Abstract
Antimicrobial resistance (AMR) is a global threat as the existing health care may become ineffective. Antibiotics, antibiotic-resistant bacteria (ARB), and antibiotic resistance genes (ARGs) considered as emerging contaminants are the three major components of AMR. India is one of the largest consumers of antibiotics with defined daily dose (DDD) of 4,950 per 1,000 population in 2015. By 2030, therapeutic and nontherapeutic use of antibiotics in veterinary animals is projected to increase by 18%. Antibiotics, ARB, and ARGs in the solid and liquid waste generated enter the environment via different pathways. The major sources of antibiotics, ARB, and ARG include domestic, hospital, and pharmaceutical industry wastewater apart from the solid/liquid waste generated from veterinary and food animals. Existing conventional wastewater treatment technologies like activated sludge process (ASP) do not ensure complete removal of antibiotics, ARB, and ARGs from wastewater. Similarly, the sludge generated find its way to agriculture land and eventually spread resistance in the environment. Once introduced in the environment, elimination of these contaminants is difficult. India’s action plan on AMR in 2017 regulates antibiotic use for human and animal and addresses environment AMR spread from all possible sources and containment. In 2020, the Government of India introduced discharge standard for 121 antibiotics in the effluents of bulk drug manufacturing industries, formulation industries, and common effluent treatment plant (CETP) receiving pharmaceutical wastewater.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- AMR
- Antibiotics
- ARB
- ARG
- Discharge standards
- Environment
- India
- Nontherapeutic
- Solid and liquid waste
- Therapeutic
- Wastewater treatment
1 Introduction
Antibiotic resistance is a major public health concern at the global level as the existing health-care services may become ineffective. To exemplify, chloramphenicol is no longer a preferred choice for treating patients with antibiotic-resistant bacterial infections [1]. By 2050, two million deaths are projected to occur in India because of antibiotic resistance [2]. In developing countries like India urbanization and population growth are in increasing trend and this could lead to high consumption of antibiotics. In 2010, India was one of the top 5 countries with the largest shares of global antibiotic consumption. According to the Center for Disease Dynamics, Economics, & Policy (CDDEP), the antibiotic consumption was 2,645 DDD per 1,000 population in the year 2000 and has increased to 4,950 DDD per 1,000 population in the year 2015 [3]. Increase in antibiotic consumption could increase the antibiotic resistance burden and failure of treatment [4]. Hence, containment of antibiotic resistance spread is important. However, it is important to understand that AMR containment is not specific to only health-care services but also to the environment.
Antibiotics, ARB, and ARGs are the three major components of antibiotic resistance. The major sources of antibiotics, ARB, and ARGs in environment are wastewater from hospital, domestic use, and pharmaceutical industries [5,6,7]. Apart from wastewater and solid/liquid waste generated from animals, nontherapeutic use also introduce antibiotics, ARB, and ARGs into the environment [5, 6]. The western part of India has 47% of nation’s pharmaceutical manufacturing units, but the effluent characteristic data including antibiotics from the manufacturing unit is scarcely available for this zone. While southern India hosts 18% of the pharmaceutical manufacturing units, concentration of the pharmaceutical compounds in the effluent is well reported compared to other zones [8]. The data is very important to understand the contribution of manufacturing units in polluting the environment with antibiotics. However, the data on contribution from animal and nontherapeutic use is limited due to lack of strict rules and stringent monitoring. Similarly, quantitative data on ARB and ARGs entering environment due to anthropogenic activities are also limited in India.
To frame policies and treatment standards for an emerging concern like antibiotics, ARB, and ARG contamination, a holistic study approach is required. The study may include data on (1) antibiotics use in hospital, veterinary antibiotics in farms, and in other nontherapeutic applications; (2) source, pathway, or fate and transport of antibiotics, ARB, and ARGs; (3) occurrences of antibiotics, ARB, and ARGs in different environment matrices; (4) efficiency of current wastewater treatment practices in removing antibiotics, ARB, and ARGs; (5) national policies and plans implemented by the Government to contain AMR spread; and (6) current status and future recommendations. This book chapter attempts at presenting the Indian scenario of the abovementioned holistic approach.
2 Antibiotic Consumption for Therapeutics Purpose
Antibiotic use is one of the major determining factors of resistance gain and transfer. In India, antibiotics are prescribed in excess [9], and consumption is increasing steadily [10]. The most commonly consumed antibiotics are β-lactams while commonly prescribed includes fluoroquinolones, macrolides, cephalosporins, tetracyclines, and co-trimoxazole apart from penicillins [11]. It was reported that global antibiotic consumption has increased by 36% of which Brazil, Russia, India, China, and South Africa (BRICS) countries contribute 76%. About 23% of overall retail sales in BRICS countries was attributed to India. Between 2010 and 2015, there was a substantial increase in consumption of cephalosporins, broad-spectrum penicillins, and macrolides [3]. Cephalosporin and broad-spectrum penicillin consumption have significantly increased from 330 and 691 to 1822 and 1,055 DDD per 1,000 population between 2000 and 2015, respectively. Similarly, the consumption of macrolides has increased from 247 in 2000 to 469 DDD per 1,000 population in 2015 (Fig. 1). The consumption of trimethoprim has dropped from 332 to 186 DDD per 1,000 population between 2000 and 2015. However, consumption of the fluoroquinolones in 2000 was 492 and increased to 1,033 DDD per 1,000 population in 2006 and has dropped to 762 DDD per 1,000 population in 2015 (Fig. 1). Pattern change in antibiotic use could be attributed to rapid economic growth, rising income, and easy availability of antibiotics.
Pattern change in antibiotic consumption in India, 2000–2015 [3]
The use of antibiotics varies due to different reasons ranging from availability [12] to self-medication. Antibiotics consumption also varies with season, and in India, the average consumption was reported to peak during September, the end of the monsoon [13]. Inappropriate consumption of antibiotics for acute diarrhea [13], viral dengue fever [14], and acute respiratory infections [12] is widely reported in India. Poor sanitation practice [13], unregulated over-the-counter private pharmacy sales [14, 15], inappropriate prescription by medical practitioners for incentives [16], and pharmacy sales in hospitals [17] are also reported in India. Over 50% of antibiotics consumed for presumed tuberculosis in Nagpur were dispensed without prescription over the counter [14]. A study conducted by Peripi et al. in Vijayawada reported that broad-spectrum antibiotics are highly prescribed by private practitioners compared to the public doctors [9], while Kumari Indira et al. observed high antibiotic prescription by rural practitioners compared to urban practitioners [12]. The prescription pattern also depends on nonclinical factors such as drug availability, type of hospital and department, and patients’ request. Drugs available in the reserve (for specific treatments) are commonly prescribed in private hospitals, while in public/government hospitals, availability at the pharmacy is the deciding factor of prescription [12]. In some situations, antibiotics are prescribed based on patients’ request. About 11–35% of doctors prescribed antibiotics due to patient pressure in a study conducted in Tamil Nadu [18]. However, irrational prescription of antibiotics is quite common in private and public sector hospitals across India [19]. Nevertheless, education on rational use of antibiotics is mandatory for both medical practitioners and patients.
3 Antibiotics Use in the Animal Food Industry
Over the years, the purpose of antibiotics use in livestock industry has changed. The antibiotics are used in animal farms as therapeutic agents, growth promoters, and prophylactic agents [20]. Antibiotics and antimicrobials constitute more than 70% of veterinary medicine [21]. Global consumption of antibiotics in 2013 in animal food industries was estimated to be 131,109 tons and is expected to increase more than 50% by the year 2030 [22]. In 2010, India was the fourth largest consumer of antibiotics in food animal, which accounts for about 3% of the global consumption. By 2030, antimicrobial consumption for animals is expected to grow by 99% in BRICS countries [23].
Antibiotics are mixed in the feed to make animals resistant against disease and to gain more weight in a short time, which increases productivity. India is one of the largest producers of milk, producing 165.4 million tons in the year 2016–2017 primarily from smallholders [24]. An easy way for a smallholder to increase the yield is to make use of uncontrolled availability of antibiotics for therapeutics and to promote growth as it is economical [25]. This could result in high concentration of antibiotics in milk products and meat and animal wastes exceeding the maximum residue limits. Lack of regulatory framework to curb the use of antibiotics in livestock and food animals is one of the reasons for resistance spread in India. It is also estimated that in 2030, the antibiotics consumption in livestock in India may increase by 18% [23]. Chicken consumption in India is rapidly increasing. To meet the growing demand addition of unregulated quantity of antibiotics to animal feed as a growth promoter in poultry is practiced. The chicken meat was reported to have antibiotic residues such as tetracyclines (sum of oxytetracycline, chlortetracycline, doxycycline) and fluoroquinolones (sum of enrofloxacin and ciprofloxacin). Concentration of tetracyclines and fluoroquinolones was in the range of 16.01 to 46.02 μg/kg and 3.37 to 196.34 μg/kg, respectively [26]. In European countries, food quality controls on chemicals including antibiotics are in place. In India, the tolerance limit for antibiotics is set for seafood under Food Safety and Standards Regulation 2011, but no such regulation is put in place for chicken meat. The chicken meat that is exported has to comply with EU standards, while the domestic consumption has no regulation [26].
Nontherapeutic use of antibiotics in food preservation, apiary, aquaculture, agriculture, poultry, and pig farming is one of the sources of antibiotic resistance gain, and it spreads to the larger environment [22, 23]. 90% of the antibiotics are administered to farm animals at subtherapeutic concentration, of which 30% constitute growth promoters. Over-the-counter sale of veterinary antibiotics as growth promoters is quite common in India [27]. Antibiotics at sublethal concentration could increase the chance of resistance acquisition and transfer in animal gut bacteria [28]. Up to 90% of the antibiotics are excreted via urine and animal feces either unchanged or as active metabolized products [29]. Apart from the antibiotic residues and ARB in animal products, animal waste in all forms (carcasses, urine, feces) serves as a medium for AMR spread in the environment.
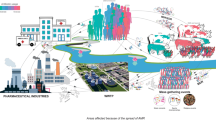
4 Source and Pathways of Antibiotics, ARB, and ARGs in the Environment
Figure 2 represents the source and pathway of antibiotics, ARB, and ARGs into the environment. Direct contamination of surface and groundwater with components of AMR is due to direct and indirect discharge of treated/untreated wastewater from residence, industries, and solid/liquid waste generated from livestock farms, aquaculture, etc. In recent years, rapid urbanization has led to the implementation of decentralized treatment plants, and the treated effluent from such plants is commonly used for landscaping, watering lawns, gardening, and irrigation. The application of treated effluent for irrigation and recreational activities could contaminate the soil and indirectly contaminate the surface and groundwater [29, 30]. The sludge generated from wastewater treatment plant (WWTP) or sewage treatment plant (STP) and solid/liquid waste from livestock farms containing antibiotics, ARB, and ARGs are widely used as soil amendment to improve the nutrient content of the agriculture soil [31, 32]. Leaching of antibiotics, ARB, and ARGs over time from amended soil may reach groundwater and contaminate it indirectly [33]. Poor sanitation practice is also reported as one of the reasons for AMR and spread [34]. According to the UNICEF monitoring data 2012, 59% of 1.1 billion people who practice open defecation in the world reside in India [35]. Open defecation contaminates the soil, surface water, and groundwater directly and indirectly.
In India, surface water serves as the source of drinking and irrigation of agriculture fields. Contamination of the water sources with antibiotics, ARB, and ARGs can disseminate resistance genes to the environment [36]. Direct and indirect human exposure to contaminated environment such as water and soil is risky. Similarly, the prevalence of antibiotic resistance in the environment can be life-threatening when there is a disease outbreak making treatment of the resistant organism’s infection difficult.
5 Antibiotics in Different Environmental Matrices
Lack of sufficient infrastructure and proper waste management practices are the main reasons for introduction of antibiotics into natural streams. The concentration of each antibiotic in different environmental matrices vary due to properties of antibiotics such as octanol-water partition coefficient, biodegradability, bioavailability, etc. (Table 1). The direct source of antibiotics in surface water results from the discharge of untreated or partially treated wastewater from hospitals, industries, and WWTPs [37]. The concentration of antibiotics in sewage is directly proportional to prescription quantity [38]. In India, antibiotics are sold as nonprescription drug with a frequency of 18% [18]. Hence, there is no control over the residual concentration ending up in sewage. High concentration of fluoroquinolones was detected in hospital effluents and in river water samples especially ciprofloxacin [38, 39]. The concentration of ciprofloxacin found in effluent from a CETP that treats wastewater from bulk drug manufacturing units was 31,000 μg/L [6].
The Yamuna River is polluted with antibiotics including ampicillin, ciprofloxacin, gatifloxacin, sparfloxacin, and cefuroxime and is continuing to receive effluents from 17 STPs and 17 storm water drains [40]. Cochin estuaries receive 250 m3 of domestic sewage because of improper waste management. This estuary is reported to have antibiotic-resistant E. coli counts higher than that reported in estuaries in France and Portugal [41, 42]. Sediments collected from Mutha River immediately after discharge point of treated wastewater from major hospitals and residential zone are enriched with genes conferring resistance to last resort antibiotics like carbapenems as well as metals and biocides [37, 43]. Contamination of Mutha River is due to the malfunctioning of WWTPs in Pune city and 50% of city’s untreated sewage being discharged into the river [38].
The discharge of conventionally treated wastewater into streams has a significant effect on all forms of surface water. Efficient tertiary treatment of wastewater, proper management of sludge, and changes in disposal ways could control antibiotic resistance transmission to different environmental matrices.
6 Seasonal and Spatial Variation of Antibiotics in the Environment Matrices
Antibiotic concentration in the environment is reported to vary with seasons. Antibiotics concentration is reported to be highest during winter season, less during summer, and least during monsoon in River Yamuna [40]. Kshipra River water was found to have high concentration of sulfamethoxazole during autumn (2.75 μg/L) and winter (2.15 μg/L) compared to summer (1.39 μg/L) and rainy season (0.04 μg/L) [48]. The change in antibiotic residue concentration could be due to change in pH. pH of the water bodies can influence the solubility of the antibiotics [50]. Sulfamethoxazole concentration in Kshipra river sediments is reported to be influenced by solubility, which in turn is influenced by pH [48]. High concentration of antibiotics in river water during winter could be due to low biodegradation rate, which is influenced by temperature and other operational parameters of the treatment plant [45]. The lower concentrations of antibiotics observed during monsoon could be due to the dilution effect of rainwater. During summer, photo degradation, temperature, and microbial activity play important role in reducing the antibiotic concentration [48].
In India, the concentration of antibiotics is comparatively more in river tributaries than in the main river because the tributaries are the immediate discharge points of wastewater. The antibiotics concentration reduces due to dilution effect when it reaches the main river [47]. Also, the contamination level of antibiotics in surface water such as river is positively correlated with land use pattern [39, 43]. This could be due to discharge of treated/untreated wastewater containing high concentration of antibiotics resulting from high domestic consumption. High concentrations of antibiotics are reported near the discharge points in surface water [51] and in rivers traversing through urbanized area as the infusion of sewage and industrial effluent are inevitable in densely populated areas [52]. There are several other physic-chemical properties such as photosensitivity, co-metabolism, nature of active metabolites/by-products, etc. that determines the spatial distribution and concentration of antibiotics in different environments. However, reports available on seasonal and spatial variation of antibiotics in different Indian environment are limited. Antibiotics in any environment could increase the chance of resistance spread, disease burden, and treatment failure from direct and indirect human exposure to resistant pathogens [36].
7 Antibiotic-Resistant Bacteria in the Environment
ARB are inherently present in the environment. ARB and antibiotics enter the environment from different sources [44] such as human and animal excreta, discharge form wastewater treatment plant, and direct sewage discharge. It is supposed that antibiotic residues that enter aquatic environment can promote resistance in aquatic microbial communities [53]. High concentration of antibiotics may select ARB in the environment [54]. Reports suggest that antibiotics at low and subminimum inhibitory concentration can also select ARB [55]. ARB mutants selected at low and subinhibitory concentrations exhibit higher stability than the ones selected at high antibiotic concentration [56].
WWTP acts as a connecting bridge between wastewater generated and aquatic environments. WWTP provides the right environment for mutation and exchange of genes resulting in resistance spread because a large number of bacteria constantly encounter antibiotics at subinhibitory concentration [56, 57]. Proportion of ARB may increase during course of wastewater treatment mainly when treatment processes involve one or more biological methods [54]. Akiba et al. reported that increased prevalence of resistant bacteria in the STPs was due to inflow of hospital wastewater. Also, the strains isolated were resistant to antibiotics quantified in the STP samples [10]. Partially treated or untreated wastewater is usually discharged into surface water leading to contamination of aquatic environment with ARB. ARB could share resistant genes with environmental bacteria. When antibiotic resistance genes are shared, the environmental ARB (eARB) could become pathogenic ARB (pARB) if the eARB carries virulence traits [58, 59]. This signifies that inadequate wastewater treatment and poor maintenance of STPs in hospitals could contribute to the spread of AMR in the environment [60, 61]. ARB are reported in different environments including hospital wastewater, untreated sewage, STPs, drinking water, river water and sediments, coastal waters, marine water, and sediments (Table 2). Bacteria exhibiting different levels of resistance are widely reported in various environments across India (Table 2). However, distribution of ARB in environment varies with season [48], and elimination of ARB from any environment can be difficult [5]. Hence, ARB introduced into the environment through anthropogenic activities are of concern.
From Table 2, it can be seen that in India, domestic/hospital wastewater and effluents from WWTPs/STPs are the widely reported sources of ARB. This is because wastewater and effluents are direct source of ARB introduction. Indirect/neglected sources of ARB include application of STP/WWTP sludge, livestock waste on land as soil amendment, open defecation, and direct dumping of animal waste in the vicinity water sources, which are widely practiced in India [29, 82,83,84]. The indirect sources ARB are least studied. Runoff water from agriculture lands and places of open defecation also serve as a medium for ARB introduction in the environment [49, 85]. However, not all the reported ARB are clinically significant.
8 Antibiotic Resistance Genes in the Environment
Some microbes have intrinsic resistance to antibiotics irrespective of antibiotic use [86]. However, excessive use of antibiotics results in resistance acquisition and transfer. Though ARGs are inherent to microbial community, the intensive use of antibiotics for human, animal, and agriculture accelerates ARG mutation and acquisition of new ARGs [53]. ARGs are widely reported in different environment including hospital wastewater, untreated sewage, STPs, drinking water, river water, and sediments (Table 3). ARGs are considered as emerging pollutants, and dissemination of ARGs in the environment is of concern. ARGs can be of genomic or plasmidic origin. However, antibiotic resistance traits are usually associated with horizontally transferable mobile genetic elements [87]. ARGs in genomic DNA are transferred to progeny, while plasmid DNA may also be transferred to different bacteria species via horizontal gene transfer (HGT) through conjugation, transduction, and transformation mechanisms [59]. ARGs are associated with MGE such as plasmids and integrons, and transposons enable easy dissemination of resistance via HGT [88]. ARGs in plasmids are self-transmissible and capable of transferring and replicating in different organisms [89].
Antibiotics stress not only selects bacteria but also results in gene mutation in resistance genes [54]. ARGs are persistent and may occur in the environment even in the absence of antibiotic selection pressure [43], and the distribution of different ARGs in environment may vary with season [27, 92]. However, ARGs proliferate irrespective of seasons during wastewater treatment [43]. The gut bacteria especially E. coli is a human commensal, antibiotic resistance indicator [69] and a reserve for antibiotic resistance genes, which can be horizontally transferred to pathogenic bacteria [93]. E. coli could reach environment matrices such as river due to discharge of partially treated and untreated domestic or hospital wastewater. ARGs encoded by plasmids in E. coli may result in resistance gene spread/transfer [69] in such environment. Bajaj et al. demonstrated co-transfer of plasmid-mediated quinolone-resistant (PMQR) gene qnrS and plasmid-mediated AmpC β-lactamase genes between E. coli strains isolated from River Ganga and quinolone susceptible E. coli J53. E. coli J53 after gene acquisition exhibited co-resistance to quinolone and β-lactams [94]. This study proves that plasmid-mediated HGT of ARG is possible between an environmental E. coli resistant to antibiotics and E. coli susceptible to antibiotics.
Mutation in RDR also determines the bacteria’s ability to resist antibiotics and may confer cross-resistance to other antibiotics [94]. Hence, it is important to understand the relationship between RDR and ARG. Lamba et al. reported correlation between expression levels of mobile genetic elements int1 and int3 and blaNDM-1 gene abundance in Enterobacteriaceae [61]. The study also reported co-carriage of int1 and blaNDM-1 gene in carbapenem -resistant Enterobacteriaceae (CRE) isolated from different sources. The co-carriage was reported to be 28%, 45%, 52%, and 57% in CRE isolated from STP, hospital, drain, and river samples, respectively, across New Delhi [61]. Co-occurrence of ARGs is quite common in MDR bacteria. In hospital setting, MDR bacterial infections are difficult to treat and sometimes are life-threatening. Chandran et al. reported co-occurrence of cephalosporin and quinolone resistance genes in E. coli isolated from hospital wastewater in Ujjain, Central India [78]. Hospital wastewater is also reported to contain MDR E. coli that are genetically diverse [78]. ARGs are inherent to resistant bacteria in the environment. However, anthropogenic activities such as discharging partially treated and untreated wastewater, application of livestock waste as soil amendments, open defection, etc. could increase ARG prevalence in the environment [85]. This could also promote resistance transfer to nonresistant/ pathogenic environmental bacteria [59].
9 Indian WWTPs Status in Eliminating Antibiotics, ARB, and ARG
Wastewater is linked to the natural environment by STPs. In India, the treatment facilities exist only for 31.5% of wastewater generated [95]. The commonly employed treatment technologies include ASP, oxidation pond, upflow anaerobic sludge blanket (UASB), sequential batch reactor, fluidized bed reactor, waste stabilization pond, Karnal Technology, rotating biological rope contactor, chrome recovery pilot plant, and aerated lagoon [96]. In India, the total capacity of treatment plants are 23,277 millions of liters per day, of which 5% is nonoperational, and 11% is under construction [95]. There is a large gap between wastewater generated and wastewater treated.
WWTPs/STPs receiving sewage and sewage mixed with hospital effluents contain antibiotics, ARB, and ARGs. The efficiency of the WWTPs in removing the AMR components decides the quality of the effluent. Hence, it is important to evaluate the performance of existing treatment technologies in removing antibiotics, ARB, and ARGs. The activated sludge process showed good removal efficiency for antibiotics like sparfloxacin and cefuroxime with 99% and 94% removal, respectively [40], and is less effective in case of low concentration antibiotics and recalcitrant antibiotics. The antibiotics removal efficiency is high when extended aeration process is employed for domestic wastewater [97]. Mutiyar and Mittal observed high removal of amoxicillin in STP involving an extended aeration ASP [97]. The antibiotic removal efficiency is also dependent on the influent wastewater quality. Prabhasankar et al. reported high antibiotic concentration in the effluent of the STPs receiving mixed wastewater from hospitals and domestic zone than that treating exclusively the hospital wastewater [1]. The high antibiotic removal rate in the STPs treating exclusively the hospital wastewater could be due to quantity of the influent wastewater and residence time. However, the antibiotics were not completely removed in the effluent of STP treating exclusively hospital wastewater [1]. Proper treatment of hospital wastewater can eliminate antibiotics by 80%, but only 40% of health-care facility has proper wastewater treatment facilities [98]. It is reported that an average of 0.53 kg of sulfamethoxazole is discharged annually from a STP in India serving an average population of 325,000 [21]. The concentrations of antibiotics in effluents from Indian STPs are higher when compared to Europe and North American countries [97]. The effluent of a CETP in Patancheru, Hyderabad, which receives wastewater from 90 drug manufacturing companies, after biological treatment is reported to have high concentration of antibiotics [6].
Mixed or not both domestic and hospital wastewater contain ARB and ARGs [99]. A STP employing UASB followed by ASP and chlorine disinfection scheme was ineffective in removing MDR E. coli [100]. Similarly, STPs receiving mixed wastewater and exclusively hospital wastewater employing ASP followed by clarification and chlorine disinfection were not effective in removing MDR E. coli [97]. The biological treatment employed in a municipal WWTP in Haridwar, removed 60–80% of fluoroquinolones, and disinfection by chlorine eliminated 96% of fluoroquinolone-resistant bacteria [54]. The effluent of a hospital wastewater treatment plant in Delhi was reported to carry carbapenem-resistant bacteria such as Pseudomonas spp., Klebsiella spp., Escherichia spp., and Acinetobacter spp., and ARG blaNDM-1 associated with int1 [101]. Similarly, effluents from 12 STPs employing aerobic sludge as treatment process across Delhi were also reported to carry ARB such as carbapenem and ESBL-resistant bacteria and ARGs such as NDM-1, CTX, OXA, and TEM. However, including anaerobic digestion and chlorination as tertiary treatment improved the removal rate of ARB and ARGs [60]. There is no complete removal of antibiotics by the existing treatment plants, and the most common removal mechanism is sorption to sludge particulates [40]. Seasonal changes and type of wastewater treatment process do not have an impact on the prevalence of resistant isolates [10]. ARB and ARGs are also not completely removed during the treatment process, and maximum percentage ends up in the sludge [60].
10 India’s Action Plans on AMR and Current Status
Antibiotic resistance is not considered a serious threat in majority of the developing countries due to lack of awareness among public. In India, antibiotic resistance made the news pages in 2010 with report on isolation of New Delhi metallo-ß-lactamase-1 (NDM-1). Ever since the NDM-1 reports in 2010 several clinical studies reporting isolation of antibiotic-resistant pathogens, and genes in clinical samples are increasing, indicating the awareness among research community. Several policies addressing effective antibiotic use and AMR containment are adopted in India since 2011 (Table 4). National antimicrobial policy for containment of AMR in India was adopted in 2011 [102], while Global Action Plan on AMR was adopted in 2014 [103].
Even though India’s action plan on AMR was adopted in 2011, the plan considered only human and animal consumption and over-the-counter sale of antibiotics as the main reason for AMR spread. This resulted in inclusion of antibiotics in schedule H1 drug, AMR prevention and containment declaration, guidelines for antimicrobial usage in treating infectious disease, and widespread campaign across India. In August 2016, the Prime Minister of India addressed the antibiotic resistance and launched “Red line campaign” to make the public aware of the importance of antibiotic use and misuse [102]. This was followed by India’s National Action Plan on AMR in 2017 that focuses on the use of antibiotics and resistance in human, animal, agriculture, food products, and environment [110]. One of the objectives of India’s National Action Plan is to reduce environmental contamination with resistance genes, resistant pathogens, and antibiotic residues arising from solid/liquid waste generated from manufacturing, use, waste treatment, and disposal [110]. Even though antibiotics are classified under schedule H1 drug, antibiotics are still sold over the counter without prescription. However, the frequency has significantly dropped compared to the earlier reported studies [113]. Following National Action Plan on AMR in 2017, research on environmental AMR in India has significantly increased and is gaining attention. About 61.3% of increase in households with toilet is reported with implementation and funding through Swachh Bharat Mission [114], and open defecation has greatly reduced. In January 2020, Indian government has published a draft comprising discharge standard for 121 antibiotics in the treated effluents of bulk drug and formulation industry and CETP treating pharmaceutical wastewater. The draft also suggests incineration of the sludge containing antibiotics residue [115].
11 Conclusion
Implemented rules and regulations for human consumption, animal use, and disposal of antibiotics must be strictly followed and regularly monitored in India. Public awareness about the importance of antibiotics to treat serious infections and AMR emergence must be created to avoid misuse of antibiotics. Recall and safe disposal of unused antibiotics from households should be implemented. AMR research in India is majorly focused on contribution of WWTPs of domestic sewage, pharmaceutical industries, and hospital effluents in environment AMR spread. There is little to no data on contribution of environmental AMR by other sources such as sewage sludge, solid/liquid waste generated by poultry, aquaculture, dairy and other livestock, agriculture run off, etc. Also a holistic approach to study the dissemination pathways of ARB and ARGs for better understanding and designing of suitable treatment technology is needed. Standards for treatment and discharge of hospital and domestic wastewater must be introduced. Similarly framing antibiotic discharge standards for sectors like poultry, aquaculture, and dairy wastewater, etc. is also necessary considering the fact that emerging contaminants like antibiotics, ARB, and ARG pose serious threat if disposed/discharged untreated. In a developing country like India, wastewater treatment capacity must be increased to treat the waste generated, and advance treatment technologies may be implemented as tertiary treatment option to ensure safe effluent discharge or reuse. Also existing conventional treatment facilities may be retrofitted with advanced tertiary treatment technologies to eliminate antibiotics, ARBs, ARGs, and the like. Alternatively, standard for reuse of reclaimed water for potable and non-potable purpose could be implemented. Quality of such water should be regularly monitored for emerging contaminants like antibiotics, ARB, and ARGs.
References
Prabhasankar VP et al (2016) Removal rates of antibiotics in four sewage treatment plants in South India. Environ Sci Pollut Res 23(9):8679–8685. https://doi.org/10.1007/s11356-015-5968-3
Laxminarayan R et al (2013) Antibiotic resistance – the need for global solutions. Lancet Infect Dis 13(12):1057–1098. https://doi.org/10.1016/S1473-3099(13)70318-9
The Center for Disease Dynamics Economics & Policy (2018) Resistance map: antibiotic resistance. https://resistancemap.cddep.org/. Accessed 14 Jun 2019
Goossens H (2009) Antibiotic consumption and link to resistance. Clin Microbiol Infect 15:12–15. https://doi.org/10.1111/j.1469-0691.2009.02725.x
Kümmerer K (2009) Antibiotics in the aquatic environment – a review – part I. Chemosphere 75(4):417–434. https://doi.org/10.1016/j.chemosphere.2008.11.086
Larsson DGJ, de Pedro C, Paxeus N (2007) Effluent from drug manufactures contains extremely high levels of pharmaceuticals. J Hazard Mater 148(3):751–755. https://doi.org/10.1016/j.jhazmat.2007.07.008
Kümmerer K (2009) Antibiotics in the aquatic environment – a review – part II. Chemosphere 75(4):435–441. https://doi.org/10.1016/j.chemosphere.2008.12.006
Philip JM, Aravind UK, Aravindakumar CT (2018) Emerging contaminants in Indian environmental matrices – a review. Chemosphere 190:307–326. https://doi.org/10.1016/j.chemosphere.2017.09.120
Peripi SB, Thadepalli VGR, Khagga M, Tripuraribhatla PK, Bharadwaj DK (2012) Profile of antibiotic consumption, sensitivity and resistance in an urban area of Andhra Pradesh, India. Singap Med J 53(4):268–272
Akiba M et al (2015) Impact of wastewater from different sources on the prevalence of antimicrobial-resistant Escherichia coli in sewage treatment plants in South India. Ecotoxicol Environ Saf 115:203–208. https://doi.org/10.1016/j.ecoenv.2015.02.018
Van Boeckel TP et al (2014) Global antibiotic consumption 2000 to 2010: an analysis of national pharmaceutical sales data. Lancet Infect Dis 14(8):742–750. https://doi.org/10.1016/S1473-3099(14)70780-7
Kumari Indira KS, Chandy SJ, Jeyaseelan L, Rashmi K, Saradha S (2008) Antimicrobial prescription patterns for common acute infections insome rural & urban health facilities of India. Indian J Med Res 128:165–171
Walsh TR, Weeks J, Livermore DM, Toleman MA (2011) Dissemination of NDM-1 positive bacteria in the New Delhi environment and its implications for human health: an environmental point prevalence study. Lancet Infect Dis 11(5):355–362. https://doi.org/10.1016/S1473-3099(11)70059-7
Morgan DJ, Okeke IN, Laxminarayan R, Perencevich EN, Weisenberg S (2011) Non-prescription antimicrobial use worldwide: a systematic review. Lancet Infect Dis 11(9):692–701. https://doi.org/10.1016/S1473-3099(11)70054-8
Kotwani A, Wattal C, Joshi PC, Holloway K (2012) Irrational use of antibiotics and role of the pharmacist: an insight from a qualitative study in New Delhi, India. J Clin Pharm Ther 37(3):308–312. https://doi.org/10.1111/j.1365-2710.2011.01293.x
Kotwani A, Wattal C, Katewa S, Joshi PC, Holloway K (2010) Factors influencing primary care physicians to prescribe antibiotics in Delhi India. Fam Pract 27(6):684–690. https://doi.org/10.1093/fampra/cmq059
Chandy SJ (2008) Consequences of irrational use of antibiotics. Indian J Med Ethics 5(4):174–175. https://doi.org/10.20529/IJME.2008.064
Sivagnanam G, Mohanasundaram J, Thirumalaikolundusubramanian P, Raaj AA, Namasivayam K, Rajaram S (2004) A survey on current attitude of practicing physicians upon usage of antimicrobial agents in southern part of India. MedGenMed, 6(2). https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1395775/. Accessed 14 Jun 2019
Kaur A et al (2018) A study of antibiotic prescription pattern in patients referred to tertiary care center in northern India. Ther Adv Infect Dis 5(4):63–68. https://doi.org/10.1177/2049936118773216
You Y, Silbergeld EK (2014) Learning from agriculture: understanding low-dose antimicrobials as drivers of resistome expansion. Front Microbiol 5. https://doi.org/10.3389/fmicb.2014.00284
Subedi B, Balakrishna K, Sinha RK, Yamashita N, Balasubramanian VG, Kannan K (2015) Mass loading and removal of pharmaceuticals and personal care products, including psychoactive and illicit drugs and artificial sweeteners, in five sewage treatment plants in India. J Environ Chem Eng 3(4, Part A):2882–2891. https://doi.org/10.1016/j.jece.2015.09.031
Boeckel TPV et al (2017) Reducing antimicrobial use in food animals. Science 357(6358):1350–1352. https://doi.org/10.1126/science.aao1495
Van Boeckel TP et al (2015) Global trends in antimicrobial use in food animals. Proc Natl Acad Sci U S A 112(18):5649–5654. https://doi.org/10.1073/pnas.1503141112
Annual Report (2017–2018) Department of Animal Husbandry, Dairying and Fisheries, Ministry of Agricultureand Farmers Welfare. http://dadf.gov.in/sites/default/filess/annual_report_17-18.pdf. Accessed 17 Nov 2019
Chauhan SL, Priyanka, Garg SR, Jadhav VJ (2019) Determination of tetracycline residues in milk by high performance liquid chromatography. Int J Curr Microbiol Appl Sci 8(2):2763–2771. https://doi.org/10.20546/ijcmas.2019.802.324
Sahu R, Saxena P, Mathur HB, Agarwal HC (2014) Antibiotics in chicken meat. Center for Science and Environment. http://re.indiaenvironmentportal.org.in/files/file/Antibiotics%20in%20Chicken_Lab%20Report_Final%2029%20July.pdf. Accessed 27 Jun 2019
Stockton B, Davies M, Meesaraganda R (2018) Zoetis and its antibiotics for growth in India. Vet Rec 183(14):432–433. https://doi.org/10.1136/vr.k4278
Jayalakshmi K, Paramasivam M, Sasikala M, Tamilam T, Sumithra A (2017) Review on antibiotic residues in animal products and its impact on environments and human health. J Entomol Zool Stud 5(3):1446–14451
Massé DI, Saady NMC, Gilbert Y (2014) Potential of biological processes to eliminate antibiotics in livestock manure: an overview. Animals 4(2):146–163. https://doi.org/10.3390/ani4020146
Kibuye FA et al (2019) Fate of pharmaceuticals in a spray-irrigation system: from wastewater to groundwater. Sci Total Environ 654:197–208. https://doi.org/10.1016/j.scitotenv.2018.10.442
Balakrishna K, Rath A, Praveenkumarreddy Y, Guruge KS, Subedi B (2017) A review of the occurrence of pharmaceuticals and personal care products in Indian water bodies. Ecotoxicol Environ Saf 137:113–120. https://doi.org/10.1016/j.ecoenv.2016.11.014
Saha S et al (2018) Assessing the suitability of sewage-sludge produced in Kolkata, India for their agricultural use. Proc Indian Natl Sci Acad 97. https://doi.org/10.16943/ptinsa/2018/49410
Singh P, Mondal T, Sharma R, Mahalakshmi N, Gupta M (2018) Poultry waste management. Int J Curr Microbiol App Sci 7(8):694–700. https://doi.org/10.20546/ijcmas.2018.708.076
Li X, Pletcher D, Walsh FC (2011) Electrodeposited lead dioxide coatings. Chem Soc Rev 40(7):3879–3894. https://doi.org/10.1039/C0CS00213E
Ambesh P, Ambesh SP (2016) Open defecation in India: a major health hazard and hurdle in infection control. J Clin Diagn Res 10(7):IL01–IL02. https://doi.org/10.7860/JCDR/2016/20723.8098
Kumar A, Pal D (2018) Antibiotic resistance and wastewater: correlation, impact and critical human health challenges. J Environ Chem Eng 6(1):52–58. https://doi.org/10.1016/j.jece.2017.11.059
Marathe NP, Pal C, Gaikwad SS, Jonsson V, Kristiansson E, Larsson DGJ (2017) Untreated urban waste contaminates Indian river sediments with resistance genes to last resort antibiotics. Water Res 124:388–397. https://doi.org/10.1016/j.watres.2017.07.060
Dhawde R, Macaden R, Saranath D, Nilgiriwala K, Ghadge A, Birdi T (2018) Antibiotic resistance characterization of environmental E. coli isolated from river Mula-Mutha, Pune District, India. Int J Environ Res Public Health 15(6). https://doi.org/10.3390/ijerph15061247
Diwan V, Tamhankar AJ, Aggarwal M, Sen S, Khandal RK, Lundborg CS (2009) Detection of antibiotics in hospital effluents in India. Curr Sci 97(12):4
Mutiyar PK, Mittal AK (2014) Occurrences and fate of selected human antibiotics in influents and effluents of sewage treatment plant and effluent-receiving river Yamuna in Delhi (India). Environ Monit Assess 186(1):541–557. https://doi.org/10.1007/s10661-013-3398-6
Divya SP, Hatha AAM (2019) Screening of tropical estuarine water in south-west coast of India reveals emergence of ARGs-harboring hypervirulent Escherichia coli of global significance. Int J Hyg Environ Health 222(2):235–248. https://doi.org/10.1016/j.ijheh.2018.11.002
Laroche E, Pawlak B, Berthe T, Skurnik D, Petit F (2009) Occurrence of antibiotic resistance and class 1, 2 and 3 integrons in Escherichia coli isolated from a densely populated estuary (seine, France). FEMS Microbiol Ecol 68(1):118–130. https://doi.org/10.1111/j.1574-6941.2009.00655.x
Dhawde R, Macaden R, Ghadge A, Birdi T (2018) Seasonal prevalence of antibiotic-resistant bacteria in the river Mula-Mutha, India. Environ Monit Assess 190(9):533. https://doi.org/10.1007/s10661-018-6911-0
Diwan V et al (2010) Antibiotics and antibiotic-resistant bacteria in waters associated with a hospital in Ujjain, India. BMC Public Health 10(1):414. https://doi.org/10.1186/1471-2458-10-414
Mohapatra S, Huang C-H, Mukherji S, Padhye LP (2016) Occurrence and fate of pharmaceuticals in WWTPs in India and comparison with a similar study in the United States. Chemosphere 159:526–535. https://doi.org/10.1016/j.chemosphere.2016.06.047
Velpandian T et al (2018) Un-segregated waste disposal: an alarming threat of antimicrobials in surface and ground water sources in Delhi. Environ Sci Pollut Res 25(29):29518–29528. https://doi.org/10.1007/s11356-018-2927-9
Gothwal R, Thatikonda S (2017) Role of environmental pollution in prevalence of antibiotic resistant bacteria in aquatic environment of river: case of Musi river, South India. Water Environ J 31(4):456–462. https://doi.org/10.1111/wej.12263
Diwan V et al (2018) Seasonal variations in water-quality, antibiotic residues, resistant bacteria and antibiotic resistance genes of escherichia coli isolates from water and sediments of the Kshipra River in Central India. Int J Environ Res Public Health 15(6):1281. https://doi.org/10.3390/ijerph15061281
Ramaswamy BR, Shanmugam G, Velu G, Rengarajan B, Larsson DGJ (2011) GC–MS analysis and ecotoxicological risk assessment of triclosan, carbamazepine and parabens in Indian rivers. J Hazard Mater 186(2):1586–1593. https://doi.org/10.1016/j.jhazmat.2010.12.037
Gao J, Pedersen JA (2005) Adsorption of sulfonamide antimicrobial agents to clay minerals. Environ Sci Technol 39(24):9509–9516. https://doi.org/10.1021/es050644c
Devarajan N et al (2016) Occurrence of antibiotic resistance genes and bacterial markers in a tropical river receiving hospital and urban wastewaters. PLoS One 11(2). https://doi.org/10.1371/journal.pone.0149211
Li D et al (2010) Antibiotic resistance characteristics of environmental bacteria from an oxytetracycline production wastewater treatment plant and the receiving river. Appl Environ Microbiol 76(11):3444–3451. https://doi.org/10.1128/AEM.02964-09
Diwan V, Chandran SP, Tamhankar AJ, Stålsby Lundborg C, Macaden R (2012) Identification of extended-spectrum β-lactamase and quinolone resistance genes in Escherichia coli isolated from hospital wastewater from Central India. J Antimicrob Chemother 67(4):857–859. https://doi.org/10.1093/jac/dkr564
Kurasam J, Sihag P, Mandal PK, Sarkar S (2018) Presence of fluoroquinolone resistance with persistent occurrence of gyrA gene mutations in a municipal wastewater treatment plant in India. Chemosphere 211:817–825. https://doi.org/10.1016/j.chemosphere.2018.08.011
Gullberg E et al (2011) Selection of resistant bacteria at very low antibiotic concentrations. PLOS Pathogens 7(7):e1002158. https://doi.org/10.1371/journal.ppat.1002158
Andersson DI, Hughes D (2012) Evolution of antibiotic resistance at non-lethal drug concentrations. Drug Resist Updat 15(3):162–172. https://doi.org/10.1016/j.drup.2012.03.005
Rizzo L et al (2013) Urban wastewater treatment plants as hotspots for antibiotic resistant bacteria and genes spread into the environment: a review. Sci Total Environ 447:345–360. https://doi.org/10.1016/j.scitotenv.2013.01.032
Singh SK, Ekka R, Mishra M, Mohapatra H (2017) Association study of multiple antibiotic resistance and virulence: a strategy to assess the extent of risk posed by bacterial population in aquatic environment. Environ Monit Assess 189(7):320. https://doi.org/10.1007/s10661-017-6005-4
Burmeister AR (2015) Horizontal gene transfer. Evol Med Public Health 2015(1):193–194. https://doi.org/10.1093/emph/eov018
Lamba M, Ahammad SZ (2017) Sewage treatment effluents in Delhi: a key contributor of β-lactam resistant bacteria and genes to the environment. Chemosphere 188:249–256. https://doi.org/10.1016/j.chemosphere.2017.08.133
Lamba M, Gupta S, Shukla R, Graham DW, Sreekrishnan TR, Ahammad SZ (2018) Carbapenem resistance exposures via wastewaters across New Delhi. Environ Int 119:302–308. https://doi.org/10.1016/j.envint.2018.07.004
Diwan V et al (2017) A three-year follow-up study of antibiotic and metal residues, antibiotic resistance and resistance genes, focusing on Kshipra – a river associated with holy religious mass-bathing in India: protocol paper. Int J Environ Res Public Health 14(6):574. https://doi.org/10.3390/ijerph14060574
Gothwal R, Shashidhar T (2017) Proliferation of ciprofloxacin resistant Bacteria in polluted sediments of Musi River, India. Soil Sediment Contam Int J 26(5):501–509. https://doi.org/10.1080/15320383.2017.1355352
Bajaj P, Singh NS, Kanaujia PK, Virdi JS (2015) Distribution and molecular characterization of genes encoding CTX-M and AmpC β-lactamases in Escherichia coli isolated from an Indian urban aquatic environment. Sci Total Environ 505:350–356. https://doi.org/10.1016/j.scitotenv.2014.09.084
Siddiqui MT, Mondal AH, Sultan I, Ali A, Haq QMR (2018) Co-occurrence of ESBLs and silver resistance determinants among bacterial isolates inhabiting polluted stretch of river Yamuna, India. Int J Environ Sci Technol 16:5611. https://doi.org/10.1007/s13762-018-1939-9
Mondal AH, Siddiqui MT, Sultan I, Haq QMR (2019) Prevalence and diversity of blaTEM, blaSHV and blaCTX-M variants among multidrug resistant Klebsiella spp. from an urban riverine environment in India. Int J Environ Health Res 29(2):117–129. https://doi.org/10.1080/09603123.2018.1515425
Skariyachan S et al (2015) Environmental monitoring of bacterial contamination and antibiotic resistance patterns of the fecal coliforms isolated from Cauvery River, a major drinking water source in Karnataka, India. Environ Monit Assess 187(5):279. https://doi.org/10.1007/s10661-015-4488-4
Mathews EB et al (2018) Occurrence and antibiotic susceptibility testing of Vibrio cholerae from district Wayanad, Kerala, India. Proc Natl Acad Sci India Sect B Biol Sci 88(2):673–678. https://doi.org/10.1007/s40011-016-0799-7
Purohit MR, Chandran S, Shah H, Diwan V, Tamhankar AJ, Stålsby Lundborg C (2017) Antibiotic resistance in an Indian rural community: a ‘one-health’ observational study on commensal coliform from humans, animals, and water. Int J Environ Res Public Health 14(4):386. https://doi.org/10.3390/ijerph14040386
Maloo A, Fulke AB, Mulani N, Sukumaran S, Ram A (2017) Pathogenic multiple antimicrobial resistant Escherichia coli serotypes in recreational waters of Mumbai, India: a potential public health risk. Environ Sci Pollut Res 24(12):11504–11517. https://doi.org/10.1007/s11356-017-8760-8
Sneha KG et al (2016) Distribution of multiple antibiotic resistant Vibrio spp across Palk Bay. Reg Stud Mar Sci 3:242–250. https://doi.org/10.1016/j.rsma.2015.11.004
Meena B et al (2015) Enterococcus species diversity and molecular characterization of biomarker genes in enterococcus faecalis in Port Blair Bay, Andaman and Nicobar Islands, India. Mar Pollut Bull 94(1):217–227. https://doi.org/10.1016/j.marpolbul.2015.02.027
Kumar S, Tripathi VR, Vikram S, Kumar B, Garg SK (2018) Characterization of MAR and heavy metal-tolerant E. coli O157:H7 in water sources: a suggestion for behavioral intervention. Environ Dev Sustain 20(6):2447–2461. https://doi.org/10.1007/s10668-017-9998-5
Mishra RK et al (2017) Bacterial diversity and antibiotic resistance in a wetland of Lakhimpur-Kheri, Uttar Pradesh, India. J Environ Biol 38(1):55–66. https://doi.org/10.22438/jeb/38/1/MS-117
Tallur PN et al (2016) Characterization of antibiotic resistant and enzyme producing bacterial strains isolated from the Arabian Sea. 3 Biotech 6(1):28. https://doi.org/10.1007/s13205-015-0332-3
Kalaiselvi K, Mangayarkarasi V, Balakrishnan D, Chitraleka V (2016) Survival of antibacterial resistance microbes in hospital-generated recycled wastewater. J Water Health 14(6):942–949. https://doi.org/10.2166/wh.2016.154
Lamba M, Graham DW, Ahammad SZ (2017) Hospital wastewater releases of Carbapenem-resistance pathogens and genes in urban India. Environ Sci Technol 51(23):13906–13912. https://doi.org/10.1021/acs.est.7b03380
Chandran SP et al (2014) Detection of carbapenem resistance genes and cephalosporin, and quinolone resistance genes along with oqxAB gene in Escherichia coli in hospital wastewater: a matter of concern. J Appl Microbiol 117(4):984–995. https://doi.org/10.1111/jam.12591
Kumar H et al (2014) Prevalence of multidrug-resistant, coagulase-positive Staphylococcus aureus in nasal carriage, food, wastewater and paper currency in Jalandhar city (north-western), an Indian state of Punjab. Environ Monit Assess 187(1):4134. https://doi.org/10.1007/s10661-014-4134-6
Tibrewal MA, Rajesh N, Rajesh V (2018) Identification and characterization of the microbial communities found in electronic industrial effluent and their potential for bioremediation. Ecotoxicol Environ Saf 164:379–387. https://doi.org/10.1016/j.ecoenv.2018.08.018
Sundaramanickam A, Suresh Kumar P, Kumaresan S, Balasubramanian T (2015) Isolation and molecular characterization of multidrug-resistant halophilic bacteria from shrimp farm effluents of Parangipettai coastal waters. Environ Sci Pollut Res 22(15):11700–11707. https://doi.org/10.1007/s11356-015-4427-5
Saha S, Saha BN, Pati S, Pal B, Hazra GC (2018) Agricultural use of sewage sludge in India: benefits and potential risk of heavy metals contamination and possible remediation options – a review. Int J Environ Technol Manag 20(3/4):183–199. https://doi.org/10.1504/IJETM.2017.089645
Mohamed Amanullah M, Sekar S, Muthukrishnan P (2010) Prospects and potential of poultry manure. Asian J Plant Sci 9(4):172–182. https://doi.org/10.3923/ajps.2010.172.182
Gupta KK, Aneja KR, Rana D (2016) Current status of cow dung as a bioresource for sustainable development. Bioresour Bioprocess 3(1):28. https://doi.org/10.1186/s40643-016-0105-9
He Y et al (2020) Antibiotic resistance genes from livestock waste: occurrence, dissemination, and treatment. NPJ Clean Water 3(1):1–11. https://doi.org/10.1038/s41545-020-0051-0
Allen HK, Donato J, Wang HH, Cloud-Hansen KA, Davies J, Handelsman J (2010) Call of the wild: antibiotic resistance genes in natural environments. Nat Rev Microbiol 8(4):251–259. https://doi.org/10.1038/nrmicro2312
Bag S et al (2018) Molecular insights into antimicrobial resistance traits of commensal human gut microbiota. Microb Ecol 77:546. https://doi.org/10.1007/s00248-018-1228-7
Lamba M, Ahammad SZ (2017) Performance comparison of secondary and tertiary treatment systems for treating antibiotic resistance. Water Res 127:172–182. https://doi.org/10.1016/j.watres.2017.10.025
Zhang X-X, Zhang T, Fang HHP (2009) Antibiotic resistance genes in water environment. Appl Microbiol Biotechnol 82(3):397–414. https://doi.org/10.1007/s00253-008-1829-z
Ahammad ZS, Sreekrishnan TR, Hands CL, Knapp CW, Graham DW (2014) Increased waterborne blaNDM-1 resistance gene abundances associated with seasonal human pilgrimages to the upper Ganges River. Environ Sci Technol 48(5):3014–3020. https://doi.org/10.1021/es405348h
Singh G, Vajpayee P, Rani N, Amoah ID, Stenström TA, Shanker R (2016) Exploring the potential reservoirs of non specific TEM beta lactamase (blaTEM) gene in the indo-Gangetic region: a risk assessment approach to predict health hazards. J Hazard Mater 314:121–128. https://doi.org/10.1016/j.jhazmat.2016.04.036
Marathe NP et al (2013) A treatment plant receiving waste water from multiple bulk drug manufacturers is a reservoir for highly multi-drug resistant integron-bearing bacteria. PLOS One 8(10):e77310. https://doi.org/10.1371/journal.pone.0077310
Bailey JK, Pinyon JL, Anantham S, Hall RM (2010) Commensal Escherichia coli of healthy humans: a reservoir for antibiotic-resistance determinants. J Med Microbiol 59(11):1331–1339. https://doi.org/10.1099/jmm.0.022475-0
Bajaj P, Kanaujia PK, Singh NS, Sharma S, Kumar S, Virdi JS (2016) Quinolone co-resistance in ESBL- or AmpC-producing Escherichia coli from an Indian urban aquatic environment and their public health implications. Environ Sci Pollut Res 23(2):1954–1959. https://doi.org/10.1007/s11356-015-5609-x
National status of waste water generation & treatment:Inventorization of Sewage Treatment Plants,Central Pollution Control Board, Ministry of Environment and Forests. http://www.sulabhenvis.nic.in/Database/STST_wastewater_2090.aspx. Accessed 17 Nov 2019
Sewage/Wastewater Treatment Technologies,National River Conservation Directorate Ministry of Jal Shakti Department of Water Resources, River Development & Ganga Rejuvenation Government of India. https://nrcd.nic.in/writereaddata/FileUpload/69307246Technologies.pdf. Accessed 5 Feb 2019
Mutiyar PK, Mittal AK (2013) Occurrences and fate of an antibiotic amoxicillin in extended aeration-based sewage treatment plant in Delhi, India: a case study of emerging pollutant. Desalin Water Treat 51(31–33):6158–6164. https://doi.org/10.1080/19443994.2013.770199
WHO, United Nations’ Children’s Fund (2015) Water, sanitation and hygiene in health care facilities Status in low- and middle-income countries and way forward. https://apps.who.int/iris/bitstream/handle/10665/154588/9789241508476_eng.pdf;jsessionid=B43B935B4DE8DF4405CF21D08970F0E1?sequence=1. Accessed 17 Nov 2019
Kümmerer K, Henninger A (2003) Promoting resistance by the emission of antibiotics from hospitals and households into effluent. Clin Microbiol Infect 9(12):1203–1214. https://doi.org/10.1111/j.1469-0691.2003.00739.x
Praveenkumarreddy Y, Akiba M, Guruge KS, Balakrishna K, Vandana KE, Kumar V (2020) Occurrence of antimicrobial-resistant Escherichia coli in sewage treatment plants of South India. J Water Sanitation Hygiene Dev 10(1):48–55. https://doi.org/10.2166/washdev.2020.051
Aggarwal A, Bhalla M, Fatima KH (2020) Detection of New Delhi metallo-beta-lactamase enzyme gene blaNDM-1 associated with the Int-1 gene in gram-negative bacteria collected from the effluent treatment plant of a tuberculosis care hospital in Delhi, India. Access Microbiol. https://doi.org/10.1099/acmi.0.000125
Gandra S, Joshi J, Trett A, Sankhil Lamkang A, Laxminarayan R (2017) Scoping report on Antimicrobial Resistance in India. Center for Disease Dynamics, Economics & Policy, Washington. https://cddep.org/wp-content/uploads/2017/11/AMR-INDIA-SCOPING-REPORT.pdf
Global Action Plan on Antimicrobial Resistance, 2014,World Health Organisation (2015). https://apps.who.int/iris/bitstream/handle/10665/193736/9789241509763_eng.pdf?sequence=1. Accessed 27 Jun 2019
National Policy for Containment of Antimicrobial Resistance in India.Ministry of Health and Family Welfare (2011). https://mohfw.gov.in/sites/default/files/3203490350abpolicy%20%281%29.pdf. Accessed 27 Jun 2019
Jaipur Declaration on Antimicrobial Resistance (2011) Ministry of health and family welfare and WHO. http://www.searo.who.int/entity/antimicrobial_resistance/rev_jaipur_declaration_2014.pdf?ua=1. Accessed 27 Jun 2019
Team C (2014) ‘Chennai declaration’: 5-year plan to tackle the challenge of anti-microbial resistance. Indian J Med Microbiol 32(3):221–228. https://doi.org/10.4103/0255-0857.129053
Antimicrobial resistance and its containment in India (2016) Ministry of health and family welfare and WHO. http://www.searo.who.int/india/topics/antimicrobial_resistance/amr_containment.pdf. Accessed 27 Jun 2019
National antimicrobial resistance research and surveillance network (AMRSN) (2015) Indian Council of Medical Research. http://pib.nic.in/newsite/PrintRelease.aspx?relid=133016. Accessed 27 Jun 2019
National Treatment Guidelines for Antimicrobial Use in Infectious Diseases (2016) National Center for Disease Control, Directorate General of Health Services. Ministry of Health & Family Welfare. http://pbhealth.gov.in/AMR_guideline7001495889.pdf. Accessed 27 Jun 2019
National Action Plan on Antimicrobial Resistance (NAP AMR 2017–2021) (2017) Ministry of Health and Family Welfare. http://www.searo.who.int/entity/antimicrobial_resistance/national-action-plans/en/. Accessed 27 Jun 2019
Delhi Declaration on Antimicrobial Resistance (2017). http://www.searo.who.int/india/topics/antimicrobial_resistance/delhi_dec_amr.pdf. Accessed 27 Jun 2019
Indian Network for Fishery and Animals Antimicrobial Resistance (INFAAR), FAO in India, Food and Agriculture Organization of the United Nations (2017). http://www.fao.org/india/news/detail-events/en/c/853974/. Accessed 27 Jun 2019
Nafade V et al (2019) Over-the-counter antibiotic dispensing by pharmacies: a standardised patient study in Udupi district, India. BMJ Global Health 4(6):e001869. https://doi.org/10.1136/bmjgh-2019-001869
Swachh Bharat Mission-Gramin, Department of Drinking Water and Sanitation, Ministry of Jal Shakti. https://sbm.gov.in/sbmReport/home.aspx. Accessed 3 May 2020
Environment Protection Amendment Rules, Ministry of Environment, Forest and Climate Change. Authority (2020) https://assets.documentcloud.org/documents/6770398/The-following-draft-of-the-notification-which.pdf. Accessed 4 May 2020
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Sasikaladevi, R., Kiruthika Eswari, V., Nambi, I.M. (2020). Antibiotic Resistance and Sanitation in India: Current Situation and Future Perspectives. In: Manaia, C., Donner, E., Vaz-Moreira, I., Hong, P. (eds) Antibiotic Resistance in the Environment . The Handbook of Environmental Chemistry, vol 91. Springer, Cham. https://doi.org/10.1007/698_2020_608
Download citation
DOI: https://doi.org/10.1007/698_2020_608
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-55064-6
Online ISBN: 978-3-030-55065-3
eBook Packages: Chemistry and Materials ScienceChemistry and Material Science (R0)