Abstract
The process of magnesium extraction by silicothermic process is in vacuum, which leads to discontinuous production. The condensation of magnesium vapor in inert gas is an important step to realize continuous magnesium production. In this paper, the condensation behavior of magnesium vapor in inert carrier gas is studied. The effects of temperatures on the condensation phenomenon, temperature in condensation zone, direct recovery rate of condensation, and microstructure of magnesium vapor were investigated. The results show that three different condensation appearance can be obtained by magnesium condensation in argon gas conditions, and the size has significant difference, and large particles of condensed magnesium are more than 500 μm, small particles of magnesium from 50 to 100 μm and powdered magnesium less than 10 μm. With the increase of temperature, the initial condensation temperature of magnesium vapor increases from 680.2 to 745.1 °C, small particles of magnesium increases, while the powdered magnesium keeps constant; the direct recovery rate of large particles of magnesium decreases from 27.1 to 15.4%, and the direct recovery rate of condensed magnesium of small particles increases; higher purity of magnesium can be obtained at different temperatures, which can provide theoretical support for continuous magnesium production process.
Access provided by Autonomous University of Puebla. Download conference paper PDF
Similar content being viewed by others
Keywords
Introduction
As the lightest structural material in industrial applications [1, 2], magnesium is widely used in metallurgy, mechanical manufacturing, aerospace, and other fields [3]. The energy consumption and pollution have been reduced with the continuous development of magnesium production by silicothermic process [4], but it still belongs to high energy consumption metallurgical process [5]; the continuous process cannot be achieved because of the existence of vacuum.
Therefore, the new technologies and processes was put forward for the existing problems in magnesium production by experts and scholars. The kinetics of magnesium reduction by silicothermic process was studied by Fu [6,7,8,9] et al.; a new process by prefabricated pellet silicothermic process was put forward, which shortened the period of magnesium production. Tian [10,11,12,13] collected magnesium powder by carbothermal reduction at 1280 °C and found that magnesium reacted inversely with CO to form magnesium oxide. The nucleation and growth process of magnesium vapor under vacuum conditions was described by Yang [14, 15], and the effects of condensation temperature, temperature gradient, and saturated vapor pressure on the morphology of magnesium crystals were explained. Xiong [16, 17] continued to study the condensation behavior and obtained the volatilization and condensation rules of pure magnesium under vacuum conditions. The Australian CSIRO Research Institute [18] uses supersonic gas injection to rapidly cool magnesium vapor to obtain condensed magnesium powder which is not easy to explode. Other experts and scholars have studied the collection of magnesium vapor condensation [19], which provides ideas for the continuous production of magnesium extracting.
All the above studies are based on vacuum conditions. In order to achieve continuous production, magnesium vapor condensation needs to be carried out in non-vacuum conditions. So the condensation behavior of magnesium vapor in argon carrier was studied using metal magnesium as raw material in this paper. The effects of temperature in constant temperature zone of magnesium vapor on initial condensation temperature, direct recovery rate, and condensation microstructure were studied experimentally in order to understand the condensation law of magnesium vapor and provide a condensation method for continuous production of magnesium.
Experiment
Experimental Materials
Condensation experiments of pure magnesium (99.9%) were carried out to obtain the condensation law of magnesium vapor.
Experimental Method
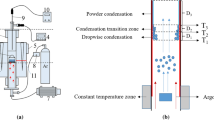
Measure the furnace temperature before placing the sample. The 15 g (±0.5 g) magnesium ingot was put into the tube furnace (in a corundum crucible) and volatilized for 2 h at 1000–1200 °C. The experimental device is shown in Fig. 1.
Pure argon (99.99%) was continuously flowed into the tube at a flow rate of 0.2 m3/h, and the magnesium vapor was fully mixed with argon and then moved to a low-temperature zone for condensation. Graphite paper (0.2 mm thick) is lined inside the tube to collect magnesium vapor condensation products, and the condensation diagram is shown in Fig. 2.
The center position of the tube furnace is 0 cm, which is the raw material placement position (that is the central position of the furnace which is the initial temperature position of the furnace temperature measurement). Magnesium vapor condenses at different positions after volatilization in argon gas flow. Large particles condense at T1–T2, small particles condense at T2–T3, and powder condense after T3 (the condensation temperature changes with different conditions). After collection, the condensate was weighed and characterized by XRD and SEM.
The formula for calculating the percentage of condensation mass is as follows:
- w i :
-
represents the mass fraction of magnesium vapor condensation in different regions,
- m i :
-
represents the mass of magnesium vapor condensation products in different regions, and
- m t :
-
represents the total mass of magnesium.
Results and Discussion
Description of Experimental Principle and Phenomena
According to Eqs. 2 and 3 [20], the relationship between saturated vapor pressure and temperature can be obtained in Fig. 3 which shows that when the temperature decreases, the saturated vapor pressure of magnesium vapor decreases gradually. When the actual partial pressure of magnesium vapor is greater than the saturated vapor pressure, the magnesium vapor begins to condense.
Different forms of condensation products appear during the evaporation condensation process of magnesium vapor in argon at 1000–1200 °C. The phenomenon of condensation products at 1100 °C is shown in Fig. 4.
Figure 4a is Mg vapor condensation in T1–T2 region, which is droplet condensation with large particles; 4 (b) is small particle condensation in T2–T3; and 4 (c) is powder condensation after T3. As can be seen from the figure, with the decrease of temperature in the condensation zone, the particle size of magnesium vapor decreases, from large droplets to small particles, and finally to powder. The reason is that the magnesium vapor moves far from the furnace center, the temperature decreases, and the magnesium vapor condenses. As the magnesium vapor continues to move, the condensation process reduces the partial pressure of magnesium vapor, the lower partial pressure leads to the direct conversion of magnesium vapor into powdered magnesium without liquefaction, so the magnesium particles size decrease gradually, which shown in Fig. 4.
Effect of Temperature in Constant Temperature Zone on Condensation Temperature of Condensation Products at Different Positions
Temperature is the main factor affecting the condensation of magnesium vapor, so the effect of temperature in constant temperature zone (1000–1200 °C) on the condensation behavior of magnesium vapor was studied experimentally. 0 cm is the central position of the furnace, and the corresponding data are the central temperature of the furnace. The temperature distribution of the tube furnace was measured which is shown in Fig. 5. The purpose of temperature measurement is to obtain the condensation temperature of magnesium vapor in different regions after obtaining the condensation products of magnesium vapor. As the distance increases, the temperature decreases.
The initial temperature of magnesium vapor condensation varies with the temperature distribution of furnace and the temperature in constant temperature zone. Figure 6 shows the variation of Mg initial temperature at different condensation zones. With the increase of temperature in constant temperature zone, the initial condensation temperature of large and small particles of condensed magnesium increases, from 680.2 °C to 745.1 °C and 599.7 °C to 659.5 °C, respectively; the initial condensation temperature of powdered condensed magnesium is maintained between 495 and 508 °C; although there is an increasing trend, it remains basically unchanged. This is because the volatilization rate of magnesium vapor increases with the increase of temperature in constant temperature zone, and the partial pressure of magnesium vapor increases at the constant flow rate of argon; after the two stages of condensation, the vapor pressure is very low, so T3 basically remains unchanged. When the temperature change in the constant temperature zone is from 1000 to 1200 °C, the initial condensation temperature of large and small particles of condensed magnesium rises 64.9 and 59.8 °C, respectively. From the relationship between saturated vapor pressure and temperature and three-phase diagram [21] of metal magnesium, it can be seen that the condensation temperature increases with the increase of saturated vapor pressure. When the vapor pressure is higher than the saturated vapor pressure, the magnesium vapor condenses. Therefore, the vapor pressure of magnesium vapor is the key factor affecting the condensation temperature, and the temperature in the constant temperature zone plays an important role in changing the vapor pressure of magnesium vapor.
Effect of Temperature in Constant Temperature Zone on the Direct Recovery Rate of Different Forms of Condensation Products
The change of temperature in the constant temperature zone not only affects the initial condensation temperature of magnesium vapor, but also affects the condensation direct recovery rate of magnesium vapor. The relationship between temperature and condensation direct recovery rate of magnesium vapor in different regions is shown in Fig. 7.
Some of the magnesium vapor is taken out of the furnace because of the condensation is in the carrier gas flow process; therefore, with the increase of temperature, the total recovery rate of magnesium is 60–67%. Magnesium vapor pressure increases at high temperature, which makes it easier to condense. With the increase of temperature, the direct recovery rate of large particles of condensed magnesium decreases from 27.1 to 15.4%, when the temperature is 1000 °C and does not reach the boiling point of magnesium, the direct recovery rate is low, the volatilization is slow, and the vapor pressure is low, so it is difficult to condensate at a constant temperature. However, the direct recovery rate of large particles of condensed magnesium decreases with the increase of temperature due to the re-evaporation of magnesium after condensation. The direct recovery rate of condensed magnesium from small particles increased from 18.7 to 26.4%.
Microstructure and Composition of Condensation Products
Figure 8 shows the microstructure and composition of magnesium vapor condensation in different regions in the flow rate of 0.2 m3/h argon at 1100 °C.
Figure 8a shows large particles of condensed magnesium with a particle size of more than 500 μm; most of them are spherical or hemispherical. Figure 8b shows a small particles of condensed magnesium with a particle size ranging from 50 to 100 μm; most of the particles are massive structure. In Fig. 8c, the particle size of powdered condensed magnesium is less than 10 μm. This figure proves that magnesium vapor with different temperatures in condensation temperature zone can collect different microstructure of magnesium. From EDS images, the purity of magnesium is higher and not oxidized. The reason for the existence of carbon element is that the magnesium metal is collected by condensation on graphite paper.
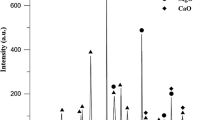
As shown in Fig. 9, X-ray diffraction analysis of three regions of large particles of condensed Mg (1), small particles of condensed Mg (2) and powder of Mg (3) at 1100 °C, the XRD diffraction peak corresponds to the characteristic peak of magnesium, which indicates that the product is pure magnesium and has good crystallization. Therefore, magnesium collected by condensation has high purity and is not oxidized in inert gas.
Conclusions
The condensation behavior of magnesium vapor was studied in inert gas at 1000–1200 °C. The effect of temperature in different constant temperature zone on condensation temperature and condensation direct recovery rate was analyzed, and the microstructure and composition of condensation products were obtained. The conclusions are as follows:
-
(1)
With the increase of the temperature in the constant temperature zone, the initial condensation temperature of large particles of magnesium increases from 680.2 to 745.1 °C and that of small particles of magnesium from 599.7 to 659.5 °C, while the powdered magnesium is kept between 485 and 508 °C, with little change.
-
(2)
With the increase of temperature, the direct recovery rate of magnesium and small particles of magnesium increases, while the direct recovery rate of large particles of magnesium decreases, and the powdered magnesium changes accordingly.
-
(3)
Three different microstructures of magnesium were obtained after magnesium condensation in argon gas flow; the results show that large particles of condensed magnesium are more than 500 μm, small particles of magnesium from 50 to 100 μm, and powdered magnesium less than 10 μm.
-
(4)
The microstructure and composition analysis showed that the condensed magnesium obtained by this method was not oxidized and has high purity, which can provide support for continuous magnesium production process.
References
Hanko G, Antrekowitsch H, Ebner P (2002) Recycling automotive magnesium scrap. JOM 54(2): 51–54.
Li R, Pan W, Sano M, et al. (2002) Kinetics of reduction of magnesia with carbon. Thermochimica Acta 390(1):145–151.
Zhu Z, Zhang L. W, Sen-Dong G. U (2012) Stress relaxation test of Hastelloy C-276 alloy and its creep constitutive equation. Chinese Journal of Nonferrous Metals 22(4):1063–1067.
Chao W, Chao Z, Shao J. Z, et al. (2015) The Effect of CaF2 on the Magnesium Production with Silicothermal Process. International Journal of Mineral Processing 142.
Wada Y, Fujii S, Suzuki E, et al. (2017) Smelting Magnesium Metal using a Microwave Pidgeon Method. Scientific Reports 7:46512.
Fu D. X, Feng N. X, Wang Y. W (2012) Study on the Kinetics and Mechanism of Grain Growth during the Thermal Decomposition of Magnesite. Bulletin of the Korean Chemical Society 33(8):2483–2488.
Fu D. X, Wang Y. W, Peng J. P, et al. (2014) Mechanism of extracting magenesium from mixture of calcined magnesite and calcined dolomite by vacuum aluminothermic reduction. Transactions of Nonferrous Metals Society of China 24(8):2677–2686.
Fu D. X, Zhang T. A, Guan L. K, et al. (2016) Magnesium Production by Silicothermic Reduction of Dolime in Pre-prepared Dolomite Pellets. JOM 68(12):3208–3213.
Fu D. X, Feng N. X, Wang Y. W, et al. (2014) Kinetics of extracting magnesium from mixture of calcined magnesite and calcined dolomite by vacuum aluminothermic reduction. Transactions of Nonferrous Metals Society of China 24(3):839–847.
Tian Y, Xu B. Q, Yang C. B, et al. (2014) Analysis of Magnesia Carbothermic Reduction Process in Vacuum. Metallurgical & Materials Transactions B 45(5):1936–1941.
Tian Y, Tao Q, Yang B, et al. (2012) Behavior Analysis of CaF2 in Magnesia Carbothermic Reduction Process in Vacuum. Metallurgical & Materials Transactions B 43(3):657–661.
Liu H, Tian Y, Yang B, et al. (2015) Condensation of Mg-vapor in vacuum carbothermic reduction of magnesia. Journal of Vacuum Science and Technology 35(7):867–871.
Tian Y, Xu B. Q, Yang C. B, et al. (2016) Study on mechanism of magnesia production by reversion reaction process in vacuum, Paper presented at the 145th TMS Annual Meeting, Nashville, TN, 14–18 February 14 2016.
Yang C. B, Yang T, Tao Q. U, et al. (2014) Magnesium vapor nucleation in phase transitions and condensation under vacuum conditions. Transactions of Nonferrous Metals Society of China 24(2):561–569.
Yang C. B, Tian Y, Qu T, et al. (2014) Analysis of the behavior of magnesium and CO vapor in the carbothermic reduction of magnesia in a vacuum. Journal of Magnesium and Alloys 2(1):50–58.
Xiong N, Tian Y, Yang B, et al. (2018) Volatilization and condensation behaviours of Mg under vacuum. Vacuum 156:463–468.
Xiong N, Tian Y, Yang B, et al. (2019) Results of recent investigations of magnesia carbothermal reduction in vacuum. Vacuum 160:213–225.
Brooks G, Trang S, Witt P, et al. (2006) The carbothermic route to magnesium. JOM 58(5):51–55.
Bin G. Y, Wang Y, Wang S. Y, et al. (2015) Effect of Argon Flow Rate on the Condensation of Magnesium Vapor from Carbothermic Reduction of Magnesia. Magnesium Technology:61–65.
Dai Y. N, Yang B (2000) The vacuum metallurgy of nonferrous metals. Metallurgical Industry Press, Beijing.
Minevich E, Moayed A, Wacksman J, et al. (1956) Principles of physical metallurgy. D.VAN nostrand company, New York.
Acknowledgements
This work was supported by National Natural Science Foundation of China under Grants (51504058; U1508217; 51404054; 51374064), the Fundamental Research Funds for the Central Universities of China (N162504003, N140204013), and the Fund of Liaoning S&T Project (201601003, LZ2014021).
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 The Minerals, Metals & Materials Society
About this paper
Cite this paper
Han, J., Zhang, T., Fu, D., Guo, J., Ji, Z., Dou, Z. (2020). Effect of Temperature on Magnesium Vapor Condensation in Inert Carrier Gas. In: Jordon, J., Miller, V., Joshi, V., Neelameggham, N. (eds) Magnesium Technology 2020. The Minerals, Metals & Materials Series. Springer, Cham. https://doi.org/10.1007/978-3-030-36647-6_47
Download citation
DOI: https://doi.org/10.1007/978-3-030-36647-6_47
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-36646-9
Online ISBN: 978-3-030-36647-6
eBook Packages: Chemistry and Materials ScienceChemistry and Material Science (R0)