Abstract
Patagonia has a great diversity of lizards, including 6 families, 11 genera, and 163 species. The majority of this diversity with documented geographical records corresponds to the Liolaemidae family (Phymaturus and Liolaemus). Latitudinally, lizard richness is higher between 37° and 39° S, decreasing gradually until latitude 54° S; longitudinally, it is higher between 69° and 71° W. The georeferenced records and the number of collected specimens have some biases, with higher values in the northern regions (up to latitude 42° S). However, there are areas up to latitude 45° S with species richness similar to others with a greater number of collected individuals. The vegetation units with the highest species richness (S) in Argentinean Patagonia are the northern and central areas reaching west of Chubut Province: Western District (S = 60) and Typical Southern Monte (S = 49), passing through areas with intermediate richness and with only one species (Wet Magellanic Steppe) or none (Evergreen shrub, Monte’s Mountains and Valleys, Western Interior Pampa, and Peat Bogs). There is a general trend toward lower species richness in vegetation units located in the extreme south of Argentina and south of latitude 41° S in Chile. We evaluated differences in lizard diversity and evenness among vegetation units through a dendrogram based on species incidences and found six clusters. Then, we compared species richness between members of each cluster with rarefaction curves. Species marginally distributed in Patagonia have a narrower altitudinal range than Patagonian endemics. Species in genus Liolaemus have small differences in altitudinal range, but species of Phymaturus have pronounced differences in altitudinal ranges. Our spatial analyses, based on intensive systematic-taxonomic activity over the last two decades, shed light into the understanding of lizard distributions in one of the regions with the greatest diversity of reptiles in the world. We also provide ecological and spatial metrics for an updated list of Patagonian lizards. We highlight that the usefulness of discretizing large volumes of information and geographic space into a synthetic framework allows using quantitative results for the study of spatial patterns of biodiversity, decision-making for design studies, use of resources, and creation of protected areas.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Biogeography and Ecological History
The biogeography of a region is defined by the study of organisms or species’ distributions and ecosystems, taking into account their relationships with the geographical space and geological periods (Cox and Moore 2010). The applications of biogeography and its constant changes through time and spatial scales, are not only limited to knowing where species inhabit, but have a very important role in public health, economic production, environmental assessment, sustainable use of resources, landscape planning, and conservation management (Spellerberg et al. 1999). In a more general context, biogeographical studies help us to understand the processes associated with the origin and maintenance of distributional patterns (Sanmartín 2012). Geological and climatic processes could affect biological characteristics through time such as reproduction (Pyron and Burbrink 2014; Liang et al. 2018), trophic level (Pianka 1986; Cooper 1997), body size (Pincheira-Donoso et al. 2008), and even the emergence of new species (Pincheira-Donoso et al. 2015). This strong relationship between species diversity and climatic and geological history makes Patagonian lizards an interesting object for biogeographical studies.
Knowledge of the geological and climatic events that occur over time in an area is essential to understand the processes that led to differences in biodiversity among different regions of the planet (Morrone 2001a). In the Patagonian region, the distribution of species’ assemblages has been shaped by two important geological-climatic events: the orogeny of the Andes (Middle-Late Miocene ~15–10 Ma) and the Pleistocene Glaciations (~ 2.5 Ma 11.7 ka) (Paruelo et al. 1998; Morrone 2001b; Albino 2011; Ruzzante and Rabassa 2011). The Andean orogeny introduced an important rain shadow toward the east, creating a cold and dry climate throughout the Patagonian steppe that promoted a reduction of South American forests in the southern sector and the expansion of taxa adapted to xeric environments (Barreda and Palazzesi 2007). Although Patagonian glaciations began at the end of the Miocene, the Patagonian ice sheet reached its full development during the early Pleistocene (Rabassa 2008). These glacial advances were represented with great impact in this region, such as the Great Patagonian Glaciation (GPG, 1–1.2 Ma) and the Last Maximum Glacial (LGM, 21 ka). In comparison with the LGM and other Pleistocene glaciations in the Patagonian Steppe, the GPG turned out to be the maximum expansion of the ice sheets (Rabassa 2008) and paleoenvironmental reconstructions allow us to know that the amount of terrestrial area in the past was very different from the current one (Ponce et al. 2011). Glaciations in Patagonia produced sea-level changes, exposing part of the continental shelf in the east and considerably increasing the available geographical space for colonization by plants and animals (Ponce et al. 2011), and promoted latitudinal movements of major ecosystems (Rabassa and Coronato 2009). The increase in extreme temperatures, aridification of the surrounding areas, decrease in precipitation, and the absence of the sea conditioner effect that displaced its coastline to the east (Rabassa and Coronato 2009) were factors that influenced the high-latitude biota of Patagonia, even in areas that were not encompassed by glaciers. However, substantial areas remained free of ice during glacial episodes and climatic conditions were less severe than those of North America (Markgraf et al. 1995). The climatic history has a strong relationship with the creation of biomes in the Patagonian region (Markgraf et al. 1995).
Biomes or ecological regions are defined as relatively large biogeographical areas distinguished by their unique climate, soils, hydrology, ecology, flora and/or fauna, and intended to serve as a geographic organizational tool for ecosystem management (Clements and Shelford 1939; Sodhi and Ehrlich 1995; Burkart et al. 1999). From an ecological historical point of view, these are strongly cohesive units, large enough to cover the ecological processes or the life history for most of the species that inhabit and interact therein (Sodhi and Ehrlich 1995). The spatial relationship between lizard species and these ecological units is one of the main axes in the analyses carried out for this chapter. Here we used discrete spatial subsamples, consisting of hexagonal, latitudinal–longitudinal, and vegetation zoning units, to (1) analyze patterns of richness, diversity, density, and number of sampling geographical records, and (2) analyze altitudinal patterns between genera and species.
2 Study Area
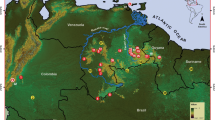
The area considered as Patagonia for this chapter is located between 33°53′48.62″ S–55°58′55.71″ S and 75°41′27.83″ W–62°06′31.39″ W, covering approximately 1,305,870 km2 (~ 7.38% of South America) and corresponding to central–southern Argentina and Chile (Fig. 8.1a). Based on a coarse classification, the study area can be divided into a cold, humid, mountainous Andean-Patagonian forest region in the west, and the Patagonian steppe region, which extends widely eastwards to the Atlantic Ocean and is mostly low, cold, dry and characterized by scattered grasses and shrubs (Cabrera 1976; León et al. 1998). Patagonia can be defined as a temperate or cool-temperate region and presents a characteristic NW-SE temperature pattern, determined mainly by the presence of the Andes. Local factors such as topography and wind affect air temperature. The climate is dry and cold in most of the territory, with an extremely variable temperature ranging from −30 °C in the winter to 45 °C degrees in the summer (Chap. 3).
(a) Patagonian region and lizard’s georeferenced records reviewed for this study. (b) Vegetation divisions used in this study following the proposal of Luebert and Pliscoff (2017) and Oyarzabal et al. (2018). (c) Lizard’s species richness by vegetation units. References: Endorheic Bolsons = BEnd, Deciduous Forest = BC, Sclerophyllous Forest = BEsc, Thorny Forest = BEsp, Laurel Forest = BL, Resinous Forest = BR, Evergreen Forest = BS, Andean—Patagonian Forests = BAP, Central District = DC, Erial Central District = DCE, Central District—Quilenbai steppe bushes = DCEAQ, Central District—Mountain steppe bushes = DCEAS, Payunia District = DLP, San Jorge Gulf District = DGSJ, Fueguino District—Wet Magellanic Steppe = DFEMH, Western District = DO, Sub Andean District—Steppe of white coiron = DSECB, Sub Andean District—Dry Magellanic Steppe = DSEMS, Valdés Península Ecotone = EPV, Rionegrino Ecotone = ER, Shrub steppe of black bush = EAMN, Steppes and pastures = EP, Altitude grass = HA, Low altitude scrub = MBA, Deciduous scrub = MC, Evergreen shrub = MS, Typical southern Monte = MAT, Monte’s Mountains and Valleys = MSB, Eastern or Transitional Monte = MODT, Western Interior Pampa = PIO, Altoandina Province = PA, Peat Bogs = T, and Without vegetation = SV
The influence of the Pacific air masses and the Andes as a topographic barrier parallel to the Pacific coast results in a strong west-east gradient of precipitation across the region (Barros et al. 2015). The rising air cools faster on the west side of the Andes, originating an annual rainfall exceeding 2000 mm on the Chilean side. On the eastern side of the Andes, the total annual precipitation decreases exponentially and most of the central portion of Patagonia receives less than 200 mm per year (Paruelo et al. 1998). Additionally, there are large areas that are annually affected by fallen snow and permanent ice fields. The different patterns of temperature, soil type, precipitation, and geological history have shaped different types of vegetation that prevail today in this broad region (Morello et al. 2012).
Patagonia had major changes since the 1890s and has undergone steady change as a result of human activity, but there has been no clear understanding of the resulting effects on biodiversity. Over the twentieth century, business activities such as oil extraction, mining, and ranching have affected different ecosystems of this area. In particular, sheep overgrazing (Bisigato and Bertiller 1997; Cesa and Paruelo 2011) has led to desertification in the vast semiarid portion of Patagonia (Ares et al. 1995; Aguiar et al. 1996). Another factor that may affect the diversity and ecological dynamics of large xerophytic areas like Patagonia (e.g., Schulze et al. 1996) is the creation of hydroelectric dams, which implies anthropic management of regional water availability and seasonal changes in rainfall (Paruelo et al. 1998) or river flow rates (Masiokas et al. 2008). An overall analysis of lizard diversity based on accurate species distributional data is essential for understanding the impact and consequences of these types of human activity (Böhm et al. 2013; Cook et al. 2013).
3 Methods
3.1 Data Collection
We carried out extensive biological surveys that began in early 1998 and continued until 2018, with field trips made in different representative areas of the Patagonian region. Throughout these decades of fieldwork, we sampled different ecoregions and completed gap areas without previous records. Each record has a voucher number with a species identity assigned, date, and place of origin. Collection sites were geographically referenced using a Garmin GPS 12TM Global Position Device. The systematic classification for families was according to Oppel (1811), Gray (1827, 1865), Frost et al. (2001), and Gamble et al. (2008). Specimens were deposited or consulted in several herpetological collections (Fig. 8.2a): LJAMM-CNP (IPEEC-CONICET), BYU (Monte L. Bean Museum, Brigham Young University), MLP (La Plata Museum), and FML (Miguel Lillo Foundation). Additional museum collections, literature vouchered, and GBIF records were obtained from AMNH (American Museum of Natural History, USA), APN-AR (Administration of National Parks Argentina), CAS (California Academy of Science, USA), CH (Colección Centro Regional Universitario Bariloche, Universidad Nacional del Comahue, Río Negro, Argentina), CHDPD (Colección personal Daniel Pincheira-Donoso), CNHM (Chicago Natural History Museum; in the present The Field Museum of Natural History, FMNH, USA), CRILaR (Centro Regional de Investigaciones Científicas y Transferencia Tecnológica, Argentina), CTALA_LB (Ministerio del Medio Ambiente de Chile), FBC (Félix Benjamín Cruz Field Collection, Argentina), IADIZA (Instituto Argentino de Investigaciones de las Zonas Áridas, Argentina), IBA-UNC (Universidad Nacional de Cuyo, Argentina), JAS-DC (José Alejandro Scolaro, Centro Nacional Patagónico, Puerto Madryn, Argentina), JMC-DC (Colección Herpetológica José Miguel Cei, Argentina), KU (Kansas University reptile collection, USA), MACN (Museo Argentino de Ciencias Naturales “Bernardino Rivadavia”, Argentina), MCN (Museo de Ciencias Naturales, Universidad Nacional de Salta, Argentina), MCZ (Museum of Comparative Zoology, Harvard University, USA), MHNG (Muséum d’histoire naturelle de la Villede De Genève, Switzerland), MLP (Museo de la Plata, Argentina), MNHN (Museum National de Histoire Naturelle, France), MNHNC (Museo Nacional de Historia Natural, Chile), MNW (Muzeum Narodowe w Warszawie, Poland), MVZ (University of California at Berkeley, USA), MZUC (Museo de Zoología de la Universidad de Concepción, Chile), NMBA (Zoologische Expedition des Naturhistorischen Museums Basel), OMNH (Osaka Museum of Natural History, Japan), PT (Proyecto Tupinambis, Félix Benjamín Cruz, Argentina), RBINS (Royal Belgian Institute of Natural Sciences, Belgium), SDNHM (San Diego Natural History Museum, USA), SDSU (San Diego State University, USA), SMNS (Staatliches Museum für Naturkunde, Germany), SSUC (Colección Patricio Sánchez Reyes de la Pontificia Universidad Católica, Chile), TCWC (Texas A & M University, USA), UNMDP (Colección Herpetológica de la Universidad Nacional de Mar del Plata, Argentina), UWBM (Burke Museum, University of Washington, USA), and ZSM (Zoologische Staatssammlung, Germany). Literature and museum records with acronyms or specific localities were quoted literally.
3.2 Spatial Analyses
We constructed a hexagonal grid with 1122 cells (White et al. 1992; White 2000) with a hexagon average area of 1163.5 km2, covering the entire territory of Patagonia (Fig. 8.3). We used hexagons rather than squares because they possess greater statistical efficiency (Olea 1984) and are more dynamically adaptable (Yfantis et al. 1987), allowing them to adjust to the boundaries of a highly irregular perimeter (such as Patagonian Pacific and Atlantic coastlines). In this approach with continuous tessellations, hexagons have the advantage over squares in that all six adjacent sides are equally distant, while squares have four closer and four more distant neighbor sides (Dengler 2009). This facilitates the comparison of different and large data sets by discretizing a major and continuous area (White 2000).
Since there are multiple zoning proposals for Patagonian ecoregions (Burkart et al. 1999; Morello et al. 2012) based on plant formations (Roig 1998) and phytogeographic provinces (Cabrera 1994), we decided to use the spatial plant units from two of the latest available works on this topic (Luebert and Pliscoff 2017 for Chile; Oyarzabal et al. 2018 for Argentina). These contributions proposed a discretization of Patagonia comprising 33 characterized vegetation units. This refined classification considers the following vegetation units: Endorheic Bolsons = BEnd, Deciduous Forest = BC, Sclerophyllous Forest = BEsc, Thorny Forest = BEsp, Laurel Forest = BL, Resinous Forest = BR, Evergreen Forest = BS, Andean—Patagonian Forests = BAP, Central District = DC, Erial Central District = DCE, Central District—Quilenbai steppe bushes = DCEAQ, Central District—Mountain steppe bushes = DCEAS, Payunia District = DLP, San Jorge Gulf District = DGSJ, Fueguino District—Wet Magellanic Steppe = DFEMH, Western District = DO, Sub Andean District—Steppe of white coiron = DSECB, Sub Andean District—Dry Magellanic Steppe = DSEMS, Valdés Península Ecotone = EPV, Rionegrino Ecotone = ER, Shrub steppe of black bush = EAMN, Steppes and pastures = EP, Altitude grass = HA, Low altitude scrub = MBA, Deciduous scrub = MC, Evergreen shrub = MS, Typical southern Monte = MAT, Monte’s Mountains and Valleys = MSB, Eastern or Transitional Monte = MODT, Western Interior Pampa = PIO, Altoandina Province = PA, Peat Bogs = T, and Without vegetation = SV (Fig. 8.1b).
We evaluated the source and overall number of lizard records, and grouped them by families, genera, and species. We used the constructed hexagonal tessellation to obtain the lizards’ sampling density (number of localities), density of individuals (number of individuals) and species richness per cell, and assessed their variation latitude, longitude, and vegetation units. We analyzed the species richness number and records by latitude-longitude and by vegetation zonification. Additionally, we performed the Shannon Wiener Index (a diversity metric) and Jaccard’s similarity index among the vegetation units. We evaluated the lizard’s assemblages per vegetation units through cluster analysis and compared species richness between cluster members with rarefaction curves, to remove the potential bias of uneven catch rates (Gotelli and Colwell 2001; Buddle et al. 2005). Finally, we analyzed the altitude ranges ordered by mean values for all genera and species of Phymaturus and Liolaemus. All figures, spatial and statistical analyses were performed with R 3.4.4 (R Core Team 2018) and vegan 2.5–2 (Oksanen et al. 2018), tmaptools 1.2–4 (Tennekes 2018), rgdal 1.3–3 (Bivand et al. 2018), rgbif 1.0.2 (Chamberlain et al. 2018), raster 2.6–7 (Hijmans 2017), maps 3.3.0 (Becker and Wilks 2018), geosphere 1.5–7 (Hijmans 2015), SDMTools 1.1–221 (VanDerWal et al. 2014), GISTools 0.7–4 (Brunsdon and Chen 2014), rgeos 0.3–28 (Bivand and Rundel 2018), maptools 0.9–2 (Bivand and Lewin-Koh 2018), sp. 1.3–1 (Pebesma and Bivand 2005), and cluster 2.0.7–1 (Maechler et al. 2018) R packages.
4 Diversification and Sampling of Lizards
For 300 years Patagonia has been the stage of herpetological discoveries by naturalists and explorers, from the abbot Juan Ignacio Molina, who made a great contribution to the Chilean fauna with his work “Saggio sulla Storia Naturale del Chili” in 1782, to J. G. Koslowsky (early twentieth century) with great contributions to the exploration of Patagonia’s herpetology. Also, great explorers like J. B. Hatcher (1896–1899), F. Meyen (1830–1832), and Charles R. Darwin himself (1833) contributed innumerable materials for the description of the Patagonian herpetofauna by A. M. G. Duméril, G. Bibron, T. Bell, R. P. Lesson, and A. F. A. Wiegmann, among others. In the middle of the twentieth century, herpetologists such as J. M. Cei and J. A. Scolaro explored and sampled large areas of Patagonia and described new species of amphibians and reptiles. In Chilean Patagonia, R. Donoso-Barros conducted numerous and important studies on the herpetofauna. Recently, Patagonia has been visited by many herpetologists and is one of the most explored areas in Argentina, with numerous new species described (see Chap. 1).
We recorded 163 species, 11 genera and 6 families of lizards, obtained from 14,985 distribution records from 2637 unique localities (Table 8.1 and Fig. 8.2). The families with the greatest genera diversity were Teiidae and Leiosauridae, followed by Liolaemidae, Phyllodactylidae, Tropiduridae, and Amphisbaenidae (Fig. 8.2b). We found the highest diversity in Liolaemus (110 species) and Phymaturus (34), followed by Diplolaemus (4), Pristidactylus (4), and Homonota (3) (Fig. 8.2c).
The number of sampled localities was highest in northwestern Patagonia, between 35°–42° S and 74°–70° W (Fig. 8.3a). The density of collected specimens was highest in northwestern Patagonia, the southern portion of Río Negro Province, and the central-northeastern strip of Chubut Province (Fig. 8.3b). The independence between high values of sampled localities vs. number of specimens collected for some cells indicates no absolute positive relationship between these two variables. Species richness was highest in northern Patagonia, west of Río Negro Province, the northeast and southeast strip of Chubut Province, and north of Santa Cruz Province (Fig. 8.3c).
Species richness gradually increased from 34° to 36° S and peaked between 37°–39° S at 49 species, and then gradually decreased toward the south (Fig. 8.4a). This pattern was clearly not correlated with the number of records, which indicated a homogeneous sampling with only a small increase in around 42° S (Fig. 8.4c). The analysis based on longitude showed that on both marine coasts (Pacific and Atlantic) there are very few locality records as well as in the Andean zone between 72° and 73° W, while the largest amount was located between 72°–65° W (Fig. 8.4d). Longitudinally, species richness showed a clear relationship with the number of records. We found the greatest species richness between 71° and 69° W, with a maximum of 75 species (Fig. 8.4b).
Cumulative spatial analysis of Patagonian lizards. References: red lines (implementing movingFun function with “moving average” from R “raster” package); (a) number of accumulated species by latitude; (b) number of accumulated species by longitude; (c) number of accumulated records by latitude; (d) number of accumulated records by longitude
5 Ecological Aspects of Lizards in Patagonia’s Ecoregions
Several studies found a relationship between lizards’ ecology, such as feeding (Celedón-Neghme et al. 2005; Corbalán and Debandi 2014), shelter (Halloy et al. 2013) and thermoregulation (Ibargüengoytía 2005; Cruz et al. 2009), and vegetation of the environments they inhabit. The combination of these aspects that are directly influenced by vegetation type might determine the presence of lizard species (Pietrek et al. 2009; Halloy et al. 2013). Hence, we decided to assess the relationship between vegetation units and lizard richness in Patagonia.
We found lizards in 29 of the 33 vegetation units. We found no lizard records in Evergreen shrub, Monte’s Mountains and Valleys, Western Interior Pampa, and Peat Bogs (Fig. 8.5). The units with the highest species richness (S) in northern and central Argentinean Patagonia, reaching western Chubut Province were the Western District (60) and Typical southern Monte (49), followed in S by Altoandina Province (40) that is restricted to Andean environments. We found low S values in northern Chilean Patagonia, pre-mountain range and south of Santa Cruz Province in Argentina: Sub Andean District—Steppe of white coiron (35), Central District—Quilenbai steppe bushes (30), Erial Central District (28), Deciduous Forest (26), and Payunia District (26). Without a clear spatial pattern, we found an even lower S for the Rionegrino Ecotone (20), Andean—Patagonian Forests and Central District—Mountain steppe bushes (16), and San Jorge Gulf District and Low altitude scrub (14). We found the lowest S values in northern Chile (Resinous Forest: 11, Sclerophyllous Forest: 10, Thorny Forest: 6, Laurel Forest: 8, and Altitude grass: 4) and in vegetation units located south of 41° S (Deciduous scrub and Without vegetation: 5, and Evergreen Forest: 4). Finally, we recorded the lowest S values for Argentina in the northern and eastern strip (Eastern or Transitional Monte: 11, Valdés Península Ecotone: 5 and Endorheic Bolsons: 3) and in the southern strip of Patagonia (Central District: 11, Shrub steppe of black bush: 6, Sub Andean District—Dry Magellanic Steppe: 5 and Fueguino District—Wet Magellanic Steppe: 1). The vegetation units and their species richness are detailed in Fig. 8.1b, c, and vegetation units by species in Table 8.1.
Bar plot with lizard species richness in increasing order for each vegetation unit. References: number of species in blue, Shannon Wiener Index in red; Endorheic Bolsons = BEnd, Deciduous Forest = BC, Sclerophyllous Forest = BEsc, Thorny Forest = BEsp, Laurel Forest = BL, Resinous Forest = BR, Evergreen Forest = BS, Andean—Patagonian Forests = BAP, Central District = DC, Erial Central District = DCE, Central District—Quilenbai steppe bushes = DCEAQ, Central District—Mountain steppe bushes = DCEAS, Payunia District = DLP, San Jorge Gulf District = DGSJ, Fueguino District—Wet Magellanic Steppe = DFEMH, Western District = DO, Sub Andean District—Steppe of white coiron = DSECB, Sub Andean District—Dry Magellanic Steppe = DSEMS, Valdés Península Ecotone = EPV, Rionegrino Ecotone = ER, Shrub steppe of black bush = EAMN, Steppes and pastures = EP, Altitude grass = HA, Low altitude scrub = MBA, Deciduous scrub = MC, Evergreen shrub = MS, Typical southern Monte = MAT, Monte’s Mountains and Valleys = MSB, Eastern or Transitional Monte = MODT, Western Interior Pampa = PIO, Altoandina Province = PA, Peat Bogs = T, and Without vegetation = SV
The Shannon Wiener diversity index (H) is one of the most commonly indices used to characterize species diversity in a community and accounts for both abundance and evenness (EH) of the species present (S) (Colwell 2009). Technically, H quantifies the uncertainty associated with predicting the identity of taxa given the number of taxa and evenness in abundances of individuals within each taxon (Gotelli and Colwell 2001; Chao and Chiu 2016). The S values of the vegetation units varied from 0 to 60 species and the average H indices among the 29 vegetation types ranged from 0 to 3.29. Vegetation units with moderate S values between 1 and 8 (DFEMH, BEnd, BS, HA, DSEMS, EPV, MC, SV, BEsp, EAMN, BL, EP) showed a gradual H increase. DFEMH unit showed only Liolaemus magellanicus presence and BEnd was represented mostly by L. darwinii. BS and HA had the same number of species, but the Eh of BS is lower and is mostly represented by L. pictus, whereas HA is mainly formed by L. kolengh. The units DSEMS, EPV, MC, and SV had five species, but showed different EH. In DSEMS we found L. sarmientoi as the most frequent species, EPV and SV had a more equitable distribution of the species that conform them, and MC is mostly represented by L. scolaroi. The vegetation units BEsp and EAMN had six species with equality components, while BL and EP had eight species, but BL was mostly represented by L. pictus. We found a greater frequency of L. lemniscatus presence for BEsc, L. pictus for BR, L. baguali and L. hatcheri for DC, Homonota darwinii for MODT, L. fitzingerii and L. kingii for DGSJ, L. curis for MBA, and L. elongatus for BAP. DCEAS and ER units showed greater evenness among the taxa present there without a dominant species. The species L. pictus and L. tenius showed high frequency for BC, L. austromendocinus for DLP, L. kingii for DCE, L. inacayali for DCEAQ, L. pictus for PA, and L. darwinii for MAT. Finally, DO and DSECB showed high evenness (Fig. 8.5).
The Jaccard similarity index (Chao et al. 2005) compares members of two datasets to see which members are shared and which are distinct, and ranges from 0 to 1 (1 = no taxon in common; 0 = all taxa in common). It is usually implemented at large spatial scales (e.g., comparing regions) to assess biodiversity considering only species’ presence-absence records (Real et al. 1996). According to these similarity comparisons, none of the 406 paired comparisons among vegetation units had the exact same taxa (Fig. 8.6). Most of the paired comparisons (217) had values of 1, indicating that in most cases vegetation units do not have any lizard species in common (Fig. 8.6). By contrast, 189 comparisons had values less than one (Fig. 8.6) and the greatest similarity was found between BL versus BR (0.16), BS (0.42) and BEsp (0.67), BR versus BS (0.43); and DGSJ versus DCEAS (0.67).
Jaccard similarity index pairwise comparisons between species and vegetation units. References: 0 = identical species, 1 = different species, Endorheic Bolsons = BEnd, Deciduous Forest = BC, Sclerophyllous Forest = BEsc, Thorny Forest = BEsp, Laurel Forest = BL, Resinous Forest = BR, Evergreen Forest = BS, Andean—Patagonian Forests = BAP, Central District = DC, Erial Central District = DCE, Central District—Quilenbai steppe bushes = DCEAQ, Central District—Mountain steppe bushes = DCEAS, Payunia District = DLP, San Jorge Gulf District = DGSJ, Fueguino District—Wet Magellanic Steppe = DFEMH, Western District = DO, Sub Andean District—Steppe of white coiron = DSECB, Sub Andean District—Dry Magellanic Steppe = DSEMS, Valdés Península Ecotone = EPV, Rionegrino Ecotone = ER, Shrub steppe of black bush = EAMN, Steppes and pastures = EP, Altitude grass = HA, Low altitude scrub = MBA, Deciduous scrub = MC, Typical southern Monte = MAT, Eastern or Transitional Monte = MODT, Altoandina Province = PA, and Without vegetation = SV
6 Spatial Patterns for Patagonian Lizard Assemblages
Organism assemblages can provide a conceptual basis for understanding the processes that determine how many, which and how species that inhabit in a particular region relate (Chase 2003). Regional and local factors can determine the patterns by which communities of organisms are assembled, as well as the resulting degree of similarity or difference between localities with similar environments (see Thompson et al. 2016; Palmeirim et al. 2017).
Hierarchical clustering is one of the most typical multivariate ordination and agglomerative analysis for biological communities (McGarigal et al. 2013). These methods start with combining two most similar sites to each other, then they proceed by combining points to points or to groups, or groups to groups using different aggregation criteria (Gardener 2014). We used the vegan R package (Oksanen et al. 2018) to implement a hierarchical clustering from the vegdist’ similar -dissimilarity structure from the “hclust” function (“stats” R base package), which needs dissimilarities as input. Function “hclust” provides several alternative clustering strategies, and we selected the Ward’s minimum variance method. This method minimizes the total within-cluster variance, merging at each step the pair of clusters with minimum between-cluster distance. We found six clusters of vegetation units (Fig. 8.7). All of these clusters had differences higher than one, and within the clusters there were differences in distance among their components. In the first cluster (in red), the most different unit of vegetation was BEsp, and the fourth cluster (cyan) showed differences between HA + SV versus DC, and EP + MP. The fifth cluster (magenta) showed differences between DCEAS + DGSJ + DCE + DO versus BAP + DSECB + DCEAQ + ER, and in the sixth group (yellow) showed differences for DLP + MAT versus BEnd + EPV + MODT (Fig. 8.7). To compare the diversity of species among the vegetation units, we considered the groups defined by this hierarchical clustering.
Species hierarchic clustering using vegetation units as clusters. References: Endorheic Bolsons = BEnd, Deciduous Forest = BC, Sclerophyllous Forest = BEsc, Thorny Forest = BEsp, Laurel Forest = BL, Resinous Forest = BR, Evergreen Forest = BS, Andean—Patagonian Forests = BAP, Central District = DC, Erial Central District = DCE, Central District—Quilenbai steppe bushes = DCEAQ, Central District—Mountain steppe bushes = DCEAS, Payunia District = DLP, San Jorge Gulf District = DGSJ, Fueguino District—Wet Magellanic Steppe = DFEMH, Western District = DO, Sub Andean District—Steppe of white coiron = DSECB, Sub Andean District—Dry Magellanic Steppe = DSEMS, Valdés Península Ecotone = EPV, Rionegrino Ecotone = ER, Shrub steppe of black bush = EAMN, Steppes and pastures = EP, Altitude grass = HA, Low altitude scrub = MBA, Deciduous scrub = MC, Typical southern Monte = MAT, Eastern or Transitional Monte = MODT, Altoandina Province = PA, and Without vegetation = SV
The species richness metric increases with sample size, and differences in richness actually may be caused by differences in sample size. To address this potential bias, we used rarefaction curves to rarefy species richness to the same number of individuals, because they are often seen as an objective solution for comparing species richness with different sample sizes. We implemented rarefaction curves and evaluated them using the interval of step sample sizes, determined by the minimum sample count achieved over each cluster. The number of expected species was between 2.42 and 4.25 for BEnd, EPV, MODT, and MAT at the same minimal subsample of 5. The number of expected species within clusters were BAP (15.88), DCEAS (15.88), DGSJ (13.56), ER (19.35), DSECB (33), DCEAQ (26.56), DCE (24.88), and DO (46.76) at the minimal subsample of 346. The number of expected species within clusters were SV (5), HA (3.80), MC (3.10), EP (5.72), and DC (6.30) at the minimal subsample of 19. The number of expected species within clusters was DFEMH (1), DSEMS (2.96), and EAMN (4.37) at the minimal subsample of 10. The number of expected species within clusters was MBA (14), BEsc (9.40), BC (23.34), and PA (29.80) at the minimal subsample of 205. The number of expected species within clusters was BEsp (6.00), BS (3.12), BL (5.55), and BR (7.50) at the minimal subsample of 34 (Fig. 8.8).
Rarefy curves with the expected species richness in random subsamples of size sample among each cluster. Vertical line is drawn at minimum sample and horizontal lines for the rarefied species richnesses. References: Endorheic Bolsons = BEnd, Deciduous Forest = BC, Sclerophyllous Forest = BEsc, Thorny Forest = BEsp, Laurel Forest = BL, Resinous Forest = BR, Evergreen Forest = BS, Andean—Patagonian Forests = BAP, Central District = DC, Erial Central District = DCE, Central District—Quilenbai steppe bushes = DCEAQ, Central District—Mountain steppe bushes = DCEAS, Payunia District = DLP, San Jorge Gulf District = DGSJ, Fueguino District—Wet Magellanic Steppe = DFEMH, Western District = DO, Sub Andean District—Steppe of white coiron = DSECB, Sub Andean District—Dry Magellanic Steppe = DSEMS, Valdés Península Ecotone = EPV, Rionegrino Ecotone = ER, Shrub steppe of black bush = EAMN, Steppes and pastures = EP, Altitude grass = HA, Low altitude scrub = MBA, Deciduous scrub = MC, Typical southern Monte = MAT, Eastern or Transitional Monte = MODT, Altoandina Province = PA, and Without vegetation = SV
The parameters referring to landscape structure (e.g., altitude) are essential in any biogeography study given the relationship between landscape structure and the ecological processes (Jongman et al. 1995). In the altitude analysis, we found a higher limit between 2100 and 2400 m a.s.l. for the genera Phymaturus, Pristidactylus, Diplolaemus, Liolaemus, and Homonota. On the other hand, Leiosaurus, Aurivela, and Amphisbaena showed an upper limit of 1400 m a.s.l., while Teius, Salvator, and Stenocercus did not exceed 800 m a.s.l. (Fig. 8.9). Within the 34 Phymaturus taxa we found a group of species above 1450 m a.s.l.: P. palluma, P. verdugo, P. roigorum, P. tromen, P. payuniae, P. maulense, P. dorsimaculatus, P. nevadoi, P. delheyi, and P. sitesi up to a maximum of 2784 m a.s.l. We identified another group of species consisting of P. vociferator, P. querque, P. zapalensis, P. somuncurensis, P. cacivioi, P. rahuensis, P. excelsus, P. desuetus, P. curivilcun, P. camilae, P. etheridgei, P. ceii, P. spurcus, P. tenebrosus, P. spectabilis, and P. sinervoi between 865 and 1450 m a.s.l. Finally, we found P. manuelae, P. videlai, P. calcogaster, P. yachanana, P. indistinctus, P. felixi, P. castillensis, and P. patagonicus between 33 and 865 m a.s.l. (Fig. 8.10). Among the 110 Liolaemus taxa we found a group of species above 2365 m a.s.l.: L. punmahuida, L. villaricensis, L. riodamas, L. thermarum, and L. antumalguen up to a maximum height of 3000 m a.s.l. Another group with a narrow range of altitude was formed by L. wiegmanni, L. yatel, L. brattstroemi, L. camarones, L. martorii, and L. multimaculatus between 0 and 64 m a.s.l. The rest of the Liolaemus taxa presented broader ranges or appeared to have restricted ranges, but those last ones actually had only few of geographical locations (Fig. 8.11).
7 Conclusions
The analyses performed for this chapter allowed us to transform an extensive review into synthetic results about the biogeography and species diversity of lizards in the vast Patagonian region. The increasing number of field samples, and the subsequent descriptions of new species during the last decades, considerably improved our understanding and knowledge of lizard species’ distributions in the extreme south of South America. The geographic distribution data presented here should be permanently updated and subject to revisions by specialists in taxonomy, systematics, and phylogeography, since there are numerous active research programs, which during the last 15 years have described, revalidated, and synonymized species as well as their distributions (mostly within Liolaemus and Phymaturus genera) (Avila et al. 2008; Abdala et al. 2012a, b; Vera-Escalona et al. 2012; Olave et al. 2014, 2015; Lobo et al. 2016; Troncoso-Palacios et al. 2016, 2018). To sum up, our spatial analyses improve our understanding of geographic distributions in one of the regions with the greatest diversity of lizards in the world and we provide ecological and spatial metrics with an updated list of taxa for Patagonian lizards. We highlight that the framework implemented here allows the analysis of large volumes of information and geographic space to obtain quantitative results that can support the study of spatial patterns of biodiversity, decision-making to design studies, use of resources, and creation of protected areas.
References
Abdala CS, Díaz Gómez JM, Juarez Heredia VI (2012a) From the far reaches of Patagonia: new phylogenetic analyses and description of two new species of the Liolaemus fitzingerii clade (Iguania: Liolaemidae). Zootaxa 3301:34–60
Abdala CS, Semhan RV, Moreno Azócar DL et al (2012b) Taxonomic study and morphology based phylogeny of the patagonic clade Liolaemus melanops group (Iguania: Liolaemidae), with the description of three new taxa. Zootaxa 3163:1–32
Aguiar MR, Paruelo JM, Sala OE, Lauenroth WK (1996) Ecosystem responses to changes in plant functional type composition: an example from the Patagonian steppe. J Veg Sci 7:381–390. https://doi.org/10.2307/3236281
Albino AM (2011) Evolution of Squamata Reptiles in Patagonia based on the fossil record. Biol J Linn Soc 103:441–457
Ares J, Beeskow A, Bertiller M et al (1995) Structural and dynamic characteristics of overgrazed lands of northern Patagonia, Argentina. In: Breymeyer A (ed) Managed grasslands. Elsevier Science Publishers B.V, Amsterdam, pp 149–175
Avila LJ, Morando M, Sites JW Jr (2008) New species of the iguanian lizard genus Liolaemus (Squamata, Iguania, Liolaemini) from Central Patagonia, Argentina. J Herpetol 42:186–196. https://doi.org/10.1670/06-244r2.1
Barreda V, Palazzesi L (2007) Patagonian vegetation turnovers during the Paleogene-early Neogene: origin of arid-adapted floras. Bot Rev 73:31–50. https://doi.org/10.1663/0006-8101(2007)73[31:PVTDTP]2.0.CO;2
Barros VR, Boninsegna JA, Camilloni IA et al (2015) Climate change in Argentina: trends, projections, impacts and adaptation. WIREs Clim Change 6:151–169. https://doi.org/10.1002/wcc.316
Becker RA, Wilks AR (2018) Maps: draw geographical maps. https://CRAN.R-project.org/package=maps. Accessed 3 Aug 2018
Bisigato AJ, Bertiller MB (1997) Grazing effects on patchy dryland vegetation in northern Patagonia. J Arid Environ 36:639–653. https://doi.org/10.1006/jare.1996.0247
Bivand R, Lewin-Koh N (2018) maptools: tools for reading and handling spatial objects. R package version 0.9-2. https://CRAN.R-project.org/package=maptools. Accessed 28 Mar 2016
Bivand R, Rundel C (2018) rgeos: interface to geometry engine – open source (GEOS). R package version 0.3-28. https://CRAN.R-project.org/package=rgeos. Accessed 28 Mar 2016
Bivand R, Keitt T, Rowlingson B (2018) rgdal: bindings for the geospatial data abstraction library. R package version 1.3-3. https://CRAN.R-project.org/package=rgdal. Accessed 28 Mar 2016
Böhm M, Collen B, Baillie JEM et al (2013) The conservation status of the world’s reptiles. Biol Conserv 157:372–385. https://doi.org/10.1016/j.biocon.2012.07.015
Brunsdon C, Chen H (2014) GISTools: Some further GIS capabilities for R. https://CRAN.R-project.org/package=GISTools. Accessed 3 Aug 2018
Buddle CM, Beguin J, Bolduc E et al (2005) The importance and use of taxon sampling curves for comparative biodiversity research with forest arthropod assemblages. Can Entomol 137:120–127. https://doi.org/10.4039/n04-040
Burkart R, Bárbaro NO, Sánchez RO, Gómez DA (1999) Ecorregiones de la Argentina. Administración de Parques Nacionales, Buenos Aires
Cabrera AL (1976) Regiones Fitogeográficas Argentinas. Enciclopedia Argentina de Agricultura y Jardinería
Cabrera AL (1994) Regiones fitogeograficas argentinas. Buenos Aires
Celedón-Neghme C, Salgado CR, Victoriano PF (2005) Preferencias alimentarias y potencial dispersor del lagarto herbívoro Phymaturus flagellifer (Tropiduridae) en Los Andes. Gayana (Concepc) 69
Cesa A, Paruelo JM (2011) Changes in vegetation structure induced by domestic grazing in Patagonia (Southern Argentina). J Arid Environ 75:1129–1135. https://doi.org/10.1016/j.jaridenv.2011.04.003
Chamberlain S, Barve V, Mcglinn D et al (2018) rgbif: Interface to the Global “Biodiversity” Information Facility API. https://CRAN.R-project.org/package=rgbif. Accessed 3 Aug 2018
Chao A, Chiu C-H (2016) Species richness: estimation and comparison. In: Balakrishnan N, Colton T, Everitt B et al (eds) Wiley StatsRef: statistics reference online. Wiley, Chichester, pp 1–26
Chao A, Chazdon RL, Colwell RK, Shen T-J (2005) A new statistical approach for assessing similarity of species composition with incidence and abundance data. Ecol Lett 8:148–159. https://doi.org/10.1111/j.1461-0248.2004.00707.x
Chase JM (2003) Community assembly: when should history matter? Oecologia 136:489–498. https://doi.org/10.1007/s00442-003-1311-7
Clements F, Shelford V (1939) Bio-cology. Wiley/Chapman & Hall, New York/London
Colwell RK (2009) Biodiversity: concepts, patterns, and measurement. In: The Princeton guide to ecology. pp 257–263
Cook CN, Possingham HP, Fuller RA (2013) Contribution of systematic reviews to management decisions: systematic reviews. Conserv Biol 27:902–915. https://doi.org/10.1111/cobi.12114
Cooper WE (1997) Correlated evolution of prey chemical discrimination with foraging, lingual morphology and vomeronasal chemoreceptor abundance in lizards. Behav Ecol Sociobiol 41:257–265. https://doi.org/10.1007/s002650050387
Corbalán V, Debandi G (2014) Resource segregation in two herbivorous species of mountain lizards from Argentina. Herpetol J 24:201–208
Cox CB, Moore PD (2010) Biogeography: an ecological and evolutionary approach. Wiley
Cruz FB, Belver L, Acosta JC et al (2009) Thermal biology of Phymaturus lizards: evolutionary constraints or lack of environmental variation? Zoology 112:425–432
Dengler J (2009) A flexible multi-scale approach for standardised recording of plant species richness patterns. Ecol Indic 9:1169–1178. https://doi.org/10.1016/j.ecolind.2009.02.002
Frost DR, Etheridge R, Janies D, Titus TA (2001) Total evidence, sequence alignment, evolution of polychrotid lizards, and a reclassification of the Iguania (Squamata: Iguania). Am Mus Novit:1–39. https://doi.org/10.1206/0003-0082(2001)343<0001:tesaeo>2.0.co;2
Gamble T, Bauer AM, Greenbaum E, Jackman TR (2008) Out of the blue: a novel, trans-Atlantic clade of geckos (Gekkota, Squamata). Zool Scr 37:355–366. https://doi.org/10.1111/j.1463-6409.2008.00330.x
Gardener M (2014) Community ecology: analytical methods using R and Excel. Pelagic Publishing Ltd
Gotelli NJ, Colwell RK (2001) Quantifying biodiversity: procedures and pitfalls in the measurement and comparison of species richness. Ecol Lett 4:379–391. https://doi.org/10.1046/j.1461-0248.2001.00230.x
Gray JE (1827) A synopsis of the genera of the saurian reptiles, in which some new genera are indicated, and the others reviewed by actual examination. Philos Mag 2:54–58. https://doi.org/10.1080/14786442708675620
Gray JE (1865) A revision of the genera and species of amphisbaenians, with the descriptions of some new species now in the collections of the British Museum. Proc Zool Soc London 1865:442–455
Halloy M, Robles C, Salica MJ et al (2013) Aportes en el estudio del comportamiento y ecología de lagartijas de los géneros Liolaemus y Phymaturus (Iguania: Liolaemini). Cuad Herpetol 27:15–26
Hijmans RJ (2015) Geosphere: spherical trigonometry. R package version 1.5-7. https://CRAN.R-project.org/package=geosphere. Accessed 28 Mar 2016
Hijmans RJ (2017) Raster: geographic data analysis and modeling. R package version 2.6-7. https://CRAN.R-project.org/package=raster. Accessed 28 Mar 2016
Ibargüengoytía NR (2005) Field, selected body temperature and thermal tolerance of the syntopic lizards Phymaturus patagonicus and Liolaemus elongatus (Iguania: Liolaemidae). J Arid Environ 62:435–448
Jongman R, Ter Braak C, Van Tongeren O (1995) Data analysis in community and landscape ecology. Cambridge University Press
León RJC, Bran D, Collantes M et al (1998) Grandes unidades de vegetación de la Patagonia extra andina. Ecol Austral:125–144
Liang M, Buckley LB, Huey RB, Du W-G (2018) A global test of the cold-climate hypothesis for the evolution of viviparity of squamate reptiles. Glob Ecol Biogeogr 27:679–689. https://doi.org/10.1111/geb.12730
Lobo F, Barrasso DA, Hibbard T, Basso NG (2016) On the evolution and diversification of an Andean clade of reptiles: combining morphology and DNA sequences of the palluma group (Liolaemidae: Phymaturus). Zool J Linnean Soc 176:648–673. https://doi.org/10.1111/zoj.12335
Luebert F, Pliscoff P (2017) Sinopsis bioclimática y vegetacional de Chile, 2nd edn. Editorial Universitaria, Santiago
Maechler M, Rousseeuw P, Struyf A et al (2018) Cluster: “Finding Groups in Data”: cluster analysis extended. https://CRAN.R-project.org/package=cluster. Accessed 3 Aug 2018
Markgraf V, McGlone M, Hope G (1995) Neogene paleoenvironmental and paleoclimatic change in southern temperate ecosystems — a southern perspective. Trends Ecol Evol 10:143–147. https://doi.org/10.1016/S0169-5347(00)89023-0
Masiokas MH, Villalba R, Luckman BH et al (2008) 20th-century glacier recession and regional hydroclimatic changes in northwestern Patagonia. Glob Planet Chang 60:85–100. https://doi.org/10.1016/j.gloplacha.2006.07.031
McGarigal K, Cushman SA, Stafford S (2013) Multivariate statistics for wildlife and ecology research. Springer Science & Business Media
Morello J, Matteucci S, Rodríguez A, Silva M (eds) (2012) Ecorregiones y complejos ecosistémicos argentinos, Primera edición. Facultad de Arquitectura, Diseño y Urbanismo, GEPAMA Grupo de Ecología del Paisaje y Medio Ambiente, Universidad de Buenos Aires, Buenos Aires
Morrone JJ (2001a) Biogeografía de América Latina y el Caribe. M&T–Manuales & Tesis SEA, Zaragoza
Morrone JJ (2001b) Review of the biogeographic provinces of the Patagonian subregion. Rev Soc Entomol Argent 60:1–8
Oksanen J, Blanchet FG, Friendly M et al (2018) Vegan: community ecology package. https://CRAN.R-project.org/package=vegan. Accessed 3 Aug 2018
Olave M, Avila LJ, Sites JW, Morando M (2014) Multilocus phylogeny of the widely distributed South American lizard clade Eulaemus (Liolaemini, Liolaemus). Zool Scr 43:323–337. https://doi.org/10.1111/zsc.12053
Olave M, Avila LJ, Sites JW, Morando M (2015) Model-based approach to test hard polytomies in the Eulaemus clade of the most diverse South American lizard genus Liolaemus (Liolaemini, Squamata). Zool J Linn Soc. https://doi.org/10.1111/zoj.12231
Olea RA (1984) Sampling design optimization for spatial functions. Math Geol 16:369–392. https://doi.org/10.1007/BF01029887
Oppel M (1811) Die Ordnungen, Familien und Gattungen der reptilien als prodom einer naturgeschichte derselben. Joseph Lindauer Verlag, München
Oyarzabal M, Clavijo J, Oakley L et al (2018) Unidades de vegetación de la Argentina. Ecol Austral 28:040–063
Palmeirim AF, Vieira MV, Peres CA (2017) Herpetofaunal responses to anthropogenic forest habitat modification across the neotropics: insights from partitioning β-diversity. Biodivers Conserv:1–15. https://doi.org/10.1007/s10531-017-1394-9
Paruelo JM, Beltran A, Jobbagy E et al (1998) The climate of Patagonia: general patterns and controls on biotic processes. Ecol Austral 8:85–101
Pebesma E, Bivand R (2005) sp: classes and methods for spatial data in R. In: R News 5 (2). http://cran.r-project.org/doc/Rnews/. Accessed 28 Mar 2016
Pianka ER (1986) Ecology and natural history of desert lizards: analyses of the ecological niche and community structure. Princeton University Press
Pietrek AG, Walker RS, Novaro AJ (2009) Susceptibility of lizards to predation under two levels of vegetative cover. J Arid Environ 73:574–577. https://doi.org/10.1016/j.jaridenv.2008.12.019
Pincheira-Donoso D, Hodgson DJ, Tregenza T (2008) The evolution of body size under environmental gradients in ectotherms: why should Bergmann’s rule apply to lizards? BMC Evol Biol 8:1–13. https://doi.org/10.1186/1471-2148-8-68
Pincheira-Donoso D, Harvey LP, Ruta M (2015) What defines an adaptive radiation? Macroevolutionary diversification dynamics of an exceptionally species-rich continental lizard radiation. BMC Evol Biol 15:153. https://doi.org/10.1186/s12862-015-0435-9
Ponce JF, Rabassa J, Coronato A, Borromei AM (2011) Palaeogeographical evolution of the Atlantic coast of Pampa and Patagonia from the last glacial maximum to the Middle Holocene. Biol J Linn Soc 103:363–379. https://doi.org/10.1111/j.1095-8312.2011.01653.x
Pyron RA, Burbrink FT (2014) Early origin of viviparity and multiple reversions to oviparity in squamate reptiles. Ecol Lett 17:13–21. https://doi.org/10.1111/ele.12168
R Core Team (2018) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna. http://www.R-project.org/. Accessed 15 Mar 2018
Rabassa J (2008) Late Cenozoic Glaciations in Patagonia and Tierra del Fuego. In: Rabassa J (ed) Developments in quaternary sciences. Elsevier, pp 151–204
Rabassa J, Coronato A (2009) Glaciations in Patagonia and Tierra del Fuego during the Ensenadan stage/age (early Pleistocene–earliest middle Pleistocene). Quat Int 210:18–36. https://doi.org/10.1016/j.quaint.2009.06.019
Real R, Vargas JM, Olmstead R (1996) The probabilistic basis of Jaccard’s index of similarity. Syst Biol 45:380–385. https://doi.org/10.1093/sysbio/45.3.380
Roig AF (1998) La vegetación de la Patagonia. In: Correa MN (ed) Flora Patagónica. Colección científica del INTA, Buenos Aires, pp 48–174
Ruzzante D, Rabassa J (2011) Palaeogeography and palaeoclimatology of Patagonia: effects on biodiversity. Biol J Linn Soc 103:221–228
Sanmartín I (2012) Historical biogeography: evolution in time and space. Evolution 5:555–568. https://doi.org/10.1007/s12052-012-0421-2
Schulze E-D, Mooney HA, Sala OE et al (1996) Rooting depth, water availability, and vegetation cover along an aridity gradient in Patagonia. Oecologia 108:503–511. https://doi.org/10.1007/bf00333727
Sodhi NS, Ehrlich PR (1995) Conservation biology for all. Oxford University Press
Spellerberg IF, Sawyer JWD, Whitten T (1999) An introduction to applied biogeography, 1st edn. Cambridge University Press, Cambridge
Tennekes M (2018) tmaptools: thematic map tools. https://CRAN.R-project.org/package=tmaptools. Accessed 3 Aug 2018
Thompson ME, Nowakowski AJ, Donnelly MA (2016) The importance of defining focal assemblages when evaluating amphibian and reptile responses to land use. Conserv Biol 30:249–258. https://doi.org/10.1111/cobi.12637
Troncoso-Palacios J, Díaz HA, Puas GI et al (2016) Two new Liolaemus lizards from the Andean highlands of Southern Chile (Squamata, Iguania, Liolaemidae). ZooKeys 632:121–146. https://doi.org/10.3897/zookeys.632.9528
Troncoso-Palacios J, Esquerré D, Urra FA et al (2018) The true identity of the new world iguanid lizard Liolaemus chillanensis Müller and Hellmich 1932 (Iguania: Liolaemidae) and description of a new species in the Liolaemus elongatus group. Zool Stud:1–19
VanDerWal J, Falconi L, Januchowski S, Shoo L, Storlie C (2014) SDMTools: Species Distribution Modelling Tools: Tools for processing data associated with species distribution modelling exercises. https://CRAN.R-project.org/package=SDMTools. Accessed 3 Aug 2018
Vera-Escalona I, D’Elía G, Gouin N et al (2012) Lizards on ice: evidence for multiple refugia in Liolaemus pictus (Liolaemidae) during the last glacial maximum in the southern andean beech forests. PLoS One 7:e48358. https://doi.org/10.1371/journal.pone.0048358
White D (2000) Global grids from recursive diamond subdivisions of the surface of an octahedron or icosahedron. Environ Monit Assess 64:93–103. http://dx.doi.org.erl.lib.byu.edu/10.1023/A:1006407023786
White D, Kimerling JA, Overton SW (1992) Cartographic and geometric components of a global sampling design for environmental monitoring. Cartogr Geogr Inf Syst 19:5–22. https://doi.org/10.1559/152304092783786636
Yfantis EA, Flatman GT, Behar JV (1987) Efficiency of kriging estimation for square, triangular, and hexagonal grids. Math Geol 19:183–205. https://doi.org/10.1007/BF00897746
Acknowledgements
We thank all curators of the consulted collections, P. F. Victoriano and J. Troncoso-Palacios for Chilean localities, past and present members of the Grupo de Herpetología Patagónica for help in field trips and laboratory tasks, in special to N. Frutos, M. L. Kozykariski, N. Feltrin (in memoriam), M. F. Breitman, C. D. Medina, J. Goldman, C. A. Durante, R. Neyro Martínez and M. Olave; F. Lobo for museum data, L. C. Belver for specimens data and D. E. Udrizar Sauthier for donation of some specimens.
Financial support for fieldwork was provided by a CONICET fellowship issued to I. Minoli, grants from ANPCYT (FONCYT PICT 2006-00506, 33789, 2011-0784, 1397), and CONICET (PEI 2001-6397, PIP 2005-6469), several small grants from Brigham Young University (including Monte L. Bean Museum, College of Agriculture and Biology, Department of Biology, Kennedy Center for International Studies) (granted to J. W. Sites Jr.), and mainly from the National Science Foundation grant “Partnership for International Research and Education” award (OISE 0530267) for support for collaborative research on Patagonian biodiversity granted to the following institutions (listed alphabetically): Brigham Young University, Centro Nacional Patagónico, Dalhousie University, Darwinion Botanical Institute, Universidad Austral de Chile, Universidad Nacional del Comahue, Universidad Nacional de Córdoba, Universidad de Concepción, and University of Nebraska (issued to J. Johnson). This chapter was written within the framework of the PUE-IPEEC-2016 22920160100044. LJA and MM are CONICET researchers, IM CONICET fellowships and CHFP CONICET technical assistant.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Minoli, I., Pérez, C.H.F., Morando, M., Avila, L.J. (2020). Biogeography, Ecology, and Spatial Patterns of Patagonian Lizards. In: Morando, M., Avila, L.J. (eds) Lizards of Patagonia. Natural and Social Sciences of Patagonia. Springer, Cham. https://doi.org/10.1007/978-3-030-42752-8_8
Download citation
DOI: https://doi.org/10.1007/978-3-030-42752-8_8
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-42751-1
Online ISBN: 978-3-030-42752-8
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)