Abstract
Gallbladder cancer (GBC) is the most common malignant tumor of the biliary tract representing 80–95% of biliary tract cancers worldwide. The disease has a striking variability in the incidence, being most common in some countries such as Chile, North India, and Japan, and less common in western countries such as the United States. Most cases are diagnosed at late stages due to vague symptoms, and only 10–30% of patients are candidates for curative resection. Given the rarity of the disease in many parts of the world, contemporary clinical data are limited to a few clinical trials. Moreover, patients with GBC are often combined with other biliary tract cancers in clinical trials. Nevertheless, the treatment of gallbladder cancer has evolved over the last decade with an increased emphasis on the use of therapies such as intensity-modulated radiotherapy (IMRT), molecular targeted therapy, and combination chemotherapy. In addition, randomized controlled trials are evaluating the roles of chemotherapy and molecular-targeted therapy in the management of the disease. In this chapter, we detail the contemporary clinical management of gallbladder cancer with a special focus on the latest developments in the field.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
Introduction
Gallbladder cancer (GBC), although considered an uncommon cancer of the gastrointestinal tract, constitutes about two thirds of the malignancies arising from the extrahepatic biliary tract in the United States. Other malignancies associated with the biliary tract including intrahepatic, perihilar and distal cholangiocarcinoma, and ampullary cancers are distinct from gallbladder cancers in their presentation and natural history and are less common. The prognosis of GBC is highly dependent on tumor stage at presentation. Many cases of GBC are identified incidentally during surgery, cholecystectomy, or pathological examination of resected gallbladders. Given the vague, nonspecific presentation, symptomatic GBC tends to present at an advanced stage, and survival is generally poor, except for the minority of cases that are identified at early stages. Unlike other extrahepatic biliary tract cancers, the presence of jaundice is associated with unresectability, often due to hilar involvement, and the prognosis is poor. Overall, 5-year survival for patients with T1 tumors approaches 50% and is lower for more advanced tumors. In this chapter, we detail the current literature on the pathophysiology, diagnosis, and management of GBC with a special focus on recent advances in the field.
Epidemiology
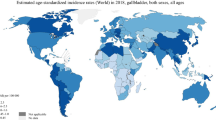
The incidence of GBC cancer varies widely worldwide depending upon the geographical location and ethnicity. High incidence rates of GBC (>15 per 100,000 women) are seen in Chile, Northern India, and Southern Pakistan followed by Japan and Thailand, while Northern America, parts of western Europe, and Mediterranean Europe have the lowest incidences (<10 per 100,000 women) [1]. Even within the US, the incidence varies depending on ethnic background with a higher incidence in American Indian, Hispanic, and Alaskan native populations, and a lower incidence in African Americans and Caucasians. In addition, the incidence of GBC increases with age, being usually seen in elderly persons (>65 years) with a female predilection [2]. In general, the incidence of GBC varies with the prevalance of two major risk factors, gallstones and typhoid infection, as well as other factors such as environmental exposure to carcinogens, liver fluke infections and patient-related factors such as intrinsic predisposition to tumorigenesis [3].
Etiology
The etiology of GBC is complex, but chronic gallbladder injury and inflammation are the most commonly associated etiological factors for GBC. Proposed risk factors for GBC are summarized in Table 11.1.
Gallstones
Gallstones are present in most (70–90%) patients with gallbladder cancer. The risk is further correlated with the size of the gallstones – the presence of gallstones >3 cm increases the risk of GBC by ten-fold compared to gallstones of size <1 cm [4]. Autopsy data also suggest that gallstones are associated with an almost seven-fold increase in the risk of GBC. Though substantial evidence favors the association between the two diseases, it is not yet clear if the association represents a direct causal link [5, 6]. The presence of gallstones is thought to induce chronic irritation and inflammation of the gallbladder mucosa, thereby leading to dysplasia of mucosal cells. These chronic inflammatory insults may act as inciting agents for oncogenic transformation by causing deoxyribonucleic acid (DNA) damage and DNA methylation defects, as well as by releasing inflammatory cytokines and growth factors leading to angiogenesis [7, 8]. Another possible explanation for the metaplasia-dysplasia transformation of gallbladder epithelium is alteration of the chemical composition of bile leading to the formation of free radical oxidation products and secondary bile acids [9]. However, environmental and genetic factors may also play key roles in the development of GBC, as about 10–25% of patients GBC do not have associated cholelithiasis [5]. The first-line imaging test for evaluation of gallstones is a right upper quadrant abdominal ultrasound (USG). On ultrasound, gallstones are typically mobile, echogenic foci with posterior acoustic shadowing as shown in Fig. 11.1.
A 79-year-old male patient being evaluated for epigastric pain demonstrates multiple echogenic foci (white arrow) layering within the gallbladder with posterior acoustic shadowing (white arrowhead) within the gallbladder (a). The same patient subsequently underwent a CT examination of the abdomen showing radiopaque gallstones (white arrow)
Chronic Inflammation
Chronic bacterial infection has been linked to GBC. Bacterial colonization may alter the bile acid milieu. The most common organisms implicated with this association are Salmonella (S. typhi and S. paratyphi) and Helicobacter (H. bilis) species [10]. Ecologically, there is a geographical correlation between the endemicity of typhoid infection and GBC, especially in Chile and North India [11, 12]. Moreover, a registry-based cohort study concluded that chronic typhoid and paratyphoid carriers showed a large excess risk (observed/expected cases) for GBC (167·0; 95% confidence interval 54·1–389) [13]. Similarly, a 12-fold increase in risk of GBC in patients with a history of typhoid infection (OR = 12.7 [CI, 1.5–598]) was reported in a case control study [14]. Retrospective case control studies from Japan and Thailand also showed an association of Helicobactor bilis with GBC (almost six-fold increase) [10].
About 10–20% of patients with primary sclerosing cholangitis (PSC) will develop hepatobiliary malignancy [15]. PSC is known to cause high frequencies of pyloric metaplasia, intestinal metaplasia/dysplasia, and invasive adenocarcinoma at rates significantly higher than the general population [15]. Retrospective studies found that GB adenocarcinomas arose out of a background of flat mucosal dysplasia, supporting the concept of a metaplasia-dysplasia-carcinoma sequence [16]. Given this strong association, it is recommended that patients with PSC should have annual gallbladder cancer surveillance (abdominal ultrasound screening) to identify gallbladder masses, and cholecystectomy should be performed if any suspicious lesions, polyps, or masses are identified [17].
Calcified/Porcelain Gallbladder
Chronic irritation and inflammation may lead to calcium deposition in the gallbladder wall. The gallbladder calcification can be of distinct types – diffuse intramural calcification (porcelain gallbladder) or isolated mucosal calcification. Calcification within the gallbladder wall is best seen on CT examination, but can also be seen on ultrasound or plain X-ray as seen in Fig. 11.2. Based on previous reports, gallbladder calcification is associated with increased risk of GBC (range 2–61%), and the risk is much higher in gallbladders with isolated mucosal calcifications leading to stippled, multiple punctate calcifications (compared to diffuse intramural calcifications) [18, 19]. Hence, gallbladders with partial calcification, stippled, or multiple punctate calcifications warrant extensive evaluation, and prophylactic cholecystectomy may be needed.
Gallbladder Polyps
Gallbladder polyps are present in 5% of the adult population and are usually asymptomatic [16]. Gallbladder polyps can be differentiated from gallstones by their typical sonographic appearance. Polyps are intraluminal, nonmobile, echogenic foci which lack posterior acoustic shadowing (Fig. 11.3). Features of polyps that predict malignancy are: size >1 cm, solitary and/or sessile masses, associated gallstones, a vascular stalk, concomitant PSC or PBC, and most importantly, rapid polyp growth [20]. Cholecystectomy is recommended in polyps with such features. By consensus guidelines, incidentally found gallbladder polyps ≤6 mm in size are considered benign and no further evaluation or follow-up is recommended. Gallbladder polyps measuring 7–9 mm are considered indeterminate and should be followed by serial ultrasonography at 12-month intervals [21]. Whether gallbladder polyps in BRCA 1/2 mutation carriers merit cholecystectomy is not conclusively proven, but given the increased risk of biliary cancers, a lower threshold for cholecystectomy is often applied to these patients.
Environmental Risk Factors/Exposure to Carcinogens
Various environmental factors such as nickel, cadmium, radon, cigarette smoking, and drugs (methyldopa and isoniazid) have been implicated in the development of GBC. Despite the fact that some studies have hypothesized an etiological role for oral contraceptive pills in GBC, the association remains unclear [16].
Congenital Abnormalities and Association with Hereditary Syndromes
About 10% of GBC patients were have an anomalous junction of the pancreaticobiliary duct leading to regurgitation of pancreatic secretions into the gallbladder. Interestingly, these anomalies are more common in patients of Asian descent, in whom the incidence of GBC is high [16]. It is hypothesized that reflux of pancreatic secretions through the common channel into the biliary tree gives rise to metaplastic and dysplastic changes in the gallbladder mucosa.
Rare cases of GBC are reported in hereditary syndromes such as Gardner Syndrome, NF-1, MEN-1, and Von-Hippel Lindau syndrome (neuroendocrine tumors of gallbladder) [22], as well as in persons with BRCA2 mutations.
Pathology of GBC
GBC usually leads to asymmetric thickening of the gallbladder wall with infiltration of surrounding structures. Most cancers originate in the gallbladder fundus (60%), followed by the body (30%) and neck (10%) [23]. Macroscopically, GBCs can be divided into papillary, tubular, and nodular forms. Tubular and nodular forms of the disease are aggressive, whereas papillary tumors are less likely to invade the liver directly and have a lower incidence of lymph-node metastasis [5].
Histologically, the majority of carcinomas of the gallbladder are adenocarcinomas (80–95%) and can be papillary, tubular, mucinous (colloid) adenocarcinoma, signet ring adenocarcinoma, cribriform carcinoma, clear cell adenocarcinoma, and hepatoid adenocarcinoma variants. Less common types of GBC include adeno-squamous carcinoma, squamous cell carcinoma, and undifferentiated or anaplastic carcinoma (2–7%) [5]. Other rare variants include small-cell carcinoma and neuroendocrine tumors. Involvement of the gallbladder by malignant melanoma, lymphoma, and sarcomas is particularly rare [24].
Two distinct types of precursor lesions are associated with GBC, flat lesions with either low- or high-grade dysplasia and adenomas [25]. Most GBCs arise from flat dysplastic lesions (metaplasia-dysplasia-carcinoma sequence) while mass-forming precursor lesions (adenoma-carcinoma pathway) are identified only in a minority of cases. It is estimated that it takes about 15 years for dysplasia to progress to carcinoma in situ and finally to GBC.
Oncogenes that were shown to be associated with GBC are KRAS (10–67%), HER2 (ERBB2), epidermal growth factor receptor (EGFR)/HER-1, and cyclins-D1 and E [26]. KRAS mutations were seen with high frequency (50–80%) in anomalous pancreatic biliary malformation patients from Japan [27]. HER2 (transmembrane receptor tyrosine kinase) amplification was reported in 33–70% of GBC cases [28]. EGFR (a member of the erbB protein family that also encodes a receptor tyrosine kinase) mutations were identified more often in GBC (70.7%) and dysplastic precancerous lesions (85.7%) than in cases with simple hyperplasia (27%) and normal gallbladder (0%) [29]. Cell cycle progression promoter cyclin D1 overexpression was seen in about 40% of cases of GBC and seemed to be associated with early venous and lymphatic invasion [26]. Tumor suppressor genes that were associated with GBC include TP53, CDKN2A, p21/CDKN1A (associated with better survival), and Fragile Histidine Triad (FHIT) (frameshift mutations, loss of function). Other studies have identified higher levels of microsatellite instability, angiogenic-inflammatory pathway gene mutations (cyclooxygenase−2 [COX-2], nitric oxide synthase [iNOS], and vascular endothelial growth factor (VEGF)), MUC-1 overexpression (associated with lymphatic spread and poor prognosis), and telomerase (hTERT) re-expression in the pathogenesis of GBC [26].
It is important to understand the mechanism of GBC metastasis for the optimal management approach. As with any other cancer, the common routes of spread of GBC are direct, lymphatic, vascular, intraperitoneal, intraductal, and neural. Locoregional spread of the disease to adjacent liver segments IV and V or surrounding organs such as the duodenum, colon, peritoneum, or anterior abdominal wall is the most common mode of spread. Peritoneal spread is more common than distant spread. Lymph node metastases typically follow the lymphatic drainage of the gallbladder – gallbladder-retro-pancreatic pathway: involvement of cystic duct and peri-choledochal nodes occurs first, followed by posterior nodes to the head of the pancreas and then to inter-aortocaval lymph nodes; gallbladder-celiac pathway: spreads through the retro-portal and right celiac lymph nodes via gastro-hepatic ligament; gallbladder-mesenteric pathway: spreads to the aortocaval lymph nodes via pancreas [30]. However, involvement of aortocaval nodes can be positive even when cystic nodes are negative [31]. Segment IV of the liver is most commonly involved in cases of vascular metastases due to the direct communicating veins [5]. Distant metastases occur in cases of retroperitoneal vein invasion and are associated with very dismal prognosis and median survival of less than 4 months. Intraductal spread is seen in about 19% of papillary carcinomas. Intraductal growth leads to obstruction of the biliary tree, resulting in early clinical presentation and an overall better prognosis [30].
Clinical Presentation
Most cases of GBC are detected incidentally during surgery for cholecystectomy, or on histologic examination of a resected polyp or may be missed only to present with recurrence during follow-up. Due to the vague or delayed symptomatic presentation of GBC, especially occurring in the background of acute cholecystitis, the disease is usually diagnosed in late stages with dismal prognosis. GBC should be considered in the differential diagnosis if an elderly patient (>65 years) presents with right hypochondrial pain, weight loss, anorexia, and jaundice. Mirizzi syndrome (right upper quadrant pain due to common hepatic duct obstruction caused by extrinsic compression from an impacted stone in the cystic duct or gallbladder), a complication of long-standing cholelithiasis, was shown to be associated with GBC in 5–28% of cases [32, 33]. Patients with advanced GBC may present with a palpable gallbladder mass, hard nodular liver, malignant ascites from carcinomatosis, and jaundice. The presence of jaundice in GBC usually portends a poor prognosis [34]. Only about 20% of the patients have disease confined to the gallbladder at the time of diagnosis. The majority (about 80%) have locoregionally advanced disease with invasion of adjacent structures or distant metastases leading to varied symptoms such as acute abdominal pain due to intestinal perforation, ascites, paraneoplastic syndromes, neuropathy, and venous thromboembolism [5]. GBC should be suspected in patients with a long-standing history of chronic cholecystitis with gallstones who have sudden weight loss and have developed new symptoms of pain.
Diagnostic Evaluation
Imaging Studies
As in any other cancer management, preoperative imaging for tumor recognition and staging play a key role in the appropriate management of GBC. Roentgenographic studies have a relatively high sensitivity for the detection of GBC in advanced stages, but imaging findings of early GBC lesions appear similar to more common benign entities. For example, it is difficult to differentiate between cholecystitis and early carcinoma because thickening of the gallbladder wall is a feature of both diseases [5]. Tumefactive sludge and adenomyomatosis are also benign entities which in some cases can mimic early GBC. Nonetheless, an US can identify findings that are suggestive of GBC such as intramural wall thickening or calcification, polyps, irregular mass, loss of the interface between the liver and gallbladder, or direct liver infiltration. The diagnostic accuracy of polyps or intramural mass in the gallbladder by US is over 80% [20]. A gallbladder mass is shown in Fig. 11.4 with corresponding CT images.
Gallbladder carcinoma with metastatic spread. An 87-year-old male with prior history of cholelithiasis and biliary colic presented with an upper abdominal mass on physical examination and obstructive jaundice. On ultrasound examination, there is a heterogeneous gallbladder mass with internal vascularity (a). Subsequent staging CT axial and coronal images (b and c, respectively) demonstrate an infiltrative gallbladder mass disrupting the enhancing gallbladder wall mucosa and invading the liver (white arrow). Innumerable liver metastases are also seen (white asterisks)
Although US may be a good initial test, it is not very helpful in evaluating extent of locally advanced disease, involved lymph nodes or staging of disease [20]. To better characterize potentially malignant gallbladder lesions, contrast-enhanced magnetic resonance imaging (MRI) is the preferred imaging modality. GBC typically is heterogeneously hyperintense on T2-weighted images and relatively iso- or hypo-intense on T1-weighted images (Fig. 11.5). All GBC show enhancement on contrast-enhanced imaging, but note that imaging appearance can overlap with chronic cholecystitis in early GBC. Focal or diffuse mural thickening of more than 1 cm is highly suggestive of GBC [35]. Early irregular enhancement along the margin and invasion into surrounding structures are also features of GBC. MRI of the abdomen is preferred over CT as the former has a better diagnostic accuracy for assessing metastatic spread to the hepatoduodenal ligament, portal vein encasement, regional lymph nodes, and liver [20]. Multiphase MRI with arterial phase images should be obtained to determine the degree of vascular involvement and anatomic course of the hepatic arteries relative to the tumor mass, which helps the surgeon in determining resectability [20].
Gallbladder carcinoma with direct invasion into the adjacent liver. A 62-year-old male with gallbladder cancer. Axial arterial phase postcontrast T1-weighted image (a), portal venous phase postcontrast T1-weighted image (b), high b-value diffusion weighted image (c), and T2-weighted image (d) all show thickened gallbladder wall infiltrating into the adjacent liver (white arrow)
For a detailed metastatic survey for the presence of distant metastases, cross-sectional imaging of the chest, abdomen, and pelvis should be obtained using multidetector contrast-enhanced CT [36]. Endoscopic ultrasound is useful for the evaluation and biopsy of regional lymph nodes and should be considered when local expertise is available. Unlike for other cancers, positron emission tomography (PET) scan is not routinely used in GBC management, but it may be selectively utilized when questionable or concerning features for regional or distant metastases are apparent on CT, MRI, or endoscopic retrograde cholangiopancreatography (ERCP).
Laboratory Evaluation
Laboratory studies are neither specific nor sensitive for the diagnosis of GBC. In stage I and II disease, the liver enzymes may not be elevated unless the biliary tract is obstructed. Elevated serum carcinoembryonic antigen (CEA) and serum carbohydrate antigen 19–9 (CA 19–9) in the setting of elevated alkaline phosphatase (ALP) should raise the suspicion of biliary tract or pancreatic malignancy, and GBC should be in the differential. However, it is important to note that serum CA 19–9 can also be raised in the setting of benign biliary obstruction or inflammation, and hence, the serum CA 19–9 should be interpreted cautiously.
Diagnostic Staging Laparoscopy
Metastases of GBC may not always be seen on diagnostic imaging studies. Moreover, the presence of lymph node (LN) disease is often difficult to determine preoperatively as abdominal CT and MRI have a detection rate of only about 24% [37]. Hence, staging laparoscopy is usually performed for better staging of the disease before proceeding to surgery. There is considerable debate about the utility of diagnostic laparoscopy. Based on the analysis of a retrospective study, staging laparoscopy is high yield if T3 disease is suspected on imaging studies or the patient had a poorly differentiated tumor or positive margins on the previously excised mass [38]. To increase sensitivity, laparoscopy can be combined with laparoscopic ultrasound to identify satellite lesions in the liver and other adjacent structures in order to determine the anatomical extension of the tumor and to evaluate for vascular invasion. Inter-aortocaval LN frozen-section evaluation during staging laparoscopy further increases the sensitivity of detection of LN metastases [39].
Staging of GBC
GBC is staged using the American Joint Committee on Cancer-Tumor Node Metastasis (AJCC TNM) staging system (Table 11.2). According to the eighth edition of the American Joint Committee on Cancer staging manual for gallbladder carcinoma (effective January 1, 2018), primary gallbladder carcinoma can be classified as T1, confined to the lamina propria (T1a) and the muscle layer (T1b) of the gallbladder; T2, extending to the serosa, further classified into T2a (peritoneal side extension) and T2b (hepatic side extension) [40]; T3, perforating the serosa or directly invading one adjacent structure such as liver, stomach, duodenum, colon, pancreas, omentum, or extrahepatic bile ducts; or T4, invading the main portal vein, the hepatic artery, or multiple extrahepatic organs. GBC disseminates via lymphatic, hematogenous, intraductal (cystic duct) and neural pathways, and intraperitoneal “drop” metastases [41]. Lymphatic spread is present in more than half of the patients at initial diagnosis and common sites of nodal metastases are nodes along the cystic duct, common bile duct, hilar, hepatic artery and/or portal vein, periaortic, portacaval, superior mesenteric and celiac arteries. The disease is classified as N1 if one to three positive nodes are involved, whereas N2 disease is four or more positive nodes. T1 or T2 primary lesions without lymph node metastasis are classified as stage IA or IB disease, respectively. Stage IIA represents T3 lesions without nodal spread. T1, T2, or T3 lesions with N1 lymph node involvement are defined as stage IIB. A T4 lesion without distant metastasis is considered stage III, and distant metastases represents stage IV. The most common sites of metastases are the liver, peritoneum, lung, and brain.
Management of GBC
GBC is an aggressive malignancy often diagnosed at late stages, and surgery is the only potentially curative option. Unfortunately, only one fourth of the patients will undergo curative surgery. Surgical options are dependent on the staging of the disease and may involve the resection of one or more adjacent organs. Given the dismal prognosis of metastatic GBC, attempts of curative surgery are limited to localized resectable disease.
Surgical Management of GBC
Achieving a tumor-free surgical margin (R0 resection) should be the primary goal of the surgery as it forms one of the important prognostic indicators.
Management of Resectable GBC When Identified as an Incidental Mass on Imaging Studies or Incidental Finding at Surgery
GBC patients should always be referred to a cancer center with available expertise in its management. As per National Comprehensive Cancer Care network (NCCN) guidelines version 1. 2017, any suspicious GBC mass need not undergo preoperative biopsy as it may lead to peritoneal spread. If the diagnosis of GBC is not conclusive, it is always prudent to have an intraoperative frozen section evaluation followed by a definitive resection in case the pathology confirms cancer. As discussed above in the section “Diagnostic Staging Laparoscopy”, staging laparoscopy is recommended and is often combined with the surgical procedure. This staging laparoscopy gives a detailed picture to the surgeon, and a stage-based surgical resection can be performed. In the event of incidental discovery of GBC during a laparoscopic surgical procedure, it should be referred promptly for definitive oncologic resection once the pathologic analysis is finalized.
For stage 0-I disease (T1aN0M0) (early GBC), simple cholecystectomy should suffice as the risk of lymph node dissemination and residual cancer is low. Retrospective studies demonstrate a >95% five-year survival in T1aNx disease treated by cholecystectomy alone. No benefit has been demonstrated for re-resection or more aggressive resection in T1a disease. However, utmost care must be taken by the surgeon to avoid any biliary spillage as it may be contaminated with malignant cells and may increase the chances of intraoperative spread of cancer cells [42]. As the primary goal of the surgery is to achieve R0 resection, in T1a disease, if simple cholecystectomy did not achieve R0 resection, the surgical resection should be extended to involve lymphadenectomy, hepatic resection (segments IVB and V), and hepatic/biliary duct resection (performed in case of positive margins in the cystic duct). In contrast, in T1b disease, lymphadenectomy and hepatic segmental resection (IVB and V) along with cholecystectomy have shown reduced recurrence rates compared to simple cholecystectomy alone (2% vs. 12.5%) [43].
Due to the involvement of deeper layers of the gallbladder and the high probability of lymphatic, perineural, and vascular metastases in stage II and III (T1b, T2, and T3; N0–1) disease, surgical resection involving extended cholecystectomy, extended lymphadenectomy (including celiac/superior mesenteric artery lymph nodes), and hepatectomy (segments IVA, V) is indicated in patients deemed to be appropriate surgical candidates [2]. It is important to note that the extent of surgery is determined by the resection required to achieve a R0 margin. Early series advocated increasingly aggressive “standard” resections for GBC with improvements in long-term outcome. However, it is likely that these aggressive resections improved outcomes by improving the R0 resection rates. Routine major hepatectomy involving the caudate lobe of the liver and extensive hepatic resection beyond the IVA and V segments are associated with higher postoperative morbidity and not associated with survival benefit [44, 45]. Hence, the surgeon should aim to achieve the R0 resection with as limited a resection as possible [43]. Having said that, major hepatectomies are indicated in case of nonachievability of R0 resection with limited resection or if the tumor invades the main vasculature of the liver [2]. Similarly, routine resection of the common bile duct does not improve outcomes but is indicated in patients with preoperative jaundice, a positive cystic duct margin, or evidence of bile duct invasion on preoperative imaging [2, 46].
Though surgery is the only potentially curative therapy for GBC, outcomes may be poor even after complete resection, particularly in stage III (T3 and/or node-positive) disease. Adjuvant chemotherapy and radiotherapy are commonly administered in margin positive resections and node positive disease. Postsurgical adjuvant therapy is discussed in detail in the section “Role of Adjuvant Therapy in the Management of GBC”.
Management of Resectable GBC When Identified on Postsurgical Pathology Review
In case of T1a disease, if the prior surgery achieved negative margins, close monitoring and periodic surveillance are recommended. In case of disease recurrence, if the patient is a surgical candidate (based on the medical comorbidities and stage of the disease), extended surgical resection to achieve R0 resection can be considered. Based on previous studies, patients who had incidental diagnosis of T1b or greater GBC based on the postsurgical pathology review required a second procedure. Despite the increased surgical risk of a second procedure, reoperation with successful R0 resections has shown significant improvements in overall survival (OS) [47,48,49,50]. Laparoscopic port site disease is often seen in patients with T2 and T3 disease and correlates with peritoneal spread. Given the correlation with peritoneal spread and the lack of benefit in OS, port site resection is not recommended during reoperation [43]. A recent multi-institutional retrospective analysis that evaluated the optimal time to reoperation concluded that surgeries performed between 4 and 8 weeks from the initial surgery are associated with better outcomes. One possible explanation for the better results for surgeries performed between 4 and 8 weeks is that the 4 weeks time frame allows the initial surgical inflammation to resolve, leading to a better appreciation of cancer spread. Moreover, waiting >8 weeks may allow disease dissemination yielding poor results.
Criteria for Unresectability of GBC
Contraindications to curative surgery include the presence of stage IV disease with liver metastasis, distant/extrahepatic metastases, peritoneal disease, malignant ascites, evidence of extensive hepatoduodenal ligament involvement, distant nodal disease (para-aortic lymph nodes), and major vessel involvement that is not amenable to vascular resection and reconstruction. It is important to note that T3 disease with direct involvement of the duodenum, colon, or liver may be amenable to surgery if an R0 en bloc resection can be achieved without significant morbidity. Although curative surgery is contraindicated in extensive stage IV disease, palliative cholecystectomy may be performed in select cases with recurrent episodes of cholecystitis, especially when other options like endoscopic stenting have failed.
Role of Surgery in Unresectable or Stage IV GBC
For patients with regional nodal involvement, radical resection yields a 5-year overall survival (OS) of 10–28% [51, 52]. In contrast, surgery may not be beneficial in prolonging survival for patients with distant metastatic nodal disease (involvement beyond the hepatoduodenal ligament, pancreaticoduodenal area, and along the common hepatic artery area) [51]. Simple cholecystectomy may be performed in GBC with extensive nodal involvement, unresectable disease, or stage IV disease for palliative purposes when other maneuvers like endoscopic stent placement or biliary bypass have failed or if the patient suffers from recurrent episodes of cholecystitis [2].
Only 20–30% of patients with GBC can undergo surgical resection as the disease is most often diagnosed at late stages when surgery may not prolong survival. For patients who are not good surgical candidates, palliative therapy with external beam radiotherapy (EBRT), systemic chemotherapy, and/or enrollment into clinical trials are available options.
Role of Adjuvant Therapy in the Management of GBC
Despite achieving R0 resection of the primary tumor, outcomes in GBC are generally poor, especially when the disease has spread to adjacent organs at the time of presentation. The risk of recurrence and metastatic spread of GBC is directly related to the T stage at the time of diagnosis. Though radical excision of the cancerous lesion followed by adjuvant therapy is the mainstay of treatment, the data supporting the adjuvant approach are conflicting.
Role of Adjuvant Radiotherapy and Chemoradiotherapy
The current literature on the role of adjuvant radiotherapy or chemoradiotherapy in the management of GBC is primarily derived from retrospective institutional and national database analyses. The lack of data from randomized trials makes it hard to determine whether there is an overall survival benefit. Nonetheless, many, but not all, retrospective analyses have shown favorable outcomes in prolonging survival, with the greatest benefits in the high stage tumors, node positive disease, and R1 resections [53, 54].
Analysis of the SEER database (1992-2002) showed that patients who received postsurgical radiotherapy had a significantly longer median survival than their counterparts who did not receive radiotherapy (14 months versus 8 months; p < 0.0001). Patients who had lymph node metastases (16 months vs. 5 months; p < 0.0001) and higher stage (T3 stage) (14 months vs. 11 months; p = 0.01) benefitted the most. In comparison, patients with stage 1 disease did not appear to benefit from radiotherapy. A recent multi-institutional retrospective analysis found survival benefits in GBC patients who received postoperative adjuvant chemotherapy (hazard ratio [HR] 0.38, 95% CI: 0.23–0.65) and chemoradiotherapy (HR 0.26, 95% CI: 0.15–0.43) [55]. The survival benefit of adjuvant therapy was specifically seen in the patients with T3/T4 disease, lymph node metastases at presentation, and who had R1 resection. Benefit for radiotherapy alone, chemotherapy alone, and chemoradiotherapy is further supported by a National Cancer Database (NCDB) analysis that showed improved 3-year overall survival (chemotherapy, HR: 0.77 [95% CI: 0.61–0.97]; radiotherapy, HR: 0.63 [95% CI 0.44–0.92]; and chemoradiotherapy, HR: 0.47 [95% CI: 0.39–0.58]). However, neither of the therapies showed a survival advantage in T1N0 stage disease [56]. Selected radiotherapy and chemoradiotherapy studies in GBC are summarized in Table 11.3. The results of these retrospective analyses are to be interpreted with caution as there might be a component of selection bias as fitter and relatively younger patients received adjuvant therapy. In contrast, an NCDB analysis showed that patients who had larger primary tumors, advanced stage, and lymph node metastases had a higher likelihood of receiving adjuvant therapy.
A recent multicenter phase II trial (Southwest Oncology Group S0809) evaluated the safety and efficacy of postoperative combined modality therapy in patients with resected EHCC or GBC [57]. Therapy consisted of four cycles of gemcitabine and capecitabine, followed by conformal radiotherapy (45 Gy to regional lymphatics and 54–59.4 Gy to the tumor bed) and concurrent capecitabine. Twenty-five patients enrolled had GBC, with most having stage III–IV disease and/or positive surgical margins. Two-year overall survival, disease-free survival, and local recurrence rates were 56%, 48%, and 8%, respectively. These data provide evidence in support of this regimen in patients with resected GBC at high risk for recurrence.
Improving Safety of Radiotherapy:
Despite the potential benefits of radiotherapy in the management of GBC, the dose of radiotherapy is limited by the proximity of the disease to vital structures including the bowel, liver, and kidneys. The risk of acute and late treatment-related adverse effects may be minimized by the use of advanced radiotherapy techniques such as three-dimensional conformal radiotherapy (3D-CRT), intensity-modulated radiotherapy (IMRT), and proton beam radiotherapy. Specifically, IMRT may reduce radiation exposure to the liver and right kidney [58]. A preliminary study evaluated the feasibility of IMRT with image guidance for target localization in ten patients with GBC [59]. The median prescription dose was 59 Gy. Treatment was well tolerated with only one patient experiencing grade 3 toxicity. Recently, a retrospective study evaluated the use of neoadjuvant chemoradiotherapy using IMRT for 28 patients with locally advanced GBC [60]. Patients received a median dose of 57 Gy in 25 fractions to the primary tumor and involved lymph nodes and 45 Gy in 25 fractions to the at-risk regional lymph nodes. Three patients (11%) experienced grade 3 acute treatment-related adverse events during chemoradiotherapy. Two patients experienced grade 3 late treatment-related adverse events after RT. An example of an IMRT treatment plan for gallbladder cancer is shown in Fig. 11.6.
Intensity-modulated radiotherapy (IMRT) plan for a patient with locally advanced, unresectable gallbladder adenocarcinoma. Treatment was delivered in 25 fractions over 5 weeks with concurrent capecitabine. The gallbladder primary tumor and involved portocaval lymph node (red volume) received a dose of 56.25 Gy, and the regional lymph nodes (cyan volume) received 45 Gy. Volumes receiving 56.25 Gy (white), 45 Gy (blue), 30 Gy (magenta), and 20 Gy (yellow) are shown
Role of Adjuvant Chemotherapy in the Management of GBC
Chemotherapy, given either alone or in combination with radiotherapy is used as adjuvant therapy following surgical resection of the primary tumor. Chemotherapy is also used in GBC patients with locally advanced unresectable disease or in patients with metastatic disease. As discussed in the section “Role of Adjuvant Radiotherapy and Chemoradiotherapy”, given the rarity of GBC, most of the data are obtained from retrospective analyses, and most studies grouped all patients with biliary tract cancer, including cholangiocarcinoma and GBC. Though the two cancer types are often analyzed in combination, subgroup analyses showed discordant responses to the therapy [61, 62]. Moreover, compared to advanced cholangiocarcinoma (median OS: 24–44 weeks), GBC is a more aggressive disease (median OS: 12 weeks) [63]. Genomic analyses of resection specimens of the two types of cancers show varying rates of driver genomic mutations, including KRAS, isocitrate dehydrogenase (IDH1/2), and fibroblast growth factor receptor 2 (FGFR2) and differences in gene expression [64]. Despite the differences in the aggressiveness and molecular pathogenesis of GBC and biliary tract cancers, randomized controlled trials usually combine the two cancers in the survival analyses, and GBC comprise a relatively small number of the patients analyzed (36% in the Advanced Biliary Cancer [ABC]-02 trial and 18% in the BILCAP trial) [65, 66].
Previous retrospective studies suggested some degree of benefit from adjuvant chemotherapy [67,68,69]. Few prospective randomized trials have been conducted, and the small number of patients with GBC enrolled in these trials compromises interpretation of the results (Table 11.4).
Encouraging results of improved median survival (53 vs. 36 months, HR 0.75, 95% CI: 0.58–0.97) were seen in the phase III BILCAP trial that evaluated capecitabine as adjuvant therapy (1250 mg/m2 D1–14 every 21 days, for 8 cycles/6 months) in biliary tract cancers including GBC (n = 79, 18% of total biliary tract patients enrolled) [65]. Patients with T1a GBC were excluded from this clinical trial. In the subgroup analysis of GBC patients, survival was numerically better in the capecitabine arm (HR of 0.84, [95% CI: 0.43–1.63]; p = 0.097). In the intention to treat analysis, median relapse-free survival (RFS) of the capecitabine group was 25 months (95% CI: 19–37 months), whereas the observational group had an RFS of 18 months (95% CI: 13–28 months). Common grade 3–4 adverse events included plantar palmar erythema (21%), fatigue (8%), diarrhea (8%), neutropenia (2%), nausea (1%), stomatitis (1%), and hyperbilirubinemia (1%). Interestingly, high rates of R1 resections (38%) were reported in this trial. The benefit of adjuvant chemotherapy was more pronounced in patients with R0 resection compared to those with R1 resection (HR: 0.73 vs. 0.90).
It is important to note that these randomized trials had only limited numbers of cases of GBC. Nonetheless, given the modest survival advantage, tolerable toxicity profile, and extrapolation from clinical trials in the metastatic setting, platinum-based compounds were often combined with gemcitabine [66]. However, the BILCAP study has established capecitabine as a new standard of care option for resected GBC.
Role of Neoadjuvant Therapy in the Management of GBC
Neoadjuvant therapy is currently being employed in locally advanced gastrointestinal malignancies with the primary aim of downstaging the disease and achieving R0 resection. There are limited data in the treatment of GBC in the neoadjuvant setting. An Indian prospective case series that evaluated the role of neoadjuvant chemotherapy with gemcitabine and cisplatin in locally advanced disease concluded that 83.3% (15/18) of patients had a good radiological response and 56.3% (9/16 patients) achieved R0 resection [70]. The study concluded that neoadjuvant chemotherapy may downsize the tumor in approximately half of patients, allowing R0 resection. Similarly, encouraging results were seen in a retrospective analysis that evaluated gemcitabine and cisplatin neoadjuvant chemotherapy in locally advanced GBC. A total of 17/37 patients (46%) could undergo R0 resection, and the patients who underwent surgery had a significantly better progression-free survival and OS [71]. A retrospective case series from India demonstrated the feasibility of administrating neoadjuvant concurrent chemoradiotherapy for locally advanced (stage III) gallbladder cancer [60, 72]. Of the 28 patients treated, 71% experienced partial or complete radiologic response, and 56% underwent R0 resection. Overall survival at 5 years was 24% for all patients and 47% for patients with an R0 resection. A single-arm prospective phase II study in Chile evaluated the feasibility of neoadjuvant chemoradiotherapy for potentially resectable gallbladder cancer [73]. Eighteen patients with stage II–IV disease received preoperative RT (45 Gy in 25 fractions) with concurrent 5-FU. Thirteen patients (72%) underwent potentially curative resection, and of these seven (54%) were alive and free of disease recurrence at a median follow-up of 2 years. To better characterize the benefit of neoadjuvant therapy in GBC, large prospective studies are needed to assess the rates of downsizing and resectability.
Management of Advanced GBC
Palliation of Obstructive Jaundice
Due to infiltration of the common hepatic duct, jaundice is a presenting complaint in 30–60% of patients with advanced GBC. In GBC patients presenting with jaundice, baseline CA 19–9 should be drawn after biliary drainage as it might be falsely elevated in patients with biliary obstruction. The CA-19-9 level can be followed every 3–4 months to determine the progression of the disease. Biliary drainage can be achieved by either percutaneous or endoscopic stenting. A randomized controlled trial evaluated percutaneous transhepatic biliary drainage vs. endoscopic stenting methods of biliary drainage in 54 patients with advanced GBC [74]. Though survival was same in both arms, the percutaneous procedure was associated with better success rates (89% vs. 41%) and lower rates of cholangitis (48 vs. 11%). Despite the lower rates of cholangitis and high success rates with percutaneous procedure, in practical settings, the percutaneous procedure is associated with higher patient discomfort due to open external drainage and comparatively higher rates of bile leak and bleeding [75].
Role of Radiotherapy and/or Chemoradiotherapy in Advanced GBC
Limited data exist on the role of radiotherapy in advanced unresectable GBC, and the available data are mainly obtained from the small number of GBC cases included in studies that evaluated all biliary tact cancers [68, 76]. Given this limited data, patients with unresectable GBC should be encouraged to participate in clinical trials.
Despite uncertainty in survival benefit, chemoradiotherapy is an acceptable choice for locoregional therapy of a locally advanced unresectable GBC in selected cases. Chemotherapy, especially fluoropyrimidine based, is frequently administered in addition to radiotherapy [68]. Systemic chemotherapy should be used as a first choice in advanced disease; and radiotherapy may be considered in patients with localized unresectable disease without metastases after initiation of first-line chemotherapy [77].
Role of Systemic Chemotherapy in Advanced GBC
Gemcitabine and fluorouracil-based therapies are commonly evaluated systemic therapies for treatment of GBC in the palliative setting. The oral fluoropyrimidine derivative, capecitabine, alone or in combination with cisplatin or oxaliplatin showed encouraging results of marginal survival benefit in unresectable GBC [78,79,80]. Similar encouraging results were reported in another study that evaluated capecitabine in combination with oxaliplatin [79]. The therapy was well tolerated, and the study concluded that of 27 unresectable GBC patients, one had complete response, 7 had partial response, and 9 had a stable disease, with a total disease control rate of 63% and median survival of 11.3 months.
Gemcitabine, either alone or in combination, has been extensively studied in patients with GBC (Table 11.5). Three phase II trials that evaluated gemcitabine alone in unresectable GBC concluded that the drug was well tolerated with response rates ranging from 0 to 30% [81,82,83]. Gemcitabine was also evaluated in combination with capecitabine in three phase II trials of metastatic, unresectable GBC [84,85,86]. Results of the three trials showed a response rate of about 30% with a median OS of 13–14 months. This combination regimen was well tolerated, with neutropenia and thrombocytopenia as the most significant toxicities.
In addition, gemcitabine was also evaluated in combination with cisplatin or oxaliplatin in phase II and III trials, and the results (response rates and median OS) were encouraging. In gemcitabine plus cisplatin combination phase II trials, response rates of 21–34.5% and median OS of 9.3–11 months were noted [87,88,89,90]. The combination was also evaluated in a phase III randomized trial (ABC-02 trial), which also demonstrated similar encouraging results making it the current standard of care therapy in unresectable GBC [91]. In this clinical trial of 410 patients with biliary tract cancers, 149 subjects had GBC. Compared to gemcitabine monotherapy, the combination therapy resulted in a better progression-free survival (8.4 vs. 6.5 months; HR: 0.72; 95% CI: 0.57–0.90; p = 0.003) and OS (11.7 vs. 8.3 months; HR:0.70; 95% CI: 0.54–0.89; p = 0.002). The combination therapy was tolerated well without significant added toxicity. In addition, the rate of tumor control was significantly increased among patients in the combination therapy group (81.4% vs. 71.8%, P = 0.049). In the GBC subgroup, 37.7% of the patients receiving combination therapy had a partial response compared to 21.4% with single agent gemcitabine. Adverse events were similar in both the cohorts. Though neutropenia was more frequent in the cisplatin–gemcitabine group, the number of neutropenia-associated infections was similar in the two groups.
The gemcitabine–oxaliplatin combination was evaluated in phase II trials [92,93,94]. The regimen was well tolerated even among patients with higher ECOG scores reflecting poor functional status. A phase III trial of 260 patients compared the combination of gemcitabine–cisplatin with gemcitabine–oxaliplatin in unresectable and metastatic GBC patients [95]. The objective response rates (23% vs. 24%) and median OS (8 vs. 9 months) were similar in both treatment cohorts; however, the combination of gemcitabine and oxaliplatin had more grade 1 or 2 peripheral neuropathy and grade 3 or 4 diarrhea.
Gemcitabine has also been evaluated in combination with fluorouracil-leucovorin and carboplatin. Patients who received the combination with fluorouracil-leucovorin had modest improvements in progression-free survival but it was not superior to gemcitabine alone [96, 97]. In a phase II trial that evaluated the combination of gemcitabine and carboplatin in a group of 20 patients with advanced GBC, 4 patients (21%) achieved a complete response and 3 (15.7%) had a partial response with an overall response rate of 36.7% [98]. Grade III and IV side effects of anemia, neutropenia, and thrombocytopenia were observed in a few patients.
Based on the above data, the combination of gemcitabine and cisplatin is the standard of care for first-line treatment of advanced GBC. The combination of gemcitabine and oxaliplatin is also a reasonable choice. Other alternative regimens include single-agent gemcitabine, gemcitabine and carboplatin, or 5-FU-based regimens. Figure 11.7 shows a flow diagram summarizing the overall management of gallbladder carcinoma.
Role of Molecular-Targeted Therapy
The molecular pathology of GBC is discussed in detail in section “Pathology of GBC”. The characteristic molecular features include mutation of KRAS, TP53, and p16/CDKN2/INK4A, as well as human epidermal growth factor receptor (HER)-2/Neu amplification. Angiogenic-inflammatory pathway gene mutations in cyclooxygenase-2 [COX-2], nitric oxide synthase [iNOS], and vascular endothelial growth factor [VEGF] are also implicated in the pathogenesis of GBC.
Tyrosine kinase inhibitors, erlotinib and lapatinib, that act by blocking the EGFR pathway (lapatinib also blocks the HER2/EGFR2 pathway) were evaluated in advanced biliary tract cancers (erlotinib) and GBC (lapatinib). These tyrosine kinase inhibitors resulted in modest benefit (~17% progression-free survival) at their best, and all responding patients had mild skin toxicity (grades 1 and 2). A multicenter, randomized phase II trial evaluated the combination of cetuximab (an EGFR inhibitor) with gemcitabine–oxaliplatin in unresectable biliary tract cancers (24% patients had GBC) [99]. The addition of cetuximab resulted in improved 4-month progression-free survival compared to the gemcitabine–oxaliplatin group (61% vs. 44%). Toxicity was comparable in both groups with slightly higher rash/hypersensitivity reactions in the cetuximab group.
Sorafenib, a tyrosine kinase inhibitor that targets VEGFR-2, 3 and platelet-derived growth factor and less potently BRAF kinases was evaluated in a phase II clinical trial involving unresectable or metastatic GBC or cholangiocarcinoma (n = 31) [100]. Twenty-nine % of the patients had stable disease and median progression-free survival was 2 months. Grade 3 or 4 toxicities were significant, affecting about two-thirds of the patients. One patient died of supraventricular tachycardia and thromboembolism.
Bevacizumab, a humanized monoclonal antibody against VEGF, was evaluated in a phase II trial in combination with gemcitabine and oxaliplatin in unresectable biliary tract cancers (28% [n = 10] of enrolled patients had GBC) [101]. In the subset of GBC patients, the median progression-free survival and OS were 6.1 and 8.5 months, respectively. Most common side effects noted were grade 3 or 4 hypertension (n = 5), proteinuria (n = 1), thrombosis (n = 2), and cardiac ischemia (n = 1). Bevacizumab was also evaluated in combination with erlotinib in a phase II trial in biliary tract cancers (n = 53; 10 patients with GBC and 43 with non-GBC) [102]. This biologic combination regimen resulted in stable disease in 51% of patients; the median OS of the entire group was 9.9 months. It is important to note that the results of the study were not stratified based on the tumor location and so cannot be extrapolated to GBC. Another study evaluated the addition of bevacizumab to gemcitabine and oxaliplatin (GEMOX) [103]. The combination was associated with a better progression-free survival compared to GEMOX therapy (6.5 vs. 3.7 months, p = 0.049). No significant difference in adverse events was noted between the groups.
Although these biologic agents are promising, more definitive randomized studies are required to define the role of antiangiogenic agents in advanced GBC, particularly among those harboring KRAS and BRAF mutations.
Role of Immunotherapy
Given the background of chronic inflammation in the pathogenesis of GBC, immunotherapy may be a potentially attractive targeted therapy [104]. A couple of tumor-related antigens have also been identified in gallbladder carcinoma – Wilms tumor 1 (WT1) and mucin-1 (MUC-1) in 68–80% and 90% of gallbladder cancers, respectively [105]. Trials of both a dendritic-based cell vaccine against WT-1 and MUC-1 antigens, as well as a randomized trial of chemotherapy and a WT1 vaccine in patients with advanced GBC have been described [106]. In addition, in the interim analysis of a phase II trial (KEYNOTE-028, NCT02054806) that evaluated the role of pembrolizumab in advanced biliary tract cancer (including GBC), 34% (n = 8) of patients with positive PD-L1 expression had a partial response or stable disease [107]. Half of the patients (52%, n = 12) had progressive disease.
The role of pembrolizumab alone (NCT03260712) in advanced GBC is being evaluated in a phase II clinical trial. This trial will hopefully provide us with more details about the role of immune-targeted therapy in advanced, inoperable GBC. A phase II clinical trial of nivolumab (NCT02829918) in patients with advanced biliary tract cancers including GBC is also currently underway. These and other clinical trials will help in defining the role of checkpoint inhibitors in patients with GBC.
Conclusion
GBC continues to represent a major challenge in gastrointestinal oncology and often has a dismal prognosis. Surgical resection remains the mainstay of treatment if diagnosed at an early stage. The roles of chemotherapy and chemoradiotherapy in the neoadjuvant and adjuvant settings remain to be defined in randomized, prospective clinical trials. Recent clinical trials have suggested a survival benefit for capecitabine as adjuvant therapy. Molecularly targeted agents that inhibit angiogenesis and EGFR and BRAF pathways are currently being evaluated in clinical trials, and patients with unresectable GBC should be highly encouraged to enroll in these trials. Despite being a distinct entity compared to other biliary tract cancers, GBCs have been frequently combined with other biliary tract cancers in therapeutic studies. This has prevented a true understanding of the most effective treatment options. Given the relative rarity of GBC, collaboration across academic and community centers with experience in the management of these cancers will be necessary to achieve continued progress in the field and to obtain better outcomes for our patients.
References
Randi G, Franceschi S, La Vecchia C. Gallbladder cancer worldwide: geographical distribution and risk factors. Int J Cancer. 2006;118(7):1591–602. https://doi.org/10.1002/ijc.21683.
Al-alem F, Mattar RE, Madkhali A, Alsharabi A, Alsaif F, Hassanain M. Incidental gallbladder cancer. In: Abdeldayem HM, editor. Updates in gallbladder diseases. Ch. 06. Rijeka: InTech; 2017.
Hundal R, Shaffer EA. Gallbladder cancer: epidemiology and outcome. Clin Epidemiol. 2014;6:99–109. https://doi.org/10.2147/CLEP.S37357.
Diehl AK. Gallstone size and the risk of gallbladder cancer. JAMA. 1983;250(17):2323–6.
Misra S, Chaturvedi A, Misra NC, Sharma ID. Carcinoma of the gallbladder. Lancet Oncol. 2003;4(3):167–76.
Nagorney DM, McPherson GA. Carcinoma of the gallbladder and extrahepatic bile ducts. Semin Oncol. 1988;15(2):106–15.
Shukla VK, Tiwari SC, Roy SK. Biliary bile acids in cholelithiasis and carcinoma of the gall bladder. Eur J Cancer Prev. 1993;2(2):155–60.
Niu X-J, Wang Z-R, Wu S-L, Geng Z-M, Zhang Y-F, Qing X-L. Relationship between inducible nitric oxide synthase expression and angiogenesis in primary gallbladder carcinoma tissue. World J Gastroenterol. 2004;10(5):725–8. https://doi.org/10.3748/wjg.v10.i5.725.
Shukla VK, Tiwari SC, Roy SK. Biliary bile acids in cholelithiasis and carcinoma of the gall bladder. European J Cancer Prevention: Official Journal of the European Cancer Prevention Organisation (ECP). 1993;2(2):155–60.
Kumar S, Kumar S, Kumar S. Infection as a risk factor for gallbladder cancer. J Surg Oncol. 2006;93(8):633–9. https://doi.org/10.1002/jso.20530.
Nervi F, Duarte I, Gomez G, Rodriguez G, Del Pino G, Ferrerio O, et al. Frequency of gallbladder cancer in Chile, a high-risk area. Int J Cancer. 1988;41(5):657–60.
Shukla VK, Khandelwal C, Roy SK, Vaidya MP. Primary carcinoma of the gall bladder: a review of a 16-year period at the university hospital. J Surg Oncol. 1985;28(1):32–5.
Caygill CP, Hill MJ, Braddick M, Sharp JC. Cancer mortality in chronic typhoid and paratyphoid carriers. Lancet (London, England). 1994;343(8889):83–4.
Strom BL, Soloway RD, Rios-Dalenz JL, Rodriguez-Martinez HA, West SL, Kinman JL, et al. Risk factors for gallbladder cancer. An international collaborative case-control study. Cancer. 1995;76(10):1747–56.
Lewis JT, Talwalkar JA, Rosen CB, Smyrk TC, Abraham SC. Prevalence and risk factors for gallbladder neoplasia in patients with primary sclerosing cholangitis: evidence for a metaplasia-dysplasia-carcinoma sequence. Am J Surg Pathol. 2007;31(6):907–13. https://doi.org/10.1097/01.pas.0000213435.99492.8a.
Hundal R, Shaffer EA. Gallbladder cancer: epidemiology and outcome. Clin Epidemiol. 2014;6:99–109. https://doi.org/10.2147/clep.s37357.
Razumilava N, Gores GJ, Lindor KD. Cancer surveillance in patients with primary sclerosing cholangitis. Hepatology (Baltimore, Md). 2011;54(5):1842–52. https://doi.org/10.1002/hep.24570.
Stephen AE, Berger DL. Carcinoma in the porcelain gallbladder: a relationship revisited. Surgery. 2001;129(6):699–703. https://doi.org/10.1067/msy.2001.113888.
Khan ZS, Livingston EH, Huerta S. Reassessing the need for prophylactic surgery in patients with porcelain gallbladder: case series and systematic review of the literature. Arch Surg (Chicago, Ill: 1960). 2011;146(10):1143–7. https://doi.org/10.1001/archsurg.2011.257.
Wiles R, Thoeni RF, Barbu ST, Vashist YK, Rafaelsen SR, Dewhurst C, et al. Management and follow-up of gallbladder polyps: Joint guidelines between the European Society of Gastrointestinal and Abdominal Radiology (ESGAR), European Association for Endoscopic Surgery and other Interventional Techniques (EAES), International Society of Digestive Surgery - European Federation (EFISDS) and European Society of Gastrointestinal Endoscopy (ESGE). Eur Radiol. 2017;27(9):3856–66. https://doi.org/10.1007/s00330-017-4742-y.
Sebastian S, Araujo C, Neitlich JD, Berland LL. Managing incidental findings on abdominal and pelvic CT and MRI, part 4: white paper of the ACR incidental findings committee II on gallbladder and biliary findings. J Am Coll Radiol: JACR. 2013;10(12):953–6. https://doi.org/10.1016/j.jacr.2013.05.022.
Mori Y, Sato N, Matayoshi N, Tamura T, Minagawa N, Shibao K, et al. Rare combination of familial adenomatous polyposis and gallbladder polyps. World J Gastroenterol: WJG. 2014;20(46):17661–5. https://doi.org/10.3748/wjg.v20.i46.17661.
Shaffer EA. Gallbladder Cancer: the basics. Gastroenterol Hepatol. 2008;4(10):737–41.
Buscemi S, Orlando E, Damiano G, Portelli F, Palumbo VD, Valentino A, et al. "Pure" large cell neuroendocrine carcinoma of the gallbladder. Report of a case and review of the literature. Inter J Surg (London, England). 2016;28(Suppl 1):S128–32. https://doi.org/10.1016/j.ijsu.2015.12.045.
Dursun N, Escalona OT, Roa JC, Basturk O, Bagci P, Cakir A, et al. Mucinous carcinomas of the gallbladder: Clinicopathologic analysis of 15 cases identified in 606 carcinomas. Arch Pathol Lab Med. 2012;136(11):1347–58. https://doi.org/10.5858/arpa.2011-0447-OA.
Bal MM, Ramadwar M, Deodhar K, Shrikhande S. Pathology of gallbladder carcinoma: current understanding and new perspectives. Pathol Oncol Res. 2015;21(3):509–25. https://doi.org/10.1007/s12253-014-9886-3.
Saetta AA. K-ras, p53 mutations, and microsatellite instability (MSI) in gallbladder cancer. J Surg Oncol. 2006;93(8):644–9. https://doi.org/10.1002/jso.20532.
Chaube A, Tewari M, Garbyal RS, Singh U, Shukla HS. Preliminary study of p53 and c-erbB-2 expression in gallbladder cancer in Indian patients manuscript id: 8962091628764582. BMC Cancer. 2006;6:126. https://doi.org/10.1186/1471-2407-6-126.
Zhou YM, Li YM, Cao N, Feng Y, Zeng F. [significance of expression of epidermal growth factor (EGF) and its receptor (EGFR) in chronic cholecystitis and gallbladder carcinoma]. Ai zheng = Aizheng =. Chin J Cancer. 2003;22(3):262–5.
Dwivedi AND, Jain S, Dixit R. Gall bladder carcinoma: Aggressive malignancy with protean loco-regional and distant spread. World J Clin Cases: WJCC. 2015;3(3):231–44. https://doi.org/10.12998/wjcc.v3.i3.231.
Vega EA, Vinuela E, Yamashita S, Sanhueza M, Cavada G, Diaz C, et al. Extended lymphadenectomy is required for incidental gallbladder Cancer independent of cystic duct lymph node status. J Gastrointest Surg. 2018;22(1):43–51. https://doi.org/10.1007/s11605-017-3507-x.
Redaelli CA, Buchler MW, Schilling MK, Krahenbuhl L, Ruchti C, Blumgart LH, et al. High coincidence of Mirizzi syndrome and gallbladder carcinoma. Surgery. 1997;121(1):58–63.
Prasad TL, Kumar A, Sikora SS, Saxena R, Kapoor VK. Mirizzi syndrome and gallbladder cancer. J Hepato-Biliary-Pancreat Surg. 2006;13(4):323–6. https://doi.org/10.1007/s00534-005-1072-2.
Regimbeau JM, Fuks D, Bachellier P, Le Treut YP, Pruvot FR, Navarro F, et al. Prognostic value of jaundice in patients with gallbladder cancer by the AFC-GBC-2009 study group. European Journal Of Surgical Oncology: The Journal of the European Society of Surgical Oncology and the British Association of Surgical Oncology. 2011;37(6):505–12. https://doi.org/10.1016/j.ejso.2011.03.135.
Rooholamini SA, Tehrani NS, Razavi MK, Au AH, Hansen GC, Ostrzega N, et al. Imaging of gallbladder carcinoma. Radiographics: A Review Publication of the Radiological Society of North America, Inc. 1994;14(2):291–306. https://doi.org/10.1148/radiographics.14.2.8190955.
Kim JH, Kim TK, Eun HW, Kim BS, Lee MG, Kim PN, et al. Preoperative evaluation of gallbladder carcinoma: efficacy of combined use of MR imaging, MR cholangiography, and contrast-enhanced dual-phase three-dimensional MR angiography. J Magn Reson Imaging: JMRI. 2002;16(6):676–84. https://doi.org/10.1002/jmri.10212.
Kokudo N, Makuuchi M, Natori T, Sakamoto Y, Yamamoto J, Seki M, et al. Strategies for surgical treatment of gallbladder carcinoma based on information available before resection. Arch Surg (Chicago, Ill: 1960). 2003;138(7):741–50.; ; discussion 50. https://doi.org/10.1001/archsurg.138.7.741.
Butte JM, Gonen M, Allen PJ, D'Angelica MI, Kingham TP, Fong Y, et al. The role of laparoscopic staging in patients with incidental gallbladder cancer. HPB (Oxford). 2011;13(7):463–72. https://doi.org/10.1111/j.1477-2574.2011.00325.x.
Agarwal AK, Kalayarasan R, Javed A, Gupta N, Nag HH. The role of staging laparoscopy in primary gall bladder cancer--an analysis of 409 patients: a prospective study to evaluate the role of staging laparoscopy in the management of gallbladder cancer. Ann Surg. 2013;258(2):318–23. https://doi.org/10.1097/SLA.0b013e318271497e.
Chun YS, Pawlik TM, Vauthey J-N. 8th edition of the AJCC Cancer staging manual: pancreas and hepatobiliary cancers. Ann Surg Oncol. 2017; https://doi.org/10.1245/s10434-017-6025-x.
Reid KM. Ramos-De la Medina a, Donohue JH. Diagnosis and surgical management of gallbladder cancer: a review. J Gastrointest Surg. 2007;11(5):671–81. https://doi.org/10.1007/s11605-006-0075-x.
Lee JM, Kim BW, Kim WH, Wang HJ, Kim MW. Clinical implication of bile spillage in patients undergoing laparoscopic cholecystectomy for gallbladder cancer. Am Surg. 2011;77(6):697–701.
Aloia TA, Járufe N, Javle M, Maithel SK, Roa JC, Adsay V, et al. Gallbladder Cancer: expert consensus statement. HPB (Oxford). 2015;17(8):681–90. https://doi.org/10.1111/hpb.12444.
Shirai Y, Wakai T, Sakata J, Hatakeyama K. Regional lymphadenectomy for gallbladder cancer: rational extent, technical details, and patient outcomes. World J Gastroenterol. 2012;18(22):2775–83. https://doi.org/10.3748/wjg.v18.i22.2775.
D'Angelica M, Dalal KM, DeMatteo RP, Fong Y, Blumgart LH, Jarnagin WR. Analysis of the extent of resection for adenocarcinoma of the gallbladder. Ann Surg Oncol. 2009;16(4):806–16. https://doi.org/10.1245/s10434-008-0189-3.
Miyazaki M, Yoshitomi H, Miyakawa S, Uesaka K, Unno M, Endo I, et al. Clinical practice guidelines for the management of biliary tract cancers 2015: the 2nd English edition. J Hepatobiliary Pancreat Sci. 2015;22(4):249–73. https://doi.org/10.1002/jhbp.233.
Pawlik TM, Gleisner AL, Vigano L, Kooby DA, Bauer TW, Frilling A, et al. Incidence of finding residual disease for incidental gallbladder carcinoma: implications for re-resection. J Gastrointest Surg. 2007;11(11):1478–86.; ; discussion 86-7. https://doi.org/10.1007/s11605-007-0309-6.
Fong Y, Jarnagin W, Blumgart LH. Gallbladder cancer: comparison of patients presenting initially for definitive operation with those presenting after prior noncurative intervention. Ann Surg. 2000;232(4):557–69.
Ouchi K, Mikuni J, Kakugawa Y. Laparoscopic cholecystectomy for gallbladder carcinoma: results of a Japanese survey of 498 patients. J Hepato-Biliary-Pancreat Surg. 2002;9(2):256–60. https://doi.org/10.1007/s005340200028.
Goetze TO, Paolucci V. Benefits of reoperation of T2 and more advanced incidental gallbladder carcinoma: analysis of the German registry. Ann Surg. 2008;247(1):104–8. https://doi.org/10.1097/SLA.0b013e318154bf5d.
Chijiiwa K, Noshiro H, Nakano K, Okido M, Sugitani A, Yamaguchi K, et al. Role of surgery for gallbladder carcinoma with special reference to lymph node metastasis and stage using western and Japanese classification systems. World J Surg. 2000;24(10):1271–6; discussion 7.
Yamamoto M, Onoyama H, Ajiki T, Yamada I, Fujita T, Saitoh Y. Surgical results of operations for carcinoma of the gallbladder. Hepato-Gastroenterology. 1999;46(27):1552–6.
Itoh H, Nishijima K, Kurosaka Y, Takegawa S, Kiriyama M, Dohba S, et al. Magnitude of combination therapy of radical resection and external beam radiotherapy for patients with carcinomas of the extrahepatic bile duct and gallbladder. Dig Dis Sci. 2005;50(12):2231–42. https://doi.org/10.1007/s10620-005-3040-8.
Todoroki T, Kawamoto T, Otsuka M, Koike N, Yoshida S, Takada Y, et al. Benefits of combining radiotherapy with aggressive resection for stage IV gallbladder cancer. Hepato-Gastroenterology. 1999;46(27):1585–91.
Kim Y, Amini N, Wilson A, Margonis GA, Ethun CG, Poultsides G, et al. Impact of chemotherapy and external-beam radiation therapy on outcomes among patients with resected gallbladder Cancer: a multi-institutional analysis. Ann Surg Oncol. 2016;23(9):2998–3008. https://doi.org/10.1245/s10434-016-5262-8.
Mitin T, Enestvedt CK, Jemal A, Sineshaw HM. Limited Use of Adjuvant Therapy in Patients With Resected Gallbladder Cancer Despite a Strong Association With Survival. J Natl Cancer Inst. 2017;109(7) https://doi.org/10.1093/jnci/djw324.
Ben-Josef E, Guthrie KA, El-Khoueiry AB, Corless CL, Zalupski MM, Lowy AM, et al. SWOG S0809: a phase II intergroup trial of adjuvant Capecitabine and gemcitabine followed by radiotherapy and concurrent Capecitabine in extrahepatic Cholangiocarcinoma and gallbladder carcinoma. J Clin Oncol: Official Journal of the American Society of Clinical Oncology. 2015;33(24):2617–22. https://doi.org/10.1200/jco.2014.60.2219.
Sun XN, Wang Q, Gu BX, Zhu YH, Hu JB, Shi GZ, et al. Adjuvant radiotherapy for gallbladder cancer: a dosimetric comparison of conformal radiotherapy and intensity-modulated radiotherapy. World J Gastroenterol. 2011;17(3):397–402. https://doi.org/10.3748/wjg.v17.i3.397.
Fuller CD, Thomas CR Jr, Wong A, Cavanaugh SX, Salter BJ, Herman TS, et al. Image-guided intensity-modulated radiation therapy for gallbladder carcinoma. Radiotherapy and Oncology: Journal of the European Society for Therapeutic Radiology and Oncology. 2006;81(1):65–72. https://doi.org/10.1016/j.radonc.2006.08.013.
Engineer R, Goel M, Chopra S, Patil P, Purandare N, Rangarajan V, et al. Neoadjuvant Chemoradiation followed by surgery for locally advanced gallbladder cancers: a new paradigm. Ann Surg Oncol. 2016;23(9):3009–15. https://doi.org/10.1245/s10434-016-5197-0.
Eckel F, Schmid RM. Chemotherapy in advanced biliary tract carcinoma: a pooled analysis of clinical trials. Br J Cancer. 2007;96(6):896–902. https://doi.org/10.1038/sj.bjc.6603648.
Yonemoto N, Furuse J, Okusaka T, Yamao K, Funakoshi A, Ohkawa S, et al. A multi-center retrospective analysis of survival benefits of chemotherapy for Unresectable biliary tract Cancer. Jpn J Clin Oncol. 2007;37(11):843–51. https://doi.org/10.1093/jjco/hym116.
Gallardo J, Rubio B, Villanueva L, Barajas O. Gallbladder Cancer, a different disease that needs individual trials. J Clin Oncol. 2005;23(30):7753–4. https://doi.org/10.1200/JCO.2005.02.7524.
Kelley RK, Bardeesy N. Biliary tract cancers: finding better ways to lump and Split. J Clin Oncol. 2015;33(24):2588–90. https://doi.org/10.1200/JCO.2015.61.6953.
Primrose JN, Fox R, Palmer DH, Prasad R, Mirza D, Anthoney DA, et al. Adjuvant capecitabine for biliary tract cancer: The BILCAP randomized study. Journal of Clinical Oncology. 2017;35(15_suppl):4006. https://doi.org/10.1200/JCO.2017.35.15_suppl.4006.
Valle J, Wasan H, Palmer DH, Cunningham D, Anthoney A, Maraveyas A, et al. Cisplatin plus gemcitabine versus gemcitabine for biliary tract Cancer. N Engl J Med. 2010;362(14):1273–81. https://doi.org/10.1056/NEJMoa0908721.
Murakami Y, Uemura K, Sudo T, Hayashidani Y, Hashimoto Y, Nakamura H, et al. Adjuvant gemcitabine plus S-1 chemotherapy improves survival after aggressive surgical resection for advanced biliary carcinoma. Ann Surg. 2009;250(6):950–6.
Sirohi B, Singh A, Jagannath P, Shrikhande SV. Chemotherapy and targeted therapy for gall bladder Cancer. Indian J Surg Oncol. 2014;5(2):134–41. https://doi.org/10.1007/s13193-014-0317-4.
Oswalt CE, Cruz AB Jr. Effectiveness of chemotherapy in addition to surgery in treating carcinoma of the gallbladder. Rev Surg. 1977;34(6):436–8.
Raj D, Singh S, Gupta N, Rathi S. Neoadjuvant chemotherapy for gall bladder cancer: Does it increase resectability? J Clin Oncol. 2016;34(4_suppl):406. https://doi.org/10.1200/jco.2016.34.4_suppl.406.
Sirohi B, Mitra A, Jagannath P, Singh A, Ramadvar M, Kulkarni S, et al. Neoadjuvant chemotherapy in patients with locally advanced gallbladder cancer. Future Oncol (London, England). 2015;11(10):1501–9. https://doi.org/10.2217/fon.14.308.
Engineer R, Wadasadawala T, Mehta S, Mahantshetty U, Purandare N, Rangarajan V, et al. Chemoradiation for unresectable gall bladder cancer: time to review historic nihilism? J Gastrointest Cancer. 2011;42(4):222–7. https://doi.org/10.1007/s12029-010-9179-3.
de Aretxabala X, Roa I, Burgos L, Cartes R, Silva J, Yañez E, et al. Preoperative chemoradiotherapy in the treatment of gallbladder cancer. Am Surg. 1999;65(3):241–6.
Saluja SS, Gulati M, Garg PK, Pal H, Pal S, Sahni P, et al. Endoscopic or Percutaneous Biliary Drainage for Gallbladder Cancer: A Randomized Trial and Quality of Life Assessment. Clin Gastroenterol Hepatol. 2008;6(8):944–50.e3. https://doi.org/10.1016/j.cgh.2008.03.028.
Pinol V, Castells A, Bordas JM, Real MI, Llach J, Montana X, et al. Percutaneous self-expanding metal stents versus endoscopic polyethylene endoprostheses for treating malignant biliary obstruction: randomized clinical trial. Radiology. 2002;225(1):27–34. https://doi.org/10.1148/radiol.2243011517.
Ben-David MA, Griffith KA, Abu-Isa E, Lawrence TS, Knol J, Zalupski M, et al. External-beam radiotherapy for localized extrahepatic cholangiocarcinoma. Int J Radiat Oncol Biol Phys. 2006;66(3):772–9. https://doi.org/10.1016/j.ijrobp.2006.05.061.
Valle JW, Borbath I, Khan SA, Huguet F, Gruenberger T, Arnold D. Biliary cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Annals of Oncology: Official Journal of the European Society for Medical Oncology. 2016;27(suppl 5):v28–37. https://doi.org/10.1093/annonc/mdw324.
Patt YZ, Hassan MM, Aguayo A, Nooka AK, Lozano RD, Curley SA, et al. Oral capecitabine for the treatment of hepatocellular carcinoma, cholangiocarcinoma, and gallbladder carcinoma. Cancer. 2004;101(3):578–86. https://doi.org/10.1002/cncr.20368.
Nehls O, Oettle H, Hartmann JT, Hofheinz RD, Hass HG, Horger MS, et al. Capecitabine plus oxaliplatin as first-line treatment in patients with advanced biliary system adenocarcinoma: a prospective multicentre phase II trial. Br J Cancer. 2008;98(2):309–15. https://doi.org/10.1038/sj.bjc.6604178.
Kim TW, Chang HM, Kang HJ, Lee JR, Ryu MH, Ahn JH, et al. Phase II study of capecitabine plus cisplatin as first-line chemotherapy in advanced biliary cancer. Annals of Ooncology: Official Journal of the European Society for Medical Oncology. 2003;14(7):1115–20.
Kubicka S, Rudolph KL, Tietze MK, Lorenz M, Manns M. Phase II study of systemic gemcitabine chemotherapy for advanced unresectable hepatobiliary carcinomas. Hepato-Gastroenterology. 2001;48(39):783–9.
Mezger J, Sauerbruch T, Ko Y, Wolter H, Funk C, Glasmacher A. Phase II study with gemcitabine in gallbladder and biliary tract carcinomas. Oncol Res Treat. 1998;21(3):232–4.
Penz M, Kornek GV, Raderer M, Ulrich-Pur H, Fiebiger W, Lenauer A, et al. Phase II trial of two-weekly gemcitabine in patients with advanced biliary tract cancer. Ann Oncol. 2001;12(2):183–6.
Knox JJ, Hedley D, Oza A, Feld R, Siu LL, Chen E, et al. Combining gemcitabine and capecitabine in patients with advanced biliary cancer: a phase II trial. J Clin Oncol Off J Am Soc Clin Oncol. 2005;23(10):2332–8. https://doi.org/10.1200/jco.2005.51.008.
Cho JY, Paik YH, Chang YS, Lee SJ, Lee DK, Song SY, et al. Capecitabine combined with gemcitabine (CapGem) as first-line treatment in patients with advanced/metastatic biliary tract carcinoma. Cancer. 2005;104(12):2753–8. https://doi.org/10.1002/cncr.21591.
Riechelmann RP, Townsley CA, Chin SN, Pond GR, Knox JJ. Expanded phase II trial of gemcitabine and capecitabine for advanced biliary cancer. Cancer. 2007;110(6):1307–12. https://doi.org/10.1002/cncr.22902.
Meyerhardt JA, Zhu AX, Stuart K, Ryan DP, Blaszkowsky L, Lehman N, et al. Phase-II study of gemcitabine and cisplatin in patients with metastatic biliary and gallbladder cancer. Dig Dis Sci. 2008;53(2):564–70. https://doi.org/10.1007/s10620-007-9885-2.
Thongprasert S, Napapan S, Charoentum C, Moonprakan S. Phase II study of gemcitabine and cisplatin as first-line chemotherapy in inoperable biliary tract carcinoma. Ann Oncol. 2005;16(2):279–81. https://doi.org/10.1093/annonc/mdi046.
Kim ST, Park JO, Lee J, Lee KT, Lee JK, Choi SH, et al. A phase II study of gemcitabine and cisplatin in advanced biliary tract cancer. Cancer. 2006;106(6):1339–46. https://doi.org/10.1002/cncr.21741.
Zhu AX, Hong TS, Hezel AF, Kooby DA. Current management of gallbladder carcinoma. Oncologist. 2010;15(2):168–81. https://doi.org/10.1634/theoncologist.2009-0302.
Valle J, Wasan H, Palmer DH, Cunningham D, Anthoney A, Maraveyas A, et al. Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer. N Engl J Med. 2010;362(14):1273–81. https://doi.org/10.1056/NEJMoa0908721.
Andre T, Tournigand C, Rosmorduc O, Provent S, Maindrault-Goebel F, Avenin D, et al. Gemcitabine combined with oxaliplatin (GEMOX) in advanced biliary tract adenocarcinoma: a GERCOR study. Ann Oncol. 2004;15(9):1339–43. https://doi.org/10.1093/annonc/mdh351.
Harder J, Riecken B, Kummer O, Lohrmann C, Otto F, Usadel H, et al. Outpatient chemotherapy with gemcitabine and oxaliplatin in patients with biliary tract cancer. Br J Cancer. 2006;95(7):848–52. https://doi.org/10.1038/sj.bjc.6603334.
Gebbia N, Verderame F, Di Leo R, Santangelo D, Cicero G, Valerio MR, et al. A phase II study of oxaliplatin (O) and gemcitabine (G) first line chemotherapy in patients with advanced biliary tract cancers. J Clin Oncol. 2005;23(16_suppl):4132. https://doi.org/10.1200/jco.2005.23.16_suppl.4132.
Sharma A, Shukla NK, Chaudhary SP, Sahoo R, Mohanti BK, Deo SVS, et al. Final results of a phase III randomized controlled trial comparing modified gemcitabine + oxaliplatin (mGEMOX) to gemcitabine+ cisplatin in management of unresectable gall bladder cancer (GBC). J Clin Oncol. 2016;34(15_suppl):4077. https://doi.org/10.1200/JCO.2016.34.15_suppl.4077.
Alberts SR, Al-Khatib H, Mahoney MR, Burgart L, Cera PJ, Flynn PJ, et al. Gemcitabine, 5-fluorouracil, and leucovorin in advanced biliary tract and gallbladder carcinoma: a north central Cancer treatment group phase II trial. Cancer. 2005;103(1):111–8. https://doi.org/10.1002/cncr.20753.
Gebbia V, Giuliani F, Maiello E, Colucci G, Verderame F, Borsellino N, et al. Treatment of inoperable and/or metastatic biliary tree carcinomas with single-agent gemcitabine or in combination with levofolinic acid and infusional fluorouracil: results of a multicenter phase II study. J Clin Oncol: Official Journal of the American Society of Clinical Oncology. 2001;19(20):4089–91. https://doi.org/10.1200/jco.2001.19.20.4089.
Julka PK, Puri T, Rath GK. A phase II study of gemcitabine and carboplatin combination chemotherapy in gallbladder carcinoma. Hepatobiliary Pancreat Dis Int: HBPD INT. 2006;5(1):110–4.
Malka D, Trarbach T, Fartoux L, Mendiboure J, de la Fouchardière C, Viret F, et al. A multicenter, randomized phase II trial of gemcitabine and oxaliplatin (GEMOX) alone or in combination with biweekly cetuximab in the first-line treatment of advanced biliary cancer: Interim analysis of the BINGO trial. J Clin Oncol. 2009;27(15S):4520. https://doi.org/10.1200/jco.2009.27.15s.4520.
El-Khoueiry AB, Rankin C, Lenz HJ, Philip P, Rivkin SE, Blanke CD. SWOG 0514: A phase II study of sorafenib (BAY 43–9006) as single agent in patients (pts) with unresectable or metastatic gallbladder cancer or cholangiocarcinomas. J Clin Oncol. 2007;25(18_suppl):4639. https://doi.org/10.1200/jco.2007.25.18_suppl.4639.
Zhu AX, Meyerhardt JA, Blaszkowsky LS, Kambadakone AR, Muzikansky A, Zheng H, et al. Efficacy and safety of gemcitabine, oxaliplatin, and bevacizumab in advanced biliary-tract cancers and correlation of changes in 18-fluorodeoxyglucose PET with clinical outcome: a phase 2 study. Lancet Oncol. 2010;11(1):48–54. https://doi.org/10.1016/s1470-2045(09)70333-x.
Lubner SJ, Mahoney MR, Kolesar JL, Loconte NK, Kim GP, Pitot HC, et al. Report of a multicenter phase II trial testing a combination of biweekly bevacizumab and daily erlotinib in patients with unresectable biliary cancer: a phase II consortium study. J Clinical Oncology: Official Journal of the American Society of Clinical Oncology. 2010;28(21):3491–7. https://doi.org/10.1200/jco.2010.28.4075.
Brechon M, Dior M, Dreanic J, Brieau B, Guillaumot MA, Brezault C, et al. Addition of an antiangiogenic therapy, bevacizumab, to gemcitabine plus oxaliplatin improves survival in advanced biliary tract cancers. Investig New Drugs. 2017; https://doi.org/10.1007/s10637-017-0492-6.
Trinchieri G. Cancer and inflammation: an old intuition with rapidly evolving new concepts. Annu Rev Immunol. 2012;30:677–706. https://doi.org/10.1146/annurev-immunol-020711-075008.
Goldstein D, Lemech C, Valle J. New molecular and immunotherapeutic approaches in biliary cancer. ESMO Open. 2017;2(Suppl 1) https://doi.org/10.1136/esmoopen-2016-000152.
Marks EI, Yee NS. Immunotherapeutic approaches in biliary tract carcinoma: current status and emerging strategies. World J Gastrointest Oncol. 2015;7(11):338–46. https://doi.org/10.4251/wjgo.v7.i11.338.
Bang YJ, Doi T, Braud FD, Piha-Paul S, Hollebecque A, Razak ARA, et al. 525 safety and efficacy of pembrolizumab (MK-3475) in patients (pts) with advanced biliary tract cancer: interim results of KEYNOTE-028. Eur J Cancer. 51:S112. https://doi.org/10.1016/S0959-8049(16)30326-4.
Mojica P, Smith D, Ellenhorn J. Adjuvant radiation therapy is associated with improved survival for gallbladder carcinoma with regional metastatic disease. Journal of Surgical Oncology 2007;96(1):8–13. https://doi.org/10.1002/jso.20831.
Cho SY, Kim SH, Park SJ, Han SS, Kim YK, Lee KW, et al. Adjuvant chemoradiation therapy in gallbladder cancer. Journal of Surgical Oncology 102(1):87–93. https://doi.org/10.1002/jso.21544.
González ME, Giannini OH, González P, Saldaña B. Adjuvant radio-chemotherapy after extended or simple cholecystectomy in gallbladder cancer. Clinical and Translational Oncology 2011;13(7):480–4. https://doi.org/10.1007/s12094-011-0685-y.
Gu B, Qian L, Yu H, Hu J, Wang Q, Shan J, et al. Concurrent Chemoradiotherapy in Curatively Resected Gallbladder Carcinoma: A Propensity Score–Matched Analysis. International Journal of Radiation Oncology*Biology*Physics 2018;100(1):138–45. https://doi.org/10.1016/j.ijrobp.2017.09.029.
Takada T, Amano H, Yasuda H, Nimura Y, Matsushiro T, Kato H, et al. Is postoperative adjuvant chemotherapy useful for gallbladder carcinoma?. Cancer 2002;95(8):1685–95. https://doi.org/10.1002/cncr.10831.
Edeline J, Benabdelghani M, Bertaut A, Watelet J, Hammel P, Joly JP, et al. Gemcitabine and Oxaliplatin Chemotherapy or Surveillance in Resected Biliary Tract Cancer (PRODIGE 12-ACCORD 18-UNICANCER GI): A Randomized Phase III Study. Journal of Clinical Oncology 2019;37(8):658–67. https://doi.org/10.1200/JCO.18.00050.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Mahipal, A. et al. (2020). Gallbladder Cancer. In: Roberts, L., Yang, J., Venkatesh, S. (eds) Evaluation and Management of Liver Masses. Springer, Cham. https://doi.org/10.1007/978-3-030-46699-2_11
Download citation
DOI: https://doi.org/10.1007/978-3-030-46699-2_11
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-46698-5
Online ISBN: 978-3-030-46699-2
eBook Packages: MedicineMedicine (R0)