Abstract
Background/Objectives:
Fat droplets in human milk (HM) are larger and surrounded by a phospholipid membrane compared with infant milk formulas (IMF). Since the physical structure of fat droplets might affect digestion and postprandial metabolism, an IMF was developed more mimicking HM lipid structure than current IMF.
Subjects/Methods:
A randomised, double-blind, crossover study was performed in 29 fasted healthy men (aged 18–25 years, BMI: 18–25 kg/m2) to compare 5-hour postprandial responses after consumption of an experimental IMF (Concept, Nuturis) with a current IMF (Control).
Results:
Postprandial triacylglycerol (TAG) concentrations tended to increase faster after intake of Concept IMF (P=0.054), but peaked 3 h after intakes at similar concentrations. ApoB48 increased steadily and peaked 3 h after consumption. Increases in plasma glucose concentrations were comparable, but peak concentrations were reached faster after consumption of Concept IMF (P<0.05). Peak insulin concentrations were higher and reached earlier after intake of Concept IMF, causing a sharper decremental glucose rebound (P<0.05) and an earlier time to nadir in non-esterified fatty acid (NEFA) concentrations (P<0.01). Changes in plasma amino acids (AA), apoB100 and apoA1 were comparable. The incremental or decremental areas under-the-curve did not differ between Concept and Control IMF. Satiety scores and changes in the satiety hormones ghrelin and peptide YY were comparable, while cholecystokinin responses were earlier and higher after consumption of Control IMF (P<0.05).
Conclusions:
This proof-of-concept study suggests that fats and carbohydrates from the Concept IMF with larger and phospholipid-coated fat droplets are more rapidly absorbed than those from the current IMF.
Similar content being viewed by others
Introduction
Human milk (HM) is considered as the gold standard for infant nutrition and the World Health Organisation therefore recommends to exclusively breastfeed babies during the first 6 months of life.1, 2 Breastfeeding is associated with several adult health benefits, including a reduced risk to develop obesity, the metabolic syndrome, and type 2 diabetes by so far unknown mechanisms.3, 4 Lipids are an important source of energy for infants and provide more than 50% of the total caloric intake from HM or infant milk formula (IMF).5, 6 Compared to IMF, fat droplets in HM are large (modal diameter of 4 μm vs 0.5 μm) and surrounded by the milk fat globule membrane (MFGM), which comprises a trilayered membrane with the typical composition of mammalian cell membranes (phospholipids, sphingolipids, cholesterol, proteins, and enzymes). The much smaller fat droplets in current IMF lack such a membrane and are mainly coated with adhering whey and casein proteins.7, 8 Although different in size and coating, fat droplet fatty acid (FA) composition in IMF is quite similar to HM, despite the usage of mainly vegetable oil sources. Still, small differences exist between HM and IMF concerning their content of FAs, while IMF virtually lacks, for instance, cholesterol and phospholipids. Functional effects of these differences are largely unknown, but provide opportunities to improve the qualitative lipid supply to infants through IMF.5, 6
The differences in size and surface composition of the fat droplets in IMF and HM might affect lipolysis rates and postprandial responses. In adult healthy males, Armand et al.9 have demonstrated that gastric administration of lipid emulsions with larger droplet sizes resulted in earlier plasma peak triacylglycerol (TAG) concentrations, possibly due to a faster gastric emptying of larger fat particles as compared with smaller particles. Michalski et al.10 reported in rats increased postprandial TAG concentrations after ingestion of an emulsified dairy product with larger MFGM-coated droplets compared with a homogenised preparation with smaller casein-coated droplets. On the other hand, postprandial plasma lipid concentrations in healthy males were not affected after the consumption of a milk drink formulation that was enriched in cow’s milk MFGM polar lipids without a change in fat droplet size.11 Since the physical structure of the fat droplet might affect digestion kinetics and consequently postprandial responses, an innovative IMF (Nuturis) was developed that mimicked more closely the structure of fat droplets in HM (that is, larger fat droplets coated with cow’s milk phospholipids derived from the MFGM7, 12). In young male C57Bl/6j mice, this IMF reduced body fat accumulation and improved metabolic profiles in adulthood.13, 14 To evaluate for the first time the metabolic effects of this newly developed IMF in humans, the primary objective of the present proof-of-concept study was to examine differences in postprandial TAG responses following consumption of two IMFs, differing only in fat droplet size and coating.
Patients and methods
Subjects
Apparently healthy, non-smoking male subjects were recruited through announcements in local newspapers and university buildings in Maastricht and met the following criteria: between 18 and 25 years of age; BMI between 20 and 25 kg/m2; no known diseases or current illnesses that might interfere with the study and no use of medication, as determined during a screening visit. All participants gave their written informed consent before screening. The medical ethical committee of the Maastricht University Medical Centre+ (MUMC+) approved the protocol and the trial is registered at the Dutch Trial Register (NTR4463).
Diet and design
A randomised, double-blind, crossover study was carried out and the two test days were separated by a washout period of at least 5 days. The sequence of the test days was determined using a computer-generated randomisation scheme. On the day preceding each test day, subjects were asked not to perform any strenuous exercise, not to consume alcohol and to consume a standardised low-fat meal (<10 g fat) for dinner. After an overnight fast of at least 10 h, an intravenous cannula was placed in an antecubital vein and a fasting blood sample was collected (T=0 min). Subjects were then asked to consume the test drinks within 7 min (4 × 100 ml shake within 1 min with 1 min intervals), which were provided as milk-shake like drinks (~400 ml) and consisted of 150 g Control product (‘Nutrilon Standaard’, a commercially available, standard IMF with small protein-coated fat droplets) or 150 g Concept product (Nuturis, an experimental IMF with large phospholipid-coated fat droplets) dissolved in 300 ml lukewarm tap water. The fat droplet size distribution of the Control and Concept products were determined as described previously.7 The composition of the two test drinks is shown in Table 1. Test drinks were prepared by a registered dietician on the morning of each test day. Subsequent blood samples were drawn after 15, 30, 45, 60, 90, 120, 180, 240 and 300 min. Subjects were not allowed to eat anything except for the test drinks, but had to drink water (150 ml) following the blood samples drawn at 120 and 240 min.
Blood sampling
An intravenous cannula was placed in an antecubital vein. At each time point, a clotting tube, an EDTA tube, an aprotinin tube and a sodium fluoride (NaF) tube (Becton, Dickinson and Company, Franklin Lakes, NY, USA) were sampled. Serum was obtained from the clotting tube by low-speed centrifugation at 1300 g for 15 min at room temperature, at least half an hour after each sampling, and was used for the analysis of lipid and (apo)lipoprotein concentrations. EDTA, aprotinin and NaF plasma were obtained by low-speed centrifugation at 1300 g for 15 min at 4 °C. EDTA plasma was used for the analysis of non-esterified free fatty acids (NEFA), insulin, amino acids and ghrelin concentrations. For ghrelin analyses, EDTA plasma was enriched with a serine protease inhibitor (PMSF) and hydrogen chloride (HCl). Cholecystokinin (CCK)-8 S and peptide YY (PYY) 3-36 concentrations were determined in aprotinin plasma and a dipeptidyl peptidase (DPP)4-inhibitor was added for the analysis of PYY3-36. NaF plasma was used for the analysis of glucose concentrations. All serum and plasma samples were snap frozen in liquid nitrogen and stored at −80 °C until analysis.
Analyses
TAG concentrations with correction for free glycerol, and total cholesterol (CHOD-PAP method; Roche Diagnostics, Mannheim, Germany), and high-density lipoprotein cholesterol (HDL-C) (CHOD-PAP method; Roche Diagnostics) after precipitation of apoB-containing lipoproteins with phosphotungstic acid and magnesium ions, were analysed in serum enzymatically (GPO-Trinder; Sigma-Aldrich Corp., St. Louis, MO, USA). Low-density lipoprotein cholesterol (LDL-C) concentrations were calculated according to the Friedewald equation.15 Apolipoprotein concentrations (apoA1 and apoB100) were analysed using highly sensitive immunoturbidimetric assays (Horiba ABX, Montpellier, France) and apoB48 concentrations were measured with an ELISA kit (Shibayagi Co., Ishihara, Japan). Total cholesterol and HDL-C concentrations were measured at baseline (T0) only. TAG, apoA1 and apoB100 concentrations were measured at T0 and after 15, 30, 45, 60 and 90 min, and at hourly intervals up to 5 h. Serum apoB48 concentrations were measured at T0 and at hourly intervals up to 5 h. Plasma glucose concentrations (Roche Diagnostic Systems, Hoffmann-La Roche, Basel, Switzerland) and plasma NEFA concentrations (NEFA kit; WAKO, Neuss, Germany) were measured enzymatically at baseline and after 15, 30, 45, 60 and 90 min and at hourly intervals up to 5 h. Plasma insulin concentrations were determined with a human insulin-specific RIA kit (Linco Research, St. Charles, MO, USA) and also measured at baseline and after 15, 30, 45, 60 and 90 min and at hourly intervals up to 5 h. Plasma amino acids concentrations were measured as described previously16 at baseline and after 15 and 30 min and at hourly intervals up to 4 h. Commercially available RIA kits were used to determine concentrations of active ghrelin, PYY3-36 (Linco Research) and of CCK8 S (Euro-Diagnostica, Malmö, Sweden). Concentrations were determined at baseline and after 15, 30, 60, 90 and 120 min. All samples from one subject were analysed within one run at the end of the study.
Statistics
This study had a statistical power of 80% at P=0.05, assuming a correlation between the tests of 10%, to detect a difference between the Control and Concept drinks of 20% of the TAG incremental AUC (iAUC) with a standard deviation (SD) of 25%, when 25 subjects were included. To compensate for potential dropouts, a total of 29 subjects were included.
Differences in baseline concentrations were analysed using analysis of variance with diet, time after intake of the IMF, and subject identification number as fixed factors. Changes from baseline were analysed using linear mixed models with diet and time as within-subject fixed factors, subject identification number as between-factor and with diet*time interaction. If the diet*time interaction term reached statistical significance, differences between the diets were tested at each individual time point using a Bonferroni correction. If the interaction term was not statistically significant, it was omitted from the model. If in that model time was significant, time points were compared to baseline values using a Bonferroni correction. To evaluate overall postprandial responses, the iAUC or decremental AUC (dAUC) were calculated using the trapezoidal rule.17 Maximal changes were calculated by subtracting baseline concentrations from the maximal (iCmax) or minimal (dCmax) concentrations. Differences in iAUC or dAUC and maximal or minimal changes were analysed using univariate analysis of variance and time to reach maximal or minimal concentrations (Tmax or Tmin) were analysed using a non-parametric Sign test. All data are presented as means±s.d. or as means±s.e.m. Tmax or Tmin were reported as medians (min-max ranges). Results were considered to be statistically significant if P<0.05. Statistical analyses were performed with blinding maintained using SPSS 20.0 for Mac Os X (SPSS, Inc., Chicago, IL, USA).
Results
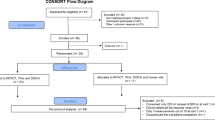
In total, 29 apparently healthy young men participated in this study. The flow of subjects through the study is shown in Supplementary Figure 1 and their baseline characteristics as measured on the first test day are presented in Table 2. All subjects completed the study.
Fasting concentrations for all parameters were comparable between both test days. Figure 1a shows postprandial TAG concentrations, which gradually increased and peaked 3 h after consumption of the test drinks (P-value for time<0.001). The postprandial pattern was comparable after intake of the Concept and the Control IMF, although there was a tendency for an earlier TAG increase after consumption of the Concept IMF (P-value diet × time=0.054). Serum apoB48 concentrations also increased over time (P-value for time<0.001), but responses were comparable after consumption of both products (Figure 1b). Figure 2a shows a significant diet*time interaction for glucose concentrations during the postprandial period (P-value diet × time=0.021). Concentrations were significantly lower after consumption of the Concept IMF compared with the Control IMF at T30, T45 and T60 (P=0.019, P=0.029 and P=0.021, respectively). In addition, maximal changes from baseline in glucose concentrations were comparable, but were reached earlier after consumption of the Concept IMF (P Tmax=0.049, Table 3). However, the absolute amplitude of glucose concentrations, that is, the difference between the maximal and the minimal glucose concentrations during the entire postprandial period, after intake of the Concept IMF (1.86±0.60 mmol/l) was slightly higher than the absolute amplitude after intake of the Control IMF (1.72±0.63 mmol/l; P=0.035). As shown in Figure 2b, postprandial insulin concentrations were comparable after consumption of both products with only a significant time effect (P for time<0.001) and maximal concentrations were again reached earlier after consumption of the Concept IMF (P Tmax<0.041 Table 3). Accordingly, maximal decreases in NEFA concentrations were also reached earlier after intake of the Concept IMF (P Tmax=0.002, Table 3), although postprandial curves were not statistically different between the two products with a significant decrease over time (P for time<0.001) (Supplementary Figure 2). Amino acid postprandial curves were comparable after both products, with a significant increase over time (P for time<0.001) and peak concentrations at 45–60 min after consumption (Supplementary Figure 3). Figure 3 shows CCK8S concentrations to follow a different postprandial curve with earlier increases after consumption of the Control IMF (P diet*time=0.012). Postprandial ghrelin curves decreased after consumption of both products (P for time<0.001) and returned to baseline concentrations after 90 min and PYY3-36 concentrations increased over time (P for time<0.001) and were comparable for both products (Supplementary Figures 4 and 5). Serum apoB100 concentrations decreased over time (P=0.001), while postprandial serum apoA1 concentrations did not change after consumption of both products (data not shown). Finally, iAUC or dAUC and maximal changes from baseline were comparable for all parameters after consumption of both products (Table 3).
(a) Serum TAG concentrations after consumption of the Concept and the Control product. Data are presented as means±s.e.m. (n=29). Changes from baseline were analysed using linear mixed models with diet and time as within-subject fixed factors and subject identification number as between-factor. (b) Serum ApoB48 concentrations after consumption of the Concept and the Control product. Data are presented as means±s.e.m. (n=29). Changes from baseline were analysed using linear mixed models with diet and time as within-subject fixed factors and subject identification number as between-factor. Control product is a standard IMF with small protein-coated lipid droplets and the Concept product is an experimental IMF with large phospholipid-coated fat droplets.
(a) Plasma glucose concentrations after consumption of the Concept and the Control product. Data are presented as means±s.e.m. (n=29). Changes from baseline were analysed using linear mixed models with diet and time as within-subject fixed factors, subject identification number as between-factor and with diet × time interaction. Differences in time to peak were analysed using a non-parametric Sign test. *Significantly different between Concept and Control product (P<0.05). (b) Serum insulin concentrations after consumption of the Concept and the Control product. Data are presented as means±s.e.m. (n=29). Changes from baseline were analysed using linear mixed models with diet and time as within-subject fixed factors and subject identification number as between-factor. Control product is a standard IMF with small protein-coated lipid droplets and the Concept product is an experimental IMF with large phospholipid-coated fat droplets.
Plasma CCK8S concentrations after consumption of the Concept and the Control product. Changes from baseline were analysed using linear mixed models with diet and time as within-subject fixed factors, subject identification number as between-factor and with diet × time interaction. Data are presented as means±s.e.m. (n=29). Control product is a standard IMF with small protein-coated lipid droplets and the Concept product is an experimental IMF with large phospholipid-coated fat droplets. *Significantly different between Concept and Control product (P<0.05)
Discussion
The present study demonstrates that consuming an IMF with large phospholipid-coated lipid droplets (that is, the Concept IMF) resulted in earlier peak concentrations of glucose and insulin, and an earlier time to nadir in NEFA concentrations. In addition, after consumption of the Concept IMF, postprandial TAG concentrations tended to increase faster and postprandial CCK curves were different compared with the Control IMF.
Size and structure of fat droplets in the Concept IMF were more similar to those in HM: fat droplets were larger and surrounded with a similar phospholipid profile as in HM.7 Effects may, therefore, have been due to the coating or lipid droplet size or to a combination of these two characteristics. This latter suggestion is supported by animal studies showing that the combination of lipid droplet size and MFGM coating is needed to have long-term beneficial effects on body fat accumulation.14 Studies in humans investigating the role of the MFGM components on postprandial metabolism are scarce. The only study so far used a milk drink rich in MFGM polar lipids and reported a tendency for reduced postprandial cholesterol concentrations without a change in postprandial TAG concentrations.11 In contrast to our data, time to peak for postprandial glucose and insulin responses were also comparable in that study, which might indicate that the presence of the ingredient MFGM does not as such influence postprandial responses. However, postprandial concentrations were determined hourly only. Since we already observed peak concentrations for glucose and insulin after 15–30 min, it is possible that Ohlsson et al.11 sampled too late to be able to detect any differences between the peak concentrations of the two milk drinks. Although still within normal ranges, we observed higher peak insulin concentrations after intake of the Concept IMF, which may explain the more profound decremental glucose rebound and the slightly higher difference between maximal and minimal glucose concentrations during the entire postprandial period after intake of the Concept IMF.
The effect of fat droplet size on postprandial characteristics has been studied in a randomised controlled trial, in which healthy subjects received intragastrically a coarse (10 μm) or a fine (0.7 μm) lipid emulsion of identical FA composition. An earlier time to peak of ~60 min in TAG concentrations was reported after administration of the emulsion with the larger fat droplets, which was explained by a faster gastric emptying of the larger fat droplets.9 We also observed a tendency for an earlier increase in TAG concentrations, an earlier time to peak in postprandial glucose and insulin concentrations, and an earlier time to nadir in NEFA concentrations after consumption of the Concept IMF, which could indicate a faster gastric emptying compared with the Control IMF. Due to the larger relative surface area, the smaller fat droplets in the Control IMF may undergo a more rapid lipolysis in the stomach resulting in the formation of more lipolytic products.18 In a semi-dynamic gastric digestion model, gastric lipolysis rates were slower for minimally processed milk emulsions which are more close to human milk and the Concept IMF compared with homogenised or homogenised/pasteurised milk emulsions more mimicking the Control IMF.19 Even though both study products initially might empty at a similar rate from the stomach, more lipolytic products, which can act as CCK-secretagogues (signal for satiation),20 may enter the duodenum after consumption of the Control IMF, resulting in higher and earlier CCK peak concentrations and an earlier feedback signal to delay gastric emptying as compared to the Concept IMF. Yet, this scenario of an overall faster gastric emptying of the Concept IMF is not supported by a similar time course observed in plasma PYY (signal for satiety) and amino acid concentrations. PYY secretory cells are located more distally in the intestine, and gastric proteolysis may be less relevant for plasma amino acid appearance compared with intestinal proteolysis of the two study products. However, other mechanisms might explain the different responses observed following consumption of the Concept IMF as well, such as access of digestive lipases to the TAG core of the fat droplets resulting in differences in fat digestion and absorption.21 Also, a difference in clotting behaviour of casein in the acidic gastric milieu due to the different coating of the two formulae tested, that is, only proteins vs partly protein and phospholipids,7 might have affected gastric emptying and thereby postprandial responses.
While there is evidence that differences in dietary fat particle size affect postprandial responses when fat is infused into the stomach or small intestine,9, 22 data are less conclusive when fat emulsions of different particle sizes are consumed orally. Peters et al. showed that consumption of a fat-free meal replacer with added rapeseed oil (5 or 9 g) with either small (0.1 μm) or large fat droplets (3 μm) did not change postprandial CCK concentrations.23 In contrast, intestinal delivery of 6 g of fine fat droplets affected satiety, gastric emptying and CCK release.24 Using magnetic resonance imaging techniques, Hussein et al.24 showed a faster gastric emptying after consumption of a 60-g fat meal containing coarse lipid droplets (6 μm mean droplet size) than after consumption of meal with fine lipid droplets (0.4 μm), but hourly-determined CCK responses were comparable after both meals. Unfortunately, TAG concentrations were not measured in that study. Steingoetter et al. assessed gastrointestinal responses after consumption of different fat emulsions using MRI and also reported a higher gastric emptying rate after consumption of a lipid emulsion containing very large droplets (52 μm) compared with smaller droplets (0.33 μm).25 However, despite this large difference in droplet size, postprandial TAG concentrations were comparable after consumption of both emulsions, while postprandial gut hormone concentrations were not determined. On the other hand, a duodenal fat infusion in healthy subjects reported an inverse relation between emulsion droplet size (0.26, 30 and 170 μm) and both plasma CCK and PYY concentrations.26
The present study has several weaknesses. First, gastric emptying was not measured. Secondly, the question remains how the effects observed can be translated to infants or to (longer-term) health effects after long-term use. Gastrointestinal conditions differ greatly between adults and infants, for example, transit time, enzyme activities, gastric pH and amounts of pancreatic lipase and bile salt are different.27 Third, postprandial responses were assessed after one single acute dose, and it remains to be determined how responses are affected when the Concept IMF would be consumed multiple times a day for a longer period.
To conclude, this proof-of-concept study suggests that fats and carbohydrates from the Concept IMF with larger and phospholipid-coated fat droplets are more rapidly absorbed than those from the current IMF, possibly due to effects on gastric emptying rate. The exact underlying mechanism, however, remains to be determined. Further, it is unknown to what extent these findings can be extrapolated to infants, and if these postprandial changes translate into (longer-term) health effects.
References
Kramer MS, Kakuma R . The Optimal Duration of Exclusive Breastfeeding—a Systematic Review. World Health Organization: Geneva, Switzerland, 2001. Accessed on June 2016.
Walker A . Breast milk as the gold standard for protective nutrients. J Pediatr 2010; 156: S3–S7.
Horta BL, Victoria CG . Long-Term Effects of Breastfeeding—a Systematic Review. World Health Organization: Geneva, Switzerland, 2013 Accessed on June 2016.
Singhal A . The role of infant nutrition in the global epidemic of non-communicable disease. Proc Nutr Soc 2016; 75: 162–168.
Delplanque B, Gibson R, Koletzko B, Lapillonne A, Strandvik B . Lipid quality in infant nutrition: current knowledge and future opportunities. J Pediatr Gastroenterol Nutr 2015; 61: 8–17.
Zou L, Pande G, Akoh CC . Infant formula fat analogs and human milk fat: New focus on infant developmental needs. Annu Rev Food Sci Technol 2016; 7: 139–165.
Gallier S, Vocking K, Post JA, Van De Heijning B, Acton D, Van Der Beek EM et al. A novel infant milk formula concept: Mimicking the human milk fat globule structure. Colloids Surf B Biointerfaces 2015; 136: 329–339.
Bourlieu C, Michalski MC . Structure-function relationship of the milk fat globule. Curr Opin Clin Nutr Metab Care 2015; 18: 118–127.
Armand M, Pasquier B, Andre M, Borel P, Senft M, Peyrot J et al. Digestion and absorption of 2 fat emulsions with different droplet sizes in the human digestive tract. Am J Clin Nutr 1999; 70: 1096–1106.
Michalski MC, Soares AF, Lopez C, Leconte N, Briard V, Geloen A . The supramolecular structure of milk fat influences plasma triacylglycerols and fatty acid profile in the rat. Eur J Nutr 2006; 45: 215–224.
Ohlsson L, Burling H, Duan RD, Nilsson A . Effects of a sphingolipid-enriched dairy formulation on postprandial lipid concentrations. Eur J Clin Nutr 2010; 64: 1344–1349.
Gallier S, Acton D, Garg M, Singh H . Natural and processed milk and oil body emulsions: Bioavailability, bioaccessibility and functionality. Food Structure; e-pub ahead of print 19 July 2016; doi:10.1016/j.foostr.2016.07.005.
Oosting A, Kegler D, Wopereis HJ, Teller IC, van de Heijning BJ, Verkade HJ et al. Size and phospholipid coating of lipid droplets in the diet of young mice modify body fat accumulation in adulthood. Pediatr Res 2012; 72: 362–369.
Baars A, Oosting A, Engels E, Kegler D, Kodde A, Schipper L et al. Milk fat globule membrane coating of large lipid droplets in the diet of young mice prevents body fat accumulation in adulthood. Br J Nutr 2016; 115: 1930–1937.
Friedewald WT, Levy RI, Fredrickson DS . Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem 1972; 18: 499–502.
van Eijk HM, Rooyakkers DR, Deutz NE . Rapid routine determination of amino acids in plasma by high-performance liquid chromatography with a 2-3 microns Spherisorb ODS II column. J Chromatogr 1993; 620: 143–148.
Brouns F, Bjorck I, Frayn KN, Gibbs AL, Lang V, Slama G et al. Glycaemic index methodology. Nutr Res Rev 2005; 18: 145–171.
Delorme V, Dhouib R, Canaan S, Fotiadu F, Carriere F, Cavalier JF . Effects of surfactants on lipase structure, activity, and inhibition. Pharm Res 2011; 28: 1831–1842.
Bourlieu C, Menard O, De La Chevasnerie A, Sams L, Rousseau F, Madec MN et al. The structure of infant formulas impacts their lipolysis, proteolysis and disintegration during in vitro gastric digestion. Food Chem 2015; 182: 224–235.
Feinle C, O'Donovan D, Doran S, Andrews JM, Wishart J, Chapman I et al. Effects of fat digestion on appetite, APD motility, and gut hormones in response to duodenal fat infusion in humans. Am J Physiol Gastrointest Liver Physiol 2003; 284: G798–G807.
van den Braak C, Thomassen G, Acton D, Ludwig T, Abrahamse E . A concept infant formula with large, phospholipid coated, droplets demonstrates slow in vitro gastric lipolysis as compared to a regular infant formula. J Pediatr Gastroenterol Nutr 2015; 60 (suppl 1), 740.
Maljaars PW, van der Wal RJ, Wiersma T, Peters HP, Haddeman E, Masclee AA . The effect of lipid droplet size on satiety and peptide secretion is intestinal site-specific. Clin Nutr 2012; 31: 535–542.
Peters HP, Bouwens EC, Schuring EA, Haddeman E, Velikov KP, Melnikov SM . The effect of submicron fat droplets in a drink on satiety, food intake, and cholecystokinin in healthy volunteers. Eur J Nutr 2014; 53: 723–729.
Hussein MO, Hoad CL, Wright J, Singh G, Stephenson MC, Cox EF et al. Fat emulsion intragastric stability and droplet size modulate gastrointestinal responses and subsequent food intake in young adults. J Nutr 2015; 145: 1170–1177.
Steingoetter A, Radovic T, Buetikofer S, Curcic J, Menne D, Fried M et al. Imaging gastric structuring of lipid emulsions and its effect on gastrointestinal function: a randomized trial in healthy subjects. Am J Clin Nutr 2015; 101: 714–724.
Seimon RV, Wooster T, Otto B, Golding M, Day L, Little TJ et al. The droplet size of intraduodenal fat emulsions influences antropyloroduodenal motility, hormone release, and appetite in healthy males. Am J Clin Nutr 2009; 89: 1729–1736.
Abrahamse E, Minekus M, van Aken GA, van de Heijning B, Knol J, Bartke N et al. Development of the digestive system-experimental challenges and approaches of infant lipid digestion. Food Dig 2012; 3: 63–77.
Acknowledgements
This research was financially supported by Nutricia Research.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
BJMvdH and DA are employed by Nutricia Research. The remaining authors declare no conflict of interest.
Additional information
Supplementary Information accompanies this paper on European Journal of Clinical Nutrition website
Rights and permissions
About this article
Cite this article
Baumgartner, S., van de Heijning, B., Acton, D. et al. Infant milk fat droplet size and coating affect postprandial responses in healthy adult men: a proof-of-concept study. Eur J Clin Nutr 71, 1108–1113 (2017). https://doi.org/10.1038/ejcn.2017.50
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ejcn.2017.50
- Springer Nature Limited
This article is cited by
-
Formula with large, milk phospholipid-coated lipid droplets in late-moderate preterm infants: a double-blind RCT
Pediatric Research (2024)
-
The effect of dietary lipid quality in early life on serum LysoPC(18:2) levels and their association with adult blood glucose levels in intrauterine growth restricted rats
Nutrition & Metabolism (2021)
-
Comparing patterns of volatile organic compounds exhaled in breath after consumption of two infant formulae with a different lipid structure: a randomized trial
Scientific Reports (2019)