Abstract
The major challenges encountered in ammonia production are the surface adsorbates and catalyst structures under conditions significant for ammonia synthesis. The discovery of scalable, active, and long-lived catalysts plays an important role in producing sustainable ammonia synthesis. Other factors that influence the ammonia production are the development of low-pressure and -temperature processes and the development of photochemical and electrochemical routes for N2 reduction based on homogeneous (molecular) and heterogeneous catalysis. In this chapter, the favouring conditions for low-pressure ammonia production are reviewed and discussed in detail. Approaches to be adapted to achieve the improved yield, increase N2/H2 conversion to ammonia, lowering the capital costs, and consumption of less energy were discussed.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
Ammonia (NH3) is the highest contributing factor in chemical industries producing more than 140 million tons of nitrogen. The main usage of NH3 is in fertilizer industry (>75%), besides it is also extensively used as a refrigerant gas, production of textiles, plastics, pesticides, and other significant chemicals. It is also used as a cleaning agent in household and industries. Even though ammonia production exists for many years, there are problems associated with its production in terms of manufacturing and instrumentation issues. Due to that, it poses a severe threat to mankind and the environment [1].
Ammonia is the main component in the chemical industry. Known and accepted innovation of the twentieth century is the production of NH3 by Haber–Bosch process [2]. More than 50% requirement of nitrogen for human use is met by ammonia production processes. Industrial production of NH3-based fertilizers is met by this process and is operated at high temperature and pressure. The information represented in Fig. 1 demonstrates how the production of NH3 correlates with the population growth during the period 1900–2015. This clearly indicates that, the NH3 generation is increased many folds to meet the demand.
An acceptable solution as a substitute of carbon is ammonia production based on a biological one. It is not possible that the existing process can be replaced by more energy efficient biological process, but renewable sources like wind or solar may give energy. If on-demand fertilizer production near the farm can be practiced, it will help to solve problems of runoffs, nitrogen pollution related to the fertilizer industry (Fig. 2) [3].
Schematic diagram showing the losses and overall efficacy of nitrogen produced, delivered and uptake by carnivorous and vegetarian diets [3]
The capital expenditure, energy consumption, and environmental impact of this well-known process have encouraged the researchers to develop a renewable and affordable process under mild conditions [4]. At high temperature and pressure, ammonia synthesis is feasible for market and also energy producing. High pressure and fast kinetics inhibit the production of the reverse reaction. Ammonia is a synthetic nitrogen fertilizer [5]. Without ammonia (fertilizer) which is hydrogen carrier and carbon-neutral liquid fuel, the availability of food for 50% population would have been a great challenge. The necessity of new catalyst is highly required as now manufacturing process for ammonia is known though high temperature and pressure are still challenge for chemical industry to solve [6, 7].
Plant life is supported mainly by ammonia, which is used for the production of protein and is also used as cleaners (glass and window, toilet bowl, waxes, oven, and drain—mainly diluted ammonium hydroxide) and in preparation of environmental impacting things like plastic, dyes, drugs, explosives, HNO3, and NH4OH. Aquatic animals generate ammonia, available in dissolved form in water bodies at or near the waste site. At hazardous waste sites (attached with soil particles), ammonia gets converted into vapor without any residuals. The nitrogen cycle is balanced from animals and plants decomposition, and by excreta of animals, ammonia is returned to the aquatic system. It is one of the important pollutants to be taken care of as a toxic, lower rate of growth, or reproduction. The production of renewable NH3 is two to three times costly than usual [8].
In this chapter, the issues faced in the production of ammonia are thoroughly discussed as well as difficulties faced in various stages of production. The Haber–Bosch process is a widely accepted method but the limitations/difficulties in this process are reviewed. The various possible alternatives for ammonia synthesis and challenges in these processes are discussed.
2 The Haber–Bosch Process
Till the twentieth century, this process was mainly used for development of production [9]. At temperature (400 ℃) and pressure (>100 bars), the pure N2 reacts with H2 over iron catalyst takes place.
The reaction is thermodynamically favored at lower reaction temperatures. This methods develops a more active catalyst allowing a reduction in stringent conditions. Production of NH3 by this process meets 1 to 2% of total energy demand world while [10, 11]. Economically beneficial and environmentally friendly methodology is much awaited. If more active catalyst that be operated at low pressure and temperature, with necessary kinetics which can produce equilibrium yield. It is considered as a great challenge to find a method for lower temperature and pressure. Whereas, N2 reduction to NH3 is performed at high temperature and pressure [12].
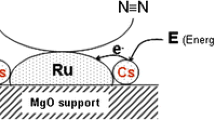
Catalysts that can replace the current one in Haber–Bosch process must be able to perform well under lower temperature and pressure condition. Ruthenium based catalyst have been identified to be a good catalyst [13,14,15,16]. Increasing temperature and decreasing pressure improved the hydrocarbons conversion and H2/CO ratio [17].
3 Manufacturing of Ammonia
Ammonia is produced on a larger scale with steam reforming on natural gas. Till date, major reasons reported are reforming, and synthesis loop. Failures and technical issues do occur frequently in the NH3 plant even after following all the standards of safety and risk assessment, which largely impacts the environment and thus market [1].
3.1 Ammonia Production
Production of ammonia is in practice by natural gas or naphtha. The four stages of ammonia production are shown in Fig. 3.
3.2 Stage 1: Catalytic Reforming
3.2.1 Desulphurization and Primary Reforming
-
Steam reforming produces synthesis gas.
-
Removal of sulfurous compounds with an increase in temperature at 400 ℃.
-
Reaction: \({\text{ZnO}} + {\text{H}}_{2} {\text{S}} \to {\text{ZnS}} + {\text{H}}_{2} {\text{O}}\).
-
Passing natural gas to the primary reformer.
-
Fed the steam with very high temperature into reformer with methane with required temperature tubes containing catalyst nickel.
-
Tubes are heated externally due to the combustion of fuel to approximately 770 ℃. CO/CO2 is formed by CH4 in the presence of catalysts H. Converted gas is synthesis gas. The range of operating pressure is more than 25 bars.
-
Process gas mixture is to be sent to the auto-thermal reformer. Outlet of reformer contains methane 6 mol%; carbon oxides 8%; carbon dioxides 6%; H2 50%; and water molecules 30%.
Dai [18] studied the prediction for numbers of years of usage of the material for tubes (which are under pressure) used in the manufacturing of ammonia at very high temperature greater than 800 ℃. With heat treatment, the life of such tubes increased to more than double the life expected. But, due to the increase in temperature, reformer furnace can explode, which is also a failure of stress corrosion and welding [19]. Ray et al. [20] investigated failure study of tubes due to high temperature. Swaminathan et al. [21] concluded that due to changes in the design of pigtail, the end of the pipe in ammonia production plant has led to shortening of pipe and added stress to it. So the possibility of expansion of tubes decreased. It led to failure of tubes in ammonia plant over the years resulting incidents like fire [22].
3.2.2 Secondary Reformer
For entering into the secondary reformer, the produced gas is cooled up to 750 ℃ and gets mix with air for supplying the required quantity of N2 for the process of synthesis gas. The reaction between methane and oxygen is highly exothermi, which favors the production of more hydrogen. The main chemical reactions that take place in the process are:
-
$$2{\text{O}}_{2} + {\text{CH}}_{4} \to 2{\text{H}}_{2} {\text{O}} + {\text{CO}}_{2}$$
-
$${\text{O}}_{2} + {\text{CH}}_{4} \to {\text{CO}}_{2} + 2{\text{H}}_{2}$$
-
$${\text{O}}_{2} + 2{\text{CH}}_{4} \to 2{\text{CO}}_{2} + 4{\text{H}}_{2}$$
-
$${\text{CO}}_{2} + {\text{H}}_{2} {\text{O}} \to {\text{CO}}_{2} + {\text{H}}_{2}$$
Heat is required in the secondary reformer in the air mixing process. Water, CO2, CO, CH4, and catalysts are required to be removed from the gas stream. Iron oxidation can be prevented by the removal of gas from the stream which may occur in shift conversion, CO2 removal, and methanation. Metal dusting possibility will be high due to the increase in the rate of carburization. Temperature and gas composition falls in the range of carburization; metallic components may be degraded in parts of reforming [23].
In newly developed techniques, the fire tube furnace can be replaced by heat exchanger reformer, so the integration of energy is of a good level [24]. Singh et al. [25] explained the study of fire in the plant of ammonia production. The pressure shell of interconnecting pipe of reformers was the responsible factor for the same. The accident stopped the plant for 15 days, and a detailed study was done for ruptured pipe and failure. Due to short-term high-temperature stress rupture, local damage took place, which led to failure. The possibility of ignorance of voids and cracks was also explained. Repair work in boiler and nozzle area gave a shock of failure of refractory. Jahromi et al. [26] presented a case study of downstream of secondary reformer failure of old waste boiler tubes.
3.3 Stage 2: Synthesis Gas Purification
3.3.1 Shift Conversion
In stage 2, the reformer will be followed by water gas shift reactors for converting CO to CO2, which can be used in the synthesis of urea. To get comfortable equilibrium in water gas shift, iron oxide at 400 ℃ is followed by 200 ℃ on a copper-based catalyst.
3.3.2 CO2 Removal
Purification of synthesis gas is taken care by removal of CO2 (reduction to 5–10 ppm) by absorption. Absorption is with hot K2CO3, Selexol (most commonly used), or MEA [27]. Simple flashing can regenerate with lower consumption of energy. Pure carbon dioxide is sent to urea plant for compression after removing water. Carbon steel walls are corroded by CO2, which also has a solution for processes. The major reason of corrosion is given in Fig. 4.
Harjac et al. [28] explained the main reasons that caused accidents/incidents of corrosion in hot K2CO3 acid gas removal plant. Pande et al. [29] presented a case study that hydrogen presence can cause destruction of pipeline, while the transfer of pipeline for CO2 from ammonia plant to urea plane.
3.3.3 Methanation
Pretreatment of removal of carbon gases is performed using methanator. This is purifying process before it enters to ammonia converter. In the design of tubes, factors to be considered are balancing heat transfer, optimization of catalyst volume, and minimization of thermal volume. In a conventional plant of ammonia production, these factors are major issues. Temperature increase or decrease may result in more leakage of carbon monoxide from shift converter, which passes from a unit of absorption, thus increases the temperature in methanator [30].
3.4 Stage 3: Compression Process
After the removal of condensed water, the mixture of gas is cooled and compressed in the centrifugal compressor at a pressure of 150–300 bar with decrease in temperature. They are operated by steam turbines with the use of self-generated steam, which is also significant approach for reduction of energy usage in the plant. Krivshich et al. [31] discussed the possibility of maximizing the reliability of compressors with dry gas seal (DGS) systems.
3.5 Stage 4: Synthesis Loop
After cooling the mixture of gas, removal of ammonia (liquid form) from separator takes place. Rapid decompression of ammonia causes production of byproducts like CH4 and H2. The gas mixture over the liquid NH3 is removed and sent back to the recovery unit. The system uses water as a solvent, and the gases remained are used as fuel in the primary reformer. Majority of ammonia is mostly used for production of urea and for storing. Metals and activated carbon exhibit high efficiency, and compounds poison the catalysts in further reactions. Anwar et al. [32] discussed the failure of synthesis loop in heat exchanger, describing the possibilities of hydrogen attack combined with fatigue loading. Shah et al. [33] gave useful designs for ammonia and urea plant for ribbon wound vessels.
3.6 Accidental Releases of Ammonia
-
Toxicity of Ammonia
Ammonia has toxicity, used on a larger scale; lower molecular weight makes it possible to form denser, so ammonia can control the hazard as it has such an important property to control it [34].
-
Dispersion into atmosphere
Preferable storage of NH3 is in iron or steel container as it can be corrosive to metals. There is possibility of flash fire, if it is stored as a liquefied gas. Depending on temperature conditions, it can be less dense and forms clouds.
-
Other Important Studies
Khan et al. [35] presented a case study of the analysis of the failure of ammonia by fitting Weibull distributions and simulation modeling technique. With the use of existing plant configurations, they estimated the availability of plant and improvement possibility by modification or change of strategy.
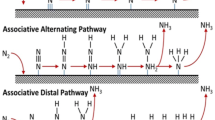
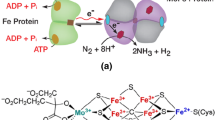
4 Electron/Proton Driven Processes for NH3 Production
4.1 Photocatalysis and Electrocatalysis
Figure 5 depicts the production of NH3 with N2RR catalysts (a direct electrocatalytic or photocatalytic) by oxidizing H2O to release electrons and protons for the N2RR in production of ammonia.
The overall reaction is \(2{\text{N}}_{2} + 6{\text{H}}_{2} {\text{O}} \to 4{\text{NH}}_{3} + 3{\text{O}}_{2}\).
A thermally catalyzed NH3 production process is coupled with electrocatalysis or photocatalysis to produce renewable H2 (See Fig. 6). Here, the reactions taking place are
In existing conventional process with the replacement of H2, which is normally derived by renewable sources. It can reduce emissions of CO2 [36,37,38,39].
4.2 Electrochemical NH3 Synthesis
With less efficiency, N2 is reduced to NH3. It is a challenge to stop contamination of ammonia with other sources. Till date, the literature supports ammonia production with contamination. To resolve the stated problem, ammonia is synthesized in the presence of argon.
4.3 Photocatalytic NH3 Synthesis
The major challenge in this process is availability of high-energy UV light requirement for reduction of nitrogen. The use of more efficient catalysts can subside the issues and enhance the production process. For a photo-electrochemical characterization, proper catalysts and more feasibility studies are required.
4.4 Electrocatalytic Oxygen Evolution Reaction
To meet the deficiency of kinetic losses, higher potentials are required in spite of catalytic materials. This is a significant challenge to overcome, and new catalysts that are able to reduce the scaling are yet awaited.
4.5 Electrocatalytic Hydrogen Evolution Reaction
Compared to NH3, one hydrogen evolution reaction (HER) method is also practiced for aqueous electrochemical systems. It controls the catalytic surface by promoting the reduction of dinitrogen. Mitigation of surface protons, while reduction of N2 and H2O in the synthesis of NH3, is an unsolved challenge to be addressed. The economy of HER can give clean H2 stream with reduction of carbon-based fuels as the hydrogen source. At lower temperature and pressure, thermal catalyst can reduce the effect on the environment by the process of ammonia production. Platinum is active for acidic or alkaline media, but comes with higher cost. Chances of improvement in acidic and alkaline media are higher in case of catalyst based on non-precious metal. Its performance is good per unit area for activities of best electrodes having a high capacity of loading. Higher magnitude than non-precious catalyst has effect for both the photocatalytic and electrocatalytic processes. In the first process, incoming light can be obstructed by high loading and in and out the transportation of water and gas can face issues which are of concern be operated in the absence of light. For both media, non-precious HER catalysts development is a challenge [40,41,42,43,44,45].
5 Grand Challenges
In identifying the optimal ammonia production process that can take place at lower temperature and pressure, there are many challenges that need to be addressed:
-
i.
Discovery of scalable, active, and long-lived catalysts for sustainable NH3 synthesis. Homogeneous redox/electrochemical/petrochemical surface processes can meet few requirements, but there is no catalyst that can fulfill all requirements.
-
ii.
Enhancement in photochemical and electrochemical approaches for N2 reduction via electron and proton transfer.
-
iii.
Development of thermal process which can take place at low pressure (<10 atm) and temperature (<200 ℃).
-
iv.
Owing the possibility of H2 production through photo-electrochemical or electrolysis process, it is necessary to find substitute catalyst that can enhance the process, which can operate at lower temperature. Such a catalyst can develop low-pressure process compatible for small-scale production.
-
v.
Enhancement in biochemical routes to N2 reduction. For sustainable energy into biological process require possible options for enzymes, including nitrogenase, on electrode surfaces.
-
vi.
Development of solar-based thermochemical methods. Nitrogen activation should be enhanced using photons generated by solar thermal process via the metal nitridation/reduction cycles.
-
vii.
Characterization of catalyst structures (chemical, physical, and electronic) and surface adsorbents for favorable ammonia synthesis. Catalyst structures can vary in terms of physical, chemical as well as electronic. The characterization of active catalyst needs to be thoroughly analyzed through numerous experimental studies.
-
viii
Process integration and optimization of catalyst design. This integration provides a breakthrough in catalyst design and provides insights in homogeneous and heterogeneous enzyme catalysis. This approach can be a test bed for more integrated approaches for controlled catalyst synthesis.
6 Critical Steps of the Reaction-Absorption Process
Absorbents at high temperature and presence of ammonia leads to hard solid structure. No impurity should be allowed. The pressure at each stage of the experiment should be checked to confirm proper operation of the recirculation cycle. Recycle of unreacted gases is presented in Fig. 7, which expresses the relationship between pressure change and pump flow. Small reciprocals are in proportion to large chemical reactions. Rate is more at a higher flow of the pump. This limit is near to the fastest reaction, and slope line indicates recycling effects [46,47,48]. The experiment confirmed that a lower pressure reaction is feasible for ammonia production. In this study, 80% conversion with fast ammonia synthesis rates are obtained. At lower pressure of 25 bars, higher level of production is possible with removal of ammonia. Characteristics of this process are reaction time, separation time, and recycle. The efficiency of the plant is controlled by the chemical reaction rate which is proportional to the time of reaction.
7 Conclusions
Low-pressure process is proposed in which N2 is produced from air using pressure swing adsorption, and H2 is produced by electrolysis of water, which reacts around 400 ℃ with catalyst. A thermally catalyzed NH3 production process is coupled with electrocatalysis or photocatalysis to produce renewable H2. Solar thermochemical looping is seen as a possible option for sustainable ammonia production, avoiding fossil CO2 emissions. The studies indicate that the viability of the absorption reaction process for the enhanced production of NH3 takes place at considerably lower pressures.
References
Kletz TA, Amyotte P (2010) Process plants: a handbook for inherently safer design.CRC Press
Logadottir A, Rod TH, Nørskov JK, Hammer B, Dahl S, Jacobsen C (2001) The Brønsted–Evans–Polanyi relation and the volcano plot for ammonia synthesis over transition metal catalysts. J Catal 197(2):229–231
Galloway JN, Cowling EB (2002) Reactive nitrogen and the world: 200 years of change. AMBIO: A J Human Environ 31(2):64–72
Deng J, Iñiguez JA, Liu C (2018) Electrocatalytic nitrogen reduction at low temperature. Joule 2(5):846–856
Kandemir T, Schuster ME, Senyshyn A, Behrens M, Schlogl R (2013) The Haber-Bosch process revisited: on the real structure and stability of “ammonia iron” under working conditions. Angew Chem Int Ed Engl 52(48):12723–12726
Ertl G (2008) Reactions at surfaces: from atoms to complexity (Nobel Lecture). Angew Chem Int Ed 47(19):3524–3535
Chorkendorff I, Niemantsverdriet JW (2003) Concepts of modern catalysis and kinetics, vol 138. Wiley Online Library
Sanchez A, Martin M (2018) Optimal renewable production of ammonia from water and air. J Clean Prod 178:325–342
Smil V (2002) Nitrogen and food production: proteins for human diets. AMBIO: A J Human Environ 31(2):126–132
Tanabe Y, Nishibayashi Y (2013) Developing more sustainable processes for ammonia synthesis. Coord Chem Rev 257(17–18):2551–2564
Bielawa H, Hinrichsen O, Birkner A, Muhler M (2001) The ammonia-synthesis catalyst of the next generation: barium-promoted oxide-supported ruthenium. Angew Chem Int Ed 40(6):1061–1063
Singh AR, Rohr BA, Schwalbe JA, Cargnello M, Chan K, Jaramillo TF, Chorkendorff I, Nørskov JK (2016) Electrochemical ammonia synthesis. The selectivity challenge. ACS Publications
Honkala K, Hellman A, Remediakis I, Logadottir A, Carlsson A, Dahl S, Christensen CH, Nørskov JK (2005) Ammonia synthesis from first-principles calculations. Science 307(5709):555–558
Milton RD, Cai R, Abdellaoui S, Leech D, De Lacey AL, Pita M, Minteer SD (2017) Bioelectrochemical Haber-Bosch Process: an ammonia-producing H2/N2 fuel cell. Angew Chem Int Ed 56(10):2680–2683
Kozuch S, Shaik S (2008) Kinetic-quantum chemical model for catalytic cycles: the Haber-Bosch process and the effect of reagent concentration. J Phys Chem A 112(26):6032–6041
Cherkasov N, Ibhadon A, Fitzpatrick P (2015) A review of the existing and alternative methods for greener nitrogen fixation. Chem Eng Process 90:24–33
Damanabi AT, Servatan M, Mazinani S, Olabi AG, Zhang Z (2019) Potential of tri-reforming process and membrane technology for improving ammonia production and CO2 reduction. Sci Total Environ 664:567–575
Dai S-H (1996) A study on the prediction of remaining life and ageing of material for pressurized tubes of industrial furnace operated at elevated temperature. Int J Press Vessels Pip 69(3):247–252
Bhaumik S, Rangaraju R, Parameswara M, Bhaskaran T, Venkataswamy M, Raghuram A, Krishnan R (2002) Failure of reformer tube of an ammonia plant. Eng Fail Anal 9(5):553–561
Ray AK, Sinha SK, Tiwari YN, Swaminathan J, Das G, Chaudhuri S, Singh R (2003) Analysis of failed reformer tubes. Eng Fail Anal 10(3):351–362
Swaminathan J, Guguloth K, Gunjan M, Roy P, Ghosh R (2008) Failure analysis and remaining life assessment of service exposed primary reformer heater tubes. Eng Fail Anal 15(4):311–331
Kletz T (2009) What went wrong?: case histories of process plant disasters and how they could have been avoided. Butterworth-Heinemann
Holland M, De Bruyn H (1996) Metal dusting failures in methane reforming plant. Int J Press Vessels Pip 66(1–3):125–133
Bharadwaj S, Schmidt L (1995) Catalytic partial oxidation of natural gas to syngas. Fuel Process Technol 42(2–3):109–127
Singh J, Basu P, Rao B (2002) Fire in secondary reformer outlet line to waste heat boiler. Process Saf Prog 21(3):205–211
Jahromi S, AliPour M, Beirami A (2003) Failure analysis of 101-C ammonia plant heat exchanger. Eng Fail Anal 10(4):405–421
Kunjunny A, Patel M, Nath N (1999) Revamping of CO ~ 2 removal section in ammonia plant at IFFCO Kalol. Fertiliser News 44:53–58
Harjac S, Atrens A, Moss C (2008) Six Sigma review of root causes of corrosion incidents in hot potassium carbonate acid gas removal plant. Eng Fail Anal 15(5):480–496
Pande JO, Tonheim J (2001) Ammonia plant NII: Explosion of hydrogen in a pipeline for CO2. Process Saf Prog 20(1):37–39
Alhabdan F, Elnashaie S (1995) Simulation of an ammonia plant accident using rigorous heterogeneous models: Effect of shift converters disturbances on the methanator. Math Comput Model 21(4):85–106
Krivshich N, Pavlyuk S, Deineka A, Kolesnik S (2003) Introduction and use of dry gas seal systems on ammonia plant compressors. Chem Pet Eng 39(9):608–611
Anwar Z, Ahmed S, Kirmani IA (1997) Expansion bellows failure of synthesis loop hot heat exchanger. Process Saf Prog 16(2):105–109
Shah M, Zhu G (1998) Burst resistant ribbon wound pressure vessels for ammonia plants. Process Saf Prog 17(2):98–103
HOLNESS DL, PURDHAM JT, NETHERCOTT JR (1989) Acute and chronic respiratory effects of occupational exposure to ammonia. Am Ind Hyg Assoc J 50(12):646–650
Khan MR, Kabir AZ (1995) Availability simulation of an ammonia plant. Reliab Eng Syst Saf 48(3):217–227
Walter MG, Warren EL, McKone JR, Boettcher SW, Mi Q, Santori EA, Lewis NS (2010) Solar water splitting cells. Chem Rev 110(11):6446–6473
McCrory CC, Jung S, Peters JC, Jaramillo TF (2013) Benchmarking heterogeneous electrocatalysts for the oxygen evolution reaction. J Am Chem Soc 135(45):16977–16987
Suntivich J, May KJ, Gasteiger HA, Goodenough JB, Shao-Horn Y (2011) A perovskite oxide optimized for oxygen evolution catalysis from molecular orbital principles. Science 334(6061):1383–1385
Callejas JF, McEnaney JM, Read CG, Crompton JC, Biacchi AJ, Popczun EJ, Gordon TR, Lewis NS, Schaak RE (2014) Electrocatalytic and photocatalytic hydrogen production from acidic and neutral-pH aqueous solutions using iron phosphide nanoparticles. ACS Nano 8(11):11101–11107
McKone JR, Sadtler BF, Werlang CA, Lewis NS, Gray HB (2013) Ni–Mo nanopowders for efficient electrochemical hydrogen evolution. ACS Catal 3(2):166–169
McEnaney JM, Soucy TL, Hodges JM, Callejas JF, Mondschein JS, Schaak RE (2016) Colloidally-synthesized cobalt molybdenum nanoparticles as active and stable electrocatalysts for the hydrogen evolution reaction under alkaline conditions. J Mater Chem A 4(8):3077–3081
McCrory CCL, Jung S, Ferrer IM, Chatman SM, Peters JC, Jaramillo TF (2015) Benchmarking hydrogen evolving reaction and oxygen evolving reaction electrocatalysts for solar water splitting devices. J Am Chem Soc 137(13):4347–4357
Lu Q, Hutchings GS, Yu W, Zhou Y, Forest RV, Tao R, Rosen J, Yonemoto BT, Cao Z, Zheng H, Xiao JQ, Jiao F, Chen JG (2015) Highly porous non-precious bimetallic electrocatalysts for efficient hydrogen evolution. Nat Commun 6(1)
Kibsgaard J, Chen Z, Reinecke BN, Jaramillo TF (2012) Engineering the surface structure of MoS2 to preferentially expose active edge sites for electrocatalysis. Nat Mater 11(11):963–969
Hsu C-L, Chang Y-H, Chen T-Y, Tseng C-C, Wei K-H, Li L-J (2014) Enhancing the electrocatalytic water splitting efficiency for amorphous MoSx. Int J Hydrogen Energy 39(10):4788–4793
Jung J, Jeong YS, Lim Y, Lee CS, Han C (2013) Advanced CO2 capture process using MEA scrubbing: Configuration of a split flow and phase separation heat exchanger. Energy Procedia 37:1778–1784
Dave N, Do T, Puxty G, Rowland R, Feron PHM, Attalla MI (2009) CO2 capture by aqueous amines and aqueous ammonia–a comparison. Energy Procedia 1(1):949–954
Ciferno JP, DiPietro P, Tarka T (2005) An economic scoping study for CO2 capture using aqueous ammonia. Final report, vol 28
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Mehta, K.P., Karri, R.R., Mubarak, N.M. (2020). Low-Pressure Ammonia Production. In: Inamuddin, Boddula, R., Asiri, A. (eds) Sustainable Ammonia Production. Green Energy and Technology. Springer, Cham. https://doi.org/10.1007/978-3-030-35106-9_7
Download citation
DOI: https://doi.org/10.1007/978-3-030-35106-9_7
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-35105-2
Online ISBN: 978-3-030-35106-9
eBook Packages: EnergyEnergy (R0)