Abstract
Malignant gliomas are the most common tumors in the central nervous system (CNS) and, unfortunately, are also the most deadly. The lethal nature of malignant gliomas is due in large part to their unique and distinctive ability to invade the surrounding neural tissue. The invasive and dispersive nature of these tumors makes them particularly challenging to treat, and currently there are no effective therapies for malignant gliomas. The brain tumor microenvironment plays a particularly important role in mediating the invasiveness of gliomas, and, therefore, understanding its function is key to developing novel therapies to treat these deadly tumors. A defining aspect of the tumor microenvironment of gliomas is the unique composition of the extracellular matrix that enables tumors to overcome the typically inhibitory environment found in the CNS. One conspicuous component of the glioma tumor microenvironment is the neural-specific ECM molecule, brain-enriched hyaluronan binding (BEHAB)/brevican (B/b). B/b is highly overexpressed in gliomas, and its expression in these tumors contributes importantly to the tumor invasiveness and aggressiveness. However, B/b is a complicated protein with multiple splice variants, cleavage products, and glycoforms that contribute to its complex functions in these tumors and provide unique targets for tumor therapy. Here we review the role of B/b in glioma tumor microenvironment and explore targeting of this protein for glioma therapy.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Proteoglycan
- Glioma dispersion
- Glycosylation
- ADAMTS4
- MMP
- Lectican
- Chondroitin sulfate
- Fibronectin
- Glioma-initiating cells
- EGF receptor
- Hyaluronan
- ECM
- TME
- BEHAB
- Glycoform
7.1 Introduction
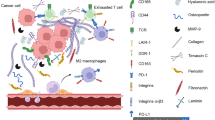
The tumor microenvironment is comprised of the cells that directly make up the tumor, neighboring normal/non-transformed cells, the extracellular matrix (ECM), and secreted molecules found within this space [5, 52, 140]. Importantly, interactions between these constituents define the molecular properties of the specific tumor [52]. High-grade gliomas are the most frequently detected and virulent form of intracranial tumors, but they are also commonly impervious to currently available treatments, including surgery as well as chemotherapy and radiation [125]. One of the primary reasons as to why gliomas are insusceptible to these therapies is due to the fact that gliomas are able to infiltrate surrounding tissues and, thus, are considered to be highly invasive [109, 126].
Many of the components within the tumor microenvironment support the dispersion and heightened motility of glioma cells. Specifically, gliomas exhibit aberrant expression patterns of cell adhesion molecules, ECM molecules, proteolytic enzymes that remodel the ECM, and growth factors [5, 140]. One such ECM molecule that is overexpressed in gliomas is the chondroitin sulfate proteoglycan (CSPG): brain-enriched hyaluronan-binding protein (BEHAB)/brevican (B/b) [42, 67]. This enhanced expression of B/b leads to an increase in the aggressiveness of the resulting tumors [35, 62, 83, 98, 134, 154]. It is important to note that that there are a number of B/b isoforms that are upregulated in glioma samples [133].
Mechanistically, B/b is cleaved by a disintegrin and metalloproteainase with thrombospondin motifs (ADAMTS)-4 [86], and this causes the abnormal adhesion of glioma cells to fibronectin, and the overall motility of the glioma cells is enhanced [62], thereby leading to tumor invasion. Moreover, as a result of an increase in the presence of B/b, fibronectin secretion is increased, as is the expression of a number of cell adhesion molecules [62]. Taken together, these molecular and structural changes to the tumor microenvironment favor glioma cell invasiveness and movement, which contributes to the resistance of gliomas to current therapeutics [109].
In this chapter we first focus on the function of B/b in normal/non-transformed cells and then delve into the molecular composition of the tumor microenvironment. Next, we dissect the specific role that B/b plays in promoting glioma cell invasion and motility. Additionally, the mechanisms underlying these processes will be discussed and will shed further light onto why the current treatments are not more effective at targeting gliomas and why targeting B/b could be a new therapeutic strategy.
7.2 Brevican: Structure and Function
7.2.1 Structure
B/b is a member of the lectican family of CSPGs along with versican, neurocan, and aggrecan [7, 113, 147]. In terms of structure, members of the lectican family are quite homologous. More specifically, these CSPGs contain a N-terminus, which is known as the hyaluronan (HA)-binding domain and link protein-like region, that mediates interactions between the lectican family members and HA (see Fig. 7.1, [104]). The binding of these CSPGs to HA is a key step in overall organization of the ECM. Within the C-terminus, there is an epidermal growth factor (EGF)-like domain that is characteristic of proteins found within the ECM, a lectin-like domain, and a complement regulatory protein-like domain [120]. It is through this lectin domain that the lecticans can bind tenascin-R, a glycoprotein present within the ECM. Furthermore, this region of CSPGs can also bind to glycolipids found on the cell surface that have been sulfated, which promotes cell adhesion [89].
BEHAB/brevican structure diversity, glycosylation, and cleavage. B/b is made as a both a secreted and GPI-linked isoform. It is routinely cleaved at a defined ADAMTS4/5 cleavage site leading to N-terminal and C-terminal fragments. In addition, work has shown that there is a lot of microheterogeneity in the glycosylation of this protein. In gliomas all forms of B/b are upregulated with the N-terminal cleavage fragment perhaps being the most critical functionally. In addition there are glioma-specific glycoforms that are generated that may provide ideal targets for glioma therapy
CSPGs consist of a core protein that is decorated with chondroitin sulfate (CS) sugar chains, which bind to the protein in the CS attachment region (Fig. 7.1). The amount of sugar units that can be added to the protein varies widely between members of the lectican family with B/b having 1–3 CS chains that adorn the protein core [7]. The core protein has been shown to hinder neurite outgrowth in cultured neuroblastoma cells [64], and the CS region of the CSPG has also been reported to inhibit overall growth and regeneration in the central nervous system (CNS) [21, 70]. A substantial body of work utilizing the bacterial enzyme, chondroitinase ABC (chABC), to digest CS chains supports these findings. In these studies, application of chABC resulted in recovery after spinal cord injury [14, 75, 136]. Additionally, administration of chABC has been reported to enhance axonal regeneration within the CNS in undamaged animals [31, 90]. Pizzorusso and colleagues used this enzyme to reopen the critical period and, thus, restore plasticity within the visual system of adult rats [105]. Taken together, both the core protein and the CS region of CSPGs inhibit growth and regeneration within the CNS and thus play a key role in restricting overall brain plasticity.
Specifically, B/b can exist in a number of different ways: a glycosylphosphatidylinositol form that is anchored to the plasma membrane [119, 120] and a form that is secreted right into the ECM [119], and additionally, B/b can be present as a glycosylated proteoglycan or as a core protein that is not glycosylated (Fig. 7.1, [146]). Interestingly, the form that is anchored to the plasma membrane was detected primarily in white matter tracts where axons are located as well glial cells that were classified as diffusely distributed throughout the brain. The form that is secreted into the ECM was highly expressed in the gray matter within the cerebral cortex, hippocampus, cerebellum, and particular thalamic nuclei [119]. Through Western blot analysis, in the adult rat brain, the full-length B/b protein runs at 145 kDa, but cleavage products have been described at 90 kDa and 50 kDa [145, 154].
Temporally, B/b expression is first detected on embryonic day 15 (E15). In all assayed regions of the CNS, the onset of B/b expression occurred after neurogenesis and instead was consistent with the generation of glial cells [66]. It has also been demonstrated that B/b expression is upregulated in response to injuries within the brain [41]. Following a stab wound to the adult rat brain, B/b was detected in regions of active gliosis [65], and, similarly, B/b expression within astrocytes was increased in response to lesions introduced into the entorhinal cortex in rats [127]. In that same vein, B/b mRNA was dramatically increased within the glial scar following cryo-injury in mice [64].
7.2.2 Function
B/b is one of the molecular constituents of the perineuronal net (PNN) that is found within the CNS. PNNs surround the cell body and proximal neurites of particular populations of neurons within the CNS. Typically, these cells are GABAergic interneurons, but they also can be found around excitatory cells. This structure serves to restrict plasticity by closing the critical period [9, 17, 23, 24, 48, 53, 58, 59, 124, 153]. Other work postulates that PNNs provide a buffering mechanism to preserve the balance of cationic charges within the extracellular milieu [17, 18, 54, 55]. Using B/b deficient mice, Bekku and colleagues revealed that B/b regulates the assembly of the proteoglycan, phosphacan, and tenascin-R at Nodes of Ranvier within the CNS [8], which is likely critical in action potential propagation. Proper B/b expression is also needed to maintain normal speeds of synaptic transmission at the calyx of Held in the medial nucleus of the trapezoid body within the brainstem, where PNNs are found in abundance [13]. Studies performed using B/b knockout mice highlight a potential role for B/b in modulating long-term potentiation in the CA1 region of the hippocampus. Of particular interest, the mice lacking B/b displayed less prominent PNNs, meaning that they were less condensed and focused at the cellular surface and instead exhibited a more diffuse expression pattern [15].
7.3 Gliomas
7.3.1 Invasion and the Tumor Microenvironment
The tumor microenvironment describes the environment around a particular tumor and consists of both cellular and non-cellular components [5, 52, 140]. It is important to note that the tumor cells and the other constituents of the microenvironment interact with one another, and this can influence the growth and spread of the tumor [52]. In gliomas their most conspicuous ability is their invasive properties within the central nervous system, which are typically very inhibitory to cellular movement.
The high mortality rate of patients with high-grade gliomas is explained by the fact that gliomas uniquely invade the central nervous system [109, 126]. The neural ECM is usually thought of as an inhibitory environment, one that is not conducive to large-scale reorganization or remodeling; this has been attributed to the high presence of CSPGs [107, 113]. Gliomas are able to circumvent this inhibitory barrier. One of the main ways gliomas are able to do this is through the secretion of molecules that facilitate cell adhesion and movement, which include fibronectin and collagen [12, 22, 30, 44, 45, 99, 106]. Other ECM molecules have been demonstrated to regulate the phenotypic characteristics of gliomas including laminin, vitronectin, and tenascin-C. Using in vitro assays, it has been reported that glioma cells produce and secrete laminin [87, 103]. Expression of the glycoprotein, vitronectin, is correlated with the glioma grade, and its expression has been linked to increased cell survival of glioma cells [131]. Similarly, tenascin-C expression is also linked to glioma grade, and its expression is thought to be involved in mediating cell adhesion, migration, and cell dispersion [57, 152]. Gliomas have also been shown to contain high levels of other ECM components like osteopontin, secreted protein acidic and rich in cysteine (SPARC), and thrombospondin [10]. Expressions of B/b, neurocan, and versican are also increased in glioma samples [100, 132, 134].
Enhanced expression of MMPs is characteristic of many tumor types, including gliomas. These enzymes degrade parts of the ECM, which then allows for the glioma cells to move through the ECM and infiltrate surrounding tissues. The MMPs that are upregulated in glioma cells include MMP-2, 3, 7, 9, 12, 13, 14, 16, 19, and 26 [33, 46, 63, 71, 73, 76, 85, 92, 106, 111, 115, 117, 118, 137, 138, 141, 148,149,150]. Other enzymes that are also responsible for the invasive properties of glioma cells are cathepsin B and urokinase-type plasminogen activator [11, 46, 71, 106, 115, 118, 141, 149] and heparanases and sulfatases [82]. In human gliomas, the overexpression of the forkhead box m1b (Foxm1b) factor leads to the enhanced invasion of glioma cells through an increase in transcription of the MMP-2 gene [32, 80].
Growth factors like epidermal growth factor (EGF), basic fibroblast growth factor (bFGF), and transforming growth factor-β (TGF-β) have been reported to mediate glioma cell invasion [28]. Glioma cells commonly display mutations or amplifications in the EGF receptor (EGFR) gene, and there is an increased presence of this receptor on the surface of the tumorigenic cells [81, 96]. Interestingly, the activation of EGFR and extracellular signal-regulated kinase (ERK) is thought to result in an increase in the expression of fibronectin [38, 130, 155], which likely underlies the increase in migration exhibited by glioma cells. Hepatocyte growth factor (HGF) is commonly overexpressed in glioma cells, and, as a result, cell migration pathways are activated, which leads to the enhanced movement of these cells [47]. Similarly, insulin-like growth factor (IGF) is also overexpressed in these tumorigenic cells, and, in specific, an increase in expression of IGFBP2 leads to an upregulation of genes that are involved in cancer cell invasion, including MMP-2 [139]. High levels of the angiogenic factor, angiopoietin-2 (Ang2), are detected in more invasive areas of gliomas, and this enhanced expression induces upregulation of MMP-2 both in vivo and in culture assays [50, 61, 69, 71]. The cell surface chemokine receptor, CXCR4, is also highly expressed in invasive glioma cells [36], and when the receptor interacts with a specific ligand, the Akt and ERK1/2 signaling pathways are activated, which affords glioma cells an increase in survival and cell division. This results in a more invasive phenotype [112, 142].
The canonical hyaluronan receptor , CD44, activates Rac1, which leads to a dramatic restructuring of the actin cytoskeleton within glioma cells. This receptor can be cleaved by ADAMTS10, and the product increases the invasive properties of glioma cells [3, 10, 91]. Rac not only facilitates the rearrangement of the actin cytoskeleton but is also known to increase cell motility [16, 110]. To demonstrate this, investigators inhibited Rac expression and found that glioma cell invasion was decreased [26, 29]. Rac does not work independently to mediate such important events; it has been reported that Rac works with the polypeptide P311 [84, 88]. The nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) is detected at high levels in glioma cells and has been hypothesized to afford these glioma cells an enhanced cell survival rate [114].
Glioma cells also exhibit changes in expression of cell adhesion molecules. For example, glioma cells express focal adhesion kinase (FAK) at higher levels than non-tumor cells [51, 56, 135], which has been linked to increases in cell proliferation [79, 151]. On the other hand, some cell adhesion molecules may exhibit decreased levels of expression in glioma cells. Expression of neural cell adhesion molecule (NCAM), for example, is reduced in glioma cells, which allows them to separate from neighboring cells and disperse into surrounding tissues [101, 116]. Cell surface integrin receptors that help join cells to one another are upregulated in glioma cells; specifically, this includes integrin α3β1, αvβ1, αvβ3, and αvβ5 [74]. Other cell adhesion molecules that display abnormal expression patterns in glioma cells include adhesion molecule on glia/β2 subunit of Na, K-ATPase (AMOG/β2), ephrin receptor tyrosine kinases (EphB2–B3), fibroblast growth factor inducible 14 receptor (Fn14), and protein tyrosine phosphatases zeta/beta [37, 94, 95, 121, 129]. Cadherin molecules also work by joining adjacent cells to one another to form structures like adherens junctions. Any changes to the structure and stability of these junctions result in an increase in the movement and invasiveness of glioma cells [4]. Glioma cells that express high levels of E-cadherin are phenotypically highly invasive [77], while cells that contain high levels of N-cadherin demonstrate the opposite, in that they are less invasive [19, 93].
7.3.2 Glioma-Initiating Cells (GICs)
Gliomas are highly resistant to current therapeutic interventions, and, as a result, patient mortality rates are still quite high [126]. The invasive properties of gliomas are key in their therapeutic resistance; however, also key is the existence of glioma-initiating cells (GICs) within these tumors. In terms of the cellular composition, GICs that are present within the tumor are molecularly distinct from other cells found within the glioma. More specifically, the GICs are capable of self-renewing and exhibit multipotency, which means that they can differentiate into any subpopulation of cells found within the CNS as well as within the tumor itself. After orthotopic transplantation, these stem cells possess the ability to form a tumor that physically matches the parental tumor [25, 39, 123].
The GICs express many of the same proteins as those that are detected within normal stem cell niches [34] including laminin [122], tenascin-C [2, 40], members of the lectican family of CSPGs [68], and phosphacan [1, 2] as well as members of the integrin family [122]. Furthermore the GICs tend to be localized near vasculature within the tumors [43, 122]. It is important to note that the vasculature may develop due to the tumor presence itself, or the GICs might possess the ability to differentiate into endothelial cells, thus forming new blood vessels [108].
7.3.3 A Key Role for B/b in the Glioma Microenvironment
As noted above the interactions with tumors are complex within the tumor microenvironment; however, B/b presents as a uniquely intriguing target within this complex environment, and its specific roles are detailed below.
7.4 The Role of B/b in Gliomas
7.4.1 B/b Expression in Gliomas
Enhanced levels of B/b expression have been detected in human glioma samples, including oligodendrogliomas, all examined grades of astrocytomas, and gliosarcomas, relative to normal brain tissue and tissues derived from non-glioma tumors [42, 67]. More specifically, this increase in B/b expression was detected within the ECM as well as the cytoplasm of glioma cells. Importantly, within higher grades of astrocytomas (grades III and IV), B/b staining was more dispersed, indicative of an increase in infiltration, compared to lower grades [83]. Glioma cell lines (e.g., 9L, CNS-1, and C6) that are propagated under normal culture conditions do not express B/b, but if they are grown as intracranial grafts, then they express B/b. This phenomenon was not noted in cells taken from noninvasive tumors [67].
In rodent and human glioma samples, B/b is cleaved, and the resulting products are a N-terminal fragment that includes the hyaluronan-binding portion of the protein core (~50–60 kDa) and a C-terminal fragment (~90–100 kDa). Full-length B/b runs at ~160 kDa [133, 154].
Gary and colleagues set forth the hypothesis that B/b modulates the invasiveness of gliomas [41]. To examine the properties and behaviors of gliomas further, investigators introduced B/b into CNS-1 cells in vitro through transfection. These cells were transfected either with a green fluorescent protein (GFP) control, full-length B/b, the C-terminus cleavage fragment of B/b, or the N-terminus cleavage fragment of B/b. These cells were then injected into rats to assess the resulting tumors. The rats that were injected with the CNS-1 cells transfected with the various forms of B/b, exhibited a lower survival rate than those rats that received the control cells. Furthermore, the B/b-derived tumors were more invasive and were highly vascular compared to the control tumors. This led to the conclusion that B/b enhances the aggressive properties of gliomas [98].
While it was established that full-length B/b is overexpressed in human glioma samples [42, 67], it was then identified that two novel isoforms of B/b were present in tumor tissues [133]. The two new isoforms were denoted as B/b∆g with a molecular mass of 150 kDa that was found within the membrane fraction and B/bsia, which had a molecular weight higher than 150 kDa and was located in both the membrane and soluble fractions. It is important to note that the benign tumors that were assayed did not express these B/b isoforms, thereby indicating that perhaps these identified forms of B/b could be used as indicators of tumor grade. Furthermore, these isoforms were specifically identified in gliomas and were not present in tissues derived from patients with epilepsy or Alzheimer’s disease. Neither isoform of B/b was found within individuals over 1 year of age. Only the B/b∆g form was faintly detected in samples harvested from embryos at 16 weeks of gestation to infants aged 19 days [133].
Through biochemical analyses, it was determined that these new isoforms of B/b were not cleavage fragments, and the peptide sequences of these forms were identical to the full-length protein, thereby indicating that these forms were derived from the same mRNA transcript. Attention was then focused on determining how these isoforms were molecularly distinct from the full-length protein. Deglycosylating enzymes were used to remove N-linked and O-linked sugars from the protein core as well as CS chains (through the use of chondroitinase), and this revealed that the B/b∆g form was underglycosylated. Further work determined that this particular isoform associates with the cell membrane in a manner distinct from other B/b forms. Importantly, this does not require calcium, interaction with the CS chains, nor the N-terminal domain of B/b, affirming that the molecular association with the membrane is unique for this specific isoform. The B/bsia form is an over-sialylated version of the protein and is generated when there is an increase in the amount of sialic acid added to O-linked carbohydrates [133].
Having established that B/b is expressed at high levels in gliomas, the next step was to ascertain which specific cellular population contained the highest amounts of B/b. Human glioblastoma tumor sections were analyzed, and B/b was found around cells that expressed Olig2 and CD133 markers, both of which are indicative of highly tumorigenic cells [20, 49]. Interestingly, these markers are also detected within GICs. To examine B/b expression in these cells, researchers utilized two GIC lines: 0627 and 0913. They determined that both B/b protein and mRNA were present in these cell lines, although the 80–90 kDa C-terminal cleavage fragment was only expressed in the 0627 cells. Interestingly, B/b knockdown did not alter any of the assayed physical properties of the glioma-initiating cells including proliferation rate, viability, adhesive properties, migration, and invasion. Based on these results, it does not appear that B/b is needed for the GICs to behave normally nor for the maintenance of their characteristic physical properties, so likely B/b works in this cell population during the later stages of glioma pathogenesis [35].
7.4.2 Cleavage of B/b in Gliomas Leads to Increased Invasiveness
To address the ability of B/b to promote invasiveness, cultured 9L cells were transfected with either the full-length protein or the N-terminal fragment described above. It is important to note that the 9L cell line is characterized as a noninvasive cell line. 9L cells that expressed either the full-length form of B/b or the N-terminal fragment displayed a higher degree of motility and invasion as compared to cells that were transfected with GFP. Of particular importance, when these cells were injected into rats, only the tumors that expressed the N-terminal cleavage fragment were able to invade surrounding brain tissue. This was not noted in tumors that expressed the full-length form of B/b, thereby suggesting that the cleavage of B/b is a key event that mediates glioma cell invasiveness in rat models [154].
This finding prompted the investigation into which molecule cleaves the lectican family member. This was addressed through the generation of an antibody against the putative cleavage site at Glu395-Ser396 within B/b [86]. The cleavage site is homologous to the well-characterized site in another CSPG, aggrecan [156]. Cleavage of aggrecan at that particular site is regulated at least partially by ADAMTS4 [128].
The resulting antibody exclusively recognizes the N-terminal fragment of B/b and is referred to as B50. Through use of the invasive CNS-1 cell line, cleavage activity was detected in culture, and most of the resulting product was detected in the media and, thus, was soluble. Investigators then aimed to determine the proper conditions for B/b cleavage by altering calcium, zinc, and sodium chloride levels in addition to pH and temperature. Administration of calcium chelators, and metalloproteinase inhibitors to the cultures, diminished the cleavage of B/b. From this work, Matthews and colleagues examined the potential role of ADAMTS4 in mediating the cleavage of B/b. They concluded that ADAMTS4 not only was expressed in CNS-1 cells but was also capable of cleaving B/b. This work pinpoints a critical role for ADAMTS4 to regulate B/b cleavage and, by extension, the invasive behavior displayed by glioma cells [86].
To directly assess if this cleavage event is necessary for the pro-invasive properties seen in glioma cells, a mutant construct in which B/b was not cleaved was introduced into CNS-1 cells. Tumor spheroids were created and then applied to organotypic slice cultures, and migration of the cells was examined. The spheroids containing the wild-type form of B/b migrated across the slice cultures more than those that expressed the mutant form of B/b that could not be cleaved. The CNS-1 cells that were transfected with wild-type B/b were implanted into rats intracranially, and the resulting tumors were more invasive, dispersed, and larger compared to those tumors that formed when the CNS-1 cells transfected with the mutant form of B/b were injected. Rats that had tumors that were more invasive exhibited a decreased survival rate compared to their counterparts [134].
7.4.3 Molecular Mechanisms: How B/b Cleavage Promotes Invasiveness
Having determined that the cleavage of B/b promotes glioma cell invasion, the mechanisms underlying this were next explored. B/b was introduced into glioma cells (U87MG, U373MG, and CNS-1 cells) through transduction in culture. Expression of B/b enhanced glioma cell adhesion to specific substrates: fibronectin, collagen, and hyaluronic acid, but this was not noted when laminin and poly-L-lysine were used. Moreover, investigators probed glioma cell motility and reported that glioma cells expressing B/b were more mobile in response to hyaluronic acid and fibronectin substrates, in comparison with control cells that did not express B/b. B/b cleavage was required for the adhesion between B/b and fibronectin and hyaluronic acid. To provide further support, the glioma cells were added to organotypic slice cultures to measure the amount of cell dispersion. Glioma cells that expressed either the full-length form of B/b or the N-terminal cleavage fragment of B/b exhibited a significant increase in cell movement compared to those cells that expressed the form of B/b that was resistant to cleavage [62].
The expression of a number of cell adhesion molecules is altered in glioma samples [4, 19, 37, 51, 56, 74, 77, 79, 93, 94, 95, 101, 121, 129, 135, 151, 157], and Hu and colleagues then focused on identifying which cell adhesion molecules might be involved in modulating glioma cell invasion. Glioma cells that expressed B/b and were plated on fibronectin displayed an increase in protein expression of β-3 integrin, a phosphorylated form of the β-3 integrin, and NCAM, in comparison with control cells that did not express B/b [62]. These results are in accordance with reports that β-3 integrin expression induces cell dispersion and spreading [143, 144]. It is known that both B/b and fibronectin are upregulated in gliomas [99, 133] compared to normal brain tissue [97, 102] and tumors that spread to the brain, but did not originate in the brain [67]. Specifically, fibronectin was found at the cell surface on glioma cells that possessed either the full-length form of B/b or the N-terminal cleavage fragment of B/b. The expressed fibronectin was organized in microfibrillar structures, which is thought to facilitate rearrangement of the ECM and promote movement of tumor cells [60, 72].
When U87MG glioma cells were incubated in conditioned media that contained either secreted full-length B/b or the N-terminal cleavage fragment of B/b, there was an increase in the amount of phosphorylated EGFR and phosphorylated ERK1/2 compared to control cells. If the glioma cells were treated with an EGFR inhibitor, then phosphorylation was inhibited, and, correspondingly, fibronectin mRNA levels decreased. Importantly, as a result of the treatment with this inhibitor, the B/b-expressing glioma cells did not adhere as well to fibronectin relative to cells that were treated with a control empty vector [62]. This work is consistent with reports that the expression of EGFR is increased in glioma cells [81, 96]. Additionally, the results presented by Hu et al. [62] corroborate previous demonstrations that the activation of EGFR and ERK induces an increase in fibronectin expression [38, 130, 155]. Precisely how fibronectin and B/b might associate with one another was directly addressed through co-immunoprecipitation and dot blot assays, in which it was shown that fibronectin binds to the N-terminal cleavage fragment of B/b, but not the full-length form of the protein. This clearly shows that the cleavage of B/b is an important event that is required for binding to fibronectin, which results in the enhancement of glioma cell movement [62].
The work presented by Hu and colleagues was supported by another set of experiments where U251 and U87 glioma cells were induced to express B/b, which resulted in an increase in the adhesion of glioma cells to fibronectin and an overall increase in motility of the glioma cells [83].
7.4.4 Impact of B/b Knockdown on Glioma Cells
To further pinpoint the critical role that fibronectin plays in mediating glioma cell motility, siRNA constructs were made to knockdown fibronectin expression. As a result, glioma cells adhered less to hyaluronan and fibronectin and additionally were less motile when plated on these substrates. Phenotypically, these glioma cells now presented the same as the control cells in terms of adhesion and motility [62].
To more thoroughly analyze how B/b is involved in regulating glioma cell behavior, U251 cells were first transduced to express B/b, and then the protein was knocked down using shDNA. Due to the knockdown of B/b, these glioma cells displayed a decrease in the rate of division and reductions in the following properties: invasiveness, migration, and dispersion or spreading distance, in direct comparison to the shDNA and mock controls. To examine resulting tumor growth in vivo, the transduced cells were introduced into nude mice. The mice that received the B/b knockdown cells developed tumors that were less infiltrative and less dispersed relative to the mice that received the control cells. This work further defined the role of B/b role in regulating glioma cell migration and invasion [83].
Dwyer and colleagues then aimed to elucidate what occurs when B/b is knocked down in intracranial gliomas. To this end, investigators transfected CNS-1 cells with B/b expression constructs at the same time as B/b knockdown constructs. After determining the knockdown efficiency, the generated CNS-1 cells were injected in the thalamus of rats. The tumors that formed after CNS-1 cells exposed to the shRNA construct specific to B/b displayed a reduction in overall volume and were less invasive compared to the tumors that developed when a control shRNA construct was introduced into the CNS-1 cells. In light of this, the survival rates of the rats injected with the B/b knockdown cells were higher than those rats that received the control cells [35]. This body of evidence suggests that B/b expression in gliomas results in increased motility and invasion [35, 83].
As stated above, B/b expression was detected in GICs [20, 35, 49], but the question as to how B/b functions in this cell population was next addressed. shRNA constructs were introduced into GICs and then were injected into the striatum of nude mice to examine the properties of the tumors that were generated as a result. The GICs that expressed the knockdown constructs to reduce B/b expression formed a tumor that was smaller in volume and was less invasive relative to the cells that contained control constructs [35]. Therefore, it does appear that B/b mechanistically functions in the same capacity in the glioma-initiating cells as in glioma cells to promote invasion, spread, and migration.
7.5 Future Directions
B/b is a key molecule present within the tumor microenvironment of gliomas that works to promote cell invasion and movement [35, 41, 62, 83, 86, 98, 133, 134, 154]. Due to the fact that glioma cells possess the ability to infiltrate the normally inhibitory ECM, patient prognosis and response to current treatment options are quite poor [126]. In addition, work suggests that B/b contributes to tumor vascularization, but the mechanism by which it does this is completely unknown [98]. Future work investigating the interaction between B/b and other cells in the tumor microenvironment such as pericytes and vascular endothelial cells is clearly necessary. In addition, future studies need to be aimed at creating treatments that specifically target the GIC population. These cells are capable of self-renewal and also are able to form all of the cells within a glioma [39, 123]. Importantly, these cells create and maintain an environment that fuels tumor development, which not only is likely responsible for driving the initial establishment of the tumor but also explains why relapses might occur [6, 27, 78]. Therefore, treatments tailored to targeting the GICs might provide promising new avenues that could lead to better patient survival rates. B/b is an intriguing target in this regard as it seems to be an important component of the stem cell niche.
A complicating factor in treating individuals with gliomas is the fact that there is a great degree of molecular heterogeneity in these types of tumors. More specifically, cell adhesion molecules, ECM constituents, enzymes, and growth factors are just some examples of molecules that may underlie glioma pathogenesis. Importantly, these molecules work together to create an intricate and complex tumor microenvironment. In light of this, the best way to devise treatments is to precisely pinpoint how these molecules work together to contribute to the development and maintenance of gliomas in addition to defining the specific role of each of these molecules. This will give us a more complete picture as to how these tumors develop, thereby, providing us with the information necessary to generate more effective treatments.
References
Abaskharoun M, Bellemare M, Lau E et al (2010a) Expression of hyaluronan and the hyaluronan-binding proteoglycans neurocan, aggrecan, and versican by neural stem cells and neural cells derived from embryonic stem cells. Brain Res 1327:6–15. https://doi.org/10.1016/j.brainres.2010.02.048
Abaskharoun M, Bellemare M, Lau E et al (2010b) Glypican-1, phosphacan/receptor protein tyrosine phosphatase-ζ/β and its ligand, tenascin-C, are expressed by neural stem cells and neural cells derived from embryonic stem cells. ASN Neuro 2:AN20100001. https://doi.org/10.1042/an20100001
Aruffo A, Stamenkovic I, Melnick M et al (1990) CD44 is the principal cell surface receptor for hyaluronate. Cell 61:1303–1313. https://doi.org/10.1016/0092-8674(90)90694-A
Asano K, Duntsch CD, Zhou Q et al (2004) Correlation of N-cadherin expression in high grade gliomas with tissue invasion. J Neuro-Oncol 70:3–15. https://doi.org/10.1023/B:NEON.0000040811.14908.f2
Balkwill FR, Capasso M, Hagemann T (2012) The tumor microenvironment at a glance. J Cell Sci 125:5591–5596. https://doi.org/10.1242/jcs.116392
Balvers R, Dirven C, Leenstra S et al (2016) Malignant glioma in vitro models: on the utilization of stem-like cells. Curr Cancer Drug Targets 17:255–266. https://doi.org/10.2174/1568009616666160813191809
Bandtlow CE, Zimmermann DR (2000) Proteoglycans in the developing brain: new conceptual insights for old proteins. Physiol Rev 80:1267–1290. https://doi.org/10.1152/physrev.2000.80.4.1267
Bekku Y, Rauch U, Ninomiya Y et al (2009) Brevican distinctively assembles extracellular components at the large diameter nodes of Ranvier in the CNS. J Neurochem 108:1266–1276. https://doi.org/10.1111/j.1471-4159.2009.05873.x
Bekku Y, Su WD, Hirakawa S et al (2003) Molecular cloning of Bral2, a novel brain-specific link protein, and immunohistochemical colocalization with brevican in perineuronal nets. Mol Cell Neurosci 24:148–159. https://doi.org/10.1016/S1044-7431(03)00133-7
Bellail AC, Hunter SB, Brat DJ et al (2004) Microregional extracellular matrix heterogeneity in brain modulates glioma cell invasion. Int J Biochem Cell Biol 36:1046–1069. https://doi.org/10.1016/j.biocel.2004.01.013
Bettinger I, Thanos S, Paulus W (2002) Microglia promote glioma migration. Acta Neuropathol 103:351–355. https://doi.org/10.1007/s00401-001-0472-x
Binder DK, Berger MS (2002) Proteases and the biology of glioma invasion. J Neuro-Oncol 56:149–158. https://doi.org/10.1023/A:1014566604005
Blosa M, Sonntag M, Jäger C et al (2015) The extracellular matrix molecule brevican is an integral component of the machinery mediating fast synaptic transmission at the calyx of Held. J Physiol 593:4341–4360. https://doi.org/10.1113/JP270849
Bradbury EJ, Moon LDF, Popat RJ et al (2002) Chondroitinase ABC promotes functional recovery after spinal cord injury. Nature 416:636–640. https://doi.org/10.1038/416636a
Brakebusch C, Seidenbecher CI, Asztely F et al (2002) Brevican-deficient mice display impaired hippocampal CA1 long-term potentiation but show no obvious deficits in learning and memory. Mol Cell Biol 22:7417–7427. https://doi.org/10.1128/mcb.22.21.7417-7427.2002
Brown MC, Cary LA, Jamieson JS et al (2005) Src and FAK kinases cooperate to phosphorylate Paxillin kinase linker, stimulate its focal adhesion localization, and regulate cell spreading and protrusiveness. Mol Biol Cell 16:4316–4328. https://doi.org/10.1091/mbc.e05-02-0131
Brückner G, Brauer K, Härtig W et al (1993) Perineuronal nets provide a polyanionic, glia associated form of microenvironment around certain neurons in many parts of the rat brain. Glia 8:183–200. https://doi.org/10.1002/glia.440080306
Brückner G, Bringmann A, Köppe G et al (1996) In vivo and in vitro labelling of perineuronal nets in rat brain. Brain Res 720:84–92. https://doi.org/10.1016/0006-8993(96)00152-7
Camand E, Peglion F, Osmani N et al (2012) N-cadherin expression level modulates integrin mediated polarity and strongly impacts on the speed and directionality of glial cell migration. J Cell Sci 125:844–857. https://doi.org/10.1242/jcs.087668
Campos B, Herold-Mende CC (2011) Insight into the complex regulation of CD133 in glioma. Int J Cancer 128:501–510. https://doi.org/10.1002/ijc.25687
Carulli D, Pizzorusso T, Kwok JCF et al (2010) Animals lacking link protein have attenuated perineuronal nets and persistent plasticity. Brain 133:2331–2347. https://doi.org/10.1093/brain/awq145
Cattaruzza S, Perris R (2005) Proteoglycan control of cell movement during wound healing and cancer spreading. Matrix Biol 24:400–417. https://doi.org/10.1016/j.matbio.2005.06.005
Celio MR, Blumcke I (1994) Perineuronal nets - a specialized form of extracellular matrix in the adult nervous system. Brain Res Rev 19:128–145
Celio MR, Spreafico R, De Biasi S et al (1998) Perineuronal nets: past and present. Trends Neurosci 21:510–515. https://doi.org/10.1016/S0166-2236(98)01298-3
Chalmers AJ (2007) Radioresistant glioma stem cells-therapeutic obstacle or promising target? DNA Repair (Amst) 6:1391–1394. https://doi.org/10.1016/j.dnarep.2007.03.019
Chan AY, Coniglio SJ, Chuang YY et al (2005) Roles of the Rac1 and Rac3 GTPases in human tumor cell invasion. Oncogene 24:7821–7829. https://doi.org/10.1038/sj.onc.1208909
Chen J, Li Y, Yu TS et al (2012) A restricted cell population propagates glioblastoma growth after chemotherapy. Nature 488:522–526. https://doi.org/10.1038/nature11287
Chicoine MR, Silbergeld DL (1997) Mitogens as motogens. J Neuro-Oncol 35:249–257. https://doi.org/10.1023/A:1005808315821
Chuang YY, Tran NL, Rusk N et al (2004) Role of synaptojanin 2 in glioma cell migration and invasion. Cancer Res 64:8271–8275. https://doi.org/10.1158/0008-5472.CAN-04-2097
Claes A, Idema AJ, Wesseling P (2007) Diffuse glioma growth: a guerilla war. Acta Neuropathol 114:443–458. https://doi.org/10.1007/s00401-007-0293-7
Corvetti L, Rossi F (2005) Degradation of chondroitin sulfate proteoglycans induces sprouting of intact Purkinje axons in the cerebellum of the adult rat. J Neurosci 25:7150–7158. https://doi.org/10.1523/jneurosci.0683-05.2005
Dai B, Kang SH, Gong W et al (2007) Aberrant FoxM1B expression increases matrix metalloproteinase-2 transcription and enhances the invasion of glioma cells. Oncogene 26:6212–6219. https://doi.org/10.1038/sj.onc.1210443
Deng Y, Li W, Li Y et al (2010) Expression of matrix metalloproteinase-26 promotes human glioma U251 cell invasion in vitro and in vivo. Oncol Rep 23:69–78. https://doi.org/10.3892/or-00000607
Denysenko T, Gennero L, Roos MA et al (2010) Glioblastoma cancer stem cells: heterogeneity, microenvironment and related therapeutic strategies. Cell Biochem Funct 28:343–351. https://doi.org/10.1002/cbf.1666
Dwyer CA, Bi WL, Viapiano MS et al (2014) Brevican knockdown reduces late-stage glioma tumor aggressiveness. J Neuro-Oncol 120:63–72. https://doi.org/10.1007/s11060-014-1541-z
Ehtesham M, Winston JA, Kabos P et al (2006) CXCR4 expression mediates glioma cell invasiveness. Oncogene 25:2801–2806. https://doi.org/10.1038/sj.onc.1209302
Foehr ED, Lorente G, Kuo J et al (2006) Targeting of the receptor protein tyrosine phosphatase β with a monoclonal antibody delays tumor growth in a glioblastoma model. Cancer Res 66:2271–2278. https://doi.org/10.1158/0008-5472.CAN-05-1221
Gaggioli C, Deckert M, Robert G et al (2005) HGF induces fibronectin matrix synthesis in melanoma cells through MAP kinase-dependent signaling pathway and induction of Egr-1. Oncogene 24:1423–1433. https://doi.org/10.1038/sj.onc.1208318
Galli R, Binda E, Orfanelli U et al (2004) Isolation and characterization of tumorigenic, stem like neural precursors from human glioblastoma. Cancer Res 64:7011–7021. https://doi.org/10.1158/00085472.CAN-04-1364
Garcion E, Halilagic A, Faissner A et al (2004) Generation of an environmental niche for neural stem cell development bythe extracellular matrix molecule tenascin C. Development 131:3423–3432. https://doi.org/10.1242/dev.01202
Gary SC, Kelly GM, Hockfield S (1998) BEHAB/brevican: a brain-specific lectican implicated in gliomas and glial cell motility. Curr Opin Neurobiol 8:576–581. https://doi.org/10.1016/S09594388(98)80083-4
Gary SC, Zerillo CA, Chiang VL et al (2000) cDNA cloning, chromosomal localization, and expression analysis of human BEHAB/brevican, a brain specific proteoglycan regulated during cortical development and in glioma. Gene 256:139–147. https://doi.org/10.1016/S0378-1119(00)00362-0
Gilbertson RJ, Rich JN (2007) Making a tumour’s bed: glioblastoma stem cells and the vascular niche. Nat Rev Cancer 7:733–736. https://doi.org/10.1038/nrc2246
Gladson CL (1999) The extracellular matrix of gliomas: modulation of cell function. J Neuropathol Exp Neurol 58:1029–1040. https://doi.org/10.1097/00005072-19991000-0001
Goldbrunner RH, Bernstein JJ, Tonn JC (1999) Cell-extracellular matrix interaction in glioma invasion. Acta Neurochir 141:295–305. https://doi.org/10.1007/s007010050301
Gondi CS, Lakka SS, Dinh DH et al (2004) Downregulation of uPA, uPAR and MMP-9 using small, interfering, hairpin RNA (siRNA) inhibits glioma cell invasion, angiogenesis and tumor growth. Neuron Glia Biol 1:165–176. https://doi.org/10.1017/S1740925X04000237
Grotegut S, Von Schweinitz D, Christofori G et al (2006) Hepatocyte growth factor induces cell scattering through MAPK/Egr-1-mediated upregulation of snail. EMBO J 25:3534–3545. https://doi.org/10.1038/sj.emboj.7601213
Guimaraes A, Zaremba S, Hockfield S (1990) Molecular and morphological changes in the cat lateral geniculate nucleus and visual cortex induced by visual deprivation are revealed by monoclonal antibodies Cat-304 and Cat-301. J Neurosci 10:3014–3024. https://doi.org/10.1523/jneurosci.10-09-03014.1990
Günther HS, Schmidt NO, Phillips HS et al (2008) Glioblastoma-derived stem cell-enriched cultures form distinct subgroups according to molecular and phenotypic criteria. Oncogene 27:2897–2909. https://doi.org/10.1038/sj.onc.1210949
Guo P, Imanishi Y, Cackowski FC et al (2011) Up-regulation of Angiopoietin-2, matrix Metalloprotease-2, membrane type 1 metalloprotease, and Laminin 5 γ 2 correlates with the invasiveness of human glioma. Am J Pathol 166:877–890. https://doi.org/10.1016/s0002-9440(10)62308-5
Gutenberg A, Brück W, Buchfelder M et al (2004) Expression of tyrosine kinases FAK and Pyk2 in 331 human astrocytomas. Acta Neuropathol 108:224–230. https://doi.org/10.1007/s00401-004-0886-3
Hanahan D, Coussens LM (2012) Accessories to the crime: functions of cells recruited to the tumor microenvironment. Cancer Cell 21:309–322. https://doi.org/10.1016/j.ccr.2012.02.022
Härtig W, Brauer K, Bigl V et al (1994) Chondroitin sulfate proteoglycan immunoreactivity of lectin-labeled perineuronal nets around parvalbumin-containing neurons. Brain Res 635:307–311. https://doi.org/10.1016/0006-8993(94)91452-4
Härtig W, Derouiche A, Welt K et al (1999) Cortical neurons immunoreactive for the potassium channel Kv3.1b subunit are predominantly surrounded by perineuronal nets presumed as a buffering system for cations. Brain Res 842:15–29. https://doi.org/10.1016/S0006-8993(99)01784-9
Härtig W, Singer A, Grosche J et al (2001) Perineuronal nets in the rat medial nucleus of the trapezoid body surround neurons immunoreactive for various amino acids, calcium-binding proteins and the potassium channel subunit Kv3.1b. Brain Res 899:123–133. https://doi.org/10.1016/S0006-8993(01)02211-9
Hecker TP, Grammer JR, Gillespie GY et al (2002) Focal adhesion kinase enhances signaling through the Shc/extracellular signal-regulated kinase pathway in anaplastic astrocytoma tumor biopsy samples. Cancer Res 62:2699–2707
Higuchi M, Ohnishi T, Arita N et al (1993) Expression of tenascin in human gliomas: its relation to histological malignancy, tumor dedifferentiation and angiogenesis. Acta Neuropathol 85:481–487. https://doi.org/10.1007/BF00230486
Hockfield S, Kalb RG, Zaremba S et al (1990) Expression of neural proteoglycans correlates with the Acquisition of Mature Neuronal Properties in the mammalian brain. Cold Spring Harb Symp Quant Biol 55:505–514. https://doi.org/10.1101/sqb.1990.055.01.049
Hockfield S, McKay R (1983) Monoclonal antibodies demonstrate the organization of axons in the leech. J Neurosci 3:369–375. https://doi.org/10.1523/jneurosci.03-02-00369.1983
Hocking DC, Chang CH (2003) Fibronectin matrix polymerization regulates small airway epithelial cell migration. Am J Physiol Cell Mol Physiol 285:L169–L179. https://doi.org/10.1152/ajplung.00371.2002
Hu B, Guo P, Fang Q et al (2003) Angiopoietin-2 induces human glioma invasion through the activation of matrix metalloprotease-2. Proc Natl Acad Sci 100:8904–8909. https://doi.org/10.1073/pnas.1533394100
Hu B, Kong LL, Matthews RT et al (2008) The proteoglycan brevican binds to fibronectin after proteolytic cleavage and promotes glioma cell motility. J Biol Chem 283:24848–24859. https://doi.org/10.1074/jbc.M801433200
Imai K, Ari H, Fukushima D et al (2015) Degradation of decorin by matrix metalloproteinases: identification of the cleavage sites, kinetic analyses and transforming growth factor- β 1 release. Biochem J 322:809–814. https://doi.org/10.1042/bj3220809
Iseki K, Hagino S, Nikaido T et al (2012) Gliosis-specific transcription factor OASIS coincides with proteoglycan core protein genes in the glial scar and inhibits neurite outgrowth. Biomed Res 33:345–353. https://doi.org/10.2220/biomedres.33.345
Jaworski DM, Kelly GM, Hockfield S (1999) Intracranial injury acutely induces the expression of the secreted isoform of the CNS-specific hyaluronan-binding protein BEHAB/brevican. Exp Neurol 157:327–337. https://doi.org/10.1006/exnr.1999.7062
Jaworski DM, Kelly GM, Hockfield S (1998) The CNS-specific hyaluronan-binding protein BEHAB is expressed in ventricular zones coincident with gliogenesis. J Neurosci 15:1352–1362. https://doi.org/10.1523/jneurosci.15-02-01352.1995
Jaworski DM, Kelly GM, Piepmeier JM et al (1996) BEHAB (brain enriched Hyaluronan binding) is expressed in surgical samples of glioma and in intracranial grafts of invasive glioma cell lines. Cancer Res 56:2293–2298
Kabos P, Matundan H, Zandian M et al (2004) Neural precursors express multiple chondroitin sulfate proteoglycans, including the lectican family. Biochem Biophys Res Commun 318:955–963. https://doi.org/10.1016/j.bbrc.2004.04.114
Koga K, Todaka T, Morioka M et al (2001) Expression of angiopoietin-2 in human glioma cells and its role for angiogenesis. Cancer Res 61:6248–6254
Laabs TL, Wang H, Katagiri Y et al (2007) Inhibiting glycosaminoglycan chain polymerization decreases the inhibitory activity of astrocyte-derived chondroitin sulfate proteoglycans. J Neurosci 27:14494–14501. https://doi.org/10.1523/jneurosci.2807-07.2007
Lakka SS, Gondi CS, Yanamandra N et al (2004) Inhibition of cathepsin B and MMP-9 gene expression in glioblastoma cell line via RNA interference reduces tumor cell invasion, tumor growth and angiogenesis. Oncogene 23:4681–4689. https://doi.org/10.1038/sj.onc.1207616
Larsen M, Artym VV, Green JA et al (2006) The matrix reorganized: extracellular matrix remodeling and integrin signaling. Curr Opin Cell Biol 18:463–471. https://doi.org/10.1016/j.ceb.2006.08.009
Laurent M, Martinerie C, Thibout H et al (2003) NOVH increases MMP3 expression and cell migration in glioblastoma cells via a PDGFR-α-dependent mechanism. FASEB J 17:1919–1921. https://doi.org/10.1096/fj.02-1023fje
Leavesley DI, Ferguson GD, Wayner EA et al (1992) Requirement of the integrin β3 subunit for carcinoma cell spreading or migration on vitronectin and fibrinogen. J Cell Biol 117:1101–1107. https://doi.org/10.1083/jcb.117.5.1101
Lee HJ, Bian S, Jakovcevski I et al (2012) Delayed applications of L1 and chondroitinase ABC promote recovery after spinal cord injury. J Neurotrauma 29:1850–1863. https://doi.org/10.1089/neu.2011.2290
Lettau I, Hattermann K, Held-Feindt J et al (2010) Matrix metalloproteinase-19 is highly expressed in astroglial tumors and promotes invasion of glioma cells. J Neuropathol Exp Neurol 69:215–223. https://doi.org/10.1097/NEN.0b013e3181ce9f67
Lewis-Tuffin LJ, Rodriguez F, Giannini C et al (2010) Misregulated E-cadherin expression associated with an aggressive brain tumor phenotype. PLoS One 5:e13665. https://doi.org/10.1371/journal.pone.0013665
Liebelt BD, Shingu T, Zhou X et al (2016) Glioma stem cells: signaling, microenvironment, and therapy. Stem Cells Int 2016:1–10. https://doi.org/10.1155/2016/7849890
Lipinski CA, Tran NL, Menashi E et al (2005) The tyrosine kinase Pyk2 promotes migration and invasion of glioma cells. Neoplasia 7:435–445. https://doi.org/10.1593/neo.04712
Liu M, Dai B, Kang SH et al (2006) FoxM1B is overexpressed in human glioblastomas and critically regulates the tumorigenicity of glioma cells. Cancer Res 66:3593–3602. https://doi.org/10.1158/0008-5472.CAN-05-2912
Louis DN, Ohgaki H, Wiestler OD et al (2007) The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol 114:97–109. https://doi.org/10.1007/s00401-007-0243-4
Lu P, Weaver VM, Werb Z (2012a) The extracellular matrix: a dynamic niche in cancer progression. J Cell Biol 196:395–406. https://doi.org/10.1083/jcb.201102147
Lu R, Wu C, Guo L et al (2012b) The role of brevican in glioma: promoting tumor cell motility in vitro and in vivo. BMC Cancer 12:607. https://doi.org/10.1186/1471-2407-12-607
Mariani L, McDonough WS, Hoelzinger DB et al (2001) Identification and validation of P311 as a glioblastoma invasion gene using laser capture microdissection. Cancer Res 61:4190–4196
Markovic DS, Vinnakota K, Chirasani S et al (2009) Gliomas induce and exploit microglial MT1-MMP expression for tumor expansion. Proc Natl Acad Sci 106:12530–12535. https://doi.org/10.1073/pnas.0804273106
Matthews RT, Gary SC, Zerillo C et al (2000) Brain-enriched hyaluronan binding (BEHAB)/brevican cleavage in a glioma cell line is mediated by a disintegrin and metalloproteinase with thrombospondin motifs (ADAMTS) family member. J Biol Chem 275:22695–22703. https://doi.org/10.1074/jbc.m909764199
McComb RD, Bigner DD (1985) Immunolocalization of laminin in neoplasms of the central and peripheral nervous systems. J Neuropathol Exp Neurol 44:242–253. https://doi.org/10.1097/00005072-198505000-00003
McDonough WS, Tran NL, Berens ME (2005) Regulation of glioma cell migration by serine-phosphorylated P3111. Neoplasia 7:862–872. https://doi.org/10.1593/neo.05190
Miura R, Aspberg A, Ethell IM et al (1999) The proteoglycan lectin domain binds sulfated cell surface glycolipids and promotes cell adhesion. J Biol Chem 274:11431–11438. https://doi.org/10.1074/jbc.274.16.11431
Moon LDF, Asher RA, Rhodes KE et al (2001) Regeneration of CNS axons back to their target following treatment of adult rat brain with chondroitinase ABC. Nat Neurosci 4:465–466. https://doi.org/10.1038/87415
Murai T, Miyazaki Y, Nishinakamura H et al (2004) Engagement of CD44 promotes Rac activation and CD44 cleavage during tumor cell migration. J Biol Chem 279:4541–4550. https://doi.org/10.1074/jbc.M307356200
Murphy G, Stanton H, Cowell S et al (1999) Mechanisms for pro matrix metalloproteinase activation. APMIS 107:38–44. https://doi.org/10.1111/j.1699-0463.1999.tb01524.x
Musumeci G, Magro G, Cardile V et al (2015) Characterization of matrix metalloproteinase-2 and -9, ADAM-10 and N-cadherin expression in human glioblastoma multiforme. Cell Tissue Res 362:45–60. https://doi.org/10.1007/s00441-015-2197-5
Nakada M, Drake KL, Nakada S et al (2006) Ephrin-B3 ligand promotes glioma invasion through activation of Rac1. Cancer Res 66:8492–8500. https://doi.org/10.1158/0008-5472.CAN-05-4211
Nakada M, Niska JA, Miyamori H et al (2004) The phosphorylation of EphB2 receptor regulates migration and invasion of human glioma cells. Cancer Res 64:3179–3185. https://doi.org/10.1158/0008-5472.CAN-03-3667
Nakamura JL (2007) The epidermal growth factor receptor in malignant gliomas: pathogenesis and therapeutic implications. Expert Opin Ther Targets 11:463–472. https://doi.org/10.1517/14728222.11.4.463
Novak U, Kaye AH (2000) Extracellular matrix and the brain: components and function. J Clin Neurosci 7:280–290. https://doi.org/10.1054/jocn.1999.0212
Nutt CL, Zerillo CA, Kelly GM et al (2001) Brain enriched hyaluronan binding (BEHAB)/brevican increases aggressiveness of CNS-1 gliomas in Lewis rats. Cancer Res 61:7056–7059
Ohnishi T, Hiraga S, Izumoto S (1998) Role of fibronectin-stimulated tumor cell migration in glioma invasion in vivo: clinical significance of fibronectin and fibronectin receptor expressed in human glioma tissues. Clin Exp Metastasis 16:729–741. https://doi.org/10.1023/A:1006532812408
Onken J, Moeckel S, Leukel P et al (2014) Versican isoform V1 regulates proliferation and migration in high-grade gliomas. J Neuro-Oncol 120:73–83. https://doi.org/10.1007/s11060-014-1545-8
Owens GC, Orr EA, Kleinschmidt DeMasters BK et al (1998) Overexpression of a transmembrane isoform of neural cell adhesion molecule alters the invasiveness of rat CNS-1 glioma. Cancer Res 58:2020–2028
Pearlman AL, Sheppard AM (1996) Extracellular matrix in early cortical development. In: Ranney Mize R, Erzurumlu RS (eds) Progress in brain research: neural development and plasticity, vol 108. Elseiver, Netherlands, pp 119–134
Pedersen P-H, Mork S, Marjenhagen K et al (1993) The migratory pattern Op fetal brain cells and glioma cells in the adult brain. J Neuropathol Exp Neurol 52:295. https://doi.org/10.1097/00005072-199305000-00139
Perkins SJ, Nealis AS, Dudhia J et al (1989) Immunoglobulin fold and tandem repeat structures in proteoglycan N-terminal domains and link protein. J Mol Biol 206:737–748. https://doi.org/10.1016/0022-2836(89)90580-9
Pizzorusso T, Medini P, Berardi N et al (2002) Reactivation of ocular dominance plasticity in the adult visual cortex. Science 298:1248–1251. https://doi.org/10.1126/science.1072699
Rao JS (2003) Molecular mechanisms of glioma invasiveness: the role of proteases. Nat Rev Cancer 3:489–501. https://doi.org/10.1038/nrc1121
Rauch U (2004) Extracellular matrix components associated with remodeling processes in brain. Cell Mol Life Sci 61:2031–2045. https://doi.org/10.1007/s00018-004-4043-x
Ricci-Vitiani L, Pallini R, Biffoni M et al (2010) Tumour vascularization via endothelial differentiation of glioblastoma stem-like cells. Nature 468:824–830. https://doi.org/10.1038/nature09557
Rich JN, Bigner DD (2004) Development of novel targeted therapies in the treatment of malignant glioma. Nat Rev Drug Discov 3:430–446. https://doi.org/10.1038/nrd1380
Ridley AJ, Schwartz MA, Burridge K et al (2003) Cell migration: integrating signals from front to Back. Science 302:1704–1709. https://doi.org/10.1126/science.1092053
Rome C, Arsaut J, Taris C et al (2007) MMP-7 (matrilysin) expression in human brain tumors. Mol Carcinog 46:446–452. https://doi.org/10.1002/mc.20293
Rubin JB, Kung AL, Klein RS et al (2003) A small-molecule antagonist of CXCR4 inhibits intracranial growth of primary brain tumors. Proc Natl Acad Sci 100:13513–13518. https://doi.org/10.1073/pnas.2235846100
Ruoslahti E (1996) Brain extracellular matrix. Glycobiology 6:489–492. https://doi.org/10.1093/glycob/6.5.489
Salhia B, Tran NL, Symons M et al (2006) Molecular pathways triggering glioma cell invasion. Expert Rev Mol Diagn 6:613–626. https://doi.org/10.1586/14737159.6.4.613
Sarkar S, Nuttall RK, Liu S et al (2006) Tenascin-C stimulates glioma cell invasion through matrix metalloproteinase-12. Cancer Res 66:11771–11780. https://doi.org/10.1158/0008-5472.CAN-050470
Sasaki H, Yoshida K, Ikeda E et al (2002) Expression of the neural cell adhesion molecule in astrocytic tumors. Cancer 82:1921–1931
Sato H, Takino T, Okada Y et al (1994) A matrix metalloproteinase expressed on the surface of invasive tumour cells. Nature 370:61–65. https://doi.org/10.1038/370061a0
Sawaya RE, Yamamoto M, Gokaslan ZL et al (1996) Expression and localization of 72 kDa type IV collagenase (MMP-2) in human malignant gliomas in vivo. Clin Exp Metastasis 14:35–42. https://doi.org/10.1007/BF00157684
Seidenbecher CI, Gundelfinger ED, Böckers TM et al (1998) Transcripts for secreted and GPI-anchored brevican are differentially distributed in rat brain. Eur J Neurosci 10:1621–1630. https://doi.org/10.1046/j.1460-9568.1998.00166.x
Seidenbecher CI, Richter K, Rauch U et al (1995) Brevican, a chondroitin sulfate proteoglycan of rat brain, occurs as secreted and cell surface glycosylphosphatidylinositol-anchored isoforms. J Biol Chem 270:27206–27212. https://doi.org/10.1074/jbc.270.45.27206
Senner V, Schmidtpeter S, Braune S et al (2003) AMOG/β2 and glioma invasion: does loss of AMOG make tumour cells run amok? Neuropathol Appl Neurobiol 29:370–377. https://doi.org/10.1046/j.1365-2990.2003.00473.x
Shen Q, Wang Y, Kokovay E et al (2008) Adult SVZ stem cells lie in a vascular niche: a quantitative analysis of niche cell-cell interactions. Cell Stem Cell 3:289–300. https://doi.org/10.1016/j.stem.2008.07.026
Singh SK, Hawkins C, Clarke ID et al (2004) Identification of human brain tumour initiating cells. Nature 432:396–401. https://doi.org/10.1038/nature03128
Spreafico R, De Biasi S, Vitellaro-Zuccarello L (1999) The perineuronal net: a weapon for a challenge. J Hist Neurosci 8:179–185. https://doi.org/10.1076/jhin.8.2.179.1834
Tanaka K, Miyata H, Sugimura K et al (2015) miR-27 is associated with chemoresistance in esophageal cancer through transformation of normal fibroblasts to cancer-associated fibroblasts. Carcinogenesis 36:894–903. https://doi.org/10.1093/carcin/bgv067
Tanaka S, Louis DN, Curry WT et al (2013) Diagnostic and therapeutic avenues for glioblastoma: no longer a dead end? Nat Rev Clin Oncol 10:14–26. https://doi.org/10.1038/nrclinonc.2012.204
Thon N, Haas CA, Rauch U et al (2000) The chondroitin sulphate proteoglycan brevican is upregulated by astrocytes after entorhinal cortex lesions in adult rats. Eur J Neurosci 12:2547–2558. https://doi.org/10.1046/j.1460-9568.2000.00109.x
Tortorella MD, Burn TC, Pratta MA et al (1999) Purification and cloning of aggrecanase-1: a member of the ADAMTS family of proteins. Science 284:1664–1666. https://doi.org/10.1126/science.284.5420.1664
Tran NL, McDonough WS, Savitch BA et al (2006) Increased fibroblast growth factor-inducible 14 expression levels promote glioma cell invasion via Rac1 and nuclear factor-κB and correlate with poor patient outcome. Cancer Res 66:9535–9542. https://doi.org/10.1158/0008-5472.CAN-06-0418
Uchiyama-Tanaka Y, Matsubara H, Mori Y et al (2002) Involvement of HB-EGF and EGF receptor transactivation in TGF-β-mediated fibronectin expression in mesangial cells. Kidney Int 62:799–808. https://doi.org/10.1046/j.1523-1755.2002.00537.x
Uhm JH, Dooley NP, Kyritsis AP et al (1999) Vitronectin, a glioma-derived extracellular matrix protein, protects tumor cells from apoptotic death. Clin Cancer Res 5:1587–1594
Varga I, Hutóczki G, Szemcsák CD et al (2012) Brevican, neurocan, tenascin-C and versican are mainly responsible for the invasiveness of low-grade astrocytoma. Pathol Oncol Res 18:413–420. https://doi.org/10.1007/s12253-011-9461-0
Viapiano MS, Bi WL, Piepmeier J et al (2005) Novel tumor-specific isoforms of BEHAB/brevican identified in human malignant gliomas. Cancer Res 65:6726–6733. https://doi.org/10.1158/0008-5472.CAN-05-0585
Viapiano MS, Hockfield S, Matthews RT (2008) BEHAB/brevican requires ADAMTS-mediated proteolytic cleavage to promote glioma invasion. J Neuro-Oncol 88:261–272. https://doi.org/10.1007/s11060-008-9575-8
Wang D, Grammer JR, Cobbs CS et al (2000) P125 focal adhesion kinase promotes malignant astrocytoma cell proliferation in vivo. J Cell Sci 113(Pt 23):4221–4230
Wang D, Ichiyama RM, Zhao R et al (2011) Chondroitinase combined with rehabilitation promotes recovery of forelimb function in rats with chronic spinal cord injury. J Neurosci 31:9332–9344. https://doi.org/10.1523/jneurosci.0983-11.2011
Wang H, Li XT, Wu C et al (2015) MiR-132 can inhibit glioma cells invasion and migration by target MMP16 in vitro. Onco Targets Ther 8:3211–3218. https://doi.org/10.2147/OTT.S79282
Wang J, Li Y, Wang J et al (2012) Increased expression of matrix metalloproteinase-13 in glioma is associate with poor overall survival of patients. Med Oncol 29:2432–2437. https://doi.org/10.1007/s12032-012-0181-4
Wang H, Shen W, Huang H et al (2003) Insulin-like growth factor binding protein 2 enhances glioblastoma invasion by activating invasion-enhancing genes. Cancer Res 63:4315
Wang M, Zhao J, Zhang L et al (2017) Role of tumor microenvironment in tumorigenesis. J Cancer 8:761–773. https://doi.org/10.7150/jca.17648
Wick W, Platten M, Weller M (2001) Glioma cell invasion: regulation of metalloproteinase activity by TGF-β. J Neuro-Oncol 53:177–185. https://doi.org/10.1023/A:1012209518843
Wu M, Chen Q, Li D et al (2008) LRRC4 inhibits human glioblastoma cells proliferation, invasion, and proMMP-2 activation by reducing SDF-1α/CXCR4-mediated ERK1/2 and Akt signaling pathways. J Cell Biochem 103:245–255. https://doi.org/10.1002/jcb.21400
Xi A, Flevaris P, Stojanovic A et al (2006) Tyrosine phosphorylation of the integrin β3 subunit regulates β3 cleavage by calpain. J Biol Chem 281:29426–29430. https://doi.org/10.1074/jbc.C600039200
Xi X, Bodnar RJ, Li Z et al (2003) Critical roles for the COOH-terminal NITY and RGT sequences of the integrin β3 cytoplasmic domain in inside-out and outside-in signaling. J Cell Biol 162:329–339. https://doi.org/10.1083/jcb.200303120
Yamada H, Watanabe K, Shimonaka M et al (1994) Molecular cloning of brevican, a novel brain proteoglycan of the aggrecan/versican family. J Biol Chem 269:10119–10126
Yamaguchi Y (1996) Brevican: a major proteoglycan in adult brain. Perspect Dev Neurobiol 3:307–317
Yamaguchi Y (2000) Lecticans: organizers of the brain extracellular matrix. Cell Mol Life Sci 57:276–289. https://doi.org/10.1007/PL00000690
Yeh WL, Lu DY, Lee MJ, Fu WM (2009) Leptin induces migration and invasion of glioma cells through MMP-13 production. Glia 57:454–464. https://doi.org/10.1002/glia.20773
Yong VW, Power C, Edwards DR (2001) Metalloproteinases in biology and pathology of the nervous system. Nat Rev Neurosci 2:502–511. https://doi.org/10.1038/35081571
Yu Q, Stamenkovic I (2000) Cell surface-localized matrix metalloproteinase-9 proteolytically activates TGF-β and promotes tumor invasion and angiogenesis. Genes Dev 14:163–176. https://doi.org/10.1101/gad.14.2.163
Zagzag D, Friedlander DR, Margolis B et al (2000) Molecular events implicated in brain tumor angiogenesis and invasion. Pediatr Neurosurg 33:49–55. https://doi.org/10.1159/000028975
Zagzag D, Shiff B, Jallo GI et al (2002) Tenascin-C promotes microvascular cell migration and phosphorylation of focal adhesion kinase. Cancer Res 62:2660–2668
Zaremba S, Guimaraes A, Kalb RG et al (1989) Characterization of an activity-dependent, neuronal surface proteoglycan identified with monoclonal antibody Cat-301. Neuron 2:1207–1219. https://doi.org/10.1016/0896-6273(89)90305-X
Zhang H, Kelly G, Zerillo C et al (1998) Expression of a cleaved brain-specific extracellular matrix protein mediates glioma cell invasion in vivo. J Neurosci 18:2370–2376. https://doi.org/10.1523/jneurosci.18-07-02370.1998
Zhang J, Zhi H, Zhou C et al (2005) Up-regulation of fibronectin in oesophageal squamous cell carcinoma is associated with activation of the Erk pathway. J Pathol 207:402–409. https://doi.org/10.1002/path.1846
Yamada H, Watanabe K, Shimonaka, M et al (1995) cDNA Cloning and the Identification of an Aggrecanase-like Cleavage Site in Rat Brevican. Biochem Biophys Res Commun 216:957–963. https://doi.org/10.1006/bbrc.1995.2713.
Sasaki H, Yoshida K, Ikeda E et al (1998) Expression of neural cell adhesion molecule in astrocytic tumors: an inverse correlation with malignancy. Cancer 82:1921–1931. https://doi.org/10.1002/(sici)1097-0142(19980515)82:10<1921::aid-cnrc16>3.0.co;2-v
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 The Editor(s) (if applicable) and The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Giamanco, K.A., Matthews, R.T. (2020). The Role of BEHAB/Brevican in the Tumor Microenvironment: Mediating Glioma Cell Invasion and Motility. In: Birbrair, A. (eds) Tumor Microenvironment . Advances in Experimental Medicine and Biology, vol 1272. Springer, Cham. https://doi.org/10.1007/978-3-030-48457-6_7
Download citation
DOI: https://doi.org/10.1007/978-3-030-48457-6_7
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-48456-9
Online ISBN: 978-3-030-48457-6
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)