appendages are destroyed, and re-epithelialization can only occur from the wound periphery. The process of cell migration can be further delayed if the burn injury cauterizes the local blood vessels, inhibiting the initial cascade of events during the inflammatory healing phase that creates a temporary fibrin matrix for cellular invasion [82]. Hence, as the coverage process may require an extremely long time frame and the dermal regenerative potential is limited, severe wound contraction and scarring are frequent outcomes. Hypertrophic scarring and impaired functionality are very common in burn patients. In some cases, keloid scars, which extend beyond the original boundaries of the injury, can be observed. In general, contracted and scarred tissues can be painful, as well as having poor functionality and cosmesis [108,109,110]. Complications associated with burn wound edema can also impact the wound healing process. Increased vessel permeability and the action of numerous inflammatory cytokines, such as prostaglandins, can lead to severe swelling that impairs the regional blood flow, particularly in the zone of stasis, thereby causing ischemic cell death [111, 112].
17.4.3 Immune System Response to Burn Injury
Suppression of the immune system has long been recognized as a major contributing factor to the high mortality rates that are associated with burn injuries. Patients with severe burns are extremely susceptible to infections that can result in death due to septic shock or multiple organ failure [113, 114]. Clinical and research findings indicate that the impaired immune response is related to the suppression of both the cellular and humoral systems. More specifically, burn patients have been reported to have reduced lymphocyte populations, including decreased numbers of T cells, B cells, and natural killer cells [115,116,117]. In general, following burn injury, there is a failure in the T-cell response related to both diminished cell number and cell functionality [118]. Dysfunctions in the neutrophil cell population and the complement system further compound the immunosuppressed condition [119].
Numerous factors appear to contribute to the impairment of the immune system in burn patients. While the precise mechanisms of action are not fully understood, the systemic inflammatory response (SIR) associated with burn injury is hypothesized to play a role in the development of the immune dysfunction [111, 120]. Research continues to be conducted in order to elucidate all of the elements that are produced in this response, including cytokines and toxic by-products such as endotoxin, tumor necrosis factors (TNF), and leukotrienes, which could be immunosuppressive in nature [121]. One compound of interest, cutaneous burn toxin (CBT), is suspected to play a major role in the inhibition of granulocyte production, thereby accounting for the reduced lymphocyte populations. CBT is composed of polymers of lipids and proteins that are formed from the damaged tissues at the burn injury site [120]. Proper debridement of the wound can help to reduce the impact of this toxin. It is also important to note that the presence of necrotic tissue, impaired blood flow, and nutritional deficiency can all have an impact on general health and immune function [122].
17.4.4 Complications
While the skin is the focus of this chapter, the systemic effects of burn injuries must also be recognized. As previously mentioned, infection and SIR are critical problems in burn patients, frequently resulting in major organ failure and death. Following injury, the release of a multitude of inflammatory cytokines and toxic by-products can severely impair all of the organ systems, including the cardiac system, renal system, and digestive system [123]. Complications can also arise from abnormally low blood pressures due to hypovolemia, attributed to a loss of blood from the damaged vessels surrounding the wound site [112]. Another major cause of mortality is the severe respiratory damage and distress associated with inhalation injuries in burn patients [124].
17.5 Conventional Treatment of Burns
17.5.1 Treatment of Minor Burns
As previously mentioned , first degree and superficial partial thickness burns will spontaneously heal within a short time period, ranging from 7 to 21 days [82, 98]. Basic first aid treatment of the wound will help to expedite the healing process and prevent infection. The injured site should be gently and thoroughly cleaned with lukewarm water and mild soap. Cool, moist compresses can be applied to reduce pain and swelling. While it is the subject of some controversy, it is generally accepted that the blisters of superficial burns should be left intact, to reduce the chances of bacterial infection and minimize discomfort. Spontaneous resorption of the blisters should commence within a week. With the exception of injuries to the face, a clean, absorbent, non-adherent dressing, such as lint-free gauze, should be applied to cover the wound. This dressing should be changed daily or as required. The application of topical antibiotics or other agents is generally not recommended, as they can impede the wound healing process and have other negative side effects [125]. Burn wounds to the face are typically left uncovered, although a thin layer of topical antibiotic can be applied for prophylactic purposes and to prevent against discomfort due to tissue dryness [106]. To control the pain that is frequently associated with superficial burns, oral analgesics may be indicated [126].
17.5.2 Primary Treatment of Severe Burns
Following stabilization , patients with deep dermal and full thickness burns should be sent to the hospital for immediate assessment and care. The maintenance of airway, breathing, and circulation is of primary importance to patient survival. During transport, the burn wound should be covered with clean towels, which can easily be removed for examination purposes. The patient should be kept warm and treated for shock [127]. Intravenous (IV) infusion should be commenced as early as possible to maintain the circulating volume and reduce the risk of damage to the cardiac, renal, and other major organ systems [128]. Upon arrival at the hospital, a team of burn care specialists will assess the severity of the burn, examining the depth of the injury and estimating the percentage of the total body surface area (TBSA) affected [129]. Morphine, delivered via IV, is often prescribed for pain management purposes [126].
Immediate burn wound treatment is required to aid in healing and reduce the risk of patient mortality. The wound site should be washed with warm saline solution and debrided to remove dead epithelial tissue and foreign contaminants. In general, as the seepage of blister fluid can provide an ideal medium for bacterial growth, blisters are evacuated and trimmed. This process also improves joint mobility and aids in the visual assessment of the burn [106]. In cases of circumferential burns, such as around the neck, torso, or limbs, a decompression escharotomy may be required to relieve the tension in the tissues. More specifically, as burn edema progresses, the inelastic eschar at the surface does not expand well, causing increased pressures that can result in ischemic conditions, impaired respiration, or tissue rupture. These situations can be prevented by the escharotomy, which involves a strategic, longitudinal incision along the length of the affected tissues [130]. Following debridement, topical antimicrobial agents, such as silver sulfadiazine, may be applied to the surface of the wound to slow or reduce the occurrence of bacterial infection [131]. As the blood supply to the burn wound is often severely impaired, the use of systemic antibiotics may be therapeutically ineffective [132]. In conservative approaches to burn treatment, an occlusive dressing or other wound covering, discussed later in the chapter, is then placed over the affected tissues prior to further treatment, such as autografting, if required.
Through advances in burn research, it is now accepted that the early surgical removal of the burned tissues is of benefit to the patient, in terms of pain management, immunocompetence, and wound healing. Excision of the necrotic tissues has been shown to reduce the total time of hospitalization, the chances of infection, the amount of surgical intervention required, and the instances of patient mortality. Improved functionality and cosmesis following wound healing have also been reported [8]. By limiting the systemic exposure to the inflammatory cytokines and toxic byproducts found in the necrotic tissues, it is possible to minimize immunosuppression and reduce the risk of major organ failure [122]. In general, the procedure involves the excision of all of the necrotic tissues, followed by the immediate application of either an autograft or a temporary wound covering. The operation is performed by tangential excision using a specialized dermatome. The necrotic tissue is removed in layers until only viable tissue remains, detected when bleeding is observed [8, 133]. Full thickness burns can be excised to either the hypodermis or the muscle fascia. While autograft acceptance is more limited on the subcutaneous adipose tissues, when successful, a superior esthetic result is achieved with this more conservative approach [8]. Overall, the major restriction to the surgical excision of burn wounds is the potential for severe blood loss, which limits the amount of tissue that can be removed at one time [9].
17.5.3 Autografting: The Current Gold Standard
The use of autologous grafted skin represents the gold standard treatment for deep dermal and full thickness burns. To date, this strategy is the only method available to achieve complete and permanent wound closure in extensive or deep burns [1, 8, 104]. Partial thickness grafts, ranging in thickness from 0.2 to 0.5 mm, are harvested from healthy donor sites using a dermatome [9]. Ideally, the mechanical properties, dimensions, and pigmentation of the skin in the donor and recipient regions are similar. As only a small portion of the dermis is extracted, the donor site defect will heal spontaneously without scar tissue formation in 2–3 weeks [107]. The autografted tissue is surgically attached to a freshly excised, uncontaminated wound site. As it is extremely thin, the graft can survive the initial ischemic period prior to vascularization via diffusion of nutrients and waste. Following the surgery, the bond between the grafted skin and the wound bed is extremely weak and must be protected from disruption due to shearing, edema, or hematoma. As healing progresses, the graft fully integrates into the wound site, inhibiting contraction and restoring functionality. In most cases, graft acceptance is high, and scar tissue formation and contraction are reduced, achieving an acceptable esthetic result [8]. However, the deep dermal hair follicles and sweat and sebaceous glands do not regenerate in the transplanted tissue. Reinnervation and limited restoration of skin sensation are possible [11]. Disadvantages of the use of autologous tissues include the creation of a donor site defect, patient risk, susceptibility to infection, and the need for multiple surgical procedures [8].
The development of new methods of permanent wound closure is mandated for the treatment of patients with severe burns involving a large surface area of the body. During a single surgical procedure, sufficient autologous tissue can often be harvested to treat burns ranging from 30% to 50% TBSA. In more extensive burns, the use of autografting is limited by a lack of donor tissue [4]. Treatment in these cases may be facilitated by a modification to the autografting technique involving the use of a tissue mesher [134]. Small linear incisions are made in the harvested tissue to allow for the expansion of the graft. In practice, the meshed skin can be expanded 1.5–9 times its original size. When expanded more than three times, it is common to cover the site initially with a meshed allograft to improve healing and provide protection during re-epithelialization [9]. While meshing allows for improved drainage and greater coverage, this skin is more prone to infection and has esthetic limitations. Due to contraction and scar tissue formation, the meshing pattern on the skin surface is permanent. Further, tissue availability is still limited using this technique. As medical knowledge and technology continue to improve, an increased number of patients are surviving from extremely large surface area burns (>90% TBSA). In these cases, autograft availability is minimal and generally insufficient. Following the completion of healing, it is possible to re-harvest the donor and previously grafted sites. This process can be repeated a limited number of times and increases patient pain and susceptibility to infection, as well as treatment time and expense [8, 9]. Temporary wound coverage materials must be utilized during the interim healing period. Ultimately, alternative means to autografting may be required to achieve complete wound closure, and severe scarring may be inevitable [135].
17.5.4 Biological Alternatives for Temporary Wound Coverage
As previously discussed, due to the limitations in the availability of autologous tissues, substitute materials to provide temporary wound coverage following early excision are required. Conventional biological approaches to achieve this goal involve the use of allografts and xenografts. However, in order to achieve permanent wound closure and healing without severe scarring and contracture, these natural materials must ultimately be replaced with autologous tissues [12].
17.5.4.1 Allografts
To date, allogenic strategies have focused on the use of cadaveric skin and amnion.
17.5.4.1.1 Cadaver Skin
Despite numerous limitations , allogenic cadaver skin remains the most successful and commonly used substitute for temporary wound coverage in severely burned patients [7]. Allogenic skin aids in the restoration of the barrier properties, thereby protecting against excessive water and electrolyte loss, infection, and pain. Moreover, allografts have been shown to initially reduce any existing bacterial contamination, stimulate re-epithelialization from the periphery, improve the ultimate esthetic appearance of the patient, and increase autograft acceptance [136]. However, in spite of the immunosuppressive nature of burn injuries, graft rejection within 1–3 weeks is inevitable. During this process, the wound site can become severely inflamed and prone to infection [137]. There are also concerns associated with the transmission of viruses, cost, and tissue availability [9]. The tissues can be utilized while fresh, preserved in glycerol at 4 °C, lyophilized, or cryopreserved. Fresh tissues have been shown to more strongly stimulate angiogenesis, have superior adherence properties, and provide greater protection against bacterial invasion. However, glycerol preservation is relatively economical, decreases the tissue antigenicity, and facilitates the establishment of tissue banking [138, 139].
17.5.4.1.2 Amnion
The use of fresh human amniotic membranes has been reported for decades and remains popular in regions of the world where the use of cadaveric skin is limited due to expense and availability [140]. Human amnion has low antigenicity and is reported to readily adhere to the wound site, reducing patient pain, accelerating the wound healing process, and repressing bacterial growth. In addition, amnion has been shown to stimulate angiogenesis, ultimately improving autograft acceptance [141]. Despite these positive attributes, the use of amnion is limited by the fragile nature of the tissues. Within several days of application, the amnion becomes fragmented and dissolves, necessitating almost daily replacement. Hence, the quantities of tissue required for large TBSA burns would be exceedingly high. Further, an occlusive dressing is required to prevent desiccation of the membranes and maintain the skin barrier properties [7, 142]. However, human amnion may be of use to expedite wound healing in superficial partial thickness burns, autologous donor sites, or highly meshed autologous tissues [143].
17.5.4.2 Xenografts
The primary motivation for the use of xenogenic tissue is the limited supply and high costs associated with allogenic cadaver skin [9]. Frozen porcine grafts are most commonly investigated due to the similarity of the tissues with the human skin [144]. Despite the potential for unlimited availability, numerous concerns restrict the use of xenografts in burn wound treatment. The primary problems are associated with the antigenic nature of the tissues. All grafts are rejected within a 14-day period [145]. There are also serious safety and sterility issues related to animal-to-human viral transmission [146]. Furthermore, xenografts have been shown to be less adherent than allografts and are associated with higher rates of infection and necrosis [136].
17.6 Burn Dressing Biomaterials and Tissue Engineering
Through the creation of unique biological substitutes, tissue engineering holds great promise for the development of novel treatment strategies for numerous diseases, disorders, and traumas. Unlike conventional therapies, through tissue engineering investigators seek to restore or regenerate the affected regions of the body, with the ultimate goal of creating functioning, healthy tissues that are fully integrated into the host system [147, 148].
To date, significant research has been conducted into the fields of burn dressing biomaterials and tissue engineering. In general, the two design strategies for skin substitutes are to create materials for either temporary wound coverage or permanent wound closure [10]. Wound coverage materials have been widely studied in both the research and clinical settings due to their greater simplicity in design. The principle behind these materials is to create an interim barrier until sufficient autologous tissues are available for grafting. Wound coverage materials have been shown to alleviate patient pain, reduce the likelihood of infection, and promote wound healing [135, 149]. In contrast, the objective of wound closure strategies is to design a material that will become incorporated into the wound site, promoting healing and restoring skin functionality without tissue autografting [135]. Such a material would be of great benefit to patients with limited autograft availability and could also eliminate the creation of donor site defects and the need for multiple surgical procedures.
17.6.1 Design Criteria
There are numerous criteria for the development of a successful skin substitute. Ideally, the construct should minimize patient pain and promote wound healing without scar tissue formation or contracture. The complete structural and functional restoration of the integumentary system and all of its components are long-term objectives. Some specific design considerations include adherence, barrier properties, mechanical properties, biodegradability and immune response, surgical handleability, and expense [107, 150,151,152].
17.6.1.1 Adherence
To reduce the occurrence of infection and minimize fluid accumulation at the tissue interface, it is extremely important for the skin substitute to readily adhere to the wound site [153]. The rapid and uniform attachment of the construct to the wound bed attenuates patient pain and creates a hypoxic environment that stimulates wound healing. Hydrophilic materials that adhere to the wound site without inflicting damage on the underlying tissues are of particular interest [107].
17.6.1.2 Barrier Properties
Wound coverage or closure materials should restore the barrier functions of the integumentary system. The construct should protect the fragile tissues at the wound site, inhibit bacterial infection, and prevent desiccation [136]. The flux of water across the material must be controlled, to prevent excessive water and electrolyte loss, while also limiting fluid accumulation at the wound site [154]. Protein loss should also be restricted, and normal rates of heat conduction should be observed across the barrier [136].
17.6.1.3 Mechanical Properties
Ideally the substitute should have mechanical properties similar to those of healthy human skin. The use of components that are too rigid could cause damage to the surrounding tissues, resulting in inflammation and subsequent device failure [155, 156]. Skin equivalents must be pliable, flexible, elastic, and durable. It is important to have a strong material, resistant to applied forces, which will conform and adhere to the wound site [7]. The material porosity is also an important design consideration. As previously mentioned, the construct must provide the barrier properties of the skin, including the controlled flux of water. However, a porous material is desirable to enable cellular infiltration and repopulation of the wound site from the surrounding tissues. To facilitate cell migration, a minimum pore size of 10 μm is required, and the material must be cell adhesive [107].
17.6.1.4 Biodegradability and Immune Response
The skin substitute should be both nontoxic and non-antigenic in nature, evoking a minimal immune response [157]. Moreover, the device should stimulate wound healing and host cell invasion from the periphery. As healing progresses, the controlled degradation and replacement of the construct with healthy host skin would be ideal [152]. This regeneration would eliminate the long-term problems associated with the restriction of growth at the wound site, a severe concern for burned children, allowing the healed regions to expand normally with the individual [135]. The products of the degradation process must also be compatible with the host system.
17.6.1.5 Surgical Handleability
To be a clinically viable alternative, the skin equivalent must be able to be sterilized, cut into a variety of shapes, and sutured into position with relative ease by the surgeons in the operating room [107].
17.6.1.6 Expense
An ideal skin substitute should be relatively inexpensive and widely available for use [152]. The materials should not be limited, and the fabrication process should be straightforward and reproducible. The construct should have long-term stability so that it can be preserved and stored for an extended time period [157].
17.6.2 Skin Substitutes
A wide variety of skin substitutes have been investigated in the laboratory and clinical settings to promote regeneration, rather than repair, following burn injury. The constructs can be classified as epidermal substitutes, dermal substitutes, or composite materials that include an epidermal and a dermal component [1].
In each of the strategies, both synthetic and natural materials have been studied. Synthetic materials can be selected that are readily available, uniform, sterilizable, biodegradable, and cost-effective. Moreover, modulating synthesis conditions can often control the physical and chemical properties of these materials [158,159,160]. Natural constructs have been shown to promote cell infiltration and host integration, reduce scar tissue formation, and, depending on processing, trigger minimal immune response. Scaffolds derived from the ECM can degrade within the body and promote matrix remodeling, thereby augmenting the regeneration of damaged or missing tissues [161, 162].
The incorporation of allogenic cells is a design feature in many skin substitutes. Cultured allogenic keratinocytes and fibroblasts derived from neonatal foreskins initiate a minimal immune response in the immunosuppressed burn patients [104]. Unlike the Langerhans cells present in the allografted tissues, these cells do not express MHC class II antigens. As a result, the production of allogenic T cells is not stimulated via this means, although the secretion of cytokines by the cells, such as α-interferon, can cause a more limited reaction [10]. Overall, due to the immunological properties of the cultured keratinocytes and fibroblasts, these cells can be included in the constructs with low concern for major immune response or sensitization.
17.6.2.1 Epidermal Substitutes
Various epidermal substitutes are available that can be used for temporary wound coverage, restoring the barrier properties of the skin. When applied to superficial partial thickness burns or autologous donor sites, these constructs can often expedite the wound healing process [135]. In deep dermal or full thickness burns, it is generally recognized that the reconstruction of the underlying dermis is required prior to the replacement of the epidermis, in order to achieve acceptable functional and cosmetic results [1].
17.6.2.1.1 Occlusive and Semi-occlusive Dressings
Many different occlusive and semi-occlusive dressings have been developed and marketed for the temporary wound coverage of superficial burns and donor sites. Although a wide variety of materials have been investigated, all of these constructs have a similar function. Occlusive dressings create a moist, hypoxic environment at the burn site. This has been shown to reduce patient pain and promote wound healing [163,164,165]. Ideally, these constructs should restore some of the barrier properties of the skin, regulating the flux of water and preventing against infection [166]. However, many of the substitutes are associated with high rates of bacterial proliferation in the moist wound bed, resulting in deep dermal damage and tissue necrosis. Further, the accumulation of fluids below the occlusive dressings can injure the surrounding tissues and lead to device separation. Other concerns are related to poor adherence and the inability to observe the wound site during the healing process [167,168,169]. Table 17.3 outlines some of the specific advantages and disadvantages of some common occlusive and semi-occlusive dressings .
17.6.2.1.2 Cultured Autologous Keratinocytes
Techniques have been developed to facilitate the expansion of autologous keratinocytes from small biopsy samples. Keratinocyte sheets can be grown in vitro on feeder cell populations of irradiated murine 3T3 fibroblasts, supplemented with growth factors [189]. This process is expensive, labor intensive, and time-consuming. The 3–5 weeks required to produce 1.8 m2 of confluent keratinocytes from a 2 cm2 biopsy sample limits the clinical applicability of this strategy [10]. Genzyme Corporation specializes in the growth of autologous keratinocytes, marketed under the trade name Epicel™ [190]. Using the enzyme dispase, the undifferentiated monolayer of cells can be removed as a sheet from the tissue culture dish. This separation process can impair the subsequent proliferation of the keratinocytes [191]. The fragile sheet can be transplanted onto the wound bed but is susceptible to damage and desiccation. Acceptance of the autologous cells is dependent on the condition of the wound bed, with a partially intact dermal layer required to avoid complete sloughing. If the grafting is successful, the resulting epidermis is unstable and has limited barrier properties [1]. Within 6 days of transplantation, the cells begin to differentiate into the normal epidermal layers. However, the absence of the DEJ can lead to blister formation and separation. With extended time, the basal lamina characteristic of the DEJ can begin to form [10]. The skin grown from these cultured cells will be incomplete, lacking a full cellular complement and all appendage structures [132].
17.6.2.1.3 Cultured Allogenic Keratinocytes
Allogenic keratinocytes have been investigated as a possible means of avoiding the culturing time delay associated with the use of autologous cells [10]. As previously discussed, the immunologic nature of the allogenic keratinocytes limits the concerns associated with host immune response [104]. The sheets could be pre-grown, cryopreserved, and stored at the hospital for immediate use. Similar to the autologous sheets, the acceptance of the allogenic cells is dependent on the state of the wound bed. Further, there are also limitations associated with the fragility of the sheets and the lack of a DEJ [1, 10]. Clinically, improved healing has been shown in superficial burns and donor sites, attributed to the cellular secretion of various stimulatory cytokines and growth factors [192]. With time, host cells can replace the donor keratinocytes in the grafted region [193].
17.6.2.1.4 Laserskin™
Laserskin™ is an epidermal substitute developed by Fidia Advanced Biopolymers that is composed of hyaluronan and autologous keratinocytes [194]. Hyaluronan has been shown to promote cellular migration, proliferation, and the formation of new blood vessels [195]. The cells are seeded in a series of small pores that are laser drilled in the hyaluronan gel (Fig. 17.5a). This device has the advantage of being able to be removed from the culture dish without the need for enzymatic digestion [10]. An intact dermal layer may be required to achieve acceptable functional and cosmetic results. Initial clinical investigations in patients with diabetic ulcers show that the construct is durable and well-accepted, with low rates of infection [161, 196].
Examples of tissue-engineered skin substitutes . (a) The epidermal substitute Laserskin™, developed by Fidia Advanced Biopolymers, is composed of autologous keratinocytes seeded in pores drilled by a laser into a hyaluronan gel. (Figure reprinted with permission from Fidia Advanced Biopolymers s.r.l.). (b) The Integra™ composite skin substitute composed of glutaraldehyde cross-linked bovine collagen type I and chondroitin 6-sulfate, covered with silicone. (Non-copyrighted source: US Food and Drug Administration)
17.6.2.2 Dermal Substitutes
The creation of a dermal substitute for the treatment of deep dermal and full thickness burns is mandated by the numerous limitations associated with the use of autologous tissues. Epidermal substitutes are contraindicated in severely burned patients due to the problems associated with skin fragility, blistering, and poor graft integration [132]. In humans, damaged dermal tissue can result in severe scar tissue formation and contracture if left untreated [104]. An engineered dermal substitute has the potential to be used in combination with cultured keratinocytes or extremely thin autografts to reconstruct the injured skin [1, 135].
17.6.2.2.1 Dermagraft™
Dermagraft™ is a dermal substitute , developed by Advanced Tissue Sciences Incorporated and now distributed by Advanced BioHealing Inc., which is composed of viable neonatal allogenic fibroblasts seeded onto a polyglactin (Vicryl) scaffold. The cells, which are expanded in vitro to confluence, secrete numerous growth factors and ECM components into the mesh [197]. A sufficient number of fibroblasts can be extracted from one foreskin to seed over 20,000 m2 of the construct [198]. Following fibroblastic cultural expansion, the device can be cryopreserved without severely impairing the cellular viability. Trials indicate that the construct promotes wound healing, angiogenesis, and re-epithelialization from the wound periphery [199]. In one specific study, it was well-tolerated as an implant, with no signs of immunological rejection observed in 400 chronic wound patients [198]. The polymeric scaffold will degrade by hydrolysis as regeneration progresses, although the rate of degradation may cause complications. As the device does not include an epidermal component, wound closure is not facilitated. However, the construct can be used to improve functionality and cosmesis in combination with meshed autografts [200, 201]. While the potential exists to seed the dermal substitute with cultured autologous keratinocytes, preliminary trials suggested that cell adhesion may be a problem, with a mean acceptance rate of only 51.2% [202].
17.6.2.2.2 AlloDerm™
AlloDerm™ is a dermal substitute developed by LifeCell that is composed of acellularized human cadaveric skin [203]. Extraction of all cellular components while preserving the dermal matrix structure, including the basal lamina of the DEJ, eliminates the immunological complications associated with the use of allogenic skin but promotes proper tissue organization during regeneration [1, 204]. Studies have indicated that AlloDerm™ readily adheres to the wound bed, promoting the invasion of cells and blood vessels and fully integrating with the host system. As it has very limited barrier properties, the scaffold must be grafted with an ultrathin autograft when it is implanted. Overall, the construct has been reported to reduce scarring and improve skin elasticity and cosmesis [205]. Due to the limited availability of allogenic tissues, acellularized xenogenic skin has also been investigated [161].
17.6.2.3 Composite Substitutes
Composite skin substitutes hold great potential for the treatment of patients with severe burns. With further research, these constructs may be able to replace all of the skin layers, eliminating the need for tissue autografting. One important design note is that it is imperative to reconstruct the basal lamina of the DEJ zone in order to create a strong and well-integrated material [10].
17.6.2.3.1 Integra™
Integra LifeSciences Corporation markets the most generally accepted composite skin substitute, originally described by Yannas and Burke (Fig. 17.5b) [206, 207]. This device has a dermal component of glutaraldehyde cross-linked bovine collagen type I and chondroitin 6-sulfate, covered with a silicone artificial epidermis [208]. When implanted, the collagen and GAG matrix, with an average pore size of 50 μm, encourage cellular invasion and fibrovascular regeneration to form a normally organized neodermis within 3–6 weeks [10, 107]. The original scaffold gradually degrades over a 30-day period [161]. The silicone membrane has good surgical handleability and regulates the flux of water at the normal regional rates observed in the skin [1]. When implanted, the average acceptance rate (AAR) is generally over 80%, lower than the 95% AAR for autografts, but comparable to that observed initially in allografts. Moreover, the substitute is well-tolerated by the immune system [135]. Within 3–4 weeks, the silastic layer can be removed, and an ultrathin (less than 0.1 mm) autograft can be placed directly on the developing dermis, with an AAR of 90% [202]. The creation of smaller donor site defects expedites and improves healing. Clinical results show that the use of Integra™ promotes wound healing, reduces contracture, and prevents hypertrophic scar formation. Superior cosmetic results are reported to the use of autografting alone, along with good joint functionality and the ability to grow in children [209]. The major disadvantages of the construct are the need for a second surgical procedure, the potential for infection, and expense [10]. In 1996, Integra™ was approved for the treatment of deep dermal and full thickness thermal burns by the FDA [152]. Boyce and Hansbrough have reported a modification to this construct, where the collagen and GAG matrix are seeded with fibroblasts and the silicon layer is replaced by cultured keratinocytes. Experimental results show that the keratinocytes fully differentiate and a complete DEJ forms in vivo [210, 211].
17.6.2.3.2 Apligraf™
Apligraf™, formerly known as Graftskin™, is manufactured by Organogenesis Incorporated. To prepare the construct, a gel of bovine collagen type I is seeded with allogenic fibroblasts [10]. Following 6 days in culture, allogenic keratinocytes are then applied and grown at the air-media interface for approximately 14 days. This process results in epidermal differentiation, including the formation of an immature stratum corneum that improves the barrier properties of the device [104]. The construct, which is FDA approved for the treatment of chronic wounds, has been shown to have good clinical results, including no signs of immunological rejection and the in vivo formation of an intact DEJ [212]. In experimental studies, when used to temporarily cover meshed autograft, Apligraf™ improves wound healing and pigmentation and reduces scar tissue formation [213]. However, this expensive product is time-consuming to manufacture and has a limited shelf life [104].
17.6.2.3.3 Biobrane™
Biobrane™, marketed by Smith & Nephew Inc., is composed of a nylon mesh that is coated in porcine collagen type I and attached to a thin silicone membrane (Fig. 17.6). This non-degradable device is recommended to expedite healing in superficial partial thickness burns and at autologous donor sites [10, 107, 214]. The outer silastic layer regulates the flux of water and prevents against bacterial contamination. As healing progresses, the device slowly separates from the skin surface [10]. It is imperative that the wound site be freshly excised and free of infection prior to the placement of the construct [215]. When used appropriately, the healing period can be reduced by up to 46% [10].
The application of a Biobrane™ graft to a debrided wound bed following a partial thickness burn injury to promote wound healing. (Figure adapted with permission from www.burnsurgery.org)
17.6.2.3.4 Transcyte™
Transcyte™, formerly known as Dermagraft-Transitional Covering™, is marketed by Smith & Nephew Inc. The device has the same basic construction as Biobrane™ but is seeded with neonatal fibroblasts. During a 17-day culturing period, the fibroblasts synthesize and secrete various growth factors and ECM components that accumulate in the mesh. The construct is then frozen and stored, killing the cells but preserving the secreted factors [10]. While this material is extremely expensive, preliminary studies indicate that it has good adhesion, promotes healing, and can remain intact for up to 6 weeks [216, 217].
17.6.3 Growth Factor Incorporation
The incorporation of cytokines or growth factors into the skin substitutes could promote regeneration and expedite healing [218,219,220]. As many interacting mediators are involved in the wound healing process (Table 17.4), research must be conducted in order to determine the optimal combination and concentrations of these factors. Additionally, it is necessary to develop a method for the sustained delivery of the factors to the wound site for the desired period. Localized application may reduce the likelihood of negative side effects associated with systemic exposure. Binding the mediators to the constructs is a potential design strategy [221]. However, researchers must show that bound stimulatory molecules remain bioactive but do not overstimulate the cells, triggering cancerous growth .
17.6.4 Epidermal Stem Cells
As previously discussed, epidermal stem cells are found in the basal epidermal layer and the bulge region of the hair follicle. These undifferentiated cells proliferate rapidly, are capable of differentiation, and have a high capacity for self-renewal [41,42,43]. The development of practical methods to isolate and culture these cells holds great promise for tissue engineering applications. Using the stem cells, it may be possible to grow autologous epidermal tissue for transplantation in a clinically acceptable time frame [45]. Potential epidermal stem cell markers include β1 integrin and keratin 19 [43].
17.7 Future Outlook
While significant progress has been made in the development of burn dressing biomaterials and tissue-engineered constructs, the complete regeneration of fully functional skin following burn injuries is currently unfeasible. Despite the disadvantages associated with autografting, the use of harvested host tissues remains the best treatment method for permanent wound closure in deep dermal and full thickness burns [1, 8, 104]. Continued research must be conducted to design new constructs or to improve the existing biomaterials in order to promote regeneration rather than repair in severe burns. A more thorough understanding of the events in integumentary organogenesis and wound healing could aid in this process. Further, constructs which more closely mimic the natural complexity of the skin should also be studied. The inclusion of appendage structures in the skin substitutes could greatly improve the device functionality. Incorporating the full epidermal and dermal cellular complement could also be of significant benefit. Seeding tissue-engineered constructs with melanocytes has been shown to improve skin pigmentation and cosmesis [236]. The addition of autologous Langerhans cells could potentially alleviate some of the problems associated with infection leading to tissue necrosis and device failure. As the number of patients who survive with severe burns over large TBSA increases, the need for an economical, effective, off-the-shelf material for permanent wound closure continues to motivate researchers in this field.
References
Balasubramani M, Kumar TR, Babu M. Skin substitutes: a review. Burns. 2001;27:534–44.
Haake A, Scott GA, Holbrook KA. Structure and function of the skin: overview of the epidermis and dermis. In: Freinkel RK, Woodley DT, editors. The biology of the skin. New York: The Parthenon Publishing Group Inc.; 2001. p. 19–45.
Pruitt BA, Mason AD. Epidemiological, demographic and outcome characteristics of burn injury. In: Herndon DN, editor. Total burn care. London: W.B. Saunders Company Ltd; 1996. p. 5–15.
Sheridan RL. Burns. Criti Care Med. 2002;30S:S500–14.
Sjoberg F, et al. Utility of an intervention scoring system in documenting effects of changes in burn treatment. Burns. 2000;26:553–9.
Nguyen TT, et al. Current treatment of severely burned patients. Ann Surg. 1996;223:14–25.
Quinn KJ, et al. Principles of burn dressings. Biomaterials. 1985;6:369–77.
Muller MJ, et al. Modern treatment of a burn wound. In: Herndon DN, editor. Total burn care. London: W.B. Saunders Company Ltd.; 1996. p. 136–47.
Berthod F, Rouabhia M. Exhaustive review of clinical alternatives for damaged skin replacement. In: Rouabhia M, editor. Skin substitute production by tissue engineering. New York: Chapman and Hall; 1997. p. 23–45.
Jones I, Currie L, Martin R. A guide to biological skin substitutes. Br J Plast Surg. 2002;55:185–93.
Boyce ST, Warden GD. Principles and practices for treatment of cutaneous wounds with cultured skin substitutes. Am J Surg. 2002;183:445–56.
Brown AS, Barot LR. Biologic dressings and skin substitutes. Clin Plast Surg. 1986;13:69–74.
Tortora GT, Anagnostakos NP. Principles of anatomy and physiology. 2nd ed. New York: Harper & Row Publishers; 1975. p. 104–14.
Burgeson RE, Christiano AM. The dermal-epidermal junction. Curr Opin Cell Biol. 1997;9:651–8.
Rouabhia M. Structural and functional complexity of the skin. In: Rouabhia M, editor. Skin substitute production by tissue engineering. Austin: R.G. Landes Company; 1997. p. 3–22.
Gupta S, Sauder DN. The skin as an immune organ: the role of keratinocyte cytokines. Univ Toronto Med J. 1995;72:118–23.
Smack DP, Korge BP, James WD. Keratin and keratinization. JAm Acad Dermatol. 1994;30:85–102.
Latkowski JA, Freedberg IM. Epidermal cell kinetics, epidermal differentiation and keratinization. In: Freedberg IM, et al., editors. Fitzpatrick’s dermatology in general medicine. New York: McGraw-Hill; 1999. p. 133–43.
Steinert PM. The two-chain coiled-coil molecule of native epidermal keratin intermediate filaments is a type I-type II heterodimer. J Biol Chem. 1990;265:8766–74.
Moll R, et al. The catalog of human cytokeratins: patterns of expression in normal epithelia, tumors and cultured cells. Cell. 1982;31:11–24.
Sun TT. Classification, expression and possible mechanisms of evolution of mammalian epithelial keratins: a unifying model. Cancer Cell. 1984;1:169–76.
Valyi-Nagy IT, et al. Undifferentiated keratinocytes control growth, morphology and antigen expression of normal melanocytes through cell-cell contact. Lab Invest. 1993;69:152–9.
Gordon PR, Mansur CP, Gilchrest BA. Regulation of human melanocyte growth, dendricity, and melanization by keratinocyte derived factors. J Invest Dermatol. 1989;92:565–72.
Martini FH, Timmons MJ, Mackinley MP. Human anatomy. New Jersey: Prentice-Hall Inc.; 2000. p. 88–107.
Nordlund JJ, Boissy RE. The biology of melanocytes. In: Freinkel RK, Woodley DT, editors. The biology of skin. The Parthenon Publishing Group: New York; 2001. p. 113–31.
Yamamoto O, Bhawan J. Three modes of melanosome transfers in Caucasian facial skin: hypothesis based on an ultrastructural study. Pigment Cell Res. 1994;7:158–69.
Boissy RE, Nordlund JJ. Biology of melanocytes. In: Arndt KA, et al., editors. Cutaneous medicine and surgery. Philadelphia: W.B. Saunders Company Ltd.; 1996. p. 1203–18.
Post PW, Rao DC. Genetic and environmental determinants of skin colour. Am J Phys Anthropol. 1977;47:399–402.
Tachibana T. The Merkel cell: recent findings and unresolved problems. Arch Histolo Cytol. 1995;58:379–96.
Fantini F, Johansson O. Neurochemical markers in human cutaneous Merkel cells. Exp Dermatol. 1995;4:365–71.
Narisawa Y, et al. Biological significance of dermal Merkel cells in the development of cutaneous nerves in human fetal skin. J Histochem Cytochem. 1992;40:65–71.
Sauder DN, Pastore S. Cytokines in contact dermatitis. Am J Contact Dermat. 1993;4:215–24.
Sauder DN. The skin as an immunologic organ. In: Soter NA, editor. Pathophysiology of dermatologic diseases. New York: McGraw-Hill Inc.; 1991. p. 101–10.
Bos JD, Kapsenberg ML. The skin immune system. Immunol Today. 1986;7:235–40.
Barratt-Boys SM, Watkins SC, Finn OJ. In vivo migration of dendritic cells differentiated in vitro. J Immunol. 1997;158:4543–7.
Romani N, Lenz A, Glassel H. Cultured human Langerhans cells resemble lymphoid dendritic cells in phenotype and function. J Invest Dermatol. 1989;93:600–9.
Groux H, Fournier N, Cottrez F. Role of dendritic cells in the generation of regulatory T cells. Semin Immunol. 2004;16:99–106.
Jones ND, et al. Differential susceptibility of heart, skin and islet allografts to T-cell mediated rejection. J Immunol. 2001;166:2824–30.
Erdag G, Sheridan RL. Fibroblasts improve performance of cultured composite skin substitutes on athymic mice. Burns. 2004;30:322–8.
Hornebeck W. Down-regulation of tissue inhibitor matrix metalloprotease-1 (TIMP-1) in aged human skin contributes to matrix degradation and impaired cell growth and survival. Pathol Biol. 2003;51:569–73.
Lavker RM, Sun T. Heterogeneity in epidermal basal keratinocytes: morphological and functional correlations. Science. 1982;15:1239–41.
Lavker RM, Sun TT. Epidermal stem cells. J Invest Dermatol. 1983;81:121S–7S.
Janes SM, Lowell S, Hutter C. Epidermal stem cells. J Pathol. 2002;197:479–91.
Barrandon Y, Green H. Three clonal types of keratinocyte with different capacities for multiplication. Proc Natl Acad Sci USA. 1987;84:2302–6.
Germain L, et al. Skin stem cell identification and culture: a potential tool for rapid epidermal sheet production and grafting. In: Rouabhia M, editor. Skin substitute production by tissue engineering. Austin: R.G. Landes Company; 2001. p. 177–210.
Brody I. The ultrastructure of the tonofibrils in the keratinization process of normal human epidermis. J Ultrastruct Res. 1960;4:264–97.
Fuchs E, Cleveland D. A structural scaffolding of intermediate filaments in health and disease. Science. 1998;279:514–9.
Fukuyama K, Kakimi S, Epstein WL. Detection of a fibrous component in keratohyalin granules of newborn rat epidermis. J Invest Dermatol. 1980;74:174–80.
Yaffe MB, Eckert RL. Involucrin, type-I and type-II keratins and filaggrin are covalently cross-linked components of the keratinocyte cornified envelope. Clin Res. 1992;40:A522.
Eckert RL, et al. Involucrin – structure and role in envelope assembly. J Invest Dermatol. 1993;100:613–7.
Hohl D. Cornified cellular envelope. Dermatologica. 1990;180:201–11.
McCall CA, Cohen JJ. Programmed cell death in terminally differentiating keratinocytes: role of endogenous endonuclease. J Invest Dermatol. 1991;97:111–4.
Wrone-Smith T, et al. Keratinocytes from psoriatic plaques are resistant to apoptosis compared with normal skin. Am J Pathol. 1997;151:1321–9.
Elias PM. Epidermal lipids, barrier function, and desquamation. J Invest Dermatol. 1983;80:44S–9S.
Weinstein GD, Boucek RJ. Collagen and elastin of human dermis. JInvest Dermatol. 1960;35:227–9.
Smith LT, Holbrook KA, Byers HP. Structure of the dermal matrix during development and in the adult. J Invest Dermatol. 1982;79:93S–104S.
Bruckner-Tuderman L, Hopfner B, Hammami-Hauasli N. Biology of anchoring fibrils: lessons from dystrophic epidermolysis bullosa. Matrix Biol. 1999;18:43–54.
Pulkkinen L, Uitto J. Mutation analysis and molecular genetics of epidermolysis bullosa. Matrix Biol. 1999;18:29–42.
Braverman IM. The cutaneous microcirculation. J Invest Dermatol Symp. 2000;5:3–9.
Metze D, Luger T. Nervous system in the skin. In: Freinkel RK, Woodley DT, editors. The biology of the skin. The Parthenon Publishing Group: New York; 2001. p. 153–76.
Skobe M, Detmar M. Structure, function and molecular control of the skin and lymphatic system. J Invest Dermatol Symp. 2000;5:14–9.
Tzaphildou M. The role of collagen and elastin in aged skin: an image processing approach. Micron. 2004;35:873–7.
Kielty CM, Shuttleworth CA. Microfibrillar elements of the dermal matrix. Microsc Res Tech. 1997;38:413–27.
Garrone R. Distribution of minor collagens during skin development. Microsc Res Tech. 1997;38:407–12.
Keene DR, Marinkovich MP, Sakai LY. Immunodissection of the connective tissue matrix in human skin. Microsc Res Tech. 1997;38:394–406.
Pasquali-Ronchetti I, Baccareni-Contri M. Elastic fiber during development and aging. Microsc Res Techn. 1997;38:428–35.
Fosang AJ, Hardingham TE. Matrix proteoglycans. In: Comper WD, editor. Extracellular matrix volume 2, molecular components and interactions. Amsterdam: Harwood Academic Publishers; 1996. p. 200–18.
Iozzo RV. Matrix proteoglycans: from molecular design to cellular function. Ann Rev Biochem. 1998;67:609–52.
Saliba MJ. Heparin in the treatment of burns: a review. Burns. 2001;27:349–58.
Savani RC, et al. The role of hyaluronan-receptor interactions in wound repair. In: Garg HG, Longaker MT, editors. Scarless wound healing. New York: Marcel Dekker, Inc.; 2000. p. 115–42.
Trowbridge JM, Gallo RL. Dermatan sulfate: new functions from an old glycosaminoglycan. Glycobiology. 2002;126:117R–25R.
Kljaer M. Role of extracellular matrix in adaptation of tendon and skeletal muscle to mechanical loading. Physiol Rev. 2004;84:649–98.
Angulo J, et al. The activation of fibroblast growth factors (FGFs) by glycosaminoglycans: influence of the sulfation pattern on the biological activity of FGF-1. Chem Biochem. 2004;5:55–61.
Martinez-Hernadez A, Amenta PS. The basement membrane in pathology. Lab Invest. 1983;48:656–77.
Woodley DT, Chen M. The basement membrane zone. In: Freinkel RK, Woodley DT, editors. The biology of the skin. The Parthenon Publishing Group: New York; 2001. p. 133–5.
Brown TA, Gil SG, Sybert VP. Defective integrin alpha 6 beta 4 in the skin of patients with junctional epidermolysis bullosa and pyloric atresia. J Invest Dermatol. 1996;107:384–91.
Larjava H. Expression of β1 integrins in normal human keratinocytes. Am J Med Sci. 1991;301:63–8.
Graf J, et al. Identification of an amino acid sequence in laminin mediating cell attachment, chemotaxis, and receptor binding. Cell. 1987;48:989–96.
Bateman JF, Lamande SR, Ramshaw JAM. Collagen superfamily. In: Comper WD, editor. Extracellular matrix volume 2, components and interactions. Harwood Academic Publishers: Amsterdam; 1996. p. 22–67.
Katz AJ. Emerging approaches to the tissue engineering of fat. Tissue Eng. 1999;26:587–603.
Widelitz RB, et al. Molecular histology in skin appendage morphogenesis. Microsc Res Tech. 1997;38:452–65.
Shakespeare P. Burn wound healing and skin substitutes. Burns. 2001;27:517–22.
Sato K, et al. Biology of the sweat glands and their disorders. I. Normal sweat gland function. J Am Acad Dermatol. 1989;20:537–63.
Hurley HJ. The eccrine sweat glands: structure and function. In: Freinkel RK, Woodley DT, editors. The biology of the skin. The Parthenon Publishing Group: New York; 2001. p. 47–76.
Speilman AL, et al. Proteinaceous precursors of human axillary odor: isolation of two novel odor-binding proteins. Experientia. 1995;15:40–7.
Botek AA, Lookingbill DP. The structure and function of sebaceous glands. In: Freinkel RK, Woodley DT, editors. The biology of the skin. The Parthenon Publishing Group: New York; 2001. p. 87–100.
Thody AJ, Shuster S. Control and function of sebaceous glands. Physiolo Rev. 1989;69:383–416.
Freinkel RK. Hair. In: Freinkel RK, Woodley DT, editors. The biology of the skin. New York: The Parthenon Publishing Group; 2001. p. 77–86.
Spradling A, Drummond-Barbosa D, Kai T. Stem cells find their niche. Nature. 2001;414:98–104.
Akiyama M, et al. Characterization of hair follicle bulge in human fetal skin; the human fetal bulge is a pool of undifferentiated keratinocytes. J Invest Dermatol. 1995;105:844–50.
Levit EK, Scher RK. Basic science of the nail unit. In: Freinkel RK, Woodley DT, editors. The biology of the skin. The Parthenon Publishing Group: New York; 2001. p. 101–12.
Kitahara T, Ogawa H. Cellular features of differentiation in the nail. Microsc Res Tech. 1997;38:436–42.
Browder LW, Erickson CA, Jeffrey WR. Developmental biology. Philadelphia: Saunders College Publishing (https://www.sdbonline.org/sites/archive/sdbeduca/BrowderContPref.html); 1991. p. 260–82.
Larsen WJ. Development of the integumentary system. In: Human embryology. 3rd ed. Philadelphia: Churchill Livingstone; 2001. p. 465–80.
Carlson BM. Integumentary, skeletal and muscular systems. In: Human embryology and developmental biology. 2nd ed. St. Louis: Mosby Inc.; 1999. p. 148–83.
Dye FJ. Human life before birth. Amsterdam: Harwood Academic Publishers; 2000. p. 97–104.
Toole BP. Hyaluronan in morphogenesis. J Intern Med. 1997;242:35–40.
Cook D. Classification of burns. In: Bosworth C, editor. Burns trauma. London: Bailliere Tindall; 1997. p. 19–34.
Monafo W. Wound care. In: Herndon DN, editor. Total burn care. London: W.B. Saunders Company Ltd.; 1996. p. 88–97.
Williams WG, Phillips LG. Pathophysiology of the burn wound. In: Herndon DN, editor. Total burn care. London: W.B. Saunders Company Ltd.; 1996. p. 63–70.
Clark RAF. Wound repair: lessons for tissue engineering. In: Lanza RP, Langer R, Chick WL, editors. Principles of tissue engineering. Austin: R.G. Landes Company; 1997. p. 737–68.
Krisner RS, Eaglstein W. The wound healing process. Wound Healing. 1993;11:629–40.
Martin P. Wound healing – aiming for perfect skin regeneration. Science. 1997;276:75–80.
Hardin-Young J, Parenteau N. Bilayered skin constructs. In: Atala A, Lanza RP, editors. Methods of tissue engineering. Academic: San Diego; 2002. p. 1177–88.
Servold SA. Growth factor impact on wound healing. Clin Podiatr Med Surg. 1991;8:937–53.
Pankurst S. Wound care. In: Bosworth C, editor. Burns trauma. London: Bailliere Tindall; 1997. p. 63–74.
Kane JB, Tompkins RG, Yarmush ML. Burn dressings. In: Ratner BD, et al., editors. Biomaterials science. San Diego: Academic; 1996. p. 360–70.
Tuan TL, Nichter LS. The molecular basis of keloid and hypertrophic scar formation. Mol Med Today. 1998;4:19–24.
Rockwell WB, Cohen IK, Ehrlich HP. Keloid and hypertrophic scars: a comprehensive review. Plast Reconstr Surg. 1989;84:827–37.
Robb EC, et al. A new model for studying the development of human hypertrophic burn scar formation. J Burn Care Rehabil. 1987;8:371–5.
Hindler F, Traber DL. Pathophysiology of the systemic inflammatory response syndrome. In: Herndon DN, editor. Total burn care. London: W.B. Saunders Company Ltd.; 1996. p. 207–16.
Kramer GC, Nguyen TT. Pathophysiology of burn shock and burn edema. In: Herndon DN, editor. Total burn care. London: W.B. Saunders Company Ltd; 1996. p. 44–52.
Erol S, et al. Changes of microbial flora and wound colonization in burned patients. Burns. 2004;30:357–61.
Kanchanapoom T, Khardori N. Management of infections in patients with severe burns: impact of multiresistant pathogens. J Burns Surg Wound Care. 2002;1:1–7.
Allgower M, Schoenenberger GA, Sparkes BG. Burning the largest immune organ. Burns. 1995;21:S7–S47.
Sparkes BG. Immunological responses to thermal injury. Burns. 1997;23:106–13.
Deveci M, et al. Comparison of lymphocyte populations in cutaneous and electrical burn patients: a clinical study. Burns. 2000;26:229–32.
Schwacha MG. Macrophages and post-burn immune dysfunction. Burns. 2003;29:1–14.
Vindenes HA, Bjerknes R. Impaired actin polymerization and depolymerization in neutrophils from patients with thermal injury. Burns. 1997;23:131–6.
Tyler MPH, et al. Dermal cellular inflammation in burns. an insight into the function of dermal microvascular anatomy. Burns. 2001;27:433–8.
Munster AM. The immunological response and strategies for intervention. In: Herndon DN, editor. Total burn care. London: W.G. Saunders Company Ltd.; 1996. p. 279–92.
Lu S, et al. Effect of necrotic tissue on progressive injury in deep partial thickness burn wounds. Chin Med J. 2002;115:323–5.
Sheridan RL, Thompkins RG. Etiology and prevention of multisystem organ failure. In: Herndon DN, editor. Total burn care. London: W.G. Saunders Company Ltd.; 1996. p. 302–12.
Sheridan RL. What’s new in burns and metabolism. J Am College of Surgeons. 2004;198:243–63.
Hartford CE. Care of out-patient burns. In: Herndon DN, editor. Total burn care. London: W.G. Saunders Company Ltd.; 1996. p. 71–80.
Jones OC. Management of pain in the burns patient. In: Bosworth C, editor. Burns trauma. Bailliere Tindall: London; 1997. p. 95–110.
Wachtel TL. Initial care of major burns. Postgrad Med. 1989;85:178–96.
Warden GD. Fluid resuscitation and early management. In: Herndon DN, editor. Total burn care. London: W.G. Saunders Company Ltd.; 1996. p. 53–60.
Ho WS, et al. Skin care in burn patients: a team approach. Burns. 2001;27:489–91.
Zhi-yang F. Local care in severe burn. In: Zhi-yang F, et al., editors. Modern treatment of severe burns. Berlin: Springer-Verlag; 1992. p. 51–63.
Ryan TJ. Wound healing and current dermatologic dressings. Clin Dermatol. 1990;8:21–9.
Boyce ST. Design principles for composition and performance of cultured skin substitutes. Burns. 2001;27:523–33.
Sheng-de G. Management of full-thickness burns. In: Zhi-yang F, et al., editors. Modern treatment of severe burns. Berlin: Springer-Verlag; 1992. p. 64–79.
Tanner JC. The mesh skin graft. Plast Reconst Surg. 1964;34:287–92.
Thompkins RG, Burke JF. Alternative wound coverings. In: Herndon DN, editor. Total burn care. London: W.B. Saunders Company Ltd.; 1996. p. 164–72.
Pruitt BA. The evolutionary development of biologic dressings and skin substitutes. J Burn Care Rehabil. 1997;18:S2–5.
Hansbrough J. Allograft (Homograft) skin. In: Wound coverage with biologic dressings and cultured skin substitutes. Austin: R.G. Landes Company; 1992. p. 21–39.
Mackie DP. The Euro skin bank: development and application of glycerol-preserved allografts. J Burn Care Rehabil. 1997;18:S7–S12.
Ben-Bassat H, et al. How long can cryopreserved skin be stored to maintain adequate graft performance? Burns. 2001;27:425–31.
Ramakrishnan KM, Jayaraman V. Management of partial-thickness burns by amniotic membranes: a cost-effective treatment in developing countries. Burns. 1997;23:S33–6.
Sawhney CP. Amniotic membrane as a biological dressing in the management of burns. Burns. 1989;15:339–42.
Hansbrough JF. Human amnion. In: Wound coverage with biologic dressings and cultured skin substitutes. Austin: R.G. Landes Company; 1992. p. 10–2.
Lin SD, et al. Amnion overlay meshed skin autograft. Burns. 1985;11:374–8.
Sullivan TP, et al. The pig as a model for human wound healing. Wound Repair Regen. 2001;9:66–76.
Hansbrough J. Xenograft (Heterograft) skin. In: Wound coverage with biologic dressings and cultured skin substitutes. Austin: R.G. Landes Company; 1992. p. 13–20.
Bach FH, et al. Uncertainty in xenotransplantation: individual benefit versus collective risk. Nat Med. 1998;4:141.
Kim B, Baez CE, Atala A. Biomaterials for tissue engineering. World J Urol. 2000;18:2–9.
Marler JJ, et al. Transplantation of cells in matrices for tissue regeneration. Adv Drug Deliv Rev. 1998;33:165–82.
Berthod F, Damour O. In vitro reconstructed skin models for wound coverage in deep burns. Br J Dermatol. 1997;136:809–16.
Gogolewski S, Pennings AJ. An artificial skin based on biodegradable mixtures of polylactides and polyurethanes for full-thickness skin wound covering. Makromol Chem, Rapid Commun. 1983;4:675–80.
Parenteau N, et al. Biological and physical factors influencing the successful engraftment of a cultured human skin substitute. Biotechnol Bioeng. 1996;52:3–14.
Sefton MV, Woodhouse KA. Tissue engineering. J Cutan Med Surg. 1998;3:S1-18–23.
Jorgensen PH, Bang C, Andreassen TT. Mechanical properties of skin graft wounds. Br J Plast Surg. 1993;46:565–9.
Jonkman MF, et al. Evaporative water loss and epidermis regeneration in partial-thickness wounds dressed with a fluid-retaining versus a clot-inducing wound covering in guinea pigs. Scand J Plast Reconstr Surg. 1989;23:29–34.
Greenwald SE, Berry CL. Improving vascular grafts: the importance of mechanical and hemodynamic properties. J Pathol. 2000;190:292–9.
Peppas NA, et al. Physiochemical foundations and structural design of hydrogels in medicine and biology. Ann Rev Biomed Eng. 2000;2:9–29.
Chvapil M. Considerations on manufacturing principles of a synthetic burn dressing: a review. J Biomed Mater Res. 1982;16:245–63.
Chen G, Ushida T, Tateishi T. Hybrid biomaterials for tissue engineering: a preparative method for PLA or PLGA-collagen hybrid sponges. Adv Mater. 2000;12:455–7.
Beumer GJ, Blitterswijk CA, Ponec M. Biocompatibility of a biodegradable matrix used as a skin substitute: an in vivo evaluation. J Biomed Mater Res. 1994;28:545–52.
Wald HL, et al. Cell seeding in porous transplantation devices. Biomaterials. 1993;14:270–8.
Ruszczak Z. Effect of collagen matrices on dermal wound healing. Adv Drug Deliv Rev. 2003;55:1595–611.
Badylak SF. The extracellular matrix as a scaffold for tissue reconstruction. Cell Dev Biol. 2002;13:377–83.
Alper JC, et al. Moist wound healing under a vapor permeable membrane. J Am Acad Dermatol. 1983;8:347–53.
Alvarez OM, Mertz PM, Eaglstein WH. The effect of occlusive dressings on collagen synthesis and re-epithelialization in superficial wounds. J Surg Res. 1983;35:142–8.
Eldad A, Kadar ST, Kushnir M. Immediate dressing of the burn wound – will it change its natural history? Burns. 1991;17:233–8.
Helfman T, Ovington L, Falanga V. Occlusive dressings and wound healing. Clin Dermatol. 1994;12:121–7.
Falanga V. Occlusive wound dressings. Arch Dermatol. 1988;124:872–6.
Queen D, et al. The preclinical evaluation of the water vapour transmission rate through burn wound dressings. Biomaterials. 1987;8:367–71.
Berardesca E, et al. Effect of occlusive dressings on the stratum corneum water holding capacity. Am J Med Sci. 1992;304:25–8.
Neal DE, et al. The effects of an adherent polyurethane film and conventional absorbent dressing in patients with small partial thickness burns. Br J Clin Pract. 1981;35:254–7.
Poulsen TD, et al. Polyurethane film (Opsite*) vs. impregnated gauze (Jelonet*) in the treatment of outpatient burns: a prospective, randomized study. Burns. 1991;17:59–61.
Eldad A, Tuchman I. The use of Omiderm® as an interface for skin grafting. Burns. 1991;17:155–8.
Staso MA, et al. Experience with Omiderm* – a new burn dressing. J Burn Care Rehabil. 1991;12:209–10.
Jonkman MF, et al. A clot-inducing wound covering with high vapour permeability: enhancing effects on epidermal wound healing in partial-thickness wounds in guinea pigs. Surgery. 1988;104:537–45.
Jonkman MF, Bruin P, Pennings AJ. Poly(ether urethane) wound coverings with high vapour permeability compared with conventional tulle gras on split-skin donor sites. Burns. 1989;15:211–6.
Bruin P, et al. A new porous polyetherurethane wound covering. J Biomed Mater Res. 1990;24:217–26.
Landrum J, Jones EB. A new dressing for burns: enclosure in a plasticized polyvinyl chloride sheet. Burns. 1976;2:86–9.
Davies JWL. Synthetic materials for covering burn wounds: progress towards perfection. Part I. Short term dressing materials. Burns. 1983;10:94–103.
Dressler DP, Barbee WK, Sprenger R. The effect of Hydron burn wound dressing on burned rat and rabbit ear wound healing. J Trauma. 1980;20:1024–8.
Brown AS. Hydron for burns. Plast Reconstr Surg. 1981;67:810–1.
Young SR, et al. Comparison of the effects of semi-occlusive polyurethane dressings and hydrocolloid dressings on dermal repair: 1. cellular changes. J Invest Dermatol. 1991;97:586–92.
Agren MS, Wijesinghe C. Occlusivity and effects of two occlusive dressings on normal human skin. Acta Derm Venereol. 1994;74:12–4.
Hansbrough J. Synthetic and biosynthetic wound coverings. In: Wound coverage with biologic dressings and cultured skin substitutes. Austin: R.G. Landes Company; 1992. p. 41–59.
Currie LJ, Sharpe JR, Martin R. The use of fibrin glue in skin grafts and tissue-engineered skin replacements: a review. Plast Reconstr Surg. 2001;108:1713–26.
DeBlois C, Cote MF, Doillon CJ. Heparin-fibroblast growth factor-fibrin complex: in vitro and in vivo applications to collagen-based materials. Biomaterials. 1993;15:665–72.
Cote MF, Doillon CJ. In vitro contraction rate of collagen in sponge-shape matrices. J Biomater Sci Polym Ed. 1992;3:301–13.
Ramshaw JAM, Werkmeister JA, Glattauer V. Collagen-based biomaterials. Biotechnol Genetic Eng Rev. 1995;13:335–82.
Doillon CJ, et al. Porosity and biological properties of polyethylene glycol-conjugated collagen materials. J Biomater Sci Polym Ed. 1994;6:715–28.
Rheinwald JG, Green H. Serial cultivation of strains of human epidermal keratinocytes: the formation of keratinizing colonies from single cells. Cell. 1975;6:331–43.
Carsin H, et al. Cultured epithelial autografts in extensive burn coverage of severely traumatized patients: a five year single-center experience with 30 patients. Burns. 2000;26:379–87.
Hafemann B, et al. Treatment of skin defects using suspensions of in vitro cultured keratinocytes. Burns. 1994;160:168–72.
Blight A, et al. The treatment of donor sites with cultured epithelial grafts. Br J Plast Surg. 1991;44:12–4.
Gielen V, et al. Progressive replacement of human cultured epithelial autograts by recipient cells as evidenced by HLA class I antigens expression. Dermatologica. 1987;175:166–70.
Lam PK, et al. Development and evaluation of a new composite Laserskin graft. J Trauma. 1999;47:918–22.
Lees VC, Fan TD, West DC. Angiogenesis in a delayed revascularization model is accelerated by angiogenic oligosaccharides of hyaluronan. Lab Invest. 1995;73:259–66.
Lobmann R, et al. Autologous human keratinocytes cultured on membranes composed of benzyl ester of hyaluronic acid for grafting in nonhealing diabetic foot lesions. a pilot study. J Diabetes Complicat. 2003;17:199–204.
Naughton G, Mansbridge J, Gentzkow G. A metabolically active human dermal replacement for the treatment of diabetic foot ulcers. Artif Organs. 1997;21:1203–10.
Naughton GK. Skin and epithelia. In: Lanza RP, Langer R, Chick WL, editors. Principles of tissue engineering. Austin: R.G. Landes Company; 1997. p. 769–82.
Gentzkow G, et al. Use of Dermagraft, a cultured human dermis, to treat diabetic foot ulcers. Diabetes Care. 1996;19:350–4.
Hansbrough JF, Dore C, Hansbrough WB. Clinical trials of a living dermal tissue replacement beneath meshed, split-thickness skin grafts on excised burn wounds. J Burn Care Rehabil. 1992;13:519–29.
Hansbrough JF, et al. Evaluation of a biodegradable matrix containing cultured human fibroblasts as a dermal replacement beneath meshed split-thickness skin grafts. Surgery. 1992;111:438–46.
Kearney JN. Clinical evaluation of skin substitutes. Burns. 2001;27:545–51.
Wainwright D. Use of an acellular allograft dermal matrix (AlloDerm) in the management of full-thickness burns. Burns. 1995;21:243–8.
Bannasch H, et al. Skin tissue engineering. Clin Plast Surg. 2003;30:573–9.
Wainwright D, et al. Clinical evaluation of an acellular allograft dermal matrix in full-thickness burns. J Burn Care Rehabil. 1996;17:124–36.
Yannas IV, Burke JF, Orgill DP. Wound tissue can utilize a polymeric template to synthesize a functional extension of skin. Science. 1982;215:174–6.
Burke JF, et al. Successful use of a physiologically acceptable artificial skin in the treatment of extensive burn injury. Ann Surg. 1981;194:413–28.
Ellis DL, Yannas IV. Recent advances in tissue synthesis in vivo by use of collagen-glycosaminoglycan copolymers. Biomaterials. 1996;17:291–9.
Sheridan RL, et al. Artificial skin in massive burns – results to ten years. Eur J Plast Surg. 1994;17:91–3.
Cooper ML, Hansbrough JF. Use of a composite skin graft composed of cultured human keratinocytes and fibroblasts and a collagen-GAG matrix to cover full-thickness wounds on athymic mice. Surgery. 1991;109:198–207.
Boyce ST, Hansbrough JF. Biologic attachment, growth and differentiation of cultured human keratinocytes on a graftable collagen and chondroitin-6-sulfate substrate. Surgery. 1988;103:421–31.
Falanga V, et al. Rapid healing of venous ulcers and lack of clinical rejection with an allogenic cultured human skin equivalent. Arch Dermatol. 1998;134:293–300.
Guerret S, et al. Long-term remodeling of a bilayered living human skin equivalent (Apligraft®) grafted onto nude mice: immunolocalization of human cells and characterization of extracellular matrix. Wound Repair Regen. 2003;11:35–45.
Klein RL, Rothman BF, Marshall R. Biobrane – a useful adjunct in the therapy of out-patient burns. J Pediatr Surg. 1984;19:846–7.
Purdue GF, et al. Biosynthetic skin substitute versus frozen human cadaver allograft for temporary coverage of excised burn wounds. J Trauma. 1987;27:155–7.
Purdue GF. Dermagraft-TC pivotal efficacy and safety study. J Burn Care Rehabil. 1997;18:S13–4.
Hansbrough J. Dermagraft-TC for partial-thickness burns: A clinical evaluation. J Burn Care Rehabil. 1997;18:S25–8.
Wang HJ, et al. Acceleration of skin graft healing by growth factors. Burns. 1996;22:10–4.
Ono I, et al. Studies on cytokines related to wound healing in donor site wound fluid. J Dermatol Sci. 1995;10:241–5.
Smith PD, et al. Efficacy of growth factors in the accelerated closure of interstices in explanted meshed human skin grafts. J Burn Care Rehabil. 2000;21:5–9.
Hansbrough JF. Biologic mediators in wound healing. In: Wound coverage with biologic dressings and cultured skin substitutes. Austin: R.G. Landes Company; 1992. p. 116–47.
Wenczak BA, Lynch JB, Nanney LB. Epidermal growth factor receptor distribution in burn wounds. Implications for growth factor-mediated repair. J Clin Invest. 1992;90:2392–401.
Kiyohara Y, et al. Improvements in wound healing by epidermal growth factor (EGF) ointment. II. Effect of protease inhibitor, nafamostat, on stabilization and efficacy of EGF in burn. J Pharmacobiodynamics. 1991;14:47–52.
Chvapil M, Gaines JA, Gilman T. Lanolin and epidermal growth factor in healing of partial-thickness pig wounds. J Burn Care Rehabil. 1988;9:279–84.
Arturson G. Epidermal growth factor in the healing of corneal wounds, epidermal wounds and partial-thickness scalds. A controlled animal study. Scand J Plast Reconstr Surg. 1984;18:33–7.
Gibran NS, et al. Basic fibroblast growth factor in the early human burn wound. J Surg Res. 1994;56:226–34.
Fu X, et al. Recombinant bovine basic fibroblast growth factor accelerates wound healing in patients with burns, donor sites and chronic dermal ulcers. Chin Med J. 2000;113:367–71.
Fu X, et al. Randomised placebo-controlled trial of use of topical recombinant bovine basic fibroblast growth factor for second-degree burns. Lancet. 1998;352:1661–4.
Hakvoort T, et al. Transforming growth factor-beta(1), -beta(2), -beta(3), basic fibroblast growth factor and vascular endothelial growth factor expression in keratinocytes of burn scars. Eur Cytokine Netw. 2000;11:233–9.
Danilenko DM, et al. Growth factors in porcine full and partial thickness burn repair. Differing targets and effects of keratinocyte growth factor, platelet-derived growth factor-BB, epidermal growth factor, and neu differentiation factor. Am J Pathol. 1995;147:1261–77.
Polo M, et al. Cytokine production in patients with hypertrophic burn scars. J Burn Care Rehabil. 1997;18:477–82.
Smith P, et al. TGF-β2 activates proliferative scar fibroblasts. J Surg Res. 1999;82:319–23.
Beer HD, et al. Expression and function of keratinocyte growth factor and activin in skin morphogenesis and cutaneous wound repair. J Invest Dermatol Symp. 2000;5:34–9.
Edmondson SR, et al. Epidermal homeostasis: the role of the growth hormone and insulin-like growth factor systems. Endocr Rev. 2003;24:737–64.
Wolf SE, et al. Insulin-like growth factor-1/insulin-like growth factor binding protein-3 alters lymphocyte responsiveness following severe burn. J Surg es. 2004;117:255–61.
Swope VB, Supp AP, Boyce ST. Regulation of cutaneous pigmentation by titration of human melanocytes in cultured skin substitutes grafted to athymic mice. Wound Repair Regen. 2002;10:378–86.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Flynn, L.E., Woodhouse, K.A. (2021). Burn Dressing Biomaterials and Tissue Engineering. In: Narayan, R. (eds) Biomedical Materials. Springer, Cham. https://doi.org/10.1007/978-3-030-49206-9_17
Download citation
DOI: https://doi.org/10.1007/978-3-030-49206-9_17
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-49205-2
Online ISBN: 978-3-030-49206-9
eBook Packages: Chemistry and Materials ScienceChemistry and Material Science (R0)