Abstract
Ion-selective electrodes (ISEs) are a subclass of electrochemical sensor that are widely applied in several fields of clinical and environmental use, physiological sensing, and process control. ISEs are classified into three types, depending on the nature of the membrane material: glass, polymer or liquid, and crystal or solid. All-solid-state ion-selective electrodes (AS-ISEs) have a solid-based selective membrane; AS-ISEs convert the activity of a specific ion to a measurable electrical signal that can be measured without the internal filling solution required by conventional ISEs. The main components of AS-ISEs are a conductive electrode, a transducer or solid contact, and an ion-selective membrane (ISM). The ISM is involved in the recognition and selection of the targeted ion; it consists of a polymeric matrix, an ionophore, a plasticizer, and ionic additives. The analytical performance of AS-ISEs is influenced by the choice of and amount of materials in the main components. This chapter will introduce the main components and the materials used for the fabrication of AS-ISEs.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
An electrochemical sensor is a qualitative and quantitative device that converts a chemical signal to a measurable electrical signal (Yogeswaran and Shen-Ming 2008). Electrochemical sensors can be divided into three classes: potentiometric, amperometric, and conductometric (Stradiotto et al. 2003). A potentiometric sensor measures an electrical potential when no current is present, while an amperometric sensor produces current when a potential is applied between two electrodes. A conductometric sensor assesses conductivity by measuring the electrical resistance of a sample solution. Ion-selective electrodes (ISEs) are potentiometric ion sensors and a subgroup of electrochemical sensors; they are widely used in various fields of biomedical, environmental, and chemical analysis, and physiological sensing (Bobacka et al. 2003; Bakker et al. 2008; Hu et al. 2016). ISEs are classified into three groups, depending on the nature of the membrane material: glass, polymeric or liquid, and crystal or solid (Fig. 16.1) (Faridbod et al. 2007).
All-solid-state ion-selective electrodes (AS-ISEs) are solid-based selective membrane electrodes that convert the activity of a specific ion to a measurable electrical signal without an internal filling solution – a liquid electrolyte that separates the sensing membrane from the inner reference electrode (Bobacka 2006; Bratovčić et al. 2009; Faridbod et al. 2008). Measuring voltage potential requires two electrodes: a reference electrode and a sensing electrode. The reference electrode potential is constant, and the sensing-electrode potential varies with the concentration of the target ions (Bratovčić et al. 2009). The potential being measured is equal to the difference in potential at the reference electrode and the sensing electrodes.
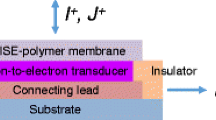
Key components of AS-ISEs are an ion-selective membrane (ISM) and a transducer or solid contact deposited on a conductive electrode made from carbon, platinum, or gold (Fig. 16.2) (Faridbod et al. 2008; Hu et al. 2016).The role of the ISM is to recognize and select the target ion, while the transducer converts the target-ion concentration to an electrical signal that can be measured against a reference electrode. A silver/silver chloride (Ag/AgCl, Cl−) electrode is usually used as the reference electrode in ISEs owing to its environmental compatibility, biocompatibility, stable potential, and redox capability (Michalska 2012). A saturated calomel (Hg/Hg2Cl2, Cl−) reference electrode has the advantage of stable potential and is not influenced by light, but is applicable only up to temperatures of about 80 °C. The aforementioned electrodes are currently commercially available; two others, the thallium chloride (Tl (Hg)/TlCl, Cl−) and the Thalamid™ electrodes are no longer used as reference electrodes owing to toxicity (Guth et al. 2009).
Polymers have been utilized as homogeneous membrane matrices in ISMs owing to good elasticity and mechanical stability (Faridbod et al. 2008). Conductive polymers are often applied as ion-to-electron transducers because of the ohmic properties integrated to a polymer that is flexible and biocompatible (Bobacka et al. 2008). Furthermore, carbon-based materials like carbon nanotubes (CNTs), graphene, and graphene derivatives (e.g., graphene oxide and reduced graphene oxide) have been used as transducers in AS-ISEs as their physical, electrical, mechanical, optical, thermal, and chemical properties make them suitable for such use (Yan et al. 2016).
This chapter will introduce readers to the materials in ion-selective membranes and all-solid-state transducers for AS-ISEs. The components of ISMs include a polymeric matrix, ionophores, plasticizers, and ionic additives, while commonly used all-solid-state transducers are conductive polymers of polypyrrole (PPy), polyaniline (PANI), and polythiophene (PT), or carbon-based materials such as carbon nanotubes and graphene.
2 Components of an Ion-Selective Membrane
An ion-selective membrane (ISM) cocktail consists of four components: a polymeric matrix, an ionophore as a recognition material, a membrane solvent or plasticizer, and ionic additives. The amount of each component can influence the physical and chemical characteristics of an ISM. Typical composition of an ISM is approximately 33% (w/w) polymeric matrix, 66% (w/w) plasticizer, 1% (w/w) ionophore, and 0.5% (w/w) ionic additives; each component of the ISM depends on the target ion (Faridbod et al. 2008).
2.1 Polymeric Matrix Materials
Polymers function as homogeneous membrane matrices for ISMs where their glass transition temperature (Tg) value should be below room temperature (Jadhav et al. 2009). The polymer membrane should be fluid enough under ambient conditions to allow for diffusion of other ISM components. Besides Tg, ISM biocompatibility is especially important for clinical applications; polyvinyl chloride (PVC) is the most commonly used polymer in ISM preparation (Fig. 16.3). Furthermore, acrylate and acrylate derivatives as well as polystyrene have been approved as polymeric matrices for biomedical applications (Faridbod et al. 2008).
2.2 Ionophores as Membrane–Active Recognition Components for Ion Carrier and Selectivity
Ionophores provide selectivity towards a target ion owing to their chemical and structural properties. The chemical property involves chemical bond strength and types of molecular interaction, while the structural property includes recognition of size and physical shape of molecular analytes. These specific characteristics influence ISEs selectivity in recognizing the target ions (Ganjali et al. 2006).
Studies have been conducted to understand how the chemical properties of ionophores lead to highly selective ISEs. Wilson et al. (2010) discovered that thioureas – compounds with N-C(S)-N functionality – will significantly improve the selectivity of ISEs towards different metal ions. The presence of intra-molecular interactions, the particular effects by conformational isomerism, and the existence of S and N atoms which act as donor sites make the thiourea derivatives as ligands more versatile (Wilson et al. 2010). Furthermore, the existence of a carbonyl group bonded to the thiourea will result in a heterocyclic compound that provides strong bonding potential as a ligand. The carbonyl group can interact with heavy metal ions such as Cu(II), Ni(II), and Hg(II) (Saeed et al. 2014). Therefore, thioureas have proved to be attractive ionophores for selective detection of heavy metal ions (Jumal et al. 2012; Ying et al. 2018).
In addition to chemical properties, the structure of an ionophore plays an important role in selectivity towards target ions. The internal cavity of an ionophore whose inner diameter is equivalent to the diameter of the target ions – but not to that of other interfering ions – makes ionophores very selective for ions of interest. In fact, the ionophore is one of the most vital components of the membrane because it is responsible for making the ISEs selective (Pięk et al. 2018).
2.3 Plasticizers as Membrane Solvent
A plasticizer is an additive in which the components of the ISM are dissolved; the plasticizer increases the plasticity and fluidity of the material to which it is added. In order to ensure high mobility of the ISM components, a 2:1 weight ratio of plasticizer to polymer is required; the ideal composition of ISMs is 60–66% (w/w) plasticizer and 30–33% polymer matrix. Tables 16.1 and 16.2 summarize the potassium-ion (K+) and calcium-ion (Ca2+) composition of an ISM, respectively. As can be seen from the tables, both K+ and Ca2+ ISMs have the same components with only a difference in the ionophores. For instance, valinomycin acts as the K+ ionophore (He et al. 2016; Vanamo and Bobacka 2014; Zhang and Zhang 2013), whereas ETH 129 (Zhao et al. 2019; Park et al. 2017; Ping et al. 2012), and ETH 5234 (ul Haque et al. 2007) act as Ca2+ ionophores. The rest of the ISM composition consists of ionic additives, plasticizers, polymer matrix, and solvent. Previous studies have used potassium tetrakis (4-chlorophenyl) borate (KTCPB) (Park et al. 2017; ul Haque et al. 2007; Shiwaku et al. 2018; Guzinski et al. 2017; Vázquez et al. 2002), potassium tetrakis[(3,5-bis[trifluoro methyl]phenyl) borate (KTFPB) (Vanamo and Bobacka 2014; Zhao et al. 2019; Ping et al. 2012), or sodium tetrakis(3,5bis[trifluoromethyl]phenyl) borate (NaTFPB) (Liu et al. 2019) as the ionic additives. The plasticizers are a membrane solvent such as bis(2-ethylhexyl) sebacate (DOS) (He et al. 2016; Guzinski et al. 2017), 2-nitrophenyl octyl ether (2-NPOE) (Ping et al. 2012; ul Haque et al. 2007; Liu et al. 2019), or bis(1-butylpentyl) adipate (BBPA) (Vázquez et al. 2002). Polyvinylchloride (PVC) has been used as the polymer matrix of the membrane (Vanamo and Bobacka 2014; Zhang and Zhang 2013; Liu et al. 2019). Looking into the weight ratio of plasticizer to polymer for K+-ISMs, Shiwaku et al. used 64.7% DOS plasticizer and 32.7% PVC (Shiwaku et al. 2018), while Vanamo and Bobacka used 62.5% and 33.3% for DOS plasticizer and PVC (Vanamo and Bobacka 2014), respectively. For Ca2+-ISMs, Zhao et al. used 65.6% 2-NPOE plasticizer and 32.8% PVC (Zhao et al. 2019), while Park et al. used 63.5% 2-NPOE plasticizer and 31.7% PVC (Park et al. 2017); both ISM cocktails follow similar ratio of plasticizer to polymer, mainly to ensure high mobility of ISM components.
2.4 Ionic Additives as Lipophilic Salts
Ionic additives, also called as lipophilic salts, provide two advantages for the ISMs: increase selectivity towards the target ion and reduce membrane ionic resistance (Morf et al. 2005). With respect to selectivity for divalent cations, a lipophilic anion improves preference for divalent over interfering monovalent cations, and at the same time reduces interference from anionic molecules (Eugster et al. 1991). Therefore, a lipophilic anion will ultimately increase the overall selectivity of ISEs for target divalent cations. Furthermore, the inclusion of ionic additives can act as an ion exchanger, which can improve selectivity when insufficient ionophore is present (Faridbod et al. 2008).
Another advantage of ionic additives is the lowering of ISM ionic resistance, easing the movement of target ions from sample solution into the membrane. Therefore, ionic additives enhance the interfacial ion-exchange kinetics and ultimately reduce the response time of ISEs (Gehrig et al. 1990). Overall, the four components of ISMs complement each other in terms of selectivity, response time, ion-exchange kinetics, fluidity and plasticity of the ISMs. The next section will discuss another important component of an ISE, the transducer.
3 Transducer Materials for AS-ISEs
Conductive polymers and nanomaterials as ion-to-electron transducers improve the performance of AS-ISEs in terms of detection limit, sensitivity, selectivity, and chemical stability (Bobacka 2006); these materials enable the miniaturization of ISEs (Hu et al. 2016). Commonly used conductive polymers are polypyrrole (PPy), polyaniline (PANI), and polythiophene (PT) (Fig. 16.4). Commonly used nanomaterials are three dimensionally–ordered macroporous (3DOM) carbon, colloid-imprinted mesoporous (CIM) carbon, carbon nanotubes (CNTs), graphene, fullerene, nanoclusters and gold nanoparticles (Liu et al. 2019).
3.1 Conductive Polymers
Conductive polymers of PPy, PANI, and PT have some key features that make them useful as ion-to-electron transducers (Bobacka 2006; Faridbod et al. 2008; Bobacka et al. 2008). First, conducting polymers can provide ohmic contact with high work function like noble metals and carbon materials. Second, conductive polymers can be deposited from solution onto the conductive electrodes either by electrochemical polymerization or by drop-casting. Third, conductive polymers electroactive property as a result of combinatory effect of electronic and ionic conductivity allows for ion to electron transduction (Faridbod et al. 2008; Bobacka et al. 2008). These are the features that make conducting polymers suitable as solid contact transducers for AS-ISEs.
-
i.
Polypyrrole (PPy)
Polypyrrole has been used as an ion-to-electron transducer in solid-contact ISEs since the beginning of the 1990s and is still used today (Bobacka et al. 2003). PPy as a solid contact enhances the performance of AS-ISEs in terms of Nernstian behavior, linear range, detection limit, and response time (Table 16.3). For example, PPy doped with Titan yellow dye (PPy/TY) as solid contact resulted in a magnesium-selective ISE (Mg+2-ISE); the solid-state electrode has a Nernstian behavior almost equivalent to that of a typical glass electrode (Gupta et al. 2004; Mosayebzadeh et al. 2014). The calibration slope and concentration range for polypyrrole- and non-polypyrrole–based solid contacts were 28.27 ± 0.40 mV per decade within the concentration range of 1.0 × 10−5 – 5.0 × 10−2 M, and 29.2 ± 0.4 mV per decade within the concentration range of 9.4 × 10−6 to 1.0 × 10−1 M, respectively (Gupta et al. 2004; Mosayebzadeh et al. 2014). The detection limits of PPy-based and non-PPy-based solid contacts were 6.28 × 10−6 M and 9.4 × 10−6, respectively, showing not much difference in performance. However, the potentiometric response of PPy-based solid-contact electrodes toward Mg+2 was found to be independent of pH from 4.5–8.0, while non–PPy–based solid contacts were independent of pH from 3.5–7.8 (Gupta et al. 2004; Mosayebzadeh et al. 2014). Polypyrrole-based Mg+2-ISEs showed fast response time compare to non-PPy-based Mg+2-ISEs, < 10 s and 13 s, respectively (Mosayebzadeh et al. 2014). The results show that a PPy solid contact is comparable to a non-solid state one.
-
ii.
Polyaniline (PANI)
Polyaniline (PANI) is utilized in ISEs fabrication owing to its stable potential, easy preparation, and low cost (Shishkanova et al. 2005). PANI has been used as a solid contact (Jiang et al. 2019), or as a component of an ion-selective membrane (Aytaç et al. 2004). In addition, PANI can exist as either film, microfiber, or nanoparticle; PANI in microfiber form enhances ISE performance owing to its hydrophobicity, which minimizes water intake into the ISM (Jiang et al. 2019). As an example, electrospun PANI-polystyrene microfiber film (e-PANI-PS) was used as a transducer in solid-contact ISEs to sense lead ion (Pb2+). The Pb2+-ISEs based on e-PANI-PS exhibited a wide linear detection range (10−8 to 10−3 mol/L), a low detection limit (~5 × 10−9 mol/ L), a Nernstian slope of 29.1 mV/decade, and a fast response time (< 10 s) (Jiang et al., 2019). Moreover, the fabricated ISEs demonstrated a lower detection limit for Pb2+ and better potential stability compared to ISEs fabricated using drop-cast PANI-PS films. The analytical performance of the e-PANI-PS microfiber transducer for Pb2+ detection is a result of the high hydrophobicity that minimizes water uptake. Furthermore, e-PANI-PS has higher capacitance and lower impedance than the drop-cast PANI-PS, which promoted fast ion-to-electron transfer at the electrode-solution interface (Jiang et al. 2019). These features make PANI one of the most used conductive polymers as a transducer for AS-ISE fabrication.
-
iii.
Polythiophene (PT)
The first polythiophene used as a solid contact in ISEs was poly(3-octylthiophene) (POT) (Bobacka et al. 2008). Poly(3,4-ethylene-dioxythiophene) (PEDOT) in its p-doped form is highly electroactive and possesses good environmental stability (less sensitive to O2 and CO2) compared to transducers based on PPy as solid contact (Bobacka 2006). Therefore, water-dispersible PEDOT with poly(styrenesulfonate) (PEDOT:PSS) (Fig. 16.5) was used as an ion-to-electron transducer in solid-contact ISEs for detection of certain ions: potassium (K+), silver (Ag+), sodium (Na+), cesium (Cs+), calcium (Ca2+), and some aromatic cations (e.g., N-methylpyridinium, bupivacaine) (Bobacka et al. 2008). However, few research reports on PEDOT:PSS use as an electrochemical sensor owing to poor adhesion of PEDOT:PSS on conductive electrode surfaces. In aqueous solution, PSS can result in swelling of PEDOT:PSS and the film can be removed from the electrode surface (Wang et al. 2014; Benoudjit et al. 2018), limiting PEDOT:PSS use as a transducer for AS-ISEs. Therefore, some studies focus on enhancing the adhesion of PEDOT:PSS on electrode surface by using polyvinyl alcohol (PVA) (Wang et al. 2014) and sodium carboxymethyl cellulose (Na-CMC) (Zhang et al. 2015) binders, or depositing PEDOT:PSS with electro-polymerization instead of by drop-casting (Benoudjit et al. 2018). The resulting improvement allows PEDOT:PSS to be an effective transducer for AS-ISEs.
3.2 Nanomaterials
Recently, nanomaterials have been suggested as transducer materials for AS-ISEs (Liu et al. 2019). Three-dimensionally ordered macroporous (3DOM) carbon (Lai et al. 2007), colloid-imprinted mesoporous (CIM) carbon (Hu et al. 2014), carbon nanotubes (CNTs) (Ganjali et al. 2010; Crespo et al. 2008), graphene (Ping et al. 2011), fullerene (Fouskaki and Chaniotakis 2008), nanoclusters (Zhou et al. 2012), and gold nanoparticles (Jaworska et al. 2011) are commonly used nanomaterials as a result of their high surface area, intrinsic hydrophobicity, and electric conductivity. Furthermore, these nanomaterial-based ISEs exhibit high potential stability and have shown more excellent resistance to O2, CO2, light, and redox interferences than do the classical conducting polymers (Liu et al. 2019). These features make these nanomaterials suitable as transducer materials for AS-ISE fabrication. Generally, nanomaterial-based solid contacts are prepared layer by layer using drop-casting methods. However, it should be noted that nanomaterials can easily detach from an electrode surface owing to poor adhesion (Liu et al. 2019). Although PVC has been successfully used as a facile and effective binder to fabricate nanomaterial-based solid contact (Hu et al. 2014), the electrical conductivity of nanomaterials might be reduced by the poor conductivity of PVC.
More recently, we utilized PEDOT:PSS with reduced graphene oxide (rGO) nanomaterial as solid contact for biosensors because the composite showed good adhesion to the electrode surface while retaining good conductivity (Ismail et al. 2019). From electrochemical analyses by cyclic voltammetry (CV), the transducer showed a high effective surface area of 0.219 mm2 and a high peak current of 0.793 mA. The high conductivity is further confirmed with electrical impedance spectroscopy (EIS), showing the composite to have low charge-transfer resistance (Rct) of 200.7 Ω (Ismail et al. 2019). The CV and EIS results suggest that rGO-PEDOT:PSS composite has the potential to be used as solid contacts for AS-ISEs.
4 Summary
This chapter introduces the materials used for fabricating the components of AS-ISEs: the ion-selective membrane (ISM) and the all-solid-state transducer. The role of an ISM is to recognize and select the target io; the ISM consists of four components: polymeric matrix, plasticizer, ionophore, and ionic additives. The physical and chemical characteristics of AS-ISEs can be influenced by the amount of each component, and the typical cocktail mix for an ISM is polymeric matrix 33% (w/w), plasticizer 66% (w/w), and ionophore 1% (w/w). Polyvinyl chloride (PVC) functions as a homogeneous membrane matrix for the other ISM components. Ionophores provide selectivity towards a target ion, which is also known as membrane-active recognition; selection of ionophore depends on the target ion. Plasticizers are responsible for controlling the physical property (plasticity or fluidity) of ISMs. Ionic additives are ion exchangers that ensure the permselectivity of the ISMs if no or insufficient ionophore is present. The solid-state transducer converts the target-ion concentration to an electrical signal that can be measured. Materials used as solid-state transducers are conductive polymers and nanomaterials. Common conductive polymers used in AS-ISEs are polypyrrole, polythiophene, and polyaniline, owing to their intrinsic characteristics. Nanomaterials such as Three-dimensionally-ordered macroporous carbon, colloid-imprinted mesoporous carbon, carbon nanotubes, graphene, fullerene, nanoclusters, and gold nanoparticles are good solid contacts for AS-ISEs owing to their ion-to-electron transduction. Reduced graphene oxide can be combined with PEDOT:PSS to serve as a solid contact for AS-ISEs.
References
Ansari R, Mosayebzadeh Z (2013) Construction of a new solid-state U (VI) ion-selective electrode based on polypyrrole conducting polymer. vi
Ansari R, Delavar AF, Mohammad-Khah A (2012) Solid-state ion selective electrode based on polypyrrole conducting polymer nanofilm as a new potentiometric sensor for Zn 2+ ion. J Solid State Electrochem 16(10):3315–3322
Ansari R, Mosayebzadeh Z, Mohammad-khah A (2013) Fabrication of a solid-state ion selective electrode based on polypyrrole conducting polymer for As (V) ion. Int J Env Anal Chem:37–41
Aytaç A, Kabasakaloğlu M, Sarı B, Talu M (2004) Ion-selective electrodes prepared with polyaniline membranes. Russ J Electrochem 40(7):732–735
Bakker E, Bhakthavatsalam V, Gemene KL (2008) Beyond potentiometry: robust electrochemical ion sensor concepts in view of remote chemical sensing. Talanta 75(3):629–635
Benoudjit A, Bader MM, Wan Salim WWA (2018) Study of electropolymerized PEDOT:PSS transducers for application as electrochemical sensors in aqueous media. Sens Bio-Sensing Res 17:18–24
Bobacka J (2006) Conducting polymer-based solid-state ion-selective electrodes. Electroanalysis 18(1):7–18
Bobacka J, Ivaska A, Lewenstam A (2003) Potentiometric ion sensors based on conducting polymers. Electroanalysis 15(5–6):366–374
Bobacka J, Ivaska A, Lewenstam A (2008) Potentiometric ion sensors. Chem Rev 108(2):329–351
Bratovčić A, Odobašić A, Ćatić S (2009) The advantages of the use of ion-selective potentiometry in relation to UV / VIS spectroscopy. Agric Conspec Sci Cus 74(3):139–142
Crespo GA, Macho S, Rius FX (2008) Ion-selective electrodes using carbon nanotubes as ion-to-electron transducers. Anal Chem 80(4):1316–1322
Eugster R, Gehrig PM, Morf WE, Spichiger UE, Simon W (1991) Selectivity-modifying influence of anionic sites in neutral-carrier-based membrane electrodes. Anal Chem 63(20):2285–2289
Faridbod F, Ganjali MR, Dinarvand R, Norouzi P (2007) The fabrication of potentiometric membrane sensors and their applications. African J Biotechnol 6(25):2960–2987
Faridbod F, Ganjali MR, Dinarvand R, Norouzi P (2008) Developments in the field of conducting and non-conducting polymer based potentiometric membrane sensors for ions over the past decade. Sensors 8(4):2331–2412
Fouskaki M, Chaniotakis N (2008) Fullerene-based electrochemical buffer layer for ion-selective electrodes. Analyst 133(8):1072–1075
Ganjali MR, Norouzi P, Rezapour M, Faridbod F, Pourjavid MR (2006) Supramolecular based membrane sensors. Sensors 6(8):1018–1086
Ganjali MR, Motakef-Kazami N, Faridbod F, Khoee S, Norouzi P (2010) Determination of Pb2+ ions by a modified carbon paste electrode based on multi-walled carbon nanotubes (MWCNTs) and nanosilica. J Hazard Mater 173(1–3):415–419
Gehrig P, Morf WE, Welti M, Pretsch E, Simon W (1990) Catalysis of ion transfer by tetraphenylborates in neutral carrier-based ion-selective electrodes. Helv Chim Acta 73(1):203–212
Gholami M, Rezayi M, Moozarm Nia P, Yusoff I, Alias Y (2015) A novel method for fabricating Fe2+ ion selective sensor using polypyrrole and sodium dodecyl sulfate based on carbon screen-printed electrode. Meas J Int Meas Confed 69:115–125
Gupta VK, Prasad R, Kumar A (2004) Magnesium-tetrazaporphyrin incorporated PVC matrix as a new material for fabrication of Mg2+ selective potentiometric sensor. Talanta 63(4):1027–1033
Guth U, Gerlach F, Decker M, Oelßner W, Vonau W (2009) Solid-state reference electrodes for potentiometric sensors. J Solid State Electrochem 13(1):27–39
Guzinski M et al (2017) PEDOT(PSS) as solid contact for ion-selective electrodes: the influence of the PEDOT(PSS) film thickness on the equilibration times. Anal Chem 89(6):3508–3516
He N, Gyurcsányi RE, Lindfors T (2016) Electropolymerized hydrophobic polyazulene as solid-contacts in potassium-selective electrodes. Analyst 141(10):2990–2997
Hu J, Zou XU, Stein A, Bühlmann P (2014) Ion-selective electrodes with colloid-imprinted mesoporous carbon as solid contact. Anal Chem 86(14):7111–7118
Hu J, Stein A, Bühlmann P (2016) Rational design of all-solid-state ion-selective electrodes and reference electrodes. Trends Anal Chem 76:102–114
Ismail NAB, Abd-Wahab F, Wan Salim WWA(2019) Cyclic voltammetry and electrochemical impedance spectroscopy of partially reduced graphene oxide PEDOT:PSS transducer for biochemical sensing, in IEEE EMBS Conference on Biomedical Engineering and Sciences, IECBES 2018 Proceedings, cv: 330–335
Jadhav NR, Gaikwad VL, Nair KJ, Kadam HM (2009) Glass transition temperature: basics and application in pharmaceutical sector. Asian J Pharm 3(2):82–89
Jaworska E, Wójcik M, Kisiel A, Mieczkowski J, Michalska A (2011) Gold nanoparticles solid contact for ion-selective electrodes of highly stable potential readings. Talanta 85(4):1986–1989
Jiang W, Liu C, Zhao Y, Waterhouse GIN, Zhang Z, Yu L (2019) A solid-contact Pb 2+ −selective electrode based on a hydrophobic polyaniline microfiber film as the ion-to-electron transducer. Synth Met 248:94–101
Jumal J, Yamin BM, Ahmad M, Heng LY (2012) Mercury ion-selective electrode with self-plasticizing poly(n–butylacrylate) membrane based on 1,2-bis-(N’–benzoylthioureido)cyclohexane as ionophore. APCBEE Procedia 3:116–123
Lai CZ, Fierke MA, Stein A, Bühlmann P (2007) Ion-selective electrodes with three-dimensionally ordered macroporous carbon as the solid contact. Anal Chem 79(12):4621–4626
Liu K, Jiang X, Song Y, Liang R (2019) Robust fabrication of nanomaterial-based all-solid-state ion-selective electrodes. RSC Adv 9(29):16713–16717
Michalska A (2012) All-solid-state ion selective and all-solid-state reference electrodes. Electroanalysis 24(6):1253–1265
Morf WE, De Rooij NF, Pretsch E (2005) Influence of cationic and anionic additives on the electrical properties of ionophore-based ion-selective membranes. J Electroanal Chem 581(2):265–274
Mosayebzadeh Z, Ansari R, Arvand M (2014) Preparation of a solid-state ion-selective electrode based on polypyrrole conducting polymer for magnesium ion. J Iran Chem Soc 11(2):447–456
Pan P et al (2016) Preparation and evaluation of a stable solid state ion selective electrode of polypyrrole/electrochemically reduced graphene/glassy carbon substrate for soil nitrate sensing. Int J Electrochem Sci 11(6):779–4793
Park J et al (2017) An autonomous lab on a chip for space flight calibration of gravity-induced transcellular calcium polarization in single-cell fern spores. Lab Chip 17(6):1095–1103
Pięk M, Paczosa-Bator B, Smajdor J, Piech R (2018) Molecular organic materials intermediate layers modified with carbon black in potentiometric sensors for chloride determination. Electrochim Acta 283:1753–1762
Ping J, Wang Y, Wu J, Ying Y (2011) Development of an all-solid-state potassium ion-selective electrode using grapheme as the solid-contact transducer. Electrochem Commun 13(12):1529–1532
Ping J, Wang Y, Ying Y, Wu J (2012) Application of electrochemically reduced graphene oxide on screen-printed ion-selective electrode. Anal Chem 84(7):3473–3479
Saeed A, Flörke U, Erben MF (2014) A review on the chemistry, coordination, structure and biological properties of 1-(acyl/aroyl)-3-(substituted) thioureas. J Sulfur Chem 35(3):318–355
Shishkanova TV, Sapurina I, Stejskal J, Král V, Volf R (2005) Ion-selective electrodes: Polyaniline modification and anion recognition. Anal Chim Acta 553(1–2):160–168
Shiwaku R et al (2018) A printed organic amplification system for wearable potentiometric electrochemical sensors. Sci Rep 8(1):1–8
Stradiotto NR, Yamanaka H, Zanoni MVB (2003) Electrochemical sensors: a powerful tool in analytical chemistry. J Braz Chem Soc 14(2):159–173
ul Haque A et al (2007) A MEMS fabricated cell electrophysiology biochip for in silico calcium measurements. Sensors Actuators B Chem 123(1):391–399
Vanamo U, Bobacka J (2014) Electrochemical control of the standard potential of solid-contact ion-selective electrodes having a conducting polymer as ion-to-electron transducer. Electrochim Acta 122:316–321
Vázquez M, Bobacka J, Ivaska A, Lewenstam A (2002) Influence of oxygen and carbon dioxide on the electrochemical stability of poly(3,4-ethylenedioxythiophene) used as ion-to-electron transducer in all-solid-state ion-selective electrodes. Sensors Actuators B Chem 82(1):7–13
Wang Z et al (2014) Facile preparation of highly water-stable and flexible PEDOT:PSS organic/inorganic composite materials and their application in electrochemical sensors. Sensors Actuators B Chem 196:357–369
Wilson D, de los Ángeles Arada M, Alegret S, del Valle M (2010) Lead(II) ion selective electrodes with PVC membranes based on two bis-thioureas as ionophores: 1, 3-bis(N’-benzoylthioureido)benzene and 1, 3-bis(N’-furoylthioureido)benzene. J Hazard Mater 181(1–3):140–146
Yan R, Qiu S, Tong L, Qian Y (2016) Review of progresses on clinical applications of ion selective electrodes for electrolytic ion tests: from conventional ISEs to graphene-based ISEs. Chem Speciat Bioavailab 28(1–4):72–77
Ying KS, Heng LY, Hassan NI, Hasbullah SA (2018) A new copper ionophore N1, N3 -bis [[3,5-bis(trifluoromethyl)phenyl] carbamothioyl] isophtalamide for potentiometric sensor. Sains Malays 47(11):2657–2666
Yogeswaran U, Shen-Ming C (2008) A review on the electrochemical sensors and biosensors composed of nanogaps as sensing material. J Optoelectron Adv Mater 12(8):290–313
Zhang L, Zhang M (2013) Screening of pretreatment parameters for novel solid-state ISE-based soil extractable potassium detection, in Proceedings of 2013 IEEE 11th International Conference on Electronic Measurement and Instruments, ICEMI 2013. 2: 947–953
Zhang H, Xu J, Wen Y, Wang Z, Zhang J, Ding W (2015) Conducting poly(3,4-ethylenedioxythiophene):poly(styrene- sulfonate) film electrode with superior long-term electrode stability in water and synergistically enhanced electrocatalytic ability for application in electrochemical sensors. Synth Met 204:39–47
Zhao L et al (2019) In vivo measurement of calcium ion with solid-state ion-selective electrode by using shelled hollow carbon nanospheres as a transducing layer. Anal Chem 91(7):4421–4428
Zhou M et al (2012) Effective solid contact for ion-selective electrodes: Tetrakis(4-chlorophenyl)borate (TB−) anions doped nanocluster films. Anal Chem 84(7):3480–3483
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Benoudjit, A.M., Shohibuddin, I.U.S., Bader, M.M., Salim, W.W.A.W. (2020). Components of All-Solid-State Ion-Selective Electrodes (AS-ISEs). In: Siddiquee, S., Gan Jet Hong, M., Mizanur Rahman, M. (eds) Composite Materials: Applications in Engineering, Biomedicine and Food Science. Springer, Cham. https://doi.org/10.1007/978-3-030-45489-0_16
Download citation
DOI: https://doi.org/10.1007/978-3-030-45489-0_16
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-45488-3
Online ISBN: 978-3-030-45489-0
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)