Abstract
The healing process of fractures depends on their biomechanical environment. The distinct knowledge and understanding of the biomechanical influence on fracture healing is the basis of the definition of the treatment strategy. The primary goal in treating a fracture is to achieve prompt and functional recovery of the injured limb. The healing process is based on the biological and mechanical situation of the fracture and its environment, defined by the interplay of rigidity and elasticity of fracture fixation. These mechanical properties directly influence the biological process of fracture healing. In cases of less rigid fixation, a callus formation can be observed that bridges the fracture gap. This healing process is supported by relative stability. Contrary to that, absolute stability aims to minimize callus formation leading to direct fracture healing. Stephan M. Perren summarized these observations in his strain theory. Strain is the deformation of elements within a material that leads to breakage if a certain degree is reached. With certain fixation methods, strain within the fracture gap can be modulated leading to different degrees of stability and different biological healing processes. Each treatment has its advantages and disadvantages that should be taken into consideration individually when defining fracture treatment strategies.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Strain theory
- Fracture healing
- Absolute and relative stability
- Biomechanics of fracture healing
- Perren’s strain theory
- Bone healing
Introduction
Fractured bone behaves differently in biological and mechanical environments. This fact influences the choice and method of treatment by the surgeon. Surgical procedures alter the biological environment, fixation of broken bone alters the mechanical environment; both directly contribute to the course of healing and are determined by the surgeon. The basic knowledge of biology and biomechanics of fracture healing are essential for all trauma surgeons since this knowledge defines the first treatment strategies (Table 2.1) [1].
This chapter serves as a summary for active clinicians rather than a pure scientific review. The primary goal of fracture fixation is to achieve prompt and if possible full function of the injured limb. The functional recovery does not only base on fracture healing but also its mechanics, biomechanics, and biology since these factors define a promising outcome for the patients. During fracture fixation, it is not always possible to achieve full mechanical and optimal biological environment. Strength and stiffness need to be sacrificed if the biological environment is aimed to be as optimal as possible. On the other hand, mechanical requirements may impair the biological environment. Osteosynthesis should not aim to permanently replace a broken bone; it should provide a temporary support allowing early functional rehabilitation with healing in a proper anatomical position [1,2,3,4]. Further, the type of implant influences the biomechanical environment: Steel with mechanical strength and ductility stands opposed to the electrochemical and biological inertness of titanium. With the treatment decision, the trauma surgeon determines a compromise between mechanical stability and the biological environment as well as the combination of technology and procedure best fit for the patient.
Bone
The bone supports and protects soft structures while enabling locomotion and mechanical functioning of the limbs. The mechanical characteristics of the bone are a combination of stiffness (little deformation under load) and strength (tolerate high load without failure). The characteristics of the bone are more similar to glass than to rubber [1]: The strong material breaks under very small deformation. The repeated displacement of fractured elements inhibits the bridging of the fracture gap. Relative stabile fixed fracture shifts the balance of mechanical versus biological functions more toward the biological environment. That leads to a sequence of biological events, mainly the formation of soft callus followed by hard callus. That leads to a reduction of strain and the deformation of the less stiff repair tissue. This lower-strain environment promotes the formation of bridging callus which subsequently increases the mechanical stability of the fracture. Full function is restored after the fracture is solidly bridged. Bone remodeling restores the original bone structure [5, 6].
Fracture
A fracture is an unphysiologic discontinuation of the bony integrity. It is the result of repetitive or a single overload. A traumatic fracture is always combined with a soft tissue damage to certain degree. The rapid separation of fracture surfaces creates a void (cavitation) that also contributes to soft tissue damages.
Mechanical and Biomechanical Effects
The loss of continuity results in deformation, pain, and impaired supportive function of the bone. Stabilization may restore function and relieve pain. This may lead to pain-free mobility and reduce complications such as complex regional pain syndromes.
With the disruption of the bone, arteries and veins are damaged. That leads to a spontaneous release of biochemical factors helping to induce bone healing. The fresh fractured bone has enough active and effective factors that support and coordinate fracture healing [7]. Biologically, surgical fracture stabilization should be nothing more than a guidance and a support in this healing process.
Blood Supply
The pure mechanical process of a fracture triggers biological reactions (callus formation and bone resorption). Equally to other tissue, the healing and remodeling depends on an intact blood supply. Table 2.2 summarizes factors that influence blood supply at the fracture site [1, 8,9,10,11].
Necrotic bone induces internal (Haversian) remodeling, allowing the replacement of dead osteocytes with temporary loss of strength (porosis). Large plate-bone contact areas show this effect just beneath the plates. A higher periosteal blood supply (e.g., with the help of a limited-contact dynamic compression plate [LC-DCP]), may reduce the amount of avascular bone. Injury to the bone reduces cortical circulation by nearly 50% [11] that attributes to a vasoconstriction in the periosteal but also the medullary vessels [12]. The healing process, however, increases blood flow in the adjacent soft tissue that nourishes the callus formation [13, 14]. This supports the statement that the approach to the fracture should damage and strip off as little soft tissue as possible. The essential angiogenesis with subsequent callus perfusion is elementary in the healing process of the fractured bone. This, however, depends on the method of treatment and the induced mechanical conditions (Table 2.3) [15,16,17,18,19,20,21,22].
Biology of Fracture Healing
Fracture healing is divided either into primary (direct) healing by internal remodeling or secondary (indirect) healing by callus formation. The biological process of osteonal bone remodeling is the basis of primary bone healing and occurs only with absolute stability. Relative stability, however, promotes secondary bone healing through flexible fixation methods. The biology behind secondary bone healing is similar to the process of embryological bone development including both intramembranous and endochondral bone formation [23]. This can be seen during the callus formation in the process of fracture healing of diaphyseal fractures (Table 2.4) [1, 24].
Biomechanics of Bone Healing
Methods of Fracture Stabilization
Surgeons interpret stability as the degree of displacement at the fracture site induced by load [25]. This degree defines the course of fracture healing. The stabilized fracture does not displace under physical load. The aim of fracture stabilization is to maintain the achieved reduction, restore stiffness at the fracture site, and minimize pain related to instability at the fracture site [26,27,28]. Absolute stability shifts the balance toward neutral mechanical fixation preventing callus formation. Relative stability shifts the balance toward the biological environment that stimulates secondary bone healing. This however can only occur if the motion at the fracture site is not too extensive preventing callus formation and delaying healing [29].
Conservative Fracture Treatment
This type of treatment requires closed reduction with subsequent stabilization to maintain reduction and reduce fragment motion. Indirect bone healing will be stimulated with a nearly uninterrupted biological environment. Traction or external splinting may achieve stabilization in conservative fracture treatment. A curved cast produces a straight bone, and a straight cast produces a bent bone [1, 30]. The pressure of the surrounding tissue reduces movement of the fragments.
Relative Stability
Relative stability allows movement of the bone fragments when physiological load is applied. Rigidity of the fixation decreases displacement. If a fixation method is considered flexible, it allows controlled interfragmentary movement under physiological load [2, 24, 31].
External Fixator
Usually, external fixators provide relative stability. The stiffness of fracture stabilization by external fixation depends on the type of implant (e.g., Schanz screws and bars), the geometrical arrangements of these elements related to one another and the bone (e.g., uniplanar, biplanar, circular), and the coupling of the implant to the bone (e.g., tensioned fine wires) [32]. Important factors that increase the stability of fixation include the following:
-
Stiffness of the connecting rods.
-
Short distance between the rod and the bone axis.
-
Number, spacing and diameter of the Schanz screws/wires, and their tension.
-
Unilateral external fixator combines axial, bending, and shearing displacements. The external fixator is the only system that allows the surgeon to modify flexibility by adjusting the implant without additional surgery (dynamization). External fixators provide quick and relative safe stability and are used when the biologic environment and the soft tissue situation allow little manipulation [33,34,35].
Intramedullary Nailing
The classic intramedullary nail achieves stability against bending and shear forces perpendicular to its long axis; it however is not immune to torque and is unable to prevent axial shortening. With low torsional stiffness and the loose coupling of intramedullary nail and bone, intramedullary nailing was indicated to simple transverse or short oblique fractures, which cannot shorten and will interdigitate to prevent rotation [36, 37]. The development of locked intramedullary nails and cannulated nails overcame many of these restrictions. Locked nail has the ability to tolerate torsional forces and improved axial loading [38]. The diameter of the nail defines the degree of stability additional to its geometry and the number of interlocking screws as well as their spatial arrangements. The advantages of the locked intramedullary nail come to the price of nonlinear stiffness of the nail-bone construction. To promote insertion of interlocking screws, the locking holes are larger than the diameter of the screws, allowing movement at the coupling, even with little load. Further insertion of interlocking screws may decrease motion as well as the use of angular-stable locking systems (e.g., expert tibial nail) [39, 40].
Internal Fixators and Bridging Plates
Multifragmented fractures that are stabilized with a plate in the manner of an external fixator provide elastic splinting. The dimensions of the implant as well as the number and the positions of the screws, the coupling between implants, and the implant and the bone define the stiffness of this fixation method. The indication for plating with the goal of relative stability includes multifragmentary fractures. If it is possible, plating with relative stability should be avoided for simple fractures since it increases the risk for delayed union or even nonunion [41].
Indirect Fracture Healing: Perren’s Strain Theory
Acceleration of bone healing is achieved with the stimulation of the formation of callus with interfragmentary movement [42, 43]. The maturing callus stiffens and reduces interfragmentary motion with the possibility of hard bony callus formation. In the early stages of fracture healing, the fracture tolerates greater deformation or higher tissue strain. Later stages when the callus is calcified, the tissue does not tolerate these deformations or strain forces. Figure 2.1 reveals the dynamics of Perren’s strain theory. Cell disruption occurs in small gap fractures, whereas in large gap fractures, the strain forces are distributed among cells with each cell experiencing less traction force [2].
Perren’s strain theory. Cell disruption occurs in small gap fractures, whereas in large gap fractures, the strain forces are distributed among cells, resulting in each cell experiencing less traction force. (Adapted from AO principles of fracture management [2], with permission from AO Foundation, Duebendorf, Switzerland)
Strain is defined as the deformation of material when a given force is applied. Since normal strain is the relative difference in length when a given load is applied, it has no dimension as is expressed by percentage. Before it fractures, intact bone has a normal strain tolerance of 2% before it fractures, compared to the strain tolerance of 100% in granulation tissue [29]. When the movement of fracture ends is too great, the local strain rises over tolerable limit of forming woven bone leading to impaired bridging by hard callus [44]. This leads to an increased volume of the soft callus resulting in a decreased local tissue strain to a level that allows bony bridging. This adaptive mechanism of increasing soft callus volume is impaired in considerable narrow fracture gaps with its movements occurring mostly at the gap leading to high-strain environment. The tolerance decreases after overloading the fracture with too much interfragmentary movement in the healing process [45]. Strain and fluid pressure have an inhomogeneous distribution within the callus. The applied load regulates the callus formation with biophysical stimuli that are sensed by the cells. Different signals are produced with these biophysical stimuli that alter extracellular matrix and tissue properties. After ossification of the callus, these signals reach a steady state and the original cortex regenerates. Excessive interfragmentary strain as well as too wide fracture gaps impair bridging by hard callus leading to the development of hypertrophic nonunions [46]. On the other hand, some mechanical stimulations are needed to form callus. This is impaired in low-strain environments after either too stiff fixation or too wide fracture gaps [44] resulting in delayed healing or nonunions.
Absolute Stability
The only effective method to abolish fracture movement is interfragmentary compression leading to a no-strain environment. This leads to direct bone healing. The fracture heals without visible callus formation. Osteonal remodeling is the consequence of this fixation method (compressive preload and friction).
Compressive Preload
The compressive preload prevents displacement of the fracture fragments leading to absolute stability if the compression is greater than traction produced by function. The static compression does not produce necrosis, neither in lag screws nor in compression plates, even in overloaded bone [47].
Friction
Friction counteracts shear forces that act tangentially and thus avoids sliding displacements. The amount of resistance to shear displacements depends on the compression-induced friction and the geometry of the surfaces in contact. Normal smooth bone surfaces produce less than 40% friction [47]. Rough surfaces allow firm fixation with additional counteracting displacements due to shear forces.
Lag Screws and Plates
The lag screw stabilizes fractures by compression after the approximation between the thread and the head of the screw. These forces exceed the time direct bone healing requires. Lag screws, however, provide small lever arm to resist functional loading. When viewed from the center of the screw, the area of compression is too small to withstand bending and shearing. This can be overcome with protection plates (neutralization plates) that protect the lag screws from these forces. Thus the plate protects, has ability to compress, and can be used as tension band, bridging of buttress. Simple transverse or short oblique fractures can be treated by a plate that is applied to one side of a fracture and then tensioned (excentric placement of screws). However, this method on a straight plate on a straight bone produces compression underneath the plate with slight tension on the opposite cortex leading to an instable situation. This problem can be overcome by overbending the plate to produce a small gap between the plate and the bone at the level of the fracture. If the plate is placed at the tension side of the bone, it acts as tension band and converts tension into compression at the far cortex producing absolute stability. The buttress is a construct that resists axial load by applying force at 90° to the axis of potential deformity. It is often combined with lag screws.
Direct Fracture Healing: Biomechanics
In the diaphysis, absolute stability is achieved by interfragmentary compression. Early functional treatment is possible within a few days of surgery. Radiological, only minor changes can be observed with minimal or no callus formation [48]. The gradual disappearance of the fracture sign shows progredient fracture healing, whereas a widening of this line may indicate insufficient stability. In the first days after surgery, minimal activity can be observed near the fracture site. The hematoma is resorbed/transformed into repair tissue. After few weeks Haversian systems remodel the bone [49] with simultaneous lamellar filling of fragments. In the following weeks, the osteons reach the fracture and cross it as soon as there is contact [50]. The newly formed osteons crossing the gap provide a micro-bridging or interdigitation.
Summary
Each tissue has elastic and rigid properties. The biomechanical function of tissues depends on the proportion of these properties. Within a tissue, elastic and rigid properties define the amount of stress this tissue can withstand. The bone has the ability to withstand a certain amount of stress as well as strain with reversible deformation (elastic deformation). However, when a certain point is reached (yield strength), single cells or cell compounds start to break and the deformation is irreversible (plastic deformation). If the tissue suffers more strain, after the maximum of stress it can withstand (tensile stress), the tissue will fracture.
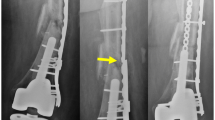
Depending on the treatment strategy, as well as the fracture pattern and properties, the degree of rigidity and elasticity within the fracture gap can be defined by the fixation method. For example, the lateral compression plate minimizes due to absolute stability within a fracture gap strain leading to minimal callus formation. Contrary, casting allows more strain leading to more callus formation due to less stable fracture fixation (Fig. 2.2). The treatment strategy depends on the fracture properties as well as the patients’ concomitant injuries. Each treatment should be evaluated and assessed individually.
(a) Stress-strain diagram with different stages of deformation. Young’s modulus is the ability of material to withstand deformation (a/b). Tensile stress is the maximal tension material can withstand. At the yield strength, elastic (reversible) deformation ends and plastic (irreversible) deformation starts. Here first fibers start to beak. (b) Different therapeutic interventions influence callus formation as well as stability as the function of strain. The most stable (rigid) fixations lead to minimal callus formation. The less flexible the fixation, the more strain is observed that leads to more callus formation. (N = Newton, m = meter, LCP = lateral compression plate, Tub plate = tubular plate, Ex. Fix. = External fixator)
References
Ito K, Perren SM. Biology and biomechanics in fracture management. In: Rüedi TP, Buckley RE, Moran CG, editors. Arbeitsgemeinschaft für Osteosynthesefragen. AO principles of fracture management. 2nd ed. Stuttgart/New York: Thieme; 2007. p. 9–31.
Stephan Perren. AO principles of fracture management. Stuttgart: Thieme; 2000.
Giannoudis P, Tzioupis C, Almalki T, Buckley R. Fracture healing in osteoporotic fractures: is it really different? A basic science perspective. Injury. 2007;38(Suppl 1):S90–9.
Jagodzinski M, Krettek C. Effect of mechanical stability on fracture healing–an update. Injury. 2007;38(Suppl 1):S3–10.
Hadjidakis DJ, Androulakis II. Bone remodeling. Ann N Y Acad Sci. 2006;1092:385–96.
Wang W, Yeung KW. Bone grafts and biomaterials substitutes for bone defect repair: a review. Bioact Mater. 2017;2(4):224–47.
Dimitriou R, Tsiridis E, Giannoudis PV. Current concepts of molecular aspects of bone healing. Injury. 2005;36(12):1392–404.
Hoppenfeld S, DeBoer P, Buckley R. Surgical exposures in orthopaedics: the anatomic approach. 4th ed. Philadelphia: Wolters Kluwer Health; 2012.
Gautier E, Cordey J, Mathys R, Rahn BA, Perren SM. Porosity and remodelling of plated bone after internal fixation: result of stress shielding or vascular damage? In: Ducheyne P, van der Perre G, Aubert AE, editors. Biomaterials and biomechanics 1983:proceedings of the fourth European conference on biomaterials. Amsterdam: Elsevier; 1984. p. 195–200.
Gautier E, Perren S, Cordey J. Effect of plate position relative to bending direction on the rigidity of a plate osteosynthesis. A theoretical analysis. Injury. 2000;31(Suppl 3):C14–20.
Grundnes O, Reikerås O. Blood flow and mechanical properties of healing bone: femoral osteotomies studied in rats. Acta Orthop Scand. 1992;63(5):487–91.
Kelly PJ, Montgomery RJ, Bronk JT. Reaction of the circulatory system to injury and regeneration. Clin Orthop Relat Res. 1990;254:275–88.
Brookes M, Revell WJ. Blood supply of bone: scientific aspects. London: Springer; 1998.
Rhinelander FW. Tibial blood supply in relation to fracture healing. Clin Orthop Relat Res. 1974;105:34-81.
Eckert-Hübner K, Claes LJ. Callus tissue differentiation and vascularization under different conditions. (Abstract from Sixth Meeting of the International Society of Fracture Repair; 23–26 Sep 1998, Strasbourg, France). J Orthop Trauma. 1999;13(4):282–3.
Kessler S, Hallfeldt K, Perren S, Schweiberer L. The effects of reaming and intramedullary nailing on fracture healing. Clin Orthop Relat Res. 1986;212:18–25.
Pfeifer R, Sellei R, Pape HC. The biology of intramedullary reaming. Injury. 2010;41(Suppl 2):S4–8.
Klein M, Rahn B, Frigg R, Kessler S, Perren SJ. Reaming versus non-reaming in medullary nailing: interference with cortical circulation of the canine tibia. Arch Orthop Trauma Surg. 1990;109(6):314–6.
Claes L, Heitemeyer U, Krischak G, Braun H, Hierholzer GJ. Fixation technique influences osteogenesis of comminuted fractures. Clin Orthop Relat Res. 1999;365:221–9.
Perren SM, Buchanan JS. Basic concepts relevant to the design and development of the Point Contact Fixator (PC-Fix). Injury. 1995;26(Suppl 2):1–4.
Tepic S, Perren SJ. The biomechanics of the PC-Fix internal fixator. Injury. 1995;26(Suppl 2):5–10.
Farouk O, Krettek C, Miclau T, Schandelmaier P, Guy P, Tscherne HJ. Minimally invasive plate osteosynthesis: does percutaneous plating disrupt femoral blood supply less than the traditional technique? J Orthop Trauma. 1999;13(6):401–6.
McKibbin B. The biology of fracture healing in long bones. J Bone Joint Surg Br. 1978;60-B(2):150–62.
Allgöwer M, Perren SM, Rüedi T. Biophysikalische Aspekte der normalen und der heilenden Knochencorticalis. Langenbecks Arch für Chirurgie. 1970;328(1–2):109–14.
Perren S. Physical and biological aspects of fracture healing with special reference to internal fixation. Clin Orthop Relat Res. 1979;138:175–96.
Perren SM, Huggler A, Russenberger M, Straumann F, Müller ME, Allgöwer M. A method of measuring the change in compression applied to living cortical bone. Acta Orthop Scand Suppl. 1969;125:7–16.
Perren S, Russenberger M, Steinemann S, Müller M, Allgöwer M. A dynamic compression plate. Acta Orthop Scand Suppl. 1969;125:31–41.
Perren SM. Optimizing the degree of fixation stability based on the strain theory. Orthopade. 2010;39(2):132–8. [Article in French].
Perren SM. Evolution of the internal fixation of long bone fractures: the scientific basis of biological internal fixation: choosing a new balance between stability and biology. Bone Joint J. 2002;84(8):1093–110.
Browner BD, Jupiter JB, Krettek C, Anderson PA. Skeletal trauma e-book: basic science management and reconstructions. 5th ed. Philadelphia: Elsevier Saunders; 2015.
Rüedi TP, Buckley RE, Moran CG; Arbeitsgemeinschaft für Osteosynthesefragen. AO principles of fracture management, 2nd ed. Vol 2 Specific fractures. Stuttgart/New York: Thieme; 2007.
Fleming B, Paley D, Kristiansen T, Pope MJ. A biomechanical analysis of the Ilizarov external fixator. Clin Orthop Relat Res. 1989;241:95–105.
Hildebrand F, Giannoudis P, Kretteck C, Pape HC. Damage control: extremities. Injury. 2004;35(7):678–89.
Pape H-C, Giannoudis P, Krettek C. The timing of fracture treatment in polytrauma patients: relevance of damage control orthopedic surgery. Am J Surg. 2002;183(6):622–9.
Pape H-C, Tornetta P, Tarkin I, Tzioupis C, Sabeson V, Olson SA. Timing of fracture fixation in multitrauma patients: the role of early total care and damage control surgery. J Am Acad Orthop Surg. 2009;17(9):541–9.
Hansen ST, Winquist RA. Closed intramedullary nailing of the femur. Küntscher technique with reaming. Clin Orthop Relat Res. 1979;138:56–61.
Bick EM. The intramedullary nailing of fractures by G. Küntscher. Translation of article in Archiv für Klinische Chirurgie, 200:443, 1940. Clin Orthop Relat Res. 1968;60:5–12.
Schandelmaier P, Krettek C, Tscherne HJ. Biomechanical study of nine different tibia locking nails. J Orthop Trauma. 1996;10(1):37–44.
Johnson KD, Tencer AF, Blumenthal S, August A, Johnston DJ. Biomechanical performance of locked intramedullary nail systems in comminuted femoral shaft fractures. Clin Orthop Relat Res. 1986;206:151–61.
Bong MR, Kummer FJ, Koval KJ, Egol KA. Intramedullary nailing of the lower extremity: biomechanics and biology. J Am Acad Orthop Res. 2007;15(2):97–106.
Piątkowski K, Piekarczyk P, Kwiatkowski K, Przybycień M, Chwedczuk BJ. Comparison of different locking plate fixation methods in distal tibia fractures. Int Orthop. 2015;39(11):2245–51.
Claes L, Augat P, Suger G, Wilke HJ. Influence of size and stability of the osteotomy gap on the success of fracture healing. J Orthop Res. 1997;15(4):577–84.
Claes LJ. Dynamisierung der Osteosynthese: Zietpunkt und Methoden. Der Unfallchirurg. Ausgabe 1/2018. Dynamisierung der Ostosynthese. 2018;121(1):3–9.
Perren SM, Cordey J. The concept of interfragmentary strain. In: Uhthoff HK, Stahl E, editors. Current concepts of internal fixation of fractures. Berlin: Springer; 1980. p. 63–77.
Goodship A, Kenwright J. The influence of induced micromovement upon the healing of experimental tibial fractures. Bone Joint J. 1985;67(4):650–5.
Schenk R, Müller J, Willenegger HJ. Experimental histological contribution to the development and treatment of pseudarthrosis. Hefte Unfallheilkd. 1968;94:15–24. [Article in German].
Perren SM, Huggler A, Russenberger M, Allgöwer M, Mathys R, Schenk R, et al. The reaction of cortical bone to compression. Acta Orthop Scand Suppl. 1969;125:19–29.
van Frank Haasnoot E, Münch TW, Matter P, Perren SM. Radiological sequences of healing in internal plates and splints of different contact surface to bone. (DCP, LC-DCP and PC-Fix). Injury. 1995;26(Suppl 2):28–36.
Schenk R, Willenegger HJ. Zum histologischen Bild der sogenannten Primärheilung der Knochenkompakta nach experimentellen Osteotomien am Hund. Experientia. 1963;19(11):593–5.
Rahn BA, Gallinaro P, Baltensperger A, Perren SM. Primary bone healing: an experimental study in the rabbit. J Bone Joint Surg Am. 1971;53(4):783–6.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Halvachizadeh, S., Pape, HC. (2020). Perren’s Strain Theory and Fracture Healing. In: Crist, B., Borrelli Jr., J., Harvey, E. (eds) Essential Biomechanics for Orthopedic Trauma. Springer, Cham. https://doi.org/10.1007/978-3-030-36990-3_2
Download citation
DOI: https://doi.org/10.1007/978-3-030-36990-3_2
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-36989-7
Online ISBN: 978-3-030-36990-3
eBook Packages: MedicineMedicine (R0)