Abstract
The skin provides a protective barrier to the body against the environment. Ineffective healing of damaged skin can cause a chronic wound which would increase the risk of infection and associated complications. The use of wound dressings to protect the wound and provide an optimal environment for wound repair is a common practice in the burn clinic. While traditional wound healing dressings have substantially changed the wound outcome, wound healing complications are still a challenge to healthcare. Advancements in tissue engineering, biomaterial sciences, and stem cell biology led to the development of novel dressings that not only dress the wounds but also actively contribute to the process of healing. This review discusses the various properties of the emerging wound dressings that are designed in attempts to improve wound care upon skin injury.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The skin functions to provide a physical barrier between the internal organs and the external environment, protecting the body from pathogens, radiation, changes in temperature, and water loss.41,47,107,145,146 Damage to the skin can impair its function, which can be caused by a variety of ways, including burns, trauma, surgeries, or lacerations. Thus, proper wound care is important to prevent infections and further associated complications, which may lead to impaired wound healing and may increase morbidity and mortality. Problems seen with wound healing are often shown in chronic wounds, characterized as wounds with slow or incomplete wound closure, which affect over 2.5 million people in the United States alone.97,112
Over several millennia, wound care has revolved around washing the wound and applying a plaster to allow the wound to heal.67,124 These plasters, which were the equivalent of current day wound dressings, were made of clay to allow the absorption of wound exudate while providing a protective barrier from the environment.109 Various methods were implemented to cover the wound mostly through trial and error in efforts to enhance wound healing. As early as 2500 BCE, wounds have been cleaned with milk or water and were treated with clay tablets during the Mesopotamian era.25 Other later treatments included using herbs, honey, and oil as has been advocated by Hippocrates of Ancient Greece.23,25 Furthermore, some bandages that were used to cover the wound consisted of various materials, such as donkey feces, which naturally contained antibiotics and were thought to improve healing outcomes by protecting the wound from infection.75,117
Currently, wound dressings are still used to promote a suitable environment for wound healing and protect the damaged tissue from the environment and bacterial infiltration. However, available commercial wound dressings in use are mostly inadequate, expensive, or may impair wound healing as in the case of adhesive wound dressings which damage the skin upon removal.80,120 An ideal wound dressing should be non-adhesive, removes exudate while maintaining a moist environment for optimal healing, prevents bacterial infection and biofilm formation, does not disrupt healing, and ideally enhances healing without promoting scar formation.54,83 Despite millennia of describing wound treatments and the high prevalence of wound care centres around the world, we continue to lack an efficient wound dressing that combines the different properties of an ideal wound dressing. In this review, we will examine some of the emerging wound dressings that are being developed as potential clinical treatments to improve wound healing in injured patients.
Skin Histology
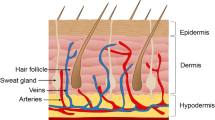
Skin is made up of the epidermal, dermal, and hypodermal (subcutaneous) layers. Each of these layers contains a different combination of cells that have various functions in the body (Fig. 1).
Epidermis
The epidermis is the outermost layer of the skin, commonly divided into four different strata (layers): stratum basale, stratum spinosum, stratum granulosum, and stratum corneum.39,142 These layers consist of mainly keratinocytes, which make up approximately 95% of the epidermis and play major roles in wound healing.122 The stratum basale, which is the innermost layer, mainly harbors keratinocyte stem cells.39 These mitotically active cells give rise to the keratinocytes in the stratum spinosum which migrate superficially to form the differentiated cells found in the stratum granulosum and corneum.61 Although keratinocytes are involved in wound healing through cross-talking with other cells such as fibroblasts and macrophages, their main role involves the synthesis of keratin which maintains the structure of the epidermis and prevents the body from environmental damage and water loss.110 Other cells found in the epidermis include melanocytes, Langerhans, and immune cells.39
Dermis
Underneath the epidermis lies the dermis, which consists of a system of dense fibrous connective tissue allowing the penetration of vascular tissues and nerve fibers, and provides elasticity and tensile strength to the skin.61 Some of the main components of the dermis include fibroblasts, macrophages and other immune cells which may be recruited due to various stimuli.61
Subcutaneous Layer
The hypodermis, or the subcutaneous layer, is mainly made up of adipocytes and harbors larger blood vessels and nerve fibers compared to the dermis.61
Wound Healing
The process of wound healing is divided into three overlapping stages: hemostasis/inflammation, proliferation, and maturation (Fig. 2). The various emerging dressings may be more appropriate for application during different stages of wound healing for optimal function.
Hemostasis/Inflammatory Phase
During the inflammatory phase, hemostasis is initiated as plasma platelets aggregate to form a clot at the surface of the wound to stop bleeding.133 This clot allows for the migration of leukocytes and lymphocytes to the wound site to promote the initiation of inflammation.50,133 Vasoconstriction is induced to minimize blood loss at the injury site. Foreign particles and cellular debris are removed by neutrophils, monocytes, and lymphocytes.79,133 These inflammatory cells are recruited by cytokines that are produced at the beginning of the inflammatory phase and enter the wound through chemotaxis. Furthermore, monocytes function to promote vasodilation and the recruitment of other phagocytes and fibroblasts by releasing cytokines and growth factors, respectively.32,79 This phase results in changes in pH which leads to the observed swelling and pain asociated with wounds.105 Consequently, the proliferation phase is initiated whereby granulation, epithelialization, angiogenesis, and contraction occur.
Proliferative Phase
During the proliferative phase, granulation tissue is formed, which consists of the vasculature, fibroblasts and inflammatory cells, and tissue matrix components such as collagen and fibronectin.71 This granulation tissue allows for the migration of the epithelial cells to form an epithelial barrier from the environment during epithelization. Furthermore, blood vessels are formed that enter the granulation tissue to allow proper blood flow and delivery of factors involved in wound healing.130 Myofibroblasts are in turn recruited to contract the wound ultimately leading to wound closure.21
Tissue Remodelling Phase
During the tissue remodeling phase, type III collagen is replaced with type I collagen fibers which crosslink and form an organized tissue, improving tensile strength and decreasing scar size.139 A disruption in the any of these phases can slow down the process of wound healing and may result in a chronic wound.91 For example, a buildup of exudate or tears in the skin upon wound dressing removal can cause skin maceration and skin damage which may impair wound healing. Furthermore, infiltration of pathogens or foreign substances into the wound may cause chronic inflammatory responses which could delay healing time.12
Wound Healing Dressings
Traditionally, wound dressings have been used mainly to protect the wound from contamination.131 They were commonly made of dry gauze (Fig. 3) or cotton wool, which are adhesive and allow the absorption of wound exudates.95,99 Hence, they required regular changes due to the excessive fluid that is absorbed by the dressing. In addition, as the gauze dressings become moist due to absorption, they adhere to the wound, which results in wound damage and pain to the patient upon frequent dressing changes.45,77,140 Currently, wound dressings in the market are designed to provide the wound with a moist environment, which has been found to improve the process of wound healing.106 However, these wound dressings tend to fail to combine the different properties favorable for an ideal wound dressing; such ideal wound dressing is described as non-adherent, antibacterial, cost-effective, allow for oxygenation of the wound, less frequent changing, protective of the wound, while maintaining a moist environment for wound healing.25 Here, we review novel wound dressings which incorporate different properties of an ideal wound dressing and classify them based on their main functional purposes. Furthermore, we classify the dressings as: (a) Concept (C), to indicate that the dressing is in the preliminary stage where no experiments in live animals were performed (b) Preclinical (PC) to indicate that the dressing underwent experiments in vivo (c) Clinical (CT) to indicate that the dressing was tested in clinical trials and (d) In practice (IP) to indicate that the dressing is available for clinical use (Table 1).
Johnson & Johnson brand military first field dressing from the National Museum of Australia. http://collectionsearch.nma.gov.au/object/108860.
Dressing as a Physical Barrier
Current Wound Dressings
Wound dressing equivalents, such as plasters, have been used primarily as physical barriers to protect the wound from the environment for several millennia.109 Over time, the materials used in dressings have changed to provide appropriate care for the wounds to ensure optimal healing. Maintaining a moist environment for the wound to heal has been established to be crucial for wound healing which modern dressings, such as hydrogel-based dressings, are designed to address while providing a physical barrier to the wound.6,11,35 Hydrogels are made of crosslinked polymers with high water content, which allow them to maintain a moist environment for the wound.44,121 In addition, their physical structure allows for moisture transmission, oxygen permeability, and exudate absorption.56
Despite having several qualities of an ideal dressing, hydrogels are low in strength, and therefore, are usually hybridized with other polymers through chemical and physical interactions to achieve ideal wound dressing properties.49 Hydrocolloids consist of cross-linked gelatin, pectin, and carboxymethyl cellulose which absorb water to form a gel.134 Like hydrogels, they maintain a moist environment which promotes fibrinolysis, angiogenesis, and overall better healing.88,134 However, they are impermeable to oxygen which has been suggested to increase epithelialization, collagen synthesis, and decrease wound pH leading to a reduction in bacterial viability.125 However, it was demonstrated that oxygen-impermeability may only be advantageous in the early stages of healing but could compromise healing during the later stages.92 Alginates are natural polysaccharides extracted from brown marine algae, consisting of linear copolymer of alpha-l-guluronic acid and beta-d-mannuronic acid.69 They are highly biodegradable and have the least harmful properties compared to other materials which make them one of the safest materials to use in skin.68,78,88 Alginates in dressings are usually crosslinked by calcium ions, resulting in a biocompatible and relatively inexpensive barrier.69 Further, the addition of calcium and/or magnesium ions supports wound healing by providing an anchor for cell adhesion.24,68,88 Alginates, especially when combined with calcium ions, have the ability to absorb large amounts of wound exudate up to 20 times their original size.134 They also allow for the incorporation of other ions such as silver to provide antibacterial and antioxidant properties, and zinc which increases the release of growth factors.2,141
Other materials used in wound dressings include collagen and chitosan. Collagen and materials derived from collagen have been used recently to fabricate modern wound dressings.26,65,113 They are used in their native form or after denaturation where they lose their triple helical form.37 Although they play an important role in hemostasis and promote wound healing, collagen-based materials are permeable to bacteria and other potential pathogens.56 Chitosan is a copolymer polysaccharide of N-acetyl-d-glucosamine and N-glucosamine units distributed to form biopolymer chains.84 As one of the most available polysaccharides, it can be used for a variety of dressings.20,88 Chitosan also has the ability to form a surface layer to protect the wound and increase water absorption in the form of a gel.52,60 Thus, chitosan provides an excellent environment for angiogenesis by activating fibroblasts and collagen deposition to increase healing rates of superficial wounds as shown in preclinical and clinical trials.10,22,52,88,135
Cellulose is made up of stable microfibrils and is readily available due to its obtainability from plants.76 Studies have shown that cellulose elicits minimal immune response and can stimulate growth factors such as platelet-derived growth factor (PDGF), fibroblast growth factor (FGF), and epidermal growth factor (EGF) which increase granulation tissue formation, vascularization, leading to enhanced wound healing.46,55,102
Foam and film dressings have been developed to specifically control moderate amount of drainage of deep and superficial wounds.12,118 They protect the wounds from external environment and can be shaped to fit different body conformations.138 Moreover, they can be used with hydrogel or alginate dressings and allow for the incorporation of antimicrobial drugs for infected wounds.134
All in all, newly developed dressings incorporate the abovementioned and related materials of similar properties with other novel materials in efforts to create innovative dressings that enhance the process of wound healing (Table 2).
Novel Wound Dressings
UrgoStart Contact (IP)
Urgo Medical Laboratories developed a suitable dressing for diabetic wounds as evaluated by Richard et al. and Edmonds et al.30,96 The dressing is impregnated with a lipidocolloid matrix containing sucrose octasulfate potassium salt, which gives it flexible and nonadherent properties.30 In a study carried out in 43 hospitals with special diabetic foot clinics in Europe on randomly assigned 240 patients, wound closure was achieved in 48% of patients in the sucrose octasulfate dressing group and 30% in the control group, showing improved wound closure of neuroischemic diabetic foot ulcer by UrgoStart Contact compared to control.30
Fibrillar Fibronectin Nanofiber Wound Dressing (PC)
Chantre et al. from SEAS and Wyss institute developed a new wound dressing that uses fibronectin protein extracted from fetal skin.14 The rationale was that wounds appearing in fetus before the third trimester leave no scars upon healing.74,100 The authors described that this dressing recruits stem cells that play a role in wound healing in a full-thickness wound mouse model. It was found in vivo that wounds treated with fibronectin dressing showed more than half of tissue regeneration in 3 weeks compared with the standard dressing. The researchers also found that the healed wounds had recovered epidermis and dermis, in addition to hair follicles and adipose tissue. Thus, the authors believe that such dressings can play an important role in regenerative medicine applications.
Soy Protein-Based Nanofiber Wound Dressing (PC)
The same group has also developed a wound dressing that contains soy-based nanofibers with estrogen-like molecules that increase cell growth.3 As estrogen helps in wound healing in pregnant women, the authors believe that this role of estrogen can help in wound healing in patients.4,5,85In vitro, this new dressing showed proliferation, migration, and infiltration of fibroblasts. In vivo, the dressing group showed accelerated re-epithelialization and less scarring of the wound compared to control.3
Eggshell Membrane Dressing (PC)
Guarderas et al., from the company Biovotec, incorporated egg shell membranes in the manufacturing of wound dressings which have healing properties similar to human placenta.42 They make use of the large number of discarded eggshells and transform them into wound dressings in a large volume due to the high availability of these membranes. These chicken egg membrane wound dressings showed 21% faster healing compared to control in the early stages of healing, but indistinguishable rates 5 days post-injury in male Sprague-Dawley rats.42
MicroMend™ (IP)
The scientists at KitoTech Medical Inc. have made a bandage called microMend™ that can replace stitches and staples.70 It can easily be applied to wounds for perfect closure. It is less painful, and there is less gap between wound edges that decreases the rate of wound infection and inflammation.70 As the bandage covers the whole wound, no further strips are required and they can easily be removed at home.70 In clinical trials, microMend was rated more favourably by patients. Furthermore, more than 80% surgeons reported microMend as a more convenient and faster way of wound closure compared to suturing.
XSTAT® (IP)
XSTAT has been approved by the FDA as a device to stop blood loss, which is a major cause of death on the battlefield.62,114 Hence, this hemostatic device dressing is used in the military. It comes in the form of syringe applicators containing more than 80 cellulose sponges with absorbent coating.62 The sponges are injected through the applicator into the wound cavity which swell up to fill the wound space and creates a temporary physical barrier to arrest blood flow.62 This dressing can be used up to 4 h which gives emergency relief to wounded patients before arriving at a trauma facility.62
Dressing as a Chemical Barrier
Skin surface has an acidic environment under normal conditions, which provides an unfavourable environment for bacterial growth.104 However, the pH of the wound increases upon exposure to the underlying tissue in the skin which buffers at a pH of 7.4.105 This, in turn, provides a suitable environment for the formation of bacterial biofilms, which lead to a risk of infection and delayed wound healing.105 Furthermore, chronic wounds have been found to have a slightly more alkaline pH 7.2–8.9 compared to the normal 6.5–8.5 pH of open wounds.94 Thus, tracking changes in pH could be useful in monitoring the progression of the wound healing process. Novel dressings are being developed to act as chemical sensors to the wound, detecting not only pH, but also other variables such as glucose concentration, bacterial infection, or temperature.
Quantitative Optical Indicator of Wound pH Dressing (C)
A wireless smart bandage for optical determination of pH as an indicator of wound status was developed by Kassal et al. (Fig. 4).57 The bandage was created by immobilizing a pH indicator dye on a commercial wound dressing and combining the pH sensing film with a new radio frequency identification (RFID)-based contact-less readout platform through a low-cost optoelectronic interface.57 The electronics store pH data quantitatively and can also transfer it wirelessly. These colour changes were compared with a laboratory pH meter and showed similar pH values. This technology is promising for the future as it is non-invasive and inexpensive. The readings can also be received on smartphones, allowing easy accessibility.

Reprinted from Ref. 57, Copyright (2017), with permission from Elsevier.
A representation of the pH and glucose concentration-sensing wireless smart bandage.
Multi-parameter Glucose and pH Sensing Dressing (C)
A novel system that uses fluorescent sensing to monitor the wound status (Fig. 5), pH, and glucose concentration during healing process was developed by Jankowska et al.51 By analyzing glucose concentrations in the wound, the healing status of the wound can be monitored as high levels of glucose concentration are associated with delayed wound healing.132 A fluorescent pH indicator dye sensitive to a pH range of 6–8, carboxynaphthofluorescein, and a metabolite-sensing enzymatic system were immobilized on a biocompatible polysaccharide matrix to develop wound monitoring coating.51 The metabolite sensing for glucose was achieved by coupled enzyme reaction in which glucose oxidase and horseradish peroxidase (HRP) react sequentially where glucose oxidase produces a substrate for HRP. Changes in concentrations are converted into a fluorescent signal, which allows for the monitoring of both the pH and glucose concentrations simultaneously. Fluorescence spectra confirmed that the coupled dye shows a strong change of the fluorescence intensity in the artificial wound exudate. With change of fluorescence intensity between pH 6.0 and 7.7, the dye showed a very strong fluorescence dependency on pH in the desired pH range.51 These findings illustrate the potential role that the wound dressing can play in monitoring glucose concentration and pH of the wound in order to assess the progression of wound healing. Moreover, the authors suggest that these sensors can also be used for other markers such lactate and uric acid.51

Reprinted from Ref. 51, Copyright (2017), with permission from Elsevier.
A schematic of the glucose and pH sensing wound dressing sending fluorescent signals upon activation.
Stretchable LED Array-Containing Hydrogel Dressing (C)
Lin et al. at MIT developed a stretchable hydrogel dressing that consists of temperature sensors, LED lights, conductive wires, semi-conductor chips, and drug-delivery components (Fig. 6).73 This dressing releases drugs according to changes in temperature of the wound and gives signal in the form of LED light when the drug is low. The dressing has the quality of stretching with the body so that the electronics embedded in the dressing remain functional.73 The authors believe that this dressing can be used inside the body in the form of glucose sensors and even neural probes in the future.

Reprinted from Ref. 73, Copyright (2016), with permission from John Wiley and Sons.
A representative photo of the stretchable LED array in hydrogel dressing.
Colour Changing Microbe-Sensitive Wound Dressing (C)
Thet et al. developed a dressing that changes colour when the wound becomes infected which can help in identifying and treating infected wounds in time.128 The dressing is made up of agarose film with fluorescent dye spread within its matrix and secretes a fluorescent dye from nanocapsules stimulated in reaction to bacterial toxins in the wound (Fig. 7). It was tested on an ex vivo porcine skin model of burn wound infection. The study showed changes in colour within 4 h depending on the bacteria strains. The authors believe that this dressing will help in timely detection and treatment of wound infection.128 Their team is working with a healthcare company, Hartmann, to develop the dressing for use in hospitals in the upcoming years.

Reprinted with permission from Ref. 128. Copyright (2015) American Chemical Society.
Schematic of the microbe-sensing wound dressing which changes color upon infection.
Impedance Sensing Bandage (PC)
Swisher et al. developed a smart bandage that detects pressure ulcers as they are formed before the skin is damaged.127 The smart bandage picks up electrical changes of the cell as it starts dying through its electrodes, which detect electrical signals that leak out from the injured cell membrane. The bandage was tested on rat skins and was able to detect different degrees of tissue damage consistently. As pressure ulcers are usually diagnosed late and cannot be cured, such non-invasive dressing could help with early detection and treatment of the pressure ulcers before the injury becomes permanent.8,127
Dressing as a Device for Exudate Management
Excessive wound exudate is commonly found in chronic wounds and remains a challenge in wound care. In order to ensure proper wound healing without skin macerations or bacterial growth, managing exudates from the damaged skin is important. Traditional dressings such as medical gauzes act to absorb the exudate from the wound, causing not only undesirable adherence, but also the dryness of the wound.54 Therefore, new dressings are being developed which act to absorb excessive wound exudate while maintaining an optimal moist environment for the wound healing process.
AQUACEL® Ag Extra™ (IP)
AQUACEL is described as a hydrofiber-foam hybrid dressing (HFHD) which contains three layers. The outermost layer is a waterproof layer that protects against the exudate layer and bacterial migration while the innermost layer is made up of sodium carboxymethylcellulose (NaCMC) fibres that form a gel when it comes in contact with exudate and keeps the environment moist.63 An increasing understanding of the role of biofilm formations in delayed wound healing has led researchers to develop AQUACEL® Ag Extra™. This dressing has a strong antimicrobial activity due to the incorporation of ionic silver and hydrofiber to manage exudates and the wound environment.82 Multiple in vitro, in vivo, and clinical trials showed the efficacy of AQUACEL® Ag Extra™ in its role of enhancing wound healing through managing exudates and decreasing the risk of infection.81,82,123
Vliwasorb® Pro (IP)
Vliwasorb is a non-adherent dressing that is capable of absorbing exudate evenly from the skin wound.34 The dressing contains sodium polyacrylate that can absorb huge amounts of exudates and can hold the exudate in its core without leakage.34,90 The dressing was evaluated to have good absorptive capacity. No pain was observed during dressing changes as there was no skin irritation and no adverse events were noted. Such superabsorbent dressing is thought to reduce and prevent complications of exudate formation and wound healing.34 These types of superabsorbent wound dressings increase patient comfort and provide faster care with lower costs.
Dressing as a Device to Control Infection
Calcium Pectinate/Silver Nanocomposite (CaP-AgNP) Hydrogel Wound Dressing (C)
The use of biologically derived material for medical use has become more prevalent as seen in the designs of novel wound dressings. Silver nanoparticles were synthesized using plant extracts of Biophytum sensitivum.7 The nanoparticles were immobilized in calcium pectinate frameworks for wound dressing application. Moreover, these biocompatible dressings have high anti-microbial properties. The use of pectin polysaccharide is highly prone to microbes so the incorporation of AgNP in calcium pectinate wound dressing is a new approach to overcome this problem. CaP-AgNP hydrogel wound dressing is thought to help in wound infection prevention, exudate management, and anti-bacterial activity.7 The dressing was shown to successfully inhibit both Gram-positive (S. aureus) and Gram-negative (E. coli) bacteria.7 Due to the combination of exudate management and antibacterial activity, this dressing has a great potential for wound dressing applications.
Cellulose-Based Self-healable Polyelectrolyte Film-Containing Wound Dressing (C)
A wound dressing made of self-healable polyelectrolyte film was prepared using chitosan with modified bacterial cellulose (BC).58 The film was prepared from a grafting technique that works at physiological pH levels (7.4) and was found to be non-toxic, flexible, and biodegradable in addition to allowing anti-microbial drug (curcumin) delivery.58 The anti-microbial activity of these films was tested and observed on Gram-positive strains—S. aureus and Gram-negative strains—E.coli.58 Pure chitosan and composite film exhibited no significant antibacterial activity, but the curcumin loaded film was able to inhibit the growth of bacteria significantly in both strain types.58 Thus, self-healable antimicrobial composite films could be potentially used as an effective wound dressing.
Gelatin/Poly(glycerol sebacate) (PGS) Membrane Drug-Releasing Dressing (C)
A membrane, which can be attached to wound dressings, was developed by Shirazaki et al. that delivers antibiotics at a controlled rate.111 Poly(glycerol sebacate) (PGS) was prepared in gelatin and dissolved in acetic acid, where an antibiotic, ciprofloxacin, was subsequently added to the solution to fabricate the gelatin/PGS membrane using electrospinning.111 Using UV-Vis spectrophotometry, the cross-linked membrane showed a 50% release of drug after 24 h and a total of 70% after 72 h, which is a proper rate of drug release for the prevention of infections.111 These results show the potential for the biodegradable gelatin/PGS membrane as an effective antibacterial wound dressing.
Bamboo Leaves-Derived Cellulose Nanocrystals Dressing (PC)
Singla et al. synthesized nanobiocomposite dressings by inserting silver nanoparticles into a matrix of cellulose nanocrystals (NC) isolated from bamboo leaves.115 The study suggests that this composite can be used for making wound dressings with anti-bacterial properties. The composite has been tested on murine wounds in vivo and showed decreased production of proinflammatory cytokines, early vascularization, increased fibroblasts proliferation, and faster epithelialization.115 The dressing maintained a moist environment for the wound and has shown enhanced regeneration of cells and tissue repair. Furthermore, when tested on diabetic wounds in vivo, the NC dressing group showed full recovery within 18 days through the regulation of growth factors and proinflammatory markers expression.116 Taken together, these results show the potential of the dressing to act as an efficient method for rapid wound closure in diabetic patients.
Procellera® (IP)
Bark et al. at Ohio State University WMC developed a bandage that uses weak electric fields to destroy microbial biofilm, thus preventing infections and assisting in healing of wounds (Fig. 8).9 The scientists incorporated silver and zinc to make a wireless electroceutical dressing (WED) which produces an electric field when it becomes wet. The dressing was shown to disrupt biofilm formation by disrupting quorum sensing and rescuing biofilm-induced E-cadherin loss.9 Furthermore, the dressing promoted faster wound closure in animal wound models and in clinical diabetic patients.9,19 The dressing can act against bacteria that have been shown to be resistant to common antibiotics.59 Thus, the dressing provides an innovative method of controlling infection in wounds in addition to promoting a proper healing environment for the wound.

Ref. 59, by permission of Oxford University Press.
Procellera®: Antimicrobial electric bandage to prevent wound infection.
Chitosan-Containing Hydrogel Dressing (C)
Mozalewska et al. developed a wound dressing that incorporates chitosan derived from shells of crustaceans into hydrogels which are commonly used to manufacture wound dressings.89 The dressing has properties of a usual hydrogel dressing and antibacterial properties due to the chitosan component. Upon crosslinking, the hydrogel was found to retain its function in addition to an increased water absorption capacity, thus, enhancing the function of the dressing in managing wound exudates.89 Preliminary data showed antibacterial characteristics of the novel chitosan-containing hydrogel dressing to Gram-positive bacteria.89 The authors believe that the dressing can be used as a replacement for classic hydrogel dressings.
Silverlon® (CT)
Silverlon® is an antimicrobial dressing that has been shown to prevent surgical site infections (SSI).1,64,129 A clinical study conducted on Silverlon®, showed that this antimicrobial dressing decreases the rate of superficial and deep prosthetic joint infections.129 Another clinical trial showed similar protective effects following colorectal surgery.64 Thus, this dressing plays a role in controlling wound infection post-surgery, decreasing cost burdens and morbidities associated with SSIs. The dressing is available for purchase, though additional studies are being performed to show support for claims that have not yet been cleared by the FDA.
Konjac Glucomannan/Silver Nanoparticle Composite Sponge (PC)
Chen et al. developed a wound dressing using an Asian plant called konjac in combination with silver nanoparticles.15 This wound dressing helped accelerate healing of wounds in rabbits and was found to have anti-microbial properties.15 Furthermore, the dressing is spongy which allows it to retain fluid and help in managing excessive exudate from the wound. Histological studies show that the dressing promotes epithelialization and fibroblast growth, the potential of the dressing in enhancing wound healing.15
Multidrug Releasing Self-assembled Films Bandage (C)
Bleeding and infection are two major causes of death from skin injuries. The Hammond lab from the Massachusetts Institute of Technology and colleagues have developed a bandage that uses a combination of two films, one of which is used for the delivery of a hemostat (thrombin) to control bleeding and the other is used for the delivery of anti-bacterial drugs (vancomycin) to control infection.48 The films are combined in a bandage in a bilayer design that allows for multidrug delivery into the wound at appropriate times.48 The authors showed that their novel bandage released thrombin in few minutes and vancomycin over 24 h which reflect their functions in the hemostatic and inflammatory phases of wound healing, respectively.48
Dressing as a Device to Enhance Wound Healing via Growth Factors
Stabilized Growth Factor-Loaded Hyaluronate-Collagen Dressing (HCD) Matrix (PC)
Growth factors have several functions in wound healing, such as recruitment of cells into the wound, stimulating cell proliferation, and regulating extracellular matrix deposition.40,126 However, growth factors that are currently used show short half-lives in vivo.18 Choi et al. developed a hyaluronate-collagen dressing (HCD) loaded with stabilized epidermal (S-EGF) and basic fibroblast (S-bFGF) growth factors and tested for its ability to improve, otherwise, impaired wound healing.18 S-EGF and S-bFGF showed longer half-lives than existing EGF and bFGF, respectively, and showed more than 90% of cell survival rate at all concentrations.18In vivo studies on diabetic mice showed that these S-EGF and S-bFGF loaded matrices had no inflammatory response at day 7 and a slight acceleration of wound healing overall.18 In another study, Choi et al. showed that their HCD matrix loaded with stabilized EGF and bFGF enhanced wound healing in diabetic ulcers due to their stability at room temperature, suggesting a potential role in enhancing wound healing in diabetic patients.17
Textile Dressing for Growth Factor/Antibiotic Drug Delivery (PC)
Mostafalu et al. reported a wound dressing that allows for drug delivery (VEGF and/or antibiotics) in a temporally and spatially controlled pattern to allow for optimal healing (Fig. 9).87 The dressing is made of fibers coated with electrically conductive ink, in addition to a hydrogel-covered core electrical heater. The dressing showed effective release of previously-loaded antibiotics and VEGF in vitro, leading to angiogenesis induction and effective control of infection.87 Furthermore, the dressing showed increased granulation tissue formation and faster wound closure in diabetic mouse models.87

Reprinted from Ref. 87, Copyright (2017), with permission from John Wiley and Sons.
Textile dressing for controlled Drug delivery. A voltage is sent via a microchip that heats up the nanofibers releasing VEGF into the wound.
Dressing as a Device to Enhance Wound Healing Through Dynamic Infusion of Cells
Cell therapy provides an effective approach to treating skin injuries and chronic wounds by accelerating cellular proliferation and migration into the wound by the host cells.33,86,144,147 Emerging wound dressing designs aim to incorporate cell therapy to allow for a dynamic management of wound healing though cell infusions such as keratinocytes or stem cells to promote rapid wound healing with minimum scarring.
Human Umbilical Cord Mesenchymal Stem Cells-Alginate Dressing (PC)
An innovative wound dressing combines human umbilical cord mesenchymal stem cells (hUCMSCs) with an alginate complex in an effort to develop a device that promotes effective wound healing.137 Human umbilical cord mesenchymal stem cells (hUCMSCs) have the ability to differentiate into multiple tissues, secrete growth factors while maintaining low-immunogenicity.137 The cells proliferated well and showed VEGF secretion when cultured in alginate gel. Furthermore, wound healing rates and neovascularization were increased in the hUCMSCs-alginate dressing group compared to control 15 days post-surgery in Balb/c mice. However, the hUCMSCs showed little migration in the alginate gel and did not reach the wound which suggests that the enhancement of wound healing could be in part due to paracrine signaling.137
Human Adipose-Derived Mesenchymal Stem Cells in Human Amnion/Pig Skin Dressing (C)
Sánchez-Sánchez et al. developed two dressings using human amnion and pig skin which have been commonly used in burn and skin injuries.101 Both dressings were infused with human adipose-derived mesenchymal stem cells (hADMSCs) which are thought to be potential factors in initiating tissue regeneration.36,101 Upon seeding hADMSCs on human amnion and pig skin, both scaffolds showed viable and proliferative hADMSCs. The seeded cells secreted interleukin 10 (IL-10) and interleukin-1β (IL-1β), which in combination contribute to proper wound healing.31,72,98,101,103
Injectable Hybrid Hydrogel for Stem Cell Delivery (PC)
An injectable hydrogel was designed with adjustable properties from hyperbranched multi-acrylated poly(ethyleneglycol) macromers (HP-PEGs) and thiolated hyaluronic acid (HA-SH) as a stem cell delivery system.143 The hydrogel system was found to not only maintain the stemness of the incorporated stem cells and their secretion ability, but also improve cell survival, angiogenesis and overall wound closure in vivo.27 Furthermore, HP-PEG infused with adipose-derived stem cells (ADSCs) lead to a thicker dermis formation and faster healing compared to control.143 Showing enhanced wound healing in a diabetic murine animal model, this injectable hydrogel may be used for stem cell delivery for skin and tissue regeneration applications.143
Conclusion and Perspectives
Despite the drastic improvements during the last four millennia, wound healing complications continue to be a global burden with hundred thousands of deaths annually and a financial challenge in health care.16,136 Considering the rapid advancements in the different fields of optic physics, microfluidics, material sciences, nanoengineering, and stem cells biology, they provide a promising role in developing novel smart dressings that act to not only serve as a passive dressing to cover wounds but also play an active role to dynamically contribute in healing. It is an exciting time to utilize these various fields of science to manufacture dressings that can combine mutliple properties and serve as physical and chemical barriers, as devices for growth factor and cellular delivery, and as antimicrobial barriers. With the emergence of such technology, the future of wound care seems promising and could significantly contribute to reducing morbidities and saving more lives.
References
Abboud, E. C., J. C. Settle, T. B. Legare, J. E. Marcet, D. J. Barillo, and J. E. Sanchez. Silver-based dressings for the reduction of surgical site infection: review of current experience and recommendation for future studies. Burns 40:S30–S39, 2014.
Agren, M. S. Zinc in wound repair. Arch. Dermatol. 135:1273–1274, 1999.
Ahn, S., C. O. Chantre, A. R. Gannon, J. U. Lind, P. H. Campbell, T. Grevesse, B. B. O’Connor, and K. K. Parker. Soy protein/cellulose nanofiber scaffolds mimicking skin extracellular matrix for enhanced wound healing. Adv. Healthc. Mater. 7:1701175, 2018.
Ashcroft, G. S., T. Greenwell-Wild, M. A. Horan, S. M. Wahl, and M. W. Ferguson. Topical estrogen accelerates cutaneous wound healing in aged humans associated with an altered inflammatory response. Am. J. Pathol. 155:1137–1146, 1999.
Ashcroft, G. S., S. J. Mills, K. Lei, L. Gibbons, M.-J. Jeong, M. Taniguchi, M. Burow, M. A. Horan, S. M. Wahl, and T. Nakayama. Estrogen modulates cutaneous wound healing by downregulating macrophage migration inhibitory factor. J. Clin. Investig. 111:1309–1318, 2003.
Atiyeh, B. S., J. Ioannovich, C. A. Al-Amm, and K. A. El-Musa. Management of acute and chronic open wounds: the importance of moist environment in optimal wound healing. Curr. Pharm. Biotechnol. 3:179–195, 2002.
Augustine, R., A. Augustine, N. Kalarikkal, and S. Thomas. Fabrication and characterization of biosilver nanoparticles loaded calcium pectinate nano-micro dual-porous antibacterial wound dressings. Progr Biomater 5:223–235, 2016.
Avijgan, M. Phytotherapy: an alternative treatment for non-healing ulcers. J. Wound Care 13:157–158, 2004.
Barki, K. G., A. Das, S. Dixith, P. D. Ghatak, S. Mathew-Steiner, E. Schwab, S. Khanna, D. J. Wozniak, S. Roy, and C. K. Sen. Electric field based dressing disrupts mixed-species bacterial biofilm infection and restores functional wound healing. Ann. Surg. 2017. https://doi.org/10.1097/SLA.0000000000002504.
Ben-Shalom, N., Z. Nevo, A. Patchornik, and D. Robinson. Novel injectable chitosan mixtures forming hydrogels. Google Patents, 2012.
Bishop, S., M. Walker, A. Rogers, and W. Chen. Importance of moisture balance at the wound-dressing interface. J. Wound Care 12:125–128, 2003.
Boateng, J. S., K. H. Matthews, H. N. Stevens, and G. M. Eccleston. Wound healing dressings and drug delivery systems: a review. J. Pharm. Sci. 97:2892–2923, 2008.
Cen, L., W. Liu, L. Cui, W. Zhang, and Y. Cao. Collagen tissue engineering: development of novel biomaterials and applications. Pediatr. Res. 63:492, 2008.
Chantre, C. O., P. H. Campbell, H. M. Golecki, A. T. Buganza, A. K. Capulli, L. F. Deravi, S. Dauth, S. P. Sheehy, J. A. Paten, and K. Gledhill. Production-scale fibronectin nanofibers promote wound closure and tissue repair in a dermal mouse model. Biomaterials 166:96–108, 2018.
Chen, H., G. Lan, L. Ran, Y. Xiao, K. Yu, B. Lu, F. Dai, D. Wu, and F. Lu. A novel wound dressing based on a Konjac glucomannan/silver nanoparticle composite sponge effectively kills bacteria and accelerates wound healing. Carbohydr. Polym. 183:70–80, 2018.
Cheng, J. Z., A. Farrokhi, A. Ghahary, and R. B. Jalili. Therapeutic use of stem cells in treatment of burn injuries. J. Burn Care Res. 39:175–182, 2018.
Choi, S. M., K. M. Lee, H. J. Kim, I. K. Park, H. J. Kang, H. C. Shin, D. Baek, Y. Choi, K. H. Park, and J. W. Lee. Effects of structurally stabilized EGF and bFGF on wound healing in type I and type II diabetic mice. Acta Biomater. 66:325–334, 2018.
Choi, S. M., H. A. Ryu, K.-M. Lee, H. J. Kim, I. K. Park, W. J. Cho, H.-C. Shin, W. J. Choi, and J. W. Lee. Development of stabilized growth factor-loaded hyaluronate-collagen dressing (HCD) matrix for impaired wound healing. Biomater. Res. 20:9, 2016.
Cole, W. Human acellular dermal matrix paired with silver-zinc coupled electroceutical dressing results in rapid healing of complicated diabetic wounds of mixed etiology: a novel case series. Wounds 28:241–247, 2016.
Dai, T., M. Tanaka, Y. Y. Huang, and M. R. Hamblin. Chitosan preparations for wounds and burns: antimicrobial and wound-healing effects. Expert Rev. Anti Infect. Ther. 9:857–879, 2011.
Darby, I. A., B. Laverdet, F. Bonté, and A. Desmoulière. Fibroblasts and myofibroblasts in wound healing. Clin. Cosmet. Investig. Dermatol. 7:301, 2014.
Dash, M., F. Chiellini, R. M. Ottenbrite, and E. Chiellini. Chitosan: a versatile semi-synthetic polymer in biomedical applications. Prog. Polym. Sci. 36:981–1014, 2011.
Daunton, C., S. Kothari, L. Smith, and D. Steele. A history of materials and practices for wound management. Wound Pract. Res. 20:174, 2012.
d’Ayala, G. G., M. Malinconico, and P. Laurienzo. Marine derived polysaccharides for biomedical applications: chemical modification approaches. Molecules 13:2069–2106, 2008.
Dhivya, S., V. V. Padma, and E. Santhini. Wound dressings: a review. Biomedicine 5:22, 2015.
Doillon, C. J., and F. H. Silver. Collagen-based wound dressing: effects of hyaluronic acid and firponectin on wound healing. Biomaterials 7:3–8, 1986.
Dong, Y., M. Rodrigues, X. Li, S. H. Kwon, N. Kosaric, S. Khong, Y. Gao, W. Wang, and G. C. Gurtner. Injectable and tunable gelatin hydrogels enhance stem cell retention and improve cutaneous wound healing. Adv. Func. Mater. 27:1606619, 2017.
Dumville, J. C., S. Deshpande, S. O’Meara, and K. Speak. Hydrocolloid dressings for healing diabetic foot ulcers. Cochrane Database Syst. Rev., 2012.
Dumville, J. C., M. O. Soares, S. O’Meara, and N. Cullum. Systematic review and mixed treatment comparison: dressings to heal diabetic foot ulcers. Diabetologia 55:1902–1910, 2012.
Edmonds, M., J. L. Lázaro-Martínez, J. M. Alfayate-García, J. Martini, J.-M. Petit, G. Rayman, R. Lobmann, L. Uccioli, A. Sauvadet, and S. Bohbot. Sucrose octasulfate dressing versus control dressing in patients with neuroischaemic diabetic foot ulcers (Explorer): an international, multicentre, double-blind, randomised, controlled trial. Lancet Diabetes Endocrinol. 6:186–196, 2018.
Eming, S. A., T. Krieg, and J. M. Davidson. Inflammation in wound repair: molecular and cellular mechanisms. J. Investig. Dermatol. 127:514–525, 2007.
Enoch, S., and D. J. Leaper. Basic science of wound healing. Surg. Oxf. Int. Ed. 26:31–37, 2008.
Erdag, G., and R. L. Sheridan. Fibroblasts improve performance of cultured composite skin substitutes on athymic mice. Burns 30:322–328, 2004.
Faucher, N., H. Safar, M. Baret, A. Philippe, and R. Farid. Superabsorbent dressings for copiously exuding wounds. Br. J. Nurs. 21:S22–S28, 2012.
Field, C. K., and M. D. Kerstein. Overview of wound healing in a moist environment. Am. J. Surg. 167:S2–S6, 1994.
Fischer, L. J., S. McIlhenny, T. Tulenko, N. Golesorkhi, P. Zhang, R. Larson, J. Lombardi, I. Shapiro, and P. J. DiMuzio. Endothelial differentiation of adipose-derived stem cells: effects of endothelial cell growth supplement and shear force. J. Surg. Res. 152:157–166, 2009.
Fleck, C. A., and R. Simman. Modern collagen wound dressings: function and Purpose. J. Am. Col. Certif. Wound Spec. 2:50–54, 2010.
Fonder, M. A., G. S. Lazarus, D. A. Cowan, B. Aronson-Cook, A. R. Kohli, and A. J. Mamelak. Treating the chronic wound: a practical approach to the care of nonhealing wounds and wound care dressings. J. Am. Acad. Dermatol. 58:185–206, 2008.
Gantwerker, E. A., and D. B. Hom. Skin: histology and physiology of wound healing. Clin. Plast. Surg. 39:85–97, 2012.
Greenhalgh, D. G. The role of growth factors in wound healing. J. Trauma 41:159–167, 1996.
Grice, E. A., H. H. Kong, S. Conlan, C. B. Deming, J. Davis, A. C. Young, G. G. Bouffard, R. W. Blakesley, P. R. Murray, and E. D. Green. Topographical and temporal diversity of the human skin microbiome. Science 324:1190–1192, 2009.
Guarderas, F., Y. Leavell, T. Sengupta, M. Zhukova, and T. L. Megraw. Assessment of chicken-egg membrane as a dressing for wound healing. Adv. Skin Wound Care 29:131–134, 2016.
Hilton, J., D. Williams, B. Beuker, D. Miller, and K. Harding. Wound dressings in diabetic foot disease. Clin. Infect. Dis. 39:S100–S103, 2004.
Hoffman, A. S. Hydrogels for biomedical applications. Adv. Drug Deliv. Rev. 64:18–23, 2012.
Hollinworth, H., and M. Collier. Nurses’ views about pain and trauma at dressing changes: results of a national survey. J. Wound Care 9:369–373, 2000.
Holm-Pedersen, P., and B. Zederfeldt. Granulation tissue formation in subcutaneously implanted cellulose sponges in young and old rats. Scand. J. Plast. Reconstr. Surg. 5:13–16, 1971.
Hopewell, J. The skin: its structure and response to ionizing radiation. Int. J. Radiat. Biol. 57:751–773, 1990.
Hsu, B. B., S. R. Hagerman, K. Jamieson, S. A. Castleberry, W. Wang, E. Holler, J. Y. Ljubimova, and P. T. Hammond. Multifunctional self-assembled films for rapid hemostat and sustained anti-infective delivery. ACS Biomater. Sci. Eng. 1:148–156, 2015.
Huang, T., H. Xu, K. Jiao, L. Zhu, H. R. Brown, and H. Wang. A novel hydrogel with high mechanical strength: a macromolecular microsphere composite hydrogel. Adv. Mater. 19:1622–1626, 2007.
Hunt, T. K., H. Hopf, and Z. Hussain. Physiology of wound healing. Adv Skin Wound Care 13:6, 2000.
Jankowska, D. A., M. B. Bannwarth, C. Schulenburg, G. Faccio, K. Maniura-Weber, R. M. Rossi, L. Scherer, M. Richter, and L. F. Boesel. Simultaneous detection of pH value and glucose concentrations for wound monitoring applications. Biosens. Bioelectron. 87:312–319, 2017.
Jayakumar, R., M. Prabaharan, P. T. Sudheesh Kumar, S. V. Nair, and H. Tamura. Biomaterials based on chitin and chitosan in wound dressing applications. Biotechnol. Adv. 29:322–337, 2011.
Jeffcoate, W. J., P. Price, and K. G. Harding. Wound healing and treatments for people with diabetic foot ulcers. Diabetes 20:S78–S89, 2004.
Jones, V., J. E. Grey, and K. G. Harding. Wound dressings. BMJ 332:777–780, 2006.
Jung, R., Y. Kim, H.-S. Kim, and H.-J. Jin. Antimicrobial properties of hydrated cellulose membranes with silver nanoparticles. J. Biomater. Sci. Polym. Ed. 20:311–324, 2009.
Kamoun, E. A., E. R. S. Kenawy, and X. Chen. A review on polymeric hydrogel membranes for wound dressing applications: PVA-based hydrogel dressings. J. Adv. Res. 8:217–233, 2017.
Kassal, P., M. Zubak, G. Scheipl, G. J. Mohr, M. D. Steinberg, and I. M. Steinberg. Smart bandage with wireless connectivity for optical monitoring of pH. Sens. Actuators B 246:455–460, 2017.
Khamrai, M., S. L. Banerjee, and P. P. Kundu. Modified bacterial cellulose based self-healable polyeloctrolyte film for wound dressing application. Carbohydr. Polym. 174:580–590, 2017.
Kim, H., S. Park, G. Housler, V. Marcel, S. Cross, and M. Izadjoo. An overview of the efficacy of a next generation electroceutical wound care device. Mil. Med. 181:184–190, 2016.
Kim, I.-Y., S.-J. Seo, H.-S. Moon, M.-K. Yoo, I.-Y. Park, B.-C. Kim, and C.-S. Cho. Chitosan and its derivatives for tissue engineering applications. Biotechnol. Adv. 26:1–21, 2008.
Kolarsick, P. A. J., M. A. Kolarsick, and C. Goodwin. Anatomy and physiology of the skin. J. Dermatol. Nurs. Assoc. 3:203–213, 2011.
Kragh, J. F., J. K. Aden, J. Steinbaugh, M. Bullard, and M. A. Dubick. Gauze vs XSTAT in wound packing for hemorrhage control. Am. J. Emerg. Med. 33:974–976, 2015.
Krejner, A., and T. Grzela. Modulation of matrix metalloproteinases MMP-2 and MMP-9 activity by hydrofiber-foam hybrid dressing-relevant support in the treatment of chronic wounds. Central-Eur. J. Immunol. 40:391, 2015.
Krieger, B. R., D. M. Davis, J. E. Sanchez, J. J. Mateka, V. N. Nfonsam, J. C. Frattini, and J. E. Marcet. The use of silver nylon in preventing surgical site infections following colon and rectal surgery. Dis. Colon Rectum 54:1014–1019, 2011.
Ksander, G., and Y. Ogawa. Collagen wound healing matrices and process for their production. Google Patents, 1990.
Lammers, G., G. S. Tjabringa, J. Schalkwijk, W. F. Daamen, and T. H. van Kuppevelt. A molecularly defined array based on native fibrillar collagen for the assessment of skin tissue engineering biomaterials. Biomaterials 30:6213–6220, 2009.
Lansdown, A. B. G. Bioactive Dressings: Old Ideas, New Technology. London: MA Healthcare, 2007.
Lee, K. Y., and D. J. Mooney. Hydrogels for tissue engineering. Chem. Rev. 101:1869–1879, 2001.
Lee, K. Y., and D. J. Mooney. Alginate: properties and biomedical applications. Prog. Polym. Sci. 37:106–126, 2012.
Leung, C. Y. P. Microstructure-based systems, apparatus, and methods for wound closure. US Patent App, 2017.
Li, J., J. Chen, and R. Kirsner. Pathophysiology of acute wound healing. Clin. Dermatol. 25:9–18, 2007.
Liechty, K. W., H. B. Kim, N. S. Adzick, and T. M. Crombleholme. Fetal wound repair results in scar formation in interleukin-10-deficient mice in a syngeneic murine model of scarless fetal wound repair. J. Pediatr. Surg. 35:866–872, 2000.
Lin, S., H. Yuk, T. Zhang, G. A. Parada, H. Koo, C. Yu, and X. Zhao. Stretchable hydrogel electronics and devices. Adv. Mater. 28:4497–4505, 2016.
Lorenz, H., and N. Adzick. Scarless skin wound repair in the fetus. West. J. Med. 159:350, 1993.
Majno, G. The healing hand: man and wound in the ancient world. Plast. Reconstr. Surg. 57:230, 1976.
Malafaya, P. B., G. A. Silva, and R. L. Reis. Natural-origin polymers as carriers and scaffolds for biomolecules and cell delivery in tissue engineering applications. Adv. Drug Deliv. Rev. 59:207–233, 2007.
Malmsjö, M., L. Gustafsson, S. Lindstedt Ingemansson, and R. Ingemansson. Negative pressure wound therapy-associated tissue trauma and pain: a controlled in vivo study comparing foam and gauze dressing removal by immunohistochemistry for substance p and calcitonin gene-related peptide in the wound edge. Ostomy-Wound Manag. 57:30–35, 2011.
Mano, J. F., G. A. Silva, H. S. Azevedo, P. B. Malafaya, R. A. Sousa, S. S. Silva, L. F. Boesel, J. M. Oliveira, T. C. Santos, A. P. Marques, N. M. Neves, and R. L. Reis. Natural origin biodegradable systems in tissue engineering and regenerative medicine: present status and some moving trends. J. R. Soc. Interface 4:999–1030, 2007.
Martin, P. Wound healing: aiming for perfect skin regeneration. Science 276:75–81, 1997.
Matsumura, H., R. Imai, N. Ahmatjan, Y. Ida, M. Gondo, D. Shibata, and K. Wanatabe. Removal of adhesive wound dressing and its effects on the stratum corneum of the skin: comparison of eight different adhesive wound dressings. Int Wound J 11:50–54, 2014.
Metcalf, D., D. Parsons, and P. Bowler. A next-generation antimicrobial wound dressing: a real-life clinical evaluation in the UK and Ireland. J. Wound Care 25:132–138, 2016.
Metcalf, D. G., D. Parsons, and P. G. Bowler. Clinical safety and effectiveness evaluation of a new antimicrobial wound dressing designed to manage exudate, infection and biofilm. Int. Wound J. 14:203–213, 2017.
Mi, F.-L., S.-S. Shyu, Y.-B. Wu, S.-T. Lee, J.-Y. Shyong, and R.-N. Huang. Fabrication and characterization of a sponge-like asymmetric chitosan membrane as a wound dressing. Biomaterials 22:165–173, 2001.
Mi, F.-L., Y.-C. Tan, H.-F. Liang, and H.-W. Sung. In vivo biocompatibility and degradability of a novel injectable-chitosan-based implant. Biomaterials 23:181–191, 2002.
Mills, S. J., J. J. Ashworth, S. C. Gilliver, M. J. Hardman, and G. S. Ashcroft. The sex steroid precursor DHEA accelerates cutaneous wound healing via the estrogen receptors. J. Investig. Dermatol. 125:1053–1062, 2005.
Morimoto, N., S. Suzuki, Y. Saso, K. Tomihata, T. Taira, Y. Takahashi, and N. Morikawa. Viability and function of autologous and allogeneic fibroblasts seeded in dermal substitutes after implantation. Wound Repair Regen. 13:A14, 2005.
Mostafalu, P., G. Kiaee, G. Giatsidis, A. Khalilpour, M. Nabavinia, M. R. Dokmeci, S. Sonkusale, D. P. Orgill, A. Tamayol, and A. Khademhosseini. A textile dressing for temporal and dosage controlled drug delivery. Adv. Funct. Mater. 27:1702399, 2017.
Moura, L. I., A. M. Dias, E. Carvalho, and H. C. de Sousa. Recent advances on the development of wound dressings for diabetic foot ulcer treatment: a review. Acta Biomater. 9:7093–7114, 2013.
Mozalewska, W., R. Czechowska-Biskup, A. K. Olejnik, R. A. Wach, P. Ulański, and J. M. Rosiak. Chitosan-containing hydrogel wound dressings prepared by radiation technique. Radiat. Phys. Chem. 134:1–7, 2017.
Münter, K.-C., S. De Lange, T. Eberlein, A. Andriessen, and M. Abel. Handling properties of a superabsorbent dressing in the management of patients with moderate-to-very high exuding wounds. J. Wound Care 27:246–253, 2018.
Nwomeh, B. C., D. R. Yager, and I. Cohen. Physiology of the chronic wound. Clin. Plast. Surg. 25:341–356, 1998.
Pandit, A. S., and D. S. Faldman. Effect of oxygen treatment and dressing oxygen permeability on wound healing. Wound Repair Regen. 2:130–137, 1994.
Parenteau-Bareil, R., R. Gauvin, and F. Berthod. Collagen-based biomaterials for tissue engineering applications. Materials 3:1863–1887, 2010.
Percival, S. L., S. McCarty, J. A. Hunt, and E. J. Woods. The effects of pH on wound healing, biofilms, and antimicrobial efficacy. Wound Repair Regen. 22:174–186, 2014.
Radhakumary, C., M. Antonty, and K. Sreenivasan. Drug loaded thermoresponsive and cytocompatible chitosan based hydrogel as a potential wound dressing. Carbohydr. Polym. 83:705–713, 2011.
Richard, J., J. Martini, M. B. Faraill, J. M’Bemba, M. Lepeut, F. Truchetet, S. Ehrler, S. Schuldiner, A. Sauvadet, and S. Bohbot. Management of diabetic foot ulcers with a TLC-NOSF wound dressing. J. Wound Care 21:142–147, 2012.
Richmond, N. A., A. D. Maderal, and A. C. Vivas. Evidence-based management of common chronic lower extremity ulcers. Dermatol. Ther. 26:187–196, 2013.
Rodero, M. P., and K. Khosrotehrani. Skin wound healing modulation by macrophages. Int. J. Clin. Exp. Pathol. 3:643, 2010.
Rogozinski, W. J. Modifiable, semi-permeable, wound dressing. Google Patents., 1993.
Rowlatt, U. Intrauterine wound healing in a 20 week human fetus. Virchows Arch A 381:353–361, 1979.
Sánchez-Sánchez, R., A. Brena-Molina, V. Martínez-López, Y. Melgarejo-Ramírez, L. Tamay de Dios, R. Gómez-García, M. L. Reyes-Frías, L. Rodríguez-Rodríguez, D. Garciadiego-Cázares, H. Lugo-Martínez, C. Ibarra, M. E. Martínez-Pardo, and C. Velasquillo-Martínez. Generation of two biological wound dressings as a potential delivery system of human adipose-derived mesenchymal stem cells. ASAIO J 61:718–725, 2015.
Sannino, A., C. Demitri, and M. Madaghiele. Biodegradable cellulose-based hydrogels: design and applications. Materials 2:353–373, 2009.
Sato, Y., T. Ohshima, and T. Kondo. Regulatory role of endogenous interleukin-10 in cutaneous inflammatory response of murine wound healing. Biochem. Biophys. Res. Commun. 265:194–199, 1999.
Schmid-Wendtner, M.-H., and H. C. Korting. The pH of the skin surface and its impact on the barrier function. Skin Pharmacol. Physiol. 19:296–302, 2006.
Schneider, L. A., A. Korber, S. Grabbe, and J. Dissemond. Influence of pH on wound-healing: a new perspective for wound-therapy? Arch. Dermatol. Res. 298:413–420, 2007.
Seaman, S. Dressing selection in chronic wound management. J. Am. Podiatr. Med. Assoc. 92:24–33, 2002.
Segre, J. A., C. Bauer, and E. Fuchs. Klf4 is a transcription factor required for establishing the barrier function of the skin. Nat. Genet. 22:356, 1999.
Sell, S. A., P. S. Wolfe, K. Garg, J. M. McCool, I. A. Rodriguez, and G. L. Bowlin. The use of natural polymers in tissue engineering: a focus on electrospun extracellular matrix analogues. Polymers 2:522–553, 2010.
Shah, J. B. The history of wound care. J. Am. Col. Certif. Wound Spec. 3:65–66, 2011.
Shetty, S., and S. Gokul. Keratinization and its disorders. Oman Med. J. 27:348–357, 2012.
Shirazaki, P., J. Varshosaz, and A. Z. Kharazi. Electrospun gelatin/poly(glycerol sebacate) membrane with controlled release of antibiotics for wound dressing. Adv. Biomed. Res. 6:105, 2017.
Siddiqui, A. R., and J. M. Bernstein. Chronic wound infection: facts and controversies. Clin. Dermatol. 28:519–526, 2010.
Silver, F., V. Sharma, D. R. Berndt, and L. E. Marn. Collagen-based wound dressing and method for applying same. Google Patents., 1993.
Sims, S. K., S. Bowling, S. P. Dituro, B. S. Kheirabadi, and F. Butler. Management of external hemorrhage in tactical combat casualty care: the adjunctive use of XStat TM compressed hemostatic sponges. J. Spec. Oper. Med. 16:19–28, 2016.
Singla, R., S. Soni, P. M. Kulurkar, A. Kumari, M. S. V. Patial, Y. S. Padwad, and S. K. Yadav. In situ functionalized nanobiocomposites dressings of bamboo cellulose nanocrystals and silver nanoparticles for accelerated wound healing. Carbohydr. Polym. 155:152–162, 2017.
Singla, R., S. Soni, V. Patial, P. M. Kulurkar, A. Kumari, Y. S. Padwad, and S. K. Yadav. In vivo diabetic wound healing potential of nanobiocomposites containing bamboo cellulose nanocrystals impregnated with silver nanoparticles. Int. J. Biol. Macromol. 105:45–55, 2017.
Sipos, P., H. Gyory, K. Hagymási, P. Ondrejka, and A. Blázovics. Special wound healing methods used in ancient Egypt and the mythological background. World J. Surg. 28:211, 2004.
Skórkowska-Telichowska, K., M. Czemplik, A. Kulma, and J. Szopa. The local treatment and available dressings designed for chronic wounds. J. Am. Acad. Dermatol. 68:e117–e126, 2013.
Solway, D. R., W. A. Clark, and D. J. Levinson. A parallel open-label trial to evaluate microbial cellulose wound dressing in the treatment of diabetic foot ulcers. Int. Wound J. 8:69–73, 2011.
Sood, A., M. S. Granick, and N. L. Tomaselli. Wound dressings and comparative effectiveness data. Adv. Wound Care (New Rochelle) 3:511–529, 2014.
Soppirnath, K. S., and T. M. Aminabhavi. Water transport and drug release study from cross-linked polyacrylamide grafted guar gum hydrogel microspheres for the controlled release application. Eur. J. Pharm. Biopharm. 53:87–98, 2002.
Sprenger, A., S. Weber, M. Zarai, R. Engelke, J. M. Nascimento, C. Gretzmeier, M. Hilpert, M. Boerries, C. Has, H. Busch, L. Bruckner-Tuderman, and J. Dengjel. Consistency of the proteome in primary human keratinocytes with respect to gender, age, and skin localization. Mol. Cell. Proteom. 12:2509–2521, 2013.
Stang, D. The use of Aquacel Ag in the management of diabetic foot ulcers. The Diabetic Foot, 2004.
Starr, A. H. Plaster or bandage for skin application. Google Patents., 1951.
Stashak, T. S., E. Farstvedt, and A. Othic. Update on wound dressings: indications and best use. Clin. Tech. Equine Pract. 3:148–163, 2004.
Steed, D. L. The role of growth factors in wound healing. Surg. Clin. N. Am. 77:575–586, 1997.
Swisher, S. L., M. C. Lin, A. Liao, E. J. Leeflang, Y. Khan, F. J. Pavinatto, K. Mann, A. Naujokas, D. Young, S. Roy, M. R. Harrison, A. C. Arias, V. Subramanian, and M. M. Maharbiz. Impedance sensing device enables early detection of pressure ulcers in vivo. Nat. Commun. 6:6575, 2015.
Thet, N. T., D. R. Alves, J. E. Bean, S. Booth, J. Nzakizwanayo, A. E. Young, B. V. Jones, and A. T. Jenkins. Prototype development of the intelligent hydrogel wound dressing and its efficacy in the detection of model pathogenic wound biofilms. ACS Appl. Mater. Interfaces 8:14909–14919, 2016.
Tisosky, A. J., O. Iyoha-Bello, N. Demosthenes, G. Quimbayo, T. Coreanu, and A. Abdeen. Use of a silver nylon dressing following total hip and knee arthroplasty decreases the postoperative infection rate. JAAOS Global Res. Rev. 1:e034, 2017.
Tonnesen, M. G., X. Feng, and R. A. Clark. Angiogenesis in wound healing. J. Investig. Dermatol. Symposium Proceedings 1:40–46, 2000.
van Rijswijk, L., and J. Beitz. The traditions and terminology of wound dressings: food for thought. J. Wound Ostomy Cont. Nurs. 25:116–122, 1998.
Velander, P., C. Theopold, T. Hirsch, O. Bleiziffer, B. Zuhaili, M. Fossum, D. Hoeller, R. Gheerardyn, M. Chen, S. Visovatti, H. Svensson, F. Yao, and E. Eriksson. Impaired wound healing in an acute diabetic pig model and the effects of local hyperglycemia. Wound Repair Regen. 16:288–293, 2008.
Velnar, T., T. Bailey, and V. Smrkolj. The wound healing process: an overview of the cellular and molecular mechanisms. J. Int. Med. Res. 37:1528–1542, 2009.
Vowden, K. Complex wound or complex patient? Strategies for treatment. Br. J. Commun. Nurs. Suppl: S6, S8, S10 passim, 2005.
Wang, W., S. Lin, Y. Xiao, Y. Huang, Y. Tan, L. Cai, and X. Li. Acceleration of diabetic wound healing with chitosan-crosslinked collagen sponge containing recombinant human acidic fibroblast growth factor in healing-impaired STZ diabetic rats. Life Sci. 82:190–204, 2008.
Wang, Y., and P. K. Maitz. Advances and new technologies in the treatment of burn injury. Adv. Drug Deliv. Rev. 123:1–2, 2018.
Wang, S., H. Yang, Z. Tang, G. Long, and W. Huang. Wound dressing model of human umbilical cord mesenchymal stem cells-alginates complex promotes skin wound healing by paracrine signaling. Stem Cells Int., 2016. https://doi.org/10.1155/2016/3269267.
Weller, C., and G. Sussman. Wound dressings update. J. Pharm. Pract. Res. 36:318–324, 2006.
Welshhans, J. L., and D. B. Hom. Soft tissue principles to minimize scarring: an overview. Facial Plast. Surg. Clin. N. Am. 25:1–13, 2017.
White, R. A multinational survey of the assessment of pain when removing dressings. Wounds uK 4:14, 2008.
Wiegand, C., T. Heinze, and U. C. Hipler. Comparative in vitro study on cytotoxicity, antimicrobial activity, and binding capacity for pathophysiological factors in chronic wounds of alginate and silver-containing alginate. Wound Repair Regen. 17:511–521, 2009.
Wysocki, A. B. Skin anatomy, physiology, and pathophysiology. Nurs. Clin. N. Am. 34:777–797, 1999.
Xu, Q., A. Sigen, Y. Gao, L. Guo, J. Creagh-Flynn, D. Zhou, U. Greiser, Y. Dong, F. Wang, H. Tai, W. Liu, W. Wang, and W. Wang. A hybrid injectable hydrogel from hyperbranched PEG macromer as a stem cell delivery and retention platform for diabetic wound healing. Acta Biomater. 75:63–74, 2018.
Yanaga, H., Y. Udoh, T. Yamauchi, M. Yamamoto, K. Kiyokawa, Y. Inoue, and Y. Tai. Cryopreserved cultured epidermal allografts achieved early closure of wounds and reduced scar formation in deep partial-thickness burn wounds (DDB) and split-thickness skin donor sites of pediatric patients. Burns 27:689–698, 2001.
Ya-Xian, Z., T. Suetake, and H. Tagami. Number of cell layers of the stratum corneum in normal skin-relationship to the anatomical location on the body, age, sex and physical parameters. Arch. Dermatol. Res. 291:555–559, 1999.
Yosipovitch, G., G. L. Xiong, E. Haus, L. Sackett-Lundeen, I. Ashkenazi, and H. I. Maibach. Time-dependent variations of the skin barrier function in humans: transepidermal water loss, stratum corneum hydration, skin surface pH, and skin temperature. J. Investig. Dermatol. 110:20–23, 1998.
You, H. J., and S. K. Han. Cell therapy for wound healing. J. Korean Med. Sci. 29:311–319, 2014.
Acknowledgments
We thank Medicine by Design-EMHSeed and Ontario Institute for Regenerative Medicine for supporting the project.
Author information
Authors and Affiliations
Corresponding author
Additional information
Associate Editor Smadar Cohen oversaw the review of this article.
Rights and permissions
About this article
Cite this article
Aljghami, M.E., Saboor, S. & Amini-Nik, S. Emerging Innovative Wound Dressings. Ann Biomed Eng 47, 659–675 (2019). https://doi.org/10.1007/s10439-018-02186-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10439-018-02186-w