Abstract
Over the last two to three decades, a large number of antibiotics from different groups have been developed to cure variety of bacterial infections in hospitals. These antibiotics, when administered orally to the patients, resulted in the detection of large amounts of antibiotics in hospital waste through excretion due to their limited metabolization in human body, coupled with the inappropriate use in the hospitals. Further, many antibiotic-resistant bacteria present in patient intestine also enters the hospital waste through excretion. The primary treatment system to treat hospital waste does not exist in developing countries. This issue is also pronounced in many developed regions and result in the release of large amount of antibiotics, antibiotic-resistant bacteria, and antibiotic resistance genes in the hospital waste. The hospital waste will create conditions that are conducive for the bacteria not only to multiply but also help to share their resistance genes with other bacteria through mobile genetic elements. Thus, the antibiotics and antibiotic-resistant bacteria that were present in the hospital waste can reach the drinking water, surface water, and marine and soil environment where they can cause variety of problems through altering the ongoing processes in the ecosystem, toxicity to various species followed by the threat to humans through food chain.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
2.1 Introduction
Antimicrobials are considered as most effective drug for the treatment of various infections in humans. In the history of medicine, penicillin was marked as the beginners of the “golden era” of antibiotics. Various antibiotics from different families were produced during 1940–1962 for the treatment of various infections caused by variety of bacteria. In the past two to three decades several new antibiotics were also developed through structural modifications of previous antibiotics to make them remain effective. These antibiotics were extensively employed in human healthcare due to their essential requirement for bacterial infection treatment, surgical involvements, and prophylactic cure and in cancer. This has resulted in the increase in global use of antibiotics due to which the usage were at the peak between 2000 and 2010 worth $40 billion per year (O’Neill 2015) with expected 36% increase in the future, which demonstrate their importance from societal and economic perspective (Van Boeckel et al. 2014). Today, antibiotics have the wide range of usage in hospitals (Cabello 2006; Sarmah et al. 2006) for the cure of various diseases in hospitals but massive development and flourishing inappropriate usage (Van Boeckel et al. 2014, 2015) have resulted in many challenges. Beside of antibiotics significance in treatment of diseases in hospitals, they are of concern due to their potential genotoxic effects. Further, the detection of large number of antimicrobials in the environment poses some serious threats including alteration in aquatic ecosystem, spread of antimicrobial resistance, and threats to public health (Daughton and Ternes 1999; Boxall 2004; Runnalls et al. 2010; Brandt et al. 2015).
2.2 Antibiotics and Their Use in Hospitals: An Overview
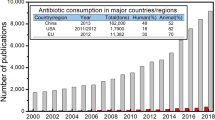
Antibiotics are designed to have such molecular structures and functions which make them to show activity against variety of bacteria which are responsible for causing many infections related to gastrointestinal tract, respiratory tract, skin infections, and many sexually transmitted diseases. Globally about 30% increase in antibiotic consumption was observed during 2000 and 2010 reaching 50–70 billion standard units, out of which about 20% was applied in the hospitals for the treatment of infections (Van Boeckel et al. 2014). Only in the USA 23 × 106 kg of antibiotics are presently in use on annual basis, out of which half of antibiotics are used for general public in hospitals and communities (Lederberg and Harrison 1998). The use of antibiotics mainly depend upon the economic status of the country and the population they hold. In 2000 the antibiotic consumption pattern was high in the USA, France, and Italy while it was reported to be low in many low- or medium-income countries. However a dramatic increase in antibiotic consumption was observed in many developing countries particularly in China, India, and Pakistan (Klein et al. 2018). This global increase in the use of antibiotics are putting burden of many untreated bacterial infections in humans, so it is the need of time not only to quantify the antibiotic use but also to focus on the reduction of antibiotic consumption in developed and developing nations. The antibiotic consumption pattern in hospitals of different countries over the period of time have been presented in Table 2.1.
2.2.1 Classification of Antibiotics
Based on the structures and functions antibiotics have been classified into broad range of groups. Within same group the antibiotics are active against the related bacteria due to similar pharmacologic and chemical properties. Several classifications of antibiotics have been developed depending upon their action of mechanism or infection cured. The most important groups of antibiotics include aminoglycosides, β-lactams (penicillin, cephalosporins, carbapenems), cephalosporins, chloramphenicol, imidazoles, lincosamides, macrolides, quinolones, rifamycins, tetracyclines, and others (Pinheiro et al. 2019). Various groups of antibiotics and examples are presented in Fig. 2.1 and explained in following sections.
2.2.1.1 Aminoglycosides
Aminoglycosides were mostly effective against Gram-negative bacteria with limited potential to work against Gram-positive bacteria. Their history linked with the discovery of streptomycin back into 1943. Aminoglycosides kill bacteria through inhibition of protein synthesis and mostly they were applied through veins instead of oral administration due to poor absorption during digestion. Streptomycin from this group was very effective against tuberculosis orcysticfibrosis. Moreover, these are commonly used prophylactically in premature infants (Hayward et al. 2019). However due to toxicity of this drug its use has also been limited today. Aminoglycosides are divided into two main groups:
-
1.
Mycin (Kanamycin, Neomycins)
-
2.
Micin (Amikacin, Gentamicin)
Aminoglycosides are derived by various species of Micromonospora and Streptomyces bacteria. Aminoglycosides produced by Streptomyces species are referred with suffix “mycin” whereas those produced from Micromonospora are referred the suffix “micin” (Farouk et al. 2015).
2.2.1.2 β-Lactams
The discovery of penicillin in 1928 led to the development of β-lactam antibiotics which consist of β-lactam ring, penicillin (amoxicillin), and cephalosporin. The β-lactams were extensively used class of antibiotics which showed activity against Gram-positive bacteria by interfering the synthesis of peptidoglycan in bacterial cell wall. In human β-lactams chemicals are most successful ever used antibiotic to cure infections due to its wide spectrum properties such as oral availability, activity, lack of toxicity, pharmacokinetics, and bactericidal action (Foster 2019). β-Lactams are further divided into two groups, which include:
-
(a)
Cephalosporins
-
(b)
Penicillin (methicillin, isoxazoyl penicillins, amoxicillin)
-
(c)
Carbapenems
-
(d)
Monobactams
Among various classes, semi-synthetic cephalosporins belong to β-lactams antibiotics which have wide applications for the treatment of human-related diseases and considered as second- or third-line therapy. According to World Health Organization (World Health Organization, 2017) cephalosporins have been declared as the most critical and highly prioritized medicine for human therapy and mainly used to combat gonorrhea infections and meningitis (BPAC 2011).
In general category, penicillin antibiotics is another β-lactam drug which have been extensively applied for curing and preventing infectious diseases. The aforementioned antibiotic is used in a drastic means for combating infections due to bacteria for many years, where its significant role in human medicine is quite popular. Penicillin are basically bicyclic organic molecules which are formed by the fusion of a β-lactam ring and a five-membered thiazolidine ring, combined at various side chains. Penicillins can be classified further into six subclasses, depending upon use, chemical structure, spectrum (extended, broad, and narrow), susceptibility to β-lactamase destruction, and source (natural, synthetic, semisynthetic). Benzyl penicillin or penicillin-G belongs to the first subclass whereas oxacillin, cloxacillin, and dicloxacillin belong to the third class (Alampanos et al. 2019).
Cephalosporin antibiotic drugs belong to the “new non-β-lactam” antibiotics which has capability to disinfect activity of Klebsiella pneumonia. Some novel cephalosporins including ceftolozane are applied in combination with tazobactam for the cure and treatment of aeruginosa infections (Peri et al. 2019).
2.2.1.3 Chloramphenicol
Chloramphenicol, also known as CAP, is a broad spectrum antibiotic group that is used for curing activity of both Gram-positive and Gram-negative bacteria (Yanovych et al. 2018). Chloramphenicol is applied to counter a broad variety of infections, which includes common cold, typhoid fever, meningitis, and bronchitis (Sharma et al. 2019). Due its side effects, this antibiotic is prohibited in many cases because of its cause of serious illnesses such as blood dyscrasias, suppression of bone marrow, gray baby syndrome, and minor effects such as headache and nausea.
2.2.1.4 Macrolides
Macrolides are a group of antibiotics medically in use for more than six decades and have the capability to inhibit a broad range of bacteria frequently used in feedlots and hospitals (Liu et al. 2014). Macrolides mainly worked against Gram-positive bacteria just like β-lactam through growth and reproduction prevention of bacteria by hindering synthesis of protein. They inhibit microbial cell growth by interfering the function of ribosome (Vázquez-Laslop and Mankin 2018). Erythromycin from this class is the most frequently used antibiotics; though bacteria has developed resistance to this group but still it is the second most prescribed group of antibiotics. Macrolide is typically a natural chemical with variety of biological activities including antiparasitic, antimalarial, and antifungal activities. A small class of natural macrolides is 12-membered macrolides, for example, pandangolide 1–4, cladospolide A and B, ozoroalide, aspergillolide, chloriolide, and balticolid (Huang et al. 2019). Macrolide chemicals have an overlapping binding site on ribosome of bacteria structurally distinct, streptogramins and lincosamides, resulting to clusters of genes encoding resistance to macrolides, called Macrolide-Lincosamide-Streptogramin resistance genes (Sutcliffe and Leclercq 2002).
2.2.1.5 Quinolones
The quinolones is an important class of synthetic antibiotics which have broad-spectrum activity against both Gram-positive and Gram-negative bacteria. Quinolones act in bactericidal manner through interference with the replication and transcription of DNA in bacteria cells. They are used in treatment of humans and food-producing animals (Zhang et al. 2017). Quinolones were mainly used to cure urinary tract infections and those which cannot be treated with other antibiotics. Besides their broad spectrum of antibacterial activity, quinolone exhibited high pharmacological properties such as antitumor, antituberculosis, antifungal, anti-HIV, antimalarial, and antiplasmodial activities. Quinolones including fluoroquinolones have efficient in vitro antiplasmodial activity against hepatic and erythrocytic stages of CQ-sensitive and CQ-resistant P. falciparum. Moreover, fluoroquinolones also exhibited excellent in vivo potency, which make them efficient candidates for the chemoprophylaxis (Fan et al. 2018). Up till now four generations/classes of quinolone antibiotics have been formulated (Hernandez-Montelongo et al. 2014) which contains various types of quinolones.
-
1.
First-generation quinolones (norfloxacin, cinoxacin)
-
2.
Second-generation quinolones (norfloxacin, ciprofloxacin, ofloxacin, enrofloxacin, and lomefloxacin)
-
3.
Third-generation quinolones (sparfloxacin, levofloxacin, moxifloxacin)
-
4.
Fourth-generation quinolones (trovafloxacin)
Currently, the most common quinolones in markets are second-generation quinolone antibiotics which include norfloxacin, ciprofloxacin, ofloxacin, enrofloxacin, gemifloxacin, lomefloxacin etc. (Gidwani and Vyas 2014) that are used in various bacterial infections. Among them, ciprofloxacin has potential to cause toxicity to central nervous, liver, and kidney toxicity and hematological toxicity in the human body. In case of third- and fourth-generation quinolone, their use is very limited because of their serious side effects. Moreover, the newly developed generation of quinolones has more genotoxic potential for instance; fourth-generation quinolones are two orders of magnitude high genotoxic as compared to third-generation quinolones (Cao et al. 2019).
2.2.1.6 Rifamycin
The rifamycin antibiotics are most commonly used in hospitals and clinics for the treatment of nontuberculous mycobacterial diseases, prophylaxis, and tuberculosis (active and latent infection) infections. Rifamycin antibiotic are complex macrocyclic molecules attained from an Actinomycete bacterium which play a preponderant role in therapeutics as antibiotics. Rifamycin combine with the RNA polymerase β-subunit which inhibit the RNA transcription, and therefore the bacterial synthesis. The side effects of the rifamycin antibiotics are associated with the discoloration of the skin and body fluids. The main class of rifamycin includes:
-
1.
Rifampin
-
2.
Rifabutin
-
3.
Rifapentine
Among semisynthetic antibiotics, two of their most applied and established rifamycin are rifampicin and rifaximi. Rifampicin is used for curing tuberculosis and inhibition of cancer cells growth in human, whereas rifaximi is applied to treat Crohn’s, diarrhea, and diverticular and intestinal infections (Jimenez-Lopez et al. 2016).
2.2.1.7 Sulfonamides
Sulfonamide is broad spectrum group of antibiotics which have a range of activities against various infections caused by Gram-positive and Gram-negative bacteria. A substantial number of this group of antibiotics was discovered based on the Prontosil which was the first sulfonamide antibiotic discovered in 1932 and made commercially available. Sulfonamide antibiotics do not kill bacteria, instead they inhibit synthesis of B vitamin folate required for nucleic acid formation, and this inhibition of folic acid results in the growth and reproduction inhibition of bacteria. The main sulfonamide type RSO2NH2 compounds have various types of pharmacological factors which includes antiviral, anticancer, and antibacterial activities; and protease inhibition, carbonic anhydrase inhibition, cyclooxygenase 2 inhibition, and diuretic action (Demir and Köksal 2019). The sulfonamides helps to treat dysentery, meningococcal meningitis, septicemia, tonsillitis, and urinary tract infections caused by Chlamydia trachomatis, E. coli, Enterobacter, Salmonella, and Shigella. The most common antibiotics in sulfonamides group are sulfamethoxazole/trimethoprim, azulfidine, and zonisamide. Their use has been limited these days due to resistance developed by bacteria against these antibiotics and their hepatotoxicity effects. New efforts have been made to develop such sulfonamide group which are potent inhibitors of the Alzheimer’s disease associated butyrylcholinestarase (Apaydin and Török 2019).
2.2.1.8 Tetracyclines
Tetracyclines are one of another broadly used antibiotic class especially in the USA and Europe, applied to cure bacterial infections of respiratory and urinary tract. Tetracyclines act in a similar way like sulfonamides and prevent synthesis of protein, followed by growth and reproduction inhibition. Tetracyclines appeared among the most prescribed antibiotics medicine for human application (Van Boeckel et al. 2014). Aminoglycosides play a key role for curing and treatment of Methicillin-resistant Staphylococcus aureus (MRSA) infections. MRSA has long been familiar as one of the main devious pathogens which is involved in nosocomial infections. It has been reported that aminoglycosides are mainly bactericidal and commonly applied synergistically in amalgamation with either glycopeptide or beta-lactam antibiotics (Goudarzi et al. 2019).
2.2.1.9 Triazole
Triazole is also known as 1,2,4-triazole and is a common pharmacophore in many drugs (Bonandi et al. 2017) which possess anti-bacterial, antifungal, antitubercular, and antitumor activities. This is the reason that triazole have wide application in clinics and hospitals to treat variety of infections and diseases (Zhang et al. 2019). Furthermore, 1, 2, 4-triazole derivatives also play an important role in the development of novel medicines by hybrid molecules. Hybrid molecules are those chemical entities which have two or more structures having different biological functions. Therefore, hybrid molecules of 1, 2, 4-triazole are marked with the dual activities, for instance the new drug may provide excellent potency against both drug-resistant bacteria and drug-sensitive (Zhang et al. 2019). The activity of various antibiotics against different infections is summarized in Table 2.2.
2.3 Misuse of Antibiotics in Hospitals
Antibiotics are critical element of today’s medicine in hospitals but they are on the top of the list of misused medicines in hospitals. Mostly antibiotics are used for the nosocomial infections and the great occurrence of infectious diseases results in the extensive practice of antibiotics in hospitals that may or may not be in an appropriate way (Wolff 1993). Due to lack of proper hygiene, misuse of antibiotics and encoded genetic elements antimicrobial resistance is becoming a great issue in the hospitals. The four major elements that are responsible for the antibiotic misuse and resistance problems in hospitals are: (1) infection, (2) patient, (3) pharmacist, and (4) physician. The role of physicians in inappropriate prescription cannot be ignored as they focus more on short-time treatment with frequent results. While the private pharmacist contribute in this problem by supplying antibiotics without verifying the physician’s prescription (Weinstein 2001). The absurd taking of antibiotics affects the whole body in different ways like allergic reactions, antibiotic resistance, antibiotic-resistant gonorrhea, and toxicity (Li 2014).
In the USA about 30% of the drug budgets in hospital is allocated for antibiotics which is correlated with the inappropriate use of antibiotics usage for more than three decades even after strict control measures. Furthermore, it was reported in 2003 that out of seven billion $ spent on antibiotics usage on annual basis, about four billion $ have been spent on the treatment of infections caused by hospital-acquired infections (Guven and Uzun 2003). Wrong prescription in hospitals increases the exposure of patients and complications (Fridkin et al. 2014), so there is a need of improved prescription of antibiotics in hospitals to offer more benefit to patients than complications. Patient outcomes can be improved by using antibiotics in an appropriate way in hospitals aiming to:
-
1.
Cure infections with an agent that requires a short time with minimum aftereffects.
-
2.
Control the spread and development of resistant microbial agents.
-
3.
Ensure better healthcare facilities on fair treatment charges.
To control the misuse of antibiotics many hospitals limit the recommendation of antibiotics by prescribing only one to two antibiotic drugs from each class. The effectiveness and appropriateness of antibiotics may be improved by the use of order forms and review of antibiotic treatment on next day of prescription (Davey et al. 2013). The computerized order form is the most effective intervention to ensure correct prescription of antibiotics. To prevent the misuse of antibiotics and enhancement of treatment it is the responsibility of doctors to correctly prescribe antibiotics to patients for good quality treatment at all stages.
2.4 Types of Wastes Generated in Hospitals
Hospital waste is categorized as medical and nonmedical waste. The medical waste is produced from various activities including first aid, emergency, operations, laboratories, and diagnosis, whereas the nonmedical waste includes the waste generated from kitchens, toilets, laundry, etc. There is high variability in the production of hospital waste depending upon hospital size, wards, services offered, country, season, etc. (Verlicchi et al. 2012; Al Aukidy et al. 2014). It was estimated that about 4 kg of the waste produced in hospitals contains at least 1 kg of the infectious waste (Babatola 2008), similarly 10–25% of the residual waste is proven to be toxic which intensify and promote disease transmission among humans (Tsakona et al. 2007).
In past gradual increase in the use of antibiotics was observed which has reached between 100,000 and 200,000 tons per year (Wise 2002) making antibiotics as potential contaminants due to their long-term and synergistic effects at low concentrations when they enter in the environment (Lombardo-Agüí et al. 2014; Oliveira et al. 2015). These antibiotics may associate with one another and can present genuine difficulties to human medicinal services. There are mainly two types of wastes generated in hospital, i.e., chemical waste and biological waste. This waste when enters into the environment from hospitals results in decreasing the efficiency of medicine and promoting bacterial resistance which has threatened the public and ecosystem health. The pathway of antibiotics released in hospitals have been presented in Fig. 2.2.
2.4.1 Chemical Waste
The most commonly detected chemical in the hospital waste are antibiotics due to their excessive use and consumption for the treatment of various types of infections. Sometimes antibiotics are ineffectively used and discharged as the active parent substance in the hospital waste. It was reported that antibiotics when given to patients they excreted 50–80% in the urine and 4–30% in the feces as parent compound (Alcock et al. 1999; Jjemba 2006; Verlicchi et al. 2012; Al Aukidy et al. 2014). On the other hand, the metabolites produced due to consumption of antibiotics remain bioactive and sometime result in the transformation to parent compound. This was observed in case of sulfonamides when N4-acetylsulfapyridine and N4-acetylsulfamethazine has changed to the parent compound (Bonvin et al. 2012). Further these antibiotics possess high stability and low volatilization which result in the persistence of these contaminants in the hospital waste.
Hospital effluents are one of the principle sources of contamination to the natural environment. After management in the hospital and excretion from patients, these antibiotic substances are released into hospital effluents. On the other hand, sometimes unused drugs are also disposed of down drains. In hospitals various antibiotics from different groups are used with high concentrations which is an indicator that major contributor of antibiotics pollution are hospitals. Many studies reported the prevalence of antibiotics in hospital waste water (Giuliani et al. 1996; Guardabassi et al. 1998; Alder et al. 2003) due to their incomplete removal in treatment plants where the antibiotics get readily adsorbed to sludge with limited biodegradation which make them to persist in the waste water and enter into surface water through effluents (Verlicchi et al. 2010). The removal efficiency of different methods used for antibiotic residues treatment in hospital waste has been presented in Table 2.3. Fluoroquinolone group of antibiotics are considered as genotoxic and these antibiotics were widely detected in hospital effluents due to limited removal with the concentrations ranging between 3000 and 87,000 ng L−1 and playing major role in the growth of antibiotic-resistant bacteria (Hartmann et al. 1998). Among various classes of antibiotics, sulfonamides and fluoroquinolones persist for longer period whereas macrolides and tetracyclines persist for comparatively longer time in the absence of sunlight. On the other hand, the other two groups of antibiotics including aminoglycosides and β-lactam showed the least persistence in the environment (Huang et al. 2011). β-Lactams and fluoroquinolones were widely used in inpatients but β-lactams seldom detected in the waste water due to its rapid cleavage on hydrolysis compared to fluoroquinolones (Bréchet et al. 2014) which is persistent and resulted in the detection with high concentrations in hospital waste water (Rodriguez-Mozaz et al. 2015). The concentration of antibiotics from various groups in hospital wastewater from different countries have been presented in Table 2.4. Throughout the most recent decades, an expanding assemblage of evidence has demonstrated that antitoxins entering the environment in this manner pose potential consequences for nontarget life forms and public health (Boxall 2004; Runnalls et al. 2010; Brandt et al. 2015).
2.4.2 Biological Waste
Antibiotics use in hospitals resulted in the prevalence of their residues in hospital waste which has emerged as global concern. This happened due to the lack of primary treatment of hospital waste water or the behavior of antibiotics in treatment plants. The antibiotic residues in hospital waste water continuously exert a strong selective pressure on ARBs and create favorable conditions (nutrient, pH, temperature, antibiotics) for antibiotic-resistant bacteria to promote gene transfer among other bacteria (Varela et al. 2014). This relationship between antibiotics, antibiotic-resistant bacteria, and rearrangement of bacterial communities has been presented in the past (Gros et al. 2013; Stalder et al. 2013; Bréchet et al. 2014; Varela et al. 2014; Baricz et al. 2018). Hospital waste holds variety of bacteria released from patient body and hospital equipment which includes many pathogens. The most common pathogens Escherichia coli and Pseudomonas aeruginosa responsible for causing urinary and respiratory tract infections has been reported by Tuméo et al. (2008). Further great number of vancomycin-resistant enterococci (VRE), multidrug-resistant, Pseudomonas aeruginosa, Enterococcus faecalis, and Enterococcus faecium were also reported in hospital effluents. It was reported that about 15% of infections in hospitals are only caused by Pseudomonas aeruginosa (Hocquet et al. 2016). So the hospital effluents containing these types of pathogens and antibiotic-resistant bacteria make these organisms to play a significant role in the evolution and propagation of these organisms and the related genes. These evidences raised an emerging concern not only to the human health but also to the environment (Jernberg et al. 2010).
The hospital effluents contain this type of biological waste and antimicrobial residues have induced the great level of concern today than ever in the history. This is evident from the report stated that infections may enhanced up to 120,000 during surgery due to decrease in the efficiency of antibiotics which have caused 6300 deaths in the USA (Teillant et al. 2015). If the spread of antimicrobial resistance will continue with the evolution of pathogens, this death rate may even rise to great numbers. Recently it has been reported that a bacterial strain isolated from livestock and patient in China is multidrug resistant and can resist to colistin (Liu et al. 2016) which is considered as “last-line” medicine for such type of pathogens (Biswas et al. 2012). These evidences suggest to step up in the hospital waste management for the protection of human and ecosystem health. The problem of hospital waste management is global but it is not properly managed in the developing countries which can result in greater effects in these countries (Tudor et al. 2005).
2.5 Environmental Burden of Hospital Waste
2.5.1 Antibiotics
The antibiotics used in hospitals ends up in the environment due to limited metabolization in human body and incomplete removal in the treatment plants. Another way of these antibiotics in the environment is through the disposal of the waste through landfills (Wang et al. 2015). This heap of antibiotic agents scattered in nature could have critical ramifications for environments and human well-being, perhaps increasing sensitivities in people and propagation of antibiotic resistance. Antimicrobials are usually detected at subtherapeutic concentrations in the environment (Kümmerer 2003) but continual exposure to these drugs increased their resistance in the environment (Kümmerer 2004). Bacterial protection from antimicrobial agents has been accounted for various environment including surface water (Ash et al. 2002), sewage (Iversen et al. 2002), drinking water (Schwartz et al. 2003), soil (Burgos et al. 2005), and marine ecosystem (Kim et al. 2004). There has been a serious threat about the presence of antimicrobial in the environment that the U.K House of Lords expressed it as: “Resistance to antibiotics constitutes a major threat to public health and ought to be recognised as such more widely than it is at present” (Wise et al. 1998). The focus should be given on the appropriate use of antimicrobials, installment of primary treatment system, followed by the improvement in the treatment processes in the hospitals. Because if they will not be appropriately prescribed by the medical practitioners and eliminated in the treatment plants, they will enter in the environment and pose threat to public and ecosystem health.
2.5.2 Development of Antibiotic Resistance in Environment
Antibiotic resistance can be developed through selective pressure caused by antimicrobials or naturally by spontaneous alteration in genes due to the lack of selective pressure in the presence of antibiotics (Blair et al. 2015). Further the antimicrobial resistance can be acquired through gaining antibiotic-resistant encoded genes from other bacteria through conjugation, transformation, and transduction. Conjugation is considered as most significant process of antibiotic resistance transmission in bacteria. The circular fragment plasmids of bacterial DNA facilitate this mechanism by independently replicating the chromosome and transfer DNA fragments through the formation of pilus when bacteria are close to each other. Transformation is also another way of transmission of antibiotic resistance through “naked” DNA from cell to cell. The free DNA originated from dead bacteria enters the receiving bacteria via cytoplasm. Genetic transfer through transduction occurs by means of a vector, mostly viruses “bacteriophages” that have the ability to infect bacteria. The virus having antibiotic-resistant coded bacterial gene transfer the genetic material to receiving bacteria. When an infecting bacteriophage transfer viral DNA to receiving bacteria, it will allow replication system of bacteria to continue to replicate infecting virus till bacterial cell expires (Alanis 2005).
In hospitals various factors are responsible for the greater resistance development in bacteria including patient’s survival in severe illness with the overuse of antibiotics, lack of effective precautionary measures, and lack of restrictions on the use of antibiotics and inappropriate prescription of antibiotics to patients. So these antibiotic-resistant bacteria and pathogens originated from the hospitals when enters into the environment can disseminate their resistance to the environmental bacteria and poses some serious consequences. This leads to severe infections resulting in the reduced effectiveness of antibiotics due to resistance genes transfer between pathogens, and more expensive and complex treatments for a longer period of time (Wellington et al. 2013). Bacteria have the ability to develop multidrug resistance through complex molecular mechanism which can increase the rate of mortality and morbidity through causing new types of infections.
2.6 Conclusion
Hospitals are serving as hotspot for the different types of antibiotics, resistance genes, and human pathogens. Antibiotics were extensively employed in the hospitals to treat various infections causing the spread of antibiotics and antibiotic-resistant bacteria from human intestine to the environment which has become a global threat. The data about the antibiotics consumption is needed to control the misuse of antibiotics and to design effective strategies. Treatment technologies needed to be installed to treat hospital waste prior to their discharge into the environment. Most importantly, antibiotic steward ship is needed to minimize the misuse and waste production in the hospital and to lower the clinical cost and improvement in the hospital treatments.
References
Akalin S (2015) Antimicrobial consumption at a university hospital in Turkey. S1 thesis, Universitas Mataram
Al Aukidy M, Verlicchi M, Voulvoulis P (2014) A framework for the assessment of the environmental risk posed by pharmaceuticals originating from hospital effluents. Sci Total Environ 493:54–64
Alampanos V, Kabir A, Furton KG, Samanidou V, Papadoyannis I (2019) Fabric phase sorptive extraction for simultaneous observation of four penicillin antibiotics residues from human blood serum prior to high performance liquid chromatography and photo-diode array detection. Microchem J 149:103964
Alanis AJ (2005) Resistance to antibiotics: are we in the post-antibiotic era? Arch Med Res 36:697–705
Alcock RE, Sweetman A, Jones KC (1999) Assessment of organic contanhnant fate in waste water treatment plants I: selected compounds and physicochemical properties. Chemosphere 38:2247–2262
Alder A, McArdell C, Golet E, Molnar E, Kuch H, Giger W (2003) Environmental exposure of antibacterial agents in hospital and municipal wastewater and river water in Switzerland [Abs.]. Proceedings of 3rd international conference on pharmaceuticals and endocrine disrupting chemicals in water pp 19–21
Apaydin S, Török M (2019) Sulfonamide derivatives as multi-target agents for complex diseases. Bioorg Med Chem Lett 29:2042–2050
Ash RJ, Mauck B, Morgan M (2002) Antibiotic resistance of gram-negative bacteria in rivers, United States. Emerg Infect Dis 8:713
Ashfaq M, Khan KN, Rasool S, Mustafa G, Saif-Ur-Rehman M, Nazar MF, Sun Q, Yu CP (2016) Occurrence and ecological risk assessment of fluoroquinolone antibiotics in hospital waste of Lahore, Pakistan. Environ Toxicol Pharmacol 42:16–22
Avci ME, Arslan F, Çiftçi Ş, Ekiz A, Tüten A, Yildirim G, Madazli R (2016) Role of spiramycin in prevention of fetal toxoplasmosis. J Matern Fetal Neonatal Med 29:2073–2076
Babatola J (2008) A study of hospital waste generation and management practice in Akure, Nigeria. Afr Res Rev 2:292–305
Baricz A, Teban A, Chiriac CM, Szekeres E, Farkas A, Nica M, Dascălu A, Oprișan C, Lavin P, Coman C (2018) Investigating the potential use of an Antarctic variant of Janthinobacterium lividum for tackling antimicrobial resistance in a One Health approach. Sci Rep 8:15272
Barry PM, Klausner JD (2009) The use of cephalosporins for gonorrhea: the impending problem of resistance. Expert Opin Pharmacother 10:555–577
Benko R, Matuz M, Doro P, Viola R, Hajdu E, Monnet D, Soos G (2009) Hungarian hospital antibiotic consumption at the regional level, 1996–2005. Infection 37:133–137
Biswas S, Brunel JM, Dubus JC, Reynaud-Gaubert M, Rolain JM (2012) Colistin: an update on the antibiotic of the 21st century. Expert Rev Anti-Infect Ther 10:917–934
Blair JM, Webber MA, Baylay AJ, Ogbolu DO, Piddock LJ (2015) Molecular mechanisms of antibiotic resistance. Nat Rev Microbiol 13:42
Blix HS, Hartug S (2005) Hospital usage of antibacterial agents in relation to size and type of hospital and geographical situation. Pharmacoepidemiol Drug Saf 14:647–649
Bonandi E, Christodoulou MS, Fumagalli G, Perdicchia D, Rastelli G, Passarella D (2017) The 1, 2, 3-triazole ring as a bioisostere in medicinal chemistry. Drug Discov Today 22:1572–1581
Bonvin F, Omlin J, Rutler R, Schweizer WB, Alaimo PJ, Strathmann TJ, McNeill K, Kohn T (2012) Direct photolysis of human metabolites of the antibiotic sulfamethoxazole: evidence for abiotic back-transformation. Environ Sci Technol 47:6746–6755
Boxall AB (2004) The environmental side effects of medication: how are human and veterinary medicines in soils and water bodies affecting human and environmental health? EMBO Rep 5:1110–1116
Brandt KK, Amézquita A, Backhaus T, Boxall A, Coors A, Heberer T, Lawrence JR, Lazorchak J, Schönfeld J, Snape JR (2015) Ecotoxicological assessment of antibiotics: a call for improved consideration of microorganisms. Environ Int 85:189–205
Bréchet C, Plantin J, Sauget M, Thouverez M, Talon D, Cholley P, Guyeux C, Hocquet D, Bertrand X (2014) Wastewater treatment plants release large amounts of extended-spectrum β-lactamase-producing Escherichia coli into the environment. Clin Infect Dis 58:1658–1665
Brown KD, Kulis J, Thomson B, Chapman TH, Mawhinney DB (2006) Occurrence of antibiotics in hospital, residential, and dairy effluent, municipal wastewater, and the Rio Grande in New Mexico. Sci Total Environ 366:772–783
Burgos J, Ellington B, Varela M (2005) Presence of multidrug-resistant enteric bacteria in dairy farm topsoil. J Dairy Sci 88:1391–1398
BPAC (2011) Appropriate use of cephalosporins. Available from: https://bpac.org.nz/bpj/2011/december/cephalosporins.aspx
Cabello FC (2006) Heavy use of prophylactic antibiotics in aquaculture: a growing problem for human and animal health and for the environment. Environ Microbiol 8:1137–1144
Casas ME, Chhetri RK, Ooi G, Hansen KM, Litty K, Christensson M, Kragelund C, Andersen HR, Bester K (2015a) Biodegradation of pharmaceuticals in hospital wastewater by a hybrid biofilm and activated sludge system (Hybas). Sci Total Environ 530:383–392
Casas ME, Chhetri RK, Ooi G, Hansen KM, Litty K, Christensson M, Kragelund C, Andersen HR, Bester K (2015b) Biodegradation of pharmaceuticals in hospital wastewater by staged Moving Bed Biofilm Reactors (MBBR). Water Res 83:293–302
Chang J (2016) Does chlortetracycline ointment aid healing of a traumatic tympanic membrane perforation? Clin Otolaryngol 41:435–436
Chang X, Meyer MT, Liu X, Zhao Q, Chen H, Chen JA, Qiu Z, Yang L, Cao J, Shu W (2010) Determination of antibiotics in sewage from hospitals, nursery and slaughter house, wastewater treatment plant and source water in Chongqing region of Three Gorge Reservoir in China. Environ Pollut 158:1444–1450
Cao D-Q, Yang W-Y, Wang Z, Hao X-D (2019) Role of extracellular polymeric substance in adsorption of quinolone antibiotics by microbial cells in excesssludge. Chem Eng J 370:684–694
Čižman M (2011) Nationwide hospital antibiotic consumption in Slovenia. J Antimicrob Chemother 66:2189–2191
Daughton CG, Ternes TA (1999) Pharmaceuticals and personal care products in the environment: agents of subtle change? Environ Health Perspect 107:907–938
Davey P, Brown E, Charani E, Fenelon L, Gould IM, Holmes A, Ramsay CR, Wiffen PJ, Wilcox M (2013) Interventions to improve antibiotic prescribing practices for hospital inpatients. Cochrane Database Syst Rev 4:CD003543
Del Rosso JQ (2015) Oral doxycycline in the management of acne vulgaris: current perspectives on clinical use and recent findings with a new double-scored small tablet formulation. J Clin Aesthet Dermatol 8:19
Demir Y, Köksal Z (2019) The inhibition effects of some sulfonamides on human serum paraoxonase-1 (hPON1). Pharmacol Rep 71:545–549
Devrajani BR, Raza S, Khushik R, Shah SZA, Mari S, Laghari S, Maheshwary N (2018) Treatment of diabetic foot infections: a prospective study highlighting the efficacy and safety of Moxifloxacin. Int J Endocrinol Metab Disord 8:32–36
Dinos GP (2017) The macrolide antibiotic renaissance. Br J Pharmacol 174:2967–2983
Fan YL, Cheng XW, Wu JB, Liu M, Zhang FZ, Xu Z, Feng LS (2018) Antiplasmodial and antimalarial activities of quinolone derivatives: an overview. Eur J Med Chem 146:1–14
Farahnik B, Zaghi S, Hendizadeh L, Gopen Q (2015) Rusty green stained temporal bone associated with exposure to tetracycline: an unusual presentation of black bone disease. J Laryngol Otol 129:276–278
Fernández AML, Rendueles M, Díaz M (2014) Sulfamethazine retention from water solutions by ion exchange with a strong anionic resin in fixed bed. Sep Sci Technol 49:1366–1378
Foster TJ (2019) Can β-lactam antibiotics be resurrected to combat MRSA? Trends Microbiol 27:26–38
Fridkin S, Baggs J, Fagan R, Magill S, Pollack LA, Malpiedi P, Slayton R, Khader K, Rubin MA, Jones M (2014) Vital signs: improving antibiotic use among hospitalized patients. Morb Mortal Wkly Rep 63:194–200
Fuchs A, Bielicki J, Mathur S, Sharland M, Van Den Anker J (2016) Antibiotic use for sepsis in neonates and children: 2016 evidence update. WHO Reviews
Farouk F, Azzazy HM, Niessen WM (2015) Challenges in the determination of aminoglycoside antibiotics, a review. Analytica Chimica Acta 890:21–43
Gidwani B, Vyas A (2014) Synthesis characterization and application of epichlorohydrin-β-cyclodextrin polymer. Colloids Surf B Biointerfaces 114:130–137
Giuliani F, Koller T, Würgler F, Widmer RM (1996) Detection of genotoxic activity in native hospital waste water by the umuC test. Mutat Res Genet Toxicol Environ Mutagen 368:49–57
Goudarzi M, Razeghi M, Dadashi M, Miri M, Hashemi A, Amirpour A, Nasiri MJ, Fazeli M (2019) Distribution of SCCmec types, tetracycline and aminoglycoside resistance genes in hospital-associated methicillin-resistant Staphylococcus aureus strains. Gene Rep 16:100454
Gros M, Rodríguez-Mozaz S, Barceló D (2013) Rapid analysis of multiclass antibiotic residues and some of their metabolites in hospital, urban wastewater and river water by ultra-high-performance liquid chromatography coupled to quadrupole-linear ion trap tandem mass spectrometry. J Chromatogr 1292:173–188
Guardabassi L, Petersen A, Olsen JE, Dalsgaard A (1998) Antibiotic resistance in Acinetobacterspp. Isolated from sewers receiving waste effluent from a hospital and a pharmaceutical plant. Appl Environ Microbiol 64:3499–3502
Guven G, Uzun O (2003) Principles of good use of antibiotics in hospitals. J Hosp Infect 53:91–96
Hartmann A, Alder AC, Koller T, Widmer RM (1998) Identification of fluoroquinolone antibiotics as the main source of umuC genotoxicity in native hospital wastewater. Environ Toxicol Chem 17:377–382
Haug JB, Berild D, Walberg M, Reikvam Å (2011) Increased antibiotic use in Norwegian hospitals despite a low antibiotic resistance rate. J Antimicrob Chemother 66:2643–2646
Hayward T, Young A, Jiang A, Crespi EJ, Coffin AB (2019) Glucococorticoid receptor activation exacerbates aminoglycoside-induced damage to the zebrafish lateral line. Hearing Res 377:12–23
Hermosilla Nájera L, Canut Blasco A, Ulibarrena Sanz M, Abasolo Osinaga E, Carlos Abecia Inchaurregui L (2003) Trends in antimicrobial utilization at a Spanish general hospital during a 5-year period. Pharmacoepidemiol Drug Saf 12:243–247
Hernandez-Montelongo J, Naveas N, Degoutin S, Tabary N, Chai F, Spampinato V, Ceccone G, Rossi F, Torres-Costa V, Manso-Silvan M (2014) Porous silicon-cyclodextrin based polymer composites for drug delivery applications. Carbohydr Polym 110:238–252
Hocquet D, Muller A, Bertrand X (2016) What happens in hospitals does not stay in hospitals: antibiotic-resistant bacteria in hospital wastewater systems. J Hosp Infect 93:395–402
Huang C, Chen T, Yan Z, Guo H, Hou X, Jiang L, Long Y (2019) Thiocladospolide E and cladospamide A, novel 12-membered macrolide and macrolide lactam from mangrove endophytic fungus Cladosporium sp. SCNU-F0001. Fitoterapia 137:104246
Huang CH, Renew JE, Smeby KL, Pinkston K, Sedlak DL (2011) Assessment of potential antibiotic contaminants in water and preliminary occurrence analysis. J Contemp Water Res Educ 120:4
Iversen A, Kühn I, Franklin A, Möllby R (2002) High prevalence of vancomycin-resistant enterococci in Swedish sewage. Appl Environ Microbiol 68:2838–2842
Jernberg C, Löfmark S, Edlund C, Jansson JK (2010) Long-term impacts of antibiotic exposure on the human intestinal microbiota. Microbiology 156:3216–3223
Jimenez-López J, Molina-García L, Rodrigues S, Santos J, Ortega-Barrales P, Ruiz-Medina A (2016) Automated determination of rifamycins making use of MPA–CdTe quantum dots. J Lumin 175:158–164
Jjemba PK (2006) Excretion and ecotoxicity of pharmaceutical and personal care products in the environment. Ecotoxicol Environ Saf 63:113–130
Kim SR, Nonaka L, Suzuki S (2004) Occurrence of tetracycline resistance genes tet (M) and tet (S) in bacteria from marine aquaculture sites. FEMS Microbiol Lett 237:147–156
Klein EY, Van Boeckel TP, Martinez EM, Pant S, Gandra S, Levin SA, Goossens H, Laxminarayan R (2018) Global increase and geographic convergence in antibiotic consumption between 2000 and 2015. Proc Natl Acad Sci U S A 115:E3463–E3470
Kovalova L, Siegrist H, Singer H, Wittmer A, McArdell CS (2012) Hospital wastewater treatment by membrane bioreactor: performance and efficiency for organic micropollutant elimination. Environ Sci Technol 46:1536–1545
Kümmerer K (2003) Significance of antibiotics in the environment. J Antimicrob J Chemother 52:5–7
Kümmerer K (2004) Resistance in the environment. J Antimicrob Chemother 54:311–320
Kümmerer K, Henninger A (2003) Promoting resistance by the emission of antibiotics from hospitals and households into effluent. Clin Microbiol Infect 9:1203–1214
Lederberg J, Harrison PF (1998) Antimicrobial resistance: issues and options. National Academies Press, Washington, DC
Li Y (2014) China’s misuse of antibiotics should be curbed. BMJ 348:g1083
Lin AYC, Yu TH, Lateef SK (2009) Removal of pharmaceuticals in secondary wastewater treatment processes in Taiwan. J Hazard Mater 167:1163–1169
Liu M, Ding R, Zhang Y, Gao Y, Tian Z, Zhang T, Yang M (2014) Abundance and distribution of Macrolide-Lincosamide-Streptogramin resistance genes in an anaerobic-aerobic system treating spiramycin production wastewater. Water Res 63:33–41
Liu P, Liu WJ, Jiang H, Chen JJ, Li WW, Yu HQ (2012) Modification of bio-char derived from fast pyrolysis of biomass and its application in removal of tetracycline from aqueous solution. Bioresour Technol 121:235–240
Liu YY, Wang Y, Walsh TR, Yi LX, Zhang R, Spencer J, Doi Y, Tian G, Dong B, Huang X (2016) Emergence of plasmid-mediated colistin resistance mechanism MCR-1 in animals and human beings in China: a microbiological and molecular biological study. Lancet Infect Dis 16:161–168
Loeffler JM, Garbino J, Lew D, Harbarth S, Rohner P (2003) Antibiotic consumption, bacterial resistance and their correlation in a Swiss university hospital and its adult intensive care units. Scand J Infect Dis 35:843–850
Lombardo-Agüí M, Cruces-Blanco C, García-Campaña AM, Gámiz-Gracia L (2014) Multiresidue analysis of quinolones in water by ultra-high perfomance liquid chromatography with tandem mass spectrometry using a simple and effective sample treatment. J Sep Sci 37:2145–2152
Lontos S, Shelton E, Angus PW, Vaughan R, Roberts SK, Gordon A, Gow PJ (2014) A randomized controlled study of trimethoprim-sulfamethoxazole versus norfloxacin for the prevention of infection in cirrhotic patients. J Dig Dis 15:260–267
Marson B, Deshmukh S, Grindlay D, Ollivere B, Scammell B (2018) A systematic review of local antibiotic devices used to improve wound healing following the surgical management of foot infections in diabetics. Bone Joint J 100:1409–1415
Michałek K, Lechowicz M, Pastuszczak M, Wojas Pelc A (2015) The use of trimethoprim and sulfamethoxazole (TMP-SMX) in dermatology. Folia Med Cracov 55:35–41
Moussavi G, Alahabadi A, Yaghmaeian K, Eskandari M (2013) Preparation, characterization and adsorption potential of the NH4Cl-induced activated carbon for the removal of amoxicillin antibiotic from water. Chem Eng J 217:119–128
Мukhin KY, Pylaeva O (2015) Zonisamide (Zonegran) in the treatment of epilepsy in adults and childern (a review and clinical case). Russkij Žurnal Detskoj Nevrologii 10:47–63
Nguyen T, Jeyakumar A (2019) Genetic susceptibility to aminoglycoside ototoxicity. Int J Pediatr Otorhinolaryngol 120:15–19
Nguyen TT, Bui XT, Luu VP, Nguyen PD, Guo W, Ngo HH (2017) Removal of antibiotics in sponge membrane bioreactors treating hospital wastewater: comparison between hollow fiber and flat sheet membrane systems. Bioresour Technol 240:42–49
Nielsen U, Hastrup C, Klausen M, Pedersen B, Kristensen G, Jansen J, Bak S, Tuerk J (2013) Removal of APIs and bacteria from hospital wastewater by MBR plus O3, O3+ H2O2, PAC or ClO2. Wat Sci Tech 67:854–862
O’Neill J (2015) Securing new drugs for future generations: the pipeline of antibiotics. Available from: https://wellcomecollection.org/works/zqv86kgr
Oliveira TS, Murphy M, Mendola N, Wong V, Carlson D, Waring L (2015) Characterization of pharmaceuticals and personal care products in hospital effluent and waste water influent/effluent by direct-injection LC-MS-MS. Sci Total Environ 518:459–478
Owusu E, Newman MJ, Kotey NK, Akumwena A, Bannerman E (2016) Susceptibility profiles of Mycobacterium ulcerans isolates to streptomycin and rifampicin in two districts of the Eastern Region of Ghana. Int J Microbiol 2016:8304524
Pakyz A, Powell JP, Harpe SE, Johnson C, Edmond M, Polk RE (2008) Diversity of antimicrobial use and resistance in 42 hospitals in the United States. Pharmacotherapy 28:906–912
Peri AM, Doi Y, Potoski BA, Harris PN, Paterson DL, Righi E (2019) Antimicrobial treatment challenges in the era of carbapenem resistance. Diagn Microbiol Infect Dis 94:413–425
Pinheiro M, Magalhães J, Reis S (2019) Antibiotic interactions using liposomes as model lipid membranes. Chem Phys Lipids 222:36–46
Plüss-Suard C, Pannatier A, Kronenberg A, Mühlemann K, Zanetti G (2011) Hospital antibiotic consumption in Switzerland: comparison of a multicultural country with Europe. J Hosp Infect 79:166–171
Polk RE, Fox C, Mahoney A, Letcavage J, MacDougall C (2007) Measurement of adult antibacterial drug use in 130 US hospitals: comparison of defined daily dose and days of therapy. Clin Infect Dis 44:664–670
Principi N, Blasi F, Esposito S (2015) Azithromycin use in patients with cystic fibrosis. Eur J Clin Microbiol Infect Dis 34:1071–1079
Putra EK, Pranowo R, Sunarso J, Indraswati N, Ismadji S (2009) Performance of activated carbon and bentonite for adsorption of amoxicillin from wastewater: mechanisms, isotherms and kinetics. Water Res 43:2419–2430
Rodriguez-Mozaz S, Chamorro S, Marti E, Huerta B, Gros M, Sànchez-Melsió A, Borrego CM, Barceló D, Balcázar JL (2015) Occurrence of antibiotics and antibiotic resistance genes in hospital and urban wastewaters and their impact on the receiving river. Water Res 69:234–242
Runnalls TJ, Margiotta-Casaluci L, Kugathas S, Sumpter JP (2010) Pharmaceuticals in the aquatic environment: steroids and anti-steroids as high priorities for research. Hum Ecol Risk Assess 16:1318–1338
Sailaja AK (2014) An overall review on rheumatoid arthritis. J Pharm Res Int 4:1138
Sarmah AK, Meyer MT, Boxall AB (2006) A global perspective on the use, sales, exposure pathways, occurrence, fate and effects of veterinary antibiotics (VAs) in the environment. Chemosphere 65:725–759
Schwartz T, Kohnen W, Jansen B, Obst U (2003) Detection of antibiotic-resistant bacteria and their resistance genes in wastewater, surface water, and drinking water biofilms. FEMS Microbiol Ecol 43:325–335
Sharma R, Akshath US, Bhatt P, Raghavarao K (2019) Fluorescent aptaswitch for chloramphenicol detection–quantification enabled by immobilization of aptamer. Sensors Actuat B Chem 290:110–117
Stalder T, Alrhmoun M, Louvet JN, Casellas M, Maftah C, Carrion C, Pons MN, Pahl O, Ploy M-C, Dagot C (2013) Dynamic assessment of the floc morphology, bacterial diversity, and integron content of an activated sludge reactor processing hospital effluent. Environ Sci Technol 47:7909–7917
Sutcliffe JA, Leclercq R (2002) Mechanisms of resistance to macrolides, lincosamides, and ketolides. In: Macrolide antibiotics. Springer, Basel, pp 281–317
Teillant A, Gandra S, Barter D, Morgan DJ, Laxminarayan R (2015) Potential burden of antibiotic resistance on surgery and cancer chemotherapy antibiotic prophylaxis in the USA: a literature review and modelling study. Lancet Infect Dis 15:1429–1437
Tsakona M, Anagnostopoulou E, Gidarakos E (2007) Hospital waste management and toxicity evaluation: a case study. Waste Manag 27:912–920
Tudor T, Noonan C, Jenkin L (2005) Healthcare waste management: a case study from the National Health Service in Cornwall, United Kingdom. Waste Manag 25:606–615
Tumbarello M, Trecarichi EM, De Rosa FG, Giannella M, Giacobbe DR, Bassetti M, Losito AR, Bartoletti M, Del Bono V, Corcione S (2015) Infections caused by KPC-producing Klebsiella pneumoniae: differences in therapy and mortality in a multicentre study. J Antimicrob Chemother 70:2133–2143
Tuméo E, Gbaguidi-Haore H, Patry I, Bertrand X, Thouverez M, Talon D (2008) Are antibiotic-resistant Pseudomonas aeruginosa isolated from hospitalised patients recovered in the hospital effluents? Int J Hyg Environ Health 211:200–204
Van Boeckel TP, Brower C, Gilbert M, Grenfell BT, Levin SA, Robinson TP, Teillant A, Laxminarayan R (2015) Global trends in antimicrobial use in food animals. Proc Natl Acad Sci U S A 112:5649–5654
Van Boeckel TP, Gandra S, Ashok A, Caudron Q, Grenfell BT, Levin SA, Laxminarayan R (2014) Global antibiotic consumption 2000 to 2010: an analysis of national pharmaceutical sales data. Lancet Infect Dis 14:742–750
Varela AR, André S, Nunes OC, Manaia CM (2014) Insights into the relationship between antimicrobial residues and bacterial populations in a hospital-urban wastewater treatment plant system. Water Res 54:327–336
Vázquez-Laslop N, Mankin AS (2018) How macrolide antibiotics work. Trends Biochem Sci 43:668–684
Verlicchi P, Al Aukidy M, Galletti A, Petrovic M, Barceló D (2012) Hospital effluent: investigation of the concentrations and distribution of pharmaceuticals and environmental risk assessment. Sci Total Environ 430:109–118
Verlicchi P, Galletti A, Petrovic M, Barceló D (2010) Hospital effluents as a source of emerging pollutants: an overview of micropollutants and sustainable treatment options. J Hydrol 389:416–428
Wang Y, Tang W, Qiao J, Song L (2015) Occurrence and prevalence of antibiotic resistance in landfill leachate. Environ Sci Pollut Res 22:12525–12533
Watkinson A, Murby E, Kolpin D, Costanzo S (2009) The occurrence of antibiotics in an urban watershed: from wastewater to drinking water. Sci Total Environ 407:2711–2723
Weinstein RA (2001) Controlling antimicrobial resistance in hospitals: infection control and use of antibiotics. Emerg Infect Dis 7:188
Wellington EM, Boxall AB, Cross P, Feil EJ, Gaze WH, Hawkey PM, Johnson-Rollings AS, Jones DL, Lee NM, Otten W (2013) The role of the natural environment in the emergence of antibiotic resistance in Gram-negative bacteria. Lancet Infect Dis 13:155–165
Wise R (2002) Antimicrobial resistance: priorities for action. J Antimicrob J Chemother 49:585–586
Wise R, Hart T, Cars O, Streulens M, Helmuth R, Huovinen P, Sprenger M (1998) Antimicrobial resistance. BMJ 317:609–610
Wolff MJ (1993) Use and misuse of antibiotics in Latin America. Clin Infect Dis 17:S346–S351
Wong KC, Brown AM, Luscombe GM, Wong SJ, Mendis K (2015) Antibiotic use for vibrio infections: important insights from surveillance data. BMC Infect Dis 15:226
World Health Organization (2017) Integrated surveillance of antimicrobial resistance in foodborne bacteria: application of a one health approach: guidance from the WHO Advisory Group on Integrated Surveillanec of Antimicrobial Resistance (AGISAR)
Yanovych D, Berendsen B, Zasadna Z, Rydchuk M, Czymai T (2018) A study of the origin of chloramphenicol isomers in honey. Drug Test Anal 10:416–422
Zhang J, Liu D, Shi Y, Sun C, Niu M, Wang R, Hu F, Xiao D, He H (2017) Determination of quinolones in wastewater by porous β-cyclodextrin polymer based solid-phase extraction coupled with HPLC. J Chromatogr B 1068:24–32
Zhang J, Wang S, Ba Y, Xu Z (2019) 1, 2, 4-Triazole-quinoline/quinolone hybrids as potential anti-bacterial agents. Eur J Med Chem 174:1–8
Zhang J, Zheng FP, Li H (2015) Lomefloxacin-induced hypoglycemia in an elderly patient with chronic kidney disease: a case report. Int J Diabetes Dev Ctries 35:374–377
Zhang W, Shen X, Wang Y, Chen Y, Huang M, Zeng Q, Wei J, Lu Q, Wang G, Deng L (2008) Antibiotic use in five children's hospitals during 2002–2006: the impact of antibiotic guidelines issued by the Chinese Ministry of Health. Pharmacoepidemiol Drug Saf 17:306–311
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Riaz, L. et al. (2020). Antibiotics Use in Hospitals and Their Presence in the Associated Waste. In: Hashmi, M. (eds) Antibiotics and Antimicrobial Resistance Genes. Emerging Contaminants and Associated Treatment Technologies. Springer, Cham. https://doi.org/10.1007/978-3-030-40422-2_2
Download citation
DOI: https://doi.org/10.1007/978-3-030-40422-2_2
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-40421-5
Online ISBN: 978-3-030-40422-2
eBook Packages: Earth and Environmental ScienceEarth and Environmental Science (R0)