Abstract
Dietary lipids play a major role in infant nutrition, development and health. As an alternative to breast milk, infant formulae (IF) are the manufactured products given to infants. However, many differences exist between breast milk fat globules that are naturally secreted by lactating mothers and the processed lipid droplets formed under pressure during homogenization and found in IF. The lipid composition and structure of the emulsion in IF could be improved to mimick breast milk fat globules. This book chapter i) describes breast milk fat globules covered by their biological membrane (MFGM), including their functions and digestion in the gastrointestinal tract of infants, ii) presents the technological steps involved in IF preparation and the blending of oils with other ingredients leading to the final composition and structure of processed lipid droplets, iii) highlights the health benefits for infants of adding dairy lipids (Fatty acids, TAG, MFGM rich in phospholipids, sphingolipids, cholesterol and glycoproteins) in IF, iv) explains the opportunities to produce food-grade ingredients enriched in bovine MFGM and to prepare processed lipid droplets in IF bio-inspired by breast milk fat globules. The next generation of IF will integrate the advantages provided by dairy lipids to improve the quality of IF and bring nutritional and heath benefits to infants worldwide.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
1 Introduction
Dietary lipids play a major role in infant nutrition, development and health. The lipids are quantitatively important as providers of energy during the early months of life, qualitatively important as providers of bioactive molecules and of major importance as structured molecules organized in the form of complex supramolecular assemblies. The biological fluid secreted by lactating mothers, i.e. human breast milk, is a complex and unique fluid that evolution adapted to satisfy neonatal needs. Lipids contribute the major portion (45–55%) of the energy contained in human milk (metabolizable energy content of human milk is about 65–70 kcal/100 mL), with a total fat intake of approximately 25 g/day in a fully breast-fed infant during the 6 months of life (Grote et al., 2016; Innis, 2011). The other sources of energy provided by breast milk are lactose and proteins. Breast milk lipids also constitute a source of bioactive molecules (e.g. the polyunsaturated fatty acids of the n-6 and n-3 series) and structural components (phospholipids, sphingolipids, cholesterol) of functional importance. Breast milk lipids act as vehicles for transport and absorption of lipid-soluble compounds such as vitamins (A, D, E and K). Lipids in human milk are extremely complex and include a wide diversity of molecular species. Although the precise physiological roles of milk lipids are not yet fully understood, they are known to modulate gastrointestinal function, lipoprotein metabolism, membrane composition and function, signaling pathways, that greatly affect infant growth, development and health.
Breast milk is the gold standard for neonatal nutrition, highly recommended as the exclusive component of the infant’s diet up to 6 months of life (AAP, 2012; WHO, 2011). However, when breast-feeding is not possible, infant formulae (IF) are the best alternative. The best way to improve IF quality and functions in infants is to mimic both the composition and structure of human breast milk. Much interest has been developed in the last decades on the qualitative importance of dietary lipids given to infants, for example, the amount of polyunsaturated fatty acids (PUFA) and the relative intake of PUFA from n-3 and n-6 series. More recently, the importance of the positional distribution of fatty acids on the 3 sn-positions of the triacylglycerol (TAG) molecules has raised since it is involved in the digestion mechanisms and has nutritional impacts for the infants. Very recently, the supramolecular organization of lipids in emulsion droplets has gained much attention due to differences existing between the large fat globules covered by a biological membrane rich in polar lipids, glycoproteins and cholesterol naturally secreted in breast milk, and the tiny processed lipid droplets covered by milk proteins and devoid of biological membrane components produced in IF. Most of IF are manufactured with blends of vegetable oils and DHA-rich oils to provide the essential fatty acids. However, the use of bovine milk lipids in IF is currently increasing.
This chapter provides information about the lipids in breast milk and in IF. The health benefits and opportunities provided by the introduction of milk lipids in IF are developed.
2 Breast Milk Fat Globules: Unique Lipid Assemblies Covered by a Biological Membrane
Human breast milk from healthy mothers is a timely-adapted and balanced source of nutrients and bioactive compounds ensuring proper growth and development of infants. Breast-feeding is, therefore, the gold standard for early nutrition of full-term infants up to 6 months of age and is highly recommended to provide the benefits of human milk components (WHO, 2011). Among all breast milk components, lipids are of high importance.
2.1 Composition of Breast Milk Lipids
Lipids are the most variable components of human milk, including quantitative and qualitative variations. The fat content of breast milk is markedly influenced by the stage of lactation (lower fat content in colostrum during early lactation compared to mature milk), the mother’s diet and genetic background (Demmelmair & Koletzko, 2018). During weaning, the fat content increases from the fore-milk (1–3 g/100 mL) to the hind-milk (5–8 g/100 mL) secreted by mothers of term infants (Saarela, Kokkonen, & Koivisto, 2005). Variations in breast milk fat content also occur over 24 h (Khan et al., 2013). Breast milk contains 2–8 g of lipids per 100 mL, with an average around 3.5–4.5 g/100 mL. About 98% of milk lipids correspond to hydrophobic molecules called triacylglycerols (TAG; tri-esters of fatty acids and glycerol). Other quantitatively minor lipids of high nutritional importance include the polar lipids that represent 0.2–1% of milk lipids and the sterols (mainly cholesterol) that account for about 0.2–0.4%. The lipids in human milk have been the focus of reviews (Demmelmair & Koletzko, 2018; Jensen, 1999; Koletzko, 2016).
2.1.1 From the Fatty Acid Composition to the Structure of Triacylglycerols
Fatty Acid Composition
Fatty acids from both colostrum and mature human milks exhibit variations. Colostrum fatty acid profile was reported to be mainly dependent on maternal nationality and age rather than mode of delivery and maternal body mass index (Fidler & Koletzko, 2000; Sinanoglou et al., 2017). Mature breast milk fatty acid profile varies greatly depending on geographical location and dietary habits of the lactating mothers and also during lactation. However, major trends can be defined regarding fatty acid composition of breast milk.
Table 15.1 shows the fatty acid composition of human breast milk. Breast milk contains fatty acids with 10–24 carbon atoms. It is rich in long-chain saturated fatty acids (34–47%), such as palmitic acid (C16:0; 15–25%), stearic acid (C18:0; 5–11%), myristic acid (C14:0; 5–9%), which constitute important sources of energy for the infants. Breast milk contains a high amount of monounsaturated fatty acids (31–43%), mainly the oleic acid (C18:1 n-9; 24–37%) and substantial amounts of polyunsaturated fatty acids (PUFA) of the n-6 series (n-6 PUFA; 12–26%) and n-3 series (n-3 PUFA; 0.8–3.6%) that are of utmost importance for infant health. The PUFA linoleic acid (LA: C18:2 n-6; 6–24%) and alpha-linolenic acid (ALA: C18:3 n-3; 0.25–3.4%) are recognized as dietary essential fatty acids because of the inability of animal tissues to introduce the necessary double bonds in the carbon chain before carbon 9. The increase in the maternal diet of LA in the last 60 years is reflected in a significant increase in the LA content of breast milk. The level of ALA has remained fairly stable during this same period, resulting in a marked increase in the LA/ALA ratio from approximately 6–8% before 1970 to 14–16% since 1980 (Ailhaud et al., 2006). LA (n-6 PUFA) and ALA (n-3 PUFA) are precursors of long-chain PUFA. The negative impact of n-6 PUFA in excess has been evidenced. Studies showed that high LA intake inhibits n-3 fatty acid synthesis in humans and DHA incorporation in tissues. The Eden Study showed that a high LA content in the colostrum could limit the benefit of colostrum DHA on cognition in children (Bernard et al., 2017).
Breast milk contains long-chain PUFA that are essential in infants for brain and retina development, i.e. the docosahexaenoic acid (DHA: C22:6n-3; traces—0.8%) and eicosapentaenoic acid (EPA: C20:5n-3; traces—0.25%), for biological membranes and nervous system, i.e. the arachidonic acid (ARA: C20:4n-6; traces —0.9%). Long-chain PUFA serve as indispensable structural components of cellular membranes and are deposited to a considerable extent in the growing brain and the retina during perinatal development. The supply of preformed long-chain PUFA with human milk lipids has been related to functional outcomes of the recipient infants such as visual acuity and development of cognitive functions during the first year of life. Breast milk in many Western countries has a ARA/DHA ratio of approximately 2/1. Many Asian and Scandinavian breast milks have much lower ARA/DHA ratios because of a higher consumption of DHA-rich fish. The content in PUFA varies during lactation. During the course of the first year of lactation, the contents of both LA (200 mg/dL) and ALA (20 mg/dL) in human milk increase by 8–38% whereas the contents of long-chain PUFA ARA (15–16 mg/dL) and DHA (7–8 mg/dL) decrease by 32–52% (Koletzko, 2016).
TAG Composition and Intramolecular Structure
Human milk fat consists of many TAG molecular species (Figs. 15.1 and 15.2). More than 100 different TAG molecules were detected in human milk fat samples (Kallio, Nylund, Bostrom, & Yang, 2017; Kim, Park, & Shim, 2015; Linderborg et al., 2014; Zhang et al., 2019). The composition of TAG in human milk can vary depending on the region, lactation and diet. The same fatty acid can be in different sn-position on the glycerol backbone, e.g. O-P-O and O-O-P (sn1-sn2-sn3; O = oleic acid, C18:1n-9; P = palmitic acid, C16:0). However, the breast milk fatty acids are not randomly distributed on the TAG molecules: a regio-specificity exists for each fatty acid. The saturated fatty acids, such as C16:0, are largely located in the sn-2 position (C16:0 >70% at sn-2 position in human milk; Table 15.2). The unsaturated fatty acids, such as C18:1n-9 and C18:2n-6 (LA), are mainly located in the sn-1 and sn-3 positions of human milk TAG (Innis, 2011; Jensen, 1999). The “Unsaturated-Saturated-Unsaturated” structure of TAG accounts for about 49% of the total TAG content in breast milk from China (Zhang et al., 2019).
Breast milk fat globules: lipid assemblies with a core of triacylglycerols covered by a biological membrane. Confocal laser scanning microscopy (CLSM) images show in red the fluid matrix of polar lipids, in black the micro-domains rich in sphingomyelin and cholesterol, and in yellow/green the glycoproteins in the milk fat globule membrane (MFGM). A schematic representation of the human MFGM is proposed. Adapted from Lopez and Ménard (2011)
Main triacylglycerol (TAG) molecules in human breast milk fat. Adapted from Zhang et al. (2019). Abbreviations of fatty acids: O= C18:1n-9; P= C16:0; Ca= C10:0; M= C14:0; La= C12:0; L= C18:2n-6; S= C18:0; Po= C16:1
The regio-distribution of the fatty acids on the three sn- positions of the glycerol backbone in TAG molecules, and more particularly the “Unsaturated-Saturated-Unsaturated” structure of dietary TAG, are of importance regarding TAG digestion and absorption in the gastrointestinal tract of infants given breast milk (Fig. 15.3) (Innis, 2011; Mu & Hoy, 2004). Indeed, gastric lipase hydrolyses most specifically the fatty acids located on the sn-3 position while pancreatic lipase has a specificity for external positions (sn-1 and sn-3). Lipids are absorbed by infants in the form of sn-2 monoacylglycerols (sn-2 MAG; Fig. 15.3) and in the form of lipid micelles containing non-esterified fatty acids from the hydrolysis of sn-1 and sn-3 positions of TAG. The esterification of palmitic acid (C16:0) on the sn-2 position of TAG in breast milk favors its intestinal absorption as sn-2 monoacylglycerols that is utilized for resynthesis of chylomicron TAG for providing energy to the infants. The non-esterified (free) saturated fatty acids released upon enzymatic hydrolysis, that were located in the sn-1 and sn-3 positions of TAG molecules, can interact in the intestine with calcium ions and form insoluble soaps that will be lost in the feces (Fig. 15.3). This would cause a considerable loss of energy for the infants. The advantages of C16:0 at the sn-2 position in human milk for fat and mineral absorption, behavior of the infants have recently been summarized in systematic and narrative reviews (Bar-Yoseph, Lifshitz, & Cohen, 2013; Miles & Calder, 2017; Petit, Sandoz, & Garcia-Rodenas, 2017). Table 15.3 shows the amount of C16:0 esterified in the sn-2 position of various sources of dietary fats (human milk fat, bovine milk fat, vegetable oils) and structured TAG.
2.1.2 Polar Lipids
Breast milk contains polar lipids, at levels accounting for 0.2–1% of total lipids (10–40 mg/100 mL) (Jensen, 1999), that are mainly located in the biological membrane surrounding the TAG core of milk fat globules (Fig. 15.1). The main polar lipids found in the human MFGM are the glycerophospholipids (phosphatidylcholine, PC; phosphatidylethanolamine, PE; phosphatidylinositol, PI; phosphatidylserine, PS) and the sphingolipids, mainly milk sphingomyelin (SM), but also cerebrosides and gangliosides (Fig. 15.4) (Lopez & Ménard, 2011; Yao et al., 2016).
Main polar lipids found in the biological membrane surrounding fat globules in human milk and bovine milk. Comparison with polar lipids in soya lecithin, that is commonly used as emulsifier in infant formulae. Adapted from Lopez et al. (2015)
Sphingolipids are based on a sphingoid backbone. In MFGM, the dominating sphingolipid is sphingomyelin (phosphocholine as head group) and in much smaller amounts glucosylceramides (glucose), lactosylceramides (lactose), and with more complex glycosyl residues gangliosides (monosaccharides, N-acetylgalactoseamine, sialic acid and others) occur. Gangliosides are exclusively located in the MFGM and act as decoy receptors for pathogens, which may prevent infections of infants, and they can modulate the behavior of immune cells (Rueda, 2007). The dietary gangliosides provided by breast milk might be important considering the ganglioside content in nervous tissue, the high requirement for the rapid brain growth in the perinatal period, and the demonstrated uptake of dietary gangliosides (Gurnida, Rowan, Idjradinata, Muchtadi, & Sekarwana, 2012; Palmano, Rowan, Guillermo, Guan, & Mc Jarrow, 2015). The role of sphingolipids in infant gut health and immunity has been reviewed (Nilsson, 2016).
The MFGM contributes to the dietary intake of breastfed infants, and provides sialic acid (gangliosides), and choline (10% provided by PC and SM) (Leermakers et al., 2015; Wang, 2012).
The individual fatty acid profile of each class of human milk polar lipids is specific (Benoit et al., 2010; Yao et al., 2016). Milk-SM contains high amount of long-chain saturated FA (C16:0, C22:0, C23:0, C24:0); PC is dominated by C16:0 and C18:1 n-9; PE by C18:1 n-9; PI and PS are rich in C18:1 n-9.
Comparing the fatty acid composition of milk total lipids and polar lipids reveals some general tendencies although there are huge differences in the fatty acid composition between the individual polar lipids than in total lipids, while C18:0 tends to be higher in polar lipids (Cilla, Quintaes, Barberá, & Alegría, 2016; Jensen, 1999; Wang et al., 2000). About 85% of the milk long-chain PUFA are contributed by TAG and only 15% are esterified on polar lipids from the MFGM (Harzer, Haug, Dieterich, & Gentner, 1983). ARA contributes up to 12% to phosphatidylethanolamine or phosphatidylinositol, but the percentage in sphingomyelin is about 0.4% and thus similar to total lipids, while DHA contributes up to 5% and 3% in phosphatidylethanolamine and phosphatidylserine, respectively (Cilla et al., 2016). The MFGM is, therefore, an interesting source of PUFA.
The nutritional importance of the MFGM lipids is not primarily based on their long-chain PUFA content, but they provide a variety of specific lipids, such as the high amount of sphingolipids, and some bioactivities in the gastro-intestinal tract that could be important and then further elucidated.
2.1.3 Sterols in Human Milk
Human milk contains 9–20 mg/100 mL cholesterol (Jensen, 1999). Various other minor sterol species exist, such as desmosterol and phytosterols (β-sitosterol, stigmasterol). However, cholesterol largely dominates in sterol composition and accounts for 94–96% of total sterol in human milk (Benoit et al., 2010; Jensen, 1999; Yao et al., 2016). Sterols are mainly located in the biological membrane surrounding fat globules in milk where they play important structural roles, mainly in the formation of ordered lipid domains (Fig. 15.1) (Lopez, Madec, & Jiménez-Flores, 2010). The amount of sterols in milk can, therefore, depend on total fat content and on the size of fat globules since it is proportional to the amount of membrane. Cholesterol is the substrate for the synthesis of bile acids, lipoproteins, vitamin D and hormones. It also acts by stabilizing the structure of cellular membranes and is incorporated into brain lipids mainly during the first months of life (Kinney, Karthigasan, Borenshteyn, Flax, & Kirschner, 1994). During the neonatal period, cholesterol found in breast milk was very early suspected to contribute to cholesterol homeostasis in the adult. It was demonstrated later by the high cholesterol content in breast milk leads to transient high total serum cholesterol concentration in infancy but low in adulthood (Owen et al., 2008).
2.2 Organization of Lipids in Breast Milk
The Milk Fat Globules
Breast milk is a natural oil-in-water (O/W) emulsion secreted by the mammary epithelial cells of the lactating mothers. Intracellular lipid droplets formed from the endoplasmic reticulum grow in size as they move toward the apical cell membrane. They are then enveloped by the apical plasma membrane and are voided into the lumen of the alveoli of the milk secreting gland (Heid & Keenan, 2005). The mechanism of milk lipid secretion leads to the formation of droplets called the milk fat globules. Sometimes, cytoplasmic remnants remain attached to fat globules (Fig. 15.1).
The size distribution of breast milk fat globules ranges from 0.3 to 15 μm with a mean diameter of about 4–5 μm (Fig. 15.1) (Lopez & Ménard, 2011). The variation in size of human milk fat globules raises unsolved questions about their specific function and metabolic fate when ingested by neonates. Nanometer-sized lipid-protein particles termed “lactosomes” have also been characterized in breast milk and the question of their potential metabolic, immune regulatory and protective role is still open (Argov-Argaman et al., 2010).
Milk fat globules are unique natural delivery systems of lipids and bioactive molecules in the gastrointestinal tract of newborns and efficient conveyers of energy. They are also involved in the protection of the neonate toward intestinal infections (Hamosh et al., 1999; Peterson, Patton, & Hamosh, 1998). Milk lipids present a very specific and complex structure at several levels of scale. Breast milk fat globules have a core rich in TAG that can be in the liquid or solid state depending on temperature, and are enveloped by a biological membrane called the milk fat globule membrane (MFGM).
Crystallization Properties of Human Milk TAG in the Core of Fat Globules
Human milk can be transferred directly from the mother to the infant during breast-feeding or expressed by the mother’s breast to be stored refrigerated or in a freezer for later feeding to her infant. Also, human milk banks collect milk from lactating mothers in order to help vulnerable and high-risk infants in neonatal intensive care units of hospitals. In this latter case, milk is frozen and heat-treated to ensure its microbiological safety. The impact of cooling breast milk has raised the attention of scientists.
Due to their high amount of long-chain saturated fatty acids (C16:0, C18:0; Table 5.2), human milk TAG can crystallize at low temperature. For example, storage of breast milk in the fridge induce the crystallization of TAG within fat globules (Lopez, Briard-Bion, Bourgaux, & Pérez, 2013). After storage in the fridge, the TAG crystals have a melting temperature above the physiological temperature of digestion in infants. Since solid TAG are not hydrolyzed by the digestive enzymes, the crystallization of milk fat that occurs upon storage of breast milk in the fridge could negatively impact the digestibility of high melting temperature TAG and the bioavailability of C16:0. It has therefore been recommended to warm breast milk around 50 °C before consumption by the infants.
The Milk Fat Globule Membrane: A Biological Membrane with a Unique Organization
The MFGM acts to stabilize physically the milk emulsion, facilitates the digestion of the fat by the infant and may play other functional and physiological roles that are not fully elucidated. The average MFGM thickness is typically 10–50 nm. The MFGM contains specific membrane proteins that are integral or peripheral (e.g. xanthine oxidase, adipophilin, fatty acid-binding protein, the mucins MUC1, MUC 4, MUC 15, lactadherin (PAS 6/7), CD36, butyrophilin), enzymes, polar lipids and cholesterol. The MFGM is a trilayer of polar lipids, where the first monolayer originates from the endoplasmic reticulum and the outer bilayer comes from the apical plasma membrane of the mammary epithelial cells (Heid & Keenan, 2005). The outer bilayer of the MFGM exhibits a heterogeneous lateral packing with a phase separation of ordered lipid domains in the gel or liquid-ordered phase that are rich in sphingomyelin and cholesterol (Fig. 15.1) (Lopez et al., 2010; Lopez & Ménard, 2011; Zou et al., 2012). The micro-domains are surrounded by a fluid matrix composed of the low melting temperature unsaturated polar lipids PC, PE, PI and PS. These ordered lipid micro-domains revealed in the MFGM were called lipid rafts by Christelle Lopez’s group (INRAE, France) by analogy with the rafts found in biological membranes (Lopez et al., 2010; Simons & Ikonen, 1997). Figure 15.1 shows images of the MFGM taken by confocal microscopy in situ in milk and present a schematic representation of the MFGM. The membrane-specific proteins are heterogeneously distributed in the MFGM, not located in the ordered lipid micro-domains, and protrude in the aqueous phase surrounding fat globules to form the glycocalyx (Hamosh et al., 1999; Lopez & Ménard, 2011).
Although the MFGM is only some nanometers thick, while the diameters of the milk fat globules are in the micrometer range, the MFGM could be very important for the physiological properties of human milk. There are good indications from in vitro studies, animal studies and observational studies that several MFGM compounds are important for infant development, including the development of cognitive functions.
2.3 Fate of Human Milk Fat Globules in the Gastrointestinal Tract of Infants: From Digestion to Absorption
The unique assembly of lipids in breast milk, in the form of fat globules of 5 μm diameter surrounded by a biological membrane (Fig. 15.1), provides bioactive components from the MFGM, a protection toward infections and an efficient digestion and absorption of lipids in the gastro-intestinal tract of infants. Milk fat globules are therefore of major significance for the growth, development and health of breast-fed infants.
The efficient absorption of milk lipids requires the digestion of milk fat globules in the gastro-intestinal tract of newborns (Fig. 15.3). The hydrolysis of the milk fat globules begins in the stomach of infants by the gastric lipase and then continues in the duodenum, where the pancreatic lipase in conjunction with colipase and the bile salt-stimulated lipase (BSSL) endogenous to human milk complete the process initiated in the stomach (Bernback, Blackberg, & Hernell, 1990; Lindquist & Hernell, 2010). The persistence of milk fat globules in the upper intestine allows the discharge of bioactive components in the distal part of the intestine and contributes to their physiological impact in breast-fed infants. In human milk, the external layers of the MFGM contain a group of glycoprotein filaments which are believed to enhance digestion by helping to bind lipase (Jensen, Ferris, & Lammikeefe, 1992). The fate of milk fat globules upon digestion in the gastrointestinal tract of newborns and the role played by milk fat globule and MFGM components (i.e. glycoproteins, free fatty acids) has raised attention of scientists for many years (Hamosh et al., 1999). However, the mechanisms of milk fat globule digestion and the identification of all the bioactives released in the gut during digestion are not yet fully known and will continue to be the focus of research studies in a near future.
3 Lipids in Infant Formulae: Composition and Structure
When mothers cannot or do not want to breast-feed their infant nor express their milk, infant formulae (IF) are the best alternative. According to the regulation, IF are the manufactured foods ingested by healthy infants during the first months of life (children under the age of 12 months), designed to satisfy by themselves the specific nutritional requirements of such infants until the introduction of appropriate complementary feeding. The composition of IF is complex and roughly based on a human mother’s milk composition at approximately 1–3 months post-partum. IF can be produced as powder or liquid and permit the bottle-feeding of babies.
Compositional and structural differences exist between human breast milk lipids and those found in IF. The composition (fatty acids, molecular species and structure of TAG) of breast milk fat globules result from the maternal diet and characteristics and to biological mechanisms while the composition of lipids in IF results from formulation. The structure and interfacial properties of breast milk fat globules result from the biological mechanisms of their secretion, while the structure of the emulsion and the interfacial properties of lipid droplets in IF result from technological processing, mainly the homogenization step that governs the size of the lipid droplets.
3.1 Manufacture of IF
IF are food products manufactured at the industrial scale by the combination of technological steps of blending, heat treatment, concentration, homogenization, spray-drying and packaging. The objective is to produce stable and reproducible end-products IF (Fig. 15.5). They are processed either as ready-to-feed (liquid) or as powdered milk. IF are oil-in-water (O/W) emulsions containing proteins, free amino acids, carbohydrates and other nutrients (minerals, vitamins, oligoelements, nutritional factors) dispersed in water.
In the manufacture of IF, homogenization is an essential technological step as it creates the O/W emulsion and prevents coalescence and formation of free fat during storage. Homogenization also prevents creaming and separation of the oil phase from water in the reconstituted IF prepared to bottle feed the infants. However, during the mechanical process of homogenization, the ingredients are blended together and submitted to pressure (from 15 to 25 MPa), that can alter their structure and functional properties. Hence, some ingredients may not be added before the homogenization step. It is, for example, the case for oils rich in n-3 and n-6 long-chain PUFA (DHA, ARA) that are highly sensitive to oxidation. In order to ensure their chemical protection towards oxidation, the long-chain PUFA-rich oils can be encapsulated in a matrix composed of modified starch or caseins, together with antioxidant molecules (e.g. vitamin C, tocopherols), and used as spray-dried powder ingredients. These encapsulated long-chain PUFA-rich oils can be added (1) in the concentrated and homogenized product just before the spray-drying step in order to limit the duration of their contact with pro-oxidant molecules (e.g. iron, copper) dispersed in the aqueous phase, or (2) by dry blending after the spray-drying process. The final IF powder is always packaged in an N2 atmosphere in order to prevent oxidation of the long-chain PUFA during the storage of the product.
3.2 Composition of Lipids in IF
According to the European Society for Pediatric Gastroenterology Hepatology And Nutrition (ESPGHAN), the minimum total lipid content in IF should be 4.4 g/100 kcal and the maximum 6 g/100 kcal (Koletzko et al., 2005). Analysis performed on commercial IF can highlight variations. For example, the lipid content in IF varies from 3.46 to 6.33 g/100 kcal (2.6–4.3 g/100 mL) (Mendonça, Araújo, Borgo, & de Rodrigues Alencar, 2017). Like human milk, fat provides approximately 40–50% of energy in IF. Lipids are mainly present in the form of TAG, but also in low amount of emulsifiers.
3.2.1 Fatty Acids and TAG
As in breast milk, the lipids in IF are mainly TAG. It is possible to combine different oils to achieve a similar average fatty acid composition as in human milk.
Over the last 40–50 years, blends of plant oils have been widely used. The most commonly used plant oils are palm oil, palm olein oil, coconut oil, soybean oil, rapeseed oil, corn oil, safflower oil, and (high oleic) sunflower oil. Each of these plant oils has a specific fatty acid composition (Table 15.4). Blends of these plant oils are the fat source for IF to provide targeted fatty acid profile (Table 15.1), mainly the saturated fatty acids such as C16:0, the monounsaturated C18:1n-9, and the polyunsaturated C18:2n-6 (LA) and C18:3n-3 (ALA). Depending on the different plant oils blended, the FA composition and TAG molecular species present in IF can vary. For example, palm olein and palm oils are sources of long-chain saturated and monounsaturated fatty acids (43% C16:0; 37% C18:1n-9) but do not contain short- or medium-chain fatty acids. On another hand, coconut oil is an excellent source of saturated medium-chain fatty acids (Table 15.4).
IF formulated without palm oil or alternative source of C16:0 have palmitate levels as low as 8% of the total fatty acids versus 26% in breast milk, whereas lauric acid (C12:0) amounts to 12–13.4% versus 5% in breast milk (Hageman, Danielsen, Nieuwenhuizen, Feitsma, & Dalsgaard, 2019). In IF with dairy fat, C16:0 levels reach 16–20% (Table 15.1) (Hageman, Danielsen, et al., 2019). In preterm infants, because of possible intestinal immaturity, facilitation of fat absorption through the inclusion of medium-chain fatty acids in the diet may be useful. Hence, most preterm and some term IF contain medium-chain TAG derived from coconut oil.
In accordance with existing regulations, all IF contain LA and ALA but in variable amounts, depending on the blends of plant oils (Table 15.5). For example, soy oil provides these PUFA. Regulation from Europe stipulates that the levels of LA and ALA should cover a ratio between 5 and 15 (EFSA, 2014). The analysis of commercial IF revealed that the LA/ALA ratio can vary from approximately 5–13 (Table 15.5). However, high LA intake have been revealed to be deleterious, and a LA/ALA ratio above 10 could induce a default in the conversion toward long-chain n-3 PUFA that is of high importance in the development of infant brain. Intake of LA levels could be reduced with a concomitant n-3 PUFA increase: 1% LA intake and ratio of 2–3 for LA/ALA should be enough to maintain a proper equilibrium for an optimal bioconversion to long-chain n-3 PUFA.
Despite long-chain PUFA such as DHA and ARA are present in breast milk and play important roles in early infant development, some, but not all, IF contain appreciable amounts of added long-chain PUFA (Table 15.5). A supply of these long-chain PUFA in IF is thought to be important since endogenous synthesis is insufficient to maintain tissue levels equivalent to breast-fed infants (Lien, Richard, & Hoffman, 2018). These long-chain PUFA, particularly DHA, are needed for optimal brain development and visual function in infants. In order to consider advances in knowledge to improve infant nutrition, the composition of IF has evolved during the last years, as shown in Table 15.5 with IF collected in supermarkets or pharmacy. An obligation of DHA supplementation at levels of 20–50 mg/100 kcal is mandatory since 2020 by European regulation for IF (EFSA, 2014). A supplementation in ARA is not imposed by Europe. Long-chain PUFA added to IF are typically from algae, fungal oils, marine oils, or egg-yolk derived lipids (Table 15.5). The addition of DHA can be performed with the addition of oils from fish (for example tuna oil) or alguae (for example Cryptheconium Cohnii oil; Schizochytrium sp. oil) (Table 15.5). The addition of arachidonic acid (ARA; C20:4 n-6) is generally performed with the addition of, for example, oil from the fungus Moetierella Alpina (Table 15.5). Irrespective of the fat blend used (plant oils, milk fat), DHA and ARA are added as separate ingredients to IF.
TAG from vegetable oils show stereospecific positioning of the fatty acids, with the saturated fatty acids typically positioned on the outer sn-1 and sn-3 positions, but less than 20% of C16:0 is esterified in the sn-2 position of TAG (Mu & Hoy, 2004). The structure of TAG in vegetable oils is therefore different to the structure of TAG in breast milk. Saturated vegetable fats such as palm oil contain high amount of C16:0 which is almost exclusively esterified at the sn-1 and sn-3 positions of TAG. The main TAG molecular species in palm oil are P-O-P, P-O-O, P-O-LA (P: palmitic acid; O: oleic acid; LA: linoleic acid). On the other hand, highly unsaturated vegetable oils not only contain unsaturated fatty acids at the sn-2 position of TAG but also an abundance of TAG with 2 or 3 unsaturated fatty acids. For example, soybean oil has about 70% of C18:2n-6 (LA) in the sn-2 position of TAG. The main TAG molecular species in soybean oil are LA-LA-LA, LA-LA-O, LA-LA-P (LA: linoleic acid; O: oleic acid).
Examination of the fatty acid profile and regio-distribution of fatty acids on TAG in IF revealed considerable differences with breast milk, mainly a lower proportion of C16:0 in the sn-2 position in IF containing only vegetable oils (Straarup, Lauritzen, Faerk, Hoy, & Michaelsen, 2006). Significant differences in TAG composition were found between human milk from Chinese mothers and IF, such as much higher medium-chain TAG and saturated TAG in IF, indicating that the IF developed by foreign manufacturers were not suitable for Chinese babies (Tu, Ma, Bai, & Du, 2017).
These TAG compositional and structural differences can impact the digestion of lipids and absorption by the infants. This is why recent research studies in the field of IF focus on the structuration of TAG, by positioning fatty acids of nutritional interest in the sn-2 position of the glycerol backbone, either by synthesis or by interesterification. Recently, TAG generated through an enzymatic process from vegetable oils or combination of vegetable oils and other oils (for example fish oil) have become available. The most common product is sn-2 palmitate also called beta-palmitate, a structured TAG with C16:0 esterified preferentially in the sn-2 position, which is used in IF currently on the market (for example INFAT® OPO, SN-2 palmitate by Advanced Lipids; Table 15.5). Beta-palmitate is the resulting product of the enzymatic interesterification of palm oil and high oleic sunflower oil, where the TAG P-O-P is transformed to O-P-O. These structured TAG make it possible to produce IF with TAG structures higher in sn-2 palmitate, often above 40% (ranging from 39 to 47%) of the total palmitic acid content (17–25%) (Bar-Yoseph et al., 2013; Miles & Calder, 2017; Sun, Wei, Su, Zou, & Wang, 2018).
3.2.2 Sterols in IF
Cholesterol is not present in the IF formulated with the blending of plant oils. The analysis of sterol contents in IF showed that those based on vegetable fats contained, on average, 0.185 mg/L of cholesterol (Claumarchirant, Matencio, Sanchez-Siles, Alegría, & Lagarda, 2015), while human breast milk contains 90–150 mg/L of cholesterol (Koletzko, 2016). IF containing a blend of vegetable oils and bovine milk fat contain higher levels of sterols, on average 0.927 mg/L (Claumarchirant et al., 2015), which is still low compared to human breast milk. However, dairy lipids (milk fat globules and MFGM) are a promising source of cholesterol in IF.
3.2.3 Lipid emulsifiers in IF
Non-dairy lipid emulsifiers and stabilizers are added to IF to ensure emulsion stability (Table 15.5). Lecithin from vegetable origin such as soy lecithin or sunflower lecithin, from fish and krill or egg yolk phospholipids, are the common emulsifiers used in IF generally recognised as safe. Monoglycerides and diglycerides (citric acid esters of mono and diglycerides; CITREM) can also be used. The emulsifiers will coat the surface of oil droplets, together with proteins (from milk: caseins, whey proteins; from rice or other vegetable origin), and effectively decrease the surface tension conferring at least short-term stability to the O/W emulsion. Moreover, the lipid emulsifiers make the emulsion more heat-resistant compared to emulsions containing lipid droplets only stabilized by proteins. Monoglycerides may also be added to IF as anti-foaming agents since excessive foaming of reconstituted milks is considered undesirable.
The nature of the emulsifier can impact the susceptibility of the lipid droplets to coalescence and break up within the gastro-intestinal tract of infants, thereby altering the total surface area of lipid exposed to the digestive lipases. The characteristics of the interfacial layer between the TAG core of the lipid droplets and the surrounding aqueous phase, including the kind of emulsifier, can impact the adsorption and activity of the digestive lipases at the oil/water interface. In vitro studies reported that the fatty acids contained in CITREM compounds are released to a large extent by gastric lipase, pancreatic lipase, pancreatic-lipase-related protein 2 and carboxyl ester hydrolase (Amara et al., 2014).
In IF formulated with plant oils, the specific components of the MFGM are generally absent. This relates to MFGM proteins, but also MFGM complex lipids (phospholipids, sphingolipids, ceramides, gangliosides) and cholesterol. However, residual milk polar lipids from the MFGM can be found in skimmed milk powder used in IF.
The metabolic impact of MFGM polar lipids surrounding breast milk TAG vs. non-dairy emulsifiers such as soy or sunflower lecithins in IF is not known.
3.3 Microstructure of Fat in IF: Processed Lipid Droplets Coated by Proteins and Non-Dairy Lipid Emulsifiers
Figure 15.6 shows the organization of fat in IF powders and after rehydration. The lipid droplets are tailored during the manufacture process, mainly during the technological process of homogenization. The objectives of homogenization are (1) to create an oil-in-water emulsion by mixing the blend of fats and the other components (proteins, minerals, emulsifiers), and (2) to form small lipid droplets that will be physically stable upon the drying process and the storage of IF powders. In-situ structural observations of IF powders performed by confocal microscopy revealed that fat can be dispersed in small spherical droplets homogeneously distributed in the powder particle (Fig. 15.6A, left). Fat can also be present in non-spherical large fat droplets and in the non-emulsified organization also called free fat (Fig. 15.6A, right). The formation of high free fat content in IF mainly occurs in the case of IF containing hydrolyzed proteins that have a low ability to stabilize the lipid droplets. The physical destabilization of the lipid droplets in the powder induces the formation of non-emulsified or free fat inside the grain powder (inner free fat) or at its periphery (surface free fat) (Fig. 15.6A, right). Free fat favors the oxidation of lipids, alters the functional properties and the quality of IF powders (e.g. flow and wetting properties, poor rehydration).
Microstructural observations of lipids (A) in infant formula powder particles (fat is in white), (B) after rehydration (fat in red, proteins in green), (C) focus on the surface composition and structure showing the adsorption of dairy proteins. Adapted from Lopez et al. (2015)
After rehydration of the IF powder, most of the standard IF exhibit tiny lipid droplets with a size distribution ranging from 0.05 to 2 μm and exhibit a large TAG/water interface, from about 20–40 m2/g fat (Lopez, Cauty, & Guyomarc’h, 2015). The size of processed lipid droplets in IF is smaller than the size of breast fat globules in human milk (Fig. 15.7). The small size of IF lipid droplets results from the pressure applied during the homogenization step. Differences in the size distribution of the lipid droplets exist between different commercial IF. They result from variations in the technological parameters used by different manufacturers or for different markets. The lipid droplets are mainly coated by dairy proteins, the casein micelles and whey proteins (Fig. 15.6). These structural parameters and interfacial composition are different from the size of fat globules in breast milk and have consequences on the mechanisms of lipid digestion in IF (Armand et al., 1996, 1999).
In some cases, IF can contain aggregates of proteins or complexes formed between lipid droplets and proteins, that may result from the thermo-induced denaturation of whey proteins occurring during the heat treatments performed for the microbial safety of IF (Fig. 15.6B; Lopez et al., 2015). Such lipoprotein complexes induced by the industrial process raise questions about the accessibility of TAG and proteins by the digestive enzymes in the gastro-intestinal tract of newborns and then about the nutritional and health impacts.
4 Dairy Lipids in Infant Formulae: Health Benefits for Infants and Potentialities
The underlying aim in the design and development of IF is to achieve a similar digestive outcome and nutritional yield to that of human milk. Scientific evidence continues to accumulate that the quality and structure of dietary lipids provided to infants has a marketed impact on health outcomes. As such, bovine milk itself is not an appropriate substitute of breast milk for infant nutrition. However, some of its components (e.g. milk fat globules, TAG, medium-chain fatty acids, C16:0, LA, ALA, MFGM, cholesterol) can be reformulated together with, for example, plant oils and structured TAG to achieve an equivalent balance to human breast milk. Bovine milk was the source of fat in IF until the 1970s and is still used in some parts of the world (Delplanque, Gibson, Koletzko, Lapillonne, & Strandvik, 2015). Since 1970s, vegetable oils have been extensively used in IF for 2 main reasons: (1) to provide high levels of mono unsaturated fatty acids and PUFA, and (2) the lower cost of vegetable oils compared to dairy fat. Palm oil has been widely used in IF to provide C16:0 to infants. However, palm oil free IF is becoming increasingly popular because of unsustainable production methods and environmental reasons (Table 15.5). On the opposite, the use of dairy lipids in IF is currently increasing (Table 15.5). Recent research studies showed that the use of dairy lipids in combination with plant oils enables a lipid profile closer to breast milk in terms of fatty acid composition, TAG structure, polar lipids and cholesterol contents with health benefits in infants.
This part of the chapter will develop the advantages provided by dairy lipids composition, TAG and MFGM, and the interest in developing lipid droplets biomimetic of breast milk fat globules. The potentialities of the addition of dairy lipids in IF are described.
4.1 Dairy FA and TAG: Specificity and Health Benefits
Bovine milk fat contains about 98% of TAG molecules, which is similar to breast milk fat. The bovine fatty acid composition comprises short-chain (C4:0–C6:0), medium-chain (C8:0–C14:0) and long-chain (>C14:0) fatty acids (Table 15.4). Bovine TAGs contain higher amount of saturated fatty acids than breast milk TAG (70% vs. 40%, respectively), including short-chain and medium-chain fatty acids. Bovine milk fat contains a lower amount of monounsaturated fatty acids (24% vs. 36%) and PUFA (2% vs. 18%) than breast milk fat.
Dairy C16:0 Content and sn-2 Position on TAG
Compared to plant oils that are widely formulated in IF, dairy fat provides (1) short- and medium-chain fatty acids, such as coconut oil and (2) high C16:0 content (about 25–30% in dairy fat), such as palm oil. Interestingly, dairy TAG have a higher percentage of C16:0 esterification at the sn-2 position compared with palm oil. Dairy TAG contain about 25–30% C16:0 and 45% of C16:0 is esterified at the sn-2 position of TAG, with 47% distributed at the sn-1 position and 8% at the sn-3 position (Table 15.3). The formulation of IF with bovine milk fat, therefore, increases the proportion of C16:0 in the sn-2 position of TAG compared to palm oil.
The fatty acid composition (i.e. medium-chain fatty acids, C16:0) and the TAG stereospecific structure (i.e. C16:0 in sn-2 position) of bovine milk fat might be beneficial for digestion and absorption of fatty acids and further outcomes. Hageman et al. (2019) performed in vitro studies to investigate the release of fatty acids from IF containing different fat blends, e.g. 100% vegetable fat versus 67% bovine milk fat and 33% vegetable fat, and compared this to human milk. These authors found that the total FA release was not affected by the composition of fat. However, different time-dependent release of individual fatty acids were observed, which might result in differences in absorption and other health effects in vivo.
The high proportion of C16:0 in sn-2 position of TAG provided by dairy TAG in IF decreases the formation of calcium soaps in the infant intestine and then contributes in a higher absorption of calcium leading to an improved bone strength.
It is important to note that, despite the supportive research studies showing the beneficial impact of sn-2 palmitate (C16:0 in the sn-2 position of TAG), guidelines on the composition of IF do not support its inclusion. This demonstrates that much more research studies in infants are needed in this area.
Dairy LA and ALA in Infant Formulae: DHA Levels in Infants
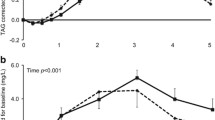
In infants, it has been shown that DHA is essential for brain development and functions. Some studies showed that infants receiving IF containing dairy lipids had an intermediate status in n-3 long-chain PUFA between breastfed infants and infants fed IF containing a mixture of vegetable oils (Courage et al., 1998). An increased number of research studies have focused on the impact of the partial replacement of plant oils by dairy fat in IF, on the DHA levels in infants. Bovine milk contains lower amount of LA and ALA than breast milk but the partial incorporation of dairy fat in IF could improve in infants the conversion pathway from ALA to n-3 long-chain PUFA.
A study performed in healthy Italian newborns showed that an IF containing a mix of dairy lipids and plant oils increased the endogenous conversion of n-3 long-chain FA from precursor ALA, leading to higher total n-3 fatty acids, DPA and DHA status in red blood cells than a plant oil-based IF. Interestingly, the DHA values were closer to those obtained with breast-feeding (Gianni et al., 2018). From inclusion at age below 3 weeks to the 4 subsequent months, the newborns exhibited a normal growth without any significant impact of dairy lipids on gastrointestinal symptoms or infant behaviour. Modifying lipid quality in IF by adding dairy lipids should, therefore, be considered as an interesting method to improve n-3 FA status in infants.
Benefits of the addition of dairy lipids in IF were observed in animal models. In young mice, it has been reported that dairy fat blend improves brain DHA level and neuroplasticity (Dinel et al., 2016). In young rats, Prof Delplanque’s group and collaborators focused on the role played by dairy fat on the DHA level of brain (Delplanque, Du, Martin, & Guesnet, 2018), which is an important goal in neonates. This group studied the impact on blood and brain DHA levels of dairy fat included in different blends of vegetable oils complying with the lipids recommendations for IF (LA: 16%; ALA: 1.6–2.5%; LA/ALA ratio: 5–10). They also evaluated the impact of pure dairy fat presenting very low levels of LA and ALA (1.9% and 0.8%, respectively), but with a proper LA/ALA ratio of 2.6 which they compared to previous ones and to rapeseed oil rich in ALA (8%). The three main findings of these studies are as follows:
-
dairy-fat-based diet (50% dairy, 50% vegetable oils) with 1.5% ALA content is more efficient than a pure vegetable oil blend with as much ALA (1.5%) and the same LA/ALA ratio of 10 to increase the brain DHA in the growing rat. Specific and complex component of dairy fat could be an explanation, such as the short and medium-chain fatty acids that are highly oxidizable after absorption and may thereby spare ALA from oxidation and favor ALA partitioning towards the desaturation and elongation pathways, increasing the long-chain n-3 PUFA levels such as those of DHA (Drouin et al., 2018).
-
dairy-fat-based diet (50% dairy, 50% vegetable oils) enriched with 2.3% ALA is even more efficient. This could be attributed to both the increased level of dietary ALA and the concomitant decrease in the LA/ALA ratio from 10 to 5 which has been recognized as an important factor driving the bioconversion of ALA into DHA, because of the competition between the parent n-3 and n-6 fatty acids for desaturation and elongation pathways.
-
dairy-fat-based diet containing pure dairy fat (100%) with only 0.8% of ALA and 1.9% of LA is as efficient as an 8% ALA rapeseed diet (22% LA) to increase the brain DHA in the growing rat, both presenting a similar very low LA/ALA ratio (less than 3) and present results comparable to the 2.3% ALA dairy/vegetable blend.
The role of the delta6-desaturase enzyme could be involved in this process and is crucial to explain these last results: ALA is the precursor of DHA but also its competitor for the last delta6-desaturase step and is regulated by substrate levels. An excess of ALA could represent the first substrate, producing increasing quantities of some n-3 long-chain fatty acids (EPA, docosa-pentaenoic acid: DPAn-3) and secondarily could limit the implication of delta6-desaturase in the second control point for DHA conversion. Explanation for rapeseed is exactly reverse and could represent an excess of precursor, which could limit the bioconversion to DHA, even reducing its level when intake is above the optimal intake (around 2.5–3% of total FA). The proof of this intra-cascade n-3 competition for delta6-desaturase has been validated. Finally, pure dairy fat, despite very low levels of PUFA (1.5–3% LA and 0.5–0.8% ALA) but associated with a very favourable LA/ALA ratio similar to rapeseed oil (maximum 3/1), was able to provide the proper conditions for a bioconversion of ALA to n-3 long-chain fatty acids and DHA necessary for the brain of young animals. Together, these observations clearly demonstrated that brain DHA levels can be substantially improved by dairy fat based-diets. The use of fats that are low in PUFA such as dairy may confer some metabolic advantages in that they allow better endogenous conversion of ALA to DHA.
4.2 MFGM: Source of Bioactive Molecules
Human milk contains complex lipids (glycerophospholipids, sphingolipids, cholesterol) and other MFGM components such as membrane-specific highly glycosylated proteins which are not provided with conventional IF based on plant oils as the lipid source.
Growing interest of supplementing IF with bovine MFGM comes from the scientific proofs of bioactivities and benefits obtained thanks to randomized infant trials, with reported impact for example on brain development and cognitive functions, immunity and gut physiology, and reduction of infections in the neonates. Several reviews have been dedicated to the supplementation of IF with bovine MFGM (Hernell, Timby, Domellof, & Lonnerdal, 2016; Timby, Domellöf, Lönnerdal, & Hernell, 2017). Although milk polar lipids and more widely MFGM components have key functions in infants, there is currently no requirement for the introduction of MFGM and individual components in IF. MFGM supplementation to IF has been proven to be safe and well-tolerated (Billeaud et al., 2014). No effects were reported on growth or long-term health outcomes. These studies stimulated further research on the preparation of MFGM-enriched ingredients. This supplementation of bovine MFGM in IF is possible thanks to the production of food-grade ingredients at the industrial scale. These two aspects are developed in the next paragraphs of the chapter.
Benefits Provided by the Supplementation of IF with Bovine MFGM
During the past 2 decades, in vitro and animal studies as well as clinical trials in infants provided scientific proofs that dietary exposure to IF containing MFGM provides beneficial effects. Although controversies exist, several of MFGM components have been related to nutritional and health-enhancing functions and the bovine MFGM has been considered as a potential nutraceutical (Spitsberg, 2005). The benefits of MFGM for infants have been reviewed (Demmelmair, Prell, Timby, & Lönnerdal, 2017). In the following section, an overview of the most recent research studies showing health benefits provided by the MFGM is proposed.
MFGM Improves Brain and Cognitive Development
The benefits provided by the supplementation in MFGM or in bioactive components from the MFGM (sialic acid, gangliosides, sphingomyelin, cholesterol, proteins) have been reported. In pre-school children consuming MFGM concentrate in chocolate formula milk for 4 months, beneficial effects on behavioral and emotional regulation were reported (Veereman-Wauters et al., 2012). In a randomized trial including infants below 2 months of age, the consumption of a MFGM-supplemented, low-energy, low-protein experimental IF showed a positive association with neurocognitive development. At 12 months of age, the cognitive score was significantly higher in the MFGM-supplemented experimental IF group than in the standard IF group but was not significantly different from that of breastfed infant group (Timby, Domellof, Hernell, Loennerdal, & Domellof, 2014). Rats receiving a diet supplemented with cholesterol displayed better performances in memory and learning than rats receiving a normal diet (Ya et al., 2013). In piglets, dietary supplementation with glycomacropeptide as a provider of sialic acid was reported to provide faster learning of difficult tasks and to improve memory (Wang et al., 2007). With regard to neurodevelopment, the ganglioside content of the MFGM might be highly relevant, considering the high ganglioside content in nervous tissue, the high requirement in the perinatal period due to the rapid brain growth, and the demonstrated uptake of dietary gangliosides (Palmano et al., 2015). Human infants fed IF supplemented with gangliosides or milk-SM displayed improved cognitive, neurobehavioral and motor development (Gurnida et al., 2012; Tanaka et al., 2013). However, whether MFGM supplementation benefits result from the action of a single or a combination of bioactive components is not yet known.
MFGM Prevents Infection
In 6–12-month-old infants in Peru, daily supplementation with MFGM enriched protein significantly decreased the duration of diarrhea episodes and the incidence of bloody diarrhea by almost 50% considering confounding factors (Zavaleta et al., 2011). In agreement with these findings, a study performed with young European pre-school infants aged 2.5–6 years, consuming daily MFGM-enriched complimentary food reported a significant decreased number of days with fever without any impact on diarrhea, constipation and cough during the four-month intervention period, compared to a corresponding supplement without MFGM components (Veereman-Wauters et al., 2012). In Swedish children recruited before 2 months of age and fed with MFGM-supplemented IF until 6 months of age, the preventive effect of MFGM on infections was also reported (Timby et al., 2015). Among infants fed the MFGM-supplemented IF, fewer episodes of acute otitis media and lower antipyretic use during the intervention were reported compared with infants fed a control IF. Glycoproteins from the MFGM (e.g. butyrophilin, mucins, lactadherin) and glycolipids (gangliosides) may participate in the defense against infections by preventing pathogen adhesion to epithelium (Fuller, Kuhlenschmidt, Kuhlenschmidt, Jimenez-Flores, & Donovan, 2013; Sprong, Hulstein, Lambers, & van der Meer, 2012) (Fig. 15.3). For some of the MFGM proteins, gastric stability has been demonstrated and they have been suggested to contribute to the protection against bacteria and viruses in the neonatal gastrointestinal tract and to affect the immune system (Hamosh et al., 1999; Peterson et al., 1998). As the proteome of bovine MFGM has been found similar to the human MFGM proteome, these findings suggest the possibility of beneficial effects of supplementing IF with bovine MFGM.
Milk sphingolipids (gangliosides) could play a protective role against bacterial toxins and bacterial development via a competition for bacteria binding sites as many bacteria adhere to epithelial cells via glycosphingolipids. Sphingolipids from the MFGM transiting in the gut may decrease pathogen adherence to the intestinal mucosa facilitating pathogen elimination (Sprong et al., 2012) and may influence the gut microbiota composition (Fig. 15.3). Sprong et al. (2012) also evidenced in vitro the bactericidal activity of digestion products of sphingolipids and more specifically of lyso-SM.
Altogether, research studies showed that the MFGM contributes to the protection of the infants from pathogens. In contrast, in a multicenter trial with French and Italian full-term neonates below 14 days of age, the safety evaluation of standard IF and IF enriched with a protein-rich MFGM fraction or a lipid-rich MFGM fraction did not reveal differences between groups in terms of diarrhea and intestinal discomfort nor in ear, respiratory and gastrointestinal infections (Billeaud et al., 2014).
MFGM and Sphingolipids Are Involved in Gut Health and Immunity
Appropriate intestinal barrier maturation is essential for absorbing nutrients and preventing pathogens and toxins from entering the body. Beneficial effects of the MFGM and MFGM components, mainly the sphingolipids, on gut epithelial barrier have been reported.
In challenging conditions against pathogenic bacteria such as Clostridium difficile or Listeria monocytogenes involving rats, authors reported that MFGM provides protection, probably by stimulating mucin secretion and preventing adherence of pathogens to the intestinal mucosa (Bhinder et al., 2017; Sprong et al., 2012). In weaned mice, providing a MFGM-rich fat feed decreased the inflammatory response to a systemic lipopolysaccharide (LPS) challenge and was associated with decreased gut permeability (Snow, Ward, Olsen, Jimenez-Flores, & Hintze, 2011). These effects may be partly related to gangliosides that inhibited degradation of tight junctions occurring during LPS-induced acute inflammation (Park, Thomson, & Clandinin, 2010). Moreover, authors reported that in mice fed a high-fat diet for 4 weeks, milk-SM had significantly lowered serum LPS compared to control, which may have been due to altered distal gut microbiota (lower fecal Gram-negative bacteria; higher Bifidobacterium) (Norris, Jiang, Ryan, Porter, & Blesso, 2016). In rat pups, 10 days feeding with MFGM-supplemented formula normalized delayed intestinal growth induced by standard IF feeding compared to suckling rats (Bhinder et al., 2017). Using neonatal piglets, authors reported that MFGM in IF accelerated the maturation of the intestinal immune system that was closer to the one observed in mother-fed piglets, induced mucosal growth without any impact on epithelial permeability (Le Huerou-Luron et al., 2018; Lemaire et al., 2018).
For further information about the role of sphingolipids in infant gut health and immunity, a very interesting review of scientific knowledge is recommended (Nilsson, 2016). Sphingolipids are important polar lipids in the MFGM but are not found in standard IF prepared with vegetable oils. Digestion of milk-SM in the gastrointestinal tract of infants generates the bioactive metabolites ceramide, sphingosine and sphingosine-1-phosphate (Fig. 15.3). The distal small intestine and colon are exposed to milk-SM and its bioactive metabolites (Nilsson, 2016; Ohlsson et al., 2010). Humans digest and absorb most of the SM in normal diets and the level of sphingolipid metabolites to which the colon is exposed can be influenced by realistic amounts of dietary SM such as milk-SM (Ohlsson et al., 2010). These compounds are both metabolic intermediates during synthesis and degradation of sphingolipids, and bioactive compounds with numerous signaling functions. After digestion of milk-SM by enzymes, its fatty acids (e.g. C24:0) are absorbed and transferred to tissues. Milk-SM is also a source of choline, known to be important in neonates for phospholipid synthesis during organ growth and for acetylcholine formation. Hence, milk-SM digestion must also be viewed in relation to choline production (Claumarchirant et al., 2016). In adult rats fed 3H-choline labeled SM, 30% of the radioactivity was in liver PC after 4 h, indicating that choline released during SM digestion is extensively reutilized for hepatic PC synthesis (Nilsson & Duan, 2006).
Milk-SM, which is a major polar lipid in the MFGM, is believed to play an important role in neonatal gut maturation during the suckling period. Milk-SM was reported to accelerate enzymatic and morphological maturation of the intestine in artificially reared rats (Motouri et al., 2003). However, it is unknown whether the effects were caused by milk-SM itself or to its metabolites. Rat studies suggest systemic effects of dietary milk-SM, including an increase of central nervous system myelination in a deficit model (Oshida et al., 2003).
Gangliosides, which are exclusively located in the MFGM, are able to modulate the behavior of immune cells. Intact gangliosides containing sialic acid have beneficial effects in the gut, e.g. reduce pro-inflammatory signaling, influence on gut bacterial flora, protective functions by their interactions with pathogens and bacterial toxins, effects on mucosal epithelial and immune functions (Miklavcic, Schnabl, Mazurak, Thomson, & Clandinin, 2012; Rueda, 2007). Hence, sphingolipids provided by the MFGM are important for mucosal functions, gut integrity and immune maturation in the neonate.
In infant pup rats the addition of bovine MFGM, whose lipids differ from human milk MFGM, to the formula made intestinal development and microbiome of formula fed rats more similar to that of breastfed rats compared to non-supplemented formulae (Bhinder et al., 2017).
Milk Polar Lipids and Sphingomyelin Affect TAG lipolysis upon Digestion
The impact of milk polar lipids, including their specificity to form sphingomyelin-cholesterol complexes (Lopez & Ménard, 2011), on the mechanisms involved in lipolysis occurring in the gastro-intestinal tract of infants is not well-known and discrepancies exist between authors. Nilsson and Duan established that milk-SM and its metabolites may influence TAG hydrolysis, cholesterol absorption, lipoprotein formation, and mucosal growth in the gut (Nilsson & Duan, 2006). The role played by the milk-SM in on the activity of the gastric lipase at the surface of lipid droplets has been demonstrated (Favé et al., 2007). The specificity of milk polar lipids rich in milk-SM in modulating the mechanisms of milk lipid digestion is of growing interest, mainly in comparison with soy lecithin that is widely used in IF (Table 15.5). Mathiassen et al. showed that exchanging soy lecithin with dairy polar lipids increased gastric lipase activity by 2.5-fold (Mathiassen et al., 2015). In mice, Lecomte et al showed that milk polar lipids introduced in an emulsion together with dairy proteins as emulsifiers resulted in a quicker elevation and clearance of plasma TAG compared to soy phospholipids (Lecomte et al., 2015). As regards to the different phases in which milk-SM can occur (gel phase below Tm, fluid phase above Tm, liquid-ordered phase in presence of cholesterol; Lopez, Cheng, & Perez, 2018), nutritional studies should further consider the biophysical properties of milk-SM to better understand its role in the mechanisms of TAG hydrolysis.
MFGM and Dairy Fat Modulate Gut Microbiota
The interaction between dietary lipids and gut microbiota is of increasing interest since it is well-known that the gut microbiome plays a crucial role in the maturation of the gastrointestinal immune defence. IF-feeding is usually associated with a higher bacterial richness and diversity and with different taxonomic composition compared with breast-feeding. Not much is known on the effect of dairy lipids on gut microbiota composition. In piglets, the composition of fecal microbiota has been reported to differ between piglets fed IF containing dairy lipids and MFGM or IF containing plant oils or mother-reared (Le Huerou-Luron et al., 2018). Supplementing IF with dairy lipids and MFGM increased Proteobacteria and Bacteroidetes while decreasing Firmicutes phyla compared with piglets receiving IF exclusively based on plant oils. The effects of milk fat and MFGM containing IF on the gut microbiota composition, and the underlying mechanisms, need to be further elucidated in infants.
4.3 Structure of the Emulsion and Interfacial Properties: Benefits of Large Droplets Coated with Milk Polar Lipids and MFGM in Infants
The organizations of lipids in human milk and in IF are different, i.e. the structure of TAG molecules, the size of lipid droplets and of the composition of their surface (MFGM vs. dairy proteins and non-dairy lipid emulsifiers, respectively) (Lopez et al., 2015; Michalski, Briard, Michel, Tasson, & Poulain, 2005) (Figs. 15.1, 15.6, and 15.7).
Pioneer studies clearly showed that dietary lipid structure affects lipolysis and the metabolic fate of fatty acids due to distinct differences in lipid digestion and absorption kinetics as well as post-prandial response (Armand et al., 1996, 1999). Any difference in postprandial lipid handling can impact lipid availability for the development of metabolic organs. This, in turn, could program metabolic homoeostasis, energy balance and metabolic response with potential impact on later life health. In recent years, studies have shown that the macrostructure of lipids in IF (i.e. the size of processed lipid droplets) and the interfacial composition (i.e. presence of MFGM and/or milk polar lipids at the surface of processed lipid droplets) are involved in metabolic programming. Animal studies highlighted the importance of the structure of lipid droplets on later adiposity and metabolism. Providing to mice pups (from post-natal day 16–42) IF with large droplets (i.e. modal diameter 6.25 μm) containing TAG from plant oils origin and covered by polar lipids from the MFGM (concept IMF Nuturis®; Nutricia Research; patent WO2010027258A1) reduced fat accumulation as well as fasting plasma leptin, resistin, glucose and lipids (TAG and total cholesterol) in adults fed a western diet, compared to the group fed standard IF (Oosting et al., 2012, 2014). The same group investigated the potential role played by large lipid droplets only, of polar lipid-coating only or the combination of both (i.e. the two parameters considered in the concept IF Nuturis®). IF with both large lipid droplet size and MFGM coating administrated during the neonatal period contributed to the observed protective effect against obesity in later life (Baars et al., 2016). Although the mechanisms remain unclear, these animal studies showed that early nutrition is associated with sustained effects on later life obesity. A clinical trial is ongoing to test the Nuturis® (NCT01609634; New Infant Formula Trial in Healthy Term Subjects on Growth, Body Composition, Tolerance and Safety; estimated study completion date: December 2019). An IF containing large, phospholipid coated lipid droplets was recently found to support adequate growth in healthy Asian infants during the first 4 months of life as compared with a standard IF (Shek, Yu, Wu, Zhu, & Chan, 2017).
Several mechanisms might explain the different responses observed following consumption of the Concept IF Nuturis®, such as access of digestive lipases to the TAG core of the fat droplets resulting in differences in fat digestion and absorption. Also, a difference in clotting behavior of casein in the acidic gastric milieu due to the different coating of the two formulae tested, that is, only proteins vs. partly protein and phospholipids, might have affected gastric emptying and thereby postprandial responses. However, the exact underlying mechanism remains to be determined. Further, it is unknown to what extent these findings can be extrapolated to infants, and if these postprandial changes translate into longer-term health effects.
Scientific proofs of the fact that the structure of the lipid droplets ingested during neonatal period programs body composition and metabolism in adulthood must be confirmed in clinical trials.
4.4 Opportunities to Produce Food-Grade Ingredients Enriched in Bovine MFGM
Technologies have become available to obtain MFGM from cow’s milk and the bovine compounds also exhibit bioactivities in human infants and adults as reported by clinical studies (Hernell et al., 2016; Timby et al., 2017).
Various dairy streams permit the recovery of MFGM and are available in sufficient amount to produce ingredients at the industrial scale (Fig. 15.8) (Dewettinck et al., 2008; Jiménez-Flores & Brisson, 2008; Vanderghem et al., 2010). MFGM can be isolated from bovine milk fat globules, e.g. after concentration in creams, shear at low temperature to break fat globules, melting and centrifugation to separate anhydrous fat from the aqueous phase, called beta serum, containing MFGM fragments and polar lipids. MFGM can also be recovered from by-products of the cow’s dairy industry, e.g. buttermilk obtained from butter making, butter serum recovered during the preparation of anhydrous milk fat, acid or neutral whey obtained from cheese production (Fig. 15.8). Treatments during dairy processing (e.g. churning, agitation, pressing of cheese curd) break the MFGM into fragments and release them in the aqueous phase.
Schematic representation of the dairy streams able to provide milk fat globule membrane components to produce ingredients. Adapted from Lopez et al. (2019)
Buttermilk, butter serum, beta serum and cheese whey are suitable sources because of their low cost and their relatively high content in MFGM components. A weak point concerning these by-products is that they have been treated at high temperatures several times for bacterial and safety reasons, inducing a denaturation of proteins, interactions between MFGM proteins and whey proteins, and interactions between sugars and proteins. These dairy streams are not produced with the same volumes (e.g. buttermilk is produced with higher volumes compared to butter serum), and do not have the same composition, in terms of dry matter, amount of MFGM and milk polar lipids, proteins. For example, butter serums exhibit a higher polar lipid content than buttermilks: 9.5 vs. 1.6%. As a result, they are not equivalent sources of MFGM and polar lipids. Moreover, the relative proportion of milk polar lipids can be altered by processing. The relative proportion of milk-SM is 23% in milk and cream, while butter serums contain a higher proportion of milk-SM (34%) and buttermilks contain a lower proportion of milk-SM (19%) (Lopez, Blot, Briard-Bion, Cirié, & Graulet, 2017). A similar relative amount of each polar lipid class was reported for milk and cheese whey. The lipid composition of industrial buttermilks and butter serums has been determined with a special emphasis on sphingolipid and ceramide isoforms (Bourlieu et al., 2018). Another variation in polar lipid composition concerns the fatty acids. It has been reported that cow diet affects the fatty acid composition of milk polar lipids recovered in by-products of the dairy industry such as buttermilks and butter serums (Lopez, Blot, et al., 2017).
The economic valorization of dairy streams containing MFGM, mainly buttermilk and butter serum, is improved by the development of technological processes able to concentrate and purify MFGM and milk polar lipids. For the last 20 years, industrial and academic research teams have focused on the development of processes able to selectively fractionate components and particularly to recover and concentrate MFGM fragments and milk polar lipids from the dairy streams (buttermilk, butter serum, cheese whey). The main challenge for these technological studies is to separate MFGM fragments and milk polar lipids from the other components (TAG, proteins, lactose and minerals). The different steps involved in the recovery of MFGM materials have been reviewed (Dewettinck et al., 2008; Gassi et al., 2016; Holzmueller & Kulozik, 2016; Singh, 2006). The objective is to selectively remove proteins (caseins and whey proteins), lactose and minerals and to isolate and concentrate MFGM and/or specific fractions such as the polar lipids. The main technological steps are e.g. acid or rennet-induced precipitation of proteins followed by centrifugation to recover the aqueous phase, filtration using membrane techniques such as microfiltration and ultrafiltration, diafiltration to remove lactose and minerals. The final products are dehydrated using spray-drying to allow their conservation. The ingredients containing MFGM and milk polar lipids are mainly found in the form of powders. These technological processes lead to the production of high added-value MFGM-enriched ingredients compared to dried by-products, e.g. buttermilk powder.
Further work is required to optimize food-grade down-stream processes for MFGM components that can be applied in the food industry.
Table 15.6 provides an overview of MFGM-containing ingredients that have been used in research studies and/or that are currently commercially available. The ingredients have a complex composition. They contain TAG, proteins, i.e. skim milk derived proteins (caseins and whey proteins) and MFGM-specific proteins, phospholipids, lactose and minerals. The relative proportions of these components depend on the technological steps used to prepare the ingredients. The total amount of proteins and the relative proportion of the different kinds of proteins can affect the functional properties of the ingredient. In these ingredients prepared mainly from by-products of the cream and cheese industry, the polar lipids and membrane-specific proteins are closely associated in the MFGM fragments released during processing of cream (Fig. 15.8) (Gallier et al., 2015; Lopez et al., 2015, 2017). Ingredients enriched in MFGM have recently become available in sufficient quantity and quality to be added in IF. Randomized clinical studies with experimental IF incorporating MFGM from bovine milk have already provided some evidence of clinical benefits.
The preparation of pure MFGM or milk polar lipids is possible from a technological point of view. Ingredients are commercially available (e.g. Lacprodan PL-75 produced by Arla Foods, PC700 produced by Fonterra). However, the utilization of these functional ingredients is difficult from an economic point of view since they are expensive. Bovine milk-SM purified from milk polar lipids is also available commercially (e.g. provided by Avanti Polar Lipids) but with a high quotation.
4.5 Opportunities to Prepare MFGM-Coated Lipid Droplets Bio-Inspired by Breast Milk Fat Globules
In recent years, IF containing MFGM-coated lipid droplets have been prepared and showed health benefits compared to protein-coated lipid droplets (Baars et al., 2016; Baumgartner, van de Heijning, Acton, & Mensink, 2017; Oosting et al., 2012, 2014). These O/W emulsions were prepared by adding ingredients containing MFGM fragments (Table 15.6) together with TAG (e.g. from vegetable oils, milk fat or a mixture of both) before the homogenization process. Such emulsions are bioinspired from milk fat globules. The biological and health functions of these emulsions are governed by the TAG/water interface, in terms of amount, composition and structure. To fully benefit the advantages provided by the MFGM, the composition and the structure of the surface of these lipid droplets are of primary importance. However, to date, information remains scarce.
The structure of the concept IF Nuturis®, that comprises processed lipid droplets of large size coated by MFGM components reported to be involved in early lipid programming, has been investigated (Gallier et al., 2015; Oosting et al., 2012, 2014). The authors used confocal microscopy with a dye having affinity for the polar lipids (i.e. Annexin-V Alexa Fluor 488) to show their presence at the TAG/water interface as a sign for the localization of MFGM components (Oosting et al., 2012, 2014). Further characterizations of the emulsion have been performed by the combination of confocal and electron microscopy (Gallier et al., 2015). The authors reported the presence of MFGM components i.e. polar lipids, glycoproteins, glycolipids and cholesterol adsorbed at the surface of the lipid droplets. Gallier and co-workers hypothesized that a thin monolayer membrane (5–10 nm) was present at the surface of the lipid droplets. However, the resolution of electron microscopy does not allow knowing if the components form a monolayer or if the trilayer organization of the MFGM can be preserved. In this study, the interactions between casein micelles and MFGM fragments in the ingredient have been observed. Moreover, the presence of casein micelles adsorbed at the surface of the lipid droplets in the concept IMF Nuturis® has been reported. The potential role played by the casein micelles adsorbed at the TAG/water interface has not been discussed, mainly as regards to the mechanisms of lipid digestion in the gastrointestinal tract of infants. By comparing the structure of the emulsion in human milk, concept IF and control IF, the authors concluded that the processed MFGM-coated lipid droplets in the concept IF are closer to the human milk fat globules than the processed protein-coated lipid droplets in the control IF.
In a more recent study, the preparation of MFGM-coated lipid droplets biomimetic of milk fat globules, the physical stability of this emulsion and its behavior as a function of pH were reported (Lopez, Cauty, et al., 2017) (Fig. 15.9). The ingredient prepared from industrial butter serum (Gassi et al., 2016) contains linear MFGM fragments of various lengths that have been released from the surface of bovine milk fat globules during phase inversion of concentrated cream. The ingredient also contains vesicles formed by MFGM and/or by MFGM polar lipids. TEM images of the emulsion showed a thin layer of MFGM components present at the surface of the lipid droplets with some MFGM fragments adsorbed at the surface of the lipid droplets and protruding in the aqueous phase (Fig. 15.9). Also, Some MFGM fragments were present in the aqueous phase of the emulsion, showing a partitioning of MFGM components between the TAG/water interface and the aqueous phase. CLSM images with specific fluorescent probes permitted to identify polar lipids and glycosylated molecules (i.e. glycoproteins and glycolipids) at the surface of the lipid droplets. The polar lipids were organized as a thin layer and as small spherical vesicles. This study also showed that the glycocalyx present around milk fat globules can be formed around processed lipid droplets coated with bovine MFGM. The description of the biomimetic emulsions and of the surface of MFGM-coated lipid droplets performed in independent studies using different ingredients leads to convergent information (Gallier et al., 2015; Lopez, Cauty, et al., 2017).
Tailoring of milk fat globule membrane (MFGM)-coated processed lipid droplets bioinspired by milk fat globules. Preparation of MFGM-containing ingredient from dairy streams and homogenization with triacylglycerols (TAG) to form the processed lipid droplets covered by MFGM fragments containing polar lipids and glycosylated compounds (glycoproteins and glycolipids) as observed using transmission electron microscopy (top) and confocal laser scanning microscopy (bottom). Adapted from Lopez, Cauty, et al. (2017)
As a conclusion, it is possible to prepare MFGM-coated lipid droplets in IF to mimic the structure of breast milk fat globules covered by the MFGM. The size of lipid droplets can be modulated by the shear applied during emulsion formation (i.e. the homogenization pressure) and both the amount and the type of tension-active molecules able to stabilize the interface formed upon homogenization. The biophysical studies performed using the combination of microscopy techniques showed that the MFGM fragments and MFGM components (e.g. polar lipids, proteins) added during the preparation of the emulsion are able to move to and adsorb at the surface of the processed lipid droplets during homogenization. The presence of MFGM fragments in the emulsions shows that they are not disrupted under pressure during homogenization under the range of pressures used. Other components such as the proteins present in the ingredient (i.e. caseins, whey proteins, membrane-specific proteins), can also be adsorbed at the surface of the lipid droplets. An important aspect to consider is the order of introduction of the ingredients during the preparation of the emulsion since it can strongly affect the composition and the structure of the lipid droplet surface as well as the functional properties of the MFGM-coated lipid droplets.
4.6 Manufacture of IF Containing Dairy Lipids
Dairy lipids can be introduced in IF in three different ways:
-
1.
anhydrous milk fat or butter oil that provides dairy TAG. Table 15.5 shows an example of IF powder, Modilac Doucéa, that contains anhydrous milk fat. Dairy TAGs need to be melted above their final melting temperature (around 40 °C) and can be blended with other oils such as plant oils. The oils are then homogenized to form the oil-in-water emulsion. See Fig. 15.5 for the preparation of IF.
-
2.
ingredients enriched in MFGM or milk polar lipids that are obtained from by-products of dairy industries. Table 15.5 shows an example of IF powder, Enfamil® Enspire™, that contains MFGM. The ingredients rich in MFGM are generally introduced in the aqueous phase before the homogenization step. MFGM can then be located at the TAG/water interface, depending on their competition with dairy proteins to stabilize the interface.
-
3.
full fat milk or cream that corresponds to the concentration of fat globules from bovine milk, and contains dairy TAG and all components of the MFGM. Table 15.5 shows examples of IF powders containing cream from bovine milk in their formulation (Biostime, Picot). Bovine milk fat globules have a large size (mean diameter around 4 μm) and, as a consequence, can phase separate during the process and be physically destabilized in the IF powder (formation of free fat highly sensitive to oxidation). To assure the physical stability of lipid droplets and the quality of IF powders towards oxidation and functional properties (e.g. wettability), milk fat globules are homogenized during the manufacture of IF together with the other oils. The processed lipid droplets formed in these IF are a mixture of TAG from milk and plants in their core, surrounded by dairy proteins and possibly MFGM components at the TAG/water interface. When cream is included as an ingredient in IF, polar lipid content is usually higher with a wide range of molecular species from different polar lipid classes specific to the bovine MFGM (SM, PC, PE, PI, PS).
In the future, processing techniques used in the manufacture of IF (homogenization, heat treatment, spray-drying) will need to be adapted to preserve the structure of milk fat globules enveloped by the MFGM and all the health benefits provided by the variety of dairy lipid components.
5 Conclusions
The partial replacement of plant oils by dairy lipids in IF, associated with the formation of MFGM coated lipid droplets, will contribute in reducing the gap with breast milk fat globules by considering both the composition and the structure. The technological opportunities exist to improve IF and the nutritional and health benefits in infants. Dairy lipids have large similarities with breast milk lipids in terms of variety of fatty acids, presence of short and medium-chain fatty acids, low levels of LA, a proper level of ALA and LA/ALA ratio which is presently better than the human breast milk, sn-2 position of palmitic acid on TAG, MFGM, cholesterol. Consequently, the use of dairy fat in IF, as well as the absolute amount of polyunsaturated LA and ALA, should be considered in the future to improve the quality of IF.
References
Ailhaud, G., Massiera, F., Weill, P., Legrand, P., Alessandri, J.-M., & Guesnet, P. (2006). Temporal changes in dietary fats: Role of n-6 polyunsaturated fatty acids in excessive adipose tissue development and relationship to obesity. Progress in Lipid Research, 45, 203–236.
Amara, S., Patin, A., Giuffrida, F., Wooster, T. J., Thakkar, S. K., Bénarouche, A., et al. (2014). In vitro digestion of citric acid esters of mono- and diglycerides (CITREM) and CITREM-containing infant formula/emulsions. Food & Function, 5, 1409–1421.
American Academy of Pediatrics (AAP). (2012). Section on breastfeeding. Breastfeeding and the use of human milk. Pediatrics, 129, e827–e841.
Anderson, R. C., MacGibbon, A. K. H., Haggarty, N., Armstrong, K. M., & Roy, N. C. (2018). Bovine dairy complex lipids improve in vitro measures of small intestinal epithelial barrier integrity. PLoS One, 13, e0190839.
Argov-Argaman, N., Smilowitz, J. T., Bricarello, D. A., Barboza, M., Lerno, L., Froehlich, J. W., et al. (2010). Lactosomes: Structural and compositional classification of unique nanometer-sized protein lipid particles of human milk. Journal of Agricultural and Food Chemistry, 58, 11234–11242.
Armand, M., Hamosh, M., Mehta, N. R., Angelus, P. A., Philpott, J. R., Henderson, T. R., et al. (1996). Effect of human milk or formula on gastric function and fat digestion in the premature infant. Pediatric Research, 40, 429–437.
Armand, M., Pasquier, B., André, M., Borel, P., Senft, M., Peyrot, J., et al. (1999). Digestion and absorption of 2 fat emulsions with different droplet sizes in the human digestive tract. The American Journal of Clinical Nutrition, 70, 1096–1106.
Baars, A., Oosting, A., Engels, E., Kegler, D., Kodde, A., Schipper, L., et al. (2016). Milk fat globule membrane coating of large lipid droplets in the diet of young mice prevents body fat accumulation in adulthood. The British Journal of Nutrition, 115, 1930–1937.
Bar-Yoseph, F., Lifshitz, Y., & Cohen, T. (2013). Review of sn-2 palmitate oil implications for infant health. Prostaglandins, Leukotrienes and Essential Fatty Acids, 89, 139–143.
Baumgartner, S., van de Heijning, B. J. M., Acton, D., & Mensink, R. P. (2017). Infant milk fat droplet size and coating affect postprandial responses in healthy adult men: A proof-of-concept study. European Journal of Clinical Nutrition, 71, 1108–1113.
Benoit, B., Fauquant, C., Daira, P., Peretti, N., Guichardant, M., & Michalski, M.-C. (2010). Phospholipid species and minor sterols in French human milks. Food Chemistry, 120, 684–691.
Bernard, J. Y., Armand, M., Peyre, H., Garcia, C., Forhan, A., De Agostini, M., et al. (2017). Breastfeeding, polyunsaturated fatty acid levels in colostrum and child intelligence quotient at age 5-6 years. The Journal of Pediatrics, 183, 43–50.e3.
Bernback, S., Blackberg, L., & Hernell, O. (1990). The complete digestion of human-milk triacylglycerol in vitro requires gastric lipase, pancreatic colipase-dependent lipase, and bile-salt stimulated lipase. The Journal of Clinical Investigation, 85, 1221–1226.
Bhinder, G., Allaire, J. M., Garcia, C., Lau, J. T., Chan, J. M., Ryz, N. R., et al. (2017). Milk fat globule membrane supplementation in formula modulates the neonatal gut microbiome and normalizes intestinal development. Scientific Reports, 7, 45274.
Billeaud, C., Puccio, G., Saliba, E., Guillois, B., Vaysse, C., Pecquet, S., et al. (2014). Safety and tolerance evaluation of milk fat globule membrane-enriched infant formulas: A randomized controlled multicenter non-inferiority trial in healthy term infants. Clinical Medicine Insights: Pediatrics, 8, CMPed.S16962.
Bourlieu, C., Cheillan, D., Blot, M., Daira, P., Trauchessec, M., Ruet, S., et al. (2018). Polar lipid composition of bioactive dairy co-products buttermilk and butterserum: Emphasis on sphingolipid and ceramide isoforms. Food Chemistry, 240, 67–74.
Cilla, A., Quintaes, K. D., Barberá, R., & Alegría, A. (2016). Phospholipids in human milk and infant formulas: Benefits and needs for correct infant nutrition. Critical Reviews in Food Science and Nutrition, 56, 1880–1892.
Claumarchirant, L., Cilla, A., Matencio, E., Sanchez-Siles, L. M., Castro-Gomez, P., Fontecha, J., et al. (2016). Addition of milk fat globule membrane as an ingredient of infant formulas for resembling the polar lipids of human milk. International Dairy Journal, 61, 228–238.
Claumarchirant, L., Matencio, E., Sanchez-Siles, L. M., Alegría, A., & Lagarda, M. J. (2015). Sterol composition in infant formulas and estimated intake. Journal of Agricultural and Food Chemistry, 63, 7245–7251.
Courage, M., Mccloy, U., Herzberg, G., Andrews, W., Simmons, B., Mcdonald, A., et al. (1998). Visual acuity development and fatty acid composition of erythrocytes in full-term infants fed breast milk, commercial formula, or evaporated milk. Journal of Developmental & Behavioral Pediatrics, 19, 9–17.
Delplanque, B., Du, Q., Martin, J.-C., & Guesnet, P. (2018). Lipids for infant formulas. OCL, 25, D305.
Delplanque, B., Gibson, R., Koletzko, B., Lapillonne, A., & Strandvik, B. (2015). Lipid quality in infant nutrition: Current knowledge and future opportunities. Journal of Pediatric Gastroenterology and Nutrition, 61, 8–17.
Demmelmair, H., & Koletzko, B. (2018). Lipids in human milk. Best Practice & Research Clinical Endocrinology & Metabolism, 32, 57–68.
Demmelmair, H., Prell, C., Timby, N., & Lönnerdal, B. (2017). Benefits of lactoferrin, osteopontin and milk fat globule membranes for infants. Nutrients, 9, 817.
Dewettinck, K., Rombaut, R., Thienpont, N., Le, T. T., Messens, K., & Van Camp, J. (2008). Nutritional and technological aspects of milk fat globule membrane material. International Dairy Journal, 18, 436–457.
Dinel, A. L., Rey, C., Bonhomme, C., Le Ruyet, P., Joffre, C., & Layé, S. (2016). Dairy fat blend improves brain DHA and neuroplasticity and regulates corticosterone in mice. Prostaglandins. Leukotrienes and Essential Fatty Acids, 109, 29–38.
Drouin, G., Catheline, D., Sinquin, A., Baudry, C., Le Ruyet, P., Rioux, V., et al. (2018). Incorporation of dairy lipids in the diet increased long-chain omega-3 fatty acids status in post-weaning rats. Frontiers in Nutrition, 5, 42.
EFSA. (2014). Scientific opinion on the essential composition of infant and follow-on formulae. EFSA Journal, 12, 24–32.
Favé, G., Leveque, C., Peyrot, J., Pieroni, G., Coste, T. C., & Armand, M. (2007). Modulation of gastric lipolysis by the phospholipid specie: Link to specific lipase-phospholipid interaction at the lipid/water interface? The FASEB Journal, 21, A1010.
Fidler, N., & Koletzko, B. (2000). The fatty acid composition of human colostrum. European Journal of Nutrition, 39, 31–37.
Fuller, K. L., Kuhlenschmidt, T. B., Kuhlenschmidt, M. S., Jimenez-Flores, R., & Donovan, S. M. (2013). Milk fat globule membrane isolated from buttermilk or whey cream and their lipid components inhibit infectivity of rotavirus in vitro. Journal of Dairy Science, 96, 3488–3497.
Gallier, S., Vocking, K., Post, J. A., Van de Heijning, B., Acton, D., Van der Beek, E. M., et al. (2015). A novel infant milk formula concept: Mimicking the human milk fat globule structure. Colloids and Surfaces B: Biointerfaces, 136, 329–339.
Gassi, J. Y., Blot, M., Beaucher, E., Robert, B., Leconte, N., Camier, B., et al. (2016). Preparation and characterisation of a milk polar lipids enriched ingredient from fresh industrial liquid butter serum: Combination of physico-chemical modifications and technological treatments. International Dairy Journal, 52, 26–34.
Gianni, M. L., Roggero, P., Baudry, C., Fressange-Mazda, C., Galli, C., Agostoni, C., et al. (2018). An infant formula containing dairy lipids increased red blood cell membrane Omega 3 fatty acids in 4 month-old healthy newborns: A randomized controlled trial. BMC Pediatrics, 18, 53.
Grote, V., Verduci, E., Scaglioni, S., Vecchi, F., Contarini, G., Giovannini, M., et al. (2016). Breast milk composition and infant nutrient intakes during the first 12 months of life. European Journal of Clinical Nutrition, 70, 250–256.
Gurnida, D. A., Rowan, A. M., Idjradinata, P., Muchtadi, D., & Sekarwana, N. (2012). Association of complex lipids containing gangliosides with cognitive development of 6-month-old infants. Early Human Development, 88, 595–601.
Hageman, J. H. J., Danielsen, M., Nieuwenhuizen, A. G., Feitsma, A. L., & Dalsgaard, T. K. (2019). Comparison of bovine milk fat and vegetable fat for infant formula: Implications for infant health. International Dairy Journal, 92, 37–49.
Hageman, J. H. J., Keijer, J., Dalsgaard, T. K., Zeper, L. W., Carriere, F., Feitsma, A. L., et al. (2019). Free fatty acid release from vegetable and bovine milk fat-based infant formulas and human milk during two-phase in vitro digestion. Food & Function, 10, 2102–2113.
Hamosh, M., Peterson, J. A., Henderson, T. R., Scallan, C. D., Kiwan, R., Ceriani, R. L., et al. (1999). Protective function of human milk: The milk fat globule. Seminars in Perinatology, 23, 242–249.
Harzer, G., Haug, M., Dieterich, I., & Gentner, P. R. (1983). Changing patterns of human milk lipids in the course of the lactation and during the day. The American Journal of Clinical Nutrition, 37, 612–621.
Heid, H. W., & Keenan, T. W. (2005). Intracellular origin and secretion of milk fat globules. European Journal of Cell Biology, 84, 245–258.
Hernell, O., Timby, N., Domellof, M., & Lonnerdal, B. (2016). Clinical benefits of milk fat globule membranes for infants and children. The Journal of Pediatrics, 173, S60–S65.
Holzmueller, W., & Kulozik, U. (2016). Isolation of milk fat globule membrane (MFGM) material by coagulation and diafiltration of buttermilk. International Dairy Journal, 63, 88–91.
Innis, S. M. (2011). Dietary triacylglycerol structure and its role in infant nutrition. Advances in Nutrition, 2, 275–283.
Jensen, R., Ferris, A., & Lammikeefe, C. (1992). Lipids in human-milk and infant formulas. Annual Review of Nutrition, 12, 417–441.
Jensen, R. G. (1999). Lipids in human milk. Lipids, 34, 1243–1271.
Jiménez-Flores, R., & Brisson, G. (2008). The milk fat globule membrane as an ingredient: Why, how, when? Dairy Science & Technology, 88, 5–18.
Kallio, H., Nylund, M., Bostrom, P., & Yang, B. (2017). Triacylglycerol regioisomers in human milk resolved with an algorithmic novel electrospray ionization tandem mass spectrometry method. Food Chemistry, 233, 351–360.
Khan, S., Hepworth, A. R., Prime, D. K., Lai, C. T., Trengove, N. J., & Hartmann, P. E. (2013). Variation in fat, lactose, and protein composition in breast milk over 24 hours: Associations with infant feeding patterns. Journal of Human Lactation, 29, 81–89.
Kim, K.-M., Park, T.-S., & Shim, S.-M. (2015). Optimization and validation of HRLC-MS method to identify and quantify triacylglycerol molecular species in human milk. Analytical Methods, 7, 4362–4370.
Kinney, H., Karthigasan, J., Borenshteyn, N., Flax, J., & Kirschner, D. (1994). Myelination in the developing human brain - Biochemical correlates. Neurochemical Research, 19, 983–996.
Koletzko, B. (2016). Human milk lipids. Annals of Nutrition & Metabolism, 69(Suppl 2), 28–40.
Koletzko, B., Baker, S., Cleghorn, G., Neto, U. F., Gopalan, S., Hernell, O., et al. (2005). Global standard for the composition of infant formula: Recommendations of an ESPGHAN coordinated international expert group. Journal of Pediatric Gastroenterology and Nutrition, 41, 584–599.
Le Huerou-Luron, I., Bouzerzour, K., Ferret-Bernard, S., Menard, O., Le Normand, L., Perrier, C., et al. (2018). A mixture of milk and vegetable lipids in infant formula changes gut digestion, mucosal immunity and microbiota composition in neonatal piglets. European Journal of Nutrition, 57, 463–476.
Lecomte, M., Bourlieu, C., Meugnier, E., Penhoat, A., Cheillan, D., Pineau, G., et al. (2015). Milk polar lipids affect in vitro digestive lipolysis and postprandial lipid metabolism in mice–3. The Journal of Nutrition, 145, 1770–1777.
Leermakers, E. T. M., Moreira, E. M., Kiefte-de Jong, J. C., Darweesh, S. K. L., Visser, T., Voortman, T., et al. (2015). Effects of choline on health across the life course: A systematic review. Nutrition Reviews, 73, 500–522.
Lemaire, M., Dou, S., Cahu, A., Formal, M., Le Normand, L., Rome, V., et al. (2018). Addition of dairy lipids and probiotic Lactobacillus fermentum in infant formula programs gut microbiota and entero-insular axis in adult minipigs. Scientific Reports, 8, 11656.
Lien, E. L., Richard, C., & Hoffman, D. R. (2018). DHA and ARA addition to infant formula: Current status and future research directions. Prostaglandins, Leukotrienes and Essential Fatty Acids, 128, 26–40.
Linderborg, K. M., Kalpio, M., Makela, J., Niinikoski, H., Kallio, H. P., & Lagstrom, H. (2014). Tandem mass spectrometric analysis of human milk triacylglycerols from normal weight and overweight mothers on different diets. Food Chemistry, 146, 583–590.
Lindquist, S., & Hernell, O. (2010). Lipid digestion and absorption in early life: An update. Current Opinion in Clinical Nutrition and Metabolic Care, 13, 314–320.
Lopez, C., Blot, M., Briard-Bion, V., Cirié, C., & Graulet, B. (2017). Butter serums and buttermilks as sources of bioactive lipids from the milk fat globule membrane: Differences in their lipid composition and potentialities of cow diet to increase n-3 PUFA. Food Research International, 100, 864–872.
Lopez, C., Briard-Bion, V., Bourgaux, C., & Pérez, J. (2013). Solid triacylglycerols within human fat globules: β crystals with a melting point above in-body temperature of infants, formed upon storage of breast milk at low temperature. Food Research International, 54, 1541–1552.
Lopez, C., Cauty, C., & Guyomarc’h, F. (2015). Organization of lipids in milks, infant milk formulas and various dairy products: Role of technological processes and potential impacts. Dairy Science & Technology, 95, 863–893.
Lopez, C., Cauty, C., & Guyomarc’h, F. (2019). Unraveling the complexity of milk fat globules to tailor bioinspired emulsions providing health benefits: The key role played by the biological membrane. European Journal of Lipid Science and Technology, 121, 1800201.
Lopez, C., Cauty, C., Rousseau, F., Blot, M., Margolis, A., & Famelart, M.-H. (2017). Lipid droplets coated with milk fat globule membrane fragments: Microstructure and functional properties as a function of pH. Food Research International, 91, 26–37.
Lopez, C., Cheng, K., & Perez, J. (2018). Thermotropic phase behavior of milk sphingomyelin and role of cholesterol in the formation of the liquid ordered phase examined using SR-XRD and DSC. Chemistry and Physics of Lipids, 215, 46–55.
Lopez, C., Madec, M.-N., & Jiménez-Flores, R. (2010). Lipid rafts in the bovine milk fat globule membrane revealed by the lateral segregation of phospholipids and heterogeneous distribution of glycoproteins. Food Chemistry, 120, 22–33.
Lopez, C., & Ménard, O. (2011). Human milk fat globules: Polar lipid composition and in situ structural investigations revealing the heterogeneous distribution of proteins and the lateral segregation of sphingomyelin in the biological membrane. Colloids and Surfaces B: Biointerfaces, 83, 29–41.
Mathiassen, J. H., Nejrup, R. G., Frøkiær, H., Nilsson, Å., Ohlsson, L., & Hellgren, L. I. (2015). Emulsifying triglycerides with dairy phospholipids instead of soy lecithin modulates gut lipase activity. European Journal of Lipid Science and Technology, 117, 1522–1539.
Mendonça, M. A., Araújo, W. M. C., Borgo, L. A., & de Rodrigues Alencar, E. (2017). Lipid profile of different infant formulas for infants. PLoS One, 12, e0177812.
Michalski, M. C., Briard, V., Michel, F., Tasson, F., & Poulain, P. (2005). Size distribution of fat globules in human colostrum, breast milk, and infant formula. Journal of Dairy Science, 88, 1927–1940.
Miklavcic, J. J., Schnabl, K. L., Mazurak, V. C., Thomson, A. B. R., & Clandinin, M. T. (2012). Dietary ganglioside reduces proinflammatory signaling in the intestine. Journal of Nutrition and Metabolism, 2012, 1.
Miles, E. A., & Calder, P. C. (2017). The influence of the position of palmitate in infant formula triacylglycerols on health outcomes. Nutrition Research, 44, 1–8.
Motouri, M., Matsuyama, H., Yamamura, J., Tanaka, M., Aoe, S., Iwanaga, T., et al. (2003). Milk sphingomyelin accelerates enzymatic and morphological maturation of the intestine in artificially reared rats. Journal of Pediatric Gastroenterology and Nutrition, 36, 241.
Mu, H. L., & Hoy, C. E. (2004). The digestion of dietary triacylglycerols. Progress in Lipid Research, 43, 105–133.
Nilsson, A. (2016). Role of sphingolipids in infant gut health and immunity. The Journal of Pediatrics, 173, S53–S59.
Nilsson, Å., & Duan, R.-D. (2006). Absorption and lipoprotein transport of sphingomyelin. Journal of Lipid Research, 47, 154–171.
Norris, G. H., Jiang, C., Ryan, J., Porter, C. M., & Blesso, C. N. (2016). Milk sphingomyelin improves lipid metabolism and alters gut microbiota in high fat diet-fed mice. The Journal of Nutritional Biochemistry, 30, 93–101.
Ohlsson, L., Hertervig, E., Jönsson, B. A., Duan, R.-D., Nyberg, L., Svernlöv, R., et al. (2010). Sphingolipids in human ileostomy content after meals containing milk sphingomyelin. The American Journal of Clinical Nutrition, 91, 672–678.
Oosting, A., Kegler, D., Wopereis, H. J., Teller, I. C., van de Heijning, B. J. M., Verkade, H. J., et al. (2012). Size and phospholipid coating of lipid droplets in the diet of young mice modify body fat accumulation in adulthood. Pediatric Research, 72, 362–369.
Oosting, A., van Vlies, N., Kegler, D., Schipper, L., Abrahamse-Berkeveld, M., Ringler, S., et al. (2014). Effect of dietary lipid structure in early postnatal life on mouse adipose tissue development and function in adulthood. British Journal of Nutrition, 111, 215–226.
Oshida, K., Shimizu, T., Takase, M., Tamura, Y., Shimizu, T., & Yamashiro, Y. (2003). Effects of dietary sphingomyelin on central nervous system myelination in developing rats. Pediatric Research, 53, 589–593.
Owen, C. G., Whincup, P. H., Kaye, S. J., Martin, R. M., Davey Smith, G., Cook, D. G., et al. (2008). Does initial breastfeeding lead to lower blood cholesterol in adult life? A quantitative review of the evidence. The American Journal of Clinical Nutrition, 88, 305–314.
Palmano, K., Rowan, A., Guillermo, R., Guan, J., & Mc Jarrow, P. (2015). The role of gangliosides in neurodevelopment. Nutrients, 7, 3891–3913.
Park, E. J., Thomson, A. B., & Clandinin, M. T. (2010). Protection of intestinal occludin tight junction protein by dietary gangliosides in lipopolysaccharide-induced acute inflammation. Journal of Pediatric Gastroenterology and Nutrition, 50, 321–328.
Peterson, J. A., Patton, S., & Hamosh, M. (1998). Glycoproteins of the human milk fat globule in the protection of the breast-fed infant against infections. Neonatology, 74, 143–162.
Petit, V., Sandoz, L., & Garcia-Rodenas, C. L. (2017). Importance of the regiospecific distribution of long-chain saturated fatty acids on gut comfort, fat and calcium absorption in infants. Prostaglandins, Leukotrienes and Essential Fatty Acids, 121, 40–51.
Rueda, R. (2007). The role of dietary gangliosides on immunity and the prevention of infection. British Journal of Nutrition, 98, S68–S73.
Saarela, T., Kokkonen, J., & Koivisto, M. (2005). Macronutrient and energy contents of human milk fractions during the first six months of lactation. Acta Paediatrica, 94, 1176–1181.
Shek, D. T. L., Yu, L., Wu, F. K. Y., Zhu, X., & Chan, K. H. Y. (2017). A 4-year longitudinal study of well-being of Chinese university students in Hong Kong. Applied Research in Quality of Life, 12, 867–884.
Simons, K., & Ikonen, E. (1997). Functional rafts in cell membranes. Nature, 387, 569–572.
Sinanoglou, V. J., Cavouras, D., Boutsikou, T., Briana, D. D., Lantzouraki, D. Z., Paliatsiou, S., et al. (2017). Factors affecting human colostrum fatty acid profile: A case study. PLoS One, 12, e0175817.
Singh, H. (2006). The milk fat globule membrane—A biophysical system for food applications. Current Opinion in Colloid & Interface Science, 11, 154–163.
Snow, D. R., Ward, R. E., Olsen, A., Jimenez-Flores, R., & Hintze, K. J. (2011). Membrane-rich milk fat diet provides protection against gastrointestinal leakiness in mice treated with lipopolysaccharide. Journal of Dairy Science, 94, 2201–2212.
Spitsberg, V. L. (2005). Invited review: Bovine milk fat globule membrane as a potential nutraceutical. Journal of Dairy Science, 88, 2289–2294.
Sprong, R. C., Hulstein, M. F. E., Lambers, T. T., & van der Meer, R. (2012). Sweet buttermilk intake reduces colonisation and translocation of Listeria monocytogenes in rats by inhibiting mucosal pathogen adherence. British Journal of Nutrition, 108, 2026–2033.
Straarup, E. M., Lauritzen, L., Faerk, J., Hoy, C. E., & Michaelsen, K. F. (2006). The stereospecific triacylglycerol structures and fatty acid profiles of human milk and infant formulas. Journal of Pediatric Gastroenterology and Nutrition, 42, 293–299.
Sun, C., Wei, W., Su, H., Zou, X., & Wang, X. (2018). Evaluation of sn-2 fatty acid composition in commercial infant formulas on the Chinese market: A comparative study based on fat source and stage. Food Chemistry, 242, 29–36.
Tanaka, K., Hosozawa, M., Kudo, N., Yoshikawa, N., Hisata, K., Shoji, H., et al. (2013). The pilot study: Sphingomyelin-fortified milk has a positive association with the neurobehavioural development of very low birth weight infants during infancy, randomized control trial. Brain and Development, 35, 45–52.
Timby, N., Domellof, E., Hernell, O., Loennerdal, B., & Domellof, M. (2014). Neurodevelopment, nutrition, and growth until 12 mo of age in infants fed a low-energy, low-protein formula supplemented with bovine milk fat globule membranes: A randomized controlled trial. The American Journal of Clinical Nutrition, 99, 860–868.
Timby, N., Domellöf, M., Lönnerdal, B., & Hernell, O. (2017). Supplementation of infant formula with bovine milk fat globule membranes. Advances in Nutrition, 8, 351–355.
Timby, N., Hernell, O., Vaarala, O., Melin, M., Lönnerdal, B., & Domellöf, M. (2015). Infections in infants fed formula supplemented with bovine milk fat globule membranes. Journal of Pediatric Gastroenterology and Nutrition, 60, 384.
Timby, N., Loennerdal, B., Hernell, O., & Domellof, M. (2014). Cardiovascular risk markers until 12 mo of age in infants fed a formula supplemented with bovine milk fat globule membranes. Pediatric Research, 76, 394–400.
Tu, A., Ma, Q., Bai, H., & Du, Z. (2017). A comparative study of triacylglycerol composition in Chinese human milk within different lactation stages and imported infant formula by SFC coupled with Q-TOF-MS. Food Chemistry, 221, 555–567.
Vanderghem, C., Bodson, P., Danthine, S., Paquot, M., Deroanne, C., & Blecker, C. (2010). Milk fat globule membrane and buttermilks: From composition to valorization. Biotechnologie, Agronomie, Société et Environnement, 14, 485–500.
Veereman-Wauters, G., Staelens, S., Rombaut, R., Dewettinck, K., Deboutte, D., Brummer, R.-J., et al. (2012). Milk fat globule membrane (INPULSE) enriched formula milk decreases febrile episodes and may improve behavioral regulation in young children. Nutrition, 28, 749–752.
Wang, B. (2012). Molecular mechanism underlying sialic acid as an essential nutrient for brain development and cognition. Advances in Nutrition, 3, 465S–472S.
Wang, B., Yu, B., Karim, M., Hu, H., Sun, Y., McGreevy, P., et al. (2007). Dietary sialic acid supplementation improves learning and memory in piglets. The American Journal of Clinical Nutrition, 85, 561–569.
Wang, L., Shimizu, Y., Kaneko, S., Hanaka, S., Abe, T., Shimasaki, H., et al. (2000). Comparison of the fatty acid composition of total lipids and phospholipids in breast milk from Japanese women. Pediatrics International, 42, 14–20.
WHO. (2011). Exclusive breastfeeding for six months best for babies everywhere. WHO: World Health Organization. Retrieved from http://www.who.int/mediacentre/news/statements/2011/breastfeeding_20110115/en/
Ya, B., Liu, W., Ge, F., Zhang, Y., Zhu, B., & Bai, B. (2013). Dietary cholesterol alters memory and synaptic structural plasticity in young rat brain. Neurological Sciences, 34, 1355–1365.
Yao, Y., Zhao, G., Xiang, J., Zou, X., Jin, Q., & Wang, X. (2016). Lipid composition and structural characteristics of bovine, caprine and human milk fat globules. International Dairy Journal, 56, 64–73.
Zavaleta, N., Kvistgaard, A. S., Graverholt, G., Respicio, G., Guija, H., Valencia, N., et al. (2011). Efficacy of an MFGM-enriched complementary food in diarrhea, anemia, and micronutrient status in infants. Journal of Pediatric Gastroenterology and Nutrition, 53, 561–568.
Zhang, X., Qi, C., Zhang, Y., Wei, W., Jin, Q., Xu, Z., et al. (2019). Identification and quantification of triacylglycerols in human milk fat using ultra-performance convergence chromatography and quadrupole time-of-flight mass spectrometery with supercritical carbon dioxide as a mobile phase. Food Chemistry, 275, 712–720.
Zou, X.-Q., Guo, Z., Huang, J.-H., Jin, Q.-Z., Cheong, L.-Z., Wang, X.-G., et al. (2012). Human milk fat globules from different stages of lactation: A lipid composition analysis and microstructure characterization. Journal of Agricultural and Food Chemistry, 60, 7158–7167.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Lopez, C. (2020). Dairy Lipids in Infant Formulae to Reduce the Gap with Breast Milk Fat Globules: Nutritional and Health Benefits Associated to Opportunities. In: Truong, T., Lopez, C., Bhandari, B., Prakash, S. (eds) Dairy Fat Products and Functionality. Springer, Cham. https://doi.org/10.1007/978-3-030-41661-4_15
Download citation
DOI: https://doi.org/10.1007/978-3-030-41661-4_15
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-41660-7
Online ISBN: 978-3-030-41661-4
eBook Packages: Chemistry and Materials ScienceChemistry and Material Science (R0)