Abstract
Electrochromic (EC) materials can change their optical properties, reversibly and persistently, when electrical voltage is applied. These materials can be integrated in multilayer devices capable of modulating the optical transmittance and heat transfer from solar radiance. EC smart windows have been used in fenestration that blocks heat on demand, with or without optical transparency. This could significantly reduce energy consumption while provide indoor comfort in buildings. Numerous inorganic EC materials are reported in this chapter. Conventional EC thin films such as tungsten oxide prepared by sputtering process has been studied extensively over the past decades. However, it failed to incorporate in practical use due to insignificant performance in solar (light and heat) modulation and manufacturing cost. Recently, great advances in the development of such materials have been reported. For example, nano composite made from indium tin oxide (ITO) nanocrystal embedded in niobium oxide glass, significantly improved the EC performance with the ability to control infrared region and visible light individually. This chapter introduces the existing EC materials, the fundamental of EC devices operation and EC thin-film characterization. Smart windows are believed to be a green technology with huge potential energy saving in building sectors in the future.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
1 Introduction
Sustainable and renewable energies have gain increased attention as people are more aware toward environment protection. While finding alternative energy to replace fossil fuels may be the ultimate solution to keep carbon footprint at minimum, the existing solution available are yet to replace fossil fuel, which is far more cost efficient (Sims et al. 2003). But a different approach such as reducing energy consumption from our daily life can be taken instead to reduce emission of greenhouse gases. One way to achieve this is through implementation of energy-efficient technologies such as “smart windows”. Smart window is one of the applications of electrochromic (EC) devices and it stand out to be the most striking and potentially most important application. This is because building with smart windows capable of varying throughput of visible light and solar energy; avoid overheating while achieve good indoor lighting conditions; reduce energy consumption for air conditioning and lighting (Granqvist 1995; Gillaspie et al. 2010).
Statistical analysis shown that global population had bloom dramatically from just 2.5 billion in 1950 to about 7.9 billion in present year (2019). The number is forecasted to continue growth and level off at a magnitude of nearly eleven billion in around year 2100 (UNDESA 2019). Change in qualities of life is happening simultaneously with the explosion of population; countries that are under developing have progressed in improving overall living standards of their citizens by providing various amenities. One of dire the consequences is, however, putting strain on the limited resources globally especially on energy, which is derived mainly from unsustainable resources – fossil fuels with ensuing injection of carbon dioxide into air. More carbon dioxides are released into the atmosphere when more energies are demanded; the cycle continues as population growth, causing global warming, sea level rising, and other chain reactions (Houghton et al. 1990).
From the total energy production, fossil fuels remained the dominant energy source since industrial era, albeit they are environmentally intensive and contributing to global warming. This situation is not expected to change soon without proper policy interventions and technological improvements. About 30 to 40% of worldwide primary energy has been spent in buildings for heating, cooling, lighting, etc. (Huovila 2007). Huge potential for saving tremendous amount of energy in buildings sector, however, a detailed study conducted in USA states that a large fraction of the energy delivered to buildings is wasted because of inefficient building technologies (Richter et al. 2008). Energy savings must be made without reducing the standard of living, but by utilizing more efficient technologies to provide the same or better performance.
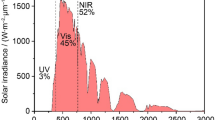
Windows are the weak links in the buildings’ energy system that frequently let in or let out too much energy, then temperature is balanced by energy guzzling of cooling or heating. Windows are very important in both architecture design and connection to outdoor. Without eliminating or making the window apertures smaller, the solution to this conundrum is implementing an energy efficient window. Clearly an energy efficient window must harmonize with nature and make good use of what nature offers in term of light and energy. To further elaborate this, sunlight is an electromagnetic radiation mainly composed of visible and infrared spectrum. An energy efficient “smart window” is capable of providing daylight to the rooms while blocking near-infrared radiation from heating (Kim et al. 2015).
Near-infrared (NIR) radiation is accounted for about half of the solar energy that is incident upon a windows but does not contribute to daylighting within a building (Runnerstrom et al. 2014). Smart window can manipulate the amount of transmission of visible and NIR transmission separately so that to save energy from lighting and air conditioning of the buildings. Motivated by the potential for significant energy savings from reduced loads of lighting, cooling and heating, many researches have been conducted in EC smart windows that dynamically control sunlight entering a building (Deb 2008; Mortimer 2011). The focus on improved energy efficiency and the development of advanced buildings drives the majority of current EC research.
In the modern society today, people are get used to the idea of building automation. Robot vacuum, dishwasher, washing machine and much more are already became part of our life. Traditionally, windows are fitted with curtains or blinds to shelter from sunlight (or heat) and provide privacy. The intuitive of energy-saving and automation lead to the development of smart window that can change from clear to dark and vice versa. To realize this, a phenomenon known as electrochromism , where the colour, transparency or other optical property of a material changes when voltage is applied, is integrated into the design of windows. In other words, electrochromism is the basis for operation of smart window that can block or unblock the sunlight with a push of a button.
Conventionally, EC smart windows are prepared by sputtering of transition metal oxide thin films as the active material (Mortimer 2011; Granqvist 2014). Unfortunately, conventional materials suffer significant drawbacks from material degradation associated with repeated ion intercalation, and adopt dark, distinct colours in their tinted states, simultaneously blocking both visible and NIR light (Granqvist 2014). For these reasons and also the cost of expenses, conventional electrochromic windows struggled to achieve widespread adoption (Gillaspie et al. 2010). A newly developed and fundamentally different class of EC device based on plasmonic electrochromism is designed to overcome those limitations and ultimately minimize the cost-performance ratio.
One of the unique features of plasmonic electrochromism that standout from conventional electrochromism is the ability to selectively control NIR absorption. Figure 9.1 shows the plasmonic EC nanocrystal smart window operates at different states; in ‘bright’ state, the smart window is transparent, allowing both NIR and visible rays to pass through freely; in ‘cool’ state, the smart window is transparent, allowing visible lights to pass through but blocking NIR rays, providing daylight to the room while keeping away from heat; in ‘dark’ state, the smart window is translucent that block both NIR and visible rays. Details of spectral selectivity will be further discussed in Sect. 9.4. Figure 9.2 below is the breakdown of various EC windows classes. EC performance is evaluated in terms of coloration efficiency, durability, and switching time, whereby performance of EC window depends on material structure and composition.
Schematic diagram of solar radiation passes through smart windows at different states (Kim et al. 2015)
2 Fundamentals of Electrochromic Device Operation and Preparation
Figure 9.3 presents a generic EC device coating with five superimposed layers – an active electrochromic electrode layer, an ion storage layer acts as counter electrode, an ion conductor layer separating the two electrodes, and two transparent conducting layers serve as electrical leads, on a single transparent substrate or positioned between 2 transparent substrates. This EC device structure is referred as thin film electrical battery whose charging state corresponds to a certain level of optical absorption and this is the most common geometry for EC devices (Niklasson and Granqvist 2007; Granqvist 2014). The working principles of EC devices are similar to electrochemical reaction in battery. Small ions such as protons (H+) or lithium ions (Li+) are often used as charge carrier in majority of EC devices because of good ion mobility.
Generic five-layer EC device design. Arrows indicate the movement of ions in an applied electric field (Granqvist 2014)
During off or bleached state, the cations are residing in the electrolyte and the ion storage layer. When the device is switched on, electric field is generated in between cathode and anode due to potential difference in which driven the cations to migrate from ion storage layer (anode), through the electrolyte, into the electrochromic layer (cathode). Ions insertion and extraction occur during the charge transfer contribute to the variability of the optical transmittance of EC film(s). This is because electrochromic materials are made up from transition metal oxides that consist of multiple oxidation states with different optical properties (Chernova et al. 2009; Wang et al. 2010). Transparent conductor layers play an important role here in conducting electricity through external circuit. Indium tin oxide (ITO) is well-known for its superior optical and electrical properties and is commonly used as transparent conductor.
In previous section, we mentioned that EC device performance is evaluated from material structure and composition which in turn determined by coating technique. Thanks to the emergence of nanotechnology, conventional inorganic EC compounds in nanostructure form opens up possibilities of enhanced properties, such as improved switching times, higher colouration efficiency, and improved stability against electrochemical cycling (Runnerstrom et al. 2014). There are numerous numbers of techniques to coat films of EC device. For example, traditional thin film preparation such as physical and chemical vapour deposition, a plethora of chemical methods (chemical bath deposition, sol-gel deposition, spin coating, Langmuir-Blodgett technique, etc.), electrochemical methods (plating, anodization) and others, and sometimes employing nanoparticles as intermediate steps as well as templating. Sputtering from physical vapour deposition is suitable for the EC films preparation because sputtering process is well understood in manufacturing. Hence, suggest that sputtering will likely be the most practical production method for smart windows in foreseeable future (Gillaspie et al. 2010). This topic mainly surrounds EC devices based on conventional materials and nanostructured of it.
On the other hand, EC device shown in Fig. 9.1 utilizes plasmonic nanomaterials which enable it to absorb NIR light at same time allow visible light to pass through. Plasmonic nanoparticles that support localized surface plasmon resonances (LSPR) have been in academic pursuit over the past decade for signal transduction and efficiency in both energy and space, as well as sensitive and specific, through miniaturization to the nanoscale (Pastoriza-Santos et al. 2018). Plasmon resonance is the phenomenon of collective oscillations of charge carriers in solids. The excess charge carriers (impurity holes or electrons) in semiconductor plasmonic nanocrystals allow themselves as intense absorption bands in the NIR region of the spectrum. In contrast to metal nanocrystal, the LSPR lies in the visible region of the spectrum and able to absorb light (Litvin et al. 2018). There are two general approaches for the preparation of nanoparticle: (1) colloidal chemistry and (2) epitaxial growth and/or nanoscale patterning through lithography-based technology (Drbohlavova et al. 2009).
2.1 Electrochromic Oxides
Perhaps EC films are the most important component inside an EC device. The unique ability to change colour and thus the transmittivity, enable the applications of EC device such as smart windows. All elements that show electrochromism with their respective oxides are transition metals. In principal, EC oxides can be categorised into two kinds, one that referred as “cathodic” that colouring under ion insertion, and the other referred as “anodic” that colouring under ion extraction. Figure 9.4 indicates the location of transition metals capable of forming oxides of these two particular categories, and interestingly, vanadium oxides are classified as intermediate or “hybrid” which are class of its own (Granqvist 1995). From Fig. 9.4, one can clearly distinguish oxides with cathodic or anodic colouration in transition metal region because electrochromism is closely related to the electronic structure of the oxides. Electrochromism can also be found in binary and ternary mixed oxide and in oxyfluorides. Also, transition metal hexacyanometallates are non-oxide with anodic electrochromism.
Cathodic colouration is found in oxides of Ti, Nb, Mo, Ta, and W, with tungsten oxide (WO3) being by far the most extensively studied one (Granqvist 2000). Take tungsten oxide (WO3) as example, upon ion injection, WO3 changes colour from a clear, transparent state to a dark blue, translucent state as tungsten ions are reduced (Granqvist 1995). Protons or cations in the electrolyte compensate the injected electrons through insertion into WO3 octahedral sites, as shown in Eq. (9.1). The injected electrons occupy the previously empty d band, giving rise to new electronic transitions. In crystalline WO3, the added electrons occupy delocalized states, whereas in amorphous WO3 electrons are localized at metal sites, resulting in polaronic absorption that can be described as an electronic charge-transfer transition between adjacent metal sites with different valency (Runnerstrom et al. 2014).
Anodic colouration is found in oxides of Cr, Mn, Fe, Co, Ni, Rh, and Ir, with nickel oxide and iridium oxide being the ones investigated in most detail (Granqvist 1995). Anodic colouration mechanism is in reverse manner compared to cathodic colouration. Take nickel oxide (NiO) as example, the electrochromic reaction can proceed via the exchange of H+ and OH− depending on the nature of the films as well on the electrolyte (Wen et al. 2014). The situation become more complicated when the films evolve during electrochemical cycling (Estrada et al. 1988; Ren et al. 2013). The electrochromic effect in NiO involves several phases and is pointed at the general applicability of the Bode reaction scheme (Wen et al. 2014); specifically the reactions in alkali electrolyte associate with Ni(OH)2 and NiOOH as shown in Eq. (9.2) in a simplified manner (Avendaño et al. 2005; Avendaño et al. 2009).
Both cathodic oxide and anodic oxide are usually employed in a typical smart window with two respective EC films; one can adjust the optical transmittance and achieve better colour neutrality than with one single EC film (Granqvist 2014). Take combination of tungsten oxide and nickel oxide as illustrated in Eqs. (9.1) and (9.2) for example, applying voltage in one direction between these two EC films will transport the ions from one to another makes both of these films coloured, while reversing the potential polarity makes both of the films bleached by return to their original properties. When there is no move of charges, the EC device shows memory effect by retaining the current state (coloured or bleached respectively). The terminology “rocking chair” has been used describe operation in smart windows as well as rechargeable battery applications (Goldner et al. 1993).
Furthermore, performance of each individual EC materials and devices is merited by colouration efficiency (CE) given in Eq. (9.3) (Gillaspie et al. 2010; Thakur et al. 2012), where Q is the electronic charge injected into the EC material per unit area, Tbleached is the transmittance in the bleached state, and Tcoloured is the transmittance in the coloured state. The CE therefore gives the change in optical density (OD) achieved by the injection of unit charge over unit area. In general, materials with higher CEs will have better durability and faster switching times, since less charge is required to produce a given optical response (Gillaspie et al. 2010).
2.2 Ion Conductor
At the central of the EC device resides the ion conductor layer which is the key component for ions transportation between cathode and anode during EC reaction. To ensure proper operation of the device, the ion conductivity of electrolyte should be larger than 10−4 S/cm (Granqvist 1995). Other requirement of electrolyte is high thermal and UV stability on top of not cause any degradations toward EC layers (Marszalek et al. 2012). Ion conductor can be generalized into two – liquid and solid electrolyte. Figure 9.5 shows the schematic diagram EC devices with different type of electrolytes.
The electrochromic device with (a) liquid and (b) solid electrolyte (Marszalek et al. 2012)
Transparent liquid electrolytes and ion-containing thin oxide films were used in early studies on EC and later the interest has switched toward polymer (solid) electrolyte mainly following the development of electrical battery technology (Granqvist 2014). Undoubtedly, the main advantages of liquid electrolyte are easy to synthesize, high ion conductivity (response time) and good stability (cycle life) (Girotto and Paoli 1999), which make it the popular candidate over the years when researching EC device. Regardless, efforts in sealing to prevent the potential of leakage and evaporation are the biggest flaws of liquid electrolyte as ion conductor in application like smart window. But the problems can be overcome by opting solid electrolyte, and it opens up the doors to all plastic and flexible EC devices too. Ionic charge transports as well as the diffusing species that enter the EC layers via electrolyte are the only major drawback in comparison to liquid electrolyte.
2.3 Transparent Conductive Oxide
Transparent conductive oxides (TCO) are semiconductors such as tin doped indium oxide, In2O3: Sn (ITO), fluorine doped tin oxide, SnO2: F (FTO) and antimony doped tin oxide, SnO2: Sb (ATO). They are known with high optical transparency in the visible region, good electrical conductivity, and high infrared reflectivity (Chuang 2010). These characteristics are found particularly useful as transparent thin-film conductors in applications like flat panel displays and solar cells, transparent heaters, heat mirrors, optoelectronic devices, and smart windows.
TCO materials suitable for transparent conductor thin films should have a carrier concentration on the order of 1020–1021 cm−3 and band-gap energy above approximately 3 eV, that is, degenerated n-type or p-type semiconductors. Furthermore, TCO thin films that are in practical use as transparent conductors are polycrystalline or amorphous and exhibit a resistivity on the order of 10−3 Ω cm or less and an average transmittance above 80% in the visible range (Minami 2013). ITO is well-known in excellent optical and electrical properties. It is a highly degenerated n-type and wide band gap (e.g., 3.5–4.3 eV) semiconductor (Kim et al. 1999). Due to its relatively low electrical resistivity and high visible transmittance, this material has been used extensively as transparent conductors. ITO thin films can be obtained readily through various growth techniques including sputtering, evaporation, chemical vapor deposition, and sol-gel (Chuang 2010).
TCO thin films on glass substrate are located at outermost layer of smart windows as shown in Fig. 9.3. The excellent electrical and optical properties of TCO allow conduction of electricity between the transparent conductors without hinder transparency of a window. When voltage is applied to the transparent conductors, ions are transferred between the ion storage film and the EC film through the ion conductor layer at the centre. Depend on the voltage polarity, the electrons are then extracted from or injected into the transparent conductors that alter the optical absorption of the EC films. The original state can be easily restored by reversing the voltage .
3 Characterization of Electrochromic Materials
There are many different techniques to choose to deposit EC metal oxide thin films. EC thin films prepared by DC magnetron sputtering are specifically chosen in this work to elaborate various characterizations of EC materials. As mentioned earlier, sputtering is well understood in manufacturing. On top of that, sputtering has good reproducibility, good substrate adherence and the possibility of depositing many different types of materials with a variety of different compositions, layers, structures and stoichiometry. The general idea of sputtering is that a target (source) of certain raw material is bombarded by accelerating ions (typically Ar+) impart a high kinetic energy to the expelled source atoms. These atoms transit through the discharge and condense onto the substrate, thus providing the film growth. Then, a series of analyses on thickness, morphology, optic, etc. to identify the microstructure and composition of deposited thin films.
3.1 Physical Characterization
3.1.1 Film Thickness by Profilometry
Deposited EC thin films from sputtering are usually measured by contact surface profilometry. The surface profilometer measures the thickness by moving a diamond tip over the step between uncoated and coated sample areas. Multiple measurements from different positions along the step are always taken and averaged.
3.1.2 Crystal Structure by X-Ray Diffraction
In a crystalline structure the atoms form regularly repeating atomic planes separated by a certain distance, d, which varies with the material. If d is shorter than the X-ray wavelength λ, the X-rays are diffracted by the uniform atomic planes and an interference pattern is seen, as described by Bragg’s law,
Where m is an integer and θ is the diffraction angle, i.e. the angle between the incident X-ray beam and the atomic plane. A spectrum with constructive inferences is obtained from scanning the diffracted beam. The peak distribution is directly related to the atomic distances. Hence, information about structure, phase composition and orientation of planes can be obtained by comparing the spectra with the database from JCDPS-International Centre for Diffraction Data (ICDD).
Non-crystalline materials with no long-range order, i.e. amorphous materials, do not show any diffraction peaks and cannot be examined by XRD. Polycrystalline materials are composed of many different crystalline grains of varying size and orientation. The size of the grains determines whether they will be detected. Crystalline samples show very distinct narrow peaks whereas the peaks from polycrystalline samples are broader. The width of the peak depends on the size of the grains, and the grain size, D, can be calculated by Scherrer’s formula (Cullity and Stock 2001):
Where k is the shape factor, λ is the wavelength of X-ray, β is the full width at half maximum of the X-ray diffraction peak, and θ is the diffraction angle. Figure 9.6 shows an example of an XRD spectrum for polycrystalline NiO measured from Siemens D5000 diffractometer.
3.1.3 Surface and Cross-Sectional Morphology by Scanning Electron Microscopy
The scanning electron microscope (SEM) which uses electrons that are reflected or knocked off the near-surface region of a sample to create an image. Various signals are giving off when the electrons interact with the atoms on the surface of sample that contain information about the surface topography and composition of the sample. Figure 9.7 shows SEM image of a ~ 500 nm thick NiO film deposited on ITO-coated glass substrate. One can deduce that the film featured triangular morphology with linear extents of ≤40 nm as shown in panel (a) and (b). The cross-sectional image in panel (c) indicates a columnar nanostructure of the deposited NiO film.
SEM images of NiO thin film. (a) and (b) are top view at different magnifications, and (c) cross-sectional view of the film on an ITO-coated glass substrate (Wen et al. 2014)
3.2 Electrochemical and Optical Characterization
Cyclic voltammetry (CV) test and optical transmittance measurement are usually conducted together to evaluate the CE in Eq. (9.3). Figure 9.8 displays an example of cyclic voltammograms of NiO-based films in KOH electrolyte for the first 15 cycles. Broad oxidation and reduction features are observed, which deduce that some minor evolution takes place during the initial cycles, but the properties are stabilized after a few cycles. Figure 9.9 presents in situ optical transmittance during the CV test as obtained in Fig. 9.8. Information obtained from these two graphical data enable the calculation of CE for this NiO films. The CE was evaluated and found to be 44±2 cm2/C at wavelength of 550 nm (Wen et al. 2014).
Cyclic voltammograms for a ~ 500 nm thick NiO film in 1 M KOH; the voltage sweep rate was 10 mV/s and arrows indicate sweep direction (Wen et al. 2014)
(a) Spectra transmittance of a ~ 500 nm thick NiO film in 1 M KOH. Inserted images refer to NiO in fully bleached and coloured states respectively, and (b) corresponding optical transmittance modulation at wavelength of 550 nm (Wen et al. 2014)
4 Spectral Selective Nano Structured Thin Films
Plasmonic electrochromism is the current approach to isolate visible and infrared radiations from solar spectrum via modulated LSPR. Research team led by Milliron is spearheading in this field with notable publications (Garcia et al. 2013; Llordés et al. 2013; Runnerstrom et al. 2014). Figure 9.10 schematically illustrates this concept with two types of nanostructure designs of the EC thin films. As shown in Fig. 9.10 a, the nanocomposite electrolyte consists of polymer hosts and transparent conducting nanoparticles such as ITO, aluminium-doped zin oxide (AZO), and lanthanum hexaboride (LaB6) (Bayrak Pehlivan et al. 2012). Electrochemical changes under different electrical potential, which alter the LSPR absorption level and in turn the electron density. This alteration enables modulation of solar infrared absorption with minor effects on the visible region. In Fig. 9.10 b, development of the electrode based on deposition of ITO nanocrystals on niobium oxide (NbOx) glass presents another approach (Llordés et al. 2013; Kim et al. 2015). Both ITO nanocrystals and NbOx glass matrix exhibit distinctly different spectral EC responses, therefore each may independently yield modulation in the NIR (ITO nanocrystals) and visible region (NbOx) (Llordés et al. 2013).
(a) Structure of nanocomposite electrolyte with nanoparticles, and (b) ITO nanocrystal-NbOx film (deposited ion a conducting glass substrate) as the working electrode in an EC structure (J. (Jialiang) Wang and Shi 2017)
5 Other Implementation of Electrochromic Thin Films
In this work, we mainly talked about electrochromic oxides incorporate in smart windows and their sole purpose in filtering lights. However, researchers have come up with creative ideas by integrating electrochromism with other usability, such as energy storage (Yang et al. 2016). Moreover, the demand of bezel-less phone screen has pushed the smartphone manufacturers in developing new technology to hide the front cameras and sensors beneath the display, without compromising the display quality nor the camera capability (Byford 2019; Gartenberg 2019). Figure 9.11 showcase the working principle of under-display camera technology by Xiaomi. Judging from the illustration, we can conclude that it is a variant of EC devices because of the cathode and anode layers. The cameras and sensors beneath the display stay hidden until the user activates the front-facing camera as shown in the demo (Porter 2019).
(a) Normal display when front camera is inactive, and (b) Transparent display when front camera is active from “transparent display-in-a-display” for under-display camera technology by Xiaomi Corp (Gartenberg 2019)
Nonetheless, it is important to note that other than electrochromism , alternative materials that exhibit photochromism or thermochromism can also be used to fabricate smart windows. For example, perovskites are materials made of mix elements with a particular crystalline structure and they can be made into solar cells. By changing the perovskites’ elemental components, their optical properties such as transparency can be altered. Researchers have successfully created modified perovskite solar window that combine all these features (Wheeler et al. 2017; Lin et al. 2018). For example, a lead-based perovskite solar window that switched from transparent to opaque when the temperature hit 60 °C while converting solar energy to electricity at the same time (Wheeler et al. 2017).
6 Conclusion
Electrochromism is one of the “green” nanotechnologies which are of large current interest (Smith and Granqvist 2011). The potential of saving energy from building sector by replacing conventional glass windows with EC smart windows is unmeasurable. This is the greatest importance for combating global warming as discussed in the beginning of this chapter. Smart windows also increased the aesthetics and comforts of modern buildings. Although tremendous research works have put into smart windows, the applicability is still far from large scale implementation. The major impediments for the installation of large-area EC windows remain fabrication cost and extended lifetime. One purposed solution is development of flexible EC devices that offer “retrofit installation” on existing glass windows to reduce overall cost. Finally, further researches on this field are necessary to realize this technology into every building and bring the concept of energy-efficient smart windows closer.
References
Avendaño E, Azens A, Niklasson GA, Granqvist CG (2005) Proton diffusion and electrochromism in hydrated NiO y and Ni1−x V x O y thin films. J Electrochem Soc 152(12):F203. https://doi.org/10.1149/1.2077308
Avendaño E, Rensmo H, Azens A, Sandell A, G. de M. Azevedo, Siegbahn H, Niklasson GA, Granqvist CG (2009) Coloration mechanism in proton-intercalated electrochromic hydrated NiO y and Ni1−x V x O y thin films. J Electrochem Soc 156(8):P132. https://doi.org/10.1149/1.3148327
Bayrak Pehlivan I, Runnerstrom EL, Li SY, Niklasson GA, Milliron DJ, Granqvist CG (2012) A polymer electrolyte with high luminous transmittance and low solar throughput: Polyethyleneimine-Lithium Bis(Trifluoromethylsulfonyl) imide with in 2O 3:Sn Nanocrystals. Appl Phys Lett 100(24):1–5. https://doi.org/10.1063/1.4728994
Byford S (2019) Oppo Unveils the World’s First under-Screen Selfie Camera - The Verge. https://www.theverge.com/2019/6/26/18759380/under-display-selfie-camera-first-oppo-announcement
Chernova NA, Roppolo M, Dillon AC, Stanley Whittingham M (2009) Layered vanadium and molybdenum oxides: batteries and electrochromics. J Mater Chem 19(17):2526. https://doi.org/10.1039/b819629j
Chuang M (2010) ITO films prepared by long-throw magnetron sputtering without oxygen partial pressure. J Mater Sci Technol 26(7):577–583. https://doi.org/10.1016/S1005-0302(10)60088-6
Cullity BD, Stock SR (2001) Elements of X-ray diffraction, 3rd edn. Prentice Hall, Upper Saddle River NJ. https://www.worldcat.org/title/elements-of-x-ray-diffraction/oclc/46437243
Deb SK (2008) Opportunities and challenges in science and technology of WO3 for electrochromic and related applications. Sol Energy Mater Sol Cells 92(2):245–258. https://doi.org/10.1016/J.SOLMAT.2007.01.026
Drbohlavova J, Adam V, Kizek R, Hubalek J (2009) Quantum dots - characterization, preparation and usage in biological systems. Int J Mol Sci 10(2):656–673. https://doi.org/10.3390/ijms10020656
Estrada W, Andersson AM, Granqvist CG (1988) Electrochromic nickel-oxide-based coatings made by reactive dc magnetron sputtering: preparation and optical properties. J Appl Phys 64(7):3678–3683. https://doi.org/10.1063/1.341410
Garcia G, Buonsanti R, Llordes A, Runnerstrom EL, Bergerud A, Milliron DJ (2013) Near-infrared spectrally selective plasmonic electrochromic thin films. Adv Opt Mater 1(3):215–220. https://doi.org/10.1002/adom.201200051
Gartenberg C (2019) Xiaomi Explains More about How Its Under-Screen Camera Actually Works - The Verge. https://www.theverge.com/circuitbreaker/2019/6/5/18654365/xiaomi-camera-under-screen-no-notch-transparent-display-technology
Gillaspie DT, Tenent RC, Dillon AC (2010) Metal-oxide films for electrochromic applications: present technology and future directions. J Mater Chem 20(43):9585–9592. https://doi.org/10.1039/c0jm00604a
Girotto EM, Marco-A. Paoli (1999) Flexible electrochromic windows: a comparison using liquid and solid electrolytes. J Braz Chem Soc 10(5):394–400. https://doi.org/10.1590/S0103-50531999000500010
Goldner RB, Haas TE, Arntz FO, Slaven S, Wong KK, Wilkens B, Shepard C, Lanford W (1993) Nuclear reaction analysis profiling as direct evidence for Lithium ion mass transport in thin film “‘rocking-chair’” structures. Appl Phys Lett 62(14):1699–1701. https://doi.org/10.1063/1.109580
Granqvist CG (1995) Handbook of inorganic electrochromic materials. Elsevier. https://doi.org/10.1016/B978-0-444-89930-9.X5000-4
Granqvist CG (2000) Electrochromic tungsten oxide films: review of progress 1993–1998. Sol Energy Mater Sol Cells 60(3):201–262. https://doi.org/10.1016/S0927-0248(99)00088-4
Granqvist CG (2014) Electrochromics for smart windows: oxide-based thin films and devices. Thin Solid Films 564:1–38. https://doi.org/10.1016/j.tsf.2014.02.002
Houghton JT, Jenkins GJ, Ephraums JJ (1990) Climate Change The IPCC Scientific Assessment. http://repositorio.cenpat-conicet.gob.ar:8081/xmlui/bitstream/handle/123456789/497/climateChange.pdf?sequence=1
Huovila P (2007) Management of Environmental Quality: An International Journal Buildings and Climate Change: Status, Challenges and Opportunities. United Nations Environment Programme. https://books.google.co.kr/books/about/Buildings_and_Climate_Change.html?id=-lgab8igWgcC&printsec=frontcover&source=kp_read_button&redir_esc=y#v=onepage&q&f=false
Kim H, Gilmore CM, Piqué A, Horwitz JS, Mattoussi H, Murata H, Kafafi ZH, Chrisey DB (1999) Electrical, optical, and structural properties of indium–tin–oxide thin films for organic light-emitting devices. J Appl Phys 86(11):6451–6461. https://doi.org/10.1063/1.371708
Kim J, Ong GK, Yang W, Leblanc G, Williams TE, Mattox TM, Helms BA, Milliron DJ (2015) Nanocomposite architecture for rapid, spectrally-selective electrochromic modulation of solar transmittance. Nano Lett 15(8):5574–5579. https://doi.org/10.1021/acs.nanolett.5b02197
Lin J, Lai M, Dou L, Kley CS, Chen H, Peng F, Sun J, Lu D, Hawks SA, Xie C, Fan C, Paul Alivisatos A, Limmer DT, Yang P (2018) Thermochromic halide perovskite solar cells. Nat Mater 17(3):261–267. https://doi.org/10.1038/s41563-017-0006-0
Litvin AP, Cherevkov SA, Dubavik A, Babaev AA, Parfenov PS, Simões Gamboa AL, Fedorov AV, Baranov AV (2018) Thin layer of semiconductor plasmonic nanocrystals for the enhancement of NIR fluorophores. J Phys Chem C 122(35):20469–20475. https://doi.org/10.1021/acs.jpcc.8b06059
Llordés A, Garcia G, Gazquez J, Milliron DJ (2013) Tunable near-infrared and visible-light transmittance in nanocrystal-in-glass composites. Nature 500(7462):323–326. https://doi.org/10.1038/nature12398
Marszalek KW, Swatowska B, Sobkow Z (2012) Ionic Conductor for Electrochromic Devices. (January). https://doi.org/10.13140/2.1.3705.2488
Minami T (2013) 88 semiconductors and semimetals transparent conductive oxides for transparent electrode applications, 1st edn. Elsevier Inc. https://doi.org/10.1016/B978-0-12-396489-2.00005-9
Mortimer RJ (2011) Electrochromic materials. Annu Rev Mater Res 41(1):241–268. https://doi.org/10.1146/annurev-matsci-062910-100344
Niklasson GA, Granqvist CG (2007) Electrochromics for smart windows: thin films of tungsten oxide and nickel oxide, and devices based on these. J Mater Chem 17(2):127–156. https://doi.org/10.1039/b612174h
Pastoriza-Santos I, Kinnear C, Pérez-Juste J, Mulvaney P, Liz-Marzán LM (2018) Plasmonic polymer nanocomposites. Nat Rev Mater 3(10):375–391. https://doi.org/10.1038/s41578-018-0050-7
Porter J (2019) Xiaomi Hits Back at Oppo with an “under-Display” Camera of Its Own - The Verge. https://www.theverge.com/2019/6/3/18650166/xiaomi-under-display-selfie-camera-weibo-teaser-mi-9-notch-hole-punch
Ren Y, Chim WK, Guo L, Tanoto H, Pan J, Chiam SY (2013) The coloration and degradation mechanisms of electrochromic nickel oxide. Sol Energy Mater Sol Cells 116:83–88. https://doi.org/10.1016/J.SOLMAT.2013.03.042
Richter B, Goldston D, Crabtree G, Glicksman L, Goldstein D, Greene D, Kammen D, Levine M, Lubell M, Savitz M, Sperling D, Schlachter F, Scofield J, Dawson J (2008) How America can look within to achieve energy security and reduce global warming. Rev Mod Phys 80(4). S1–109. https://doi.org/10.1103/RevModPhys.80.S1
Runnerstrom EL, Llordés A, Lounis SD, Milliron DJ (2014) Nanostructured Electrochromic smart windows: traditional materials and NIR-selective plasmonic nanocrystals. Chem Commun 50(73):10555–10572. https://doi.org/10.1039/c4cc03109a
Sims REH, Rogner HH, Gregory K (2003) Carbon emission and mitigation cost comparisons between fossil fuel, nuclear and renewable energy resources for electricity generation. Energy Policy 31(13):1315–1326. https://doi.org/10.1016/S0301-4215(02)00192-1
Smith GB, Granqvist CG (2011) Green nanotechnology : solutions for sustainability and energy in the built environment. CRC Press. https://www.crcpress.com/Green-Nanotechnology-Solutions-for-Sustainability-and-Energy-in-the-Built/Smith-Granqvist/p/book/9781420085327
Thakur VK, Ding G, Ma J, Lee PS, Lu X (2012) Hybrid materials and polymer electrolytes for electrochromic device applications. Adv Mater 24(30):4071–4096. https://doi.org/10.1002/adma.201200213
UNDESA (2019) World Population Prospects: Population Division Database. https://population.un.org/wpp/Graphs/Probabilistic/POP/TOT/900
Wang JM, Sun XW, Jiao Z (2010) Application of nanostructures in electrochromic materials and devices: recent Progress. Materials 3(12):5029–5053. https://doi.org/10.3390/ma3125029
Wang, Julian (Jialiang), and Donglu Shi (2017) Spectral selective and Photothermal Nano structured thin films for energy efficient windows. Appl Energy 208(August): 83–96. doi:https://doi.org/10.1016/j.apenergy.2017.10.066
Wen RT, Niklasson GA, Granqvist CG (2014) Electrochromic nickel oxide films and their compatibility with potassium hydroxide and Lithium perchlorate in propylene carbonate: optical, electrochemical and stress-related properties. Thin Solid Films 565:128–135. https://doi.org/10.1016/j.tsf.2014.07.004
Wheeler LM, Moore DT, Ihly R, Stanton NJ, Miller EM, Tenent RC, Blackburn JL, Neale NR (2017) Switchable photovoltaic windows enabled by reversible photothermal complex dissociation from methylammonium lead iodide. Nat Commun 8(1):1722. https://doi.org/10.1038/s41467-017-01842-4
Yang P, Sun P, Mai W (2016) Electrochromic energy storage devices. Mater Today 19(7):394–402. https://doi.org/10.1016/J.MATTOD.2015.11.007
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Tan, X.Y., Wang, H., Kim, T.G. (2020). Electrochromic Smart Windows: An Energy-Efficient Technology. In: Siddiquee, S., Gan Jet Hong, M., Mizanur Rahman, M. (eds) Composite Materials: Applications in Engineering, Biomedicine and Food Science. Springer, Cham. https://doi.org/10.1007/978-3-030-45489-0_9
Download citation
DOI: https://doi.org/10.1007/978-3-030-45489-0_9
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-45488-3
Online ISBN: 978-3-030-45489-0
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)