Abstract
Colorectal cancer (CRC) is the second most common cancer and second leading cause of death from cancer in Canada. Screening efforts should be tailored depending on risk factors such as family history, age, and history of polyps. Testing of hereditary colorectal cancer syndromes with immunohistochemistry allows for appropriate screening of syndrome-related malignancies and genetic counselling. Here we review screening, multidisciplinary treatment, and surveillance of colon cancer based on current evidence.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Adjuvant chemotherapy
- Colon cancer
- Screening
- Colorectal polyps
- Crohn’s disease
- FAP
- Locally advanced
- Lynch
- MAP
- Ulcerative colitis
Introduction
Colorectal cancer (CRC) is the second most common cancer in Canada, with an estimated 26,900 new cases diagnosed in 2020 [1]. It is also the second leading cause of death from cancer in Canada with an estimated 9700 deaths (5300 men and 4400 women) in 2020 [1, 2]. Although the age-standardized incidence for CRC has been declining in males and females, this decline appears to be confined to older adults as the incidence has been rising in those younger than age 50 [1].
The most common stage of CRC at the time of diagnosis is stage III [1]. There is a strong association between cancer stage at time of diagnosis and survival (Table 6.1).
The current recommended staging system is the American Joint Committee on Cancer (AJCC) eighth edition.
Screening and Surveillance for Average and High-Risk Patients
Screening
Special Notes
-
There is good quality evidence that population screening using either FOBT or flexible sigmoidoscopy reduces colorectal cancer mortality [4, 5] (Table 6.2).
-
FOBT has been shown to reduce relative risk of CRC mortality by 16% [4, 5].
-
FIT has been shown to have superior sensitivity in detecting CRC and advanced adenoma when compared to gFOBT [6]. It is also anticipated that the reduction in CRC-related death through FIT screening is at least equivalent to that through gFOBT. However, direct comparison between gFOBT and FIT in terms of CRC-related mortality is lacking.
-
A randomized trial from Norway showed that population screening with flexible sigmoidoscopy decreased colorectal cancer mortality (11.7/100,000 deaths per person-years absolute risk reduction) [7].
-
At least four randomized controlled trials and ten observational studies have shown that screening with flexible sigmoidoscopy reduces incidence and mortality in distal, but not proximal colorectal cancer [8].
-
A systematic review and meta-analysis showed decreased mortality for proximal cancers with colonoscopy compared to flexible sigmoidoscopy based on observational data [8].
-
Colonoscopy is recommended by the American College of Gastroenterology for screening, although there are no randomized trials demonstrating a reduction in mortality [9].
-
A population-based study in Ontario of 2,412,077 people demonstrated that the colonoscopy rate was inversely proportional to death from CRC [10]. A case–control study in Ontario has demonstrated a significant association between colonoscopy and fewer deaths from CRC; specifically left-sided cancers [11].
-
Colonoscopy is the most sensitive of available screening options at detecting cancer or polyps and is thus an acceptable modality; however, it is associated with the highest risk and cost.
-
A shorter interval between testing or repeat colonoscopy should be performed if the first colonoscopy is sub-optimal.
-
Quality indicators for colonoscopy:
-
Cecal intubation rate > 90%, adequate bowel preparation, post polypectomy bleeding rate of <0.5%, and perforation rate of <0.1% [12, 13].
-
Polypectomy and adenoma detection rates (ADR) are also important quality indicators. Some studies have suggested ADR ≥ 25% may be associated with lower incidence of interval cancer [14]; however, there is no consensus on what the appropriate target should be [12, 13].
-
There is insufficient evidence to suggest a minimum withdrawal time from the cecum of 6 min improves quality of endoscopy or improves ADR [10, 11]. However, shorter mean withdrawal times have been independently associated with lower ADR [14].
-
Surveillance
Special Notes
-
Table 6.3 is adapted from Ontario ColonCancerCheck Guidelines.
-
Patients with multiple colorectal adenomas (>10) should be considered for germline genetic testing of APC, MUTYH, and MMR.
-
Above surveillance interval assumes (1) no family history of CRC in a first-degree relative with an age of onset <60, (2) colonoscopy was complete and adequate, and all visible polyps were completely removed.
Hereditary Colorectal Cancer Syndromes
Lynch Syndrome and Microsatellite Instability
-
Lynch syndrome is the most common hereditary CRC syndrome with a lifetime colorectal cancer risk of 40–80% (Table 6.4). This genetic disease results from mutations in DNA mismatch repair (MMR) genes leading to microsatellite instability (MSI).
-
MSI is identified in approximately 15% of all CRC and is a feature of Lynch syndrome.
-
Majority of cases of MSI are sporadic, due to methylation of an MMR gene, rather than a germline mutation found in Lynch syndrome. Revised Bethesda Guidelines provide criteria for testing of individuals at risk for Lynch syndrome [16].
-
MSI may be screened for in all colorectal cancers via PCR or Immunohistochemistry (IHC) for defective MMR.
Revised Bethesda Guidelines
-
CRC diagnosed in a patient < age 50.
-
Synchronous or metachronous CRC or other Lynch-related tumor.
-
CRC diagnosed in a first-degree relative with a Lynch-related tumor, one diagnosed < age 50.
-
CRC diagnosed in two or more first- or second-degree relatives with Lynch-related tumors.
-
CRC with MSI-high (MSI-H) histology in patient < age 60:
-
Tumor infiltrating lymphocytes.
-
Crohn’s-like lymphocytic reaction.
-
Medullary growth pattern.
-
Mucinous/Signet ring differentiation.
-
Special Notes
-
In stage II patients, IHC testing should be considered as MSI-H status has been shown to predict lack of benefit from fluorouracil-based adjuvant chemotherapy [7, 18].
-
Extracolonic manifestations of Lynch syndrome include cancers of the uterus (30–60%), ovary (4–12%), urinary tract (5–12%), stomach (8–10%), small bowel, pancreas (4%), biliary tract, brain, and skin [15].
-
Testing guidelines based on age and family history miss a significant proportion of patients with MSI-H tumors. Universal testing of patients with CRC is a more sensitive method of identifying MSI-H patients and may be more cost-effective than traditional guidelines [19,20,21].
-
The proposed ASCO/ESMO guidelines suggest (1) universal testing of all patients with CRC or (2) testing of all patients <70 and patients >70 who fulfill any of the revised Bethesda guidelines [19].
-
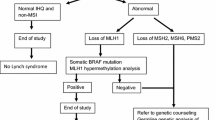
Tumor testing for MMR deficiency with IHC ± MSI:
-
If loss of MLH1/PMS2 protein expression is observed in the tumor, analysis of BRAF V600E mutation and/or analysis of methylation of the MLH1 promoter should be carried out first to rule out a sporadic case.
-
If tumor is MMR deficient and somatic BRAF mutation is not detected or MLH1 promoter methylation is not identified, testing for germline mutations is indicated.
-
If loss of any of the other proteins (MSH2/MSH6/PMS2) is identified, test for corresponding genes to the absent protein (e.g., MSH2, MSH6, EPCAM, PMS2, MLH1).
-
Full germline testing for Lynch should include DNA sequencing and large re-arrangement analysis.
-
Polyposis Syndromes
Familial Adenomatous Polyposis (FAP)
-
>100–1000s of adenomas distributed in the colon and rectum at presentation.
-
Accounts for <1% of all CRC cancers. Polyps often manifest in adolescents or young adults.
-
Extracolonic manifestations of FAP: gastric and duodenal polyps, desmoid tumors, thyroid and brain tumors, congenital hypertrophy of the retinal pigmented epithelium (CHRPE), supernumerary teeth, osteomas, and epidermoid cysts.
-
Duodenal/ampullary adenocarcinomas follow CRC as the major cause of cancer death in patients with FAP.
-
Desmoid tumors are found in up to 30% of patients with FAP and are the third most common cause of death in FAP. They peak around age 30 or 2–3 years after surgery. Depending on the location and symptoms, management includes observation (10% resolve spontaneously), medical therapy (NSAIDS, tamoxifen, vinblastine/methotrexate, or chemotherapy), or surgical resection.
Attenuated Familial Adenomatous Polyposis (AFAP)
-
10–99 colorectal adenomas at presentation, preponderance for right colon. Polyps tend to develop later in life compared to FAP.
MUTYH-Associated Polyposis (MAP)
-
Autosomal recessive inheritance, phenotype characterized by <100 adenomas. Average age of onset mid-50s. Up to 1/3 of biallelic MUTYH-mutation carriers may develop CRC in the absence of colorectal polyposis. Heterozygote individuals are also at a slightly increased risk of CRC (Table 6.4).
Germline Testing for APC and MUTYH [15]
-
Should be considered in all patients with multiple colorectal adenomas (>10).
-
APC germline testing should include DNA sequencing and large re-arrangement analysis.
Management
Primary Localized Colon Cancer
Special Notes
-
Polyps
-
Endoscopic management of sessile and pedunculated polyps is appropriate provided they are removed as a single specimen and lack high-risk features [28,29,30].
-
High-risk features of malignant polyps include poorly differentiated histology, lymphovascular invasion, tumor budding, piecemeal excision, and positive margin [28, 29].
-
Data regarding surveillance following successful endoscopic resection is lacking. Repeat endoscopic evaluation for local recurrence is recommended 3–6 months post resection. There is no defined role for routine imaging (Table 6.5); however, in high-risk patients not undergoing resection, enrollment in a surveillance program may be considered [28,29,30].
-
Given that lymph node involvement has been reported in 5–17% of malignant polyps [28,29,30,31], practice at the University of Toronto has included radiographic staging at diagnosis.
-
-
Adjuvant Treatment
-
Adjuvant chemotherapy should begin within 8 weeks of surgery. If delayed beyond 12 weeks, there is limited to no clinical benefit [32, 33].
-
The benefit of adjuvant chemotherapy is clearest in patients with stage III disease where ~30% decrease in risk of recurrence and mortality has been demonstrated [34].
-
The role of adjuvant chemotherapy among patients with high-risk stage II disease (perforation, obstruction, nodal harvest <12 nodes, T4, poorly differentiated histology) is more controversial [34].
-
When adjuvant chemotherapy is administered for stage II disease, oxaliplatin is often omitted due to adverse side effects and unclear benefit. Additionally, as noted previously, MIS-H status predicts lack of benefit from fluorouracil-based adjuvant chemotherapy in stage II disease [17, 18].
-
Six months of adjuvant therapy remains the standard of care; however, given the small absolute difference in DFS and the reduced rates of toxicity, adjuvant therapy may be limited to 3 months in patients with T1-T3 and N1 disease [35].
-
-
Technical Considerations
-
A minimally invasive approach is recommended in all suitable patients. Evidence suggests that the principal benefits are reduction in length of stay and postoperative pain with equivalent oncological outcomes [28, 36,37,38,39,40].
-
Several retrospective studies and one prospective randomized trial have evaluated the use of robotic surgery. While feasibility and safety compared to laparoscopy has been demonstrated, to date there is no convincing evidence to favor the use of robotics over conventional laparoscopic techniques [28, 44,45,46,47].
-
Routine extended lymphadenectomy is not standard of care. At present, no randomized trials have compared complete mesocolic excision surgery to conventional colectomy [28].
-
Quality Indicators:
-
-
Surveillance
-
If a preoperative assessment was not performed, colonoscopy should be performed within 6 months of surgery or as soon as possible after the completion of adjuvant therapy. Frequency of colonoscopies thereafter should be dictated by the findings [24, 48].
-
Of patients who recur, 80% are within the first 2–2.5 years, and 95% recur by 5 years [48]
-
Any new and persistent or worsening symptoms warrant the consideration of a recurrence.
-
The general practice at the University of Toronto is to perform CT of the chest/abdomen/pelvis every 6 to 12 months for the first 2 years then annually up to 5 years.
-
The American Society of Clinical Oncology (ASCO) 2013 endorsement of CCO practice guidelines suggests considering CT chest/abdomen every 6–12 months for 3 years in patients at a higher risk of recurrence [48].
-
The intensity of postoperative surveillance should depend on the likelihood that additional therapy would be recommended in the setting of recurrent disease.
-
Management of Patient Populations at High Risk for Colon Cancer
Special Notes
-
Lynch syndrome: Segmental resection may be considered in cases of significant comorbidity, advanced age, or advanced disease. Detailed discussion of risk/benefits and need for close endoscopic surveillance should be emphasized if segmental resection is to be performed.
-
FAP: The choice between colectomy + IRA and TPC-IPAA must be balanced with patient age, degree of rectal polyposis, wish to bear children, risk of developing desmoids, and possibly the site of mutation in the APC gene.
-
AFAP/MAP: Preservation of the rectum may be considered when rectal clearance is possible (Table 6.6). The risk of recurrence in rectal stump must be balanced against the alteration in function with proctocolectomy and pelvic pouch.
-
IBD: Nomenclature and management of dysplasia in IBD is evolving. Recent SCENIC [49] guidelines advocate chromoendoscopy for surveillance. Consider referral to an IBD center if dysplasia is identified on random biopsy. Endoscopic management of dysplasia associated mass lesions (DALM) should be done at expert centers.
Locally Advanced Colon Cancer or Locoregional Recurrence
Special Notes
-
Histologically negative margins should be the goal of en bloc resection [50, 51]. Relevant margins should be marked on the specimen by the surgeon.
-
Neoadjuvant chemoradiotherapy may improve resectability and negative margin rates (Table 6.7) [52, 53].
Colon Cancer with Distant Metastases
Special Notes
-
Resection of the primary tumor should be considered in symptomatic patients or in those with potentially resectable metastatic disease.
-
First-line chemotherapy should be strongly considered in asymptomatic patients with unresectable metastatic disease (Table 6.8).
-
If a synchronous metastasis is resectable, the timing of surgery and chemotherapy should be individualized for each patient. Options include synchronous or staged colectomy with metastasectomy vs. neoadjuvant chemotherapy followed by synchronous or staged colectomy and metastasectomy vs. colectomy followed by chemotherapy and staged metastasectomy or vice versa.
-
Patients with unresected primaries should be followed as up to 20% need surgical resection during the course of their treatment.
-
Bevacizumab administration has been associated with delayed wound healing and GI perforation [54, 58, 59]. The bevacizumab product monograph states it should be discontinued ≥28 days before elective surgery and should not be initiated for ≥28 days after surgery.
-
However, while patients on bevacizumab therapy undergoing surgery have been shown to experience significant morbidity and mortality, the risk of complications has not been detectably associated with time since exposure in population-based studies [59].
-
There may be a survival advantage in resection of the primary tumor in patients with unresectable metastatic disease [60]. Randomized trials investigating this topic are ongoing [61, 62].
Landmark Publications (Table 6.9)
Referring to Medical Oncology (See Tables 6.7 and 6.8)
-
1.
High-risk stage II.
-
2.
Stage III, IV.
-
3.
Locally advanced or recurrent disease.
Referring to Radiation Oncology (See Tables 6.7 and 6.8)
-
1.
Consider for locally advanced or recurrent disease.
-
2.
Palliative management of symptomatic lesions with unresectable metastatic disease.
Referring to Multidisciplinary Cancer Conference (MCC)
-
1.
Locally advanced or recurrent disease.
-
2.
Metastatic disease in fit patients (synchronous and metachronous).
Toronto Pearls
-
Neoadjuvant chemoradiotherapy for locally advanced or recurrent colon cancer may improve resectability and negative margin rates. Careful preoperative planning and multidisciplinary approach are necessary to achieve the goal of R0 resection.
References
Canadian Cancer Statistics Advisory Committee. Canadian Cancer Statistics 2020. Toronto: Canadian Cancer Society; 2020. Available at: https://www.cancer.ca/~/media/cancer.ca/CW/cancer%20information/cancer%20101/Canadian%20cancer%20statistics%20supplementary%20information/2020/2020_cancer-specific-stats.pdf?la=en. Accessed 21 June 2020.
Canadian Cancer Society’s Steering Committee on Cancer Statistics. Canadian cancer statistics 2014. Toronto: Canadian Cancer Society; 2014.
Colorectal Cancer Staging and Survival. Toronto: Canadian Partnership Against Cancer; 2010.
Hewitson P, Glasziou P, Watson E, et al. Cochrane systematic review of colorectal cancer screening using the fecal occult blood test (hemoccult): an update. Am J Gastroenterol. 2008;103(6):1541–9.
Holme O, Bretthauer M, Fretheim A, Odgaard-Jensen J, Hoff G. Flexible sigmoidoscopy versus faecal occult blood testing for colorectal cancer screening in asymptomatic individuals. Cochrane Database of Syst Rev. 2013;9:CD009259.
Tinmouth J, Vella ET, Baxter NN, et al. Colorectal cancer screening in average risk populations: evidence summary. Can J Gastroenterol Hepatol. 2016;2016:2878149.
Holme O, Loberg M, Kalager M, Bretthauer M, Hernan MA, Aas E, et al. Effect of flexible sigmoidoscopy screening on colorectal cancer incidence and mortality: a randomized clinical trial. JAMA. 2014;312(6):606–15.
Brenner H, Stock C, Hoffmeister M. Effect of screening sigmoidoscopy and screening colonoscopy on colorectal cancer incidence and mortality: systematic review and meta-analysis of randomised controlled trials and observational studies. BMJ. 2014;348:g2467.
Rex DK, Johnson DA, Anderson JC, et al. American College of Gastroenterology guidelines for colorectal cancer screening 2009 [corrected]. Am J Gastroenterol. 2009;104(3):739–50.
Rabeneck L, Paszat LF, Saskin R, Stukel TA. Association between colonoscopy rates and colorectal cancer mortality. Am J Gastroenterol. 2010;105(7):1627–32.
Baxter NN, Goldwasser MA, Paszat LF, et al. Association of colonoscopy and death from colorectal cancer. Ann Intern Med. 2009;150(1):1–8.
Tinmouth J, Kennedy EB, Baron D, Burke M, Feinberg S, Gould M, et al. Colonoscopy quality assurance in Ontario: systematic review and clinical practice guideline. Can J Gastroenterol Hepatol. 2014;28(5):251–74.
Lieberman DA, Rex DK, Winawer SJ, Giardiello FM, Johnson DA, Levin TR, et al. Guidelines for colonoscopy surveillance after screening and polypectomy: a consensus update by the US Multi-Society Task Force on Colorectal Cancer. Gastroenterology. 2012;143(3):844–57.
Shaukat A, Rector TS, Church TR, et al. Longer withdrawal time is associated with a reduced incidence of interval cancer after screening colonoscopy. Gastroenterology. 2015;149:952–7.
Dubé C, McCurdy BR, Bronstein T, et al. ColonCancerCheck: recommendations for post polypectomy surveillance. 2019. https://www.cancercareontario.ca/en/content/coloncancercheck-recommendations-post-polypectomy-surveillance.
Umar A, Boland CR, Terdiman JP, et al. Revised Bethesda guidelines for hereditary nonpolyposis colorectal cancer (Lynch syndrome) and microsatellite instability. J Natl Cancer Inst. 2004;96(4):261–8.
Ribic CM, Sargent DJ, Moore MJ, et al. Tumor microsatellite-instability status as a predictor of benefit from fluorouracil-based adjuvant chemotherapy for colon cancer. N Engl J Med. 2003;349(3):247–57.
Sargent DJ, Marsoni S, Monges G, et al. Defective mismatch repair as a predictive marker for lack of efficacy of fluorouracil-based adjuvant therapy in colon cancer. J Clin Oncol. 2010;28(20):3219–26.
Stoffel EM, Mangu PB, Gruber SB, Hamilton SR, Kalady MF, Lau MW, et al. Hereditary colorectal cancer syndromes: American Society of Clinical Oncology clinical practice guideline endorsement of the familial risk-colorectal cancer: European society for medical oncology clinical practice guidelines. J Clin Oncol. 2015;33(2):209–17.
Matloff J, Lucas A, Polydorides AD, Itzkowitz SH. Molecular tumor testing for Lynch syndrome in patients with colorectal cancer. J Natl Compr Cancer Netw. 2013;11(11):1380–5.
Perez-Carbonell L, Ruiz-Ponte C, Guarinos C, Alenda C, Paya A, Brea A, et al. Comparison between universal molecular screening for Lynch syndrome and revised Bethesda guidelines in a large population-based cohort of patients with colorectal cancer. Gut. 2012;61(6):865–72.
Jeffery M, Hickey BE, Hider PN. Follow-up strategies for patients treated for non-metastatic colorectal cancer. Cochrane Database Syst Rev. 2007;1:002200.
Figueredo A, Rumble RB, Maroun J, et al. The members of the Gastrointestinal Cancer Disease Site Group. Follow-up of patients with curatively resected colorectal cancer. Practice Guideline Report #2–9. June 16, 2010.
Members of the Colorectal Cancer Survivorship Group. Follow-up care, surveillance protocol, and secondary prevention measures for survivors of colorectal cancer. Toronto: Cancer Care Ontario; 2012 [Being Updated 2018 Jun]. Program in Evidence-based Care Evidence-Based Series No.: 26–2 Version 2.
Kuebler JP, Wieand HS, O’Connell MJ, et al. Oxaliplatin combined with weekly bolus fluorouracil and leucovorin as surgical adjuvant chemotherapy for stage II and III colon cancer: results from NSABP C-07. J Clin Oncol. 2007;25(16):2198–204.
Andre T, Boni C, Mounedji-Boudiaf L, et al. Multicenter international study of oxaliplatin/5-Fluorouracil/Leucovorin in the Adjuvant Treatment of Colon Cancer (MOSAIC) Investigators. Oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment for colon cancer. N Engl J Med. 2004;350(23):2343–51.
Twelves C, Wong A, Nowacki MP, et al. Capecitabine as adjuvant treatment for stage III colon cancer. N Engl J Med. 2005;352(26):2696–704.
Vogel JD, Eskicioglu C, Weiser MR, et al. The American Society of Colon and Rectal Surgeons clinical practice guidelines for the treatment of colon cancer. Dis Colon Rectum. 2017;60:999–1017.
Williams JG, Pullan RD, Hill J, et al. Management of the malignant colorectal polyp: ACPGBI position statement. Color Dis. 2013;15(Suppl 2):1–38.
Rutter MD, Chattree A, Barbour JA, et al. British Society of Gastroenterology/ Association of Coloproctologists of Great Britain and Ireland guidelines for the management of large non-pedunculated colorectal polyps. Gut. 2015;64:1847–73.
Suzuki T, Sadahiro S, Mukoyama S, et al. Risk of lymph node and distant metastases in patients with early invasive colorectal cancer classified as Haggitt’s level 4 invasion: image analysis of submucosal layer invasion. Dis Colon Rectum. 2003;45(2):203–8.
Biagi JJ, Raphael MJ, Mackillop, et al. Association between time to initiation of adjuvant chemotherapy and survival in colorectal cancer: a systematic review and meta-analysis. JAMA. 2011;305(22):2335–42.
Lima IS, Yasui Y, Scarfe A, Winget M. Association between receipt and timing of adjuvant chemotherapy and survival for patients with stage III colon cancer in Alberta. Canada Cancer. 2011;117:3833–40.
Meyers B, Cosby R, Quereshy F, Jonker D. Adjuvant systemic chemotherapy for stage II and III colon cancer following complete resection. Toronto: Cancer Care Ontario; 2015. [In Review 2017 Nov]. Program in Evidence-based Care Evidence-based Series No.: 2-29 Version 2.
Grothey A, Sobrero AF, Shields AF, et al. Duration of adjuvant chemotherapy for stage III colon cancer. N Engl J Med. 2018;378:1177–88.
Smith A, Rumble RB, Langer B, et al. Laparoscopic surgery for cancer of the colon. Toronto: Cancer Care Ontario; 2005. Program in Evidence-Based Care. Evidence-based Series #2-20-2.
Clinical Outcomes of Surgical Therapy Study Group. A comparison of laparoscopically assisted and open colectomy for colon cancer. N Engl J Med. 2004;350(20):2050–9.
Jayne DG, Guillou PJ, Thorpe H, et al. Randomized trial of laparoscopic-assisted resection of colorectal carcinoma: 3-year results of the UK MRC CLASICC Trial Group. J Clin Oncol. 2007;25(21):3061–8.
Lacy AM, Garcia-Valdecasas JC, Delgado S, et al. Laparoscopy-assisted colectomy versus open colectomy for treatment of non-metastatic colon cancer: a randomised trial. Lancet. 2002;359(9325):2224–9.
Kuhry E, Schwenk W, Gaupset R, et al. Long-term outcome of laparoscopic surgery for colorectal cancer: a cochrane systematic review of randomised controlled trials. Cancer Treat Rev. 2008;34(6):498–504.
Amri R, Bordeianou LG, Sylla P, Berger DL. Association of radial margin positivity with colon cancer. JAMA Surg. 2015;150:890–8.
Le Voyer TE, Sigurdson ER, Hanlon AL, et al. Colon cancer survival is associated with increasing number of lymph nodes analyzed: a secondary survey of intergroup trial INT-0089. J Clin Oncol. 2003;21(15):2912–9.
Smith AJ, Driman DK, Spithoff K, et al. Guideline for optimization of colorectal cancer surgery and pathology. J Surg Oncol. 2010;101(1):5–12.
Park JS, Choi GS, Park SY, Kim HJ, Ryuk JP. Randomized clinical trial of robot-assisted versus standard laparoscopic right colectomy. Br J Surg. 2012;99:1219–26.
Lorenzon L, Bini F, Balducci G, et al. Laparoscopic versus robotic-assisted colectomy and rectal resection: a systematic review and meta-analysis. Int J Color Dis. 2016;31(2):161–73.
Juo YY, Hyder O, Haider AH, Camp M, Lidor A, Ahuja N. Is minimally invasive colon resection better than traditional approaches?: First comprehensive national examination with propensity score matching. JAMA Surg. 2014;149(2):177–84.
Pinar I, Fransgaard T, Thygesen LC, et al. Long-term outcomes of robot-assisted surgery in patients with colorectal cancer. Ann Surg Oncol. 2018;25(13):3906–12.
Meyerhardt JA, Mangu PB, Flynn PJ, Korde L, Loprinzi CL, Minsky BD, et al. Follow-up care, surveillance protocol, and secondary prevention measures for survivors of colorectal cancer: American Society of Clinical Oncology clinical practice guideline endorsement. J Clin Oncol. 2013;31(35):4465–70.
Laine L, Kaltenbach T, Barkun A, et al. SCENIC international consensus statement on surveillance and management of dysplasia in inflammatory bowel disease. Gastrointest Endosc. 2015;81:489–501.
Bowne WB, Lee B, Wong WD, et al. Operative salvage for locoregional recurrent colon cancer after curative resection: an analysis of 100 cases. Dis Colon Rectum. 2005;48(5):897–909.
Smith AJ, Driman DK, Spithoff K, McLeod R, Hunter A, Rumble RB, et al. Optimization of surgical and pathological quality performance in radical surgery for colon and rectal cancer: margins and lymph nodes. Toronto: Cancer Care Ontario; 2008. [In review 2013 Dec]. Program in Evidence-based Care Evidence-based Series No.: 7–14.
Cukier M, Smith AJ, Milot L, et al. Neoadjuvant chemoradiotherapy and multivisceral resection for primary locally advanced adherent colon cancer: a single institution experience. Eur J Surg Oncol. 2012;38(8):677–82.
Hallet J, Zih FS, Lemke M, et al. Neo-adjuvant chemoradiotherapy and multivisceral resection to optimize R0 resection of locally recurrent adherent colon cancer. Eur J Surg Oncol. 2014;40(6):706–12.
Hurwitz H, Fehrenbacher L, Novotny W, et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med. 2004;350(23):2335–42.
Giantonio BJ, Catalano PJ, Meropol NJ, et al. Bevacizumab in combination with oxaliplatin, fluorouracil, and leucovorin (FOLFOX4) for previously treated metastatic colorectal cancer: results from the Eastern Cooperative Oncology Group Study E3200. J Clin Oncol. 2007;25(12):1539–44.
Saltz LB, Clarke S, Diaz-Rubio E, et al. Bevacizumab in combination with oxaliplatin-based chemotherapy as first-line therapy in metastatic colorectal cancer: a randomized phase III study. J Clin Oncol. 2008;26:2013–9. Erratum appears in J Clin Oncol. 2009;27(4):653.
Van Cutsem E, Kohne CH, Hitre E, et al. Cetuximab and chemotherapy as initial treatment for metastatic colorectal cancer. N Engl J Med. 2009;360(14):1408–17.
Hapani S, Chu D, Wu S. Risk of gastrointestinal perforation in patients with cancer treated with bevacizumab: a meta-analysis. Lancet Oncol. 2009;10:559–68.
Baxter N, Fischer HD, Richardson D, et al. A population-based study of complications after colorectal surgery in patients who have received Bevacizumab. Dis Colon Rectum. 2018;61(3):306–13.
Faron M, Pignon JP, Malka D, Bourredjem A, Douillard JY, Adenis A, et al. Is primary tumour resection associated with survival improvement in patients with colorectal cancer and unresectable synchronous metastases? A pooled analysis of individual data from four randomised trials. Eur J Cancer. 2015;51(2):166–76.
Jorine't Lam-Boer J, Mol L, Verhoef C, et al. The CAIRO4 study: the role of surgery of the primary tumour with few or absent symptoms in patients with synchronous unresectable metastases of colorectal cancer – a randomized phase III study of the Dutch Colorectal Cancer Group (DCCG). BMC Cancer. 2014;14:741.
Rahbari NN, Lordick F, Fink C, et al. Resection of the primary tumour versus no resection prior to systemic therapy in patients with colon cancer and synchronous unresectable metastases (UICC stage IV): SYNCHRONOUS – a randomised controlled multicentre trial (ISRCTN30964555). BMC Cancer. 2012;12:142.
Fleshman J, Sargent DJ, Green E, et al. Laparoscopic colectomy for cancer is not inferior to open surgery based on 5-year data from the COST Study Group trial. Ann Surg. 2007;246(4):655–62. discussion 662–4.
Green BL, Marshall HC, Collinson F, et al. Long-term follow-up of the Medical Research Council CLASICC trial of conventional versus laparoscopically assisted resection in colorectal cancer. Br J Surg. 2013;100(1):75–82.
Colon Cancer Laparoscopic or Open Resection Study Group, Buunen M, Veldkamp R, Hop WC, Kuhry E, Jeekel J, et al. Survival after laparoscopic surgery versus open surgery for colon cancer: long-term outcome of a randomised clinical trial. Lancet Oncol. 2009;10(1):44–52.
Deijen CL, Vasmel JE, de Lange-de Klerk ESM, et al. Ten-year outcomes of a randomised trial of laparoscopic versus open surgery for colon cancer. Surg Endosc. 2016;31(6):2607–15.
Lacy AM, Delgado S, Castells A, et al. The long-term results of a randomized clinical trial of laparoscopy-assisted versus open surgery for colon cancer. Ann Surg. 2008;248(1):1–7.
Yothers G, O'Connell MJ, Allegra CJ, et al. Oxaliplatin as adjuvant therapy for colon cancer: updated results of NSABP C-07 trial, including survival and subset analyses. J Clin Oncol. 2011;29(28):3768–74.
Andre T, Boni C, Navarro M, et al. Improved overall survival with oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment in stage II or III colon cancer in the MOSAIC trial. J Clin Oncol. 2009;27(19):3109–16.
Tournigand C, Andre T, Bonnetain F, Chibaudel B, Lledo G, Hickish T, et al. Adjuvant therapy with fluorouracil and oxaliplatin in stage II and elderly patients (between ages 70 and 75 years) with colon cancer: subgroup analyses of the Multicenter International Study of Oxaliplatin, Fluorouracil, and Leucovorin in the Adjuvant Treatment of Colon Cancer trial. J Clin Oncol. 2012;30(27):3353–60.
André T, de Gramont A, Vernerey D, et al. Adjuvant Fluorouracil, Leucovorin, and Oxaliplatin in stage II to III Colon Cancer: updated 10-year survival and outcomes according to BRAF mutation and mismatch repair status of the MOSAIC Study. J Clin Oncol. 2015;33:4176–87.
Twelves C, Scheithauer W, McKendrick J, Seitz JF, Van Hazel G, Wong A, et al. Capecitabine versus 5-fluorouracil/folinic acid as adjuvant therapy for stage III colon cancer: final results from the X-ACT trial with analysis by age and preliminary evidence of a pharmacodynamic marker of efficacy. Ann Oncol. 2012;23(5):1190–7.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Khorasani, S. et al. (2020). Colon Cancer. In: Wright, F., Escallon, J., Cukier, M., Tsang, M., Hameed, U. (eds) Surgical Oncology Manual. Springer, Cham. https://doi.org/10.1007/978-3-030-48363-0_6
Download citation
DOI: https://doi.org/10.1007/978-3-030-48363-0_6
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-48362-3
Online ISBN: 978-3-030-48363-0
eBook Packages: MedicineMedicine (R0)