Abstract
‘Everything is everywhere, but the environment selects’
– Lourens Baas Becking
This quote by Dutch botanist Lourens Baas Becking, when extended to microbiology, implies the activities of microorganisms are defined by the environment in which they find themselves, with biotic and abiotic factors alike impacting their activity, metabolism and viability. This is definitely true of Bdellovibrio bacteriovorus-and-like-organisms (BALOs), which are influenced directly by both their environment and the prey metabolic activities, stimuli that can have drastic impacts on the predatory activities of these strains. The goal of this chapter, therefore, is to delve deeper into, and gain a better understanding of, the correlation between predatory bacteria and their environment. Towards this end, we discuss here many of the factors and conditions (biotic and abiotic) that impact BALOs and their activities, including the osmolality, oxygen, serum albumin and indole. We hope through this discussion young scientists will gain a significant understanding of the current hurdles holding BALOs back from many real-world applications, such as in treatment of bacterial infections or within wastewater treatment systems, and encouragement to find solutions.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
1 Bdellovibrio bacteriovorus-and-Like Organisms, Collectively: Bacterial Predators with Much Potential
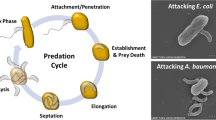
Bdellovibrio bacteriovorus-and-like organisms, collectively referred to as BALOs, are bacterial predators that attack and consume other Gram-negative bacterial species. BALOs have been isolated from habitats all over the world in various abundances and with different adaptations, most notably their differing tolerance to salt concentrations (Amat and Torrella 1989; Chauhan et al. 2009; Fry and Staples 1976; Jurkevitch et al. 2000; Schoeffield and Williams 1990) (Fig. 1). Isolates from samples taken thousands of kilometres away from each other maybe similar enough to potentially be the same BALO species, whereas BALO isolates within a single sample may differ tremendously from one another (Snyder et al. 2002), with some strains possessing a much broader predation spectrum than others. For instance, the type strain is B. bacteriovorus HD100, an intraperiplasmic predator that is capable of attacking over 100 different human pathogens, including strains of Acinetobacter, Klebsiella and Salmonella (Dashiff et al. 2011a; Im et al. 2017b; Sun et al. 2017). Similarly, the epibiotic predator Micavibrio aeruginosavorus has broad spectrum activity, albeit much more restricted than B. bacteriovorus, against a number of pathogenic strains (Dashiff et al. 2011a). However, the prey range for Peredibacter starrii, another intraperiplasmic predatory strain, is restricted to only Pseudomonads (Stolp and Starr 1963).
Examples of the different environmental habitats occupied by BALO species and their measured populations within each. It should be noted that these values were based on plaque-forming units using top agar plates, which is inherently biased as the prey was pre-selected and does not represent the complete predatory complement. Quantitative PCR analyses imply these values may be orders of magnitude higher. (Van Essche et al. 2009; Zheng et al. 2008)
Although differences exist between these strains and their activities, some characteristics are true for all three. Most prominently and long known is their dependency on magnesium and calcium. The presence of these ions has been linked to diverse functions necessary for predation to occur successfully. In their absence, for instance, predator-prey attachment rates are much lower (Starr and Seidler 1971) and the bdelloplasts appear to be significantly less stable (Seidler and Starr 1969). Moreover, the activity of their extracellular lytic enzymes is greatly reduced if these ions are not provided (Huang and Starr 1973). These requirements are not as stringent for all strains as B. bacterivorous 109 J seems to be able to recycle Ca2+ from prey cells (Huang and Starr 1973). Moreover, marine BALOs additionally require potassium for high motility and attachment rates (Marbach and Shilo 1978).
Given their propensity to attack Gram-negative pathogens, several groups have considered applying BALOs as a therapeutic to reduce or remove these harmful bacteria, as reviewed in several articles (Choi et al. 2017; Dwidar et al. 2012b). BALOs also mitigate plant (Barel et al. 2005; McNeely et al. 2017) and animal pathogens (Cao et al. 2014, 2018; Li et al. 2014), with all of these studies hinting at the potential application of predators as biocontrol agents within the agricultural and aquacultural sectors to reduce spoilage and loss in productivity.
However, recent research has found BALOs face many hurdles, impediments that may be biotic or abiotic in nature. From the presence of sugars or salts within the media to the production of secondary metabolites by prey strains, researchers are currently defining the limitations of predation while also seeking ways to overcome these hurdles or, as in the case of Vampirovibrio chlorellavorus, to employ them a means of controlling undesired predatory activities (Bagwell et al. 2016; Ganuza et al. 2016).
2 Abiotic Factors Impacting the Predatory Activities of Bdellovibrio bacteriovorus and Other BALOs
2.1 Oxygen
As strict aerobes, it comes as no surprise that BALOs and their predatory activities strongly correlate with the availability of oxygen (Kadouri and Tran 2013; Schoeffield et al. 1996; Varon and Shilo 1968). In one of the first studies on this topic, Varon and Shilo (1968) measured the predator-prey attachment rate when either agitated or stationary. In the agitated cultures, the attachment rates at 20 min were greater than 70% but hovered only near 20% in the stationary tubes. This was evaluated further by Kadouri and Tran (2013) where the activities of three BALO strains, i.e., B. bacteriovorus HD100, B. bacteriovorus 109 J and M. aeruginosavorus, were measured under different oxygen concentrations (0 to 100%). Similar with Varon and Shilo (1968), the BALO strains did not effectively attack planktonic bacteria under microaerobic or anaerobic conditions, but prey biofilms were reduced by as much as 60% in the former environment. These results suggest predation of surface attached prey is still possible when oxygen levels are low, but not when the environment is anaerobic.
One predatory strain, B. bacteriovorus W, however, can attack planktonic prey even when the oxygen partial pressure is very low (3–5 mm Hg) (Burger et al. 1968), a level where many anaerobes are also capable of growing. As several obligate (aerotolerant) anaerobes, including Prevotella intermedia and some strains of Fusobacterium nucleatum, may be used as prey by BALOs (Dashiff and Kadouri 2011; Van Essche et al. 2011), B. bacteriovorus W represents a class of predators that may be better adapted for survival within low-oxygen environments and biocontrol of these anaerobic pathogens.
Aside from controlling predation, oxygen also impacts the long-term survival of predatory strains, as illustrated in the study by Schoeffield et al. (1996) where the viabilities of several different BALO species, representing both halotolerant and non-halotolerant species, were measured over several days when under either aerobic or anaerobic conditions. Their study found anaerobic conditions led to significantly faster losses with both classes of predators. However, these results conflict with those of Williams and Falkler (1984), where predatory bacteria were isolated from the anaerobic region (13 m depth) within the Miles River. In fact, they found the oxygen concentration had no impact on the predatory numbers, with similar numbers of isolates at each of the depths tested (i.e., 0.5–13 m). Whether these differences are due to the bacterial strain, their overall concentration (which was several log higher in the lab) or some unidentified environmental factors that contribute to stabilizing the predator under anaerobic conditions remains to be elucidated.
2.2 Temperature
Predatory strains that have been studied to date generally have mesophilic preferences with optimal temperatures between 28 °C and 35 °C, although limited predatory activities have been seen in the range of 10–45 °C (Atterbury et al. 2011; Fratamico and Whiting 1995; Varon and Shilo 1968). As with oxygen, Varon and Shilo (1968) studied predator-prey attachment at different temperatures, spanning from 4 °C to 45 °C. As the temperature rose from 4 °C to 25 °C, the percentage of B. bacteriovorus 109 cells that were attached to prey increased in a fairly linear manner from 1% to 64%, and remained somewhat steady thereafter until 35 °C, which was the maximum permissible temperature. Increasing the temperature further reduced the number of attachment events significantly, with only 7% of predators attached to prey at 45 °C. These results were corroborated by Fratamico and Whiting (1995), who measured prey viabilities at set times over 24 h at temperatures between 4 °C and 37 °C. In both studies, predation and attachment was optimal at or near 37 °C and decreased as the temperature was lowered, with 4 °C showing no predation (Fratamico and Whiting 1995) and only 1% of predators attached to prey cells (Varon and Shilo 1968).
Although the majority of BALO studies use mesophilic predatory strains, this does not preclude their presence in hotter or colder environs. As potential proof that thermophilic BALOs exist, predatory strains were reportedly found in significant numbers (~ 1% of total counts) in two hot spring microbial mats where the surface temperatures were 57 ± 2 °C and 91 ± 3 °C (Sangwan et al. 2015). A draft genome of this BALO strain was constructed and homologues for Bd0108 and Bd0105, two genes required for intraperiplasmic stages of predation, were not found, hinting this strain has a highly specialized genome to cope with the atypical conditions within this environment. Although the authors were unsuccessful in culturing the predators, they did manage to capture images of them attacking E. coli and found an unusual predation mechanism; the predator was epibiotic but was attached side-on with the prey cell, as opposed to the polar attachment seen with M. aeruginosavorus. Along with other culture-independent studies that revealed a significantly higher BALO-diversity in soil (Davidov et al. 2006), fresh water (Li et al. 2015) and saltwater habitats (Li et al. 2015; Pineiro et al. 2007b) than originally expected, the above study highlights a potentially untapped diversity of BALOs that grow at extreme temperatures, and should encourage BALO researchers to consider other environments, such as glacial pools or the deep ocean, when seeking novel predatory strains.
2.3 pH
Attachment of the predator to its prey is most effective when the pH is between 6 and 9.3 based on Varon and Shilo (1968), with a maximum at the slightly basic pH of 8. At lower pH levels, attachment rates and predator motility were heavily impaired (Fratamico and Whiting 1995; Varon and Shilo 1968) but was still possible at a pH of 5.6. Dashiff et al. (2011b) evaluated this further by measuring the predatory viabilities at different pH values. They reported that incubating either B. bacteriovorus 109 J or M. aeruginosavorus at a pH of 4 or lower for 24 h completely kills (<1 PFU/ml) both predators while, for M. aeruginosavorus, no loss was seen when incubated in DNB media at a pH of 5. Host-independent (HI) variants of B. bacteriovorus 109 J, which grow axenically, were much more sensitive than the wild-type (host-dependent) strain; 1 h at pH 4 completely eradicated the HI population (> 7-log loss) while the wild-type population dropped by only 3-log.
The sensitivity of predatory strains to acidic pHs was used by one group to control Vampirovibrio chlorellavorus, a predatory non-photosynthetic cyanobacterium, and its predation of Chlorella HS26, an algae used to produce lipids for biodiesel (Ganuza et al. 2016). Shifting the pH to 3.5 for only 15 min with the small addition of acetate (0.5 g/L) reduced the V. chlorellavorus viability by 2-log without significantly affecting that of Chlorella HS26. Using this cost-effective protocol, they were able to protect open ponds of Chlorella HS26 from predation, extending their longevity and overall productivity.
2.4 Salinity and Osmolality
Given their pervasive presence throughout nature, it is not surprising researchers have found some BALO strains prefer low levels of salt while others are more suited for growth in seawater, where the osmolality is around 1000 mOsm/kg. For instance, the best studied strain, B. bacteriovorus HD100, was isolated from soil (Stolp and Starr 1963), prefers freshwater and is generally unable to predate when the osmolality is greater than 250 mOsm/kg, or approximately 0.82% NaCl (Im et al. 2017b). If the salinity was reduced slightly to 0.65% (200 mOsm/kg), predation was as effective as in HEPES buffer as based on the 24-h prey viabilities. The loss of activity seen with osmolalities of between 250 and 350 mOsm/kg was not due a reduced B. bacteriovorus HD100 viability; the 24-h values were not significantly different from those within HEPES, where the osmolality was typically around 40 mOsm/kg.
On the opposite side of the BALO spectrum one finds Halobacteriovorax spp., including B. litoralis and B. marinus, which are ubiquitous in saltwater environments and require NaCl concentrations of 0.5% or greater for optimal predation rates (Koval et al. 2015). Predatory bacteria have also been isolated from estuaries, where midline salinities are found (Pineiro et al. 2007a, 2013; Williams and Falkler 1984) and, in fact, certain clades of Bacteriovorax are only found in less saline waters and disappear as the river mixes with and enters ocean waters (Pineiro et al. 2013).
In a separate but related study, Kandel et al. (2014) identified predatory strains within fresh and saltwater zero discharge systems (ZDS) over a seven-month period. These ZDS mesocosms, where the water is continuously recycled, were developed to rear fish and use microbial activities to remove nitrogen, sulphate and organic materials. An analysis of both freshwater and saltwater ZDS found relatively equal numbers of Bacteriovorax/Bacteriolyticum within each (between 104 and 105) and a similar concentration of Bdellovibrio spp. within the freshwater ZDS. Interestingly, within the saltwater ZDS, where the salt concentration was 20 ppt (approximately 600 mOsm/kg), Bdellovibrio were still found at an average concentration of around 103 PFU/ml. Phylogenetic analysis identified a relatively large number of sequences that were somewhat related to B. bacteriovorus HD100 but, since the maximum likelihood tree had low bootstrap values, reliable annotation is difficult and further analysis needs to be done to confirm the heritage of these strains. However, their results strongly imply halo-tolerant Bdellovibrio species do exist within nature and, as of yet, remain an uncharacterized group of BALOs.
2.5 Environmental Factors and Niche Partitioning
In a recent study, the distribution and abundance of three BALO families (Peredibacteraceae, Bdellovibrionaceae and Bacteriovoracaceae) was investigated in perialpine lakes (Paix et al. 2019). The spatially separated, seasonally changing coexistence of these families suggests that each have different strategies for their respective environmental niches, with depth and temperature reportedly as the main factors. Similar observations could be made for soil and rhizosphere isolates, which display a locally separated coexistence of various BALO strains, each with a different prey spectra, in relatively close proximity to one another (Jurkevitch et al. 2000). Both studies show a clear adaptation or selection of BALOs that is driven by environmental factors, leading to niche partitioning of the different predatory species and strains within a local environment. However, the causes are still under investigation. For instance, it cannot be ruled out that a predator may “follow” a prey organism into a given niche that is beneficial to the prey and slowly adapt to that environment over time.
2.6 Susceptibility of BALOs to Some Environmental Factors May Be Mitigated When in a Bdelloplast
When present intraperiplasmically, i.e., within the periplasm of a prey, predators may be protected from some environmental conditions and survive significantly longer than free attack-phase BALOs. This was proven to be true for anoxic conditions and/or at elevated temperatures (Schoeffield et al. 1996). Similarly, during winter, when the overall temperatures drop, some BALOs “hibernate” in estuarine sediments within bdelloplasts, and these sediments later on serve as a reservoir to recolonize the above waters during the warmer seasons (Williams 1988). Prey biofilms also offer protection against environmental stresses, as illustrated by different studies showing Bacteriovorax spp. survival rates under naturally occurring, unfavourable habitat conditions (temperature/salinity) improved significantly when associated with biofilms (Kelley et al. 1997; Williams et al. 1995, 2009). In each case, Bacteriovorax was less susceptible when associated with a biofilm rather than as attack-phase planktonic cells. All of these studies illustrate a potential survival mechanism used by BALOs to reduce the impacts of salinity and temperature. As discussed in the following sections, however, being within a prey does not provide blanket protection against all conditions, though.
3 Biotic Factors that Impact the Predatory Activities of Bdellovibrio bacteriovorus and Other BALOs
In addition to the abiotic factors listed above, research over the past decade has identified a range of biotic elements from bacteria and eukaryotes that impact predation. The former is showing us that predation may not be as straight-forward as previously thought, with prey secreting factors that inhibit predation and non-prey bacteria offering some benefits, while the latter (eukaryotic factors) is important if application of BALOs as living antibiotic is to be realized.
3.1 Bacterial Factors
Prey Metabolic Activities
The prey metabolic activities may also influence the local environment in a way that is less suitable for the predator, such as through acidification of the medium. As discussed above, the pH can have a tremendous impact on predation. Within their study, Dashiff et al. (2011b) found the addition of either glycerol or glucose to co-cultures of E. coli and B. bacteriovorus 109 J blocked predation. They initially evaluated if these carbohydrates alone killed the predator and found this was not the case. Intriguingly, neither was able to block predation when the prey cells were heat killed, suggesting the metabolic activity of E. coli was responsible for the inhibitions seen. Further evaluation found the cause was the media pH, which dropped from pH 6.5 to less than pH 4 within the first 5 h as the carbohydrates were consumed by the prey. As was reported by Varon and Shilo (1968), this pH was both inhibitory and lethal, leading to a significant and rapid killing of B. bacteriovorus 109 J (Dashiff et al. 2011b). Importantly, the same experiments performed in buffered media did not give the same results. This suggests there may be microenvironments within nature that inhibit bacterial predation due to the activities of the prey cells within them.
Another example of prey activity that actively hinders predation was recently reported by Duncan et al. (2018) in their article discussing B. bacteriovorus HD100’s ability to predate on Vibrio cholera. V. cholerae is highly motile which puts stress (literally) on the predator when it is attempting to attack it; the predator is dragged along while attached to the prey as V. cholerae continues to swim. Although predation was still relatively successful (99.4% killing with a wild-type, motile V. cholerae over 14 h with an initial MOI of 0.1), the non-motile V. cholerae ΔmotY mutant was more susceptible to predation by B. bacteriovorus 109J.
Prey Secondary Metabolites
Substances produced by potential prey bacteria can also significantly impact BALO activities. Indole is a secondary metabolite produced by various bacteria and reported to be involved in quorum sensing (Lee and Lee 2010). This molecule, which was not toxic towards B. bacteriovorus HD100, slowed predation when present at a concentration of 0.25–1 mM and completely blocked it when added to a final concentration of 2 mM (Dwidar et al. 2015). Through activity assays and transcriptomics, the authors found that indole represses expression of many flagellar genes, compromising the predator’s motility during the attack-phase, i.e., they stop swimming. Moreover, indole interfered with the growth of B. bacteriovorus HD100 within bdelloplast, bringing it to a halt and preventing further development. As such, the bdelloplast offers no apparent protection against indole.
Another secondary metabolite that impacts predation is cyanide. Strains of Pseudomonas and Chromobacterium are cyanogenic and produce significant quantities of cyanide when amino acids are available (Askeland and Morrison 1983; Freeman et al. 1975; Gallagher and Manoil 2001; Michaels et al. 1965; Mun et al. 2017). When this occurs and the cyanide concentration was below 100 μM, predation with B. bacteriovorus HD100 slowed down but it’s viability was stable (Mun et al. 2017), much like what is seen with indole. Increasing the cyanide concentration to 200 μM or higher completely blocked predation and led to a slight but statistical drop (~50%) in the B. bacteriovorus HD100 viabilities. This concentration (202 μM) was achieved with C. piscinae when incubated in dilute nutrient broth (1:10 diluted NB) while much higher concentrations (600–800 μM) were obtained when NB was used, illustrating the small amount of amino acids needed to achieve resistance in this strain. Another similarity between cyanide and indole is the bdelloplast-associated predatory strains were also susceptible, i.e., being within the prey did not protect the intraperiplasmic predator. As with indole, they were just as sensitive to cyanide as attack-phase cells (Dwidar et al. 2015; Mun et al. 2017).
Antibiotics
Strains of Streptomyces are known for their ability to produce a wide-range of antibiotics (Procopio et al. 2012) but, as Gram-positive bacteria, they are not prey for known BALOs. In the study by Varon and Shilo (1968), it was reported three protein synthesis inhibitors, i.e., streptomycin, chloramphenicol and puromycin, produced by strains of Streptomyces all blocked predation with B. bacteriovorus 109. A deeper analysis found the predator still attached to the prey when exposed to these antibiotics but invasion did not occur, suggesting de novo protein synthesis is needed after attachment to the prey. In contrast, attachment and invasion both occurred when ampicillin, a β-lactam antibiotic that inhibits cell wall synthesis, was tested. A subsequent study reported treatment of B. bacteriovorus with penicillin, a different β-lactam antibiotic, leads to the stable formation of spheroplasts (Thomashow and Rittenberg 1978). Both lysozyme and D-cycloserine, another antibiotic produced by Streptomyces that inhibits cell wall synthesis (Kuehl et al. 1955), had similar effects on this predator (Thomashow and Rittenberg 1978), implying B. bacteriovorus is tolerant to cell wall-targeting antibiotics. Another important finding was penicillin did not have the same effect when used in combination with chloramphenicol, i.e., there was no spheroplast formation. As with the protein inhibitors mentioned above, this illustrates de novo protein and peptidoglycan synthesis both occur during the attack-phase.
A more recent study evaluated the use of B. bacteriovorus HD100 in the presence of violacein (Im et al. 2017a). Violacein, a bisindole compound formed through a condensation reaction involving two tryptophan molecules (Hoshino et al. 1987), is produced by a wide-range of Gram-negative bacteria (Choi et al. 2015a, b). As an antibiotic, the spectrum of violacein primarily encompasses Gram-positive strains where it appears to attack the cellular membrane, causing loss in integrity and leakage of the cellular components (ATP, protein, etc.) (Aruldass et al. 2018). As BALOs are Gram-negative bacteria, the limited spectrum of violacein these two antibacterials were combined and used together (Im et al. 2017a). They demonstrated the specificity of both, i.e., violacein against only Gram-positive and B. bacteriovorus HD100 against only Gram-negative, and that they did not interfere with the activity of the other. Moreover, when used together, their combined activities reduced the viability of mixed cultures (i.e., four different pathogens) by 4-log, and was much more effective than the combined use of gentamycin and chloramphenicol. Consequently, that study showed predatory bacteria can successfully be used alongside antibiotics that specifically target Gram-positive bacterial strains.
Bacterial Proteins
In contrast with the above inhibitors, Serratia marcescens employs a different class of biological defence to protect it from the epibiotic predator, M. aeruginosavorus. Garcia et al. (2018) reported S. marcescens expresses and secretes PrtS, a serralysin family metalloprotease, which protects it from predation. Not only did PrtS reduce predation of S. marcescens by more than 95%, it similarly protected E. coli, reducing M. aeruginosavorus predation by as much as 98%. However, experiments with the intraperiplasmic B. bacteriovorus 109 J found this protease affords no protection, implying its inhibitory activity may be specific for epibiotic predators. Another important characteristic of PtrS is, rather than working against the predator like many of the factors discussed here, this enzyme hydrolyzes some yet unidentified surface protein(s) in the outer membrane of the prey (E. coli and S. marcescens) but does not impact their ability to grow. This study also suggests the recognition mechanisms used by B. bacteriovorus and M. aeruginosavorus are likely distinct, with the latter recognizing a specific outer membrane protein within its prey that is susceptible to proteolytic hydrolysis.
On the other hand, extracellular proteins may also be beneficial to B. bacteriovorus, particularly its own, as shown recently in work done with S. aureus biofilms (Im et al. 2018). As a Gram-positive bacterium, S. aureus is not a prey for B. bacteriovorus (Im et al. 2017a; Monnappa et al. 2014), although Iebba et al. (2014), using unconventional methods, allegedly claims otherwise. Monnappa et al. (2014) found proteases secreted by a host-independent variant of B. bacteriovorus (HIB) extensively hydrolysed the surface proteins of planktonic S. aureus cells. In two subsequent studies, Dwidar et al. (2017) and Im et al. (2018) demonstrated wild-type attack-phase B. bacteriovorus HD100 also respond to extracellular amino acids and secrete the same proteases in response. In the first study, B. bacteriovorus HD100 was found to secrete proteases in both a time- and dose-dependent manner when incubated alone in different nutrient media preparations (HEPES, 0.2x NB, 1x NB and 5x NB). Moreover, the B. bacteriovorus HD100 gene expression patterns in 1x NB mimicked those seen during intraperiplasmic phase, as reported by Karunker et al. (2013). In Im et al. (2018), this was expanded to studies with S. aureus biofilms. Although this bacterium is not a prey for B. bacteriovorus HD100, results which were confirmed once more in that study, the authors found the predator benefitted from interacting with S. aureus biofilms, specifically by hydrolysing proteins present within the extracellular polymeric substances (EPS). The extracellular proteases responsible were produced de novo by attack-phase B. bacteriovorus HD100 when they encountered the S. aureus biofilms, while the supply of amino acids translated into significantly higher ATP pools within the predators and improved killing rates. Together, these three studies (Dwidar et al. 2017; Im et al. 2018; Monnappa et al. 2014) prove predatory bacteria gain a clear benefit from extracellular amino acids, even if they are from non-prey biofilms and their EPS layers.
3.2 Eukaryotic Factors
Predators Are not Harmful Towards Eukaryotes
In 1996, Lederberg coined the term ‘living antibiotic’ in an article where he mentioned bacteriophage and BALOs may be developed as new therapeutic agents (Lederberg 1996). Since that time, work by different groups reports BALOs actively predate a large number of human pathogens, including drug-resistant strains (Dashiff et al. 2011a; Im et al. 2017a; Sun et al. 2017), and their biofilms (Dwidar et al. 2012a, 2013; Kadouri et al. 2007). Later work also demonstrated BALOs are not harmful towards human cells (Monnappa et al. 2016), neither inducing strong cytokine responses nor leading to any observed increase in cell death. One reason for their mild nature is their unique lipid A, which contains α-D-mannopyranose residues instead of phosphate, making it the first example of a lipid A that lacks negatively charged groups (Schwudke et al. 2003). Due to this change in structure, the B. bacteriovorus HD100 lipid A did not induce strong immunogenic responses, i.e., cytokines, from human macrophage cells.
More than not being harmful towards eukaryotic cells, several studies demonstrated BALOs actually protected animal cells from pathogens. Boileau et al. (2011) reported B. bacteriovorus 109 J lowered Moraxella bovis attachment to Madin-Darby bovine kidney cells by sixfold. Similarly, the study by Dwidar et al. (2013) showed B. bacterivorous HD100 protected mammalian cells from a strain of Pseudomonas. In that study, when the predator was not present, a biofilm of Pseudomonas sp. DSM 50906 killed human cells located beneath it, leading to an “footprint” zone of clearing. With the addition of B. bacterivorous HD100, though, the human cells within this zone were healthy. Regarding non-prey pathogens, as mentioned above, BALO proteases hydrolysed S. aureus surface proteins, which reduced the ability of this pathogen to invade human epithelial cells by 80% (Monnappa et al. 2014).
Although the above studies illustrate the gentle, and potentially helpful, nature of BALOs towards host cells, the same cannot be said for the host impact on BALOs and their activities. This is discussed further in the following section.
Serum Albumin, a Proteinaceous Inhibitor Within in Blood Sera
As noted in Sect. 2.4, predation is inhibited by the osmolality. As the value for blood sera in most higher organisms hovers around 300 mOsm/kg (Hall et al. 2012), this would limit the activity of BALOs. However, this is not the only inhibiting factor associated with blood sera. As reported by Im et al. (2017b), human serum albumin also coats B. bacteriovorus HD100 and prevents it from attacking its prey. Blood sera contains several different proteins but albumin is the most common one, present at a concentration of approximately 35–53 mg/ml (Choi et al. 2004). Using both an immunoassay, i.e., dot blot analyses with antibodies specific for albumin, and FITC-labelled bovine serum albumin, they unequivocally demonstrated that albumin is binding to and coating the predator, not the prey, and that a subsequent treatment with proteinase K restored their ability to attack. All of these suggest mammalian serum albumin proteins bind a component present on the surface of B. bacteriovorus HD100 and block its ability to bind and/or recognize its prey. Although albumin also binds other bacterial strains, all previously reported strains were Gram-positive (de Chateau et al. 1996, Johansson et al. 2002; Willcox et al. 1993), making B. bacteriovorus HD100 the first clear example of a Gram-negative bacterium to be bound by this class of proteins.
Their study also showed the impact of albumin varied somewhat based on the prey. Whereas predation of both E. coli and Salmonella enterica were inhibited by human serum albumin, a clinical isolate of K. pneumoniae was still attacked slightly, but significantly (4.5-fold reduction), even when albumin was present (Im et al. 2017b). Similarly, in the study by Baker et al. (2017), long-term experiments using a different strain of K. pneumoniae saw a temporary, approximately 4-log reduction 32–78 h after initiating predation. The results with K. pneumoniae in both studies indicate that predation, though heavily impaired, may still occur with some select pathogenic strains.
4 Conclusions – A Move Towards Using Native BALOs?
Predatory microorganisms are a remarkable group of bacteria that possess a very distinctive lifestyle. The unique properties possessed by BALOs make them a potential alternative to chemical antibiotics against diverse human and animal pathogens, including multi-drug resistant strains. It should come as no surprise, therefore, that as research into their activities has progressed, there continues to be a clear move towards their use as living antibiotics within higher organisms, such as cows, rabbits, rats and zebrafish (Atterbury et al. 2011; Boileau et al. 2016; Shatzkes et al. 2017; Willis et al. 2016). However, discrepancies exist between the observed in vitro and in vivo predatory activities with most of the in vivo results being underwhelming, i.e., only mild reductions in the pathogen viability or only a slight benefit. The only in vivo applications so far where BALOs have consistently been effective are within aquaculture (Cao et al. 2014, 2015; Guo et al. 2017).
As presented in this chapter, BALOs and their activities are negatively impacted by different environmental and biotic factors, many of which may contribute to the less than ideal results seen in the in vivo studies. As such, effort should be given (1) to identify the limitations of and hurdles to be overcome for these bacteria, as they pertain to their use within animal hosts, and (2) to seek out other predatory strains that are inherently resistant to the offending factors currently holding back breakthroughs in in vivo experiments.
Much effort has been given to the first, as shown by the many studies referenced here, but the second is an area that has not been extensively tapped, yet holds much promise. A case in point is the natural preference of Halobacteriovorax spp. for higher osmolalities, which may make them a better choice for use within the blood sera if one with the proper prey spectrum can be isolated. As these strains have structurally different lipid A molecules than B. bacteriovorus (Beck et al. 2010; Jayasimhulu et al. 2007), though, host cell responses would need to be evaluated to determine if they, like B. bacteriovorus HD100 and other isolates (Monnappa et al. 2016), do not elicit strong cytokine responses. In addition, as discussed briefly in the beginning of this chapter, BALOs are fairly ubiquitous throughout nature, yet most studies have limited their characterization to three main strains, B. bacteriovorus HD100, B. bacteriovorus 109 J and M. aeruginosavorus. With a plethora of different predators in nature, and possibly extreme environments, an untapped resource still exists and should be explored, particularly within an environmental setting that befits their application. As illustrated in Fig. 2, the predator and prey are not the only condition that governs their respective activities; the environmental setting needs to be considered as well. Consequently, finding an active predator within a certain locale dictates that the predator is likely adapted to the conditions within that location. This is exemplified in the successful aquaculture studies mentioned above – the predators were not the three powerhouse strains but, rather, were isolated from the environments in question, an aspect of those studies that helped to ensure their success. Extending this perspective to other concerns, for example, gut dysbiosis within humans, rather than using soil organisms, i.e., B. bacteriovorus HD100 and B. bacteriovorus 109 J, researchers should perhaps identify and characterize BALOs found within the guts of mammals (Schwudke et al. 2001).
References
Amat AS, Torrella F. Isolation and characterization of marine and salt pond halophilic Bdellovibrios. Can J Microbiol. 1989;35:771–8.
Aruldass CA, Masalamany SRL, Venil CK, Ahmad WA. Antibacterial mode of action of violacein from Chromobacterium violaceum UTM5 against Staphylococcus aureus and methicillin-resistant Staphylococcus aureus (MRSA). Environ Sci Pollut Res Int. 2018;25:5164–80.
Askeland RA, Morrison SM. Cyanide production by Pseudomonas fluorescens and Pseudomonas aeruginosa. Appl Environ Microbiol. 1983;45:1802–7.
Atterbury RJ, Hobley L, Till R, Lambert C, Capeness MJ, Lerner TR, et al. Effects of orally administered Bdellovibrio bacteriovorus on the well-being and Salmonella colonization of young chicks. Appl Environ Microbiol. 2011;77:5794–803.
Bagwell CE, Abernathy A, Barnwell R, Milliken CE, Noble PA, Dale T, et al. Discovery of bioactive metabolites in biofuel microalgae that offer protection against predatory Bacteria. Front Microbiol. 2016;7:516.
Baker M, Negus D, Raghunathan D, Radford P, Moore C, Clark G, et al. Measuring and modelling the response of Klebsiella pneumoniae KPC prey to Bdellovibrio bacteriovorus predation, in human serum and defined buffer. Sci Rep. 2017;7:8329.
Barel G, Sirota A, Volpin H, Jurkevitch E. Fate of predator and prey proteins during growth of Bdellovibrio bacteriovorus on Escherichia coli and Pseudomonas syringae prey. J Bacteriol. 2005;187:329–35.
Beck S, Muller FD, Strauch E, Brecker L, Linscheid MW. Chemical structure of Bacteriovorax stolpii lipid A. Lipids. 2010;45:189–98.
Boileau MJ, Clinkenbeard KD, Iandolo JJ. Assessment of Bdellovibrio bacteriovorus 109J killing of Moraxella bovis in an in vitro model of infectious bovine keratoconjunctivitis. Can J Vet Res. 2011;75:285–91.
Boileau MJ, Mani R, Breshears MA, Gilmour M, Taylor JD, Clinkenbeard KD. Efficacy of Bdellovibrio bacteriovorus 109J for the treatment of dairy calves with experimentally induced infectious bovine keratoconjunctivitis. Am J Vet Res. 2016;77:1017–28.
Burger A, Drews G, Ladwig R. Host range and infection cycle of a newly isolated strain of Bdellovibrio bacteriovorus. Arch Mikrobiol. 1968;61:261–79.
Cao H, He S, Lu L, Yang X, Chen B. Identification of a Proteus penneri isolate as the causal agent of red body disease of the cultured white shrimp Penaeus vannamei and its control with Bdellovibrio bacteriovorus. Antonie Van Leeuwenhoek. 2014;105:423–30.
Cao H, An J, Zheng W, He S. Vibrio cholerae pathogen from the freshwater-cultured whiteleg shrimp Penaeus vannamei and control with Bdellovibrio bacteriovorus. J Invertebr Pathol. 2015;130:13–20.
Cao HP, Yang YB, Lu LQ, Yang XL, Ai XH. Effect of copper sulfate on Bdellovibrio growth and bacteriolytic activity towards gibel carp-pathogenic Aeromonas hydrophila. Can J Microbiol. 2018;64:1054–8.
Chauhan A, Fortenberry GZ, Lewis DE, Williams HN. Increased diversity of predacious Bdellovibrio-Like Organisms (BLOs) as a function of eutrophication in Kumaon Lakes of India. Curr Microbiol. 2009;59:1–8.
Choi S, Choi EY, Kim DJ, Kim JH, Kim TS, Oh SW. A rapid, simple measurement of human albumin in whole blood using a fluorescence immunoassay (I). Clin Chim Acta. 2004;339:147–56.
Choi SY, Kim S, Lyuck S, Kim SB, Mitchell RJ. High-level production of violacein by the newly isolated Duganella violaceinigra str. NI28 and its impact on Staphylococcus aureus. Sci Rep. 2015a;5:15598.
Choi SY, Yoon KH, Lee JI, Mitchell RJ. Violacein: properties and production of a versatile bacterial pigment. Biomed Res Int. 2015b;2015:465056.
Choi SY, Im H, Mitchell RJ. Violacein and bacterial predation: promising alternatives for priority multidrug resistant human pathogens. Future Microbiol. 2017;12:835–8.
Dashiff A, Kadouri DE. Predation of oral pathogens by Bdellovibrio bacteriovorus 109J. Mol Oral Microbiol. 2011;26:19–34.
Dashiff A, Junka RA, Libera M, Kadouri DE. Predation of human pathogens by the predatory bacteria Micavibrio aeruginosavorus and Bdellovibrio bacteriovorus. J Appl Microbiol. 2011a;110:431–44.
Dashiff A, Keeling TG, Kadouri DE. Inhibition of predation by Bdellovibrio bacteriovorus and Micavibrio aeruginosavorus via host cell metabolic activity in the presence of carbohydrates. Appl Environ Microbiol. 2011b;77:2224–31.
Davidov Y, Friedjung A, Jurkevitch E. Structure analysis of a soil community of predatory bacteria using culture-dependent and culture-independent methods reveals a hitherto undetected diversity of Bdellovibrio-and-like organisms. Environ Microbiol. 2006;8:1667–73.
de Chateau M, Holst E, Bjorck L. Protein PAB, an albumin-binding bacterial surface protein promoting growth and virulence. J Biol Chem. 1996;271:26609–15.
Duncan MC, Forbes JC, Nguyen Y, Shull LM, Gillette RK, Lazinski DW, et al. Vibrio cholerae motility exerts drag force to impede attack by the bacterial predator Bdellovibrio bacteriovorus. Nat Commun. 2018;9:4757.
Dwidar M, Hong S, Cha M, Jang J, Mitchell RJ. Combined application of bacterial predation and carbon dioxide aerosols to effectively remove biofilms. Biofouling. 2012a;28:671–80.
Dwidar M, Monnappa AK, Mitchell RJ. The dual probiotic and antibiotic nature of Bdellovibrio bacteriovorus. BMB Rep. 2012b;45:71–8.
Dwidar M, Leung BM, Yaguchi T, Takayama S, Mitchell RJ. Patterning bacterial communities on epithelial cells. PLoS One. 2013;8:e67165.
Dwidar M, Nam D, Mitchell RJ. Indole negatively impacts predation by Bdellovibrio bacteriovorus and its release from the bdelloplast. Environ Microbiol. 2015;17:1009–22.
Dwidar M, Im H, Seo JK, Mitchell RJ. Attack-phase Bdellovibrio bacteriovorus responses to extracellular nutrients are analogous to those seen during late Intraperiplasmic growth. Microb Ecol. 2017;74:937–46.
Fratamico PM, Whiting RC. Ability of Bdellovibrio-Bacteriovorus 109j to lyse gram-negative food-borne pathogenic and spoilage bacteria. J Food Prot. 1995;58:160–4.
Freeman LR, Angelini P, Silverman GJ, Merritt C Jr. Production of hydrogen cyanide by Pseudomonas fluorescens. Appl Microbiol. 1975;29:560–1.
Fry JC, Staples DG. Distribution of Bdellovibrio-Bacteriovorus in sewage works, river water, and sediments. Appl Environ Microbiol. 1976;31:469–74.
Gallagher LA, Manoil C. Pseudomonas aeruginosa PAO1 kills Caenorhabditis elegans by cyanide poisoning. J Bacteriol. 2001;183:6207–14.
Ganuza E, Sellers CE, Bennett BW, Lyons EM, Carney LT. A novel treatment protects Chlorella at commercial scale from the predatory bacterium Vampirovibrio chlorellavorus. Front Microbiol. 2016;7:848.
Garcia CJ, Pericleous A, Elsayed M, Tran M, Gupta S, Callaghan JD, et al. Serralysin family metalloproteases protects Serratia marcescens from predation by the predatory bacteria Micavibrio aeruginosavorus. Sci Rep. 2018;8:14025.
Guo YB, Pan Q, Yan SQ, Chen YH, Li MJ, Chen D, et al. Bdellovibrio and like organisms promoted growth and survival of juvenile abalone Haliotis discus hannai Ino and modulated bacterial community structures in its gut. Aquacult Int. 2017;25:1625–43.
Hall NH, Isaza R, Hall JS, Wiedner E, Conrad BL, Wamsley HL. Serum osmolality and effects of water deprivation in captive Asian elephants (Elephas maximus). J Vet Diagn Investig. 2012;24:688–95.
Hoshino T, Kondo T, Uchiyama T, Ogasawara N. Studies on the biosynthesis of Violacein .1. Biosynthesis of Violacein – a novel rearrangement in tryptophan-metabolism with a 1,2-shift of the indole ring. Agric Biol Chem Tokyo. 1987;51:965–8.
Huang JCC, Starr MP. Effects of calcium and magnesium ions and host viability on growth of Bdellovibrios. Antonie Van Leeuwenhoek. 1973;39:151–67.
Iebba V, Totino V, Santangelo F, Gagliardi A, Ciotoli L, Virga A, et al. Bdellovibrio bacteriovorus directly attacks pseudomonas aeruginosa and Staphylococcus aureus cystic fibrosis isolates. Front Microbiol. 2014;5:280.
Im H, Choi SY, Son S, Mitchell RJ. Combined application of bacterial predation and violacein to kill polymicrobial pathogenic communities. Sci Rep. 2017a;7:14415.
Im H, Son S, Mitchell RJ, Ghim CM. Serum albumin and osmolality inhibit Bdellovibrio bacteriovorus predation in human serum. Sci Rep. 2017b;7
Im H, Dwidar M, Mitchell RJ. Bdellovibrio bacteriovorus HD100, a predator of gram-negative bacteria, benefits energetically from Staphylococcus aureus biofilms without predation. ISME J. 2018;12:2090–5.
Jayasimhulu K, Hunt SM, Kaneshiro ES, Watanabe Y, Giner JL. Detection and identification of Bacteriovorax stolpii UKi2 Sphingophosphonolipid molecular species. J Am Soc Mass Spectrom. 2007;18:394–403.
Johansson MU, Frick IM, Nilsson H, Kraulis PJ, Hober S, Jonasson P, et al. Structure, specificity, and mode of interaction for bacterial albumin-binding modules. J Biol Chem. 2002;277:8114–20.
Jurkevitch E, Minz D, Ramati B, Barel G. Prey range characterization, ribotyping, and diversity of soil and rhizosphere Bdellovibrio spp. isolated on phytopathogenic bacteria. Appl Environ Microbiol. 2000;66:2365–71.
Kadouri DE, Tran A. Measurement of predation and biofilm formation under different ambient oxygen conditions using a simple gasbag-based system. Appl Environ Microbiol. 2013;79:5264–71.
Kadouri D, Venzon NC, O’Toole GA. Vulnerability of pathogenic biofilms to Micavibrio aeruginosavorus. Appl Environ Microbiol. 2007;73:605–14.
Kandel PP, Pasternak Z, van Rijn J, Nahum O, Jurkevitch E. Abundance, diversity and seasonal dynamics of predatory bacteria in aquaculture zero discharge systems. FEMS Microbiol Ecol. 2014;89:149–61.
Karunker I, Rotem O, Dori-Bachash M, Jurkevitch E, Sorek R. A global transcriptional switch between the attack and growth forms of Bdellovibrio bacteriovorus. PLoS One. 2013;8:e61850.
Kelley JI, Turng BF, Williams HN, Baer ML. Effects of temperature, salinity, and substrate on the colonization of surfaces in situ by aquatic bdellovibrios. Appl Environ Microbiol. 1997;63:84–90.
Koval SF, Williams HN, Stine OC. Reclassification of Bacteriovorax marinus as Halobacteriovorax marinus gen. nov., comb. nov. and Bacteriovorax litoralis as Halobacteriovorax litoralis comb. nov.; description of Halobacteriovoraceae fam. nov. in the class Deltaproteobacteria. Int J Syst Evol Microbiol. 2015;65:593–7.
Kuehl FA, Buhs RP, Putter I, Ormond R, Lyons JE, Chaiet L, et al. D-4-Amino-3-Isoxazolidone, a new antibiotic. J Am Chem Soc. 1955;77:2344–5.
Lederberg J. Smaller fleas ... ad infinitum: therapeutic bacteriophage redux. Proc Natl Acad Sci USA. 1996;93:3167–8.
Lee JH, Lee J. Indole as an intercellular signal in microbial communities. FEMS Microbiol Rev. 2010;34:426–44.
Li H, Chen C, Sun Q, Liu R, Cai J. Bdellovibrio and like organisms enhanced growth and survival of Penaeus monodon and altered bacterial community structures in its rearing water. Appl Environ Microbiol. 2014;80:6346–54.
Li N, Chen H, Williams HN. Genome-wide comparative analysis of ABC systems in the Bdellovibrio-and-like organisms. Gene. 2015;562:132–7.
Marbach A, Shilo M. Dependence of marine Bdellovibrios on potassium, calcium, and magnesium-ions. Appl Environ Microbiol. 1978;36:169–77.
McNeely D, Chanyi RM, Dooley JS, Moore JE, Koval SF. Biocontrol of Burkholderia cepacia complex bacteria and bacterial phytopathogens by Bdellovibrio bacteriovorus. Can J Microbiol. 2017;63:350–8.
Michaels R, Hankes LV, Corpe WA. Cyanide formation from glycine by nonproliferating cells of Chromobacterium violaceum. Arch Biochem Biophys. 1965;111:121–5.
Monnappa AK, Dwidar M, Seo JK, Hur JH, Mitchell RJ. Bdellovibrio bacteriovorus inhibits Staphylococcus aureus biofilm formation and invasion into human epithelial cells. Sci Rep. 2014;4:3811.
Monnappa AK, Bari W, Choi SY, Mitchell RJ. Investigating the responses of human epithelial cells to predatory bacteria. Sci Rep. 2016;6:33485.
Mun W, Kwon H, Im H, Choi SY, Monnappa AK, Mitchell RJ. Cyanide production by Chromobacterium piscinae shields it from Bdellovibrio bacteriovorus HD100 predation. MBio. 2017;8
Paix B, Ezzedine JA, Jacquet S. Diversity, dynamics, and distribution of Bdellovibrio and like organisms in Perialpine Lakes. Appl Environ Microbiol. 2019;85
Pineiro SA, Stine OC, Chauhan A, Steyert SR, Smith R, Williams HN. Global survey of diversity among environmental saltwater Bacteriovoracaceae. Environ Microbiol. 2007a;9:2441–50.
Pineiro SA, Stine OC, Chauhan A, Steyert SR, Smith R, Williams HN. Global survey of diversity among environmental saltwater Bacteriovoracaceae. Environ Microbiol. 2007b;9:2441–50.
Pineiro S, Chauhan A, Berhane TK, Athar R, Zheng G, Wang C, et al. Niche partition of Bacteriovorax operational taxonomic units along salinity and temporal gradients in the Chesapeake Bay reveals distinct estuarine strains. Microb Ecol. 2013;65:652–60.
Procopio RE, Silva IR, Martins MK, Azevedo JL, Araujo JM. Antibiotics produced by Streptomyces. Braz J Infect Dis. 2012;16:466–71.
Sangwan N, Lambert C, Sharma A, Gupta V, Khurana P, Khurana JP, et al. Arsenic rich Himalayan hot spring metagenomics reveal genetically novel predator-prey genotypes. Environ Microbiol Rep. 2015;7:812–23.
Schoeffield AJ, Williams HN. Efficiencies of recovery of Bdellovibrios from brackish-water environments by using various bacterial species as prey. Appl Environ Microbiol. 1990;56:230–6.
Schoeffield AJ, Williams HN, Turng B, Fackler WA Jr. A comparison of the survival of Intraperiplasmic and attack phase Bdellovibrios with reduced oxygen. Microb Ecol. 1996;32:35–46.
Schwudke D, Strauch E, Krueger M, Appel B. Taxonomic studies of predatory bdellovibrios based on 16S rRNA analysis, ribotyping and the hit locus and characterization of isolates from the gut of animals. Syst Appl Microbiol. 2001;24:385–94.
Schwudke D, Linscheid M, Strauch E, Appel B, Zahringer U, Moll H, et al. The obligate predatory Bdellovibrio bacteriovorus possesses a neutral lipid a containing alpha-D-mannoses that replace phosphate residues: similarities and differences between the lipid as and the lipopolysaccharides of the wild type strain B. bacteriovorus HD100 and its host-independent derivative HI100. J Biol Chem. 2003;278:27502–12.
Seidler FJ, Starr MP. Factors affecting intracellular parasitic growth of Bdellovibrio Bacteriovorus developing within Escherichia Coli. J Bacteriol. 1969;97:912.
Shatzkes K, Tang C, Singleton E, Shukla S, Zuena M, Gupta S, et al. Effect of predatory bacteria on the gut bacterial microbiota in rats. Sci Rep. 2017;7
Snyder AR, Williams HN, Baer ML, Walker KE, Stine OC. 16S rDNA sequence analysis of environmental Bdellovibrio-and-like organisms (BALO) reveals extensive diversity. Int J Syst Evol Microbiol. 2002;52:2089–94.
Starr MP, Seidler RJ. Bdellovibrios. Annu Rev Microbiol. 1971;25:649.
Stolp H, Starr MP. Bdellovibrio Bacteriovorus Gen. Et Sp. N., a predatory, Ectoparasitic, and Bacteriolytic microorganism. Antonie Van Leeuwenhoek. 1963;29:217–48.
Sun Y, Ye J, Hou Y, Chen H, Cao J, Zhou T. Predation efficacy of Bdellovibrio bacteriovorus on multidrug-resistant clinical pathogens and their corresponding biofilms. Jpn J Infect Dis. 2017;70:485–9.
Thomashow MF, Rittenberg SC. Penicillin-induced formation of osmotically stable spheroplasts in nongrowing Bdellovibrio bacteriovorus. J Bacteriol. 1978;133:1484–91.
Van Essche M, Sliepen I, Loozen G, Van Eldere J, Quirynen M, Davidov Y, et al. Development and performance of a quantitative PCR for the enumeration of Bdellovibrionaceae. Environ Microbiol Rep. 2009;1:228–33.
Van Essche M, Quirynen M, Sliepen I, Loozen G, Boon N, Van Eldere J, et al. Killing of anaerobic pathogens by predatory bacteria. Mol Oral Microbiol. 2011;26:52–61.
Varon M, Shilo M. Interaction of Bdellovibrio Bacteriovorus and host bacteria .I. kinetic studies of attachment and invasion of Escherichia Coli B by Bdellovibrio Bacteriovorus. J Bacteriol. 1968;95:744.
Willcox MD, Patrikakis M, Loo CY, Knox KW. Albumin-binding proteins on the surface of the Streptococcus milleri group and characterization of the albumin receptor of Streptococcus intermedius C5. J Gen Microbiol. 1993;139:2451–8.
Williams HN. A study of the occurrence and distribution of Bdellovibrios in estuarine sediment over an annual cycle. Microbial Ecol. 1988;15:9–20.
Williams HN, Falkler WA Jr. Distribution of bdellovibrios in the water column of an estuary. Can J Microbiol. 1984;30:971–4.
Williams HN, Schoeffield AJ, Guether D, Kelley J, Shah D, Falkler WA. Recovery of Bdellovibrios from submerged surfaces and other aquatic habitats. Microbial Ecol. 1995;29:39–48.
Williams HN, Turng BF, Kelley JI. Survival response of Bacteriovorax in surface biofilm versus suspension when stressed by extremes in environmental conditions. Microb Ecol. 2009;58:474–84.
Willis AR, Moore C, Mazon-Moya M, Krokowski S, Lambert C, Till R, et al. Injections of predatory Bacteria work alongside host immune cells to treat Shigella infection in zebrafish larvae. Curr Biol. 2016;26:3343–51.
Zheng G, Wang C, Williams HN, Pineiro SA. Development and evaluation of a quantitative real-time PCR assay for the detection of saltwater Bacteriovorax. Environ Microbiol. 2008;10:2515–26.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Im, H., Bäcker, L.E., Mitchell, R.J. (2020). Environmental and Biotic Factors Impacting the Activities of Bdellovibrio bacteriovorus. In: Jurkevitch, E., Mitchell, R. (eds) The Ecology of Predation at the Microscale. Springer, Cham. https://doi.org/10.1007/978-3-030-45599-6_6
Download citation
DOI: https://doi.org/10.1007/978-3-030-45599-6_6
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-45598-9
Online ISBN: 978-3-030-45599-6
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)